Abstract
Purpose
To address the lack of prospective data on the real-life clinical application of trans-arterial radioembolization (TARE) in Europe, the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) initiated the prospective observational study CIRSE Registry for SIR-Spheres® Therapy (CIRT).
Materials and Methods
Patients were enrolled from 1 January 2015 till 31 December 2017. Eligible patients were adult patients treated with TARE with Y90 resin microspheres for primary or metastatic liver tumours. Patients were followed up for 24 months after treatment, whereas data on the clinical context of TARE, overall survival (OS) and safety were collected.
Results
Totally, 1027 patients were analysed. 68.2% of the intention of treatment was palliative. Up to half of the patients received systemic therapy and/or locoregional treatments prior to TARE (53.1%; 38.3%). Median overall survival (OS) was reported per cohort and was 16.5 months (95% confidence interval (CI) 14.2–19.3) for hepatocellular carcinoma, 14.6 months (95% CI 10.9–17.9) for intrahepatic cholangiocarcinoma. For liver metastases, median OS for colorectal cancer was 9.8 months (95% CI 8.3–12.9), 5.6 months for pancreatic cancer (95% CI 4.1–6.6), 10.6 months (95% CI 7.3–14.4) for breast cancer, 14.6 months (95% CI 7.3–21.4) for melanoma and 33.1 months (95% CI 22.1–nr) for neuroendocrine tumours. Statistically significant prognostic factors in terms of OS include the presence of ascites, cirrhosis, extra-hepatic disease, patient performance status (Eastern Cooperative Oncology Group), number of chemotherapy lines prior to TARE and tumour burden. Thirty-day mortality rate was 1.0%. 2.5% experienced adverse events grade 3 or 4 within 30 days after TARE.
Conclusion
In the real-life clinical setting, TARE is largely considered to be a part of a palliative treatment strategy across indications and provides an excellent safety profile.
Level of evidence
Level 3.
Trial registration
ClinicalTrials.gov NCT02305459.
Electronic supplementary material
The online version of this article (10.1007/s00270-020-02642-y) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, Metastasis, Observational study, Registries, Therapeutic embolization, Liver, Yttrium-90, Radioisotope brachytherapy, Trans-arterial radioembolization
Introduction
Current guidelines for the treatment of primary liver malignancies (e.g. hepatocellular carcinoma (HCC), intra-hepatic cholangiocarcinoma (ICC)) and hepatic metastases, e.g. from colorectal cancer (mCRC), propose trans-arterial radioembolization (TARE, also known as selective internal radiation therapy (SIRT)) as an optional treatment modality for patients with liver dominant disease not suitable for surgical or ablative therapies, or who experienced no response, significant side effects or intolerance when treated with systemic therapies. [1–7].
At the time of the study’s conception in 2014, available studies on TARE consisted of large cohort series and smaller experimental trials [8–17]. In the meantime, several large-scale randomized controlled trials on TARE in mCRC and HCC have been completed and published [18–22], as well as large prospective and retrospective studies on HCC, ICC and mCRC [23–30]. As more centres in Europe included TARE in their armamentarium of treatments for liver malignancies, there was a need for a multicentre, prospective data collection on the use of TARE in clinical practice beyond high-expertise centres, where countries with different health-care systems were able to contribute to evaluate how TARE is used in standard clinical practice in Europe [31]. A recent multicentre prospective observational study in the UK describes the outcome of TARE in clinical practice for mCRC and ICC [32, 33], and a large-scale prospective observational study on TARE is currently being conducted in the USA (NCT02685631). Physicians and patients will benefit from the insights provided by real-world data from European countries and from patients with other liver malignancies beside HCC, mCRC and ICC. Data on less established uses of TARE such as metastatic liver disease from tumour entities such as breast cancer, malignant melanoma, or pancreatic cancer would be needed to uncover potential benefits of these specific patient groups [34–36].
To further improve the understanding of the real-life clinical application of TARE in Europe, the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) initiated the prospective CIRSE Registry for SIR-Spheres® Therapy (CIRT) for patients treated with TARE with Y90 resin microspheres (SIR-Spheres® Y-90 resin microspheres, Sirtex Medical Pty Limited; St. Leonards, NSW, Australia). Besides data on how TARE is embedded in the real-life clinical practice (primary objective), CIRT collected data on safety, effectiveness (overall survival (OS), progression-free survival (PFS), liver-specific PFS and imaging response), quality of life (QOL) and details concerning the treatment application.
This manuscript specifically discusses data concerning real-life application of TARE, therapeutic outcome (in terms of overall survival) and safety (in terms of 30-day mortality and morbidity) for all indications. Future manuscripts will include further analysis of the CIRT data considering, e.g. dosimetry data, PFS, hepatic-PFS, imaging response and QOL, as well as subgroup analyses per indication, including less evaluated indications like liver metastases from NET, breast cancer, pancreatic cancer and melanoma.
Materials and Methods
Study Design
CIRT is a prospective, multicentre, single-device, observational study of patients with hepatic malignancies treated with TARE with Y90 resin microspheres as standard of care. As observational study, CIRT did not prescribe or encourage the use of TARE in a particular patient group, but observed its use in the real-life clinical setting. Sites were invited to participate if TARE was in their armamentarium of treatment options to treat hepatic malignancies, and if they met the minimum selection criteria of at least 40 treatments in total, with a minimum of ten procedures within 12 months prior to invitation. From August 2014 to April 2017, 68 sites from 12 countries were invited to participate, of whom 27 included patients, representing five countries in the European Union, Switzerland, Turkey and Israel (see Supplement 1 and Supplement 2).
A detailed manuscript on the methodology of CIRT has been previously reported [37].
Patient inclusion criteria were: the patient was 18 years or older, diagnosed with primary or metastatic liver malignancies, scheduled to be treated with TARE with Y90 resin microspheres. There were no specific exclusion criteria. All included patients signed the informed consent form. Patient recruitment occurred between 1 January 2015 and 31 December 2017. Follow-up data was collected until 31 December 2019; patients were followed up for 24 months or until study exit. Specific follow-up intervals were left to the discretion of the medical teams. It was recommended that patient follow-up data would be collected every 3 months. In case follow-up evaluations were not performed at the site of the TARE treatment, sites were encouraged to obtain follow-up information from referring physicians.
Assessments
The real-life usage of TARE is determined by evaluating the intention of the treatment per indication, and how the TARE treatment was embedded in between prior and post-interventional hepatic and systemic therapies. Overall survival was measured from day of TARE treatment until date of death. Safety outcomes are described as 30-day morbidity and mortality rates according to the Common Terminology Criteria for Adverse Events, version 5.0. Monitored serious adverse events (SAEs, grade 3 and 4) were abdominal pain, fatigue, fever, nausea, vomiting, gastrointestinal ulceration, gastritis, radiation cholecystitis and radioembolization-induced liver disease (REILD). Patient characteristics, prior treatments and volumetric data were collected around time of treatment. Post-TARE treatments and safety data were collected at every follow-up. Survival status was collected as information became available.
Bias
As observational study, CIRT is sensitive to selection bias. This was addressed by contractually agreeing with study sites that all consecutive cases would be included. Regular remote monitoring by the CIRSE Clinical Research Department was done to verify if sites included all of their cases and to address missing data and data queries. However, it was not possible to perform source document verification.
Statistical Analysis
Data regarding the primary endpoint, safety and overall survival (OS) data are presented by summaries and descriptive statistics. Overall survival is presented graphically as Kaplan–Meyer curves and median time-to-event per indication with 95% confidence intervals (CI) being provided. Cox multiple regression is used to assess the impact of the covariates for OS and hazard ratios (HR), 95% CI and p-values are provided for all covariates. Covariates were chosen prior to data analysis and were published in a methodology manuscript [37]. P-values of < 0.05 are considered statistically significant. Patients who had withdrawn consent or are lost to follow up are censored at the last time they were documented as being alive (OS). All available data are used, and no imputations of missing data are made. Where missing data were observed, it was explained in the summary tables.
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Data from 1050 patients were included in the study. Twenty-three patients were excluded (see Supplement 3). The treated cohort (1027) consisted of 542 (52.8%) patients with primary liver tumours (HCC 422 (41.2%), ICC 120 (11.6%)) and 485 (47.2%) with metastatic liver disease (mCRC 237 (23.1%), neuroendocrine (NET) 58 (5.6%), breast 47 (4.6%) and pancreatic cancer and melanoma 32 each (3.1%), and other metastases 79 (7.7%)). 64.9% of the patients were male, and the median age was 65 years (interquartile range (IQR) 56–72).
Patient Characteristics and Real-Life Application
Primary Liver Tumours
For patients with primary liver tumours, the Eastern Cooperative Oncology Group (ECOG) status was 0 in 58.5% of the patients, with 8.3% having ECOG 2 or higher (Table 1). The presence of ascites was observed in 14.3% of the patients, while cirrhosis was more frequently observed in patients with HCC, 71.1% versus 12.5% in ICC. On the other hand, in patients with ICC, extra-hepatic disease was observed in 30% of the cases versus 9.5% of HCC patients. In patients with HCC, unilateral (right-sided) liver tumours were found in 50.2% of the cases, compared to 27.5% in the ICC cohort, which saw more bilobar liver tumours (59.2%). In total, portal vein thrombosis was found in 30.5% of the cases.
Table 1.
Patient characteristics–primary tumours
| Category | Subcategory | HCC (n = 422) | ICC (n = 120) | All (n = 542) |
|---|---|---|---|---|
| ECOG status | 0–fully active | 252 (59.7%) | 65 (54.2%) | 317 (58.5%) |
| 1–restricted | 136 (32.2%) | 41 (34.2%) | 177 (32.7%) | |
| 2 or higher | 34 (8.1%) | 11 (9.2%) | 45 (8.3%) | |
| Missingb | – | 3 (2.5%) | 3 (0.6%) | |
| Extra-hepatic disease | No | 382 (90.5%) | 84 (70.0%) | 466 (86.0%) |
| Yes | 40 (9.5%) | 36 (30.0%) | 76 (14.0%) | |
| Ascites | No | 357 (84.6%) | 107 (89.2%) | 464 (85.7%) |
| Yes | 65 (15.4%) | 13 (10.8%) | 78 (14.3%) | |
| Cirrhosis | No | 122 (28.9%) | 105 (87.5%) | 227 (41.9%) |
| Yes | 300 (71.1%) | 15 (12.5%) | 315 (58.1%) | |
| Location of liver tumours | Bilobar | 159 (37.7%) | 71 (59.2%) | 230 (42.4%) |
| Left only | 51 (12.1%) | 16 (13.3%) | 67 (12.4%) | |
| Right only | 212 (50.2%) | 33 (27.5%) | 245 (45.2%) | |
| Number of liver tumours | 1 | 110 (26.1%) | 32 (26.7%) | 142 (26.2%) |
| 2–5 | 154 (36.5%) | 35 (29.2%) | 189 (34.9%) | |
| 6–9 | 23 (5.5%) | 10 (8.3%) | 33 (6.1%) | |
| 10 or more | 55 (13.0%) | 10 (8.3%) | 65 (12.0%) | |
| Uncountable | 80 (19.0%) | 33 (27.5%) | 113 (20.8%) | |
| Portal vein | Patent | 282 (66.8%) | 95 (79.2%) | 377 (69.6%) |
| Segmental thrombosis | 82 (19.4%) | 14 (11.7%) | 96 (17.8%) | |
| Lobar thrombosis | 38 (9.0%) | 7 (5.8%) | 45 (8.3%) | |
| Main thrombosis | 20 (4.7%) | 4 (3.3%) | 24 (4.4%) | |
| Total tumour to liver percentage | Median | 9.2% | 12.8% | 10.0% |
| Q1, Q3 | 3.4%, 20.2% | 7.9%, 21.5% | 4.4%, 20.4% | |
| Missing | 67 (15.9%) | 23 (19.2%) | 90 (16.7%) |
In the primary liver cancer cohorts, median time from diagnosis to TARE was 188 days (IQR 71–590) for HCC and 201 (IQR 65–468) for ICC (Table 2). 60.0% received TARE with palliative intentions (non-curative, e.g. to prolong freedom from or relief of cancer-related symptoms); tumour downsizing was intended in 29.9% of the cases. In the HCC cohort, prior systemic treatments were provided in 10.7% of the patients, while 44.8% receive some form of prior locoregional treatments such as trans-arterial chemoembolization (TACE) (23.0%) or surgery (17.1%). In contrast, ICC patients received prior systemic treatment in 60.8% of the cases [39.2% received combined regimens based on gemcitabine (see Supplement 4)] and locoregional treatments in 34.2%, primarily in the form of surgical procedures (26.7%). Less than 10% of the primary cancer patients received systemic therapies in a concomitant setting. Following TARE, 31.4% received further systemic treatment: in patients with HCC, 18.9% and 4.5% underwent treatment with a tyrosine kinase inhibitor (TKI) and/or other treatments, respectively. Locoregional treatments were applied in 18.4% of the primary liver cases.
Table 2.
Patient characteristics—metastatic tumours
| Category | Subcategory | mCRC (n = 237) | NET (n = 58) | Breast (n = 47) | Pancreatic (n = 32) | Melanoma (n = 32) | Other liver metastases (n = 79)a | All (n = 485) |
|---|---|---|---|---|---|---|---|---|
| ECOG status | 0–fully active | 140 (59.1%) | 38 (65.5%) | 29 (61.7%) | 20 (62.5%) | 15 (46.9%) | 41 (51.9%) | 283 (58.4%) |
| 1–restricted | 75 (31.6%) | 14 (24.1%) | 16 (34.0%) | 8 (25.0%) | 14 (43.8%) | 32 (40.5%) | 159 (32.8%) | |
| 2 or higher | 18 (7.6%) | 5 (8.6%) | 2 (4.3%) | 3 (9.4%) | 1 (3.1%) | 6 (7.6%) | 35 (7.2%) | |
| Missingb | 4 (1.7%) | 1 (1.7%) | – | 1 (3.1%) | 2 (6.3%) | – | 8 (1.6%) | |
| Extra-hepatic disease | No | 139 (58.6%) | 25 (43.1%) | 17 (36.2%) | 17 (53.1%) | 25 (78.1%) | 33 (41.8%) | 256 (52.8%) |
| Yes | 98 (41.4%) | 33 (56.9%) | 30 (63.8%) | 15 (46.9%) | 7 (21.9%) | 46 (58.2%) | 229 (47.2%) | |
| Ascites | No | 228 (96.2%) | 54 (93.1%) | 44 (93.6%) | 29 (90.6%) | 31 (96.9%) | 75 (94.9%) | 461 (95.1%) |
| Yes | 9 (3.8%) | 4 (6.9%) | 3 (6.4%) | 3 (9.4%) | 1 (3.1%) | 4 (5.1%) | 24 (4.9%) | |
| Cirrhosis | No | 235 (99.2%) | 58 (100%) | 45 (95.7%) | 32 (100%) | 32 (100%) | 77 (97.5%) | 479 (98.8%) |
| Yes | 2 (0.8%) | – | 2 (4.3%) | – | – | 2 (2.5%) | 6 (1.2%) | |
| Location of liver tumours | Bilobar | 158 (66.7%) | 51 (87.9%) | 37 (78.7%) | 23 (71.9%) | 32 (100%) | 56 (70.9%) | 357 (73.6%) |
| Left only | 23 (9.7%) | – | 4 (8.5%) | 1 (3.1%) | – | 5 (6.3%) | 33 (6.8%) | |
| Right only | 55 (23.2%) | 7 (12.1%) | 6 (12.8%) | 8 (25.0%) | – | 18 (22.8%) | 94 (19.4%) | |
| Missing | 1 (0.4%) | – | – | – | – | – | 1 (0.2%) | |
| Number of liver tumours | 1 | 21 (8.9%) | 2 (3.4%) | 7 (14.9%) | 1 (3.1%) | 3 (9.4%) | 8 (10.1%) | 42 (8.7%) |
| 2–5 | 52 (21.9%) | 9 (15.5%) | 4 (8.5%) | 6 (18.8%) | 5 (15.6%) | 12 (15.2%) | 88 (18.1%) | |
| 6–9 | 25 (10.5%) | – | 3 (6.4%) | 1 (3.1%) | 2 (6.3%) | 9 (11.4%) | 40 (8.2%) | |
| 10 or more | 58 (24.5%) | 14 (24.1%) | 7 (14.9%) | 10 (31.3%) | 15 (46.9%) | 18 (22.8%) | 122 (25.2%) | |
| Uncountable | 81 (34.2%) | 33 (56.9%) | 26 (55.3%) | 14 (43.8%) | 7 (21.9%) | 32 (40.5%) | 193 (39.7%) | |
| Portal vein | Patent | 234 (98.7%) | 58 (100%) | 43 (91.5%) | 32 (100%) | 30 (93.8%) | 75 (94.9%) | 472 (97.3%) |
| Segmental thrombosis | 3 (1.3%) | – | 3 (6.4%) | – | 1 (3.1%) | 2 (2.5%) | 9 (1.9%) | |
| Lobar thrombosis | – | – | – | – | 1 (3.1%) | 1 (1.3%) | 2 (0.4%) | |
| Main thrombosis | – | – | 1 (2.1%) | – | – | 1 (1.3%) | 2 (0.4%) | |
| Total tumour to liver percentage | Median | 8.9% | 20.8% | 7.8% | 6.6% | 10.7% | 10.9% | 10.5% |
| Q1, Q3 | 3.8%, 18.3% | 8.5%, 40.0% | 4.0%, 18.6% | 3.8%, 14.0% | 5.0%, 18.8% | 4.0%, 25.6% | 4.5%, 21.8% | |
| Missing | 40 (18.9%) | 17 (29.3%) | 18 (38.3%) | 6 (24.0%) | 12 (37.5%) | 12 (15.2%) | 105 (21.6%) |
Metastatic Liver Tumours
In patients with metastatic liver tumours, ECOG 0 was observed in 58.4% of the patients (Table 3). Extra-hepatic disease was present in 52.8% of the patients, but ascites and cirrhosis were observed in 4.9% and 1.2% of the patients, respectively. Most of the patients (73.6%) had bilobar tumour burden with a liver to tumour percentage of 10.5%. Portal vein thrombosis was observed in 2.6% of the cases.
Table 3.
Real-life application—primary tumours
| Category | Subcategory | HCC (n = 422) | ICC (n = 120) | All (n = 542) |
|---|---|---|---|---|
| Time since primary diagnosis (days) | Median | 188 | 201 | 191 |
| Q1, Q3 | 71, 590 | 65, 468 | 70, 652 | |
| Missing | 4 (0.9%) | 2 (1.7%) | 6 (0.1%) | |
| Intention of treatmentc | Ablation | 17 (4.0%) | 7 (5.8%) | 24 (4.4%) |
| Bridge to liver surgery | 3 (0.7%) | 3 (2.5%) | 6 (1.1%) | |
| Bridge to liver transplant | 23 (5.5%) | 2 (1.7%) | 25 (4.6%) | |
| Downsizing / down-staging | 137 (32.5%) | 25 (20.8%) | 162 (29.9%) | |
| Palliative | 242 (57.3%) | 83 (69.2%) | 325 (60.0%) | |
| Prior TARE hepatic procedures | Yes | 189 (44.8%) | 41 (34.2%) | 230 (42.4%) |
| No | 233 (55.2%) | 79 (65.8%) | 312 (57.6%) | |
| Surgical (any)a | 72 (17.1%) | 32 (26.7%) | 104 (19.2%) | |
| Ablation (any) | 62 (14.7%) | 7 (5.8%) | 69 (12.7%) | |
| TACE (any) | 97 (23.0%) | 2 (1.7%) | 99 (18.3%) | |
| Vascular (any) | 15 (3.6%) | 1 (0.8%) | 16 (3.0%) | |
| Abdominal radiotherapy (any) | 7 (1.7%) | 5 (4.2%) | 12 (2.2%) | |
| Prior systemic therapy | Yes | 45 (10.7%) | 73 (60.8%) | 118 (21.8%) |
| No | 377 (89.3%) | 47 (39.2%) | 424 (78.2%) | |
| Concomitant chemotherapyb | Yes | 32 (7.6%) | 11 (9.2%) | 43 (7.9%) |
| No | 390 (92.4%) | 109 (90.8%) | 499 (92.1%) | |
| Post-TARE systemic therapy | Yes | 125 (29.6%) | 45 (37.5%) | 170 (31.4%) |
| No | 262 (62.1%) | 63 (52.5%) | 325 (60.0%) | |
| Missingd | 35 (8.3%) | 12 (10.0%) | 47 (8.7%) | |
| Post-TARE hepatic procedures | Yes | 80 (19.0%) | 20 (16.7%) | 100 (18.4%) |
| No | 307 (72.7%) | 88 (73.3%) | 395 (72.9%) | |
| Missingd | 35 (8.3%) | 12 (10.0%) | 47 (8.7%) | |
| Surgical (any)a | 3 (0.7%) | 4 (3.3%) | 7 (1.3%) | |
| Ablation (any) | 11 (2.6%) | 4 (3.3%) | 5 (2.8%) | |
| TACE (any) | 34 (8.1%) | 1 (0.8%) | 35 (6.5%) | |
| Vascular (any) | 7 (1.7%) | 2 (1.7%) | 9 (1.7%) | |
| Abdominal radiotherapy (any) | 13 (3.1%) | 6 (5.0%) | 19 (3.5%) |
aPatients can have multiple prior and post-TARE hepatic procedures
bConcomitant if systemic therapy start date is within 4 weeks of first TARE treatment start date and up to 8 weeks after first TARE end date (where end date is within 42 days of first TARE in case of two sessions)
cIntention of TARE is for first treatment
dMissing data include data from patients that were lost to follow up or deceased before the first follow-up could be included (n = 47)
Median time from diagnosis of the liver metastases to TARE was 579 days (IQR 253–1089) for the complete metastatic cohort, ranging between 84 days (IQR 56–315) for melanoma metastases to 1242 days (IQR 441–2196) in NET (Table 4). Similar to the primary liver tumour cohort, the intention of TARE was palliative in 77.3% of the patients and downsizing of the tumour in 15.3%. 88.0% of the patients received systemic treatment, and 33.6% received locoregional treatment prior to TARE. 13.2% of the patients received systemic treatments in a concomitant setting. After TARE, systemic treatment was applied in 35.1% of the patients. 13.8% received locoregional treatments.
Table 4.
Real-life application—metastatic tumours
| Category | Subcategory | mCRC (n = 237) | NET (n = 58) | Breast (n = 47) | Pancreatic (n = 32) | Melanoma (n = 32) | Other liver metastases (n = 79) | All (n = 485) |
|---|---|---|---|---|---|---|---|---|
| Time since metastatic diagnosis (days) | Median | 438 | 1242 | 1089 | 514 | 84 | 437 | 579 |
| Q1, Q3 | 230, 785 | 441, 2196 | 386, 2297 | 258, 850 | 56, 315 | 281, 877 | 253, 1089 | |
| Missing | 39 (16.5%) | 12 (20.7%) | 7 (14.9%) | 7 (21.9%) | 1 (3.1%) | 11 (13.9%) | 77 (15.9%) | |
| Intention of treatmentc | Ablation | 18 (7.6%) | 5 (8.6%) | 3 (6.4%) | 2 (6.3%) | – | 6 (7.6%) | 34 (7.0%) |
| Bridge to liver surgery | 2 (0.8%) | – | – | – | – | – | 2 (0.4%) | |
| Bridge to liver transplant | – | – | – | – | – | – | 0 (0.0%) | |
| Downsizing/down-staging | 41 (17.3%) | 4 (6.9%) | 4 (8.5%) | 9 (28.1%) | 3 (9.4%) | 13 (16.5%) | 74 (15.3%) | |
| Palliative | 176 (74.3%) | 49 (84.5%) | 40 (85.1%) | 21 (65.6%) | 29 (90.6%) | 60 (75.9%) | 375 (77.3%) | |
| Prior TARE hepatic procedures | Yes | 86 (36.3%) | 27 (46.6%) | 11 (23.4%) | 14 (43.8%) | 1 (3.1%) | 24 (30.4%) | 163 (33.6%) |
| No | 150 (63.3%) | 31 (53.4%) | 36 (76.6%) | 18 (56.3%) | 31 (96.9%) | 55 (69.6%) | 322 (66.2%) | |
| Missing | 1 (0.4%) | – | – | – | – | – | 1 (0.2%) | |
| Surgical (any)a | 67 (28.3%) | 15 (25.9%) | 5 (10.6%) | 5 (15.6%) | – | 16 (20.3%) | 108 (22.2%) | |
| Ablation (any) | 27 (11.4%) | 4 (6.9%) | 2 (4.3%) | 6 (18.8%) | 1 (3.1%) | 6 (7.6%) | 46 (9.5%) | |
| TACE (any) | 3 (1.3%) | 2 (3.4%) | 2 (4.3%) | 1 (3.1%) | – | – | 8 (1.6%) | |
| Vascular (any) | 3 (1.3%) | 3 (5.2%) | 1 (2.1%) | – | – | – | 7 (1.4%) | |
| Abdominal radiotherapy (any) | 6 (2.5%) | 3 (5.2%) | 4 (8.5%) | 3 (9.4%) | – | 5 (6.3%) | 21 (4.3%) | |
| Prior systemic therapy | Yes | 226 (95.4%) | 47 (81.0%) | 47 (100%) | 27 (84.4%) | 13 (40.6%) | 67 (84.8%) | 427 (88.0%) |
| No | 11 (4.6%) | 11 (19.0%) | – | 5 (15.6%) | 19 (59.4%) | 12 (15.2%) | 64 (12.0%) | |
| Concomitant chemotherapyb | Yes | 31 (13.1%) | 7 (12.1%) | 6 (12.8%) | 4 (12.5%) | 4 (12.5%) | 12 (15.2%) | 64 (13.2%) |
| No | 206 (86.9%) | 51 (87.9%) | 41 (87.2%) | 28 (87.5%) | 28 (87.5%) | 67 (84.8%) | 421 (86.8%) | |
| Post-TARE systemic therapy | Yes | 87 (36.7%) | 16 (27.6%) | 20 (42.5%) | 7 (21.9%) | 12 (37.5%) | 28 (35.4%) | 170 (35.1%) |
| No | 106 (44.7%) | 34 (58.6%) | 21 (44.7%) | 19 (59.4%) | 17 (53.1%) | 42 (53.2%) | 239 (49.3%) | |
| Missingd | 44 (18.6%) | 8 (13.8%) | 6 (12.8%) | 6 (18.7) | 3 (9.4%) | 9 (11.4%) | 76 (15.7%) | |
| Post-TARE hepatic procedures | Yes | 35 (14.8%) | 10 (17.2%) | 3 (6.4%) | 5 (15.6%) | 5 (15.6%) | 9 (11.4%) | 67 (13.8%) |
| No | 159 (67.1%) | 40 (69.0%) | 38 (80.9%) | 21 (65.6%) | 24 (75.0%) | 61 (77.2%) | 333 (70.5%) | |
| Missingd | 43 (18.1%) | 8 (13.8%) | 6 (12.8%) | 6 (18.7) | 3 (9.4%) | 9 (11.4%) | 76 (15.7%) | |
| Surgical (any)a | 10 (4.2%) | 1 (1.7%) | – | 1 (3.1%) | – | – | 12 (2.5%) | |
| Ablation (any) | 11 (4.6%) | – | 1 (2.1%) | – | 1 (3.1%) | 1 (1.3%) | 14 (2.9%) | |
| TACE (any) | 6 (2.5%) | – | – | – | 1 (3.1%) | 1 (1.3%) | 8 (4.2%) | |
| Vascular (any) | 2 (0.8%) | 1 (1.7%) | – | – | 3 (9.4%) | 15 (1.6%) | ||
| Abdominal radiotherapy (any) | 10 (4.2%) | 7 (12.1%) | 2 (4.3%) | 4 (12.5%) | – | 4 (5.1%) | 27 (5.6%) |
aPatients can have multiple prior and post-TARE hepatic procedures
bConcomitant if systemic therapy start date is within 4 weeks of first TARE treatment start date and up to 8 weeks after first TARE end date (where end date is within 42 days of first TARE in case of two sessions)
cIntention of TARE is for first treatment
dMissing data include data from patients that were lost to follow up or deceased before the first follow-up could be included (n = 76)
Overall Survival
During the observation period, 495 (48.2%) patients died and 349 (33.9%) were lost to follow up. 26 (2.5%) patients had less than 2 years of follow-up but no recorded reason for non-completion. 157 (15.3%) patients were alive and completed the 2-year follow-up period (see Supplement 5).
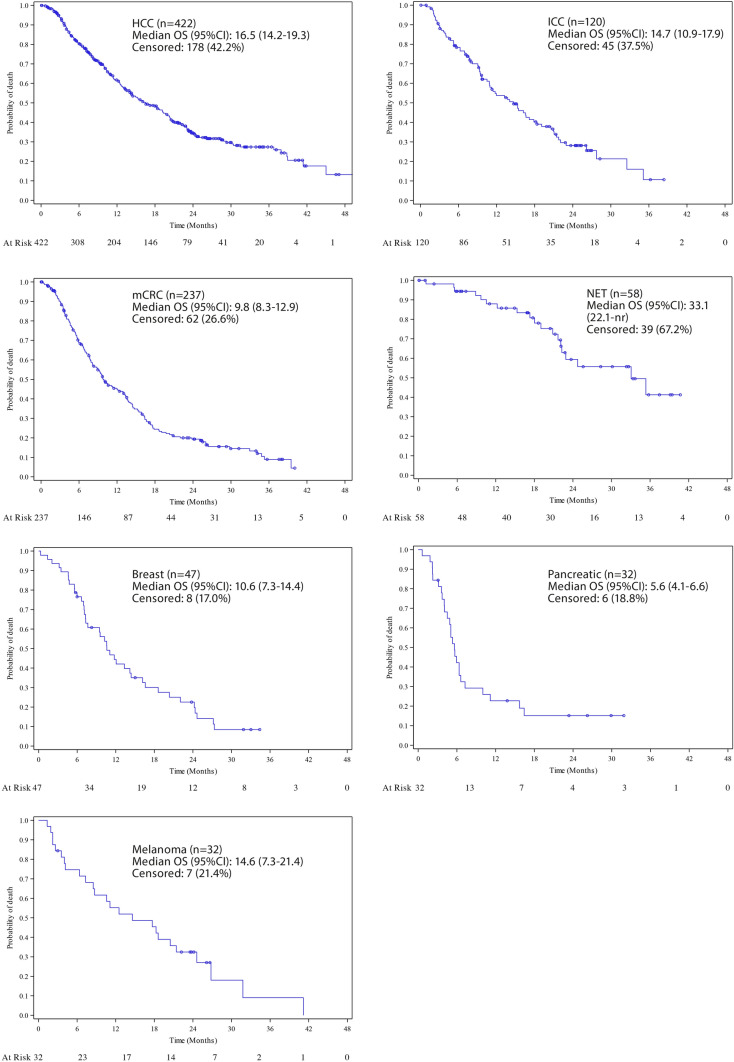
Median overall survival for patients following TARE was 16.5 months (95% CI 14.2–19.3) for HCC and 14.7 months (95% CI 10.9–17.9) for ICC. For liver metastases, median OS for mCRC was 9.8 months (95% CI 8.3–12.9), 5.6 months (95% CI 4.1–6.6) for pancreatic cancer metastases, 10.6 months (95% CI 7.3–14.4) for breast cancer, 14.6 months (95% CI 7.3–21.4) for melanoma and 33.1 months (95% 22.1–nr) for neuroendocrine tumours (see Fig. 1).
Fig. 1.
Kaplan–Meier curves per indication of overall survival in months after TARE, including at risk patients per interval
For the whole cohort, covariate analyses showed that extra-hepatic disease and ECOG status ≥ 0 were associated with a lower survival rate (HR 1.372, 95% CI 1.149–1.638, p < 0.0001; HR 1.513, 95% CI 1.280–1.789, for ECOG 1 and HR 1.624, 95% CI 1.217–2.168 for ECOG 2, p < 0.0001, respectively), as well as the presence of cirrhosis (HR 1.304, 95% CI 1.063–1.599, p = 0.0128) and ascites (HR 1.344, 95% CI 1.035–1.746, p = 0.0039). Unilateral malignancies had a better survival outcome than bilobar malignancies (HR 0.790, 95% CI 0.589–1.059 for left liver lobe tumours and HR 0.694, 95% CI 0.572–0.843 for right liver lobe tumours, p = 0.0024), and a higher tumour burden was negatively associated with survival (HR 1.414, 95% CI 1.143–1.750, p = 0.0195 was found for a tumour to liver percentage of more than 20%). Prior systemic chemotherapy (yes/no) did not qualify as a significant prognostic factor (p = 0.2068); however, the number of chemotherapy lines prior to TARE was found to be statistically significant (increased HR compared to no prior chemotherapy, p < 0.0001). Covariates as sex, number of liver tumours and prior hepatic procedures did not produce any significant differences in results (p-values > 0.05, see Table 5).
Table 5.
Covariate analysis
| Covariate | Level | Events (%) | HR estimatea | 95% CI | p valueb |
|---|---|---|---|---|---|
| ECOG | 0-Fully active | 58.7% (352/600) | 1.000 | ||
| 1-Restricted | 73.2% (246/336) | 1.513 | [1.280, 1.789] | < 0.0001 | |
| 2 or higher | 70.0% (56/80) | 1.624 | [1.217, 2.168] | ||
| Extra-hepatic disease prior to treatment | No | 59.0% (426/722) | 1.000 | ||
| Yes | 76.7% (234/305) | 1.372 | [1.149, 1.638] | < 0.0001 | |
| Cirrhosis | No | 65.6% (463/706) | 1.000 | ||
| Yes | 61.4% (197/321) | 1.304 | [1.063, 1.599] | 0.0128 | |
| Ascites | No | 63.6% (588/925) | 1.000 | ||
| Yes | 70.6% (72/102) | 1.344 | [1.035, 1.746] | 0.0039 | |
| Tumour to liver percentage | Less than 10% | 59.0% (242/410) | 1.000 | ||
| 10%–20% | 62.0% (127/205) | 1.137 | [0.914, 1.413] | 0.0195 | |
| Greater than 20% | 66.8% (147/220) | 1.414 | [1.143, 1.750] | ||
| Unknown | 75.0% (144/192) | 1.098 | [0.879, 1.373] | ||
| Location of liver tumours | Bilobar | 71.4% (419/587) | 1.000 | ||
| Left only | 57.0% (57/100) | 0.790 | [0.589, 1.059] | 0.0024 | |
| Right only | 54.3% (184/339) | 0.694 | [0.572, 0.843] | ||
| Prior chemotherapy: number of lines | 0 | 57.4% (296/516) | 1.000 | ||
| 1 | 64.4% (123/191) | 1.176 | [0.931, 1.485] | < 0.0001 | |
| 2–5 | 76.8% (172/224) | 1.855 | [1.493, 2.303] | ||
| 6 or more | 72.5% (66/91) | 1.355 | [1.010, 1.818] |
aA hazard ratio above 1 implies a higher rate of non-survival for that category compared to the reference category (for which the hazard ratio is 1.000). Selection of covariates based on a stepwise procedure. Variables that did not qualify (p > 0.05) were: sex (p = 0.2800), prior systemic therapy (p = 0.2664), prior hepatic procedures (p = 0.0895) and number of liver tumours (p = 0.0964)
bP values are from global Wald test
Safety
Across the entire cohort, the 30-day mortality rate of patients that received TARE was 1.0% (n = 10, (see Supplement 5). Serious adverse events (SAE, grade 3 and 4) within 30 days of treatment were found in less than 2.5% of the patients. SAEs such as gastritis, gastrointestinal ulcerations, radiation cholecystitis and radioembolization-induced liver disease (REILD) occurred in less than 0.3% of the total patient cohort.
Discussion
The results reported here derive from the largest prospective study on TARE to date and provide a good representation of the European application of TARE in its diverse clinical context. This study provides valuable information on the real-life clinical application and outcomes of TARE in indications for which guidelines are available and used (HCC, ICC, mCRC), as well as insights in the less established use of TARE in liver metastases of NET, breast cancer, pancreatic cancer and melanoma.
The data indicate that in the real-life clinical setting, TARE is largely considered to be a part of a palliative treatment strategy, across indications. That is to say to prolong freedom from or relief of cancer-related symptoms. The relatively low number of patients receiving any systemic therapy (33.1%) or loco-regional treatments (16.3%) after TARE suggests that TARE is used as “last meaningful treatment” rather than being planned as an early consolidation in the scope of various treatment options, suggesting that TARE is used according to most of the current European guidelines [1–5]. Our reported safety data confirming a favourable toxicity profile of TARE may support the consideration of its use earlier in the armamentarium.
Considering the timing of the TARE treatment in relation to prior systemic therapies, our study reported that the majority of the metastatic liver malignancies (mCRC, NET, breast and pancreatic) were treated with TARE after one or more systemic therapy line (Supplement 2). For mCRC, studies have shown that good results can still be achieved in heavily pre-treated patients (see below) [38–40]. In NET, TARE can be considered for patients not responding to systemic therapies or have undergone prior peptide receptor radionuclide therapy (PRRT), TACE or bland embolization, which is reflected in the long median time from metastatic diagnosis to TARE (1242 days, Table 4) [35, 41, 42]. Due to the high OS generally found in NET patients, care should be taken in applying TARE in NET, as treatment-related deaths have been observed in this patient population [43]. For hepatic breast cancer malignancies, all patients in this study were reported to have received prior systemic therapy and most of them received TARE with palliative intent, which have shown to delay progression and decrease tumour size [44–46]. The timing of TARE in pancreatic and melanoma liver metastases is less well understood [47–49]. A Finnish retrospective study on TARE in melanoma patients with hepatic metastases achieved a median OS for TARE of 18.7 months as a first-line treatment compared to chemotherapy (10.5 months), which is reflected in our reported median time from diagnosis to TARE of 84 days [50].
For our primary cohorts, TARE was provided considerably earlier in the treatment pathway (median 188 days (IQR 71–590) for HCC and median 201 days (IQR 65–468) for ICC), suggesting fewer prior hepatic treatments or systemic therapies. Indeed, current guideline recommendations on HCC suggest TARE fairly early in the treatment pathway [4, 7]. For ICC, TARE is recommended after at least 1 line of systemic therapy in locally advanced and metastatic ICC [2]. A recent retrospective study by Bargellini et al. suggests no significant differences between OS between chemotherapy naïve patients and patients who received prior first-line chemotherapy (with and without progression) [30]. A phase 2 trial by Edeline et al. found a median OS of 22 months in chemotherapy naïve patients treated with glass TARE and concomitant chemotherapy, suggesting that administering TARE early in the treatment pathway of unresectable ICC could be beneficial [51]. In our results, prior systemic therapy was provided to 60.8% of the patients, suggesting that sites may have different approaches concerning the place of TARE in the treatment pathway of patients with ICC.
It is encouraging that the median OS for the different cohorts found in our study is consistent with findings of other studies: in mCRC treated with TARE, White et al. reported a pooled weighted OS of 9.6 months (23 studies, n = 2517, 95% CI 8.9–10.4) [33], which is consistent with our findings (OS 9.8 months, 95% CI 8.3–12.9). For the smaller cohorts neuroendocrine, breast, pancreatic and melanoma liver metastases, the median OS found in this study were comparable with the median OS found in other studies (breast, a systematic review of 12 studies (n = 452) found an OS of 11.3 months [52]; neuroendocrine, a systematic review of 18 studies (n = 870) found a median OS of 27.6 months [42]; pancreatic, OS 5.5 months [48]; melanoma, OS 19.9 months [53] and 18.7 months [50]). This supports the fact that the real-life clinical application of TARE in metastatic liver tumours is in accordance with current evidence and strengthens the expectations regarding survival for patients treated with TARE for these indications.
For primary tumours, systematic reviews from Al-Adra et al. and Boehm et al. reported a median OS of 15.5 months (range 7–22.2 months) and 13.9 months (95% CI 9.5–18.3), respectively [54, 55]. Our ICC cohort presented an OS well within the expected range of survival for patients with ICC treated with TARE (14.7, 95% CI 10.9–17.9). For HCC, RCTs such as SARAH, SIRveNIB and SORAMIC found median OS of 8.0, 8.8 and 12.1 months, respectively [18, 22, 56], while retrospective studies found 12.9 and 12.8 months [12, 57]. Our relatively high median OS (16.5 months) can be explained by our high number of Child-Turcotte-Pugh (CTP) A (81.4%) versus CTP B (18.0%) (see supplement 3) paralleling the data presented by Salem et al. [58] and Sangro et al. [29].
Our study confirms previous findings that independent of indication, prognostic factors commonly associated with an increased survival rate are ECOG 0, reduced tumour burden, lack of cirrhosis and ascites, low number of chemotherapy lines prior to TARE and no extra-hepatic disease [16, 57, 59–62]. Kurilova et al. have shown that in mCRC patients in the salvage setting, 1-year OS can range from 10% to 90% based on independent baseline parameters (number of extra-hepatic disease sites, carcinoembryonic antigen, albumin, alanine aminotransferase level, tumour differentiation level and the sum of the two largest tumour diameters) [38]. Damm et al. have developed a scoring system for patients with mCRC consisting of a combination of tumour load, CEA or CA19-9 levels and Karnofsky index to improve patient selection for TARE [39]. In HCC, the presence of portal vein thrombosis has been identified as a negative prognosticator for survival and will be evaluated in a subsequent subgroup analysis [63, 64]. Potential other prognostic factors such as time from (metastatic) diagnosis to treatment and tumour markers were not evaluated at this time.
Limitations of this study are the observational design, whereas potentially important confounding factors could not be controlled. The relatively high number of patients that were lost to follow up can introduce bias regarding the interpretation of OS. A potential explanation might be the fact that TARE requires a comprehensive infrastructure with patients being referred to specialised centres for the treatment while being followed up by their local physician. Follow-up information was in those cases obtained by contacting the referring physician or, if this was not possible, the patient was considered as lost to follow up. While it was outside of the scope of the study to improve the necessary infrastructure for interventional radiology to follow up on their patients, this study provides an opportunity to reflect on the necessity for interventional radiologists to initiate follow-up standards and order relevant imaging after TARE. The CIRSE initiative Standards of Quality Assurance in Interventional Oncology is an initiative to improve quality assurance in interventional oncology, amongst which post-intervention follow-ups and imaging are one of the quality standards [70] Another limitation has been the timing of the study. In the last years, research on TARE has provided insights in the importance of biomarkers, genetic information and tumour absorbed dose on the oncological outcomes [39, 65–69]. As CIRT was designed before these insights were accepted and applied, data on these outcomes have not been included in the objectives of the study. Finally, this analysis did not take into account the potential differences of national guidelines, reimbursement policies and standards of practice.
Conclusion
This large-scale prospective observational study confirmed that TARE is safe and effective in the real-life clinical setting across various indications. In the real-life clinical setting, TARE is largely considered to be a part of a palliative treatment strategy and less as a component of early consolidation. Real-life OS is comparable to the results from prior clinical trials. Careful patient selection, also in the salvage setting, has been shown to be essential in the treatment liver malignancies with TARE. As new therapies like immune-oncology become available and synergistic treatment concepts get further accepted, TARE will likely become more and more integrated in the standard armamentarium of oncological treatment regimen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors want to thank the patients, the CIRT investigators and site staff involved in the study. We specifically want to thank the local study nurses that contributed significantly to the quality of the collected data through feedback and comments during the data collection phase. The CIRT study was funded by an independent investigator-initiated research grant from SIRTEX Medical Europe GmbH (Bonn, Germany). CIRSE, the Cardiovascular and Interventional Radiological Society of Europe, is responsible for the independent execution of the CIRT study and has sole ownership of the data. Statistical analysis was performed by Anders Nordlund from TrialFormSupport, Lund, Sweden. The electronic data capturing system was developed and supported by ConexSys Inc (Lincoln, RI, United States). Necessary server infrastructure was developed and maintained by ITEA GmbH (Vienna Austria). The authors thank the CIRSE Central Office and the staff of the CIRSE Clinical Research department for their support during the design and setup of the study and drafting of the manuscript.
On behalf of the CIRT Steering Committee: Jean-Pierre Pelage, Caen University and Medical Center, Department of Diagnostic Imaging and Interventional Radiology, avenue de la Cote de Nacre, 14033, Caen, France. Derek M Manas, Newcastle upon Tyne Hospitals NHS Foundation Trust, Department of Hepatobiliary Surgery, Freeman Rd NE7 7DN, Newcastle upon Tyne, United Kingdom. Frank T Kolligs, HELIOS Klinikum Berlin-Buch, Department of Internal Medicine and Gastroenterology, Schwanebecker Chaussee 50, 13125, Berlin, Germany. Samer Ezziddin, Universitätsklinikum des Saarlandes, Department of Nuclear Medicine, Kirrberger Str. Gebäude 50, 66421, Homburg, Germany. Ralph Peters (technical advisor), SIRTEX Medical Europe GmbH, Joseph-Schumpeter-Allee 33, 53227 Bonn, Germany.
On behalf of the CIRT Principal Investigators: Thomas Albrecht, Vivantes Klinikum Neukölln, Department for Radiology and Interventional Therapy, Rudower Str. 48, 12351, Berlin, Germany. Olivier D’Archambeau, University Hospital Antwerp, Department of Radiology, Wilrijkstraat 10, 2650, Antwerp, Belgium. Tugsan Balli, Çukurova University, Radiology Department, Balcalı Hospital, 01330, Adana, Turkey. Sadik Bilgic, Ankara University, Department of Radiology, Medical Faculty, Cebeci, 06590, Ankara, Turkey. Alan Bloom, Hadassah- Hebrew University Medical Center, Department of Radiology, Jerusalem, Israel. Roberto Cioni, University of Pisa, Diagnostic and Interventional Radiology, Department of Translational Research and New Technologies in Medicine, Via U. Foscolo 5, 50059, Vinci, Pisa, Italy. Roman Fischbach, Asklepios Klinik Altona, Department of Radiology and Neuroradiology, Paul-Ehrlich-Straße 1, 22763, Hamburg, Germany. Patrick Flamen, Institute Jules Bordet, Université Libre de Bruxelles, Nuclear Medicine Department, 121 Boulevard de Waterloo, 1000, Brussels, Belgium. Laurent Gerard, University Hospital of Liege, Division of Radiology, domaine du Sart-Tilman B35, 4000, Liège, Belgium. Gerd Grözinger, Eberhard Karls University, Department of Diagnostic and Interventional Radiology, Hoppe-Seyler-Str. 3, D-72076, Tübingen, Germany. Marcus Katoh, Helios Hospital Krefeld, Department of Diagnostic and Interventional Radiology, Lutherplatz 40, 47805, Krefeld, Germany. Michael Koehler, University Hospital Muenster, Department of Clinical Radiology, Albert-Schweitzer-Strasse 33, 48129, Muenster, Germany. Jan Robert Kröger, University of Cologne, Faculty of Medicine and University Hospital Cologne, Department of Radiology, Kerpener Str. 62, 50937, Cologne, Germany. Christiane Kuhl, University Hospital Aachen, Department of Radiology, Pauwelsstr. 30, 52074, Aachen, Germany. Franco Orsi, European Institute of Oncology, Interventional Radiology Division, Via Ripamonti 435, 20100, Milan, Italy. Murat Ozgun, St. Franziskus Hospital, Department of Radiology, Hohenzollernring 70, 48145, Muenster, Germany. Peter Reimer, Academic Teaching Hospital the University of Freiburg, Städtisches Klinikum Karlsruhe, Institute for Diagnostic and Interventional Radiology, Moltkestrasse 90, 76133, Karlsruhe, Germany. Maxime Ronot, APHP, University Hospitals Paris Nord Val de Seine, Department of Radiology, 100 bd général Leclerc, 100 bd général Leclerc, Beaujon, Clichy, Hauts-de-Seine, France. Axel Schmid, University Hospital Erlangen, Department of Radiology, Maximiliansplatz 1, 91054, Erlangen, Germany. Alessandro Vit, Azienda Ospedaliero Universitaria, SOC Diagnostica Angiografica e Radiologia Interventistica, via Grazzano 150/C, 33100, Udine, Italy
Abbreviations
- CI
Confidence interval
- CIRSE
Cardiovascular and Interventional Radiological Society of Europe
- CIRT
CIRSE registry for SIR-spheres therapy
- ECOG
Eastern cooperative oncology group
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- ICC
Intrahepatic cholangiocarcinoma
- IQR
Interquartile range
- mCRC
Metastatic colorectal cancer
- NET
Neuroendocrine tumour
- OS
Overall survival
- PFS
Progression-free survival
- PRRT
Peptide receptor radionuclide therapy
- QOL
Quality of life
- RCT
Randomized controlled trials
- REILD
Radioembolization-induced liver disease
- SAE
Serious adverse event
- SIRT
Selective internal radiation therapy
- TACE
Trans-arterial chemoembolization
- TARE
Trans-arterial radioembolization
- TKI
Tyrosine kinase inhibitor
Funding
The CIRT study was funded by an independent investigator-initiated research grant from SIRTEX Medical Europe GmbH (Bonn, Germany). CIRSE, the Cardiovascular and Interventional Radiological Society of Europe, is responsible for the independent execution of the CIRT study and has sole ownership of the data.
Compliance with Ethical Standards
Conflict of interest
JB and GM received speaker fees and proctor fees from SIRTEX Medical, BP received proctor fees from SIRTEX Medical, MP reported grants from Bayer and SIRTEX during the course of the study, OP is shareholder of GOGITh-SAS, NdJ is an employee of CIRSE, BS received grants, personal fees and non-financial support from SIRTEX Medical and BMS, grants and personal fees from Onxeo, personal fees and non-financial support from Astra Zeneca, Bayer, Ipsen, and BTG, and personal fees from Lilly, Eisai and Roche. DA is on the advisory board of Terumo, Boston Scientific, SIRTEX Medical Europe and Biocompatibles. RC, RG, TH, NS, TP and GM declared no conflict of interest. All authors report no conflict of interest directly related to the submitted work.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
For this type of study, consent for publication is not required. However, consent for publication was obtained for every individual person’s data included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Niels de Jong, Email: dejong@cirse.org.
On behalf of the CIRT Steering Committee:
Jean-Pierre Pelage, Derek M. Manas, Frank T. Kolligs, Samer Ezziddin, and Ralph Peters
On behalf of the CIRT Principal Investigators:
Thomas Albrecht, Olivier D’Archambeau, Tugsan Balli, Sadik Bilgic, Alan Bloom, Roberto Cioni, Roman Fischbach, Patrick Flamen, Laurent Gerard, Gerd Grözinger, Marcus Katoh, Michael Koehler, Jan Robert Kröger, Christiane Kuhl, Franco Orsi, Murat Ozgun, Peter Reimer, Maxime Ronot, Axel Schmid, and Alessandro Vit
References
- 1.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016 doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 2.Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016 doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 3.Giammarile F, Bodei L, Chiesa C, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2011 doi: 10.1007/s00259-011-1812-2. [DOI] [PubMed] [Google Scholar]
- 4.Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018 doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Dufour JF, Greten TF, Raymond E, et al. Clinical practice guidelines EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma european organisation for research and treatment of cancer. J Hepatol. 2012 doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®) Colon Cancer, Version 4.2020. NCCN website. 15 June 2020. Accessed 24 August 2020. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- 7.Benson AB, D’Angelica MI, Abbott DE, et al. NCCN Clinical practice guidelines in oncology (NCCN Guidelines ®) Hepatobiliary Cancers, Version 5.2020. NCCN website. 4 August 2020. Accessed 24 August 2020. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- 8.Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Dis. 2014 doi: 10.1055/s-0034-1394142. [DOI] [PubMed] [Google Scholar]
- 9.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010 doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013 doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 11.Hilgard P, Hamami M, El Fouly A, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010 doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 12.Sangro B, Carpanese L, Cianni R, et al. Survival after Yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology. 2011 doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 13.Mancini R, Carpanese L, Sciuto R, et al. A multicentric phase II clinical trial on intra-arterial hepatic radiotherapy with 90Yttrium SIR-spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: Preliminary results on toxicity and response rates. In Vivo (Brooklyn). 2006. [PubMed]
- 14.Sofocleous CT, Garcia AR, Pandit-Taskar N, et al. Phase i trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014 doi: 10.1016/j.clcc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Gulec SA, Pennington K, Wheeler J, et al. Yttrium-90 microsphere-selective internal radiation therapy with chemotherapy (Chemo-SIRT) for colorectal cancer liver metastases: An in vivo double-arm-controlled phase II trial. Am J Clin Oncol Cancer Clin Trials. 2013 doi: 10.1097/COC.0b013e3182546c50. [DOI] [PubMed] [Google Scholar]
- 16.Martin LK, Cucci A, Wei L, et al. Yttrium-90 radioembolization as salvage therapy for colorectal cancer with liver metastases. Clin Colorectal Cancer. 2012 doi: 10.1016/j.clcc.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendlisz A, Van Den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 18.Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 19.Wasan HS, Gibbs P, Sharma N, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017 doi: 10.1016/S1470-2045(17)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma RA, Wasan HS, Van Hazel GA, et al. Overall survival analysis of the FOXFIRE prospective randomized studies of first-line selective internal radiotherapy (SIRT) in patients with liver metastases from colorectal cancer. J Clin Oncol. 2018 doi: 10.1200/jco.2017.35.15_suppl.3507. [DOI] [Google Scholar]
- 21.Chauhan N, Mulcahy MF, Salem R, et al. Therasphere yttrium-90 glass microspheres combined with chemotherapy versus chemotherapy alone in second-line treatment of patients with metastatic colorectal carcinoma of the liver: Protocol for the EPOCH phase 3 randomized clinical trial. J Med Internet Res. 2019 doi: 10.2196/11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: Selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018 doi: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 23.Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is Yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann Surg Oncol. 2015 doi: 10.1245/s10434-014-4164-x. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for 90Y resin microspheres. J Gastrointest Oncol. 2015 doi: 10.3978/j.issn.2078-6891.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rognoni C, Ciani O, Sommariva S, et al. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: Systematic review and meta-analyses. Oncotarget. 2016 doi: 10.18632/oncotarget.11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levillain H, Duran Derijckere I, Ameye L, et al. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: a multicenter study. Eur J Nucl Med Mol Imaging. 2019 doi: 10.1007/s00259-019-04427-z. [DOI] [PubMed] [Google Scholar]
- 27.Riby D, Mazzotta AD, Bergeat D, et al. Downstaging with radioembolization or chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-08486-7. [DOI] [PubMed] [Google Scholar]
- 28.Cucchetti A, Cappelli A, Mosconi C, et al. Improving patient selection for selective internal radiation therapy of intra-hepatic cholangiocarcinoma: a meta-regression study. Liver Int. 2017 doi: 10.1111/liv.13382. [DOI] [PubMed] [Google Scholar]
- 29.Sangro B, Maini CL, Ettorre GM, et al. Radioembolisation in patients with hepatocellular carcinoma that have previously received liver-directed therapies. Eur J Nucl Med Mol Imaging. 2018 doi: 10.1007/s00259-018-3968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bargellini I, Mosconi C, Pizzi G, et al. Yttrium-90 radioembolization in unresectable intrahepatic cholangiocarcinoma: results of a multicenter retrospective study. Cardiovasc Intervent Radiol. 2020 doi: 10.1007/s00270-020-02569-4. [DOI] [PubMed] [Google Scholar]
- 31.Sposito C, Mazzaferro V. The SIRveNIB and SARAH trials, radioembolization vs. sorafenib in advanced HCC patients: reasons for a failure, and perspectives for the future. HepatoBiliary Surg Nutr. 2018. 10.21037/hbsn.2018.10.06 [DOI] [PMC free article] [PubMed]
- 32.White J, Carolan-Rees G, Dale M, et al. Analysis of a national programme for selective internal radiation therapy for colorectal cancer liver metastases. Clin Oncol. 2019;31(1):58–66. doi: 10.1016/j.clon.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 33.White J, Carolan-Rees G, Dale M, et al. Yttrium-90 transarterial radioembolization for chemotherapy-refractory intrahepatic cholangiocarcinoma: a prospective. Observational Study. J Vasc Interv Radiol. 2019;30(8):1185–1192. doi: 10.1016/j.jvir.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Kuei A, Saab S, Cho SK, Kee ST, Lee EW. Effects of Yttrium-90 selective internal radiation therapy on non-conventional liver tumors. World J Gastroenterol. 2015 doi: 10.3748/wjg.v21.i27.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbier CE, Garske-Román U, Sandström M, Nyman R, Granberg D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-015-3264-6. [DOI] [PubMed] [Google Scholar]
- 36.Puippe G, Pfammatter T, Schaefer N. Arterial therapies of non-colorectal liver metastases. Visz Gastrointest Med Surg. 2015;31(6):414–422. doi: 10.1159/000441689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helmberger T, Arnold D, Bilbao JI, et al. Clinical application of radioembolization in hepatic malignancies: Protocol for a prospective multicenter observational study. J Med Internet Res. 2020 doi: 10.2196/16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurilova I, Beets-Tan RGH, Flynn J, et al. Factors affecting oncologic outcomes of 90Y radioembolization of heavily pre-treated patients with colon cancer liver metastases. Clin Colorectal Cancer. 2019 doi: 10.1016/j.clcc.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damm R, Seidensticker R, Ulrich G, et al. Y90 Radioembolization in chemo-refractory metastastic, liver dominant colorectal cancer patients: outcome assessment applying a predictive scoring system. BMC Cancer. 2016 doi: 10.1186/s12885-016-2549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sofocleous CT, Violari EG, Sotirchos VS, et al. Radioembolization as a salvage therapy for heavily pretreated patients with colorectal cancer liver metastases: factors that affect outcomes. Clin Colorectal Cancer. 2015 doi: 10.1016/j.clcc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barat M, Cottereau A-S, Kedra A, et al. The Role of Interventional Radiology for the Treatment of Hepatic Metastases from Neuroendocrine Tumor: An Updated Review. J Clin Med. 2020 doi: 10.3390/jcm9072302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia Z, Wang W. Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: a systematic review. Eur J Radiol. 2018 doi: 10.1016/j.ejrad.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Zuckerman DA, Kennard RF, Roy A, Parikh PJ, Weiner AA. Outcomes and toxicity following Yttrium-90 radioembolization for hepatic metastases from neuroendocrine tumors—a single-institution experience. J Gastrointest Oncol. 2019 doi: 10.21037/jgo.2018.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bale R, Putzer D, Schullian P. Local treatment of breast cancer liver metastasis. Cancers (Basel). 2019 doi: 10.3390/cancers11091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon AC, Gradishar WJ, Kaklamani VG, et al. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy. J Vasc Interv Radiol. 2014 doi: 10.1016/j.jvir.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakobs TF, Hoffmann RT, Fischer T, et al. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008 doi: 10.1016/j.jvir.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Rowcroft A, Loveday BPT, Thomson BNJ, Banting S, Knowles B. Systematic review of liver directed therapy for uveal melanoma hepatic metastases. HPB. 2020 doi: 10.1016/j.hpb.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Gibbs P, Do C, Lipton L, et al. Phase II trial of selective internal radiation therapy and systemic chemotherapy for liver-predominant metastases from pancreatic adenocarcinoma. BMC Cancer. 2015 doi: 10.1186/s12885-015-1822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michl M, Haug AR, Jakobs TF, et al. Radioembolization with yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases: Efficacy, safety and prognostic factors. Oncol. 2014 doi: 10.1159/000355821. [DOI] [PubMed] [Google Scholar]
- 50.Tulokas S, Mäenpää H, Peltola E, et al. Selective internal radiation therapy (SIRT) as treatment for hepatic metastases of uveal melanoma: a Finnish nation-wide retrospective experience. Acta Oncol (Madr). 2018 doi: 10.1080/0284186X.2018.1465587. [DOI] [PubMed] [Google Scholar]
- 51.Edeline J, Touchefeu Y, Guiu B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2019.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feretis M, Solodkyy A. Yttrium-90 radioembolization for unresectable hepatic metastases of breast cancer: a systematic review. World J Gastrointest Oncol. 2020 doi: 10.4251/wjgo.v12.i2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing M, Prajapati HJ, Dhanasekaran R, et al. Selective internal yttrium-90 radioembolization therapy (90Y-SIRT) versus best supportive care in patients with unresectable metastatic melanoma to the liver refractory to systemic therapy: Safety and efficacy cohort study. Am J Clin Oncol Cancer Clin Trials. 2017 doi: 10.1097/COC.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 54.Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015 doi: 10.1016/j.ejso.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015 doi: 10.1002/jso.23781. [DOI] [PubMed] [Google Scholar]
- 56.Ricke J, Sangro B, Amthauer H, et al. The impact of combining Selective Internal Radiation Therapy (SIRT) with Sorafenib on overall survival in patients with advanced hepatocellular carcinoma: the soramic trial palliative cohort. J Hepatol. 2018 doi: 10.1016/s0168-8278(18)30424-0. [DOI] [Google Scholar]
- 57.Abdallah MA, Wongjarupong N, Hassan MA, et al. The efficacy, safety, and predictors of outcomes of transarterial radioembolization for hepatocellular carcinoma: a retrospective study. Expert Rev Gastroenterol Hepatol. 2020 doi: 10.1080/17474124.2020.1777856. [DOI] [PubMed] [Google Scholar]
- 58.Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1000-patient 15-year experience. Hepatology. 2018 doi: 10.1002/hep.29691. [DOI] [PubMed] [Google Scholar]
- 59.Abouchaleh N, Gabr A, Ali R, et al. 90 Y radioembolization for locally advanced hepatocellular carcinoma with portal vein thrombosis: Long-term outcomes in a 185-patient cohort. J Nucl Med. 2018 doi: 10.2967/jnumed.117.199752. [DOI] [PubMed] [Google Scholar]
- 60.Reimer P, Virarkar MK, Binnenhei M, Justinger M, Schön MR, Tatsch K. Prognostic factors in overall survival of patients with unresectable intrahepatic cholangiocarcinoma treated by means of yttrium-90 radioembolization: results in therapy-naïve patients. Cardiovasc Intervent Radiol. 2018 doi: 10.1007/s00270-017-1871-2. [DOI] [PubMed] [Google Scholar]
- 61.Köhler M, Harders F, Lohöfer F, et al. Prognostic factors for overall survival in advanced intrahepatic cholangiocarcinoma treated with yttrium-90 radioembolization. J Clin Med. 2019;9(1):56. doi: 10.3390/jcm9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hickey R, Lewandowski RJ, Prudhomme T, et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: safety and survival outcomes from a 531-patient multicenter study. J Nucl Med. 2016 doi: 10.2967/jnumed.115.166082. [DOI] [PubMed] [Google Scholar]
- 63.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008 doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 64.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012 doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Lahti SJ, Xing M, Zhang D, Lee JJ, Magnetta MJ, Kim HS. KRAS status as an independent prognostic factor for survival after yttrium-90 radioembolization therapy for unresectable colorectal cancer liver metastases. J Vasc Interv Radiol. 2015 doi: 10.1016/j.jvir.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 66.Ziv E, Bergen M, Yarmohammadi H, et al. PI3K pathway mutations are associated with longer time to local progression after radioembolization of colorectal liver metastases. Oncotarget. 2017 doi: 10.18632/oncotarget.15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Hoven A, Rosenbaum C, Elias S, et al. Insights into the dose-response relationship of hepatic radioembolization with resin yttrium-90 microspheres: a prospective cohort study in patients with colorectal cancer liver metastases. J Vasc Interv Radiol. 2016 doi: 10.1016/j.jvir.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 68.Hermann A-L, Dieudonné A, Ronot M, et al. Relationship of tumor radiation–absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90 Y in the SARAH study. Radiology. 2020 doi: 10.1148/radiol.2020191606. [DOI] [PubMed] [Google Scholar]
- 69.Sofocleous CT, Vasiniotis Kamarinos N. Tumor-absolbed dose: the missing link in radioembolization. Radiology. 2020 doi: 10.1148/radiol.2020202354. [DOI] [PubMed] [Google Scholar]
- 70.Adam A, De Baère T, Bilbao JI, et al. Standards of quality assurance in interventional oncology, First Edition. CIRSE–Cardiovascular and Interventional Radiological Society of Europe. 2018. ISBN 978-3-9502501-5-2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.