Abstract:
The use of cardiopulmonary bypass (CPB) contributes significantly to intraoperative anemia. The use of a prescriptive circuit that is tailored to the patient size could significantly reduce priming volumes, resulting in less hemodilution. The purpose of this study was to determine whether a prescriptive circuit resulted in decreased hemodilution, reduced blood product usage, and improved outcomes. In total, 204 patients prospectively received the prescriptive protocol between March 2019 and November 2019. This protocol was composed of three circuit sizes: small [body surface area (BSA) ≤ 1.85 m2], medium (BSA 1.86–2.30 m2), and large (BSA ≥ 2.31 m2). Data for CPB and post-bypass transfusions were collected, along with postoperative outcomes. These patients were then 1:2 propensity score matched to 401 patients who were retrospectively reviewed who had undergone cardiac surgery using a one-sized CPB circuit. The prescriptive protocol cohort had more patients with renal disease, whereas the conventional cohort had more history of hypertension. Intraoperative results show the prescriptive circuit had lower mean prime volume and total prime volume after reverse autologous prime (1,084 mL vs. 1,798 mL, p < .0001; 725 mL vs. 1,181 mL, p < .0001). Ultrafiltration was higher in the prescriptive group (872 vs. 645 mL, p < .0001), which likely balanced the increased use of del Nido cardioplegia in the prescriptive group (1,295 vs. 377 mL, p < .0001). The drop in hematocrit (HCT) from baseline was less in the prescriptive group (15.1 ± 4.91 vs. 16.2 ± 4.88, p = .0149), whereas the postoperative HCT was higher (32.79 ± 4.88 vs. 31.68 ± 4.99, p = .0069). Transfusion of packed red cells did not change between the two groups. Implementation of a prescriptive circuit did not reduce on-bypass or intraoperative blood product usage. However, there was a significant reduction in on-bypass hemodilution and increased postoperative HCT.
Keywords: cardiopulmonary bypass (CPB), blood transfusion, blood conservation, equipment, statistics, propensity matching
The use of blood product transfusions during cardiac surgery has long been considered a necessity and accounts for the consumption of 10–15% of the nation’s blood supply (1). Although blood products are a useful, life-saving resource, research shows that its use can also lead to significant postoperative complications and poor short- and long-term outcomes (2–4). As a result, significant focus has been placed on the development of multidisciplinary blood management programs that focus on reducing the need for blood product transfusions. Efforts to avoid transfusion triggers are largely focused on decreasing intraoperative hemodilution caused by the use of large amounts of intravenous fluids (IVF) during cardiac surgery, which have been linked to increased morbidity and mortality(5). One of the main sources of intraoperative hemodilution during cardiac surgery is the cardiopulmonary bypass (CPB) machine because of the significant amount of crystalloid solution required to prime the circuit. CPB-related hemodilution has been associated with numerous adverse outcomes, including ischemic organ injury, acute kidney injury, prolonged intensive care unit (ICU) stays, and increased morbidity and mortality risks (6,7). In cases of severe hemodilution, the patient is more likely to receive a blood transfusion, which can result in a compounding effect and put the patient at the highest risk for complications (8).
CPB-induced hemodilution and blood product usage can be reduced by implementing multiple perfusion strategies, such as minimizing circuit size, assisted venous drainage, cell salvage, pump blood salvage, modified ultrafiltration, and retrograde autologous prime (RAP) (9–11). The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologist have recommended miniaturized circuits a class I(A) and vacuum-assisted venous drainage (VAVD) as a class II(B) recommendation in their updated guidelines for blood conservation (1). These strategies have been further supported by the American Society for Extracorporeal Technology Standards and Guidelines for Perfusion Practice (Standard 9.2) and additional blood management recommendations, including matching the size of the CPB circuit to the patient size (12). The use of prescriptive circuits has long been standard of care in pediatric perfusion as a means for reducing hemodilution and perioperative blood requirements. However, this technique has not been universally implemented in the adult perfusion setting. With increased focus on blood conservation and reducing prime volumes, the ability to customize a circuit to best fit the size and metabolic needs on an individual patient has the potential to improve clinical care. Studies on the use of prescriptive circuits in the adult population have reported reductions in packed red blood cell (PRBC) transfusion and increased nadir hematocrit (HCT) on bypass (13,14). However, these studies are retrospective, and a prospective review is necessary.
At our institution, in an effort to improve blood conservation and on-bypass hemodilution, the perfusion team transitioned from a one-size venous reservoir bag (OS-VRB) system to a prescriptive circuit that would allow for selection of the oxygenator and arterial and venous loop (A-V loop) based on the patient’s body surface area (BSA). This quality improvement project sought to determine whether implementation of a prescriptive protocol resulted in an increase in nadir HCT on bypass, reduced the need for intraoperative blood product transfusions, and improved patient outcomes.
MATERIALS AND METHODS
This study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston and the Memorial Hermann Clinical Innovation and Research Institute (HSC-MS-18-1028). Additional approval was obtained from the Institutional Review Board at the University of Nebraska Medical Center (081-19-ET).
Patient Population
From March to November 2019, data were prospectively collected for 199 patients who underwent cardiac surgery by the Department of Cardiothoracic and Vascular Surgery at the McGovern Medical School at The University of Texas Health Science Center at Houston (UTHealth) at Memorial Hermann Hospital at Texas Medical Center. The study included all patients ≥18 years who underwent CPB using the prescriptive circuit, hard-shell circuit protocol (PC-HS). Patients for whom the protocol was not followed and patients who were emergently transferred to our institution were excluded from the analysis. The conventional group was retrospectively reviewed from the 2018 patient database of patients who underwent CPB with a OS-VRB perfusion circuit by the same surgical team and institution. The OS-VRB was 2:1 propensity matched (n = 398) to the prescriptive group.
Perfusion Circuit
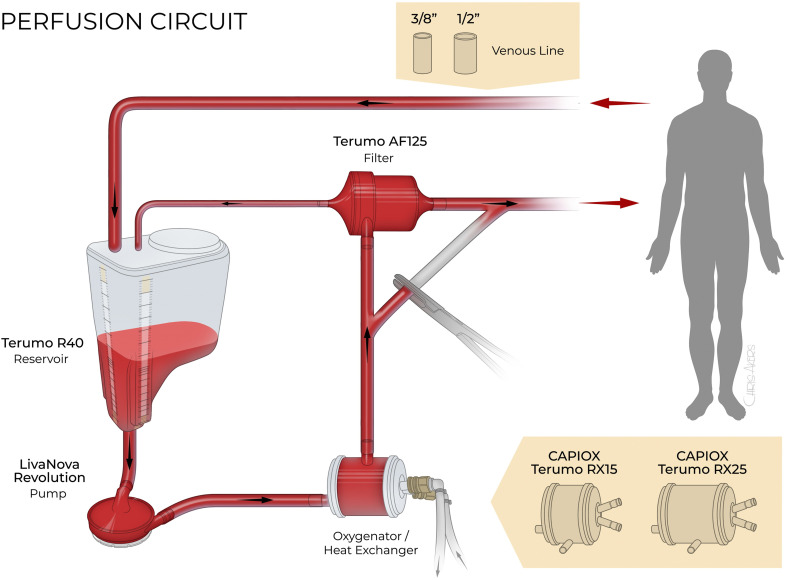
The heart–lung machine used in bypass procedures for the conventional and prescriptive groups was a LivaNova S5® with the LivaNova Revolution® centrifugal pump (LivaNova, Arvada, CO). The PC-HS circuit consisted of a Capiox® RX15 and RX25, R40 (4,000 mL) reservoir, Capiox® AF125 arterial filter, and custom X-Coating® tubing pack (Terumo Cardiovascular Group, Ann Arbor, MI). The custom tubing pack used a 3/8 × 3/8″ A-V loop. If the case required a 1/2″ venous line, the 3/8″ venous line was removed at the surgical table and a 1/2″ internal diameter tubing of the same length was connected to the reservoir and primed from the table. During the study period, modifications were made to the custom tubing pack that reduced the tubing length. For cases requiring retrograde cerebral perfusion (RCP), an additional custom tubing pack (RCP harness) was added to the circuit. The OS-VRB perfusion circuit comprised the Medtronic Affinity Fusion™ oxygenator, MVR1600 reservoir bag, Affinity® AF100 arterial filter, and a custom Medtronic Balance® Biosurface tubing pack with a 1/2 × 3/8″ A-V loop (Medtronic, Minneapolis, MN). A custom RCP harness was added in cases requiring RCP. The LivaNova Vanguard 4:1 Cardioplegia System was used for both groups.
Prescriptive Protocol
Before the patient enters the operating room, the perfusionist would review the chart for the most recent height (cm) and weight (kg) and calculate BSA (m2). Using BSA (m2), the oxygenator and A-V loop were selected using the prescriptive protocol (Figure 1). For small patients with a BSA ≤ 1.85 m2, the RX15 (Terumo Cardiovascular Group) oxygenator and 3/8 × 3/8″ A-V loop were selected with a crystalloid prime volume of 1,118 mL in version 1 and 935 mL in version 2. The medium-sized circuit for patients with a BSA 1.86–2.30 m2 consisted of the RX25 (Terumo Cardiovascular Group) and 3/8 × 3/8″ A-V loop with 1,233 mL of prime in version 1 and 1,050 mL in version 2. The largest circuit was for the patient with a BSA ≥ 2.31 m2 and consisted of the RX25 (Terumo Cardiovascular Group) with a 3/8 × 1/2″ A-V loop that resulted in 1,374 mL prime for version 1 and 1,191 mL in version 2. The RCP harness added an additional 338 mL prime in version 1 and 277 mL in version 2. If the patient required the largest circuit with a 3/8 × 1/2″ A-V loop, the RCP harness venous line would also be upsized to 1/2″ tubing at the surgical table, resulting in an additional 433 and 337 mL of prime. The prescriptive protocol was developed using BSA and blood flow (BF) requirements that would allow the perfusionist to exceed normal BF at a 2.4 cardiac index (L/min/m2) if the patient required a higher perfusion rate without exceeding the manufacturers’ recommended flow rate for the oxygenator. The OS-VRB system required 1,800 mL of prime and the RCP harness if needed added 200 mL. The starting prime volumes for both groups reflected the minimum crystalloid prime volume before RAP.
Figure 1.
Prescriptive perfusion circuit. Illustration of the prescriptive circuit showing the options for a 3/8″ or ½″ venous line and the Capiox® RX15 or RX 25 (Terumo Cardiovascular Group).
CPB Management
The CPB circuit for both groups was primed with Lactated Ringer’s solution (B. Braun Medical Inc., Bethlehem, PA) and circulated before initiation of heparin. VAVD was available for all prescriptive circuit cases and was used at the perfusionist’s discretion. Similarly, in the OS-VRB group, the perfusionist had the option for adding kinetic-assisted venous drainage to augment venous return. All patients received standard CPB management with a target perfusion pressure for 55–75 mmHg. Heparin was administered at a dose of 300 units/kg to achieve an activated clotting time (ACT) ≥ 480 seconds. For isolated coronary artery bypasses and valve replacements, the patient’s temperatures were allowed to drift to 33°C. For cases requiring longer pump times, target nasopharyngeal temperatures ranged from 28 to 32°C. Cases that required deep hypothermic circulatory arrest and RCP used a rapid cool method with a target nasopharyngeal temperature below 20°C, for an average of 10–15 minutes of cooling. Reverse autologous priming is used on every case, depending on patient parameters, such as hemodynamic stability. This was determined after aortic cannulation by the anesthesia team. Additional fluid management strategies included ultrafiltration to remove excess volume and increase HCT, the use of pressors to control mean arterial pressure, and, in rare instances, the use of colloids to increase oncotic pressure. A strict transfusion trigger was not used. However, if the HCT was ≤18%, the perfusionist and anesthesiologist would begin assessing other clinical parameters to determine the need for a unit of PRBC. During the course of CPB, arterial blood gases were run 5 minutes after initiation and every 20 minutes after until termination of bypass on a GEM Premier 3500 (Instrumentation Laboratory, Bedford, MA) and ACTs were collected at the same interval using an ACT Plus® (Medtronic, Minneapolis, MN). Following aortic cross-clamping, either Buckberg cardioplegia solution or del Nido cardioplegia (CPG) was administered, depending on the surgeon’s preference, at 2–3°C. Buckberg solution was delivered at a 4:1 ratio (blood:CPG) arresting dose of 1,000 mL with maintenance antegrade doses at a 12:1 ratio of 200–400 mL every 15–20 minutes. Continuous retrograde cardioplegia is given at either 12:1 or cold straight blood. del Nido was administered at a 1:4 ratio (blood:CPG), arresting doses ranged from 800 to 1,200 mL based on the patient’s size. If cross-clamp exceeded 60 minutes, del Nido was redosed at the same ratio with volumes ranging from 200 to 400 mL. Following termination of bypass, heparin reversal was achieved with a protamine dose of 1 mg/unit of heparin. During this time, blood remaining in the circuit was either returned to the patient before decannulation or chased to the cell saver with 2 L of lactated Ringer’s solution and returned to the patient as a washed unit before leaving the operating room.
Data Collection
Data for the conventional group were obtained from our institutions’ STS database, and missing perfusion data were then found using retrospective chart review. For the prescriptive group, general patient information including age, BSA, body mass index (BMI), and gender; and preoperative baseline laboratory values for blood urea nitrogen (BUN), glomerular filtration rate (GFR), serum creatinine (SCr), and HCT were collected and recorded by the perfusionist before the start of the case. For both groups, perfusion data that were collected included pre-bypass HCT, the last HCT drawn perioperatively before initiating bypass, prime volume, RAP volume, nadir HCT on bypass, total crystalloid added during bypass, the number of units of PRBC and fresh frozen plasma (FFP), ultrafiltration volume, and urine output on bypass. In addition, the number of PRBC units, FFP, platelets, and cryoprecipitate given by anesthesia post-CPB were recorded, and the total blood loss was estimated. Measures of postoperative outcomes included the number of postoperative PRBC, FFP, platelet, and cryoprecipitate units; length of stay in the ICU; length of stay admission to discharge; time on ventilator; HCT at arrival in the ICU; HCT at 24 hours postop; and return to the operating room for reoperation.
Statistical Analysis
The propensity to receive perfusion by prescriptive circuit vs. conventional circuit pump was estimated by screening preoperative patient characteristics against perfusion type using Spearman rank correlation methods. Variables identified by the correlation screen and variables considered likely to be reasonably related to both treatment assignment and bleeding risk were included in a non-parsimonious logistic regression model with treatment assignment as the dependent variable. Variables included in the model were elective operation status, diagnosis of hypertension, renal disease history, perfusion setup for del Nido cardioplegia, female gender, age at surgery, BMI, HCT at baseline, history of prior sternotomy, and currently undergoing coronary artery bypass graft surgery. Untransformed score (logit) was used for matching to retain normality of the score distribution. Patients receiving conventional circuit perfusion were matched 2:1 to patients receiving prescriptive circuit perfusion to maximize statistical power because the prescriptive circuit was introduced recently, with only 204 cases performed using this technique. Matching was by nearest neighbor greedy matching. The effect of propensity matching on reduction of selection bias was assessed by standardized mean difference reduction among the covariate set. All computations were performed using SAS software version 9.4 (SAS Software, Cary, NC), with propensity score matching using the propensity score matching procedure, version 15.1. All data were summarized as mean (±SD).
RESULTS
The preoperative characteristics of the unmatched and propensity-matched cohorts are reported in Table 1. Most of the preoperative variables examining age, BSA, BMI, BUN, GFR, SCr, gender, chronic kidney disease (CKD) stage calculated using GFR, history of diabetes, emergency status, and redo sternotomy were not found to be statistically different. There were a significantly higher number of cases with a history of hypertension in the conventional group (88% vs. 67%, p < .0001). The prescriptive group had significantly more cases with a history of renal disease.
Table 1.
Preoperative characteristics of matched cohort.
| Matched Cohort (n = 605) | |||
|---|---|---|---|
| Variable | Prescriptive Circuit (n = 204) | Conventional Circuit (n = 401) | p-Value* |
| Age (years) | 61.3 (±12.3) | 61.9 (±23.3) | .8599 |
| BSA (m2) | 2.1 (±.28) | 2.1 (±.30) | .5008 |
| BMI (kg/m2) | 30.8 (±6.9) | 30.0 (±6.7) | .2096 |
| BUN (mg/dL) | 21.9 (±14.2) | 20.4 (±12) | .0885 |
| Pre-GFR | 72.2 (±26.5) | 72.8 (±27.2) | .7963 |
| SCr-baseline (mg/dL) | 1.3 (±1.3) | 1.3 (±1.1) | .4089 |
| Female | 148 (32%) | 128 (32%) | .7255 |
| CKD stage | |||
| 1 | 98 (48%) | 183 (46%) | .5209 |
| 2 | 64 (31%) | 118 (29%) | |
| 3 | 19 (9%) | 52 (13%) | |
| 3.5 | 9 (4%) | 28 (7%) | |
| 4 | 6 (3%) | 9 (2%) | |
| 5 | 8 (4%) | 11 (3%) | |
| DM | 68 (33%) | 160 (40%) | .1151 |
| HTN | 137 (67%) | 351 (88%) | <.0001* |
| HX RD | 41 (20%) | 23 (6%) | <.0001* |
| Emergent | 16 (8%) | 28 (7%) | .7000 |
| Redo stern. | 24 (12%) | 40 (10%) | .4986 |
DM, diabetes mellitus; HTN, hypertension; Hx RD, history of renal disease; MOD-SEV, moderate to severe; stern, sternotomy.
Statistical Significance. Data are Mean ± SD.
For intraoperative and postoperative measures (Table 2), there was no significant difference between the groups regarding the type of procedure, pump time, cross-clamp time, circulatory arrest time, lowest temperatures, need for reoperation, urine output on CPB, or a need for extracorporeal membrane oxygenation. There was a significant increase in the number of patients who received del Nido cardioplegia in the prescriptive group, with 50% of cases vs. 32% in the conventional group (p < .0001). In addition, more cases required the use of an intra-aortic balloon pump (IABP) in the control group, with it being used on 9% of cases vs. the prescriptive group, which only used IABP in 3% of cases (p = .0121).
Table 2.
Intraoperative and postoperative results.
| Matched Cohort (n = 605) | |||
|---|---|---|---|
| Variable | Prescriptive Circuit (n = 204) | Conventional Circuit (n = 401) | p-Value* |
| CABG | 102 (50%) | 207 (52%) | .7061 |
| Valve | 80 (39%) | 154 (38%) | .8463 |
| Aortic | 47 (23%) | 85 (21%) | .6040 |
| Other | 6 (3%) | 12 (3%) | .9720 |
| Multiple procedures | 30 (15%) | 56 (14%) | .8052 |
| del Nido | 101 (50%) | 128 (32%) | <.0001* |
| ECMO | 6 (3%) | 7 (2%) | .3377 |
| IABP | 7 (3%) | 36 (9%) | .0121* |
| Re-operation | 26 (13%) | 46 (11%) | .6474 |
| CPB time (min) | 123 (±56.1) | 120 (±48.9) | .6376 |
| Cross-clamp (min) | 77 (±35.6) | 78.3 (±34.3) | .5642 |
| CA time (min) | 4.1 (±10.1) | 4.0 (±10.7) | .6988 |
| Lowest temperature (°C) | 30.2 (±5.7) | 30.3 (±5.6) | .9705 |
| Starting prime volume (cc) | 1,084 (±141) | 1799 (±103) | <.0001* |
| Total prime volume (cc) | 725 (±349) | 1,182 (±382) | <.0001* |
| RAP (cc) | 419 (±316) | 649 (±349) | <.0001* |
| Crystalloid (cc) | 551 (±847.32) | 114 (±586) | <.0001* |
| Fluid balance CPB (cc) | 933 (±1,221) | 940 (±1,003) | .9502 |
| del Nido volume (cc) | 1,295 (±425) | 377 (±629) | <.0001* |
| Ultrafiltrate (cc) | 872 (±1,041) | 646 (±1,131) | <.0001* |
| Urine output CPB (cc) | 286 (±343) | 262 (±201) | .5423 |
| Ventilator (min) | 1,187 (±3,144) | 2030 (6,148) | .9604 |
| LOS postop (days) | 9.9 (±8.8) | 10 (±7.) | .3931 |
| SCr day 1 | 1.2 (±.77) | 1.6 (±1.4) | <.0001* |
CABG, coronary artery bypass graft; CA, circulatory arrest; ECMO, extracorporeal membrane oxygenation; RAP, reverse autologous prime; LOS, length of stay.
Statistical significance. Data are given in mean ± SD.
There were significant differences between the groups in variables pertaining to CPB-related volumes. There was a significant decrease in the prescriptive groups with a starting mean prime volume of 1,084 mL (±141) and total prime volume of 725 (±349) mL compared with the conventional group that had a starting prime volume of 1,798 (±102) mL and total prime volume of 1,181 (±381) mL (p < .0001). Retrograde autologous volume was higher in the conventional group than in the prescriptive group at 648 (±348) mL for conventional and 419 (±316) mL for prescriptive group, (p < .0001). del Nido volume was higher in the prescriptive group 1,295 (±425) mL vs. 377 (±629) mL in the conventional group (p < .0001). The addition of crystalloid solution on bypass and ultrafiltration volume was higher in the prescriptive group with 551 (±847) mL of crystalloid added and an average volume removal of 872 (±1,041) mL compared to the conventional group, which only saw 113 (±586) mL and 645 (±1,131), respectively (p < .0001). Of the postoperative measures, no difference was found in the examined length of stay and ventilator time. However, SCr postop day 1 was significantly lower in the prescriptive group 1.21 (±.77) and 1.63 (±1.35) in the conventional group (p < .0001).
No difference was found between the two groups’ baseline HCT, pre-bypass HCT, and lowest HCT on CPB. (Table 3) The drop in HCT from baseline to lowest on pump was found to be significantly less in the prescriptive circuit group 15.1 points (±4.9) than 16.2 (±4.9) in the conventional group (p = .0149). However, the drop in HCT from pre-bypass to lowest on pump was not significant. The immediate postoperative HCT was found to be significantly higher in the prescriptive group than the conventional group, with an average HCT of 32.8% (±4.5) vs. 31.7% (±4.9) (p = .0069). The HCT at 24 hours was significant in the unmatched cohort but lost significance once matched.
Table 3.
HCT and CPB hemodilution.
| Matched Cohort (n = 605) | |||
|---|---|---|---|
| Variable | Prescriptive Circuit (n = 204) | Conventional Circuit (n = 401) | p-Value* |
| HCT-baseline | 36.8 (±6.2) | 37.6 (±6.5) | .1836 |
| HCT-pre-bypass | 33.7 (±6.4) | 33.6 (±6.3) | .6961 |
| Nadir HCT (%) | 21.7 (±4.6) | 21.3 (±4.6) | .2502 |
| HCT drop (BASE:CPB) | 15.1 (±4.9) | 16.2 (±4.9) | .0149* |
| HCT drop (PRE:CPB) | 11.5 (±6.1) | 12.2 (±4.4) | .4720 |
| HCT-postop (%) | 32.8 (±4.5) | 31.7 (±5.0) | .0069* |
| HCT-ICU 24 hr (%) | 28.5 (±4.5) | 28.3 (±10.8) | .0552 |
BASE, baseline HCT; PRE, pre-bypass HCT; postop, postoperative.
Statistical significance. Data are presented as mean ± SD.
As shown in Table 4, there was no significant difference in on-bypass PRBC transfusions, post-bypass PRBC, FFP, platelets, or cryoprecipitate, across all total intraoperative products. We saw a significant increase in the number of FFP units given on bypass in the prescriptive group (.08 ± .36) compared with the conventional group (.03 ± .22), (p = .0128). Similarly, we found no significant difference across all blood products used in the ICU.
Table 4.
Transfusion requirements during CPB and total intraoperative and postoperative phases.
| Matched Cohort (n = 605) | |||
|---|---|---|---|
| Variable | Prescriptive Circuit (n = 204) | Conventional Circuit (n = 401) | p-Value* |
| PRBC-CPB (units) | .48 (±.97) | .51 (±1.14) | .6953 |
| FFP-CPB (units) | .08 (±.36) | .03 (±.22) | .0128* |
| PRBC POST-CPB (units) | .66 (±1.75) | .77 (±1.72) | .9264 |
| FFP POST-CPB (units) | .79 (±1.99) | .67 (±1.52) | .8946 |
| PLT POST-CPB (units) | .48 (±1.1) | .45 (±.91) | .5956 |
| Cryoprecipitate (units) | .31 (±.91) | .25 (±.76) | .6093 |
| Tot. intraoperative PRBC (units) | 1.14 (±2.17) | 1.28 (±2.35) | .9175 |
| Tot. intraoperative FFP (units) | .87 (±2.04) | .70 (±1.59) | .5293 |
| Tot. intraoperative prod. (units) | 2.79 (±5.45) | 2.68 (±4.92) | .3934 |
| PRBC ICU (units) | 1.35 (±4.24) | 1.48 (±3.99) | .1686 |
| FFP-ICU (units) | .57 (±2.51) | .57 (±2.40) | .7112 |
| PLT-ICU (units) | .22 (±1.04) | .34 (±1.51) | .1416 |
| Tot. ICU prod. (units) | 3.08 (±3.08) | 2.52 (±8.09) | .1770 |
PLT, platelets; POST, post-bypass; tot, total; prod, products.
Statistical significance. Data are presented as mean ± SD.
DISCUSSION
Implementation of the prescriptive circuit resulted in a significant reduction in prime volume; however, we did not see a significant change in our lowest HCT, although we did see an increasing trend. The nadir on-bypass HCT in this study represented the lowest overall measure during the bypass period. It would be beneficial to know at what time the lowest level was recorded because this would allow us to determine if the lowest HCT was due to CPB priming volume, the addition of cardioplegic solutions, or other operative factors.
An additional factor that could have impacted the nadir HCT on bypass was the significant increase in cases that received del Nido cardioplegia and the significant increase in del Nido volume administered to the prescriptive group. del Nido added an average of 1,295.49 (±425.31) mL in the prescriptive group vs. 377.44 (±629.39) mL in the matched control group. This is a significant amount of crystalloid and can lead to more hemodilution on bypass. Because of the possible impact del Nido made in this cohort, examination of the del Nido stratification is warranted.
In addition to nadir HCT on bypass, the drop in HCT from baseline to bypass and pre-bypass to bypass was also examined as a measure of CPB hemodilution. The HCT drops from baseline to lowest on pump significantly decreased from 16.2 points in the conventional group to 15.1 points in the prescriptive, indicating a reduction in bypass-related hemodilution. However, the HCT drop from pre-bypass to nadir on pump was not found to be significant. This could be because of the differences in the measurement of the pre-bypass HCT between the two groups. Before this study, pre-bypass HCT was not measured at a standardized time; it could have been drawn at any time between entrance into the operating room and initiation of bypass. For the prescriptive group, the pre-bypass HCT measurement was standardized to be drawn immediately before initiation because this would provide an HCT that would account for HCT changes due to IVF administered by anesthesia.
Despite the implementation of the prescriptive circuit, we saw no significant change in on-bypass PRBC transfusions, total intraoperative transfusions, or postoperative transfusions. The only area that we found a significant difference was units of FFP on bypass in the prescriptive group. During the study, we did not specify the trigger for administration of FFP on bypass. Our current clinical practice usually only adds FFP on bypass if there is concern of possible AT3 deficiency, due to inability to maintain a therapeutic ACT, or if volume is inadequate to achieve desired flow and hemodilution of coagulation factors is a concern. Therefore, we were unable to determine if the difference in FFP transfusion rates was the result of increased AT3 deficiency among patients or the combination of decreased circulating volume and increased hemodilution of coagulation factors.
Our current departmental trigger for the use of PRBCs is an HCT of 18%. However, we do not have strict transfusion protocol, and decisions to transfuse are based on patient parameters at that time such as hemodynamic stability, reservoir volume, and risk of coagulopathies. Although this process allows for clinicians their autonomy, it lends a great deal of inconsistency in transfusion triggers across all cardiac surgery departments. In addition, anesthesia serves as the gatekeeper for blood products at our institution, and changes in personnel and/or departmental protocol could have affected transfusion triggers. Adoption of a strict, multidisciplinary protocol could yield more significant reduction in blood product usage.
To determine the effect of the prescriptive circuit on postoperative hemodilution outcomes, we compared the HCT immediately after arrival in the ICU. We found a significant increase in postoperative HCT in the prescriptive group, with an average HCT of 32.79% vs. the control group which had an average of 31.68%. A second measure of postoperative hemodilution compared the HCT between the two groups 24 hours after being transported to the ICU. Although it was not found to be significant once propensity matching was completed, it provides evidence of a strong trend toward increased 24-hour postoperative HCT and warrants further investigation with a larger sample size. Despite the increased postoperative HCT, transfusion rates in the ICU did not improve.
One of the limitations of this study is the use of a retrospective cohort that relied on chart review. Although multiple sources were used to find missing data, some information was not recorded that could have affected the results. For example, in the conventional group, the addition of crystalloid solution during CPB often had no value, and there was no way to determine whether the value was missing or whether there was no crystalloid added. This could have significantly affected measures pertaining to intraoperative hemodilution. In addition, we found that some data points had variability in the conventional cohort, such as the measurement of the pre-bypass HCT, as mentioned earlier. A second limitation is the study size. Studies that have reported significant changes in on-bypass HCT and blood product transfusions were retrospective chart reviews with a large population over many years (14–16). Our study examined the use of prescriptive circuits early in its adoption and involved a complete overhaul of the CPB circuit from a closed bag system to an open reservoir system, and it is believed that further reductions in tubing length and increased perfusionist comfort with the new circuit and protocol could result in more significant improvement. In addition, the use of a larger control group could result in better matching to the intervention group and, thus, further reduce bias. Another limitation was the exclusion of anesthesia IV fluids. In this study, we attempted to keep anesthesia blinded over concern that by requesting the total IV fluids from anesthesia, it would alert them to the study and, possibly, alter their clinical decisions. As reported by Campbell and colleagues, pre-CPB fluid contributes significantly to hemodilutional anemia and blood transfusion requirements (17). Therefore, future iterations of this study would benefit from the inclusion of IVF as a contributor to intraoperative anemia.
Last, the use of del Nido cardioplegia significantly increased in the intervention group, with 50% of the cases using it for cardiac arrest and myocardial protection. This increase was due to increased adoption as the preferred cardioplegic solution by members of the surgical team, and it was not able to be controlled for in this study. Although studies have shown that overall transfusion rates are not significantly different between del Nido and standard cardioplegia, the association between del Nido volume and CPB hemodilution in this study warrants further review(18,19). In summary, implementation of the prescriptive circuit did not reduce on-bypass or intraoperative blood product usage. However, there was a significant reduction in the HCT drop on bypass and increased HCT postoperatively, indicating a reduction in on-bypass hemodilution. Our findings show that prescriptive CPB circuits can reduce on-bypass hemodilution; however, its effectiveness in reducing blood product usage warrants further study.
REFERENCES
- 1.Society of Thoracic Surgeons Blood Conservation Guideline Task Force; Ferraris VA, Brown JR, et al. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. [DOI] [PubMed] [Google Scholar]
- 2.Engoren MC, Habib RH, Zacharias A, et al. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–6. [DOI] [PubMed] [Google Scholar]
- 3.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. [DOI] [PubMed] [Google Scholar]
- 4.Loor G, Koch CG, Sabik JF III, et al. Implications and management of anemia in cardiac surgery: Current state of knowledge. J Thorac Cardiovasc Surg. 2012;144:538–46. [DOI] [PubMed] [Google Scholar]
- 5.Pradeep A, Rajagopalam S, Kolli HK, et al. High volumes of intravenous fluid during cardiac surgery are associated with increased mortality. HSR Proc Intensive Care Cardiovasc Anesth. 2010;2:287–96. [PMC free article] [PubMed] [Google Scholar]
- 6.Habib RH, Zacharias A, Schwann TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: Should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–50. [DOI] [PubMed] [Google Scholar]
- 7.Ranucci M, Aloisio T, Carboni G, et al. Acute kidney injury and hemodilution during cardiopulmonary bypass: A changing scenario. Ann Thorac Surg. 2015;100:95–100. [DOI] [PubMed] [Google Scholar]
- 8.Loor G, Rajeswaran J, Li L, et al. The least of 3 evils: Exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg. 2013;146:1480–7. [DOI] [PubMed] [Google Scholar]
- 9.Ranucci M, Conti D, Castelvecchio S, et al. Hematocrit on cardiopulmonary bypass and outcome after coronary surgery in nontransfused patients. Ann Thorac Surg. 2010;89:11–7. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann B, Kaufmann C, Stiller M, et al. Positive impact of retrograde autologous priming in adult patients undergoing cardiac surgery: A randomized clinical trial. J Cardiothorac Surg. 2018;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao S, Li Y, Diao X, et al. Vacuum-assisted venous drainage in adult cardiac surgery: A propensity-matched study. Interact Cardiovasc Thorac Surg. 2020;2:236–42. [DOI] [PubMed] [Google Scholar]
- 12.Baker RA, Bronson SL, Dickinson TA, et al. Report from AmSECT’s international consortium for evidence-based perfusion: American society of extracorporeal technology standards and guidelines for perfusion practice: 2013. J Extra Corpor Technol . 2013;45:156–66. [PMC free article] [PubMed] [Google Scholar]
- 13.Lahanas A, Argerakis PW, Johnson KA, et al. A retrospective comparison of blood transfusion requirements during cardiopulmonary bypass with two different small adult oxygenators. Perfusion. 2013;28:541–5. [DOI] [PubMed] [Google Scholar]
- 14.Bronson SL, Riley JB, Blessing JP, et al. Prescriptive patient extracorporeal circuit and oxygenator sizing reduces hemodilution and allogeneic blood product transfusion during adult cardiac surgery. J Extra Corpor Technol. 2013;45:167–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Blessing JM, Riley JB. Lean flow: Optimizing cardiopulmonary bypass equipment and flow for obese patients-A technique article. J Extra Corpor Technol . 2017;49:30–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Ariyaratnam P, Mclean LA, Cale A, et al. Mini-extracorporeal circulation technology, conventional bypass and prime displacement in isolated coronary and aortic valve surgery: A propensity-matched in-hospital and survival analysis. Interact Cardiovasc Thorac Surg. 2018;27:13–9. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JA, Holt DW, Shostrom VK, et al. Influence of intraoperative fluid volume on cardiopulmonary bypass hematocrit and blood transfusions in coronary artery bypass surgery. J Extra Corpor Technol. 2008;40:99–108. [PMC free article] [PubMed] [Google Scholar]
- 18.Stammers AH, Tesdahl EA, Mongero LB, et al. Does the type of cardioplegic technique influence hemodilution and transfusion requirements in adult patients undergoing cardiac surgery? J Extra Corpor Technol. 2017;49:231–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Stammers AH, Tesdahl EA, Mongero LB, et al. Does the type of cardioplegia used during valve surgery influence operative nadir hematocrit and transfusion requirements? Perfusion. 2018;33:638–48. [DOI] [PubMed] [Google Scholar]