Abstract
Purpose
To test the hypothesis that hypoalbuminemia is independently associated with increased risk of acute kidney injury (AKI).
Methods
A meta-analysis was performed of observational clinical studies evaluating the relationship between serum albumin level and the occurrence of AKI by multivariate methods. Additionally, the impact was assessed of lower serum albumin on mortality in patients who developed AKI. Eligible studies were sought by multiple methods, and adjusted odds ratios (OR) were quantitatively combined using a random effects model.
Results
Seventeen clinical studies with 3,917 total patients were included: 11 studies (6 in surgical or intensive care unit patients and 5 in other hospital settings) evaluating the influence of serum albumin on AKI incidence and 6 studies describing the relationship between serum albumin and mortality among patients who had developed AKI. Lower serum albumin was an independent predictor both of AKI and of death after AKI development. With each 10 g L−1 serum albumin decrement, the odds of AKI increased by 134%. The pooled OR for AKI was 2.34 with a 95% confidence interval (CI) of 1.74–3.14. Among patients who had developed AKI, the odds of death rose 147% (pooled OR 2.47, 95% CI 1.51–4.05) with each 10 g L−1 serum albumin decrement.
Conclusions
This meta-analysis provides evidence that hypoalbuminemia is a significant independent predictor both of AKI and of death following AKI development. Serum albumin determinations may be of utility in identifying patients at increased risk for AKI or for death after AKI. Controlled studies are warranted to assess interventions aimed at correcting hypoalbuminemia.
Keywords: Hypoalbuminemia, Serum albumin, Acute kidney injury, Mortality, Risk factors, Meta-analysis
Introduction
Acute kidney injury (AKI) is associated with significant morbidity and mortality in critically ill patients [1, 2]. Risk factors leading to this complication are under active investigation [3]. Among independent risk factors for AKI currently identified are age, body mass index, baseline renal function, acute circulatory or respiratory failure, liver disease, infection, peripheral vascular occlusive disease, chronic obstructive pulmonary disease, chronic heart failure, lymphoma or leukemia, prior invasive procedures, and higher-risk surgery [1, 4]. While hypoalbuminemia has been well established as a potent independent risk factor for morbidity and mortality [5], its role, if any, as a predictor specifically of AKI remains poorly defined. There is also the possibility that hypoalbuminemia may augment the risk of AKI. Analyzing existing observational studies focused on the epidemiology of AKI may make it feasible to evaluate these questions.
This meta-analysis was designed to test the hypothesis that hypoalbuminemia is independently associated with increased risk of AKI. Additionally, the impact of hypoalbuminemia on survival of patients developing AKI was assessed.
Methods
Study selection
The primary criterion for clinical study selection was the availability of data on AKI incidence in relation to serum albumin level. Additionally, clinical studies with data on mortality as related to serum albumin levels in patients who had developed AKI were sought. Observational studies are susceptible to the influence of confounding variables, and that influence can be minimized through multivariate analysis. Accordingly, only clinical studies evaluating the impact of serum albumin by multivariate methods were eligible for inclusion in the meta-analysis [5]. Otherwise, no restrictions were placed on study design, including the definition of AKI adopted by the investigators. Eligibility was not based on either time period or reporting language.
Search strategy
Eligible studies were identified by computer searches of MEDLINE, EMBASE, the Cochrane Library, and the abstracts from the annual meetings of the Society for Critical Care Medicine, the American Thoracic Society, and the American Society of Anesthesiologists, and the annual International Symposium on Intensive Care and Emergency Medicine. Reference lists of primary study publications and review articles were also examined. The searches were conducted between March and August 2009. Search terms included the following: acute renal failure, acute kidney injury, mortality, survival, death, serum albumin, and hypoalbuminemia (see "Appendix").
Data extraction
For each study, data were extracted on year reported, study design, number of centers conducting the study, number of patients, clinical indication, patient age and gender, mean baseline serum albumin and creatinine concentrations, method of multivariate analysis, number and types of covariates used to adjust the effect size of serum albumin, the definition of AKI applied, the adjusted odds ratios (OR) for AKI and mortality after AKI development and their standard errors or 95% confidence intervals (CI).
Statistical analysis
Based on a previous meta-analysis of hypoalbuminemia as an outcome predictor [5], significant between-study heterogeneity was anticipated. In order to accommodate such expected heterogeneity, individual study-adjusted OR per 10 g L−1 decrement in serum albumin were quantitatively combined under a random effects model [6]. For studies in which multivariate analysis was performed by Cox regression, the effect size measure was the hazard ratio. Though defined differently from the OR, the hazard ratio has been shown to be similar to the OR both theoretically and empirically [5]. Accordingly, in this report, the term OR is used to denote both the OR and the hazard ratio.
OR is sometimes reported with reference to serum albumin cutoffs such as <35 g L−1 rather than to continuous decrements. In such instances, the median serum albumin values below and above the cutoff were used to re-express the reported OR based on per 10 g L−1 decrements [6]. Those medians were calculated from individual patient serum albumin values, when reported. When such individual patient values were presented as data points in graphical displays, the data were derived from the graphs.
Heterogeneity was evaluated for statistical significance by Cochran Q test and magnitude by calculation of the I 2 statistic. Publication bias was assessed by linear regression of standardized effect as a function of precision. Prospective design and larger patient population were assumed to be indicative of higher study quality. More recent studies were considered to be more closely representative of contemporary clinical practice. Statistical analysis was performed using the software program Comprehensive Meta Analysis version 2.2.048 (Biostat; Englewood, NJ, USA).
Results
Included studies
The process of study selection is depicted in Fig. 1. After initial screening and examination of retrieved full clinical study reports, 51 candidate reports were selected for possible inclusion. Of those, 34 were excluded upon detailed review. By far the most common basis for exclusion, accounting for 23 of the 34 excluded reports, was lack of multivariate analysis. The typical data presentation in those studies was a univariate comparison of mean serum albumin levels between patients who did or did not develop AKI. Other grounds for exclusion were assessment of end points other than AKI in four reports, predictors other than serum albumin in three, or patients with end-stage renal disease or chronic kidney disease in four studies.
Fig. 1.
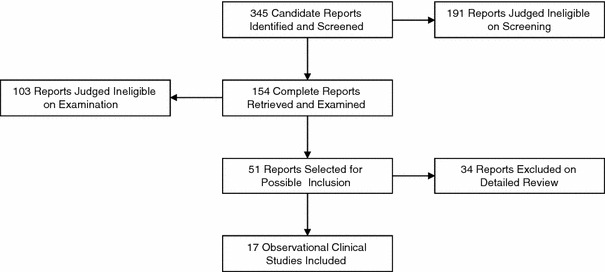
Study selection process
Seventeen studies reported from 1989 to 2009 with 3,917 total patients fulfilled all inclusion criteria, including multivariate analysis of hypoalbuminemia as an independent outcome predictor, and were included in the meta-analysis [7–23]. None was unpublished.
Eleven of the studies, six in surgical or intensive care unit (ICU) patients and five in other hospital environments, addressed the role of hypoalbuminemia in AKI development (Table 1). The remaining six studies dealt with the relationship between hypoalbuminemia and mortality among patients who had already developed AKI (Table 2).
Table 1.
Studies on predictors of AKI development
| Study | Indication/population | Design | AKI definition | Albumina (g L−1) |
|---|---|---|---|---|
| Surgery or ICU | ||||
| Rich et al. [7] | Cardiac surgery | R | SCr increase ≥1.0 mg dL−1 or BUN ≥ 20 mg dL−1 | 38 |
| Létourneau et al. [13] | BMT patients admitted to ICU | R | SCr doubling or increase to >200 μmol L−1 | 27.1 |
| Kim et al. [14] | Burn ICU patients | R | SCr increase to ≥2 mg dL−1 | 23.7 |
| Chawla et al. [15] | Medical and surgical ICU admissions | P | >75% SCr increase if baseline SCr ≤ 2.0 mg dL−1 or >50% increase if baseline > 2.0 mg dL−1 | 30 |
| Boyle et al. [17] | First orthotopic heart transplant | P | Dialysis | 38 |
| Cabezuelo et al. [18] | Consecutive orthotopic liver transplants | R | Persistent ≥50% SCr increase | 35.6 |
| Other hospital indications | ||||
| Rich and Crecelius [8] | Cardiac angiography | P | ≥44 μmol L−1 SCr increase within 48 h | 40.7 |
| Contreras et al. [9] | Hospital i.v. amikacin treatment | P | ≥0.5, ≥1.0, or >1.5 mg dL−1 SCr increase if ≤1.9, 2.0-4.9, or ≥5.0 mg dL−1 baseline, respectively | 34.4 |
| Drawz et al. [21] | Consecutive medical, surgical, and obstetric hospital admissions | Rb | ≥0.5, ≥1.0, or ≥1.5 mg dL−1 SCr increase if ≤1.9, 2.0-4.9, or ≥5.0 mg dL−1 baseline, respectively | 35 |
| Park et al. [22] | Transarterial chemoembolization in hepatocellular carcinoma | R | AKIN criteria (creatinine) | 35.7 |
| Hung et al. [23] | Stevens-Johnson syndrome and toxic epidermal necrolysis | R | RIFLE GFR criteria | 37.7 |
AKI Acute kidney injury; AKIN acute kidney injury network; BMT bone marrow transplantation; BUN blood urea nitrogen; GFR glomerular filtration rate; ICU intensive care unit; P prospective; R retrospective; RIFLE risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage kidney disease; SCr serum creatinine
aMean baseline serum albumin level
bMulticenter
Table 2.
Studies on predictors of mortality after AKI development
| Study | Indication/population | Design | AKI definition | Albumina (g L−1) |
|---|---|---|---|---|
| Chertow et al. [10] | ATN | Pb | ≥1.0 mg dL−1 SCr increase over 24–48 h | 27 |
| Obialo et al. [11] | Renal failure, ATN, or acute tubulointerstitial nephritis | R | ≥2.0 mg dL−1 SCr increase | 31 |
| Lins et al. [12] | Consecutive adults with AKI | P | SCr > 2 mg dL−1 or ≥50% increase | 32 |
| Dharan et al. [16] | Consecutive hospitalized patients with AKI | P | ≥0.5 or 1.0 mg dL−1 SCr increase if ≤1.9 or 2.0-4.9 mg dL−1 baseline, respectively | – |
| Mahajan et al. [19] | Consecutive ICU patients with AKI | R | >2 mg dL−1 SCr increase or 25% if CKD | – |
| Sezer et al. [20] | Hospital-diagnosed AKI | P | ≥1.5 mg dL−1 SCr increase within 48 h | 33.8 |
AKI Acute kidney injury, ATN acute tubular necrosis, CKD chronic kidney disease, ICU intensive care unit, P prospective, R retrospective, SCr serum creatinine, - not reported
aMean baseline serum albumin level
bPlacebo arm of a multicenter randomized controlled trial
All 17 studies were specifically designed to identify predictors/risk factors of AKI development or death after AKI. Baseline serum albumin level was evaluated as a candidate predictor/risk factor in all studies. In 9 of the 11 studies on AKI development, none of the study patients presented with pre-existing AKI at baseline. In the other two such studies, 93 [13] and 92% [18] of the patients were free of pre-existing AKI at baseline.
Study characteristics
The median number of patients per study was 184 with an interquartile range (IQR) of 104–236, and six studies involved >200 patients. Eight of the 17 included studies (47%) were prospective and 9 retrospective (53%) in design. Mean patient age ranged from 43 to 78 years, averaging 57 years with a standard deviation (SD) of 12 years. The mean percentage of male patients was 64% (SD 13%). Mean baseline serum albumin averaged 33.3 g L−1 (SD 4.8 g L−1). For the 11 studies on AKI predictors, mean baseline serum creatinine was on average 1.15 mg dL−1 (SD 0.170 mg dL−1), compared with 4.62 mg dL−1 (SD 0.384 mg dL−1) in the 6 studies on patients who had developed AKI.
The presence of chronic kidney disease (CKD) in the patient population was indicated for 11 studies. There were no CKD patients in six of those studies, and the percentages with CKD in the remaining five studies ranged from 4.8 to 40.2%.
In 14 of the 17 studies, the definition of AKI was based exclusively upon serum creatinine (SCr), either a fixed increase in 9 studies or a graded increase depending on baseline SCr in 4 studies (Tables 1, 2). In the four remaining studies, AKI was defined in accordance with an increase in either SCr or blood urea nitrogen, the RIFLE glomerular filtration rate (GFR) criteria [24], the AKIN creatinine criteria [25], or the need for dialysis.
No information on usage of resuscitation fluids was provided in the reports of 14 studies, although in one of those studies shock patients were excluded [9]. In one study, fluids were administered at physicians’ discretion [10], while in another no formal protocol for fluid management was implemented [8]. In a study on burn patients, the Parkland formula for fluid resuscitation was followed [14].
The multivariate analytical methods employed were logistic regression in 15 studies (88%) and Cox regression in 2 (12%). The effects of serum albumin were adjusted for a median of three covariates (IQR 2–5). The most frequent covariates were gender, which was assessed in all 17 studies, age in 16, SCr in 15, diabetes in 10, hypertension in 9, and sepsis, congestive heart failure, and need for mechanical ventilation in 8 studies each. Among the less frequently evaluated covariates were malignancy in six studies, and inflammation, New York Heart Association class, and chronic liver disease in two studies each. After model development in two studies [16, 21], a separate validation sample was used for testing.
Albumin and AKI
Lower serum albumin was a significant independent predictor of AKI development. With each 10 g L−1 decrement in serum albumin the odds of AKI increased by 134% (pooled OR, 2.34; CI, 1.74–3.14). The odds of AKI development were more than doubled in association with lower albumin among the six studies of surgical or ICU patients and nearly tripled among studies in other hospital settings. Serum albumin was the first or second most powerful independent predictor of AKI in 7 of the 11 studies evaluating this end point (Fig. 2).
Fig. 2.
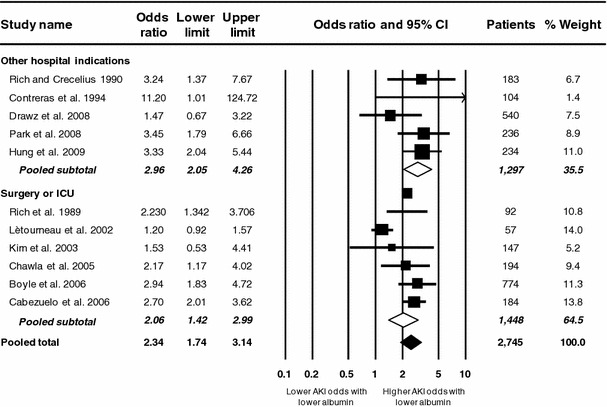
Meta-analysis of adjusted odds ratio for acute kidney injury (AKI) per 10 g L−1 decrement in serum albumin. Data points scaled in proportion to meta-analytic weight. Error bars indicate 95% confidence interval (CI). ICU Intensive care unit
Significant heterogeneity was present (p < 0.001), confirming the appropriateness of the random effects model. Approximately two-thirds of the total variance was attributable to heterogeneity (I 2 67.9%). There was no evidence of publication bias (p = 0.21). Heterogeneity persisted among studies on surgical and ICU patients (p = 0.001; I 2 75.7%) but not on other indications (p = 0.32; I 2 14.9%).
The increase in odds of AKI associated with lower serum album was greater among studies with prospective design and larger size (Table 3). Significant heterogeneity was absent in both the prospective (p = 0.56; I 2 0%) and larger (p = 0.33; I 2 13.3%) studies, but remained present in the retrospective (p < 0.001; I 2 76.5%) and smaller (p = 0.002; I 2 71.2%) studies. The differences between recent and older studies and between studies adopting AKI definitions based on fixed versus graded SCr increases were relatively minor (Table 3).
Table 3.
Sensitivity analyses of studies on AKI predictors
| Parameter | n | Pooled adjusted OR (95% CI) |
|---|---|---|
| Design | ||
| Retrospective | 7 | 2.14 (1.47-3.13) |
| Prospective | 4 | 2.79 (1.99-3.93) |
| Size (patients) | ||
| ≤200 | 7 | 2.11 (1.43-3.13) |
| >200 | 4 | 2.86 (2.11-3.89) |
| Year reported | ||
| ≤2000 | 3 | 2.58 (1.67-3.97) |
| >2000 | 8 | 2.23 (1.58-3.16) |
| AKI definition | ||
| Fixed SCr increase | 4 | 1.96 (1.11-3.49) |
| Graded SCr increasea | 3 | 2.04 (1.13-3.71) |
| Otherb | 4 | 2.90 (2.24-3.76) |
AKI Acute kidney injury; BUN blood urea nitrogen; CI confidence interval; GFR glomerular filtration rate; n number of studies; OR odds ratio; RIFLE risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function and end-stage kidney disease; SCr serum creatinine
aDetermined by baseline SCr
bSCr or BUN increase, RIFLE GFR criteria, AKIN creatinine criteria, or dialysis
Albumin and mortality
Lower serum albumin was a significant independent risk factor for mortality among patients who had developed AKI (Fig. 3). The odds of death rose 147% (pooled OR 2.47; 95% CI 1.51–4.05) with each 10 g L−1 serum albumin decrement. Significant heterogeneity was also present with respect to this end point (p < 0.019; I 2 62.9%). Due to the comparatively small number of included studies evaluating mortality after AKI development, however, reliable subgroup sensitivity analyses were judged not to be feasible.
Fig. 3.
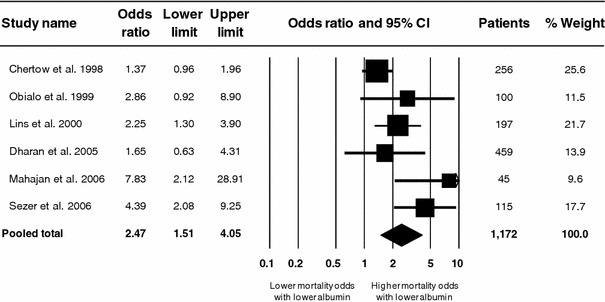
Meta-analysis of adjusted odds ratio for mortality per 10 g L−1 serum albumin decrement in patients who had developed AKI. Graphic conventions and abbreviations as in Fig. 2. Error bars indicate 95% confidence interval (CI)
Discussion
This meta-analysis provides evidence for the hypothesis that hypoalbuminemia is an independent risk factor for AKI. Hypoalbuminemia was also independently associated with increased risk of mortality among patients developing AKI. These associations were highly consistent in direction, as judged by OR > 1 in each and every included study (Figs. 2, 3). The pooled OR of 2.34 (95% CI 1.74–3.14) for AKI suggests that the kidney may be particularly vulnerable to hypoalbuminemia, since in a previous meta-analysis [5] the pooled OR for total complications (1.89, CI 1.59–2.24) was smaller.
One limitation of this meta-analysis was reliance on observational studies. Although studies without multivariate analysis were excluded, there remains the possibility of confounding by latent unrecognized or unmeasured variables affecting outcome. Hence, the observed independent associations cannot be considered proof of a causative role. Another limitation was the lack of available information on resuscitation protocols applied.
It could be postulated that albumin merely serves as a negative biomarker of inflammation. That possibility was investigated in two studies included in this meta-analysis. In one of those studies, hypoalbuminemia was an independent risk factor for AKI (adjusted OR 2.17, 95% CI 1.17–4.02), while systemic inflammatory response syndrome was not [15]. In the other study, the independent risk factors for AKI included low serum albumin (adjusted OR 3.33, 95% CI 2.04–5.44), but not C-reactive protein, eosinophil count, or white blood cell count [23]. In a previous meta-analysis that was not focused specifically on AKI [5], hypoalbuminemia remained an independent risk factor for morbidity and mortality when inflammatory markers were evaluated as covariates.
While the direction of the hypoalbuminemia effect on AKI and post-AKI death was remarkably consistent, the presence of heterogeneity suggests that a single underlying effect size cannot adequately explain the data observed. At least three sources of heterogeneity were apparent: study quality, clinical indication, and definition of AKI. However, higher quality studies, by the dual criteria of prospective design and larger size, were free of significant heterogeneity, as were studies in hospital indications outside surgery or the ICU. The lack of a uniformly accepted definition of AKI (formerly acute renal failure, ARF) before the introduction of the RIFLE [24] or AKIN [25] criteria must be considered a major limitation for every analysis including older studies in the area.
The meta-analysis suggests that determinations of serum albumin may be of value in identifying patients at higher risk for AKI as well as death following the development of AKI. A separate question is whether the demonstrated associations between hypoalbuminemia and AKI and death following AKI development might also provide a rationale for modifying clinical management with the aim of restoring more nearly normal serum albumin levels. That question can only be answered affirmatively if serum albumin contributes to these outcomes causally rather than serving simply as a marker of other pathophysiologic processes.
In the current analysis, hypoalbuminemia effects were potent, since the odds both of AKI and death following AKI were more than doubled. A dose-response relationship was evident in the studies included in the meta-analysis, since those odds progressively increased with each 10 g L−1 decrement in serum albumin. A dose-response relationship was also apparent in a study of 438 cadaver renal transplant recipients [26]. In patients receiving >0.8 g kg−1 exogenous albumin, the incidence of delayed graft function was reduced and the frequency of graft survival increased compared with those receiving lower albumin doses.
Several lines of evidence also suggest mechanisms by which serum albumin may protect the kidney. One study included in the meta-analysis showed that declining albumin levels were associated with increased incidence of i.v. amikacin nephrotoxicity, suggesting that the specific ligand-binding properties of albumin may mediate renoprotection in patients treated with nephrotoxic drugs [9]. Albumin also mitigated the nephrotoxicity of interleukin-2 (IL-2) in a randomized trial of 107 patients who had developed vascular leak due to IL-2 [27]. The frequency of oliguria was 2.8% in the albumin group compared with 32.5% in patients receiving normal saline. In a large study on chronic renal insufficiency, albumin was found to be an independent predictor of GFR [28].
Albumin at approximately 1% of its serum concentration increased the survival of cultured renal tubular cells, and the effect was independent of either bound lipid or colloid osmotic pressure [29, 30]. This renoprotective action of albumin was mediated by its capabilities for scavenging reactive oxygen species, preventing oxidative damage, and binding and delivering protective lysophosphatidic acid [29, 30]. Consistent with direct renoprotection is the effectiveness of albumin in the preservation of human transplantable kidneys up to 50 h [31]. Albumin appears to play a major role in maintaining renal perfusion as well as glomerular filtration and medullary fluid reabsorption. Improved perfusion may be the consequence of prolonged potent renal vasodilatation caused by reaction of serum albumin with oxides of nitrogen to form S-nitroso-albumin [32] or by binding of platelet-activating factor [33]. Although increasing albumin concentration above 30 g L−1 decreases GFR secondary to the resulting increase in oncotic pressure, reduction of serum albumin below the critical value 30 g L−1 in the perfusion media was found to decrease renal plasma flow and GFR in the isolated perfused rat kidney [34]. Furthermore fluid transport in the medulla appears to be dependent on albumin concentration in peritubular capillaries [35].
Albumin has further been shown to stimulate renal tubular cell DNA synthesis via signaling pathways involving Ca2+, protein kinase C, epidermal growth factor receptor, mitogen-activated protein kinases, and nuclear factor-κB [36, 37]. Enhanced DNA synthesis has been interpreted as a mechanism by which albumin maintains proximal tubular integrity and function [36, 37].
The present meta-analysis was focused on serum albumin level as a predictor rather than on the effects of administering exogenous albumin. Nevertheless, studies of albumin administration are relevant to the question of whether low serum albumin may play a causal role for poor renal outcomes [9, 38]. A large multicenter RCT comparing 4% albumin with crystalloid for fluid resuscitation failed to demonstrate any difference in outcome parameters including renal function, but proved that albumin itself was safe [39].
In three randomized trials of hypoalbuminemic patients with spontaneous bacterial peritonitis, albumin infusion reduced the odds of renal impairment by 70–79% compared either with no albumin [40, 41] or hydroxyethyl starch 200/0.5 [42]. Although only one of the three trials was powered to show statistical significance [40], the magnitudes of the albumin effects closely coincided in all three.
In a prospective study of 21 consecutive patients with hepatorenal syndrome (HRS), complete responses defined as 1.5 mg dL−1 SCr or lower were achieved in 77% of patients receiving albumin as an adjunct to terlipressin versus 25% of those treated with terlipressin alone [43]. Survival was also improved in the albumin recipients. Based on studies of extracorporeal albumin dialysis (EAD), HRS appears to be another indication in which the benefit of albumin may derive from its specific ligand-binding properties. EAD is designed to take advantage of toxin binding by albumin. In a randomized trial, EAD improved survival of hypoalbuminemic HRS patients and reduced levels of both creatinine and bilirubin [44]. Animal evidence exists of direct nephrotoxicity by unconjugated bilirubin [45, 46]. Nevertheless, systemic toxicity arising from unbound ligands such as uremic toxins in hypoalbuminemic patients might be a major indirect contributor to renal dysfunction.
The results in HRS also illustrate the apparent capacity of albumin to improve kidney function even in the presence of pre-existing renal impairment. Further supporting this concept is a randomized crossover trial of patients with nephrotic syndrome, in which albumin increased urine output and sodium excretion [47].
Taken together, this meta-analysis provides evidence for hypoalbuminemia as an independent risk factor for AKI and for post-AKI death. Currently available data support a causal role for serum albumin in maintaining kidney integrity and function. Hence, controlled clinical studies are warranted to evaluate the effects on renal function of interventions aimed at correcting hypoalbuminemia. Further evaluation of serum albumin in identifying patients at increased risk for AKI and for death after AKI would also be justified.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Appendix: search strategy
Search “acute kidney injury” OR AKI OR “acute renal failure” OR ARF
Search mortality OR survival OR death
Search “serum albumin” OR hypoalbuminemi* OR hypoalbuminaemi*
Search #1 AND #2 AND #3
References
- 1.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PG. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 3.de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 4.Joannidis M, Metnitz PG. Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin. 2005;21:239–249. doi: 10.1016/j.ccc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237:319–334. doi: 10.1097/00000658-200303000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 7.Rich MW, Keller AJ, Schechtman KB, Marshall WG, Jr, Kouchoukos NT. Increased complications and prolonged hospital stay in elderly cardiac surgical patients with low serum albumin. Am J Cardiol. 1989;63:714–718. doi: 10.1016/0002-9149(89)90257-9. [DOI] [PubMed] [Google Scholar]
- 8.Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150:1237–1242. doi: 10.1001/archinte.150.6.1237. [DOI] [PubMed] [Google Scholar]
- 9.Contreras AM, Ramírez M, Cueva L, Alvarez S, de Loza R, Gamba G. Low serum albumin and the increased risk of amikacin nephrotoxicity. Rev Invest Clin. 1994;46:37–43. [PubMed] [Google Scholar]
- 10.Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH. Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. The Auriculin Anaritide Acute Renal Failure Study Group. J Am Soc Nephrol. 1998;9:692–698. doi: 10.1681/ASN.V94692. [DOI] [PubMed] [Google Scholar]
- 11.Obialo CI, Okonofua EC, Nzerue MC, Tayade AS, Riley LJ. Role of hypoalbuminemia and hypocholesterolemia as copredictors of mortality in acute renal failure. Kidney Int. 1999;56:1058–1063. doi: 10.1046/j.1523-1755.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- 12.Lins RL, Elseviers M, Daelemans R, Zachee P, Gheuens E, Lens S, De Broe ME. Prognostic value of a new scoring system for hospital mortality in acute renal failure. Clin Nephrol. 2000;53:10–17. [PubMed] [Google Scholar]
- 13.Létourneau I, Dorval M, Bélanger R, Légaré M, Fortier L, Leblanc M. Acute renal failure in bone marrow transplant patients admitted to the intensive care unit. Nephron. 2002;90:408–412. doi: 10.1159/000054728. [DOI] [PubMed] [Google Scholar]
- 14.Kim GH, Oh KH, Yoon JW, Koo JW, Kim HJ, Chae DW, Noh JW, Kim JH, Park YK. Impact of burn size and initial serum albumin level on acute renal failure occurring in major burn. Am J Nephrol. 2003;23:55–60. doi: 10.1159/000066299. [DOI] [PubMed] [Google Scholar]
- 15.Chawla LS, Abell L, Mazhari R, Egan M, Kadambi N, Burke HB, Junker C, Seneff MG, Kimmel PL. Identifying critically ill patients at high risk for developing acute renal failure: a pilot study. Kidney Int. 2005;68:2274–2280. doi: 10.1111/j.1523-1755.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 16.Dharan KS, John GT, Antonisamy B, Kirubakaran MG, Jacob CK. Prediction of mortality in acute renal failure in the tropics. Ren Fail. 2005;27:289–296. [PubMed] [Google Scholar]
- 17.Boyle JM, Moualla S, Arrigain S, Worley S, Bakri MH, Starling RC, Heyka R, Thakar CV. Risks and outcomes of acute kidney injury requiring dialysis after cardiac transplantation. Am J Kidney Dis. 2006;48:787–796. doi: 10.1053/j.ajkd.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Cabezuelo JB, Ramírez P, Ríos A, Acosta F, Torres D, Sansano T, Pons JA, Bru M, Montoya M, Bueno FS, Robles R, Parrilla P. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69:1073–1080. doi: 10.1038/sj.ki.5000216. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan S, Tiwari S, Bharani R, Bhowmik D, Ravi S, Agarwal SK, Tiwari SC. Spectrum of acute renal failure and factors predicting its outcome in an intensive care unit in India. Ren Fail. 2006;28:119–124. doi: 10.1080/08860220500530395. [DOI] [PubMed] [Google Scholar]
- 20.Sezer MT, Demir M, Gungor G, Senol A. Predictors of mortality in patients with acute renal failure. Acta Medica (Hradec Kralove) 2006;49:183–188. [PubMed] [Google Scholar]
- 21.Drawz PE, Miller RT, Sehgal AR. Predicting hospital-acquired acute kidney injury—a case-controlled study. Ren Fail. 2008;30:848–855. doi: 10.1080/08860220802356515. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Chung HC, Lee JS, Lee BM, Kim DM, Hwang JC, Jo MW, Noh M, Shin JW. Acute kidney injury after transarterial chemoembolization for hepatocellular carcinoma: a retrospective analysis. Blood Purif. 2008;26:454–459. doi: 10.1159/000157322. [DOI] [PubMed] [Google Scholar]
- 23.Hung CC, Liu WC, Kuo MC, Lee CH, Hwang SJ, Chen HC. Acute renal failure and its risk factors in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Nephrol. 2009;29:633–638. doi: 10.1159/000195632. [DOI] [PubMed] [Google Scholar]
- 24.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawidson IJ, Sandor ZF, Coorpender L, Palmer B, Peters P, Lu C, Sagalowsky A, Risser R, Willms C. Intraoperative albumin administration affects the outcome of cadaver renal transplantation. Transplantation. 1992;53:774–782. doi: 10.1097/00007890-199204000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Pockaj BA, Yang JC, Lotze MT, Lange JR, Spencer WF, Steinberg SM, Topalian SL, Schwartzentruber DJ, White DE, Rosenberg SA. A prospective randomized trial evaluating colloid versus crystalloid resuscitation in the treatment of the vascular leak syndrome associated with interleukin-2 therapy. J Immunother. 1994;15:22–28. doi: 10.1097/00002371-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 29.Levine JS, Koh JS, Triaca V, Lieberthal W. Lysophosphatidic acid: a novel growth and survival factor for renal proximal tubular cells. Am J Physiol. 1997;273:F575–F585. doi: 10.1152/ajprenal.1997.273.4.F575. [DOI] [PubMed] [Google Scholar]
- 30.Iglesias J, Abernethy VE, Wang Z, Lieberthal W, Koh JS, Levine JS. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol. 1999;277:F711–F722. doi: 10.1152/ajprenal.1999.277.5.F711. [DOI] [PubMed] [Google Scholar]
- 31.Burleson RL, Jones DB, Yenikomshian AM, Cornwall C, DeVoe C, DeRito J. Clinical renal preservation by cryoperfusion with an albumin perfusate: renal perfusion with albumin. Arch Surg. 1978;113:688–692. doi: 10.1001/archsurg.1978.01370180030003. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann MA, Castelli I, Pargger H, Drop LJ. Nitric oxide dose-response study in the isolated perfused rat kidney after inhibition of endothelium-derived relaxing factor synthesis: the role of serum albumin. J Pharmacol Exp Ther. 1995;273:855–862. [PubMed] [Google Scholar]
- 33.Gerkens JF. Reproducible vasodilatation by platelet-activating factor in blood- and Krebs-perfused rat kidneys is albumin-dependent. Eur J Pharmacol. 1990;177:119–126. doi: 10.1016/0014-2999(90)90261-4. [DOI] [PubMed] [Google Scholar]
- 34.Zamlauski-Tucker M, Cohen JJ. Effect of substrate-free albumin on perfused rat kidney function. Ren Physiol. 1988;10:352–360. doi: 10.1159/000173144. [DOI] [PubMed] [Google Scholar]
- 35.MacPhee PJ, Michel CC. Fluid uptake from the renal medulla into the ascending vasa recta in anaesthetized rats. J Physiol. 1995;487:169–183. doi: 10.1113/jphysiol.1995.sp020869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon R, Brunskill NJ. Activation of mitogenic pathways by albumin in kidney proximal tubule epithelial cells: implications for the pathophysiology of proteinuric states. J Am Soc Nephrol. 1999;10:1487–1497. doi: 10.1681/ASN.V1071487. [DOI] [PubMed] [Google Scholar]
- 37.Lee YJ, Han HJ. Albumin-stimulated DNA synthesis is mediated by Ca2+/PKC as well as EGF receptor-dependent p44/42 MAPK and NF-κB signal pathways in renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;294:F534–F541. doi: 10.1152/ajprenal.00408.2007. [DOI] [PubMed] [Google Scholar]
- 38.Joannidis M, Druml W, Forni LG, Groeneveld AB, Honore P, Oudemans-van Straaten HM, Ronco C, Schetz MR, Woittiez AJ. Prevention of acute kidney injury and protection of renal function in the intensive care unit Expert opinion of the Working Group for Nephrology, ESICM. Intensive Care Med. 2010;36:392–411. doi: 10.1007/s00134-009-1678-y. [DOI] [PubMed] [Google Scholar]
- 39.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 40.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 41.Chen T-A, Tsao Y-C, Chen A, Lo G-H, Lin C-K, Yu H-C, Cheng L-C, Hsu P-I, Tsai W-L. Effect of intravenous albumin on endotoxin removal, cytokines, and nitric oxide production in patients with cirrhosis and spontaneous bacterial peritonitis. Scand J Gastroenterol. 2009;44:619–625. doi: 10.1080/00365520902719273. [DOI] [PubMed] [Google Scholar]
- 42.Fernández J, Monteagudo J, Bargallo X, Jiménez W, Bosch J, Arroyo V, Navasa M. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627–634. doi: 10.1002/hep.20829. [DOI] [PubMed] [Google Scholar]
- 43.Ortega R, Ginès P, Uriz J, Cárdenas A, Calahorra B, De Las Heras D, Guevara M, Bataller R, Jiménez W, Arroyo V, Rodés J. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–948. doi: 10.1053/jhep.2002.35819. [DOI] [PubMed] [Google Scholar]
- 44.Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J, Hickstein H, Loock J, Löhr J-M, Liebe S, Emmrich J, Korten G, Schmidt R. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277–286. doi: 10.1053/lv.2000.6355. [DOI] [PubMed] [Google Scholar]
- 45.Elias MM, Comin EJ, Grosman ME, Galeazzi SA, Rodriguez Garay EA. Inhibitory effect of unconjugated bilirubin on p-aminohippurate transport in rat kidney cortex slices. Biochim Biophys Acta. 1982;693:265–272. doi: 10.1016/0005-2736(82)90431-X. [DOI] [PubMed] [Google Scholar]
- 46.Elias MM, Comin EJ, Grosman ME, Galeazzi SA, Rodriguez Garay EA. Possible mechanism of unconjugated bilirubin toxicity on renal tissue. Comp Biochem Physiol A Comp Physiol. 1987;87:1003–1007. doi: 10.1016/0300-9629(87)90027-2. [DOI] [PubMed] [Google Scholar]
- 47.Fliser D, Zurbrüggen I, Mutschler E, Bischoff I, Nussberger J, Franek E, Ritz E. Coadministration of albumin and furosemide in patients with the nephrotic syndrome. Kidney Int. 1999;55:629–634. doi: 10.1046/j.1523-1755.1999.00298.x. [DOI] [PubMed] [Google Scholar]


