Abstract
The SARS-CoV-2/COVID-19 pandemic has spread across the globe and affected millions of individuals as of the efficient virus transmission potential mediated via multiple virus shedding routes. The presence of SARS-CoV-2 in the stool samples and its prolonged shedding in environmental compartments like sewage and wastewater signifies a potential threat adding to the transmission cycle of this novel virus. The potential role played by the asymptomatic COVID-19 patients in transmitting the disease via the fecal-oral route is now under investigation. Hence, in the present scenario, wastewater-based epidemiology, and sewage surveillance may provide valuable insights into the prevalence of SARS-CoV-2 among the human population and could serve as a sensitive surveillance system and a crucial early warning tool. Further studies are required to determine the survival of SARS-CoV-2 in the environment, transmissibility through wastewater, and the potential to infect humans via the fecal-oral route. Appropriate frameworks with regards to evaluation and analysis of SARS-CoV-2 will help implement appropriate intervention strategies and necessary sanitation practices to ensure virus free clean water supply to have a check on the further spread of this pandemic virus.
Keywords: COVID-19, SARS-CoV-2, Sewage, Wastewater, Public health, Surveillance
Graphical abstract
Highlights
-
•
The SARS-CoV-2 RNA has been detected from the sewage and wastewater.
-
•
Sewage surveillance and wastewater testing for SARS-CoV-2 could serve as a sensitive surveillance system and early warning tool.
-
•
Novel computational surveillance for analyzing wastewater based epidemiology (WBE) is being explored.
-
•
The survivability, transmissibility and infectivity of SARS-CoV-2 in sewage are vital issues and need to be addressed properly.
-
•
Ample wastewater/sewage treatment protocols and sanitation practices must be adopted to curb this pandemic virus.
The ongoing coronavirus disease 2019 (COVID-19) pandemic that began in December 2019 is caused by a novel coronavirus identified as severe acute respiratory syndrome virus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) (Gorbalenya et al., 2020). The Huanan seafood market in Wuhan, China, was primarily believed to be the origin of this virus (Chan et al., 2020). The complex transmission mechanisms and non-availability of specific targeted drugs and diagnostics led to the worldwide spread of this virus (Dhama et al., 2020), affecting more than 215 countries and territories with more than 8 million laboratory-confirmed cases and approximately 0.4 million deaths. Most of the SARS-CoV-2 infections are mild but some progress to severe clinical manifestations with acute respiratory distress syndrome (ARDS) (Harapan et al., 2020; Rodriguez-Morales et al., 2020) and the disease progression, in part, may be associated with immune responses (Keam et al., 2020). In this artcle, the key finding on the existence SARS CoV-2 in sewage and wastewater is reviwed from the published literature. For this, the literature search was carried out using the keywords SARS-CoV-2, Sewage, Wastewater, Public Health in the Pubmed and Google Scholar, Pubmed search using SARS-CoV-2 and Sewage as key words showed 57 articles, whereas SARS-CoV-2 and wastewater keywords resulted in 108 articles. The key findings from the most relevant articles were discussed in this article to highlight the public health concern.
The virus transmission is established through direct close contact with COVID-19 patients (symptomatic as well as asymptomatic) via their secretions, mainly respiratory droplets produced as a result of coughing or sneezing and indirectly via contact with the virus contaminated droplets (Harapan et al., 2020; Zou et al., 2020). Reports confirm the presence of COVID-19 on more than two dozen cruise ships in different parts of the world that could be one more source of large waterbodies contamination which may not have any significance due to the dilution of virus particles in large waterbodies such as occean (Salducci et al., 2020; Dahl et al., 2020; Chen et al., 2020a). A recent study comparing the SARS CoV-2 detection in the wastewater and river showed the virus was detectable only in the wastewater but not in the river waster (Haramoto et al., 2020).
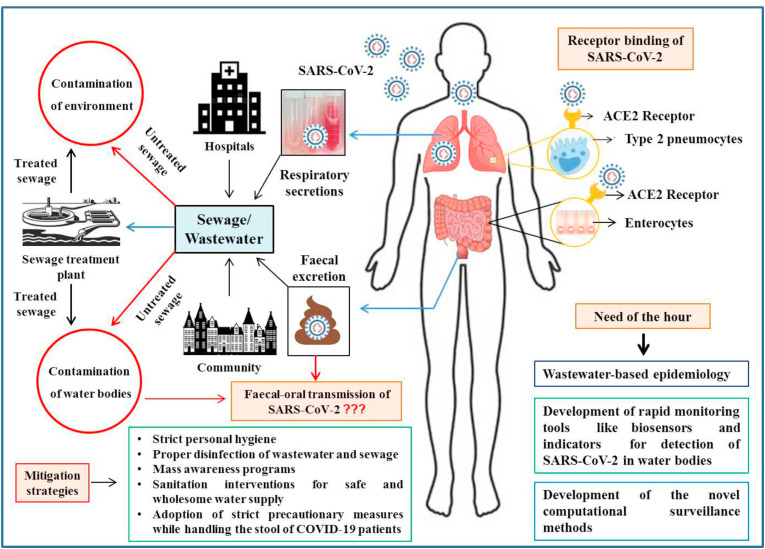
The virus could survive on surfaces, clothes, and fomites for different time intervals like 3 hours in the air, 4 hours on copper, 24 hours on cardboard along with 2–3 days on stainless steel and plastic (ECDC 2020; van Doremalen et al., 2020). However, as viruses cannot replicate outside the host cell, their detection in environmental samples/surfaces and sewage suggests their excretion by infected humans or animals (O'Brien and Xagoraraki, 2019). For host cell entry, SARS-CoV-2 uses the receptor angiotensin-converting enzyme 2 (ACE2), which is highly expressed in the gastrointestinal tract (enterocytes) and other internal organs, like the testes, kidneys, and heart (Harmer et al., 2002; Xu et al., 2020; Zheng et al., 2020a), but has medium to low expression in the lungs (type 2 pneumocytes), liver, blood, spleen, and brain (Li et al., 2020; Lukassen et al., 2020). The higher expression of ACE2 in the gastrointestinal tract (GIT) is also supported by SARS-CoV-2 shedding for a longer duration from the stool samples of patients with COVID-19 (D'Amico et al., 2020). Consequently, diarrhea has been observed in 2–50% (average, 10.4%) of COVID-19 patients, with or without respiratory symptoms (D'Amico et al., 2020). Although current evidence suggests the involvement of the GIT in SARS-CoV-2 infection, evidence for the direct involvement of the liver is lacking. Further studies are required to understand the mechanism underlying viral shedding via the fecal route, and to investigate whether SARS-CoV-2 is a hepato- or cholangio-tropic virus that can be secreted into the bile (Ding and Liang, 2020).
The involvement of the GIT has been confirmed by detecting the virus in 53% (9 out of 17) stool samples of COVID-19 cases, although with a lower viral load compared to respiratory samples, throat, and sputum (Pan et al., 2020). The viral RNA has been detected in fecal samples from both symptomatic and asymptomatic patients (Gao et al., 2020; Holshue et al., 2020; Zhang et al., 2020a; D'Amico et al., 2020). Furthermore, the viable virus has also been detected in stool samples from patients with COVID-19 (Wang et al., 2020; Wu et al., 2020). Additionally, shedding of the virus in the feces for several days after the disappearance of all respiratory symptoms has been reported in patients with COVID-19 (Wu et al., 2020). Peccia et al. (2020) amid COVID-19 pandemic detected the viral RNA of SARS-CoV-2 in sewage sludge in the New Haven, Connecticut, USA over 2 months of time. Notably, the higher virus concentration was noted in sludge prior to peak of infection cases in community that indicated shedding of the virus in larger numbers before onset of clinical signs.
Viral shedding can occur via the fecal route in patients whose oropharyngeal samples tested negative for SARS-CoV-2 and up to 7 days after negative conversion in pharyngeal swabs (Chen et al., 2020b). A study reported the median duration of viral shedding in the stool was 22 days in comparison to the 18 and 16 days in respiratory airways and serum samples, respectively (Zhang et al., 2020b). Hence, to prevent the possibility of a false negative result during the initial screening, stool samples should be tested to complement other tests for enabling the early diagnosis of COVID-19. Moreover, the fecal sample collection is non-invasive and can be performed by the patients themselves (Bonato et al., 2020). Taking into consideration the possibility of prolonged viral shedding in cured patients, laboratory-confirmed cases of COVID-19 should only be declared as negative when both the oropharyngeal and fecal samples test negative, to prevent the possibility of fecal-oral transmission (Ali et al., 2020).
The SARS-CoV-2 RNA has been detected in the sewage or wastewater samples (Ahmed et al., 2020; Young et al., 2020; Wang et al., 2020). This contamination could be because of viral excretion from symptomatic, asymptomatic, or pre-symptomatic cases (Lodder et al., 2020). Wang et al. (2020) have reported that sewage samples from the inlet of the pre-processing disinfection equipment and the outlet of pre-processing disinfection pools tested positive for SARS-CoV-2 RNA in a hospital setting. Still, samples from the final outlet of the last sewage disinfection pool tested negative for the viral RNA. Moreover, all the sewage samples collected from the hospital were cultured and found to be negative for the virus, indicating that sewage cannot lead to the transmission of SARS-CoV-2 (Wang et al., 2020). However, as the findings of Wang et al. (2020) indicated the absence of a virus in the final outlet, the role of sewage water and sewage-contaminated drinking water in SARS-CoV-2 transmission further needs assessment. The evidence of shedding of live virus in stool is weak, and for which purpose more studies are suggested, however municipal wastewater from the affected communities may contain the virus (Wang et al., 2020; Zhang et al., 2020b). In wastewater-based epidemiology (WBE), SARS-CoV-2 was detected in 50% samples (6/12) from influent untreated wastewaters in the world's worst-affected country, Italy (La Rosa et al., 2020a). Recently, it has been speculated with an apprehension of SARS-CoV-2 transmission through irrigation system that relies in the majority of places through usage of sewage water especialy the less developed countires. Amid ongoing COVID-19 pandemic and emerging evidences of SARS-CoV-2 presence in the wastewater one must be hesitant to reuse the unprocessed or partially processed waterwater for irrigation (Polo et al., 2020; Siddiqui et al., 2020).
Furthermore, a study reported the detection of SARS-CoV-2 RNA in sewage samples before reporting COVID-19 cases, suggesting the possibility of virus monitoring before the occurrence of cases by the health surveillance system (Medema et al., 2020). However, the hypothesis of detecting SARS-CoV-2 RNA in the sewage long before the report of first case seems to be a rare event since it will be very difficult for the virus to circulate undetected in the community. Another study conducted in Paris on wastewater samples revealed the increase in viral genome units, which was reflected in the rise in the number of fatal COVID-19 cases (Wurtzer et al., 2020). In this context, WBE may provide insights into the prevalence of SARS-CoV-2 and other viruses, as it may contain several viruses shed by symptomatic and asymptomatic individuals (Xagoraraki and O'Brien, 2020). Additionally, WBE has proved of use as an early warning system for disease outbreaks caused by poliovirus, norovirus, and hepatitis A virus (Hellmér et al., 2014; Asghar et al., 2014). Moreover, WBE may serve as a crucial monitoring approach as it covers a broad array of compounds and pathogens present in the wastewater (Daughton, 2020). However, the sensitivity of WBE may depend upon the population size of sewer catchment, proportion of asymptomatic, presymptomatic, and mildly symptomatic individuals. Viral particles or RNA in sewage samples represents a collection of virus shed by many people in the community; hence it is equivalent to pooled testing. Moreover, global investigators highlighted that sewage surveillance could reveal the true scale of the COVID-19 pandemic and helps in designing crucial policy makings to contain the virus (Mallapaty, 2020).
Waterborne infections have posed significant public health concerns in recent years since owing to their potential to cause various disease outbreaks, including virus origin (La Rosa et al., 2020b). Hence, for a timely exploration of such possibilities in COVID-19 and better application of preventive strategies, deliberation on possibilities or otherwise, need to be undertaken. Waterborne viruses often enter and contaminate water sources through contaminated feces, and the same has been proposed for COVID-19 (Hart and Halden, 2020; Lodder et al., 2020). Its survival in water, transmissibility through water, and potential to infect the consumers are vital issues that need to be explored for SARS-CoV-2. Although enveloped viruses like coronaviruses (CoVs) have different structural and survival characteristics in water compared to non-enveloped viruses especially the waterborne enteric viruses like norovirus, enterovirus, hepatitis A virus or adenovirus which are excreted in large amounts in feces and are responsible for major water-related outbreaks (WHO 2017; Bonadonna and La Rosa, 2019), timely investigation and current evidence necessitate future strategies for prevention of any possible eventuality (La Rosa et al., 2020). Considering the current global emergency of COVID-19 and frequent detection of SARS-CoV-2 in sewage water (Amirian 2020; Heller et al., 2020), leniency in any such aspect can prove dangerous. Additionally, wastewater management is considered a practical approach for tracking the transmission of waterborne viruses (Hart and Halden 2020). Thorough and regular monitoring of crucial reservoirs will help identify peaks in viral concentrations or indicators, which may be used as early signs of an epidemic.
Additionally, community fecal pollution represents the public and animal health status of a society (O'Brien and Xagoraraki, 2019). In view of the possible role of asymptomatic and undiagnosed COVID-19 patients in transmitting the disease via the fecal-oral route, strict wastewater surveillance should be implemented as a preventive measure, as the SARS-CoV-2 virus present in water, soil, and other environmental compartments will finally accumulate in the wastewater and sewage (Bogler et al., 2020). In addition to that, the virus can also get accumulated in the groundwater, surface water, and other natural water compartments as a result of leaking sewers or insufficient wastewater treatment (Bogler et al., 2020). The conventional and centralized water treatment methods that utilize filtration and disinfection may also inactivate the COVID-19 virus (EPA 2020).
As of now, trivial information is existing on the treatement methodologies applied in wastewater treatment plants. Bhatt et al. (2020) reviewed and emphasized the treatment options for eliminating viruses in wasterwater and thus reducing the risk of SARS-CoV-2 transmission through wasterwater, a neglected but possible source of infection. The treatment of biomedical wastewater treatment is an instant challenge where need arises of developing disinfection data and boosting disinfection amounts while treating biomedical wasterwater and employing suitable disinfection methodology. COVID-19 pandemic to stop plausible transmission. A list of disinfectants including benzalconium chloride, sodium dichloro isocyanurate, peracetic acid, performic acid, chloramines, chlorine dioxide are showing good virucidal activities. Adopting such measures would be beneficial in stopping the COVID-19 pandemic plausible transmission (Kataki et al., 2021). In a related study, Zaneti et al. (2020) looked at the consequecnes of COVID-19 and highlighted the risk of SARS-CoV-2 infection among personnels working in wastewater treatment plants using a surrogate pathogen (SARS-CoV-1). The results are suggestive of virus transmission among workers.
Moreover, to prevent transmission via sewage, the current conventional methods of sewage treatment need to be analyzed, re-evaluated, and replaced with advanced methods that can prevent the spread of SARS-CoV-2 through the environment (Núñez-Delgado, 2020). In this context, novel computational surveillance methods are being used for analyzing wastewater based epidemiology of COVID-19 at national and global levels and temperature, average in-sewer travel time, and per-capita water use have been identified as critical variables (Hart and Halden 2020). Besides, many new economic and rapid monitoring tools are being developed for the detection of SARS-CoV-2 in sewage and wastewater, including ELISA, paper-based indicator methods, and biosensors (Orive et al., 2020). These tools will reveal the accurate picture of the ongoing pandemic associated with a specific population linked to a wastewater treatment plant (Orive et al., 2020). Sewage and wastewater surveillance can help in the screening of entire population. it is non-invasive, as well as it can help in diagnosis well before the detection of clinical cases (Medema et al., 2020; Randazzo et al., 2020). Several studies points presence of multiple pathogens in sewage sludge, counting even SARS-CoV-2. Integrated point-of-care biosensor system could improve the wastewater-based epidemiology for establishing early warning (Mao et al., 2020). Therefore, WBE can be used as an early warning tool that can rapidly detect COVID-19 outbreaks. However, the sensitivity of WBE for detecting SARS-CoV-2 is less as compared to norovirus due to the low viral load in wastewater (Hata and Honda, 2020). Viability of CoVs in wastewater is the issue concerning the wider transmission and spread of these viruses and posing high public health concerns. Data on the survivability of SARS-CoV-2 in sewage r wastewater is lacking at the moment. However, a few studies were successful in isolating infective SARS-CoV-2 from urine (Sun et al., 2020) and stool samples (Wang et al., 2020; Zhang et al., 2020b). The failure of isolating infective SARS-CoV-2 from stool or wastewater samples does not imply the absence of infective virions, instead, it indicates the difficulty of isolating intact enveloped virions (Bogler et al., 2020). In addition to this, the concentration of SARS-CoV-2 needs to be even higher to isolate infective virions from stool or wastewater samples as compared to RNA detection (Wölfel et al., 2020). This could very well define why wastewater can get tested positive for the SARS-CoV-2 RNA, but cannot isolate the infective virions. Based on structural characteristics of SARS-CoV-2 it is assumed to behave differently in aqueous environments (Quilliam et al., 2020) but still considerable persistence in various environments (van Doremalen et al., 2020) provides clues regarding risks of transmission (Amirian 2020; Quilliam et al., 2020).
Based on the available literature, evidence on the presence of SARS-CoV-2 in surface or groundwater sources and transmission through contaminated drinking-water is rising (La Rosa et al., 2020b). Although the possibility of fecal-oral transmission is not yet established for SARS-CoV-2, a thorough investigation is needed to ascertain the role of water and related sanitation interventions in the spread of COVID-19 via this route. Presence of SARS-CoV-2 in the stools (Chen et al., 2020b; D'Amico et al., 2020; Gu et al., 2020; Holshue et al., 2020; Pan et al., 2020; Wang et al., 2020; Wu et al., 2020; Zhang et al., 2020a) and persistence in surroundings (Kampf et al., 2020) suggests the possibility to contaminate the environment especially water. Though respiratory droplet infection through close contact is the primary and dominant route of transmission of SARS-CoV-2 but the potential of fomite-based (Cai et al., 2020; van Doremalen et al., 2020), vertical (Chen et al., 2020c) and faeco-oral transmission (Gu et al., 2020) cannot be ruled out as considerable evidence are accumulating (Amirian 2020). This respiratory infection is now widening its dimensions of spread through various transmission routes with current evidence supporting probable faeco-oral transmission to have dare consequences (Amirian 2020; Gu et al., 2020).
Additionally, viral shedding in the stool necessitates the adoption of a modified strategy for screening the COVID-19 patients (found negative in nasopharyngeal swabs) by focusing on this route of viral shedding and environmental contamination, including that of water bodies and sewage. The involvement of enteric transmission may add a new sphere to global risk. It may further pose a risk for the public health workers, sanitation workers, and drinking water suppliers and consumers if the necessary measures are not practiced (CDC 2020; Lodder et al., 2020). Further, since due to the presence of SARS-CoV-2 in stools, sewage and wastewater, the possibility of faecal-oral transmission increases, there is need for evaluating all the probable routes from feces to the mouth of the susceptible population and analyzing available studies using frameworks of environmental dynamics and viral persistence or survivability and transmission in shaping future strategies for pandemic management (Heller et al., 2020). One such framework analysis by Heller et al. (2020) proposed the possible transmission of SARS-CoV-2 from feces to the mouth of human beings via water and contact surfaces. Hence considering this framework of possible SARS-CoV-2 faecal-oral transmission routes, novel interventions safeguarding clean water supply and adequate sanitation for controlling the COVID-19 pandemic should be added to current mitigation strategies (Heller et al., 2020). The wastewater plumbing system harbors several pathogenic microorganisms. Some of these viruses such as SARS-CoV-2, under some circumstances, might have the potential to enable airborne transmission (Gormley et al., 2020). In 2003, a superspreading event of SARS-CoV was reported in Hong Kong that was transmitted by “virus laden droplets” generated from the empty U-bends in bathrooms (McKinney et al., 2006). The proposed route of airborne transmission was found to be aided by bathroom ventilation that drew contaminated air into the room (McKinney et al., 2006; Gormley et al., 2020). The virus-laden air can even get transported to the adjacent buildings with the help of prevailing winds (McKinney et al., 2006). The feasibility of this airborne transmission route was evaluated in a full-scale experimental study using wastewater plumbing test-rig (Gormley et al., 2017). The authors used Pseudomonas putida as the model organism that was flushed into the wastewater plumbing system. Findings indicated the transmission of viable organism between the rooms situated at different floors through the airflow system. This subsequently contributed to the contamination of surfaces within the system and rooms via droplet fallout (Gormley et al., 2017). Thereore, the possibility of SARS-CoV-2 transmission via virus-laden droplets generated from the wastewater plumbing system cannot be ruled out. With the current literature providing evidences for viral persistence in environment, possible transmission via contaminated waste surfaces and aerosols from wastewater systems (Nghiem et al., 2020), frameworks are being drafted to deduce probable routes of transmission (Heller et al., 2020) with focus on adopting appropriate pandemic mitigation strategies that can help in prevention and control. Though the wastewater surveillance is very useful, there is an urgent need to develop a standardized and optimized protocol for the detection of SARS-CoV-2 in wastewater which can be used globally for the surveillance (Kitajima et al., 2020). While there is no evidence for SARS COV-2 transmission through feco-oral route via contaminated drinking water, it is a major public health concern in underprivileged societies such an rural and urban slums where there is no wastewater treatment infrastructures, no clean drining water, high population density, and poor hygiene (Arslan et al., 2020).
The super spreading nature of SARS-CoV-2 is pointing towards the evaluation of the potential of wastewater and sewage for waterborne transmission. The explorative wastewater-based epidemiology and sewage surveillance investigations for SARS-CoV-2 are now the need of the hour. Such studies can provide the actual virus burden in communities and to take appropriate control measures to prevent further spread of this virus. Additionally, strict personal hygiene (hand hygiene) and precautionary measures must be adopted while handling the stools of COVID-19 positive patients along with proper disinfection of wastewater and sewage from healthcare settings, elderly houses, quarantine facilities, and containment areas. Future research to ascertain the role of fecal-oral route in the transmission of SARS-CoV-2 must include environmental factors to determine whether the virus remains viable in the conditions that would facilitate such transmissions or not. A thorough study on the enteric involvement and viral shedding in the feces is necessary to investigate whether concentrations of SARS-CoV-2 RNA in fecal samples correlate with the disease severity and gastrointestinal symptoms. In the future, if the fecal-oral transmission hypothesis is confirmed, immediate sanitation interventions for safe and wholesome water supply must be ensured to contain this pandemic.
Besides, frequent hand washing and personal hygiene measures must be adopted along with mass awareness programs. In this context, reinforcement of provisions related to safe drinking water and adequate sanitation is of utmost importance. It would be helpful to design prevention and control strategies, including treatment of diarrhea, and conduct wastewater-based epidemiological studies, which would help alert the population catered to by a specific sewage treatment plant. Besides, regular monitoring of wastewater for various pathogens is essential even after this pandemic ends to maintain public health and address the threat of waterborne diseases. Frameworks of environmental dynamics and the persistence and transmission of SARS-CoV-2 from waste water and sewage need to be evaluated for designing appropriate interventional strategies to contain the spread of virus while ensuring clean water supply and proper waste water disposal. Ample sanitation practices are also needed for curbing further spread of this pandemic virus. Wastewater-based epidemiology and sewage surveillance can be alternative or complementary to other mechanisms of surveillance to understand the prevalence and disease spread.
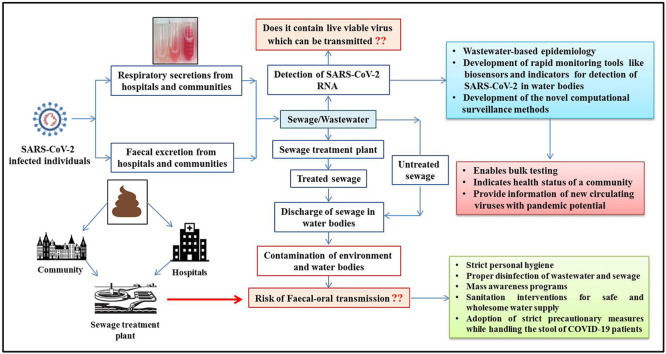
An overview of the modes of sewage and wastewater contamination by excretions and secretions of COVID-19 patients along with strategies to counter it are presented in Fig. 1 .
Fig. 1.
Potential mechanisms of sewage and wastewater contamination by excretions and secretions of COVID-19 patients along with mitigation strategies to counter it.
Funding
This compilation is a review article written by its authors and required no substantial funding to be stated.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities. YSM acknowledges the Guru Angad Dev Veterianry and Animal Scince University (GADVASU), Ludhiana and ICAR-Education Division, New Delhi for National Fellowship.
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Zaid M., Saqib M.A.N., Ahmed H., Afzal M.S. SARS-CoV-2 and the hidden carriers - sewage, feline, and blood transfusion. J. Med. Virol. 2020 doi: 10.1002/jmv.25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirian E.S. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health [published online ahead of print, 2020 Apr 23] Int. J. Infect. Dis. 2020;95:363–370. doi: 10.1016/j.ijid.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan M., Xu B., Gamal El-Din M. Transmission of SARS-CoV-2 via fecal-oral and aerosols-borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743:140709. doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., El-Bassioni L., Akande A.O., Al-Maamoun E., Zaidi S., Adeniji A.J., Burns C.C., Deshpande J., Oberste M.S. Lowther SA. Environmental surveillance for polioviruses in the global polio eradication initiative. J. Infect. Dis. 2014;210(Suppl. 1):S294. doi: 10.1093/infdis/jiu384. 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A., Arora P., Prajapati S.K. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: a review with emphasis on SARS-CoV-2. J Environ Chem Eng. 2020 Oct;8(5):104429. doi: 10.1016/j.jece.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler A., Packman A., Furman A., Gross A., Kushmaro A., Ronen A., Dagot C., Hill C., Vaizel-Ohayon D., Morgenroth E., Bertuzzo E. Rethinking wastewater risks and monitoring in light of the COVID-19 pandemic. Nature Sustainability. 2020:1. doi: 10.1038/s41893-020-00605-2. 0. [DOI] [Google Scholar]
- Bonadonna L., La Rosa G. A review and update on waterborne viral diseases associated with swimming pools. Int. J. Environ. Res. Publ. Health. 2019;16(2):166. doi: 10.3390/ijerph16020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato G., Dioscoridi L., Mutignani M. Faecal-oral transmission of SARS-COV-2: practical implications. Gastroenterology. 2020;S0016–5085(20):30449. doi: 10.1053/j.gastro.2020.03.066. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Sun W., Huang J., Gamber M., Wu J., He G. Indirect virus transmission in cluster of COVID-19 cases, wenzhou, China, 2020. Emerg. Infect. Dis. 2020;26(6):1343–1345. doi: 10.3201/eid2606.200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2020. Water and COVID-19 FAQs.https://www.cdc.gov/coronavirus/2019-ncov/php/water.html 12th May 2020. [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.M., Jyan H.W., Chien S.C. Containing COVID-19 among 627,386 persons in contact with the diamond princess cruise ship passengers who disembarked in taiwan: big data analytics. J. Med. Internet Res. 2020;22(5) doi: 10.2196/19540. Published May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., Yang J., Ye G., Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Dahl E. Coronavirus (Covid-19) outbreak on the cruise ship diamond princess. Int. Marit. Health. 2020;71(1):5‐8. doi: 10.5603/MH.2020.0003. [DOI] [PubMed] [Google Scholar]
- D'Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin. Gastroenterol. Hepatol. 2020;S1542–3565(20):30481. doi: 10.1016/j.cgh.2020.04.001. X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. The international imperative to rapidly and inexpensively monitor community-wide COVID-19 infection status and trends. Sci. Total Environ. 2020;726:138149. doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccines Immunother. 2020;16:1–7. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Liang T.J. Is SARS-CoV-2 also an enteric pathogen with potential fecal-oral transmission: a COVID-19 virological and clinical review. Gastroenterology. 2020;S0016–5085(20):30571. doi: 10.1053/j.gastro.2020.04.052. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . ECDC; Stockholm: 2020. European Centre for Disease Prevention and Control. Disinfection of Environments in Healthcare and Non-healthcare Settings Potentially Contaminated with SARS-CoV-2.https://www.ecdc.europa.eu/en/publications-data/disinfection-environments-covid-19 17th March 2020. [Google Scholar]
- EPA What is EPA's role in ensuring drinking water remains safe? 2020. https://www.epa.gov/coronavirus/what-epas-role-ensuring-drinking-water-remains-safe 16th May 2020.
- Gao Q.Y., Chen Y.X., Fang J.Y. Novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2019;21(3):125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., deGroot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D., Sidorov I.A., Sola I., Ziebuhr J. 2020. Severe Acute Respiratory Syndrome-Related Coronavirus: the Species and its Viruses – a Statement of the Coronavirus Study Group. [DOI] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob Health. 2020 May;8(5):e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A., Rodriguez-Gil C. Pathogen cross-transmission via building sanitary plumbing systems in a full scale pilot test-rig. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential. Fecal-Oral Transmission Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan H., Itoh N., Yufika A., Winardi W., Keamg S., Te H., Megawati D., Hayati Z., Wagner A., Mudatsir M. Coronavirus disease 2019 (COVID-19): a literature review. J. Infect. Public Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R. Potential sensitivity of wastewater monitoring for SARS-CoV-2: comparison with norovirus cases. Environ. Sci. Technol. 2020;54:6451–6452. doi: 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020;729:138919. doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80(21):6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. Washington state 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataki S., Chatterjee S., Vairale M.G., Sharma S., Dwivedi S.K. Concerns and strategies for wastewater treatment during COVID-19 pandemic to stop plausible transmission. Resour. Conserv. Recycl. 2021 Jan;164:105156. doi: 10.1016/j.resconrec.2020.105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keam S., Megawati D., Patel S., Tiwari R., Dhama K., Harapan H. Immunopathology and immunotherapeutic strategies in SARS-CoV-2 infection. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P. First detection of SARS-CoV-2 in untreated wastewaters in Italy [published online ahead of print, May 23] Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;S2468–1253(20):30087. doi: 10.1016/S2468-1253(20)30087-X. X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020 doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580(7802):176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Mao K., Zhang H., Yang Z. An integrated biosensor system with mobile health and wastewater-based epidemiology (iBMW) for COVID-19 pandemic. Biosens. Bioelectron. 2020;169:112617. doi: 10.1016/j.bios.2020.112617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at amoy gardens. J. Environ. Health. 2006 May;68(9):26–30. quiz 51-2. Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ Sci Technol Lett. 2020 May 20:acs.estlett.0c00357. doi: 10.1021/acs.estlett.0c00357. [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chemical and Environ. Eng. 2020;1:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Delgado A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total Environ. 2020;727:138647. doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E., Xagoraraki I. A water-focused one-health approach for early detection and prevention of viral outbreaks. One Health. 2019;7:100094. doi: 10.1016/j.onehlt.2019.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732:139298. doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020 doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo D., Quintela-Baluja M., Corbishley A., Jones D.L., Singer A.C., Graham D.W., Romalde J.L. Making waves: wastewater-based epidemiology for COVID-19 - approaches and challenges for surveillance and prediction. Water Res. 2020;186:116404. doi: 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam R.S., Weidmann M., Moresco V., Purshouse H., O'Hara Z., Oliver D.M. COVID-19: the environmental implications of shedding SARS-CoV-2 in human faeces. Environ. Int. 2020;140:105790. doi: 10.1016/j.envint.2020.105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav. Med. Infect. Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salducci M., La Torre G. COVID-19 emergency in the cruise's ship: a case report of conjunctivitis. Clin. Ter. 2020;171(3) doi: 10.7417/CT.2020.2212. e189‐e191. [DOI] [PubMed] [Google Scholar]
- Siddiqui R., Khamis M., Ibrahim T., Khan N.A. Irrigation system and COVID-19 recurrence: a potential risk factor in the transmission of SARS-CoV-2. ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00570. [DOI] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S.B., Liu X., Dai J., Li X., Huang S., Huang X., Luo L., Wen L., Zhuo J., Li Y., Wang Y., Zhang L., Zhang Y., Li F., Feng L., Chen X., Zhong N., Yang Z., Huang J., Zhao J., Li Y.M. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microb. Infect. 2020 Dec;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020 May 12;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . fourth ed. incorporating the 1st addendum; 2017. Guidelines for Drinking-Water Quality.https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/ [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.M., Moulin L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. MedRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [Google Scholar]
- Xagoraraki I., O'Brien E. Wastewater-based epidemiology for early detection of viral outbreaks. In: O'Bannon D., editor. Women in Water Quality. vol. 75. Springer Nature Switzerland; 2020. [DOI] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I., Chan M., Vasoo S., Wang L.F., Tan B.H., Lin R.T.P., Lee V.J.M., Leo Y.S., Lye D.C. Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. J. Am. Med. Assoc. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneti R.N., Girardi V., Spilki F.R., Mena K., Westphalen A.P.C., da Costa Colares E.R., Pozzebon A.G., Etchepare R.G. Quantitative microbial risk assessment of SARS-CoV-2 for workers in wastewater treatment plants. Sci. Total Environ. 2020 Sep 3;754:142163. doi: 10.1016/j.scitotenv.2020.142163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J. Med. Virol. 2020 doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., Song Y., Zhen W., Feng Z., Wu G., Xu J. Isolation of 2019-nCoV from a stool specimen of a laboratory-confrmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2:123–124. [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]