Graphical abstract
Keywords: Hydrogen sulfide, Burn, Skin wound, Psoriasis, Systemic sclerosis, Melanoma
Highlights
-
•
Three hydrogen sulfide (H2S) production enzymes including CSE, CBS and 3-MST exist in the skin.
-
•
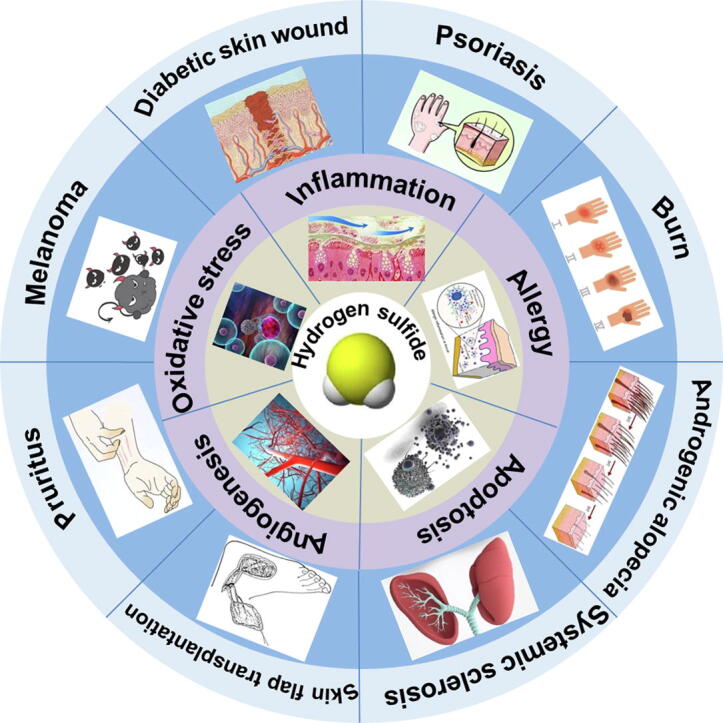
H2S regulates burn, diabetic skin wound, psoriasis, systemic sclerosis, melanoma, and pruritus.
-
•
H2S regulates oxidative stress, inflammation, angiogenesis and apoptosis in skin diseases.
-
•
Some ideal characteristics of H2S-based therapeutics for topical delivery are preferred.
-
•
Therapeutic potential of H2S for skin disorders will be further proposed in clinical trials.
Abstract
Background
Hydrogen sulfide (H2S) is now recognized as a vital endogenous gasotransmitter with a variety of biological functions in different systems. Recently, studies have increasingly focused on the role of H2S in the skin.
Aim of Review
This review summarizes recent progress and provides perspectives on H2S in the treatment of dermatological diseases.
Key Scientific Concepts of Review
Three H2S production enzymes, cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulfur transferase (3-MST), are all present in the skin, and it is likely that different cell types in the skin express them differently. Previous studies have demonstrated that H2S protects against several dermatological diseases, such as burns, diabetic skin wounds, psoriasis, skin flap transplantation, systemic sclerosis, melanoma, and pruritus. The mechanism might be related to the regulation of oxidative stress, inflammation, angiogenesis, apoptosis, and allergic reactions. H2S-based therapeutics require certain characteristics for topical delivery, for example, controlled release, appropriate physicochemical properties, good storage stability, acceptable odor, and advanced delivery systems. H2S-induced S-sulfhydration on proteins are potential novel targets for therapeutic intervention and drug design for the skin, which may lead to the development and application of H2S-related drugs for dermatological diseases.
Introduction
Hydrogen sulfide (H2S) had long been considered as a toxic gas that pollutes the environment and induces occupational disease. However, in 1989, scientists discovered the existence of endogenous H2S in the brains of rats and humans [1], [2], which opened up research on the physiological functions of H2S. Since then, H2S has been regarded as a biological mediator rather than only an environmental toxin [3]. With extensive in-depth study, H2S has been found to have a variety of biological functions in different systems, such as cardiovascular, neurological, reproductive, and endocrine systems [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. H2S is now recognized as the third endogenous gasotransmitter after nitric oxide (NO) and carbon monoxide (CO). In recent years, studies have increasingly focused on the role of H2S in the skin. In this review, we summarize recent progress and perspectives of H2S in the treatment of dermatological diseases.
Endogenous enzymes for hydrogen sulfide production in the skin
Endogenous H2S is mainly produced from L-cysteine by catalysis of cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS). But in mitochondria, H2S is synthesized by 3-mercaptopyruvate sulfur transferase (3-MST) in the presence of the substrate β-mercaptopyruvate pyruvate [5].
Protein quantification of forearm skin samples from healthy adults has shown that both CSE and 3-MST are expressed in the skin [15]. But another study found only CSE expression in melanoma cells and human melanoma tissues [16], which might be related to the source of melanoma cells. CSE expression increases at the later stages of healing in the granulation tissue of wounds [17], and this is significantly attenuated in ob/ob mice [18]. It is also worth noting that CSE deficiency delays wound healing [19]. However, compared with wild-type mice, the healing of burn wounds is unaffected if 3-MST is deficient [20].
One study showed that H2S promotes neovascularization after hind limb ischemia in CBS mutant mice via the peroxisome proliferator-activated receptor-γ (PPAR-γ)/vascular endothelial growth factor (VEGF) axis [21]. Many articles have demonstrated that CBS is also expressed in the skin. In 1976, CBS was found in cultured skin fibroblasts obtained from a patient with homocystinuria [22], [23]. One group found lower CBS activities in cultured human skin fibroblasts of young patients with the atherosclerotic or venous disease compared to healthy controls [24]. CBS-deficient mice have wrinkled skin with hyperkeratosis of the epidermis and thinning of the dermis [25]. Another study verified that dermal fibroblasts from Down syndrome individuals have higher CBS expression than control cells. CBS localization is both cytosolic and mitochondrial [26].
Taken together, the expression of these enzymes in the skin has been poorly studied and is incompletely understood. Most likely, CSE, CBS, and 3-MST are all present and are likely expressed by different skin cell types.
Implications of hydrogen sulfide in dermatology
Burns
One study verified that H2S levels in the plasma increase after scald burn injuries in wild-type mice, but the levels are unchanged in 3-MST-deficient mice. Although 3-MST deletion has no obvious effect on skin wound healing after a burn, 3-MST deficiency aggravates liver, renal, and cardiac dysfunctions [20]. In any case, increasing 3-MST expression or activity might be beneficial to attenuate severe complications after a burn. However, 3-MST is only one of the endogenous enzymes for H2S generation in the skin.
Exogenous H2S supplementation may also have the potential to affect burns. In one study, immediately after 5% of the total body surface area (TBSA) was given a deep partial-thickness scald in rats, the H2S donor sodium hydrosulfide (NaHS) was intraperitoneally injected [27]. The tissues in the base of the wound were collected after the burn to isolate macrophages, and it was found that NaHS increased the secretion of basic fibroblast growth factor (bFGF) and transforming growth factor β1 (TGF-β1), but decreased the release of tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) in cultured macrophages. These data suggest that H2S has the potential to promote growth factor secretion and wound healing.
However, the role of H2S in inflammation during burns remains controversial. One study found that H2S levels in the plasma significantly increased after 30% TBSA burn induction in male BALB/c mice using 95 °C water for 8 s. More importantly, intraperitoneal injection of NaHS (10 mg/kg) aggravated burn-related systemic inflammation [28]. However, whether elevated H2S levels after a burn resulting from damaged cells or antagonize acute damage remains unknown. To complicate matters, another study has provided completely different results. After 40% TBSA of adult male C57BL/6 mice was exposed to 95 °C water for 10 s, the H2S content in the plasma decreased. NaHS (2 mg/kg) administration 1 h after the burn significantly decreased the levels of TNF-α, IL-6, and IL-8, but increased the levels of IL-10 in the plasma [29]. This indicates that H2S supplementation was able to inhibit inflammation post-burn, potentially providing a new strategy to improve the immune response and promote burn healing.
The above contradictory effects of H2S on inflammation after burns might be ascribed to the burn degree of injury, the course of the burn, or the dosage or treatment time of H2S donors. Some researchers have even proposed that H2S may promote inflammation in the early stage of a burn but inhibit inflammation and promote healing in the late stage of a burn [30]. Another study has also shown an important role for the pro-inflammatory action of H2S during a burn [31]. Therefore, the real role of H2S in burns might be closely associated with the detailed properties of the burn injury. Due to the biphasic effects of H2S on burns, H2S supplementation in the late, not early, stage of a burn may be helpful to accelerate healing.
Diabetic skin wounds
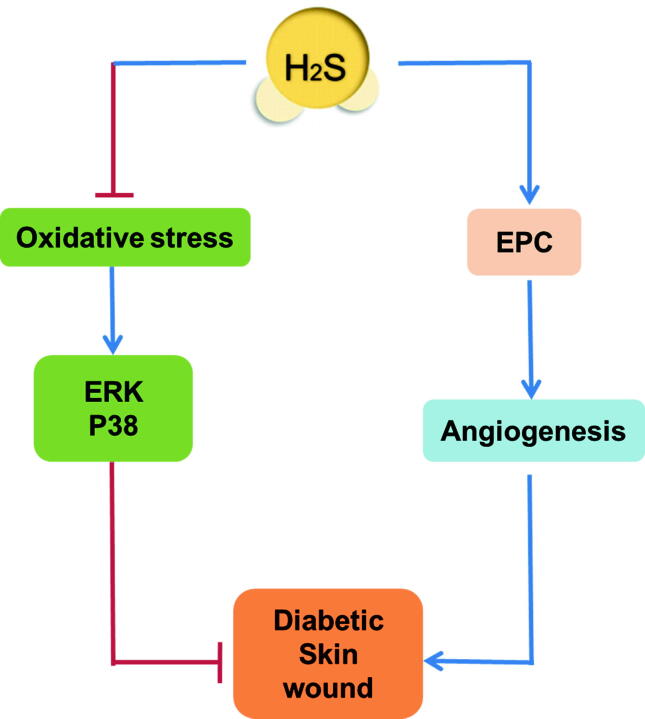
Diabetes mellitus (DM) is a common disease throughout the world, and delayed wound healing is a major complication that causes great harm to patients. The exact mechanism of delayed wound healing in DM is not fully understood, but it may be related to sustained inflammation, excessive oxidative stress, and impaired angiogenesis. Changes in the balance of H2S play an important role in the pathogenesis of β-cell dysfunction that occurs in response to type 1 and type 2 diabetes [32]. We know that H2S regulates inflammation, oxidative stress, and angiogenesis [33], [34], [35], suggesting a potential role for H2S in promoting diabetic wound healing. It has been found that CSE expression and H2S levels in granulation tissue of wounds in ob/ob mice are significantly lower than those in controls, suggesting that H2S production is impaired in DM wounds. Moreover, intraperitoneal injection of NaHS attenuates neutrophil and macrophage infiltration, inhibits TNF-α and IL-6 secretion, and accelerates wound healing in the ob/ob mice [18]. 3-MP (the substrate of 3-MST) supplementation increases H2S production, facilitates wound healing, induces dermal microvessel relaxation, and increases mitochondrial bioenergetic function in rats [36]. The above research demonstrates that the enhancement of endogenous H2S production or exogenous H2S supplementation promotes diabetic trauma healing. During DM, reactive oxygen species (ROS) increase to activate mitogen-activated protein kinase (MAPK), including extracellular regulated protein kinases1/2 (ERK1/2) and p38, to induce neutrophil NETosis, and finally to delay wound healing via neutrophil extracellular traps (NETs). In db/db diabetic mice, wound healing is significantly delayed, while the levels of NETs and NETosis are increased. Na2S promotes wound healing by reducing NETs and NETosis levels, which might be due to ROS suppression followed by ERK1/2 and p38 phosphorylation inhibition in a NETosis model of neutrophils induced by phorbol 12-myristate 13-acetate (PMA) (Fig. 1) [37]. Another study also confirmed that both NaHS and 4-hydro-xythiobenzamide promote wound healing in db/db diabetic mice [38]. Moreover, the endothelial progenitor cell (EPC) function of db/db diabetic mice is significantly decreased after CSE inhibitor administration or CSE silencing. NaHS significantly restores the EPC function to improve refractory wound lesions in diabetic mice (Fig. 1). Furthermore, the angiogenesis of EPC is also enhanced by NaHS via angiopoietin-1 activation [38]. This shows a direct pathway in the promotion of diabetic wound healing by H2S. After topical application of 2% sodium bisulfide ointment, wound healing is accelerated, and superoxide dismutase (SOD), heme oxygenase-1 (HO-1), VEGF, and intercellular adhesion molecule-1 in neo-granulation tissues are significantly increased in streptozotocin-induced diabetic rats [39]. This suggests that an H2S donor might be a novel agent for diabetic skin ulcerations. Considering the few adverse reactions, developing H2S-related preparations to be applied locally on the skin is desirable.
Fig. 1.
Possible mechanisms of hydrogen sulfide (H2S) in the healing of diabetic skin wounds. Increasing endogenous H2S production or exogenous H2S supplementation attenuates oxidative stress and inhibits extracellular regulated protein kinases (ERK) 1/2 and p38 phosphorylation to accelerate diabetic skin wound healing. H2S also improves angiogenesis by restoring endothelial progenitor cell (EPC) function to promote skin wound healing.
Delayed wound healing in DM is also associated with keratinocyte damage and dysfunction. In one study, HaCaT cells, a cell line of human skin keratinocytes, were exposed to methylglyoxal (MGO) to establish a diabetic wound healing model in vitro [40]. An N-mercapto-based H2S donor, a novel controllable H2S-releasing molecule, enhanced cell viability, inhibited cell apoptosis, alleviated intracellular ROS content, increased mitochondrial membrane potential, and ultimately promoted cell adhesion and migration in MGO-stimulated HaCaT cells [40]. Other researchers have designed microparticles (NaHS@MPs) that provide an in situ depot for the sustained release of exogenous H2S using an emulsion method. NaHS@MPs continuously releases H2S to inhibit oxidative stress, suppress ERK1/2 and p38, promote the proliferation and migration of epidermal/endothelial cells, improve angiogenesis, and accelerate the healing of full-thickness wounds in diabetic mice [41]. These novel H2S-related biomaterials have great potential for the healing of diabetic wounds.
Psoriasis
Psoriasis is a heritable inflammatory skin disease [42]. Although a large number of studies have confirmed that H2S is a “double-edged sword” that can promote or inhibit inflammation [5], [14], [43], it is not clear whether H2S plays an important role in the occurrence and development of psoriasis. One group found that H2S levels in serum from patients with chronic progressive psoriasis are only about half of those from healthy controls, while TNF-α, IL-6, and IL-8 levels are about two-fold. More importantly, H2S levels in the serum are negatively correlated with the severity of psoriasis [44]. However, the causal relationship between H2S levels and psoriasis remains unknown.
Keratinocyte over-proliferation is considered to be a vital pathophysiological characteristic of psoriasis [45]. The levels of IL-6 and IL-8 increase after TNF-α stimulation in human HaCaT keratinocytes, and this is alleviated by exogenous H2S supplementation in a concentration-dependent manner. The mechanism might be related to the inhibition of ERK, p38, and nuclear factor-κB (NF-κB) activation [44]. Another study also found that both NaHS and GYY4137, as two common H2S donors, increase inducible nitric oxide synthase (iNOS) expression and promote NO secretion depending on Akt activation, thereby inhibiting ERK activation and decreasing VEGF production to attenuate the proliferation of human keratinocytes (Fig. 2) [46]. NaHS also decreases cell numbers in S phase, increases those in G2/M phase, inhibits colony formation, suppresses cell adhesion to plastic dishes, and impairs the viability of adherent cells. The mechanism may be related to the blockage of the Raf/MAPK/ERK signaling pathway and the reduction of β4, α2, and α6 integrins expression [47]. Taken together, H2S can inhibit keratinocyte growth, proliferation, and adhesion. H2S-releasing compounds might therefore be promising therapeutic agents for psoriasis.
Fig. 2.
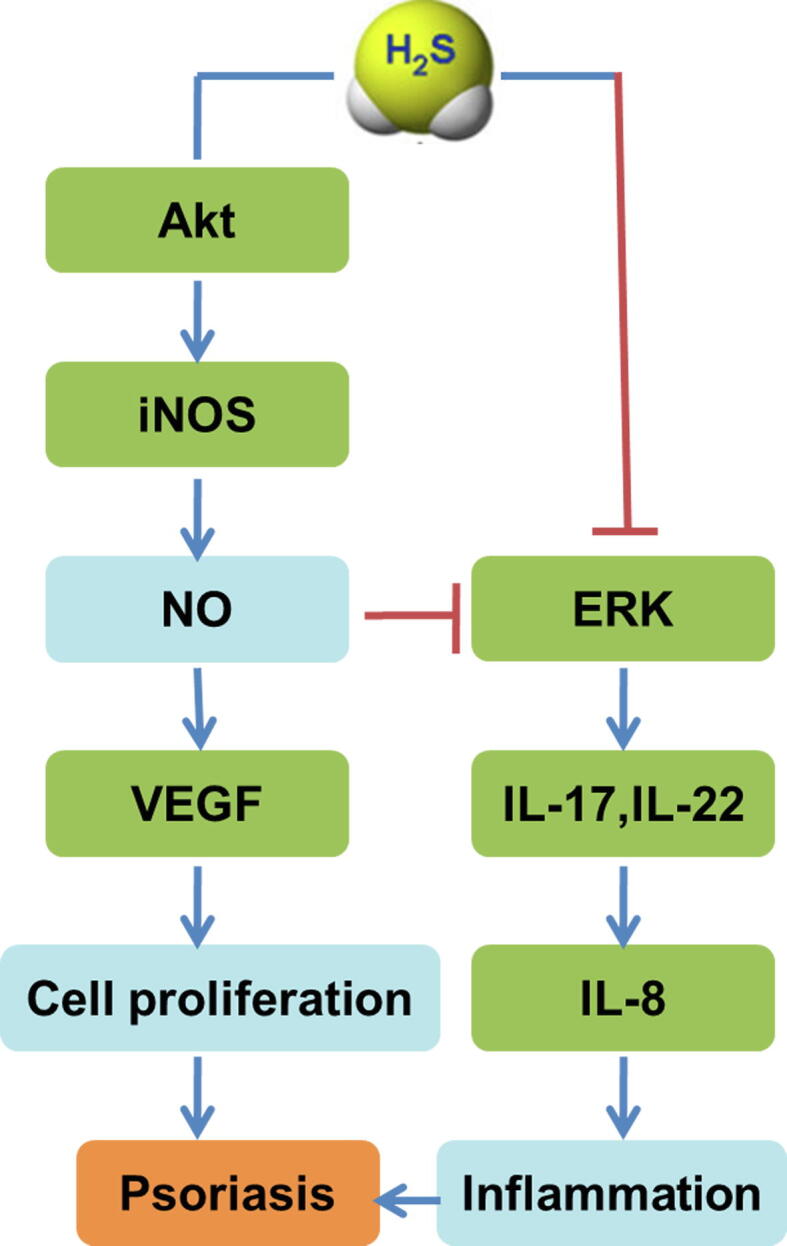
Possible mechanisms of hydrogen sulfide (H2S) action against psoriasis. Inflammation and over-proliferation of keratinocytes are vital pathophysiological mechanisms of psoriasis. H2S increases inducible nitric oxide synthase (iNOS) expression and promotes nitric oxide (NO) secretion depending on Akt activation, thereby inhibiting extracellular regulated protein kinases (ERK) activation and decreasing vascular endothelial growth factor (VEGF) production to attenuate the proliferation of human keratinocytes. H2S also inhibits IL-17 and IL-22 production and decreases IL-8 secretion to attenuate inflammation via ERK phosphorylation inhibition in human keratinocytes.
Previous research has verified that T cell infiltration in lesions is the initiating factor of keratinocyte proliferation. IL-17 and IL-22, which are produced from Th1/Th17 lymphocytes, promote keratinocytes to secrete IL-8, and finally enhance inflammation, which is a critical event in the pathogenesis of psoriasis [48]. H2S inhibits IL-17 and IL-22 production and decreases IL-8 secretion to attenuate inflammation via ERK phosphorylation inhibition in human keratinocytes (Fig. 2) [49]. Although many studies have confirmed the ability of H2S to regulate T cell activity in the immune system [50], [51], [52], the real role of H2S in T cells of the skin needs further study.
A complex interaction between keratinocytes and T cells is involved in psoriasis. However, whether H2S can regulate the interaction between keratinocytes and T cells to inhibit and even block the pathways for keratinocytes proliferation or differentiation requires further investigation.
Skin flap transplantation
Skin flap transplantation is a common manner of reconstructive surgery. However, the obstruction of local microcirculation and blood flow reperfusion injury after successful transplantation limit the application of skin flaps. Some researchers have found that H2S pretreatment alleviates apoptosis in mouse-derived fibroblasts after hypoxia-reoxygenation in an in vitro model of cutaneous tissue transplantation [53]. This suggests that H2S administration before ischemia significantly protects against reperfusion injury, indicating the potential applicability of H2S in skin transplantation.
Recently, one team intuitively investigated the effects of H2S on tissue necrosis and skin microcirculation by stereofluorescence microscopy and intravital fluorescence microscopy in an axial pattern ear flap model of hairless mice. They found that GYY4137 significantly dilates capillaries, increases functional capillary density, reduces auricular tissue necrosis, and improves microcirculation [54]. In summary, H2S improves microcirculation and maintains the vitality of transplanted skin flaps. H2S administration is therefore a potential means to improve the success rate and therapeutic effect of skin flaps.
Systemic sclerosis
Systemic sclerosis (SSC) is a serious connective tissue disease with unknown etiology. It is characterized by multiple organ fibrosis owing to inflammation, vascular injury, and excessive collagen deposition. Skin sclerosis is one of the main manifestations of SSC. One study found that H2S concentrations in plasma significantly decrease in a bleomycin-induced mouse model of SSC [55]. After NaHS treatment, the pathological structure of the skin is improved, the dermis becomes thinner and the lipid layer thicker, and inflammatory cell infiltration and collagen deposition are alleviated. The mechanism may be related to the reduction of extracellular matrix accumulation via TGF-β1 inhibition [55]. This provides a new modality for the clinical treatment of SSC-related organ fibrosis.
Melanoma
H2S shows a bimodal pharmacological character in cancer, in that it both inhibits metabolite biosynthesis and increases their concentrations to a certain threshold to exert anticancer effects [56]. Previous studies have demonstrated an emerging role for H2S in various cancers including colon cancer, ovarian cancer, urothelial carcinoma, renal cell carcinoma, and prostate cancer [57], [58]. In dermatology, H2S is a potential chemopreventive agent against melanoma development.
Melanoma is a common skin cancer with high mortality. Compared with normal human epidermal melanocytes, CSE expression is increased in melanoma cells. Immunohistochemical analysis of human melanoma samples shows that the highest levels of CSE expression are in primary tumors. However, CSE expression is decreased in metastatic lesions and almost absent in non-lymph node metastases, indicating that the occurrence and metastasis of melanoma may be related to the H2S pathway [16]. H2S donors can inhibit melanoma cell proliferation by regulating the cell cycle, promoting apoptosis, inhibiting NF-κB activation and downstream anti-apoptotic-related protein expression, and reducing AKT and ERK phosphorylation [16]. The inhibitory effect of H2S on melanoma may offer a promising alternative to existing therapies.
Acetyl deacylasadisulfide (ADA), a novel H2S-releasing compound, inhibits human melanoma cell proliferation and invasion by inducing apoptosis. Further studies have confirmed that ADA reduces NF-κB nuclear translocation and activation, decreases anti-apoptotic protein expression, and inhibits Akt and ERK phosphorylation. In vivo, B16/F10 melanoma cells were injected into C57BL/6 mice via the tail vein, and ADA administration reduced the volume of lung metastatic foci in inoculated mice in a concentration-dependent manner [59]. These studies suggest that the H2S donor ADA significantly inhibits the progression of melanoma and could represent an important lead compound for the design and development of novel anti-metastatic agents.
A “combination therapy” based on the H2S pathway may be a potential treatment for melanoma. One group successfully designed several H2S-releasing non-steroidal anti-inflammatory drugs (H2S-NSAIDs) with the properties of H2S release and cyclooxygenase (COX) inhibition. For example, ATB-346 [2-(6-methoxynapthalen-2-yl)-propionic acid 4-thiocarbamoyl phenyl ester] significantly inhibits melanoma proliferation via NF-κB and Akt suppression [60]. Naproxen-4-hydroxybenzodithioate (NAP-HBTA) exhibits an inhibitory effect on melanoma cell proliferation. Further experiments have shown that NAP-HBTA enhances apoptosis and inhibits cell motility, invasion, and cell colony formation. NAP-HBTA also significantly suppresses melanoma growth and progression in mice. The mechanism may be related to the inhibition of chemokine (C-X-C motif) ligand 1 (CXCL1), matrix metalloprotein (MMP)-2, and MMP-13 expression [61]. Considering the good pharmacokinetic properties of naproxen, a naproxen H2S-derivative might alleviate the toxicity of H2S to organs, which provides a new method for the prevention and treatment of melanoma and other diseases.
Other diseases
Androgenic alopecia is a chronic, progressive condition affecting millions of individuals worldwide. Sodium thiosulfate (STS), known to generate H2S, accelerates hair growth in the “telogen model” of mice, suggesting that H2S stimulates telogen hair follicles to reenter the anagen phase of hair growth [62]. H2S may represent a novel and beneficial remedy for hair loss in androgenic alopecia.
Pruritus is a distressing physiological self-protective mechanism similar to pain. However, the role of H2S in pruritus remains controversial. Some scholars have found that subcutaneous injection of NaHS or Na2S increases scratching behavior in a μ-opioid receptor-dependent and histamine-independent manner in mice [63], while others have reported that Na2S significantly ameliorates histamine- or compound 48/80 (C48/80)-induced pruritus in male BALB/c mice [64]. Further studies are needed to clarify the exact effect and detailed mechanism of H2S on pruritus.
Conclusions and future perspectives
Over the last few decades, significant progress has been achieved in delineating the potential effect of H2S on dermatological diseases such as burns, diabetic skin wounds, psoriasis, skin flap transplantation, systemic sclerosis, and melanoma. The regulation of oxidative stress, inflammation, angiogenesis, apoptosis, and allergic reactions might be responsible for the distinct roles of H2S in the skin. However, the specific signaling molecules involved in the above processes need to be further explored. Recently, a novel post-translational modification of specific cysteine residues of target proteins induced by H2S, named S-sulfhydration (or S-persulfidation), has been proposed [5], [65], [66]. As of now, specific proteins with S-sulfhydration as well as their physiological function in dermatology are not well known.
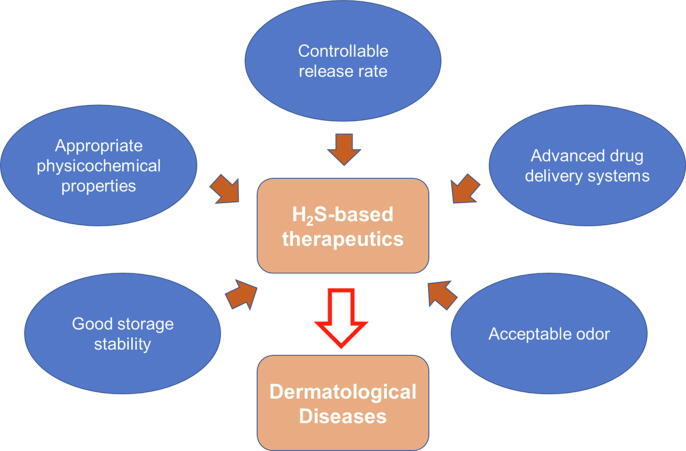
H2S-based therapeutics have been evolving to deliver H2S to desired locations at appropriate concentrations and with appropriate pharmacokinetics. Instead of taking H2S-based therapeutics orally, the application of small molecules on the skin may provide targeted local therapy for skin disorders and decrease the risk of off-target side effects. Successful topical drug delivery of H2S-based therapeutics allows the molecules to be conveyed directly to the site of action, providing relevant therapeutic concentrations and avoiding unnecessary systemic circulation, first-pass hepatic metabolism, and off-target side effects. Some characteristics of H2S donors should be improved. First, controllable release rates of H2S are essential, since its release directly affects the concentration of H2S in the target site and thus its pharmacological functions. Second, topical delivery is often challenging because organs exposed to the environment possess effective barrier functions that hinder the entry of xenobiotics. Third, targeted intraepidermal delivery using advanced formulation strategies is desired, since it not only fine-tunes the release of an H2S donor from the small formulation “depot” before being triggered by certain environments but also enables improved cutaneous delivery via specific transport pathways with minimal risk of systemic exposure. Other aspects such as appropriate physicochemical properties, good storage stability, acceptable odor (odorless or without unpleasant smell) are also important for drugability (Fig. 3). In future studies, the therapeutic potential of H2S for skin disorders will be explored in clinical trials.
Fig. 3.
Ideal characteristics of hydrogen sulfide (H2S)-based therapeutics for dermatological diseases. Some characteristics of H2S-based therapeutics for topical delivery would be ideal; for example, these molecules should present the controllable release of H2S, appropriate physicochemical properties, good storage stability, and acceptable odor. These features could serve as reference points to evaluate existing donors and guide future drug design. Formulating H2S-based therapeutics for dermatological diseases into advanced drug delivery systems that increase cutaneous bioavailability and decrease systemic exposure is preferred.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China, grant number 81703099 (SY), and the Natural Science Foundation of Nantong City, grant number MS12017015-7 (LZ).
Role of funding source
The funding sources had no role in the study design, collection, analysis, or interpretation of the data, the writing of the report, or the decision to submit the article for publication.
Compliance with ethics requirements
Not applicable.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Goodwin L.R., Francom D., Dieken F.P., Taylor J.D., Warenycia M.W., Reiffenstein R.J. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol. 1989;13(2):105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- 2.Savage J.C., Gould D.H. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr. 1990;526(2):540–545. doi: 10.1016/s0378-4347(00)82537-2. [DOI] [PubMed] [Google Scholar]
- 3.Szabo C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem Pharmacol. 2018;149:5–19. doi: 10.1016/j.bcp.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng G., Liu J., Liu S., Song Q., Liu L., Xie L. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br J Pharmacol. 2018;175(8):1126–1145. doi: 10.1111/bph.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng G., Zhao S., Xie L., Han Y., Ji Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br J Pharmacol. 2018;175(8):1146–1156. doi: 10.1111/bph.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng G., Ma Y., Xie L., Ferro A., Ji Y. Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. Br J Pharmacol. 2015;172(23):5501–5511. doi: 10.1111/bph.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14(5):329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 8.Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C., Brace L. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160(1–2):132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 11.Wang R., Szabo C., Ichinose F., Ahmed A., Whiteman M., Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci. 2015;36(9):568–578. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura H. Hydrogen sulfide and polysulfides as biological mediators. Molecules. 2014;19(10):16146–16157. doi: 10.3390/molecules191016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo C., Papapetropoulos A. International union of basic and clinical pharmacology. CII: pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol Rev. 2017;69(4):497–564. doi: 10.1124/pr.117.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z., Polhemus D.J., Lefer D.J. Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ Res. 2018;123(5):590–600. doi: 10.1161/CIRCRESAHA.118.311134. [DOI] [PubMed] [Google Scholar]
- 15.Kutz J.L., Greaney J.L., Santhanam L., Alexander L.M. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J Physiol. 2015;593(9):2121–2129. doi: 10.1113/JP270054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panza E., De Cicco P., Armogida C., Scognamiglio G., Gigantino V., Botti G. Role of the cystathionine gamma lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015;28(1):61–72. doi: 10.1111/pcmr.12312. [DOI] [PubMed] [Google Scholar]
- 17.Goren I., Kohler Y., Aglan A., Pfeilschifter J., Beck K.F., Frank S. Increase of cystathionine-gamma-lyase (CSE) during late wound repair: Hydrogen sulfide triggers cytokeratin 10 expression in keratinocytes. Nitric Oxide. 2019;87:31–42. doi: 10.1016/j.niox.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H., Lu S., Chai J., Zhang Y., Ma X., Chen J. Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J Diabetes Complications. 2017;31(9):1363–1369. doi: 10.1016/j.jdiacomp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106(51):21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad A., Druzhyna N., Szabo C. Effect of 3-mercaptopyruvate Sulfurtransferase Deficiency on the Development of Multiorgan Failure, Inflammation, and Wound Healing in Mice Subjected to Burn Injury. J Burn Care Res. 2019;40(2):148–156. doi: 10.1093/jbcr/irz007. [DOI] [PubMed] [Google Scholar]
- 21.Majumder A., Singh M., George A.K., Behera J., Tyagi N., Tyagi S.C. Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-beta-synthase mutant mice via PPAR-gamma/VEGF axis. Physiol Rep. 2018;6(17) doi: 10.14814/phy2.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths R., Tudball N. Studies on the use of skin fibroblasts for the measurement of cystathionine synthase activity with respect to homocystinuria. Clin Chim Acta. 1976;73(1):157–162. doi: 10.1016/0009-8981(76)90317-x. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths R., Tudball N. Studies on normal and mutant cystathionine beta-synthase from cultured skin fibroblasts. Biochem Soc Trans. 1976;4(4):630–631. doi: 10.1042/bst0040630. [DOI] [PubMed] [Google Scholar]
- 24.Nordstrom M., Kjellstrom T. Age and cystathionine beta-synthase activity in cultured fibroblasts from patients with arterial and venous vascular disease. Atherosclerosis. 1998;139(2):231–236. doi: 10.1016/s0021-9150(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 25.Robert K., Maurin N., Ledru A., Delabar J., Janel N. Hyperkeratosis in cystathionine beta synthase-deficient mice: an animal model of hyperhomocysteinemia. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(2):1072–1076. doi: 10.1002/ar.a.20082. [DOI] [PubMed] [Google Scholar]
- 26.Panagaki T., Randi E.B., Augsburger F., Szabo C. Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc Natl Acad Sci USA. 2019;116(38):18769–18771. doi: 10.1073/pnas.1911895116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Xu D.B., Wang H.J. Effects of hydrogen sulfide on the secretion of cytokines in macrophages of deep partial-thickness burn wound in rats. Zhonghua Shao Shang Za Zhi. 2016;32(7):408–412. doi: 10.3760/cma.j.issn.1009-2587.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Sio S.W., Moochhala S., Bhatia M. Role of hydrogen sulfide in severe burn injury-induced inflammation in mice. Mol Med. 2010;16(9–10):417–424. doi: 10.2119/molmed.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng J., Lin X., Fan H., Li C. Hydrogen sulfide attenuates the inflammatory response in a mouse burn injury model. Mol Med Rep. 2013;8(4):1204–1208. doi: 10.3892/mmr.2013.1610. [DOI] [PubMed] [Google Scholar]
- 30.Akter F. The role of hydrogen sulfide in burns. Burns. 2016;42(3):519–525. doi: 10.1016/j.burns.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia M. H(2)S and substance P in inflammation. Methods Enzymol. 2015;555:195–205. doi: 10.1016/bs.mie.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2012;17(1):68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan L.L., Qin M., Liu X.H., Zhu Y.Z. The Role of Hydrogen Sulfide on Cardiovascular Homeostasis: An Overview with Update on Immunomodulation. Front Pharmacol. 2017;8:686. doi: 10.3389/fphar.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul B.D., Snyder S.H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem Pharmacol. 2018;149:101–109. doi: 10.1016/j.bcp.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mistry R.K., Brewer A.C. Redox regulation of gasotransmission in the vascular system: A focus on angiogenesis. Free Radic Biol Med. 2017;108:500–516. doi: 10.1016/j.freeradbiomed.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coletta C., Modis K., Szczesny B., Brunyanszki A., Olah G., Rios E.C. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by DL-alpha-lipoic acid. Mol Med. 2015;21:1–14. doi: 10.2119/molmed.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C.T., Chen L., Chen W.L., Li N., Chen M.J., Li X. Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis. Mol Cell Endocrinol. 2019;480:74–82. doi: 10.1016/j.mce.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Liu F., Chen D.D., Sun X., Xie H.H., Yuan H., Jia W. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014;63(5):1763–1778. doi: 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G., Li W., Chen Q., Jiang Y., Lu X., Zhao X. Hydrogen sulfide accelerates wound healing in diabetic rats. Int J Clin Exp Pathol. 2015;8(5):5097–5104. [PMC free article] [PubMed] [Google Scholar]
- 40.Yang C.T., Zhao Y., Xian M., Li J.H., Dong Q., Bai H.B. A novel controllable hydrogen sulfide-releasing molecule protects human skin keratinocytes against methylglyoxal-induced injury and dysfunction. Cell Physiol Biochem. 2014;34(4):1304–1317. doi: 10.1159/000366339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin W.C., Huang C.C., Lin S.J., Li M.J., Chang Y., Lin Y.J. In situ depot comprising phase-change materials that can sustainably release a gasotransmitter H2S to treat diabetic wounds. Biomaterials. 2017;145:1–8. doi: 10.1016/j.biomaterials.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 42.van Vugt L.J., van den Reek J., Hannink G., Coenen M.J.H., de Jong E. Association of HLA-C*06:02 status with differential response to ustekinumab in patients with psoriasis: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(6):708–715. doi: 10.1001/jamadermatol.2019.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br J Pharmacol 2019 Jan 18. doi: http://doi.org/10.1111/bph.14579. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 44.Alshorafa A.K., Guo Q., Zeng F., Chen M., Tan G., Tang Z. Psoriasis is associated with low serum levels of hydrogen sulfide, a potential anti-inflammatory molecule. Tohoku J Exp Med. 2012;228(4):325–332. doi: 10.1620/tjem.228.325. [DOI] [PubMed] [Google Scholar]
- 45.Albanesi C., Madonna S., Gisondi P., Girolomoni G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol. 2018;9:1549. doi: 10.3389/fimmu.2018.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merighi S., Gessi S., Varani K., Fazzi D., Borea P.A. Hydrogen sulfide modulates the release of nitric oxide and VEGF in human keratinocytes. Pharmacol Res. 2012;66(5):428–436. doi: 10.1016/j.phrs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Gobbi G., Ricci F., Malinverno C., Carubbi C., Pambianco M., Panfilis G. Hydrogen sulfide impairs keratinocyte cell growth and adhesion inhibiting mitogen-activated protein kinase signaling. Lab Invest. 2009;89(9):994–1006. doi: 10.1038/labinvest.2009.61. [DOI] [PubMed] [Google Scholar]
- 48.Dainichi T., Kitoh A., Otsuka A., Nakajima S., Nomura T., Kaplan D.H. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018;19(12):1286–1298. doi: 10.1038/s41590-018-0256-2. [DOI] [PubMed] [Google Scholar]
- 49.Mirandola P., Gobbi G., Micheloni C., Vaccarezza M., Di Marcantonio D., Ruscitti F. Hydrogen sulfide inhibits IL-8 expression in human keratinocytes via MAP kinase signaling. Lab Invest. 2011;91(8):1188–1194. doi: 10.1038/labinvest.2011.76. [DOI] [PubMed] [Google Scholar]
- 50.Yang R., Qu C., Zhou Y., Konkel J.E., Shi S., Liu Y. Hydrogen sulfide promotes Tet1- and Tet2-Mediated foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity. 2015;43(2):251–263. doi: 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang M., Haase C., Viljanen J., Xu B., Ge C., Kihlberg J. Cutting edge: processing of oxidized peptides in macrophages regulates T cell activation and development of autoimmune arthritis. J Immunol. 2017;199(12):3937–3942. doi: 10.4049/jimmunol.1700774. [DOI] [PubMed] [Google Scholar]
- 52.Yang R., Yu T., Liu D., Shi S., Zhou Y. Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res Ther. 2018;9(1):62. doi: 10.1186/s13287-018-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson P.W., Singh S.P., Belkin D., Nagineni V., Weinstein A.L., Weissich J. Hydrogen sulfide protects against ischemia-reperfusion injury in an in vitro model of cutaneous tissue transplantation. J Surg Res. 2010;159(1):451–455. doi: 10.1016/j.jss.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Grambow E., Augustin V.A., Struder D., Kundt G., Klar E., Vollmar B. The effects of hydrogen sulfide on microvascular circulation in the axial pattern flap ear model in hairless mice. Microvasc Res. 2018;120:74–83. doi: 10.1016/j.mvr.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Yin X., Gao L., Feng S., Song K., Li L. The protective effect of hydrogen sulfide on systemic sclerosis associated skin and lung fibrosis in mice model. Springerplus. 2016;5(1):1084. doi: 10.1186/s40064-016-2774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szabo C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat Rev Drug Discov. 2016;15(3):185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akbari M., Sogutdelen E., Juriasingani S., Sener A. Hydrogen sulfide: emerging role in bladder, kidney, and prostate malignancies. Oxid Med Cell Longev. 2019;2019:2360945. doi: 10.1155/2019/2360945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu H., Blake S., Chan K.T., Pearson R.B., Kang J. Cystathionine beta-synthase in physiology and cancer. Biomed Res Int. 2018;2018:3205125. doi: 10.1155/2018/3205125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Cicco P., Panza E., Armogida C., Ercolano G., Taglialatela-Scafati O., Shokoohinia Y. The hydrogen sulfide releasing molecule acetyl deacylasadisulfide inhibits metastatic melanoma. Front Pharmacol. 2017;8:65. doi: 10.3389/fphar.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Cicco P., Panza E., Ercolano G., Armogida C., Sessa G., Pirozzi G. ATB-346, a novel hydrogen sulfide-releasing anti-inflammatory drug, induces apoptosis of human melanoma cells and inhibits melanoma development in vivo. Pharmacol Res. 2016;114:67–73. doi: 10.1016/j.phrs.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Ercolano G., De Cicco P., Frecentese F., Saccone I., Corvino A., Giordano F. Anti-metastatic properties of naproxen-HBTA in a murine model of cutaneous melanoma. Front Pharmacol. 2019;10:66. doi: 10.3389/fphar.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maekawa M., Ohnishi T., Balan S., Hisano Y., Nozaki Y., Ohba H. Thiosulfate promotes hair growth in mouse model. Biosci Biotechnol Biochem. 2019;83(1):114–122. doi: 10.1080/09168451.2018.1518705. [DOI] [PubMed] [Google Scholar]
- 63.Wang X.L., Tian B., Huang Y., Peng X.Y., Chen L.H., Li J.C. Hydrogen sulfide-induced itch requires activation of Cav3.2 T-type calcium channel in mice. Sci Rep. 2015;5:16768. doi: 10.1038/srep16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues L., Ekundi-Valentim E., Florenzano J., Cerqueira A.R., Soares A.G., Schmidt T.P. Protective effects of exogenous and endogenous hydrogen sulfide in mast cell-mediated pruritus and cutaneous acute inflammation in mice. Pharmacol Res. 2017;115:255–266. doi: 10.1016/j.phrs.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2(96):ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paul B.D., Snyder S.H. H2S: a novel gasotransmitter that signals by sulfhydration. Trends Biochem Sci. 2015;40(11):687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]