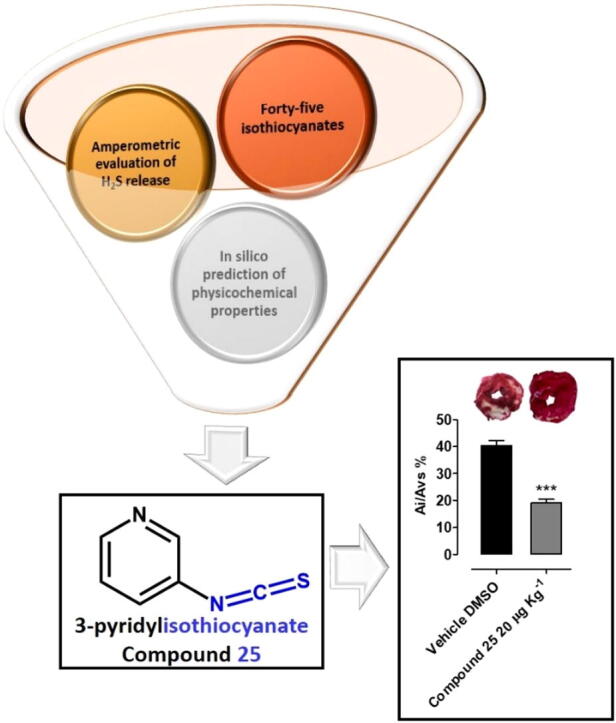
Graphical abstract
Keywords: Hydrogen sulfide, H2S releasing compounds, Isothiocyanates, In silico prediction, Cardioprotection
Abstract
Introduction
The gasotransmitter hydrogen sulphide (H2S), an endogenous ubiquitous signalling molecule, is known for its beneficial effects on different mammalian systems. H2S exhibits cardioprotective activity against ischemia/reperfusion (I/R) or hypoxic injury.
Methods
A library of forty-five isothiocyanates, selected for their different chemical properties, has been evaluated for its hydrogen sulfide (H2S) releasing capacity. The obtained results allowed to correlate several factors such as steric hindrance, electronic effects and position of the substituents to the observed H2S production. Moreover, the chemical-physical profiles of the selected compounds have been studied by an in silico approach and from a combination of the obtained results, 3-pyridyl-isothiocyanate (25) has been selected as the most promising one. A detailed pharmacological characterization of its cardioprotective action has been performed.
Results
The results herein obtained strongly indicate 3-pyridyl-isothiocyanate (25) as a suitable pharmacological option in anti-ischemic therapy. The cardioprotective effects of compound 25 were tested in vivo and found to exhibit a positive effect.
Conclusion
Results strongly suggest that isothiocyanate-based H2S-releasing drugs, such as compound 25, can trigger a ‘‘pharmacological pre-conditioning” and could represent a suitable pharmacological option in antiischemic therapy.
Introduction
The gasotransmitter hydrogen sulphide (H2S), an endogenous ubiquitous signalling molecule, is known for its beneficial effects on different mammalian systems [1]. H2S is mainly produced from l-Cysteine via the catalytic activity of two pyridoxal-5′-phosphate dependent enzymes, known as cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) [2]. A third pathway, where 3-mercaptopyruvate sulphur transferase (3-MST) and cysteine aminotransferase (CAT) [3], [4], [5] cooperate, is also responsible for H2S biosynthesis. Endogenous H2S is involved in many physio-pathological processes and the role of H2S has been mostly studied by inhibiting its physiological production [6], [7] or using exogenous sources of H2S.
H2S exhibits cardioprotective activity against ischemia/reperfusion (I/R) or hypoxic injury. The mechanisms of action accounting for this cardioprotective activity involve mitochondrial ATP-sensitive potassium channels (mitoKATP) [8], anti-apoptotic responses [9] and inhibition of type-5 phosphodiesterase [10]. Furthermore, H2S behaves as an antioxidant molecule, able to activate the Nrf-2-mediated machinery and reduce the reactive oxygen species, suggesting that also this effect may be involved in the protective properties against I/R injury [11].
Due to the intriguing biological activities of H2S in the cardiovascular function, compounds able to generate exogenous H2S, are viewed as promising cardioprotective agents. In this scenario, different classes of H2S-donors have been described in the literature, such as GYY4137 [12], thiadiazolidin-3,5-diones [13], arylthioamides [14], iminothioethers [15], mercaptopyruvate [16], dithioates [17]. Furthermore, also natural [18], [19] and synthetic [20] isothiocyanates are known to generate H2S with a slow kinetic and in an l-Cysteine dependent manner. In addition, hybrid molecules bringing an isothiocyanate portion were developed and investigated in neurodegenerative diseases [21], [22], [23].
Despite their numerous properties, natural isothiocyanates have some limitations: most of them, including sulforaphane, are volatile oils and particularly unstable at room temperature. In fact, they are spontaneously converted into several inactive intermediates with relatively high degradation rates [24].
Starting from these findings [25], [26], we selected a series of natural and synthetic isothiocyanates, aiming at defining a structure-activity relationship correlating their structure and H2S-releasing properties. We selected forty-five aliphatic and aromatic isothiocyanates, commercially available, variously substituted both in terms of steric hindrance and electronic properties. Aromatic derivatives included compounds with electron-withdrawing and electron-donating substituents in ortho, meta and para positions with respect to the -SCN moiety. Pyridine (25) and naphthalene (37) derivatives were selected as ring equivalents of phenyl isothiocyanate (1). Among the selected compounds, 3-pyridyl-isothiocyanate (25) showed the highest H2S releasing ability. This compound has been employed in a different research field as starting material for the synthesis of cationic polymers for targeted delivery [27], [28]. Here we describe it as an efficient H2S donor that was thoroughly studied for its pharmacological activity in different experimental models of I/R injury in rats.
Materials and methods
Substances
All isothiocyanates were commercial products purchased from Fluorochem (Hadfield, UK).
Amperometric approach
The H2S-generating properties of the tested compounds have been evaluated by an amperometric approach, through an Apollo-4000 Free Radical Analyzer (WPI) detector and H2S-selective mini-electrodes at room temperature, in phosphate buffer solution at pH 7.4 in the absence or in the presence of l-cysteine 4 mM, as reported previously [18]. The generation of H2S was observed for 30 min.
Molecules preparation and prediction of physicochemical properties
The three-dimensional structures of the selected molecules were built in Maestro molecular modelling environment (Maestro release 2018) and minimized using MacroModel software (MacroModel, Schrödinger, LLC, New York, NY, 2018) as previously described [28], [29]. Furthermore, LigPrep (LigPrep, Schrödinger, LLC, New York, NY, 2018) application was used to refine the chemical structures. The resulting compounds, saved as sdf file, were investigated for their physicochemical properties. This investigation was performed by the webserver FAFDrugs4.0 (http://fafdrugs4.mti.univ-paris-diderot.fr/ access May 2019) [30].
Compound 25 intracellular H2S release measurement
H9c2 were cultured up to about 90% confluence and 24 h before the experiment cells were seeded onto a 96 well clear bottom black plate at a density of 72 × 103 per well. After 24 h, the medium was replaced and cells were incubated for 30 min with a 100 μM solution of the fluorescent dye WSP-1 (Washington State Probe, 1,3′-methoxy-3-oxo-3H-spiro[isobenzofuran-1,9′-xanthen]-6′-yl 2-(pyridine-2-yl-disulfanyl benzoate) that is highly sensitive for H2S detection [31], [32]. Then, the supernatant was removed and replaced with different solutions of compound 25 dissolved in standard buffer (HEPES 20 mM; NaCl, 120 mM; KCl, 2 mM; CaCl2·2H2O, 2 mM; MgCl2·6H2O, 1 mM; glucose, 5 mM; and pH 7.4, at room temperature) at three increasing concentrations (30, 100, and 300 μM). The change in fluorescence (expressed as fluorescence index measured at λ = 465–515 nm) was monitored every 5 min for 60 min, by means of a spectrofluorometer. On the bases of previous experiments [15], [33], 4-carboxyphenylisothiocyanate (4-CPI, Sigma-Aldrich) 300 μM was used as slow H2S donor reference compound. Six different experiments (n = 6) were performed, each carried out in three replicates. The results are expressed as mean ± SEM.
Animal procedures and ethical statements
All the procedures involving animals were carried out following the guidelines of the European Community Council Directive 86-609 and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki, EU Directive 2010/63/EU for animal experiments). All the experiments were authorized by the Ethical Committee of the University of Pisa and by the Italian Ministry of Health (authorization number 45972/2016). All the animals were housed in humidity- and temperature-controlled rooms (22 °C and 50%, respectively) with 12 h light/dark cycles, water, and food availability ad libitum.
Measurement of coronary flow
Adult male normotensive Wistar rats (300–350 g) were anaesthetized with an overdose of sodium thiopental (100 mg·kg−1 i.p.); hearts were quickly excised, rapidly mounted in a Langendorff apparatus (Radnoti, Monrovia, USA) and perfused with Krebs solution (NaHCO3, 25 mM; NaCl, 118.1 mM; KCl, 4.8 mM; MgSO4, 1.2 mM; CaCl2·2H2O, 1.6 mM; KH2PO4, 1.2 mM; and glucose, 11.5 mM) gassed with clioxicarb at 37 °C at constant pressure (70–80 mmHg). As reported previously [34], coronary flow (CF) was volumetrically recorded every 5 min, expressed as mL·min−1, and normalized to the heart weight (g). After a 20 min equilibration period, some hearts were selected to determine the effects of compound 25 on CF of hearts precontracted with angiotensin II (AngII). These hearts were perfused with AngII 0.1 μM, and at the onset of a stable coronary spasm (observed as a reduction in the CF), cumulatively increasing concentrations of compound 25 (1, 3, 10 and 30 μM, for 20 min) were perfused (in the constant presence of AngII 0.1 μM). Changes in CF are expressed as percentage (%) of the basal CF. Experiments were carried out on hearts from six animals (n = 6) for each different treatment.
Ex vivo ischemia/reperfusion injury
Male Wistar rats (260–350 g) were randomized in 8 groups and were treated with an intra peritoneal (i.p.) injection of different increasing doses of compound 25 180 µg·kg−1, 60 µg·kg−1, 20 µg·kg−1 and 6.7 µg·kg−1 or diazoxide 40 mg.Kg−1 (a mitoKATP opener) or 4-CPI 240 µg.Kg−1 or vehicle (DMSO) or 5-hydroxy decanoic acid (5-HD) 10 mg·kg−1 for 20 min followed by compound 25 20 µg·kg−1. After 2 h, all the animals were anaesthetized with sodium thiopental (100 mg·kg−1 i.p.) and heparinized (100UI i.p.) to prevent blood clotting. After opening the chest, hearts were quickly excised, mounted on a Langendorff apparatus as reported previously. A water-filled latex balloon connected to a pressure transducer (Bentley Trantec, mod 800, UgoBasile, Comerio, Italy) was introduced into the left ventricle via the mitral valve and the volume was adjusted to achieve a stable left ventricular end-diastolic pressure of 5–10 mmHg during initial equilibration. After 30 min of equilibration, hearts were subjected to 30 min of global ischemia (no flow). Thereafter, hearts were perfused for 120 min. Functional parameters were continuously recorded during the whole experiment. At the end of reperfusion hearts were removed from the Langendorff apparatus and left ventricles were isolated and submitted to morphometric assays. Experiments were carried out on hearts from six animals (n = 6) for each different treatment.
Morphometric analysis of the ischemic area
The potential cardioprotective effects of compound 25 have been evaluated by the possible reduction of the size of injured areas in compound 25-treated hearts submitted to I/R. The left ventricle was cut in 2 mm-wide slices which were immersed in a 1% aqueous solution of 2,3,5-triphenyltetrazolium chloride (TTC) for 20 min at 37 °C and then in a 10% aqueous solution of formaldehyde. After 24 h, ventricular slices were photographed and analysed to highlight necrotic areas due to the ischemic process (visible as a white or light pink color) and the healthy areas (visible as a strong red due to the TTC reaction).
LDH activity measurement
The LDH was measured for dynamic monitoring of cellular damage with the experiments lasting 120 min of reperfusion. Coronary effluent samples were collected at the last 5 min of the pre-ischemic phase and every 5 min during the first 30 min of reperfusion and then every 10 min—a total of 13 samples were collected per heart. The flow rate was measured each time an LDH sample was collected. The samples were stored on ice until the end of each experiment. The LDH was assessed by a spectrophotometric method, by adding 27.6 mM pyruvate and 4.8 mM NADH, and measuring the conversion of NADH to NAD+ at the wavelength of λ = 340 nm. The amount of released LDH has been expressed in enzymatic mU·g−1 released in 120 min of reperfusion (without the small amount recorded in the pre-ischemic phase), resulting from the AUC analysis (area under the curve of the LDH amount recorded) and related to 1 g of the heart weight.
Acute in vivo myocardial infarction
The cardioprotective effects of compound 25 were evaluated in vivo, in an experimental model more closely resembling the clinical condition of acute myocardial infarction. The experimental protocol for coronary occlusion-reperfusion was performed as described previously [35], with minor modifications. Two hours before the experimental procedure, rats received an i.p. injection (about 0.3 mL) of compound 25 (20 µg·kg−1) or diazoxide 40 mg·Kg−1 (a mitoKATP opener) or 4-CPI 240 µg·Kg−1 or vehicle (DMSO). Then, rats were anaesthetized with sodium thiopental (70 mg·kg−1, i.p.). The trachea was intubated and connected to a rodent ventilator (mod. 7025 UgoBasile, Comerio, Italy) for artificial ventilation with room air (stroke volume, 1 mL/100 g body weight; 70 S/min). Electrocardiogram (ECG) was continuously measured by lead II (Mindray, PM5000, 2 Biological Instruments, Varese, Italy). The chest was opened by a left thoracotomy. A 6–0 surgical needle was passed around the left anterior descending coronary artery (LAD), located between the base of the pulmonary artery and left atrium. The ends of the suture were passed through a polypropylene tube (PE50) to form a snare, allowing reversible artery occlusion. The acute infarction protocol consisted of 30 min occlusion/120 min reperfusion; successful occlusion was confirmed by observing regional cyanosis downstream of the ligature, and by ST elevation in the ECG recording. At the end of reperfusion, rats were euthanized by an overdose of sodium thiopental, hearts were quickly excised, mounted on a Langendorff apparatus and perfused for 10 min with Krebs solution at 37 °C to clean coronary blood vessels. Then, the atria and right ventricle were removed from the hearts. The left ventricular tissue was dried, frozen at − 20 °C, and cut into 4–5 transverse slices from apex to base of equal thickness (about 2 mm). The slices were submitted to morphometric analysis. Experiments were carried out on hearts from six animals (n = 6) for each different treatment.
Results
Amperometric determination of H2S release
The compounds selected comprehend both aliphatic and aromatic isothiocyanates characterized by different substituents (electron donating/withdrawing and different steric hindrance groups). All the compounds were incubated at the concentration of 1 mM in the presence or in the absence of l-Cys 4 mM to evaluate their H2S releasing properties. The results about the amount of H2S produced are expressed as Cmax in µM (maximal concentration of H2S at the steady state) and are listed in Table 1.
Table 1.
Structures and parameters of Cmax of the forty-five isothiocyanates (1 mM) were determined after incubation in the assay buffer at physiological pH and temperature, in the absence or in presence of 4 mM l-Cysteine.
| Compd | Structure | H2S-release without l-cysteine 4 mM(Cmax μM) | H2S-release + l-cysteine (Cmax μM) |
|---|---|---|---|
| 1 | 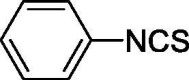 |
1.214 ± 0.145 | 10.029 ± 1.206 |
| 2 | 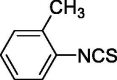 |
1.041 ± 0 | 2.396 ± 0.515 |
| 3 | 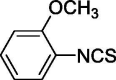 |
2.386 ± 0.974 | 5.270 ± 0.557 |
| 4 | 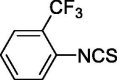 |
0.364 ± 0 | 4.154 ± 0.537 |
| 5 | 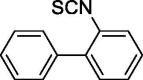 |
0 | 0.29 ± 0.02 |
| 6 | 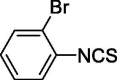 |
3.617 ± 1.030 | 13.335 ± 1.863 |
| 7 | 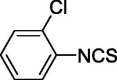 |
0 | 18.309 ± 2.220 |
| 8 | 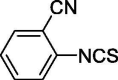 |
0 | 6.33 ± 1.51 |
| 9 |  |
0 | 0.16 ± 0.01 |
| 10 | 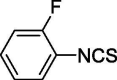 |
5.351 ± 0 | 23.406 ± 3.506 |
| 11 | 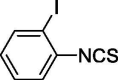 |
0 | 1.93 ± 0.14 |
| 12 | 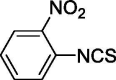 |
0.614 ± 0.267 | 20.066 ± 0.337 |
| 13 | 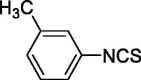 |
0.411 ± 0 | 5.732 ± 0.929 |
| 14 | 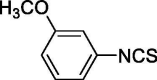 |
0.865 ± 0.303 | 6.422 ± 0.551 |
| 15 | 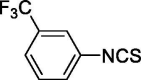 |
0.168 ± 0 | 19.028 ± 1.328 |
| 16 | 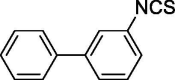 |
0 | 0.15 ± 0.02 |
| 17 | 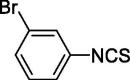 |
5.355 ± 1.521 | 17.750 ± 2.961 |
| 18 | 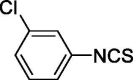 |
0 | 21.575 ± 1.220 |
| 19 | 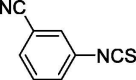 |
0 | 29.29 ± 1.85 |
| 20 | 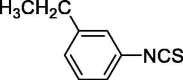 |
0 | 1.2 ± 0.9 |
| 21 | 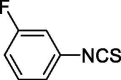 |
3.594 ± 0 | 36.919 ± 4.399 |
| 22 | 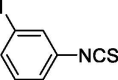 |
0 | 2.47 ± 0.14 |
| 23 | 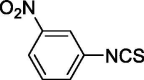 |
0.912 ± 0.432 | 22.158 ± 1.857 |
| 24 | 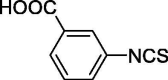 |
0.59 ± 0.38 | 30.32 ± 1.37 |
| 25 | 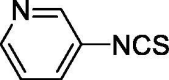 |
8.719 ± 1.555 | 65.436 ± 4.356 |
| 26 |  |
0.612 ± 0 | 9.064 ± 0.444 |
| 27 |  |
1.550 ± 0.441 | 7.411 ± 0.126 |
| 28 |  |
0.786 ± 0 | 32.007 ± 3.404 |
| 29 | 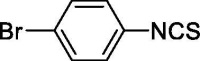 |
5.350 ± 1.000 | 37.713 ± 4.866 |
| 30 | 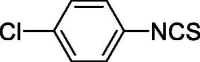 |
1.876 ± 0 | 17.576 ± 2.997 |
| 31 | 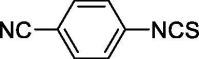 |
0 | 16.58 ± 0.32 |
| 32 | 0 | 3.87 ± 0.42 | |
| 33 | 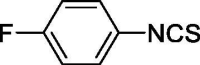 |
3.777 ± 0 | 19.517 ± 1.194 |
| 34 | 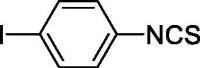 |
0 | 1.19 ± 0.27 |
| 35 | 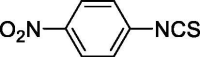 |
0.635 ± 0.369 | 24.880 ± 4.449 |
| 36 | 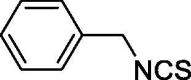 |
0 | 1.246 ± 0.891 |
| 37 | 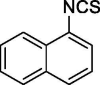 |
0.205 ± 0.005 | 0.36 ± 0.03 |
| 38 | 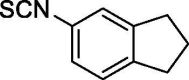 |
0 | 2.08 ± 0.05 |
| 39 |  |
0.674 ± 0.286 | 1.124 ± 1.015 |
| 40 | 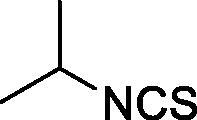 |
0 | 0 |
| 41 | 2.541 ± 0.585 | 12.973 ± 0.981 | |
| 42 | 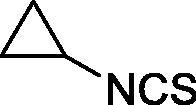 |
1.389 ± 0.586 | 0 |
| 43 | 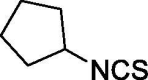 |
4.092 ± 0.413 | 0 |
| 44 | 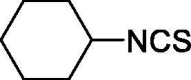 |
0.161 ± 0 | 0.461 ± 0 |
| 45 | 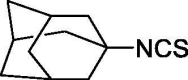 |
0 | 0 |
The incubation in the assay buffer without l-cysteine led to a negligible or very low release of H2S (Cmax < 2 μM; Table 1) for most of the compounds. Following l-cysteine addition, the H2S release is, generally, more significant with the highest Cmax value of 65.436 µM (compound 25).
Referring to the l-cysteine evoked H2S release, the structure-activity relationships, relative to the selected compounds, can be described as follows. As a general trend, the aromatic compounds were more efficient donors with respect to the aliphatic ones. The greater ability to release H2S is associated with the presence on the aromatic ring of electron-withdrawing groups with a fairly linear trend observable in the series of ortho- meta- and para- substituted compounds:
o-CH2CH3< o-I< o-CH3< o-CF3< o-OCH3< o-CN< H< o-Br< o-Cl< o-NO2< o-F
m-CH2CH3< m-I< m-CH3< m-OCH3< H< m-Br< m-CF3< m-Cl< m-NO2< m-CN< m-F
p-I< p-CH2CH3< p-OCH3< p-CH3< H< p-CN< p-Cl< p-F< p-NO2< p-CF3< p-Br
The ability to release H2S follows the order ortho < meta < para for all the compounds with the only exception of chloro-, fluoro- and iodo-substituted derivatives that follow the order para < ortho < meta. The poor H2S releasing profile of the o-, m- and p-iodo-phenylisothiocyanates results from the combination of steric factors (particularly important for the ortho- and meta-derivatives) and reactivity features. Aryl iodide, in fact, are very prone to C-S cross-coupling with aromatic and aliphatic thiols.
The most efficacious H2S donor was compound 25, 3-pyridyl-isothiocyanate, where the electron-deficient nature of the pyridine ring, attributed to the electron-withdrawing, inductive, and mesomeric effects of the nitrogen atom, accounts for the high H2S release. The molecular mechanism responsible for thiol activated H2S release from isothiocyanate has been recently elucidated in detail [36]. It involves an intramolecular cyclization of the cysteine-ITC intermediate crucial for the H2S releasing. The strongly electrophilic nature of the –SCN moiety is amplified by the presence of electron-withdrawing groups on the aromatic ring thus making the central carbon more prone to undergo a nucleophilic attack.
In silico evaluation of physicochemical properties
The drug-like profile represents a critical property for assuring the advancement of a drug candidate into preclinical studies and clinical trials. Notably, at the beginning of drug discovery trajectory it is particularly important to prioritize potential hit compounds that could be suitable starting points for searching novel drug candidates. Accordingly, it is a common procedure, during the drug discovery pipeline, consider some molecular descriptors for selecting potential drug candidates with satisfactory physicochemical properties and ADMET (absorption, distribution, metabolism, excretion, and toxicity) profile [29], [37], [38], [39]. Accordingly, we performed an in silico analysis to determine which derivatives could be submitted to further cellular and in vivo studies. The calculation of the physicochemical properties, regarding the molecules reported in Table 1, was performed by means of the online server FAFDrugs4.0 and the output is reported in Table 2.
Table 2.
In silico drug-like profile of the molecules presented in this work.
| Compd | MWa | logPb | logSwc | tPSAd | Lipinskie | Solubility (mg L−1) | Solubility FIf | VEBERg | EGANh | 4_400i | 3_75j |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 135.19 | 3.40 | −3.07 | 44.45 | 0 | 6267 | Good | Good | Good | Good | Bad |
| 2 | 149.21 | 3.69 | −3.28 | 44.45 | 0 | 5587 | Good | Good | Good | Good | Bad |
| 3 | 165.21 | 3.29 | −3.05 | 53.68 | 0 | 7811 | Good | Good | Good | Good | Bad |
| 4 | 203.18 | 4.21 | −3.88 | 44.45 | 0 | 4191 | Good | Good | Good | Good | Bad |
| 5 | 211.28 | 4.95 | −4.51 | 44.45 | 0 | 2332 | Reduced | Good | Good | Good | Bad |
| 6 | 214.08 | 4.01 | −3.94 | 44.45 | 0 | 4144 | Good | Good | Good | Good | Bad |
| 7 | 169.63 | 3.95 | −3.63 | 44.45 | 0 | 4492 | Good | Good | Good | Good | Bad |
| 8 | 160.20 | 3.04 | −2.98 | 68.24 | 0 | 8150 | Good | Good | Good | Good | Bad |
| 9 | 163.24 | 4.12 | −3.54 | 44.45 | 0 | 4747 | Good | Good | Good | Good | Bad |
| 10 | 153.18 | 3.42 | −3.20 | 44.45 | 0 | 6272 | Good | Good | Good | Good | Bad |
| 11 | 261.08 | 3.97 | −4.21 | 44.45 | 0 | 3872 | Good | Good | Good | Good | Bad |
| 12 | 180.18 | 3.15 | −3.09 | 90.27 | 0 | 8282 | Good | Good | Good | Good | Bad |
| 13 | 149.21 | 3.72 | −3.30 | 44.45 | 0 | 5482 | Good | Good | Good | Good | Bad |
| 14 | 165.21 | 3.68 | −3.30 | 53.68 | 0 | 6109 | Good | Good | Good | Good | Bad |
| 15 | 203.18 | 4.37 | −3.98 | 44.45 | 0 | 3789 | Good | Good | Good | Good | Bad |
| 16 | 211.28 | 4.99 | −4.53 | 44.45 | 0 | 2274 | Reduced | Good | Good | Good | Bad |
| 17 | 214.08 | 4.12 | −4.01 | 44.45 | 0 | 3866 | Good | Good | Good | Good | Warning |
| 18 | 169.63 | 3.36 | −3.26 | 44.45 | 0 | 6514 | Good | Good | Good | Good | Bad |
| 19 | 160.20 | 3.13 | −3.04 | 68.24 | 0 | 7700 | Good | Good | Good | Good | Bad |
| 20 | 163.24 | 4.16 | −3.56 | 44.45 | 0 | 4629 | Good | Good | Good | Good | Bad |
| 21 | 153.18 | 3.53 | −3.26 | 44.45 | 0 | 5852 | Good | Good | Good | Good | Bad |
| 22 | 261.08 | 4.94 | −4.82 | 44.45 | 0 | 2101 | Reduced | Good | Good | Good | Bad |
| 23 | 180.18 | 3.26 | −3.16 | 90.27 | 0 | 7672 | Good | Good | Good | Good | Warning |
| 24 | 179.20 | 2.96 | −2.94 | 84.58 | 0 | 9426 | Good | Good | Good | Good | Good |
| 25 | 136.17 | 2.25 | −2.38 | 57.34 | 0 | 12,637 | Good | Good | Good | Good | Warning |
| 26 | 149.21 | 3.92 | −3.43 | 44.45 | 0 | 4833 | Good | Good | Good | Good | Bad |
| 27 | 165.21 | 3.58 | −3.23 | 53.68 | 0 | 6507 | Good | Good | Good | Good | Bad |
| 28 | 203.18 | 4.44 | −4.03 | 44.45 | 0 | 3625 | Good | Good | Good | Good | Bad |
| 29 | 214.08 | 4.03 | −3.96 | 44.45 | 0 | 4092 | Good | Good | Good | Good | Bad |
| 30 | 169.63 | 3.91 | −3.61 | 44.45 | 0 | 4606 | Good | Good | Good | Good | Bad |
| 31 | 160.20 | 3.06 | −2.99 | 68.24 | 0 | 8048 | Good | Good | Good | Good | Bad |
| 32 | 163.24 | 4.35 | −3.68 | 44.45 | 0 | 4107 | Good | Good | Good | Good | Bad |
| 33 | 153.18 | 3.44 | −3.21 | 44.45 | 0 | 6194 | Good | Good | Good | Good | Bad |
| 34 | 261.08 | 4.22 | −4.37 | 44.45 | 0 | 3308 | Good | Good | Good | Good | Bad |
| 35 | 180.18 | 3.62 | −3.38 | 90.27 | 0 | 6115 | Good | Good | Good | Good | Warning |
| 36 | 149.21 | 3.16 | −2.89 | 44.45 | 0 | 8333 | Good | Good | Good | Good | Bad |
| 37 | 185.24 | 4.34 | −4.03 | 44.45 | 0 | 3303 | Reduced | Good | Good | Good | Bad |
| 38 | 185.24 | 4.34 | −4.03 | 44.45 | 0 | 3303 | Reduced | Good | Good | Good | Bad |
| 39 | 99.15 | 2.41 | −1.84 | 44.45 | 0 | 15,731 | Good | Good | Good | Good | Warning |
| 40 | 101.17 | 2.37 | −1.89 | 44.45 | 0 | 15,217 | Good | Good | Good | Good | Warning |
| 41 | 143.25 | 3.98 | −2.91 | 44.45 | 0 | 7838 | Good | Good | Good | Good | Bad |
| 42 | 99.15 | 2.12 | −1.72 | 47.69 | 0 | 17,678 | Good | Good | Good | Good | Warning |
| 43 | 127.21 | 2.83 | −2.35 | 44.45 | 0 | 12,185 | Good | Good | Good | Good | Warning |
| 44 | 141.23 | 3.38 | −2.78 | 44.45 | 0 | 8770 | Good | Good | Good | Good | Bad |
| 45 | 193.31 | 4.24 | −3.64 | 44.45 | 0 | 5056 | Good | Good | Good | Good | Bad |
MW: molecular weight; blogP: octanol/water partition coefficient calculated by means of XLOGP3 program; clogSw: aqueous solubility; dtPSA: topological polar surface area; eLipinski: number of violations of Lipinski’s rule of five (up to 1 violation is allowed); fSolubility FI: solubility calculated by using the Forecaster Index (FI); gVEBER: oral bioavailability calculated by Veber rules; hEGAN: oral bioavailability calculated by Egan rules; i4_400: safety profile calculated by the GSK 4_400 rules; j3_75: safety profile calculated by the Pfizer 3_75 rules.
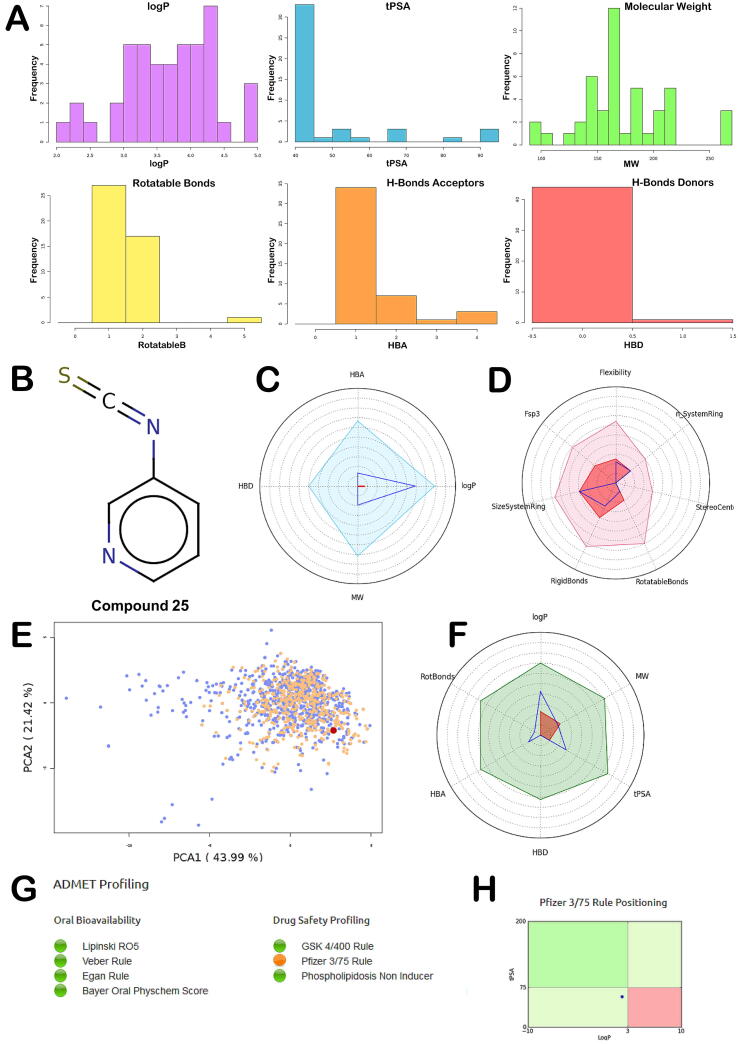
The calculation of the physicochemical properties of the molecules, along with some filters commonly used in pharma companies highlighted a conserved trend indicating that the selected compounds possess a satisfactory drug-like profile, although some properties can be improved including chemical complexity. According to this calculation all the compounds presented no violation of the Lipinski rules of five [40]. The analysis of the main PhysChem descriptors is reported in Fig. 1A.
Fig. 1.
(A) Main PhysChem descriptors analysis; (B) Chemical structure of compound 25; (C) Radar plot positioning compound’s values within the selected filter ranges. Compound values (blue line) should fall within the Lipinski rule of five filter area (light blue) (pale blue and red); (D) Radar plot visualizing compound complexity. It involves the number of system ring, stereocenters, rotatable and rigid bonds, the flexibility (ration between rotatable and rigid bonds), the carbon saturation (fsp3 ratio), and the maximum size of system rings; (E) Graph regarding oral bioavailability taking into account the model obtained with 466 orally bioavailable compounds extracted from the DrugBank database and 916 orally bioavailable compounds extracted from eDrugs library. The graph is obtained by applying the PCA (Principal Component Analysis) of the 15 principal physicochemical descriptors of these molecules (blue). Then, the compound analyzed (pink) is projected, in the same conditions, on the same chemical space; (F) Radar plot representing an oral absorption estimation: The compounds values are materialized by the blue line, which should fall within the optimal green area. The white area is the extreme maximum zone while the red one is the extreme minimum zone. These zones are obtained with the following descriptors ranges: logP (−2 to 5), Molecular Weight (150–500), tPSA (20–150), Rotatable Bonds (0–10), H-Bonds Acceptors (0–10) and Donors (0–5); (G) ADMET profiling for compound 25; (H) Pfizer 3_75 rule positioning. The output was generated by FAFDrugs4 webserver.
The computational analysis revealed that for solubility issue compounds 5, 16, 22, 37 and 38 cannot be considered for further investigation. Moreover, also compounds 11, 15, 17, 28 and 34, presenting a calculated solubility under 4000 mg·L−1, were not considered for additional pharmacological tests. The remaining compounds, although with different values, showed satisfactory solubility according to the different descriptors used in the calculation (Table 2). Supplementary analysis considering two filters used in Pharma companies (GSK 4_400 filter is related to the evaluation of logP and MW and Pfizer 3_75 filter is related to the evaluation of logP and tPSA) allowed us to identify compounds with the best predicted drug-like profile. In particular, the GSK 4_400 filter indicating that compounds with a logP less than 4 and a MW less than 400 Da could present a more favorable drug-like profile [41]. Instead, the Pfizer 3_75 filter suggesting that compounds possessing a high logP value (>3) and low tPSA value (<75) are approximately 2.5 times more likely to be toxic [42]. Accordingly, all the compounds with a “bad” profile regarding these two filters were deprioritized from further investigation. Following this screening protocol, we identified a small subset of compounds (23, 24, 25, 35, 39, 40, 42, 43) characterized by an acceptable drug-like profile. To select the candidates to submit to the pharmacological evaluation, we coupled the output of the in silico analysis with the data regarding the capability to release H2S. From this evaluation three compounds (23, 25 and 35) were identified as suitable to proceed towards further tests; but the better H2S releasing properties and drug-like profile of 25 (Fig. 1B–H), with respect to the other two compounds, prompted us to select it for a complete pharmacological characterization.
Intracellular H2S release in H9c2 cells
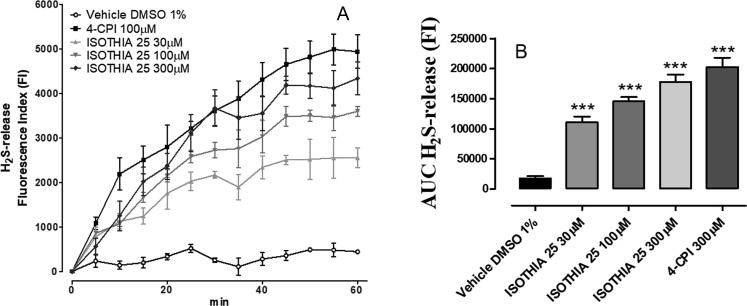
The H2S generation was detected in H9c2 cells by spectrofluorometric measurements using the WSP-1 probe, which specifically and irreversibly interacts with H2S. The fluorescence produced by this interaction was quantitatively recorded by a spectrofluorometric approach which showed that the addition of the vehicle did not cause any significant increase of fluorescence. In contrast, the addition of compound 25 at the concentration of 30, 100 and 300 µM to H9c2, preloaded with the fluorescent dye, led to a time and concentration-dependent increase of fluorescence (FI, fluorescence index), indicating a significant generation of H2S (P < 0.001 vs vehicle). 4-CPI 300 µM was used as reference H2S-donor and showed a comparable H2S-release than compound 25 300 µM (Fig. 2).
Fig. 2.
(A) Graph shows the WSP-1 fluorescence increase evoked by the administration of vehicle (DMSO 1%), 300 μM 4-CPI, 30, 100 and 300 μM compound 25 on H9c2 cells. (B) The histograms show the total amount of H2S released by vehicle (DMSO 1%), 300 μM 4-CPI, 30, 100 and 300 μM compound 25, during 60 min, expressed as AUC. Data are expressed as mean ± standard error. Six different experiments were carried out, each in triplicate. *** = significantly different from the vehicle (P < 0.001).
Effects on the coronary flow in Angiotensin-II precontracted rat hearts
The basal CF in Langendorff-perfused rat hearts was 10.54 ± 0.42 mL/min/g. As expected, the perfusion with Angiotensin II (AngII, 0.1 μM) caused a significant reduction (about 25%) of the coronary flow in isolated rat hearts when compared to the basal CF. In the constant presence of AngII, the “add-on” perfusion with 1, 3, 10, 30 μM compound 25 led to a concentration related increase of CF up to the complete recovery of the basal coronary flow and, thus, abolishing the AngII-mediated vasoconstriction at the maximum concentration tested. 4-CPI 30 µM, used as reference H2S-donor [11], led to a slightly higher increase in the coronary flow value of about 110% (Fig. 3).
Fig. 3.
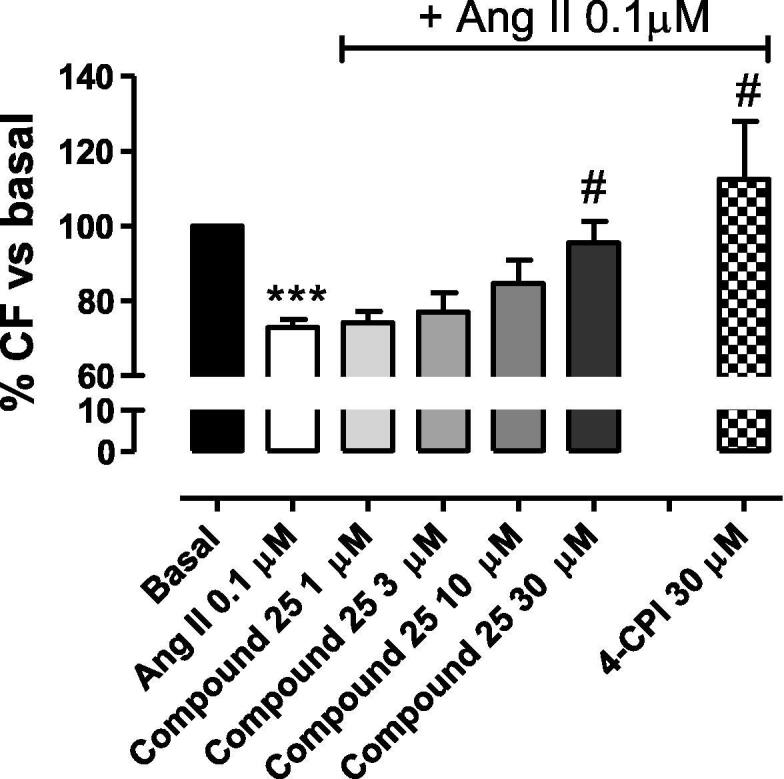
Effects on coronary flow. The histograms show the changes of CF (expressed as a % of the basal CF) after the perfusion with 1, 3, 10, 30 µM compound 25 in the constant presence of Ang II 0.1 µM. 4-CPI 30 µM has been used as reference drug. Data are expressed as mean ± standard error. Six different experiments were carried out. *** = significantly different from basal CF (P < 0.001); # = significantly different from Ang II 0,1 µM (P < 0.05).
Effects on isolated rat heart subjected to I/R injury
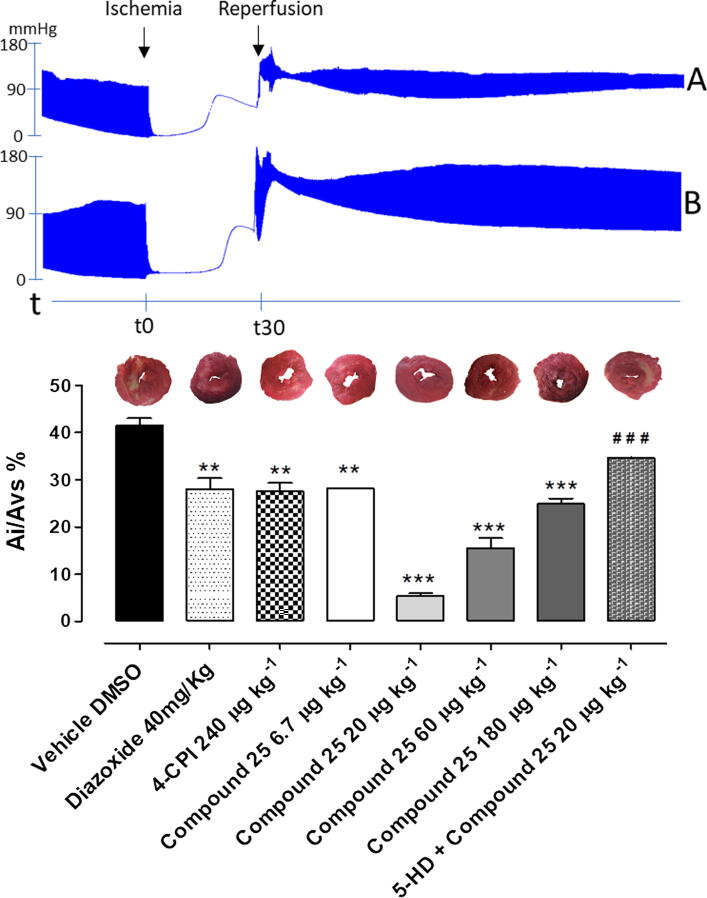
I/R caused marked functional damage to isolated heart of vehicle-treated rats, with a significant reduction of myocardial vital tissue, evaluated by morphometric analysis showing about 40% of ischemic area, expressed as ratio of ischemic area to total left ventricular area (Ai/ALV%). The treatment with 4-CPI 240 µg·kg−1 reduced the damaged tissue of about 10% compared to vehicle. A comparable cardioprotective effect has been measured as reduction of the ischemic area in hearts of compound 25 180 µg·kg−1-treated rats (equimolar dose of 4-CPI 240 µg·kg−1). Surprisingly, the reduction of compound 25 dose, further limited the I/R injury damage, exerting the maximum cardioprotective effect at 20 µg·kg−1. Lower dose (compound 25 6.7 µg·kg−1) lead to a reduction of the cardioprotective effect. In order to study the involvement of mitoKATP channels in the cardioprotective effects evoked by compound 25, 5-HD 10 mg·kg−1 has been administered 20 min before the treatment with compound 25 20 µg·kg−1: 5-HD 10 mg·kg−1 clearly limited the cardioprotective effect of the isothiocyanate, since the ischemic area was significant more extended compared to the treatment with compound 25 20 µg·kg−1 alone (Ai/ALV% 34.6 ± 0.1 vs 5.3 ± 0.5; data expressed as mean ± SEM). This result strongly suggests that mitoKATP is likely to be involved in the cardioprotective effects of H2S. To further confirm the involvement of mitoKATP channels, administration of diazoxide 40 mg/Kg (a mitoKATP opener) imitated the ability of cardioprotective and anti-ischemic effect of compound 25 (Fig. 4).
Fig. 4.
The histograms show the morphometric analysis of the ischemic area observed in ventricular slices of rat hearts expressed as Ai/ALV% after I/R-induced injury. The bars refer to the different pharmacological pre-treatments: vehicle, 40 mg·Kg−1 diazoxide, 240 µg·kg−1 4-CPI, 6.7, 20, 60, 180 µg·kg−1 compound 25 and 5-HD 10 mg·kg−1 + 20 µg·kg−1 compound 25. Data are expressed as mean ± standard error. Six different experiments were carried out. * = significantly different from vehicle (***P < 0.001; **P < 0.01). Representative pictures of rat left ventricle slices and the infarct size for each treatment are reported upon the histograms. Representative changes of functional parameters of vehicle (A) and compound 25 20 µg·kg−1 have been reported.
LDH activity measurement
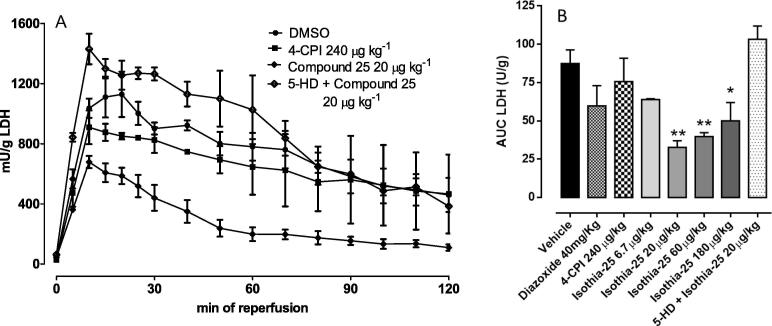
The LDH activity was measured in the coronary effluent collected during the preischemic and reperfusion periods. There were no differences between the various groups in LDH released during the preischemic period (data not shown). However, during reperfusion, LDH rose progressively in the groups treated with vehicle and with 5-HD 10 mg·kg−1 + compound 25 20 µg·kg−1. In the perfusate of hearts from rat given compound 25 at different doses, the amount of LDH released during the reperfusion period was markedly reduced, reflecting the pattern of the Ai/ALV%. Compound 25 20 µg·kg−1 was the most effective dose tested in reducing the LDH release. Diazoxide slightly reduced the amount of LDH in perfusate of hearts (Fig. 5).
Fig. 5.
(A) LDH release profile in rat perfused heart preparations subjected to ischemia–reperfusion obtained from rat treated with vehicle, 240 µg·kg−1 4-CPI, 20 µg·kg−1 compound 25 and 10 mg·kg−1 5-HD + 20 µg·kg−1 compound 25. (B) Bar graph shows the AUC related to LDH curves of all treatments (vehicle, 40 mg·Kg−1 diazoxide, 240 µg·kg−1 4-CPI, 6.7, 20, 60, 180 µg·kg−1 compound 25 and 5-HD 10 mg·kg−1 + 20 µg·kg−1 compound 25). Data are expressed as mean ± standard error. Six different experiments were carried out. * = significantly different from vehicle (*P < 0.05; **P < 0.01). # = significantly different from 20 µg·kg−1 compound 25 (## P < 0.01).
Cardioprotective effect of compound 25 in vivo
Rats were pretreated with vehicle or compound 25 20 µg·kg−1 2 h before 30 min of coronary occlusion and 2 h of reperfusion. In rats treated with compound 25 20 µg·kg−1, there was a significant reduction in myocardial ischemic area, measured as Ai/ALV% (40.3 ± 1.8 vs 19.0 ± 1.4, P < 0.001, data expressed as mean ± SEM). These findings indicate that compound 25 exerts cardioprotective effect on I/R-induced cardiac injury. Furthermore, also the cardioprotective effects of diazoxide and 4-CPI, mitoKATP opener and the H2S-donor reference drug respectively, have been evaluated. Both the compounds promoted a significant cardioprotection reducing the ischemic area of about 15% (Fig. 6).
Fig. 6.
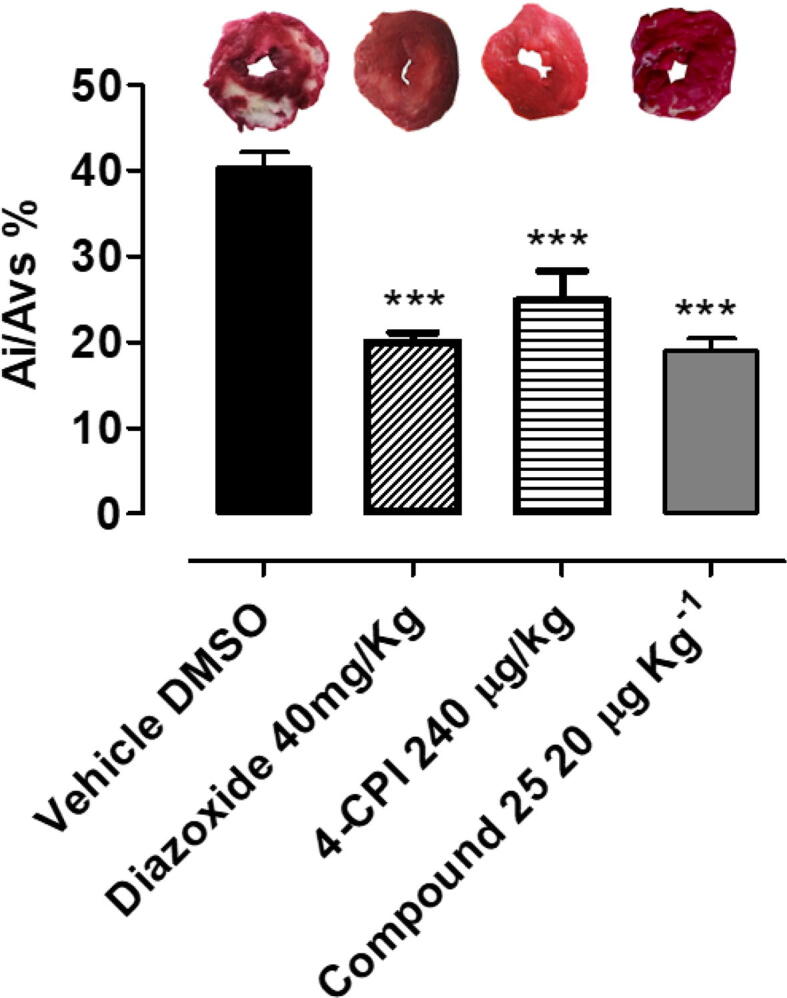
Morphometric quantification of ischemia/reperfusion (I/R)-induced injury observed in ventricular slices of rat hearts, after acute myocardial infarction in vivo, after treatment with vehicle, 40 mg·Kg−1 diazoxide, 240 µg·kg−1 4-CPI, 20 µg·kg−1 compound 25. Data are expressed as mean ± standard error. Six different experiments were carried out. *** = significantly different from vehicle (P < 0.001). Representative pictures of rat left ventricle slices and the infarct size for each treatment are reported upon the histograms.
Discussion
Infarction and myocardial ischemia are main causes of mortality in Western countries, and the identification of innovative pharmacological treatments to limit I/R-induced cardiac injury remains a challenging issue. The discovery of H2S as an endogenous gasotransmitter and the comprehension of its pivotal role in regulating cardiovascular function and in mediating cardioprotective effect, offered novel promising perspectives in this field of research [43]. In particular, H2S-releasing molecules, such as GYY4137 and 4-CPI, have shown significant protective effects in experimental models of myocardial I/R, suggesting that H2S-donors can actually be viewed as a promising class of anti-ischemic drugs [44]. In this paper, we evaluated the H2S-releasing properties of a small library of isothiocyanates, since this chemical moiety has been reported to exhibit biological effects clearly related with the release of H2S [45]. To investigate how the structure of the isothiocyanates affects their H2S releasing properties, several derivatives were selected and the H2S generation was amperometrically measured.
Isothiocyanates show l-Cys-dependent H2S-releasing properties. Accordingly, in this study we confirmed that the incubation of all the isothiocyanates led to the formation of negligible amounts of H2S in the absence of l-Cysteine. Contrarily, in the presence of l-Cysteine, the incubation of the tested compounds led to a slow release of H2S. Presently, the H2S-releasing properties derive from a combination of several factors such as steric hindrance, electronic effects and position of the substituents in association with the water solubility of the compounds. The aliphatic isothiocyanates (39–45) show a very little release of H2S, when compared to the aromatic ones. Only compound 41 (hexyl isothiocyanate), identified as one of the components of the extracts from several Brassicacee, showed a more relevant ability of H2S releasing (Cmax = 12.973 μM). The relevance of the conjugation between -SCN moiety and the aromatic ring is demonstrated by the dramatic lack of H2S releasing properties of compound 36 (benzyl isothiocyanate). As a clear consequence of the steric hindrance, the presence of any substituent in ortho to -SCN moiety caused a dramatic fall in the H2S production (compounds 2, 3, 4, 5, 8, 9, 11). On the other hand, the exceptions of compounds 6, 7, 10 and 12, demonstrate that the presence of electron-withdrawing groups partially compensate this steric effect. When analyzing meta- and para-substituted compounds we have a clear overview of the electronic effects deprived from the steric ones. The meta- and para- derivatives, electron-donating substituted (13, 14, 16, 20, 26, 27, 32) and electron-withdrawing substituted (15, 17, 18, 19, 21, 23, 24, 28, 29, 30, 31, 33, 35) are, respectively, worse and better H2S donors with respect to 1. The poor H2S donor profile of the o-, m- and p-iodo substituted derivatives (11, 22, 34) is due not only to steric and reactivity factors but also to the low water solubility of the three derivatives (Table 2). The scarce water solubility may also account for the negligible H2S production of compounds 5, 16, 37 and 38. On the other hand, the excellent H2S release of compound 25 arises certainly from the electron deficient nature of the pyridine ring but even the high water solubility of the compound plays an important role.
The observed behavior is strongly supported by the recently described molecular mechanism responsible for H2S release from isothiocyanates [36]. It implies a nucleophilic attack by the cysteine thiol group on the isothiocyanate moiety central carbon, leading to an ITC-cysteine adduct. An electron poor ring (such as pyridine or an electron withdrawing substituted aromatic ring) facilitates the intramolecular nucleophilic addition of the amino group leading to a 4,5-dihydrothiazole intermediate that produces H2S.
Irrespective of the mechanism of reaction, such an organic thiol-dependency of the H2S-releasing process is viewed as a particularly advantageous property, because it allows these compounds to behave as “smart” H2S-donors, able to release the gasotransmitter only in a biological environment.
Starting from the H2S-generation properties, in silico methods have been used in order to select the best candidate to be investigated about the cardioprotective properties.
The drug-like profile represents a critical property for assuring the advancement of a drug candidate into preclinical studies and clinical trials. Furthermore, the use of in silico methods to establish the potential drug-like profile for a given set of molecules, prioritizing only the most promising compounds, can limit the costs and time required for selecting potential drug candidates, also reducing the use of animals for the in vivo studies.
Based on this evaluation, compound 25 has been identified as suitable to proceed towards further pharmacological investigations considering both physicochemical properties, drug-like features and H2S-releasing rate.
The H2S-releasing properties of compound 25 have been firstly described by an amperometric method performed in phosphate buffer, in the presence or in the absence of l-Cys. Since the H2S-release in buffer without l-Cys is negligible, compound 25, is expected to be relatively stable in water, but behaves as H2S-generating agent in biological systems where it can interact with endogenous organic thiols. Accordingly, as clearly highlighted in the experiments carried out with the fluorometric dye WSP‐1, compound 25 was able to release H2S inside cells in a concentration‐dependent manner, and this observation led to the conclusion that compound 25 shows the ability to cross the H9c2 cell membrane and release H2S at the intracellular level.
As reported for other H2S donor, in this model we evaluated the intracellular release of H2S and it is not surprising that there is not a proportional correlation between the concentration of compound 25 and the levels of the intracellular H2S release. Indeed, the H2S release could be influenced by different factors. First, the test compound must cross the cell membrane and enter the cellular environment; these processes strongly depend on the physicochemical properties of the compound. Then, the test compound (that is a thiol-dependent H2S-donor) must react with the intracellular free thiols; this reaction is strongly influenced by the compound structure and by the stoichiometric rate compound/free thiols. Therefore, a linear relationship between the extracellular concentration of the H2S-donor and the intracellular release of H2S is not expected and can be only evaluated empirically.
Since H2S plays a pivotal role in the regulation of the vascular tone and in the cardio-protection process, the effect at the cardiac level of compound 25 has been further investigated in both ex vivo and in in vivo experimental models.
Compound 25 promoted a clear vasorelaxing effect in the coronary vascular bed and effectively counteracted the coronary vasoconstriction induced by AngII.
In an ex vivo experimental model of myocardial I/R (Langendorff-perfused rat hearts), i.p. pre-administration of compound 25, led to significant and evident limitation of tissue injury (the maximum effect has been measured at the dose of 20 µg·kg−1), although no clear dose-dependency was observed. Starting from the lower dose of 6.7 µg·kg−1 a dose-dependent cardioprotection can be observed up to 20 µg·kg−1. But further increasing doses of compound 25, failed to show increasing cardioprotective effects, rather increase of the ischemic area can be observed, thus indicating a weaker cardioprotective effect. This behavior can be explained by the hormesis that can be represented by a “U-shape curve”. Indeed, the hormetic effect of both H2S and isothiocyanates is widely described in the literature [46].
Regarding the vasorelaxing effect and the cardioprotection of 4-CPI and compound 25, the different activity of the two compounds is explained by the different targets. H2S promotes vasodilation mainly by activating pharmacological targets expressed on the surface of the vascular smooth muscle cell membranes. For instance, such a vasodilation effect involves the activation of sarcolemmal KATP, Kv7 and BKCa channels. As concerns the cardioprotective effects, they are mainly due to the activation of mitochondrial targets (i.e. to the triggering of ischemic preconditioning mechanisms). Therefore, pharmacokinetic factors (linked to the physicochemical features of the molecule) are likely to account for the differences observed in the concentration evoking vasodilator and cardioprotective effects.
Furthermore, 4-CPI 240 µg/kg exhibited a clear cardioprotective effect, but 72 µg/kg of 4-CPI failed to produce cardioprotective effects. The dose of 180 µg/kg compound 25 (equimolar dose of 4-CPI 240 µg/kg) had a similar cardioprotective effects with the 240 µg/kg of 4-CPI. However, the concentration of 20 µg/kg compound 25 exerted the maximum cardioprotective effect, while 72 µg/kg of CPI failed to produce cardioprotective effects. Although the two isothiocyanates can release H2S, they seem to behave in a different manner. The different efficacy and effect in the cardioprotection may be due to the physicochemical properties of the two compounds. The cardioprotection is mediated by intracellular channels (mitoKATP is a main actor in mediating the cardioprotection effect of H2S). Thus, the compounds have to cross the membrane and probably 4-CPI is able to exert a more efficient vasorelaxing effect, but it is worse in crossing the membrane failing to reach the mitochondrial specific target.
Further investigations about the molecular mechanism on the cardioprotective properties of compound 25 have been carried out by evaluating the involvement of mitoKATP channels whose activation has been previously reported to mediate beneficial effect in I/R injury models. Testai and colleagues [34] demonstrated that H2S and H2S-donors are able to activate mitoKATP channels suggesting that they may be a main target of the anti-ischemic effects evoked by this gasotransmitter. Indeed, in our experimental model, the mitoKATP-blocker 5-HD (10 mg·Kg−1) almost completely abolished the effects of compound 25, strongly suggesting that mitoKATP is likely to be involved in the cardioprotective effects of H2S.
The “bi-modal” curve is reflected also by the measurement of LDH activity in the perfusate effluent, confirming the tissue damage highlighted by the morphometric analysis.
Finally, the cardioprotective effects of compound 25 were tested in vivo, in an experimental model of acute myocardial infarction in rats, more closely resembling the clinical pattern of myocardial infarction. Also, in this model, compound 25 (20 µg·kg−1) exhibited cardioprotective effects.
Conclusion
A library of forty-five isothiocyanates was evaluated for its ability to release H2S and then, by an in silico approach, compound 25 was identified as the most promising one. This derivative was thoroughly characterized for its cardioprotective function by means of ex vivo and in vivo assays. In conclusion, our results strongly suggest that isothiocyanate-based H2S-releasing drugs, such as compound 25, can trigger a “pharmacological pre-conditioning” and could represent a suitable pharmacological option in anti-ischemic therapy.
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
Funding
This study was supported by the Italian Ministry of University and Research (MIUR) PRIN 2017XP72RF - Hydrogen Sulfide in the Vascular inflamm-Aging: role, therapeutic Opportunities and development of novel pharmacological tools for age-related cardiovascular diseases (SVAgO).
Declaration of Competing Interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Vincenzo Calderone, Email: caliendo@unina.it.
Giuseppe Caliendo, Email: vincenzo.calderone@unipi.it.
References
- 1.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14(5):329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 2.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26(3):243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11(4):703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto R., Otsuguro K., Yamaguchi S., Ito S. Contribution of cysteine aminotransferase and mercaptopyruvate sulfurtransferase to hydrogen sulfide production in peripheral neurons. J Neurochem. 2014;130(1):29–40. doi: 10.1111/jnc.12698. [DOI] [PubMed] [Google Scholar]
- 5.Stipanuk M.H. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 6.Brancaleone V., Esposito I., Gargiulo A., Vellecco V., Asimakopoulou A., Citi V. D-Penicillamine modulates hydrogen sulfide (H2S) pathway through selective inhibition of cystathionine-gamma-lyase. Br J Pharmacol. 2016;173(9):1556–1565. doi: 10.1111/bph.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corvino A., Severino B., Fiorino F., Frecentese F., Magli E., Perissutti E. Fragment-based de novo design of a cystathionine gamma-lyase selective inhibitor blocking hydrogen sulfide production. Sci Rep. 2016;6:34398. doi: 10.1038/srep34398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng B., Yang J., Qi Y., Zhao J., Pang Y., Du J. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313(2):362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 9.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105(4):365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa A.D., Garlid K.D., West I.C., Lincoln T.M., Downey J.M., Cohen M.V. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97(4):329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 11.Testai L., Marino A., Piano I., Brancaleone V., Tomita K., Di Cesare Mannelli L. The novel H2S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoKATP channels and reduction of oxidative stress. Pharmacol Res. 2016;113(Pt A):290–299. doi: 10.1016/j.phrs.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Rose P., Dymock B.W., Moore P.K. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol. 2015;554:143–167. doi: 10.1016/bs.mie.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Severino B., Corvino A., Fiorino F., Luciano P., Frecentese F., Magli E. 1,2,4-Thiadiazolidin-3,5-diones as novel hydrogen sulfide donors. Eur J Med Chem. 2018;143:1677–1686. doi: 10.1016/j.ejmech.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 14.Martelli A., Testai L., Citi V., Marino A., Pugliesi I., Barresi E. Arylthioamides as H2S donors: L-cysteine-activated releasing properties and vascular effects in vitro and in vivo. ACS Med Chem Lett. 2013;4(10):904–908. doi: 10.1021/ml400239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barresi E., Nesi G., Citi V., Piragine E., Piano I., Taliani S. Iminothioethers as hydrogen sulfide donors: from the gasotransmitter release to the vascular effects. J Med Chem. 2017;60(17):7512–7523. doi: 10.1021/acs.jmedchem.7b00888. [DOI] [PubMed] [Google Scholar]
- 16.Mitidieri E., Tramontano T., Gurgone D., Citi V., Calderone V., Brancaleone V. Mercaptopyruvate acts as endogenous vasodilator independently of 3-mercaptopyruvate sulfurtransferase activity. Nitric Oxide. 2018;75:53–59. doi: 10.1016/j.niox.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Ercolano G., De Cicco P., Frecentese F., Saccone I., Corvino A., Giordano F. Anti-metastatic properties of naproxen-HBTA in a murine model of cutaneous melanoma. Front Pharmacol. 2019;10:66. doi: 10.3389/fphar.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Citi V., Martelli A., Testai L., Marino A., Breschi M.C., Calderone V. Hydrogen sulfide releasing capacity of natural isothiocyanates: is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014;80(8–9):610–613. doi: 10.1055/s-0034-1368591. [DOI] [PubMed] [Google Scholar]
- 19.Lucarini E., Micheli L., Trallori E., Citi V., Martelli A., Testai L. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2 S release in vivo. Phytother Res. 2018;32(11):2226–2234. doi: 10.1002/ptr.6159. [DOI] [PubMed] [Google Scholar]
- 20.Martelli A., Testai L., Citi V., Marino A., Bellagambi F.G., Ghimenti S. Pharmacological characterization of the vascular effects of aryl isothiocyanates: is hydrogen sulfide the real player? Vascul Pharmacol. 2014;60(1):32–41. doi: 10.1016/j.vph.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Sestito S., Daniele S., Pietrobono D., Citi V., Bellusci L., Chiellini G. Memantine prodrug as a new agent for Alzheimer's Disease. Sci Rep. 2019;9(1):4612. doi: 10.1038/s41598-019-40925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapposelli S., Gambari L., Digiacomo M., Citi V., Lisignoli G., Manferdini C. A Novel H2S-releasing Amino-Bisphosphonate which combines bone anti-catabolic and anabolic functions. Sci Rep. 2017;7(1):11940. doi: 10.1038/s41598-017-11608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sestito S., Pruccoli L., Runfola M., Citi V., Martelli A., Saccomanni G. Design and synthesis of H2S-donor hybrids: a new treatment for Alzheimer's disease? Eur J Med Chem. 2019;184 doi: 10.1016/j.ejmech.2019.111745. [DOI] [PubMed] [Google Scholar]
- 24.Franklin S.J., Dickinson S.E., Karlage K.L., Bowden G.T., Myrdal P.B. Stability of sulforaphane for topical formulation. Drug Dev Ind Pharm. 2014;40(4):494–502. doi: 10.3109/03639045.2013.768634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martelli A., Citi V., Testai L., Brogi S., Calderone V. Organic isothiocyanates as hydrogen sulfide donors. Antioxid Redox Signal. 2020;32(2):110–144. doi: 10.1089/ars.2019.7888. [DOI] [PubMed] [Google Scholar]
- 26.Calderone V., Martelli A., Testai L., Citi V., Breschi M.C. Using hydrogen sulfide to design and develop drugs. Expert Opin Drug Discov. 2016;11(2):163–175. doi: 10.1517/17460441.2016.1122590. [DOI] [PubMed] [Google Scholar]
- 27.Zuber G, Frisch B, Creusat G, Thomann JS. Cationic polyethylenimine derivatives for delivering pharmacological molecules 2011, WO 201112095.
- 28.Elzes M.R., Si G., Engbersen J.F.J., Paulusse J.M.J. Thiourea-functional bioreducible poly(amido amine)s in gene delivery. ACS Symp Ser. 2019;1309(5):93–117. [Google Scholar]
- 29.Gasser A., Brogi S., Urayama K., Nishi T., Kurose H., Tafi A. Discovery and cardioprotective effects of the first non-Peptide agonists of the G protein-coupled prokineticin receptor-1. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0121027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaccagnini L., Brogi S., Brindisi M., Gemma S., Chemi G., Legname G. Identification of novel fluorescent probes preventing PrP(Sc) replication in prion diseases. Eur J Med Chem. 2017;127:859–873. doi: 10.1016/j.ejmech.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 31.Lagorce D., Sperandio O., Baell J.B., Miteva M.A., Villoutreix B.O. FAF-Drugs3: a web server for compound property calculation and chemical library design. Nucleic Acids Res. 2015;43(W1):W200–W207. doi: 10.1093/nar/gkv353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng B., Chen W., Liu C., Rosser E.W., Pacheco A., Zhao Y. Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging. Chemistry. 2014;20(4):1010–1016. doi: 10.1002/chem.201303757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martelli A., Citi V., Calderone V. Vascular effects of H2S-donors: fluorimetric detection of H2S generation and ion channel activation in human aortic smooth muscle cells. Methods Mol Biol. 2019;2007:79–87. doi: 10.1007/978-1-4939-9528-8_6. [DOI] [PubMed] [Google Scholar]
- 34.Citi V., Piragine E., Pagnotta E., Ugolini L., Di Cesare Mannelli L., Testai L. Anticancer properties of erucin, an H2 S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1) Phytother Res. 2019;33(3):845–855. doi: 10.1002/ptr.6278. [DOI] [PubMed] [Google Scholar]
- 35.Testai L., D'Antongiovanni V., Piano I., Martelli A., Citi V., Duranti E. Different patterns of H2S/NO activity and cross-talk in the control of the coronary vascular bed under normotensive or hypertensive conditions. Nitric Oxide. 2015;47:25–33. doi: 10.1016/j.niox.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Testai L., Strobykina I., Semenov V.V., Semenova M., Pozzo E.D., Martelli A. Mitochondriotropic and cardioprotective effects of triphenylphosphonium-conjugated derivatives of the diterpenoid isosteviol. Int J Mol Sci. 2017;18(10):2060. doi: 10.3390/ijms18102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y., Yang X., Lu Y., Liang D., Huang D. Isothiocyanates as H2S donors triggered by cysteine: reaction mechanism and structure and activity relationship. Org Lett. 2019;21(15):5977–5980. doi: 10.1021/acs.orglett.9b02117. [DOI] [PubMed] [Google Scholar]
- 38.Sirous H., Chemi G., Gemma S., Butini S., Debyser Z., Christ F. Identification of novel 3-hydroxy-pyran-4-one derivatives as potent HIV-1 integrase inhibitors using in silico structure-based combinatorial library design approach. Front Chem. 2019;7:574. doi: 10.3389/fchem.2019.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirous H., Chemi G., Campiani G., Brogi S. An integrated in silico screening strategy for identifying promising disruptors of p53-MDM2 interaction. Comput Biol Chem. 2019;83:107105. doi: 10.1016/j.compbiolchem.2019.107105. [DOI] [PubMed] [Google Scholar]
- 40.Chemi G., Gemma S., Campiani G., Brogi S., Butini S., Brindisi M. Computational tool for fast in silico evaluation of hERG K(+) channel affinity. Front Chem. 2017;5:7. doi: 10.3389/fchem.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 42.Gleeson M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J Med Chem. 2008;51(4):817–834. doi: 10.1021/jm701122q. [DOI] [PubMed] [Google Scholar]
- 43.Hughes J.D., Blagg J., Price D.A., Bailey S., Decrescenzo G.A., Devraj R.V. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg Med Chem Lett. 2008;18(17):4872–4875. doi: 10.1016/j.bmcl.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 44.Salloum F.N. Hydrogen sulfide and cardioprotection–mechanistic insights and clinical translatability. Pharmacol Ther. 2015;152:11–17. doi: 10.1016/j.pharmthera.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Citi V., Piragine E., Testai L., Breschi M.C., Calderone V., Martelli A. The role of hydrogen sulfide and H2S-donors in myocardial protection against ischemia/reperfusion injury. Curr Med Chem. 2018;25(34):4380–4401. doi: 10.2174/0929867325666180212120504. [DOI] [PubMed] [Google Scholar]
- 46.Martelli A., Piragine E., Citi V., Testai L., Pagnotta E., Ugolini L. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br J Pharmacol. 2020;177(4):824–835. doi: 10.1111/bph.14645. [DOI] [PMC free article] [PubMed] [Google Scholar]