Graphical abstract
Keywords: Hydrogen sulfide, Diabetes mellitus, Insulin resistance, Cystathionine γ-lyase, Cystathionine β-synthase
Abstract
Background
Insulin resistance and impaired insulin secretion lead to disorders of glucose metabolism, which contributes to the development of diabetes. Hydrogen sulfide (H2S), a novel gasotransmitter, is found to play important roles in regulation of glucose metabolism homeostasis.
Aim of Review
This study aimed to summarize and discuss current data about the function of H2S in insulin secretion and insulin resistance regulation as well as the underlying mechanisms.
Key Scientific Concepts of Review
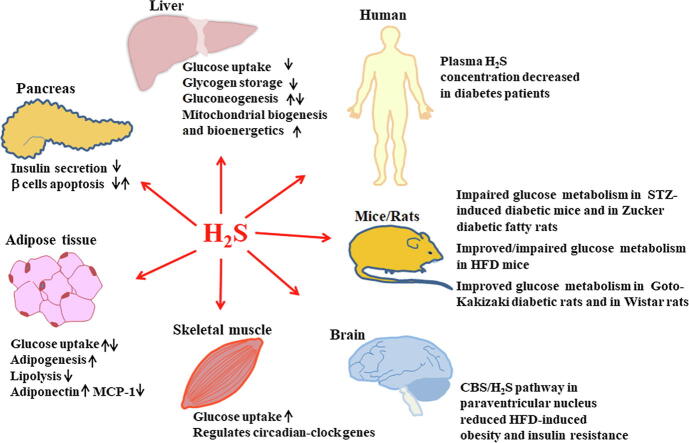
H2S could be endogenously produced in islet β cells, liver, adipose, skeletal muscles, and the hypothalamus, and regulates local and systemic glucose metabolism. It is reported that H2S suppresses insulin secretion, promotes or reduces the apoptosis of islet β cells. It plays important roles in the regulation of insulin sensitivity in insulin responsive tissues. H2S inhibits glucose uptake and glycogen storage, and promotes or inhibits gluconeogenesis, mitochondrial biogenesis and mitochondrial bioenergetics in the liver. In adipose tissue, several investigators indicated that H2S promoted glucose uptake in adipocytes, while other studies reported that H2S inhibits this process. H2S has also been shown to promote adipogenesis, inhibit lipolysis, and regulate adiponectin and MCP-1 secretion from adipocytes. In skeletal muscle, H2S increases glucose uptake and improves insulin sensitivity. It is also observed that H2S modulates circadian-clock genes in muscle. Hypothalamic CBS/H2S pathway reduces obesity and improves insulin sensitivity via the brain-adipose interaction. Most studies indicated plasma H2S levels decreased in diabetic patients. However, the mechanisms by which H2S regulates systemic glucose metabolism remain unclear. Whether H2S acts as a new promising target for diabetes mellitus treatment merits further studies.
Introduction
Diabetes mellitus is increasing in prevalence and has emerged as a global health challenge [1], [2]. Insulin resistance is an abnormal state with the impaired sensitivity of tissues, such as skeletal muscle, adipose and liver tissues, to the action of insulin. This condition is compensated by hyperinsulinemia resulting from pancreatic β‐cell dysfunction and contributes to the development of diabetes [3], [4], [5]. The pathogenesis of insulin resistance and β-cells dysfunction remain importantly scientific issues. Studies showed that the novel gaseous signaling molecule, hydrogen sulfide (H2S), could be produced in insulin-synthesizing pancreatic β cells and in target organs of insulin, such as liver, adipose and muscle [6], [7], [8], [9], [10]. Plasma H2S concentration is decreased in diabetic subjects compared with the control (45.1 ± 15.5 μmol/L versus 54.0 ± 26.4 μmol/L reported by Suzuki et al.; 10.5 [4.8, 22.0] μmol/L versus 38.9 [29.7, 45.1] μmol/L expressed in median plasma H2S concentration [25th, 75th percentiles] reported by Whiteman et al.) [11], [12], [13]. Moreover, H2S is found to regulate insulin sensitivity and insulin secretion [10]. 10–100 μmol/L H2S donor NaHS that may produce about 3–35 μmol/L H2S has important physiological effects on insulin-dependent metabolism, while 200–1000 μM NaHS that possibly releases about 70–350 μmol/L H2S leads to toxic effects [14], [15], [16]. Therefore, regulating H2S level has emerged as a promising strategy in the treatment of diabetes mellitus. Herein, we have summarized and discussed current data about the function of H2S in insulin secretion and insulin resistance regulation as well as the underlying mechanisms.
Endogenous production and elimination of H2S
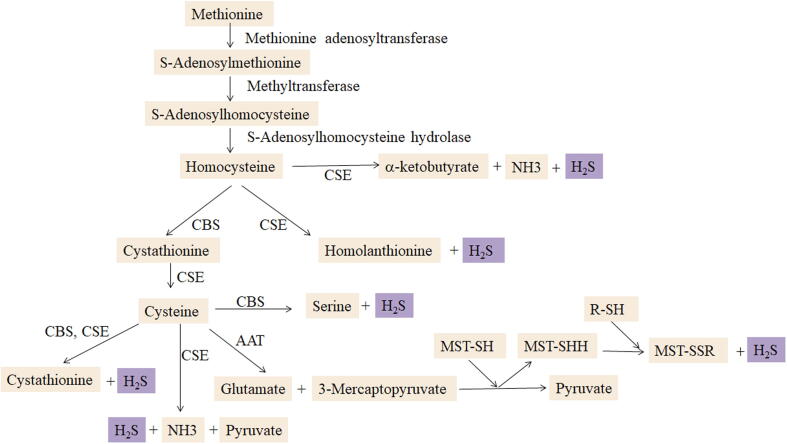
H2S is colorless, characterized by a rotting egg odor, transparent and water-soluble. It can be generated from L-cysteine and/or homocysteine catalyzed by two enzymes, cystathionine beta-synthase (CBS) and cystathionine gamma-lyase (CSE) (Fig. 1). Production of H2S from 3-mercaptopyruvate is catalyzed by 3-mercaptopyruvate sulfurtransferase (3-MST) [6]. Cysteine aminotransferase (CAT) could also catalyze H2S generation in several tissues. The distribution of these enzymes is tissue specific. H2S is mainly produced via CSE in the pancreas, adipose tissue, liver, cardiovascular system and respiratory system, while H2S production in the central nervous system is predominantly catalyzed by CBS. In order to maintain the physiological levels of H2S, the production and elimination of H2S are precisely regulated and controlled. Oxidation, methylation, scavenging and expiration are responsible for H2S elimination. For example, H2S is oxidized in mitochondria to thiosulfate, then to sulfite or sulfate, and ultimately excreted in urine by the kidney. H2S methylation occurs mainly in the cytosol. H2S can also be scavenged by methemoglobin and other proteins and exhaled through the lung.
Fig. 1.
Endogenous H2S generation in mammals. H2S, hydrogen sulfide; CSE, cystathionine γ-lyase; CBS, cystathionine β-synthase; 3-MST, 3-mercaptopyruvate sulfurtransferase; AAT, aspartate aminotransferase.
H2S in islet β cells
Endogenous H2S production in islet β-cells
An endogenous generation of H2S via CBS and/or CSE was identified in pancreatic β-cells from several species (Table 1). A different H2S dominant synthase was identified in the pancreatic β cells depending on the species. A small amount of CBS was expressed by HIT-T15 Syrian hamster pancreatic β-cells with no CSE mRNA transcripts shown [17]. Both CBS and CSE were detected in mouse pancreases, mouse and rat pancreatic islets and MIN6 mouse islet β cells [18], [19], [20], [21]. INS-1E rat insulinoma cells expressed CSE [22]. CSE inhibition by CSE siRNA or DL-propargylglycine (PPG) significantly depleted H2S production from cultured INS-1E cells [22]. PPG also significantly reduced H2S production in rat pancreatic islets [18]. These results suggested that CSE was the dominant enzyme catalyzing H2S production in INS-1E rat insulinoma cell and in rat islet β cell. The distribution patterns of the two enzymes in the mouse pancreas are not identical. In immunohistochemistry results of the pancreas taken from male ICR mice, CBS existed in pancreatic exocrine and endocrine islet cell cytoplasm. CSE was primarily expressed in exocrine cell cytoplasm and showed faint levels in the islets [20]. No protein expression of 3-MST was detected by Western blot in mouse pancreases [21], but the contribution of 3-MST to H2S production in pancreatic β-cells from other species has not been investigated. These studies indicated that H2S dominant synthases in pancreatic β cells are species-specific.
Table 1.
Expression of H2S-synthesizing enzymes in pancreases and islet β cells.
| H2S-synthesizing enzyme | Expression | Cells/Model | Refs. |
|---|---|---|---|
| CBS | mRNA and protein were detected (low levels) | HIT-T15 cells | [17] |
| mRNA was detected (low levels) | Rat pancreatic islets | [18] | |
| mRNA was detected | MIN6 cells | [19] | |
| mRNA and protein were detected | Mouse pancreatic islets | [19] | |
| mRNA and protein were detected (mRNA was moderately expressed) | Mouse pancreatic islets | [20] | |
| protein was detected | Moue pancreases | [20], [21] | |
| CSE | No mRNA expression was detected | HIT-T15 cells | [17] |
| mRNA and protein were detected (H2S dominant synthase) | INS-1E cells | [22] | |
| mRNA and protein were detected (H2S dominant synthase) | Rat pancreatic islets | [18] | |
| mRNA was detected | MIN6 cells | [19] | |
| mRNA was detected | Mouse pancreatic islets | [19] | |
| mRNA and protein were detected (low levels) | Mouse pancreatic islets | [20] | |
| protein was detected | Mouse pancreases | [20], [21] | |
| 3-MST | No protein expression was detected | Mouse pancreases | [21] |
Hyperglycemia regulates endogenous H2S in pancreatic β-cells
How high glucose regulates CSE/H2S pathway is controversial. High glucose (20 mmol/L) was found to suppress H2S production in INS-1E cells by downregulating CSE promoter activity and mRNA level, as glucose activates p38-MAPK/specificity protein 1 (SP1) pathway [22], [23] (Fig. 2). Insulin secretion was promoted in high glucose-stimulated INS-1E cells [22], indicating that high glucose (20 mmol/L)-induced H2S/CSE inhibition might lead to stimulating a long-term insulin release. On the contrary, high glucose (10 or 20 mmol/L) was observed to induce H2S production in MIN6 cells by upregulating CSE expression. Similar phenomenon occurred in mouse islets [20]. The difference of the effect of glucose on H2S/CSE pathway between mouse pancreatic β-cells and INS-1E cells may be explained in part by different glucose levels during the pre-incubation period before treatment, different H2S responses based on animal species or cell types, and other experimental conditions. Therefore, further studies will be needed to explore the effect of hyperglycemia on endogenous H2S in pancreatic β-cells and its mechanisms.
Fig. 2.
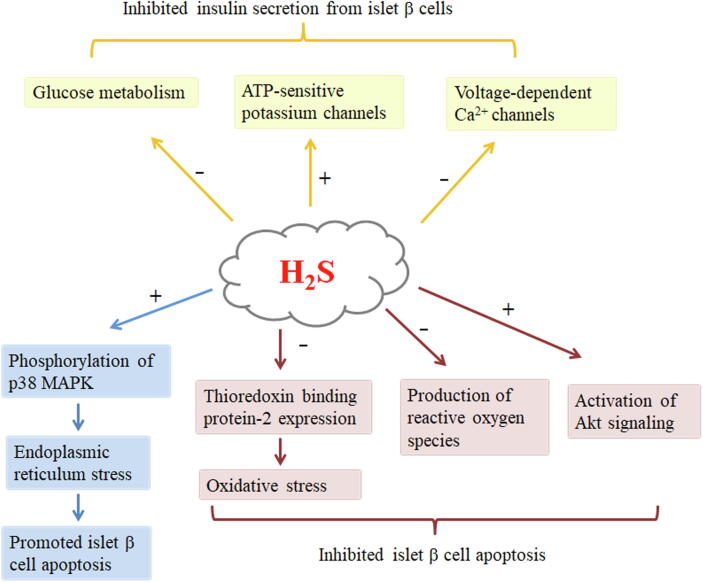
. H2S inhibits insulin secretion and regulates apoptosis of islet β cells. H2S inhibits insulin secretion from islet β cells through stimulating of KATP channels, inactivating VDCC and suppressing glucose metabolism. H2S promotes islet β cell apoptosis through phosphorylation of p38 MAPK and subsequent enhances ER stress. H2S inhibits islet β cell apoptosis through reducing thioredoxin binding protein-2 expression, inhibiting reactive oxygen species production and activating Akt signaling pathway. + indicates increase, − indicates inhibition.
H2S inhibits insulin secretion from pancreatic β-cells
Absolute or relative deficiency of insulin secretion caused by the loss or dysfunction of pancreatic β-cells contributes to the occurrence and development of diabetes mellitus. Several studies investigated the effects of H2S on insulin secretion by pancreatic β-cells (Table 2).
Table 2.
H2S regulates insulin secretion and islet β cells apoptosis.
| Action | Cells/Model | H2S gas/donor application (concentration) | Effects | Refs. |
|---|---|---|---|---|
| Insulin section | ||||
| In vitro study | INS-1E cells | H2S gas (100 μM) | Inhibited | [22] |
| HIT-T15 cells | NaHS (100 μM) | Inhibited | [17] | |
| MIN6 cells | NaHS (10, 100 and 1000 μM) | Inhibited | [19] | |
| Isolated mouse islets | NaHS (100 and 300 μM) | Inhibited | [24] | |
| Isolated mouse islets | NaHS (100 and 1000 μM) | Inhibited | [19] | |
| In vivo study | Zucker diabetic fatty rats | – | Inhibited | [18] |
| Nonfasting wild-type mice | NaHS (39 μmol/kg) | Inhibited | [26] | |
| Islet β cell apoptosis | ||||
| In vitro study | INS-1E cells | H2S gas (100 μM) | Promoted | [25] |
| Isolated mouse islets | NaHS (100 μM) | Inhibited | [20] | |
| Isolated mouse islets | NaHS (100 μM) | Inhibited | [28] | |
| MIN6 cells | NaHS (100 μM) | Inhibited | [28] | |
| In vivo study | Streptozotocin-induced diabetic mice | – | Promoted | [26] |
| High fat diet-induced obese mice | – | Inhibited | [21] | |
Wu et al. [18] demonstrated that compared with control rats, impaired insulin secretion, reduced serum insulin level, elevated pancreatic CSE protein levels and H2S production, hyperglycemia and insulin resistance were showed in Zucker diabetic fatty (ZDF) rats. Intraperitoneal injection of PPG to inhibit CSE activity and pancreatic H2S production could restore normal pancreatic insulin secretion, upregulate the serum insulin, and improve high blood glucose as well as decrease hemoglobin A1c (HbA1c) in ZDF rats. These findings indicated that abnormally increased pancreatic H2S production might lead to the occurrence of impaired insulin secretion in diabetes. After the overexpression of CSE with adenovirus to increase H2S production in INS-1E cells, insulin secretion at low glucose (5 mmol/L) did not change, whereas insulin secretion induced by high glucose (16 mmol/L) was significantly reduced [22]. Similar effects were observed in Syrian hamster pancreatic β-cells (HIT-T15) treated with exogenous (sodium hydrogen sulfide, NaHS) or endogenous (CBS overexpression) H2S [17]. Additionally, NaHS inhibited high glucose-induced insulin release by mouse pancreatic islets and MIN6 cells. However, NaHS failed to regulate insulin release induced by 3 mmol/L glucose [19]. High glucose caused more insulin release by freshly isolated pancreatic islets of CSE-deficient mice than that by littermate wild-type mouse islets [24]. H2S donor suppressed high glucose-induced insulin release in wild-type islets [24]. These data suggest that both endogenous H2S generated from pancreatic β-cells and exogenous H2S inhibit insulin secretion in the presence of high glucose, while the inhibition of pancreatic H2S synthesis promotes insulin secretion, suggesting that abnormal H2S synthesis is the cause and impaired insulin secretion is the effect.
Such inhibition involves several mechanisms as follows (Fig. 2). First, H2S decreases insulin release in islet β-cells via ATP-sensitive potassium (KATP) channels activation [17], [18], [22]. H2S at 100 μmol/L elevated KATP channel currents in islet β-cells from both ZDF and Zucker lean (ZL) rats, which were abolished by gliclazide, a KATP channel antagonist. Under resting conditions, KATP currents intensity was lower in ZDF islet β cells than in ZL β cells [18]. Patch-clamp whole-cell recording assay showed that treated INS-1E cells with high glucose (16 mmol/L) decreased KATP currents compared with cells by 5 mmol/L glucose treatment. H2S (100 μmol/L) application of INS-1E cells did not affect whole-cell KATP currents induced by 5 mmol/L glucose, but markedly upregulated KATP channel currents stimulated by 16 mmol/L glucose. Application of PPG or CSE siRNA to lower the cell H2S content reduced KATP currents, which was abolished by H2S donor supplementation. It was also found that H2S facilitated KATP channel activity in INS-1E cells by upregulating single-KATP-channel open probability, but not the open time of single channels, single-channel conductance, linear current–voltage relation of single-channel currents, or ATP sensitivity of KATP channels. The results suggest that H2S directly acts on KATP channel proteins [22]. Similarly, treatment of HIT-T15 cells with H2S decreased intracellular K+ concentration, indicating that H2S opens cell K+ channel to cause K+ outflow. Following K+ outflow, the HIT-T15 cell membrane was hyperpolarized by NaHS (100 μmol/L) treatment. This caused the voltage-gated Ca2+ channels to shut down, which in turn prevented Ca2+ from flowing into cells and lowered intracellular Ca2+ ion concentration. Glibenclamide, a KATP channel antagonist, reversed these effects and the inhibition of H2S on insulin release [17]. These results demonstrate that H2S-activated KATP channels act as an important mechanism by which H2S inhibits insulin secretion.
Several studies reported that diazoxide, an activator of KATP channels, could not completely abolish the inhibitory effect of NaHS on insulin release, suggesting that the underlying mechanism involves a KATP channel-independent pathway [19]. Kaneko et al. found that H2S inhibited glucose-stimulated [Ca2+]i oscillation in mouse pancreatic β-cells [19]. The H2S donors NaHS and ACS67 decreased L-type voltage-dependent Ca2+ channel (VDCC) currents in the wild-type islet β-cells, whereas an L-type VDCC antagonist nifedipine inhibited it [24]. Moreover, the inhibitory effect of NaHS on L-type VDCCs was reversible. NaHS administration delayed the channel recovery from depolarization-caused inactivation but failed to alter the channel current–voltage relationship curve. Application of CSE inhibitor PPG to mouse islet β-cells promoted the basal activity of L-type VDCCs. Moreover, CSE-deficient islet β cells showed higher density of L-type VDCC current than that in wild-type β-cells. Nifedipine co-application enhanced the inhibitory role of NaHS in insulin secretion. L-type VDCC activator Bay K-8644 increased insulin secretion stimulated by high glucose, which was counteracted by NaHS or nifedipine addition [24]. These results demonstrate that H2S reduces insulin secretion at least partially by inhibiting L-type VDCCs in islet β-cells. Therefore, NaHS suppresses insulin release through KATP-channel-dependent mechanisms and -independent mechanisms. KATP channels activated by H2S resulted in islet β cell hyperpolarization and decreased L-type VDCC activity, suggesting KATP-channel-dependent mechanisms. Data demonstrating that H2S directly suppressed L-type VDCC suggest KATP-channel-independent mechanisms involved in H2S action [24] (Fig. 2).
H2S regulates apoptosis of islet β-cells
Impaired islet β-cell survival arising from an imbalance between cell proliferation and death constitutes one of the major pathogenic factors for diabetes. The pattern of islet β-cell death in the development of diabetes is mainly apoptosis. H2S regulation of islet β-cell apoptosis is controversial (Table 2). Endogenously produced H2S by CSE overexpression or exogenously produced H2S (100 μmol/L, within physiologically relevant concentration [15]) incubation for 12 h induced INS-1E cell apoptosis, which may not be associated with the changed redox status [25]. The study further indicated that H2S increased the phosphorylation of p38-MAPK, and then enhanced endoplasmic reticulum (ER) stress, demonstrated by increased expression of Bip, CHOP and SREBP-1c, thus inducing apoptosis of β-cell. (Fig. 2). The phenomenon of H2S-induced INS-1E cell apoptosis suggests a suppressive effect of H2S on insulin secretion by decreasing β-cell mass. Type 1 diabetes was formed in wild-type mice 24 days after the first intraperitoneal administration of streptozotocin (STZ), while CSE knockout delayed it. CSE inhibitor PPG treatment protected against hypoinsulinemia and hyperglycemia in mice subjected to STZ. Moreover, STZ markedly promoted CBS mRNA expression, H2S generation and islet β cell apoptosis in pancreatic tissue of wild-type mice, while CSE knockout partly abolished STZ-induced islet β-cell apoptosis [26], [27]. Treatment of INS-1E cells with STZ increased H2S generation and decreased the viability of the cells, while PPG co-treatment partially reversed the decreased cell viability. Mechanistically, STZ induced KATP channel currents in wild-type islet β cells, which was blocked by PPG treatment or CSE knockout. Glucose intolerance, hyperglycemia as well as low insulin secretion were observed in the wild-type mice with NaHS administration [26]. Collectively, the results demonstrated that the H2S produced by CSE accelerated diabetes in STZ mice through inducing β-cell apoptosis and activating KATP channels. Inhibition of the CSE/H2S pathway is thought to be a novel strategy for protection against diabetes mellitus.
In contrast, several studies reported a protective role of H2S in regulating pancreatic β-cell apoptosis. NaHS (100 μmol/L) inhibited high glucose (20 mmol/L)-stimulated cell apoptosis and elevated the glutathione concentration in mouse islet β cell line MIN6 [20], [28], indicating that H2S might protect pancreatic β-cells from glucotoxicity. The inhibitory effect of H2S on pancreatic β-cell apoptosis was also confirm in cultured islets [28]. Interestingly, H2S suppressed fatty acid-, cytokines- or H2O2-induced β-cell apoptosis, while H2S did not prevent mouse islets and MIN6 cells from apoptosis stimulated by thapsigargin and tunicamycin, both of which led to ER stress. It indicates that the anti-oxidative capacity and the activation of Akt signaling but not ER stress may be involved in H2S-mediated prevention from β-cell apoptosis (Fig. 2). In addition, Thioredoxin binding protein-2 (TBP-2) plays an important role in β-cell apoptosis and the onset and development of diabetes. TBP-2 expression was increased in the pancreas from high fat diet (HFD)-fed CSE-deficient mice which showed elevated β-cell apoptosis, decreased pancreatic insulin contents, and glucose intolerance [21]. The administration of the H2S donor NaHS abolished 20 mmol/L glucose exposure-enhanced TBP-2 expression in CSE knockout pancreatic β cells. These data suggested that the CSE/H2S pathway protected against the occurrence of HFD-induced diabetes mellitus by inhibiting TBP-2-mdidated β-cell apoptosis (Fig. 2). However, CSE knockout delayed the onset of STZ-induced type 1 diabetes, decreased hypoinsulinemia and hyperglycemia, and reduced islet β-cell apoptosis [26]. These findings put forward the possibility that H2S induces apoptosis of extremely impaired β-cells and inhibits apoptosis of not extremely impaired β-cells. While, the effects of the endogenous H2S on β-cell functions may depend on the age or stage/type of diabetes.
Hydrogen sulfide regulates insulin sensitivity
Insulin resistance acts as a fundamental pathology of type 2 diabetes [5]. Insulin resistance means that the responsiveness of target tissues to insulin is reduced, leading to reduced glucose uptake to skeletal muscle and adipose and elevated hepatic glucose output. Previous studies have shown that H2S is important for regulating insulin sensitivity in the liver, adipose and skeletal muscle.
H2S and liver glucose metabolism
Hepatic insulin resistance refers to the failure of insulin to inhibit glycogenolysis and gluconeogenesis in the liver to maintain normal plasma glucose levels [5], [29]. The enzymes responsible for endogenous H2S, CSE, CBS, and 3-MST are found in the liver and contribute to liver H2S production. The effect of diabetes mellitus and its related conditions on H2S production system in the liver is controversial. Compared with non-diabetic rats, H2S production and CSE and CBS mRNA levels in the liver were increased in STZ diabetic rats, while insulin treatment reversed these effects [27]. Alternatively, H2S formation and CSE activity and protein expression in the liver were suppressed in STZ-induced type 1 diabetic rats [30]. Livers taken from insulin sensitizer metformin-treated SJL mice (100 mg/kg b.w. per day) exhibited an increased H2S concentration [31]. Further study will be needed to explore the regulatory mechanism of diabetes mellitus and its related conditions on the production of H2S in the liver.
H2S regulates glucose uptake, glycogen storage and gluconeogenesis
H2S function in liver glucose metabolism has been studied extensively (Table 3). One view is that H2S suppresses glucose uptake and glycogen storage but promotes gluconeogenesis. NaHS (10, 30, and 100 μmol/L) inhibited glucose uptake and impaired glycogen storage in HepG2 cells by inhibiting glucokinase activity [32]. CSE overexpression decreased glycogen content in HepG2 cells, whereas CSE knockout increased glycogen content in liver tissues. Primary hepatocytes from CSE knockout mice showed increased glucose consumption but reduced glucose production via gluconeogenesis and glycogenolysis pathways. H2S promoted the rate of gluconeogenesis and increased glycogenolysis in HepG2 cells, while CSE-deficient hepatocytes showed a decreased rate of gluconeogenesis and glucose release. The research of Untereiner et al. also confirmed that H2S increased the hepatic glucose production and gluconeogenesis [33].
Table 3.
H2S regulates glucose uptake, glycogen storage, gluconeogenesis and mitochondrial function in liver.
| Action | Cells/Model | H2S gas/donor application (concentration) | Effects | Refs. |
|---|---|---|---|---|
| Glucose uptake | ||||
| In vitro study | HepG2 cells/primary mouse hepatocytes | NaHS (10, 30 and 100 μM)/– | Inhibited | [32] |
| Glycogen storage | ||||
| In vitro study | HepG2 cells | NaHS (10, 30 and 100 μM) | Inhibited | [32] |
| In vivo study | Liver from mice under nonfastingor 6-h fasting condition | – | Inhibited | [32] |
| Gluconeogenesis | ||||
| In vitro study | HepG2 cells/primary mouse hepatocytes | NaHS (10, 30 and100 μM)/– | Promoted | [32] |
| Primary mouse hepatocytes | NaHS (30 μM) | Promoted | [33] | |
| HepG2 cells/primary mouse hepatocytes | NaHS (50 μM)/– | Promoted | [34] | |
| HepG2 cells/primary mouse hepatocytes | NaHS (100 μM)/– | Inhibited | [35] | |
| In vivo study | Pyruvate tolerance test on overnight-fasted mice | NaHS (39 and 63 μM/kg) | Promoted | [33] |
| Pyruvate tolerance test on high fat diet-fed mice | NaHS (50 μM/kg/day) | Inhibited | [35] | |
| Mitochondrial function | ||||
| Biogenesis | Primary moue hepatocytes | NaHS (30 μM) | Promoted | [38] |
| Bioenergetics | HepG2 cells | NaHS (0.01 and 0.1 μM) | Promoted | [39] |
Mechanistically, the fact that H2S attenuated AMP-activated protein kinase (AMPK) activation was involved in H2S-induced inhibition of glucose uptake by HpG2 cells. Further study indicated that H2S promoted glucose production via upregulated phosphoenolpyruvate carboxykinase (PEPCK) activity but decreased glucokinase (GK) activity [32]. In addition, H2S-induced upregulation of peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and phosphoenolpyruvate carboxykinase expression through the glucocorticoid receptor (GR) pathway, upregulation of PGC-1α expression through the cAMP/PKA pathway, and the activation and upregulation of PGC-1α S-sulfhydration may explain, at least in part, CSE-generated H2S regulation of hepatic glucose production [33]. And H2S upregulates FBPase and G6Pase expression might contribute mechanistically as well (by sulfhydration). Furthermore, H2S sufhydrated pyruvate carboxylase at cysteine 265, and increased its activity, thus promoting gluconeogenesis in hepatocytes [34]. These results demonstrated that H2S in the liver reduced glucose uptake and promoted glucose output, contributing to hyperglycemia and insulin resistance. Downregulation of hepatic H2S synthesis or CSE activity is a potential approach to preventing the development of insulin resistance.
In contrast, another view is that H2S inhibits hepatic gluconeogenesis. Guo et al. [35] reported that CSE deficiency promoted hepatic gluconeogenesis. Knockdown of CSE in HepG2 cells increased glucose production under insulin stimulation, while NaHS (100 μM) attenuated CSE knockdown-induced glucose production. Furthermore, CSE knockout inhibited insulin and AMPK signaling pathways and increased nuclear accumulation of Forkhead box protein O1 (FoxO1), thus promoting hepatic gluconeogenesis. But NaHS treatment reversed the effects. CSE knockout mice exhibited exacerbated HFD-induced obesity and insulin resistance, while administration of NaHS to wild-type mice alleviated HFD-caused metabolism disorders. These data suggest that H2S inhibits hepatic gluconeogenesis through a FoxO1-dependent mechanism, thus attenuating HFD-induced insulin resistance.
H2S regulates hepatic mitochondrial biogenesis and mitochondrial bioenergetics
The mitochondria are crucial in regulating energy homeostasis, β-oxidation of fatty acids, and reactive oxygen species (ROS) production. Mitochondrial dysregulation occurs in insulin resistance [36], [37]. The amount of mitochondria was decreased in CSE-deficient hepatocytes compared with control [38]. NaHS treatment restored the mitochondrial content in CSE-knockout hepatocytes. The expressions of NRF-1, NRF-2, Tfam and peroxisome proliferator-activated receptor-γ coactivator-related protein (PPRC) were each downregulated in CSE-knockout hepatocytes. NaHS incubation increased PPRC levels but reduced PGC-1β expression in mouse hepatocytes. NaHS induced PPRC sulfhydration, while CSE knockout reduced it. However, the sulfhydration of PGC-1β was not detected. PGC-1α silencing reduced H2S donor-induced hepatic mitochondrial biogenesis. The same phenomenon was observed in PPRC-silence hepatocytes. The effect of H2S donor-stimulated mitochondrial biogenesis was completely blocked by knockdown of both PGC-1α and PPRC genes in wild-type and CSE-knockout hepatocytes. These data suggested that the endogenous H2S-stimulated hepatic mitochondrial biogenesis through PGC-1α and PPRC pathway [38]. NaHS sulfhydrated ATP synthase alpha subunit at cysteine 244 and 294, and activated this enzyme, thereby stimulating mitochondrial bioenergetics in HepG2 cells and in the liver [39] (Table 3).
Hydrogen sulfide and adipose tissue function
Adipose tissue serves as the main energy storage organ. Adipocytes uptake and storage much triglycerides under the energy sufficiency conditions, but consume them during fasting or energy insufficiency conditions. During fasting or stress conditions, triglycerides undergo lipolysis by lipases including adipocyte triglyceride lipase (ATGL), hormone sensitive lipase (HSL) and monoglyceride lipase (MGL). The products of triglyceride hydrolysis, glycerol and free fatty acids (FFA), are released into the blood and taken up by other tissues. For example, FFA is transported to skeletal muscles and heart to provide energy for these organs. They serve as energy substrates while glycerol is taken up by the liver. Adipose tissue is also thought to be an active endocrine organ. Cytokines and FFA released from adipocytes regulate systemic metabolic process. Several adipokines are produced and released from adipocytes, such as leptin, adiponectin and resistin. Maintaining normal adipose tissue function helps to maintain systemic metabolic homeostasis. Adipose tissue dysfunction is an important cause of metabolic diseases, such as diabetes, obesity and atherosclerosis [40], [41]. How H2S modulates adipose tissue function is discussed below (Table 4).
Table 4.
H2S regulates glucose uptake, adipogenesis and lipolysis in adipocytes.
| Action | Cells/Model | H2S gas/donor application (concentration) | Effects | Refs. |
|---|---|---|---|---|
| Glucose uptake | ||||
| 3T3-L1 adipocytes | H2S gas (10, 25 and 50 μM) | Promoted | [45] | |
| 3T3-L1 adipocytes | Na2S (10 and 100 μM) | Promoted | [44] | |
| 3T3-L1 adipocytes | NaHS (25, 50 and 100 μM) | Promoted | [45] | |
| 3T3-L1 adipocytes | NaHS (100 μM) | Promoted | [43] | |
| 3T3-L1 adipocytes | NaHS (100, 500 and 1000 μM) | Inhibited | [48] | |
| Adipogenesis | ||||
| 3T3-L1 adipocytes | H2S gas (100 μM) | Promoted | [43] | |
| 3T3-L1 adipocytes | NaHS (30 μM) | Promoted | [50] | |
| 3T3-L1 adipocytes | NaHS (50 μM) | Promoted | [49] | |
| 3T3-L1 adipocytes | GYY4137 (50 μM) | Promoted | [49] | |
| 3T3-L1 adipocytes | GYY4137 (100 μM) | Promoted | [43] | |
| Lipolysis | ||||
| 3T3-L1 adipocytes | NaHS (50 μM) | Inhibited | [49] | |
| 3T3-L1 adipocytes | GYY4137 (50 μM) | Inhibited | [49] | |
| Primary rat adipocytes | GYY4137 (1000 μM) | Inhibited | [57] | |
| Adipokines or inflammatory factors secretion | ||||
| 3T3-L1 adipocytes | NaHS (50 μM) | Attenuated high glucose-induced decrease in adiponectin and the increase in MCP-1 section | [58] | |
| High fat diet-induced diabetic mice | SG-1002 (20 mg/kg/day) | Increased the plasma adiponectin level | [59] | |
Hydrogen sulfide exists in adipose tissues
H2S was first reported in 2009 to be produced in rat adipose tissues, such as epididymal adipose, perirenal adipose, and brown adipose tissues [42]. RT-PCR analysis showed that epididymal adipose, perirenal adipose and brown adipose tissues all expressed the mRNA of CSE and CBS, the two H2S-producing enzymes. CSE protein expression was also detected in these adipose tissues. After the adipose tissues were pretreated with the CSE inhibitor PPG, endogenous H2S generation was decreased to less than 20%, indicating that CSE was a key enzyme to catalyze this gasotransmitter generation from adipose tissues. In addition, CSE/H2S pathway existed both in pre-adipocytes and adipocytes [42], [43]. The study also demonstrated that in rat visceral adipose, CSE/H2S level increased with age [42].
H2S regulates insulin sensitivity in adipose tissue
Several studies reported that H2S directly regulated insulin sensitivity in adipocytes. H2S improved high glucose-induced insulin resistance [44]. Manna et al. demonstrated that H2S and its precursor L-cysteine upregulated insulin signaling pathways. They found that both H2S and L-cysteine increased phosphatidylinositol 3,4,5-trisphosphate (PIP3), downstream phosphorylated Akt level as well as glucose uptake by cultured 3T3-L1 cells in the presence of 25 mmol/L glucose. H2S mediated the effect of L-cysteine on PIP3 and glucose uptake. Furthermore, H2S activated PI3K but inhibited PTEN. L-cysteine, H2S or PIP3 upregulated Akt and PKCζ/λ phosphorylation, glucose transporter type 4 (GLUT4) activity, and glucose utilization in high glucose-stimulated cells. These results suggested that PIP3 participated in the effect of L-cysteine or of H2S on glucose utilization. PKCζ phosphorylation was more effective than AKT2 for glucose uptake in L-cysteine-, H2S-, and PIP3-incubated adipocytes incubated with high glucose. These results suggested that H2S or L-cysteine improved glucose metabolism by upregulating the cellular PIP3 level, which was mediated by suppressing PTEN/NF-κB pathway as well as activating PI3K/PIP3/Akt/PKCζ/λ insulin signaling in adipocytes [44].
Xue et al. [45] indicated that both NaHS and H2S gas solution treatment significantly increased glucose uptake in adipocytes. Application of NaHS to cultured adipocytes incubated with 5.5 mM glucose or 25 mM glucose increased phosphorylated insulin receptor (p-IR), p-PI3K and p-Akt levels. Insulin receptor (IR) knockdown abolished the effect of NaHS, which indicated that IR mediated the promotion of H2S on glucose utilization. Furthermore, H2S donor activated IR in purified IR protein. Chronic treatment of NaHS (30 μmol/kg/day) was found to improve insulin resistance in Goto-Kakizaki diabetic rats. This study suggested that NaHS/H2S improved insulin resistance through activating the insulin receptor signaling pathway [45].
Adipogenesis participates in obesity and insulin resistance regulation [46]. CSE expression and H2S generation were upregulated during the differentiation of 3T3L1 preadipocytes to mature adipocytes. Treatment with H2S or upregulated CSE increased triglyceride deposition in adipocyte differentiation, while inhibiting CSE activity with PPG reduced intracellular triglyceride deposition. H2S donor GYY4137 inhibited PDE activity and promoted adipocyte differentiation. CSE/H2S pathway upregulated PPARγ level in the process of adipocyte differentiation. H2S sulfhydrated cysteine-139 in PPARγ, and then induced activation of PPARγ, which enhanced glucose uptake and lipid storage. Mutation of cysteine 139 abolished sulfhydration of PPARγ and upregulated PPARγ activity. In vivo experiments further confirmed that H2S improved insulin resistance and promoted lipid accumulation by sulfhydrating PPARγ in high-fat-diet-induced obese mice. These data suggested that under physiological conditions or at the early stage of obesity, the CSE/H2S pathway converted glucose into triglyceride storage in adipocytes by sulfhydrating PPARγ, upregulating its activity and maintaining systemic glucose metabolism homeostasis [43].
Manna et al. revealed that 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] upregulated GLUT4 protein expression, GLUT4 translocation and glucose uptake inhibited by high glucose in 3T3L1 adipocytes. The 1,25-(OH)2D3-caused GLUT4 translocation and glucose uptake were dependent on 1,25-(OH)2D3-upregulated CSE activity and H2S formation. Inhibiting CSE activation using PPG or knocking down CSE using siRNAs blocked the positive function of 1,25-(OH)2D3 upon GLUT4 activation and glucose uptake. These results indicated that CSE/H2S pathway in adipocytes might mediate the improvement of 1,25-(OH)2D3 on glucose utilization impaired by high glucose in 3T3L1 adipocytes [47].
In contrast, Huang et al. reported that H2S mediated tumor necrosis factor-α (TNF-α)-stimulated insulin resistance. The study showed that treatment with TNF-α increased H2S concentration of cultures medium from 3T3-L1 adipocytes, and decreased glucose consumption and uptake. TNF-α also increased CSE protein expression and activity. CSE inhibitors PPG and β-cyano-L-alanine (BCA) attenuated TNF-α-induced H2S generation in the cells, and improved TNF-α-caused impaired glucose consumption and uptake. Treatment with H2S (100, 500 and 1000 mM) inhibited glucose consumption and uptake in adipocytes. The results indicated the H2S mediated TNF-α-induced insulin resistance in adipocytes [48].
Above all, these studies suggested that H2S directly regulated insulin sensitivity in adipocytes. How H2S regulates insulin sensitivity in adipocytes needs to be further studied.
H2S promotes adipogenesis in adipocytes
Adipogenesis is the process of conversion of preadipocytes into adipocytes, contributing to increasing numbers of adipocytes in adipose tissue and adipose tissue expansion [46]. Tsai et al. reported that H2S promoted adipogenesis in 3T3L1 adipocytes [49]. They found that CSE, CBS and 3-MST levels were increased in the process of 3T3L1 cell differentiation. Treating 3T3L1 adipocytes during differentiation with GYY4137 or NaHS elevated the expression of adipogenesis-associated genes and increased lipid accumulation in adipocytes. Treating the cells with the CSE inhibitor, PPG, CSE small interference RNAs, the CBS inhibitor, aminooxyacetic acid, or CBS small interference RNAs decreased the expression of adipogenesis-related genes. Aminooxyacetic acid or PPG inhibited lipid droplet formation in mature adipocytes. These results indicated that H2S promoted adipogenesis likely by increasing fatty acid binding protein 4 expression [49]. In addition, Cai et al. recently reported the effects of the CSE/H2S pathway on lipid storage in adipocytes. They found that treatment with an H2S donor or CSE overexpression sulfhydrated PPARγ at cysteine 139 to induce PPARγ activation, thereby promoting adipogenesis in adipocytes [43]. In accordance with these results, Yang et al. [50] indicated that CSE/H2S levels increase in the process of adipocyte differentiation. H2S sulfhydrated PPARγ to induce its activation and promote adipogenesis. Studies in genetic mouse models also confirmed positive regulation of H2S on adipogenesis. Lower body weight and less adipose tissue were observed in CSE knockout mice and CBS knockout mice [51].
H2S inhibits lipolysis in adipocytes
Lipolysis causes triglyceride hydrolysis to release glycerol and FFA. Lipolysis increases in obesity and excessive FFA are released into the bloodstream, contributing to local oxidative stress, inflammation and ectopic fat deposition in target tissues, which leads to insulin resistance and diabetes [52], [53], [54]. Lipolysis is regulated by multiple endogenous factors, including catecholamines, insulin, and cytokines [55], [56]. Studies have investigated whether H2S regulates lipolysis. Geng and colleagues studied the effect of H2S on lipolysis in adipocytes. The CSE inhibitor PPG elevated lipolysis, while H2S precursor L-cysteine and H2S donor GYY4137 inhibited lipolysis in adipocytes. PPG upregulated phosphorylation of PKA substrate and HSL as well as perilipin 1 expression, while H2S donor GYY4137 reduced these protein expressions. Results from in vivo studies indicated that PPG upregulated lipolysis and GYY4137 suppressed adipose lipolysis in HFD-fed mice. However, both PPG and GYY4137 improved insulin sensitivity in HFD mice. These findings suggested the CSE/H2S pathway inhibited lipolysis through PKA-perilipin/HSL signaling pathway and improved insulin sensitivity [57].
Lipolysis inhibition by H2S was confirmed by Tsai et al. They reported that both GYY4137 and NaHS inhibited CL-316, 243 (β-adrenoceptor agonist)-stimulated lipolysis in mature 3T3-L1 adipocytes, while the CBS inhibitor AOAA or CSE inhibitor PPG promoted it, suggesting that endogenous and exogenous H2S inhibit lipolysis [49].
Role of H2S in regulation of adipokines and inflammatory factors in adipose
Adipokines secreted from adipose tissue, like leptin and adiponectin, regulate systemic glucose and lipid metabolic homeostasis. Pan and colleagues found high glucose (25 mmol/L) reduced CSE expression in differentiated 3T3-L1 adipocytes. CSE overexpression or supplementation with NaHS abolished the elevated monocyte chemoattractant protein 1 (MCP-1) and reduced adiponectin secretion from mature 3T3-L1 adipocytes induced by high glucose. These findings suggested that inhibiting the CSE/H2S pathway at least partly mediated the regulation of high glucose in adipocyte inflammation [58]. Barr found that in HFD-induced diabetic cardiomyopathy mice, HFD feeding induced lower levels of circulating H2S and adiponectin. Treatment with an orally active H2S donor, SG-1002, increased the plasma H2S level and restored adiponectin levels. These results indicated that H2S might increase adiponectin secretion from adipose tissue in HFD-induced diabetic mice [59]. Further studies in humans indicated the relationship between plasma H2S and adiponectin levels. In 36 healthy volunteers, plasma H2S levels positively correlated with the adiponectin concentration [60].
H2S and skeletal muscle
Skeletal muscle serves as one of the chief insulin responsive organs and regulates systemic glucose metabolism. CBS and CSE are abundant in human skeletal muscles similar to their expressions in human kidney and liver [61], [62], [63]. CBS, CSE and 3-MST were detected in rat skeletal muscle, but their expressions were much lower than those in rat kidney and liver tissue [64]. The levels of three H2S synthases as well as H2S production capacity were also detectable in mouse skeletal muscle [65]. Skeletal muscles in both ovine and bovine expressed very little 3-MST. Therefore, the expression of the H2S synthases in skeletal muscle varies between species.
H2S might promote glucose uptake in the muscle. Administration of NaHS to L6 myotubes promoted glucose uptake and upregulated phosphorylated insulin receptor (IR), PI3K and AKT levels, which were blocked by the IR inhibitor [45]. Either PI3K inhibitor or IR knockdown attenuated NaHS-induced upregulation of glucose uptake. Moreover, NaHS could directly activate IR. Chronic NaHS administration improved insulin resistance accompanied by elevated phosphorylated PI3K and Akt levels in the muscles from Goto-Kakizaki (GK, a spontaneous type 2 diabetic model) diabetic rats. The same phenomenon was found in Wistar rats [45]. These results indicated that H2S might promote glucose uptake in skeletal muscles through sensitizing the IR-PI3K-Akt signaling pathway, thus ameliorating insulin resistance. Moreover, the promotion of glucose uptake by H2S was further confirmed in C2C12 mouse myotubes [66]. NaHS administration (10 or 20 μM; 6 h) increased glutathione (GSH) biosynthesis, glucose uptake and utilization, but decreased ROS generation. In contrast, inhibition of endogenous H2S in C2C12 myotubes by CSE knockdown reduced GSH biosynthesis, GLUT4 level and glucose uptake, but increased ROS generation [66]. Persistent hyperglycemia is one of the main causes of oxidative stress. NaHS treatment improved diaphragm contractility and ultrastructural damage in diabetic rat, which might be related to the inhibition of oxidative damage and cell apoptosis in diaphragmatic muscle [67]. These results indicated that H2S might be crucial for maintaining glucose homeostasis in the muscle.
H2S regulates circadian clock in the skeletal muscle. Disruption of core clock genes (CCG) contributes to the progression of metabolic disorders [68]. H2S synthesis enzymes (CBS, CSE and 3-MST), antioxidant genes, CCG and clock-controlled genes were all decreased in muscle of HFD-induced mice, but markers of oxidative stress and expression of oxidative stress related genes were increased [65]. In cultured mouse myoblast cell line C2C12, high glucose or palmitate treatment decreased CSE/H2S level and CCG expression, but increased oxidative stress. TNF and MCP-1 treatment displayed similar effects. Inhibiting CSE and glutamate cysteine ligase catalytic subunit (GCLC) with siRNA or pharmacological inhibitors decreased H2S production, Bmal1 and Clock mRNA expression, and increased oxidative stress. Application of H2S donor NaHS or GSH precursor improved expression of CCG [65]. These results suggest that H2S may be an important endogenous regulator of the circadian clock and metabolic disorder.
H2S and hypothalamus
CBS and 3-MST are the major endogenous enzymes in the brain that produce H2S [69], [70], [71], [72], [73]. CBS/H2S pathway in the hypothalamic paraventricular nucleus (PVN) was found to improve obesity and insulin sensitivity via the brain-adipose interaction [74]. CBS was detected in thyrotropin-releasing hormone (TRH) and CRH producing neurons. Hypothalamus CBS protein levels was downregulated in HFD-induced obese rats and in db/db obese mice. Overexpression of PVN CBS decreased food intake and improved obesity and insulin sensitivity in high-fat-diet rats. Moreover, CBS overexpression in PVN upregulated pre-TRH level and downregulated adreno-cortico-tropic-hormone (ACTH) and corticosterone content in the blood. The activation of mTORC1 signaling pathway is involved in the upregulation of CBS/H2S in pre-TRH level. Further results [74] indicated that leptin activated FOXO3a to increase CBS expression and H2S production in the hypothalamus, regulating neuroendocrine hormone (TRH and ACTH) to impact systemic glucose metabolism and energy homeostasis. However, the roles of H2S in regulating glucose metabolism control by the hypothalamus and its mechanism need to be further studied.
Roles of H2S in glucose metabolism in vivo
Endogenous H2S plays vital roles in regulating pancreatic β-cells, liver, adipose, skeletal muscle and hypothalamus function to modulate glucose metabolism. How H2S regulates glucose metabolism in vivo has attracted great attention. Guangdong and colleagues found that CSE knockout mice showed a delayed onset of STZ-induced diabetes and the reduced apoptotic β-cell death. STZ upregulated pancreatic H2S generation, and the treatment of PPG improved glucose metabolism disorder in STZ wild type mice. But NaHS injection impaired glucose metabolism [26]. The inhibition of H2S production with PPG upregulated serum insulin levels, and decreased blood glucose in Zucker diabetic fatty (ZDF) rats, indicating that inhibiting H2S production improved hyperglycemia [18]. In contrast, other studies reported that middle-aged CSE-deficient mice showed increased blood glucose levels and impaired glucose intolerance [21]. Xue et al. showed that chronic NaHS administration improved glucose metabolism disorder in Goto-Kakizaki diabetic rats. Application of NaHS (60 μmol/kg·day, 10 weeks) improved insulin sensitivity in Wistar rats [45]. Guo et al. indicated that CSE knockout promoted HFD-induced mouse obesity and insulin resistance and increased hepatic gluconeogenesis. A low dose of NaHS treatment (25 μmol/kg/d, intraperitoneally for 12 weeks) improved HFD-induced obesity and insulin resistance but a high dose of NaHS treatment (50 μmol/kg/d, intraperitoneally for 12 weeks) promoted HFD–induced obesity and insulin resistance [35]. Treatment with H2S gas buffer, or the H2S donor GYY4137 improved insulin resistance in HFD-induced obese mice [43]. Geng et al. reported that both upregulation and downregulation of H2S level could improve insulin resistance in HFD mice [57]. Therefore, the roles of H2S in glucose metabolism in vivo are not consistent, and need to be further investigated.
H2S in humans
Most studies indicated plasma H2S levels decreased in diabetic patients. Compared with lean subjects, overweight and type 2 diabetes patients had decreased blood H2S concentrations. The median plasma H2S levels (25th, 75th percentiles) in lean, overweight and type 2 diabetes subjects were 38.9 (29.7, 45.1) μmol/L, 22.0 (18.6, 26.7) μmol/L and 10.5 (4.8, 22.0) μmol/L, respectively [12]. There was a negative correlation between blood H2S level and fasting glucose. The negative correlations between blood H2S level and insulin sensitivity were also observed. Adiposity emerged as an independent predictor of blood H2S. Similarly, Jain et al. and other groups reported that plasma H2S levels were reduced in type 2 diabetes patients compared to control (45.1 ± 15.5 μmol/L versus 54.0 ± 26.4 μmol/L reported by Suzuki et al.) [11], [13], [75]. H2S synthesis enzyme expression and H2S production were also decreased in diabetic conditions. CSE protein expression and activity in peripheral blood mononuclear cells (PBMCs) from type 1 diabetes patients were decreased significantly compared with normal subjects. A significantly negative correlation was seen between CSE protein expression and HbA1c levels in the type 1 diabetes patients [30]. Xiaonan et al. reported that the leukocyte-derived H2S production rate was much lower in both obese and overweight hypertensive than in normal weight hypertensive patients. H2S production from leukocyte was negatively correlated with the levels of homeostasis model assessment of insulin resistance in overweight and obese hypertensive patients. CSE expression in leukocytes of patients with high insulin levels was decreased compared with patients with high insulin levels. The production rate of leukocyte-derived H2S positively correlated with the plasma IL-10 levels [75]. High glucose inhibited CSE/H2S levels in peripheral blood monocytes [76]. These results indicated that the plasma H2S concentration decreased in diabetic patients. Whether this decrease contributes to the development of diabetes in humans needs to be further investigated.
Conclusions and future perspectives
In summary, H2S could be endogenously produced from islet β cells, the liver, adipose, skeletal muscles and the hypothalamus, and regulates local and systemic glucose metabolism. It inhibits insulin secretion from islet β cells and promotes or suppresses β-cell apoptosis. It regulates insulin sensitivity in insulin responsive tissues. In the liver, H2S inhibits glucose uptake and glycogen storage, and promotes or inhibits gluconeogenesis, mitochondrial biogenesis and mitochondrial bioenergetics. Several research groups reported that H2S promoted glucose uptake in adipocytes while others reported H2S inhibited this process. It is also reported that H2S promotes adipogenesis, inhibits lipolysis, and regulates adipokine secretion from adipocytes. H2S increases glucose uptake, modulates CCG in myotubes and muscle from HFD mice, inhibits oxidative damage and cell apoptosis in diaphragmatic muscle. CBS/H2S pathway in the paraventricular nucleus improved obesity and insulin sensitivity via the brain-adipose interaction. Plasma H2S levels are downregulated in diabetes patients compared with controls.
Although numerous studies have been performed to explore how H2S regulates insulin resistance, β-cell function, and glucose metabolism, the results are sometimes controversial and many important issues need to be further studied. This discrepancy might be due to several mechanisms. For example, varying H2S concentrations may cause different effects. Low concentrations of H2S may play a protective role while high concentrations of H2S may cause toxic effects. Differences under experimental conditions, species-specificity, and specificity of H2S-synthesizing enzyme inhibitors and donors might also contribute to this discrepancy. The roles of tissue-specific H2S in systemic glucose metabolism have been far from clear. The molecular mechanisms through which H2S regulates β cell function and insulin resistance require further study. How H2S regulates glucose metabolism in human remains largely unknown and needs further investigation.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by National Natural Science Foundation of China (81770422 to YH, 81670395 to HJ, 81921001 to WK, and 81622004 to HJ), Beijing Natural Science Foundation (7182168 to YH, 7191012 to HJ, and 7171010 to JD), Open Foundation from Beijing Key Laboratory of Hypertension Research to YH, and Beijing Hospitals Authority Youth Programme (QML20170302 to HZ).
Biographies

Heng Zhang was awarded his PhD degree in Physiology by Peking University, China in 2013, for his work on the effect of intermedin on insulin resistance and obesity. Then, he works as a doctor of endocrinology at Affiliated Beijing Chaoyang Hospital of Capital Medical University. In 2017, he was selected into the Beijing Hospitals Authority Youth Programme. He now focuses on the basic and clinical research of obesity, insulin resistance and adipose metabolism.

Yaqian Huang is an associate professor in the Department of Pediatrics at Peking University First Hospital, China. She had obtained her PhD degree in Physiology from Peking University in 2013. Since then, she has been working at Peking University First Hospital. She is currently the vice president of Youth Committee of Chinese Pediatric Cardiology Society, Chinese Medical Association. Her research interest is focused on sulfur-containing gaseous signal molecules.

Selena Chen is master’s student in the Division of Biological Sciences at the University of California, San Diego, in the laboratory of Dr. Bryan Sun. Her research focuses on understanding the genetic regulators of skin development and what molecular pathways are disrupted during skin disease. She was awarded her Bachelor of Science in Biochemistry and Cellular Biology at the University of California, San Diego in 2018.

Chaoshu Tang is a professor at the Department of Physiology and Pathophysiology, Peking University Health Science Centre, China. He is a Chief Scientist of National “973” Key Basic Research Project. He has been engaged in scientific research and teaching of cardiovascular physiology and pathological mechanism for a long time. Professor Tang has won the second prize of National Science and Technology Progress (1998), the first prize of Ministry (1991, 1997, 2003 and 2005), and the second and third prizes and many other awards.

Guang Wang is a professor and the director of Department of Endocrinology, Affiliated Beijing Chaoyang Hospital of Capital Medical University, and vice president of Huairou Hospital, China. He has been engaged in the basic and clinical research of endocrinologic and metabolic diseases for many years. His research interests focus on the insulin resistance, β cell dysfunction and type 2 diabetes. In 2016, he was selected as the seventh “National Excellent Scientific and Technological Worker”. And he has been successfully selected into the Beijing high-level health personnel training plan and the “Municipal Candidates” of Beijing Million Talents Project.

Junbao Du is a professor at the Department of Pediatric Cardiology, Peking University First Hospital, China. He is majored in endogenous hydrogen sulfide research. Dr. Du is a Changjiang Scholar of China, Distinguished Young Scholars of NSFC and outstanding Young-middle Aged Expert Approved by Ministry of Health of China. He serves as a PI of Gasotransmitters and Cardiovascular Diseases Laboratory, Key Lab. of Cardiovascular Sciences, the Ministry of Education, P. R. China. He is also the president of Pediatric Cardiology Committee of Cardiovascular Physicians Society, Chinese Medical Doctors Association and a committee member of Asian Pacific Pediatric Cardiology Society.

Hongfang Jin is a professor of Pediatric Cardiology, Peking University First Hospital, China. She is majored in endogenous hydrogen sulfide research. She is an Excellent Young Scholars of National Natural Science Foundation (NSFC), and an Excellent Young Scholar in National Youth Top-notch Talent Support Program of China. Professor Jin is the president of Basic Research Committee of Chinese Pediatric Cardiology Society, vice-president of Pediatric Committee of Cardiovascular Society of Chinese Medical Doctor Association.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Guang Wang, Email: drwg6688@aliyun.com.
Junbao Du, Email: junbaodu1@126.com.
Hongfang Jin, Email: jinhongfang51@126.com.
References
- 1.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo R.A., Ferrannini E., Groop L., Henry R.R., Herman W.H., Holst J.J. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 4.Vetere A., Choudhary A., Burns S.M., Wagner B.K. Targeting the pancreatic β-cell to treat diabetes. Nat Rev Drug Discov. 2014;13(4):278–289. doi: 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- 5.Czech M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang C., Li X., Du J. Hydrogen sulfide as a new endogenous gaseous transmitter in the cardiovascular system. Curr Vasc Pharmacol. 2006;4(1):17–22. doi: 10.2174/157016106775203144. [DOI] [PubMed] [Google Scholar]
- 7.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y.H., Lu M., Hu L.F., Wong P.T., Webb G.D., Bian J.S. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal. 2012;17(1):141–185. doi: 10.1089/ars.2011.4005. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4–10. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2012;17(1):68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S.K., Bull R., Rains J.L., Bass P.F., Levine S.N., Reddy S. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010;12(11):1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteman M., Gooding K.M., Whatmore J.L., Ball C.I., Mawson D., Skinner K. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53(8):1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K., Sagara M., Aoki C., Tanaka S., Aso Y. Clinical implication of plasma hydrogen sulfide levels in Japanese patients with type 2 diabetes. Intern Med. 2017;56(1):17–21. doi: 10.2169/internalmedicine.56.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson K.R., Healy M.J., Qin Z., Skovgaard N., Vulesevic B., Duff D.W. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R669–R680. doi: 10.1152/ajpregu.00807.2007. [DOI] [PubMed] [Google Scholar]
- 15.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J., Chan A., Ali S., Saha A., Haushalter K.J., Lam W.L. Hydrogen sulfide–mechanisms of toxicity and development of an antidote. Sci Rep. 2016;6:20831. doi: 10.1038/srep20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali M.Y., Whiteman M., Low C.M., Moore P.K. Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J Endocrinol. 2007;195(1):105–112. doi: 10.1677/JOE-07-0184. [DOI] [PubMed] [Google Scholar]
- 18.Wu L., Yang W., Jia X., Yang G., Duridanova D., Cao K. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest. 2009;89(1):59–67. doi: 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko Y., Kimura Y., Kimura H., Niki I. L-cysteine inhibits insulin release from the pancreatic beta-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes. 2006;55(5):1391–1397. doi: 10.2337/db05-1082. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko Y., Kimura T., Taniguchi S., Souma M., Kojima Y., Kimura Y. Glucose-induced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS Lett. 2009;583(2):377–382. doi: 10.1016/j.febslet.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto M., Yamaoka M., Takei M., Ando T., Taniguchi S., Ishii I. Endogenous hydrogen sulfide protects pancreatic beta-cells from a high-fat diet-induced glucotoxicity and prevents the development of type 2 diabetes. Biochem Biophys Res Commun. 2013;442(3–4):227–233. doi: 10.1016/j.bbrc.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Yang W., Yang G., Jia X., Wu L., Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol. 2005;569(Pt 2):519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L., Yang G., Tang G., Wu L., Wang R. Rat pancreatic level of cystathionine γ-lyase is regulated by glucose level via specificity protein 1 (SP1) phosphorylation. Diabetologia. 2011;54(10):2615–2625. doi: 10.1007/s00125-011-2187-4. [DOI] [PubMed] [Google Scholar]
- 24.Tang G., Zhang L., Yang G., Wu L., Wang R. Hydrogen sulfide-induced inhibition of L-type Ca2+ channels and insulin secretion in mouse pancreatic beta cells. Diabetologia. 2013;56(3):533–541. doi: 10.1007/s00125-012-2806-8. [DOI] [PubMed] [Google Scholar]
- 25.Yang G., Yang W., Wu L., Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem. 2007;282(22):16567–16576. doi: 10.1074/jbc.M700605200. [DOI] [PubMed] [Google Scholar]
- 26.Yang G., Tang G., Zhang L., Wu L., Wang R. The pathogenic role of cystathionine γ-lyase/hydrogen sulfide in streptozotocin-induced diabetes in mice. Am J Pathol. 2011;179(2):869–879. doi: 10.1016/j.ajpath.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusuf M., Kwong Huat B.T., Hsu A., Whiteman M., Bhatia M., Moore P.K. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem Biophys Res Commun. 2005;333(4):1146–1152. doi: 10.1016/j.bbrc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi S., Kang L., Kimura T., Niki I. Hydrogen sulphide protects mouse pancreatic β-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br J Pharmacol. 2011;162(5):1171–1178. doi: 10.1111/j.1476-5381.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel B.M., Goyal R.K. Liver and insulin resistance: new wine in old bottle!!! Eur J Pharmacol. 2019;862:172657. doi: 10.1016/j.ejphar.2019.172657. [DOI] [PubMed] [Google Scholar]
- 30.Manna P., Gungor N., McVie R., Jain S.K. Decreased cystathionine-γ-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients. J Biol Chem. 2014;289(17):11767–11778. doi: 10.1074/jbc.M113.524645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiliński B., Wiliński J., Somogyi E., Piotrowska J., Opoka W. Metformin raises hydrogen sulfide tissue concentrations in various mouse organs. Pharmacol Rep. 2013;65(3):737–742. doi: 10.1016/s1734-1140(13)71053-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Yang G., Untereiner A., Ju Y., Wu L., Wang R. Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology. 2013;154(1):114–126. doi: 10.1210/en.2012-1658. [DOI] [PubMed] [Google Scholar]
- 33.Untereiner A.A., Wang R., Ju Y., Wu L. Decreased gluconeogenesis in the absence of cystathionine gamma-lyase and the underlying mechanisms. Antioxid Redox Signal. 2016;24(3):129–140. doi: 10.1089/ars.2015.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju Y., Untereiner A., Wu L., Yang G. H2S-induced S-sulfhydration of pyruvate carboxylase contributes to gluconeogenesis in liver cells. BBA. 2015;1850(11):2293–2303. doi: 10.1016/j.bbagen.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Guo W., Li D., You Y., Li W., Hu B., Zhang S. Cystathionine γ-lyase deficiency aggravates obesity-related insulin resistance via FoxO1-dependent hepatic gluconeogenesis. FASEB J. 2019;33(3):4212–4224. doi: 10.1096/fj.201801894R. [DOI] [PubMed] [Google Scholar]
- 36.Koliaki C., Roden M. Alterations of mitochondrial function and insulin sensitivity in human obesity and diabetes mellitus. Annu Rev Nutr. 2016;36:337–367. doi: 10.1146/annurev-nutr-071715-050656. [DOI] [PubMed] [Google Scholar]
- 37.Morino K., Petersen K.F., Dufour S., Befroy D., Frattini J., Shatzkes N. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115(12):3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Untereiner A.A., Fu M., Módis K., Wang R., Ju Y., Wu L. Stimulatory effect of CSE-generated H2S on hepatic mitochondrial biogenesis and the underlying mechanisms. Nitric Oxide. 2016;58:67–76. doi: 10.1016/j.niox.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Módis K., Ju Y., Ahmad A., Untereiner A.A., Altaany Z., Wu L. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol Res. 2016;113(Pt A):116–124. doi: 10.1016/j.phrs.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusminski C.M., Bickel P.E., Scherer P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15(9):639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 42.Feng X., Chen Y., Zhao J., Tang C., Jiang Z., Geng B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem Biophys Res Commun. 2009;380(1):153–159. doi: 10.1016/j.bbrc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 43.Cai J., Shi X., Wang H., Fan J., Feng Y., Lin X. Cystathionine γ lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. BBA. 2016;1861(5):419–429. doi: 10.1016/j.bbalip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Manna P., Jain S.K. Hydrogen sulfide and L-cysteine increase phosphatidylinositol 3,4,5- trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J Biol Chem. 2011;286(46):39848–39859. doi: 10.1074/jbc.M111.270884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue R., Hao D.D., Sun J.P., Li W.W., Zhao M.M., Li X.H. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal. 2013;19(1):5–23. doi: 10.1089/ars.2012.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghaben A.L., Scherer P.E. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 47.Manna P., Jain S.K. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem. 2012;287(50):42324–42332. doi: 10.1074/jbc.M112.407833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C.Y., Yao W.F., Wu W.G., Lu Y.L., Wan H., Wang W. Endogenous CSE/H2 S system mediates TNF-α-induced insulin resistance in 3T3-L1 adipocytes. Cell Biochem Funct. 2013;31(6):468–475. doi: 10.1002/cbf.2920. [DOI] [PubMed] [Google Scholar]
- 49.Tsai C.Y., Peh M.T., Feng W., Dymock B.W., Moore P.K. Hydrogen sulfide promotes adipogenesis in 3T3L1 cells. PLoS One. 2015;10(3):e0119511. doi: 10.1371/journal.pone.0119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang G., Ju Y., Fu M., Zhang Y., Pei Y., Racine M. Cystathionine gamma-lyase/hydrogen sulfide system is essential for adipogenesis and fat mass accumulation in mice. Biochim Biophys Acta, Mol Cell Biol Lipids. 2018;1863(2):165–176. doi: 10.1016/j.bbalip.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Gupta S., Kruger W.D. Cystathionine beta-synthase deficiency causes fat loss in mice. PLoS One. 2011;6(11):e27598. doi: 10.1371/journal.pone.0027598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delarue J., Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10(2):142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 53.Duncan R.E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H.S. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anthonsen M.W., Rönnstrand L., Wernstedt C., Degerman E., Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273(1):215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J., Hupfeld C.J., Taylor S.S., Olefsky J.M., Tsien R.Y. Insulin disrupts beta-adrenergic signaling to protein kinase A in adipocytes. Nature. 2005;437(7058):569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 56.Qiao L., Kinney B., Schaack J., Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 2011;60(5):1519–1527. doi: 10.2337/db10-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng B., Cai B., Liao F., Zheng Y., Zeng Q., Fan X. Increase or decrease hydrogen sulfide exert opposite lipolysis, but reduce global insulin resistance in high fatty diet induced obese mice. PLoS One. 2013;8(9):e73892. doi: 10.1371/journal.pone.0073892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan Z., Wang H., Liu Y., Yu C., Zhang Y., Chen J. Involvement of CSE/H2S in high glucose induced aberrant secretion of adipokines in 3T3-L1 adipocytes. Lipids Health Dis. 2014;13:155. doi: 10.1186/1476-511X-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barr L.A., Shimizu Y., Lambert J.P., Nicholson C.K., Calvert J.W. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide. 2015;46:145–156. doi: 10.1016/j.niox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain S.K., Micinski D., Lieblong B.J., Stapleton T. Relationship between hydrogen sulfide levels and HDL-cholesterol, adiponectin, and potassium levels in the blood of healthy subjects. Atherosclerosis. 2012;225(1):242–245. doi: 10.1016/j.atherosclerosis.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veeranki S., Tyagi S.C. Role of hydrogen sulfide in skeletal muscle biology and metabolism. Nitric Oxide. 2015;46:66–71. doi: 10.1016/j.niox.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Islam K.N., Polhemus D.J., Donnarumma E., Brewster L.P., Lefer D.J. Hydrogen sulfide levels and nuclear factor-erythroid 2-related factor 2 (NRF2) activity are attenuated in the setting of critical limb ischemia (CLI) J Am Heart Assoc. 2015;4(5):e001986. doi: 10.1161/JAHA.115.001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vellecco V., Mancini A., Ianaro A., Calderone V., Attanasio C., Cantalupo A. Cystathionine β-synthase-derived hydrogen sulfide is involved in human malignant hyperthermia. Clin Sci (Lond) 2016;130(1):35–44. doi: 10.1042/CS20150521. [DOI] [PubMed] [Google Scholar]
- 64.Du J.T., Li W., Yang J.Y., Tang C.S., Li Q., Jin H.F. Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress. Chin Med J (Engl) 2013;126(5):930–936. [PubMed] [Google Scholar]
- 65.Parsanathan R., Jain S.K. Hydrogen sulfide regulates circadian-clock genes in C2C12 myotubes and the muscle of high-fat-diet-fed mice. Arch Biochem Biophys. 2019;672:108054. doi: 10.1016/j.abb.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parsanathan R., Jain S.K. Hydrogen sulfide increases glutathione biosynthesis, and glucose uptake and utilization in C2C12 mouse myotubes. Free Radic Res. 2018;52(2):288–303. doi: 10.1080/10715762.2018.1431626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang R., Jia Q., Guo X., Liu X., Ma S., Gao Q. Protective effects of hydrogen sulfide on diaphragmatic muscle of Type 1 diabetic rats and its anti-apoptotic mechanisms. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(11):1173–1178. doi: 10.11817/j.issn.1672-7347.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Stenvers D.J., Scheer F.A.J.L., Schrauwen P., la Fleur S.E., Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15(2):75–89. doi: 10.1038/s41574-018-0122-1. [DOI] [PubMed] [Google Scholar]
- 69.Pozo M., Claret M. Hypothalamic Control of systemic glucose homeostasis: the pancreas connection. Trends Endocrinol Metab. 2018;29(8):581–594. doi: 10.1016/j.tem.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11(4):703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 71.Kimura Y., Shibuya N., Kimura H. Sulfite protects neurons from oxidative stress. Br J Pharmacol. 2019;176(4):571–582. doi: 10.1111/bph.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu L.F., Lu M., Hon Wong P.T., Bian J.S. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15(2):405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- 73.Khademullah C.S., Ferguson A.V. Depolarizing actions of hydrogen sulfide on hypothalamic paraventricular nucleus neurons. PLoS One. 2013;8(5):e64495. doi: 10.1371/journal.pone.0064495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng F., Han J., Lu H., Cui C., Yang J., Cui Q. Cystathionine beta synthase-hydrogen sulfide system in paraventricular nucleus reduced high fatty diet induced obesity and insulin resistance by brain-adipose axis. Biochim Biophys Acta, Mol Basis Dis. 2018;1864(10):3281–3291. doi: 10.1016/j.bbadis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 75.Sun X., Chen Y., Zeng Q., Huang X., Cai J. Reduction of leukocyte-derived H2S linked to abnormal glycolipid metabolism in hypertensive subjects. Clin Exp Hypertens. 2017;39(5):427–434. doi: 10.1080/10641963.2016.1267193. [DOI] [PubMed] [Google Scholar]
- 76.Jain S.K., Manna P., Micinski D., Lieblong B.J., Kahlon G., Morehead L. In African American type 2 diabetic patients, is vitamin D deficiency associated with lower blood levels of hydrogen sulfide and cyclic adenosine monophosphate, and elevated oxidative stress? Antioxid Redox Signal. 2013;18(10):1154–1158. doi: 10.1089/ars.2012.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]