Abstract
Purpose:
Some but not all randomized controlled trials (RCTs) of soy isoflavones showed their beneficial effect on arterial stiffness, a predictor of cardiovascular events, dementia, and all-cause mortality, independent of traditional risk factors. To test the hypothesis that supplementation of soy isoflavones reduces arterial stiffness, we performed a systematic review and meta-analysis of RCTs of soy isoflavones on arterial stiffness.
Methods:
The protocol of this systematic review was registered with PROSPERO (CRD42019126128) and written in accordance with PRISMA. The PubMed, Embase, and clinicaltrials.gov databases were searched using the following criteria: human subjects, soy isoflavones as intervention, and arterial stiffness as primary outcome. A random-effects meta-analysis was used to pool estimates across studies. Standardized mean difference (SMD) was used to synthesize quantitative results.
Results:
Among 998 articles retrieved, 8 articles met our criteria. Duration of intervention was relatively short (maximum of 12 weeks). Outcome measurements extracted were pulse wave velocity (PWV), systemic arterial compliance (SAC), augmentation index (AI), and cardio-ankle vascular index (CAVI). Soy isoflavones reduced arterial stiffness compared to placebo (Standardized Mean Difference: −0.33, 95% confidence interval: −0.47, −0.19). Subgroup analyses showed no difference between treatment effects for intervention duration (<6weeks vs. ≥6weeks) or gender (women only vs. men only vs. combined). Sensitivity analysis showed no difference in the effect of soy isoflavones between PWV, CAVI, SAC, and AI.
Conclusion:
Supplementation of soy isoflavones reduced arterial stiffness. Longer-duration trials with larger number of participants are warranted.
Keywords: soy isoflavones, arterial stiffness, meta-analysis, randomized controlled trials
Introduction
Arterial stiffness, also known as the loss of arterial elasticity, is closely associated with biological aging and therefore affects primarily middle to older populations [1]. As the human body ages, structural changes occur within the arterial walls, including fragmentation of elastin fibers and increased deposition of collagen, partly due to repeated cycles of mechanical stress [1]. The physical stiffening of arteries has major health implications for its connection to various adverse cardiovascular and other health outcomes such as coronary heart disease (CHD), stroke, hypertension, heart failure, chronic kidney disease (CKD), dementia, and all-cause mortality [2–4].
Soy isoflavones, a class of phytoestrogens, are one of the richest sources of isoflavones in human diet [5]. Recent meta-analyses have suggested an inverse association of soy intake with cardiovascular risk factors such as blood pressure and cholesterol [6–9]. A recent observational study has also documented an inverse association of dietary consumption of soy isoflavones with arterial stiffness [10], which is a predictor of future cardiovascular events [3, 11, 12], incident dementia [2], and all-cause mortality [3, 12] independent of traditional cardiovascular risk factors.
Randomized controlled trials (RCTs) have been conducted in recent years to study the effect of soy isoflavones on arterial stiffness [13–20]. However, the quantity of studies conducted remains limited and results have been inconsistent with some studies showing either a positive effect [13–15,18, 20] or no effect [16, 17, 19]. A previous systematic review published in 2011 examined dietary and nutrient interventions, including soy isoflavones, as a means of reducing arterial stiffness. Based on four RCTs of soy or isoflavones, they concluded that soy or isoflavones supplementation was one of a few methods of reducing arterial stiffness [21]. Since this publication, several RCTs of soy isoflavones on arterial stiffness have been reported.
The purpose of this systematic review and meta-analysis was to evaluate the effect of soy isoflavones on arterial stiffness through the qualitative and quantitative analysis of relevant RCTs. We hypothesized that the supplementation of soy isoflavones through its various forms, compared to a placebo, would reduce arterial stiffness.
Methods
Literature Search and Study Selection
The systematic review was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the protocol was registered with PROSPERO (CRD42019126128) [22]. A search in PubMed, Embase, clinicaltrials.gov, and referenced articles was performed using one or more of the following search terms for isoflavones (flavones, flavonoids, genistein, coumestrol, pterocarpans, daidzein, equol, soy, soya), arterial stiffness (vascular stiffness, elasticity, arterial pressure, blood pressure, pulse wave analysis, wave reflections, augmentation index, arterial compliance, cardio-ankle vascular index, carotid femoral pulse wave velocity, brachial ankle pulse wave velocity, pulse pressure, pulse wave velocity), and RCTs (randomized controlled trial, controlled clinical trials as topic, clinical trial, clinical study, placebos, double-blind method) (Online Resource Table 1). The search was limited to studies published between January 1966 through February 2019 in human subjects and in the English language.
Each of the selected studies were included in the systematic review process if they met the following criteria: participants were human subjects, primary treatment intervention was soy isoflavones, primary outcome was arterial stiffness, and the study was a RCT. A reference list of included articles and similar systematic reviews was also searched. However, no additional articles were included.
A review of titles, abstracts and full-texts of each article was conducted by five investigators (AS, BM, CC, DS, XZ) using a two-step method. The first step was a general approach as titles and abstracts that did not meet the inclusion criteria were screened out. The second step was more comprehensive as full texts of the remaining articles were thoroughly read and also excluded if they did not meet the inclusion criteria. Any discrepancies that arose during the screening process were discussed by all the investigators.
Quality Assessment
This assessment comprised of seven criteria including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and a category for other bias [23]. For each study, investigators assigned either high risk of bias, low risk of bias, or unclear risk of bias for each above-mentioned criteria.
Data Collection and Synthesis
Information extracted from each article included year of publication, study location, study design, total sample size (number of women, men), study population characteristics (e.g., postmenopausal status, age, hypertensive status), intervention duration (weeks), intervention dose, washout period (weeks), and the mean and standard deviation/error of arterial stiffness outcome measurements both at baseline and after placebo and treatment intervention. Outcome data were extracted for pulse wave velocity (PWV), systemic arterial compliance (SAC), augmentation index (AI), and cardio-ankle vascular index (CAVI). However, attempts to reach out to authors of one study to clarify information and obtain additional results were not met with a response [13].
A series of calculations were executed to standardize the data in terms of standard deviation (SD) as some articles reported standard error of mean (SE) or confidence intervals (CI) [23]. Converting SE to SD was calculated by and converting 95% CI to SD was calculated by . The mean change from baseline to after placebo or treatment was calculated by , and the standard deviation of change was calculated by [23]. For one article (Tormala 2008), data was given separately in terms of equol producers and non-equol producers [19]. Therefore, combining both data into one mean and standard deviation was calculated by and [23].
The standardized mean difference (SMD) was used to synthesize the results, which was , where [23]. Because the outcome measurements were assessed using different methodologies, results across all of the studies were standardized to a uniform scale before being combined. Therefore, the SMD demonstrated the size of the treatment effect relative to the variability observed for each study. A summary SMD statistic was calculated by a random effects model. For the systemic arterial compliance outcome, we multiplied its SMD by −1 to make the direction of results consistent with the other three outcomes. Therefore, negative values represented a positive effect of the treatment intervention. Heterogeneity across studies was assessed and the I2 statistic was calculated to describe the percentage of variation across each study that may be due to heterogeneity than chance. A forest plot was generated for each of the four outcomes individually before the data was pooled together for overall analyses. In addition, a funnel plot was generated to identify potential biases or systematic heterogeneity.
To assess whether the effect of soy isoflavones on arterial stiffness differed by various study characteristics, a subgroup analysis was conducted by intervention duration (<6 weeks vs. ≥6 weeks) and gender (women only vs. men only vs. combined). The SMD, 95% CI, and I2 statistics were calculated and a forest plot was generated. A sensitivity analysis was also conducted to determine if there was a difference in overall SMD between the four measurements of outcomes. For the three studies with two outcome measurements, data for one measurement of outcome was removed at a time to determine its effect on overall results [16–18]. The removal process was then applied to the other outcome measurement. All analysis was conducted by Cochrane’s Review Manager 5 program with a p-value <0.05 [23].
Results
The initial search yielded a total of 998 citations (548 in PubMed, 449 in Embase, and 1 in clinicaltrials.gov) (Fig. 1). After an initial exclusion of 90 duplicate articles, 908 citations were reviewed. Of the 908 remaining articles, 896 were excluded for the following reasons: not human subjects (n=127); unrelated to soy isoflavones (n=474); unrelated to arterial stiffness (n=184); and not a randomized controlled clinical trial (n=111). During the screening of the remaining twelve full-text articles, an additional four were excluded because the outcome studied was unrelated to arterial stiffness. These exclusions left eight articles for full review and assessment.
Fig 1.
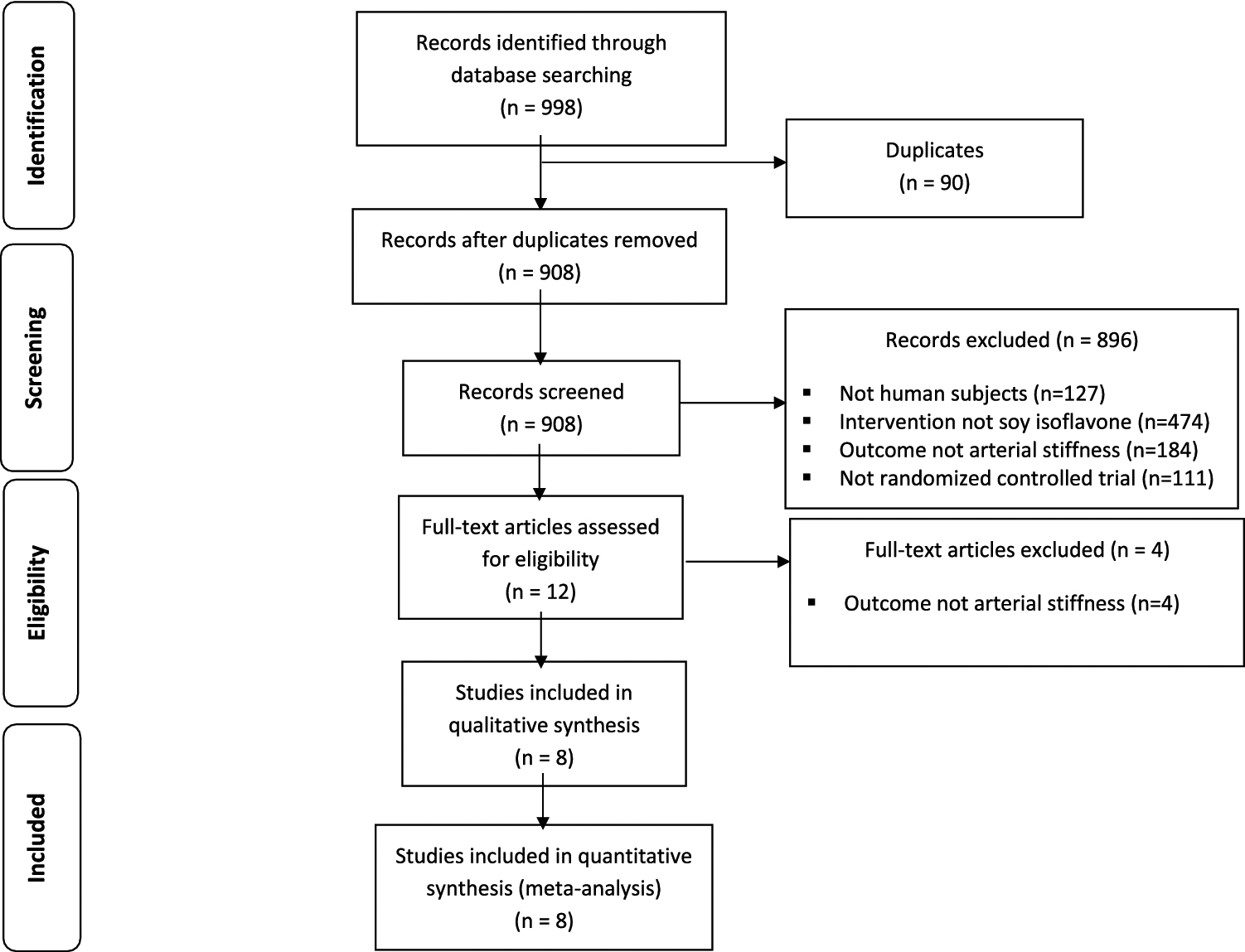
Flowchart documenting the process of article selection through identification, screening, exclusion, and inclusion phases.
The study characteristics, outcome measurements and results are summarized in Tables 1 and 2. Seven studies were crossover design and had washout periods lasting 0 to 4 weeks [13–16, 18–20] while one study was parallel design [17]. Two studies included only women [14, 19], one study included only men [13], and five studies included both women and men [15–18, 20]. Across all studies, there were 276 women and 209 men with participants primarily in their 40s and 50s. Though our publication search parameters were large (1966–2019), included studies were all published between 1997 and 2017. Two studies were based in the United States, three in Australia, while one study in each of Finland, Japan, and the United Kingdom. Intervention duration ranged from 24 hours to 12 weeks. Intervention dose ranged from 10 mg to 85 mg. Soy isoflavones were administered through either tablet, powder (beverage), or food (nuts) form.
Table 1:
Study Characteristics
| Study (First Author, Year Published) | Study Location | Study Designa | Sample size (women/men) | Study Population | Age (yr) | Blood Pressure level | Intervention Duration (weeks) | Intervention Dose | Washout Period (Weeks) |
|---|---|---|---|---|---|---|---|---|---|
| Nestel, 1997 [14] | Adelaide, Australia | PC, CO | n=21 (21/0) | Menopausal and post-menopausal women | Mean=54.0 | Normal | 5 days | Tablet containing 80 mg soy isoflavone (aglycone) with 45 mg of genistein | 0 |
| Teede, 2001 [17] | Clayton, Australia | DB, PC, P | n=213 (108/105) | Healthy men and post-menopausal women | 50.0–75.0 | Normal | 12 | Soy protein isolate containing 40 g soy protein, 118 mg soy isoflavones | N/A |
| Teede, 2003 [IS] | Melbourne, Australia | DB, PC, CO | n=80 (34/46) | Healthy men and post-menopausal women | 45.0–75.0 | Normal | 6 | Two daily soy isoflavone tablets containing 40 mg of genistein and daidzein (80 mg total) | 1 |
| Tormala, 2008 [19] | Helsinki, Finland | PC, CO | n=40 (40/0) | Post-menopausal women | Mean=57.7 | Normal | 8 | Soy drink containing 112 mg soy isoflavones expressed as aglycone | 4 |
| Usui, 2013 [20] | Kyoto, Japan | DB, PC, CO | n=54 (38/16) | Overweight or obese men and post-menopausal women | Mean=59.4 | Metabolic Syndromec | 12 | 10 mg S-equol tablets (expressed as aglycones equivalent) containing daidzein, genistein, and glycitein | 0 |
| Reverri, 2015 [15] | California, USA | PC, CO | n=17 (12/5) | Post-menopausal women and men at cardiometabolic risk | >45.0 | Metabolic Syndromec | 4 | Soy nuts containing 101 aglycone equivalent (55 mg of genistein, 42 mg of daidzein, and 4 mg of glycitein) | 2 |
| Hazim, 2016 [13] | Norwich, United Kingdom | DB, PC, CO | n=28 (0/28) | Healthy equol producing (n=14) and non-equol producing men (n=14) | 50.0–75.0 | N/A | 24 hours | Soy isoflavone capsule containing 80-mg aglycone equivalents of daidzein and genistein | 1 |
| Richter, 2017 [16] | Pennsylvania, USA | PC, CO | n=20 (11/9) | Pre- and post-menopausal women and men with moderately elevated resting blood pressure | 35.0–60.0 | Normal | 6 | Soya protein isolate containing 85 mg of soy isoflavones (expressed as aglycone units) | 2 |
DB, double-blind; PC, placebo-controlled; P, parallel design; CO, crossover design
High normal: Systolic Blood Pressure: 130–139 millimeter of mercury (mmHg) or Diastolic Blood Pressure: 85–89mmHg
Metabolic syndrome criteria: Systolic Blood Pressure >130 mmHg or Diastolic Blood Pressure> 85 mmHg
Table 2:
Study Outcome Results
| Study (First Author, Year Published) | Measurement of Outcomea | Outcome | Placebo | Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baselinee | After Interventione | Baselinee | After Interventione | |||||||
| Mean | SD/SE/CI | Mean | SD/SE/CI | Mean | SD/SE/CI | Mean | SD/SE/CI | |||
| Nestel, 1997 [14] | SAC | Mear difference in systemic arterial compliarce significantly improved at the erd of the treatment period compared with the placebo period (p=0.011) assessed using paried t-test analysis | SAC: 0.57 | SAC: 0.33b | SAC: 0.81 | SAC: 0.40b | SAC: 0.67 | SAC: 0.33b | SAC: 0.99 | SAC: 0.54b |
| Teede, 2001 [17] | SAC, PWV | No significant treatment effect on arterial compliance as assessed by MANOVA ircorpratingSAC and PWV (p=0.1) | SAC: 0.56 PWV: 9.70 |
SAC: (0.49–0.63)d PWV: 0.20c |
SAC: 0.57 PWV: 9.40 |
SAC: (0.52–0.61)d PWV: 0.20c |
SAC: 0.53 PWV: 10.10 |
SAC: (0.49–0.58)d PWV: 0.20c |
SAC: 0.57 PWV: 9.60 |
SAC: (0.52–0.63)d PWV: 0.20c |
| Teede, 2003 [18] | SAC, PWV | Isoflavone intervention significantly improved arterial stiffness with systemic arterial compliance compared to placebo (P=0.04) and with PWV (p=0.02) assessed using repeated-measures ANOVA, Bonferroni correction | SAC: 0.56 PWV: 8.53 |
SAC: 0.02c PWV: 0.10c |
SAC: 0.54 PWV: 8.71 |
SAC: 0.02c PWV: 0.20c |
SAC: 0.52 PWV: 8.69 |
SAC: 0.02c PWV: 0.20c |
SAC: 0.57 PWV: 8.39 |
SAC: 0.02c PWV: 0.20c |
| Tormala, 2008 [19] | AI | No significant treatment effect on augmentation index assessed using paired t-test | EPS AI: 25.90 Non-EPs AI: 29.60 |
EPsAI: 1.10c Non-EPs AI: 0.90c |
EPS AI: 23.80 Non-EPs AI: 29.50 |
EPs AI: 5.10c Non-EPs AI: 1.50c |
EPs AI: 25.90 Non-EPs AI: 29.60 |
EPs AI: 1.10c Non-EPs AI: 0.90c |
EPs AI: 23.40 Non-EPs AI: 29.10 |
EPs AI: 1.40c Non-EPs AI: 1.20c |
| Usui, 2013 [20] | CAVI | Natural equol intervention significantly improved CAVI score (P < 0.01) compared to the placebo group assessed using paired t-test | CAVI: 7.80 | CAVI: 0.20c | CAVI: 7.90 | CAVI: 0.20c | CAVI: 8.00 | CAVI: 0.20c | CAVI: 7.70 | CAVI: 0.20c |
| Reverri, 2015 [15] | AI | Change in augmentation index (%) significantly improved between intervention compared to the control (P=0.03) assessed using a linear mixed model | AI: 23.80 | AI: 16.50b | AI: 23.50 | AI: 4.10c | AI: 25.20 | AI: 20.30b | AI: 19.70 | AI: 4.10c |
| Hazim, 2016 [13] | PWV | PWV significantly improved in Equol producers at 24 h [PWV change from 0 h: isoflavone, −0.2+/− 0.2 m/s; placebo, 0.6+/− 0.2 m/s; P <0.01) assessed using independent t-tests | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Richter, 2017 [16] | Al, PWV | No significant treatment effect on augmentation index (p=0.59) or PWV (p=0.84) assessed using MIXED procedure | AI: 24.70 PWV: 7.80 |
AI: 2.70c PWV: 0.20c |
AI: 27.20 PWV: 7.7 |
AI: 1.60c PWV: 0.20c |
AI: 24.70 PWV: 7.80 |
AI: 2.70c PWV: 0.20c |
AI: 27.50 PWV: 7.8 |
AI: 1.50c PWV: 0.20c |
SAC, systemic arterial compliance; PWV, pulse-wave velocity; AI, augmentation index; CAVI, cardio-ankle vascular index; EPs, equol producers; Non-EPs, non-equol producers
standard deviation
standard error of mean
confidence interval
Units: SAC (mm/Hg), PWV (m/s), AI (%), CAVI (unit)
The funnel plot of each of the four subgroup measurements of outcomes (PWV, SAC, AI, and CAVI) showed a low risk of publication bias as most data points were precise and close to the center. (Fig. 2) The primary result of the quality assessment showed an overall low risk of bias for each study. One study had three criteria with a high risk of bias [15] while the other seven studies had either one [16, 17, 20] or two [13, 14, 18, 19] out of the seven criteria (Fig. 3). The one parallel design trial had a higher risk of performance bias [17]. One study in particular only reported positive results for a subgroup (equol producers) at a specific time period rather than complete results over the entirety of the intervention period [13].
Fig 2.
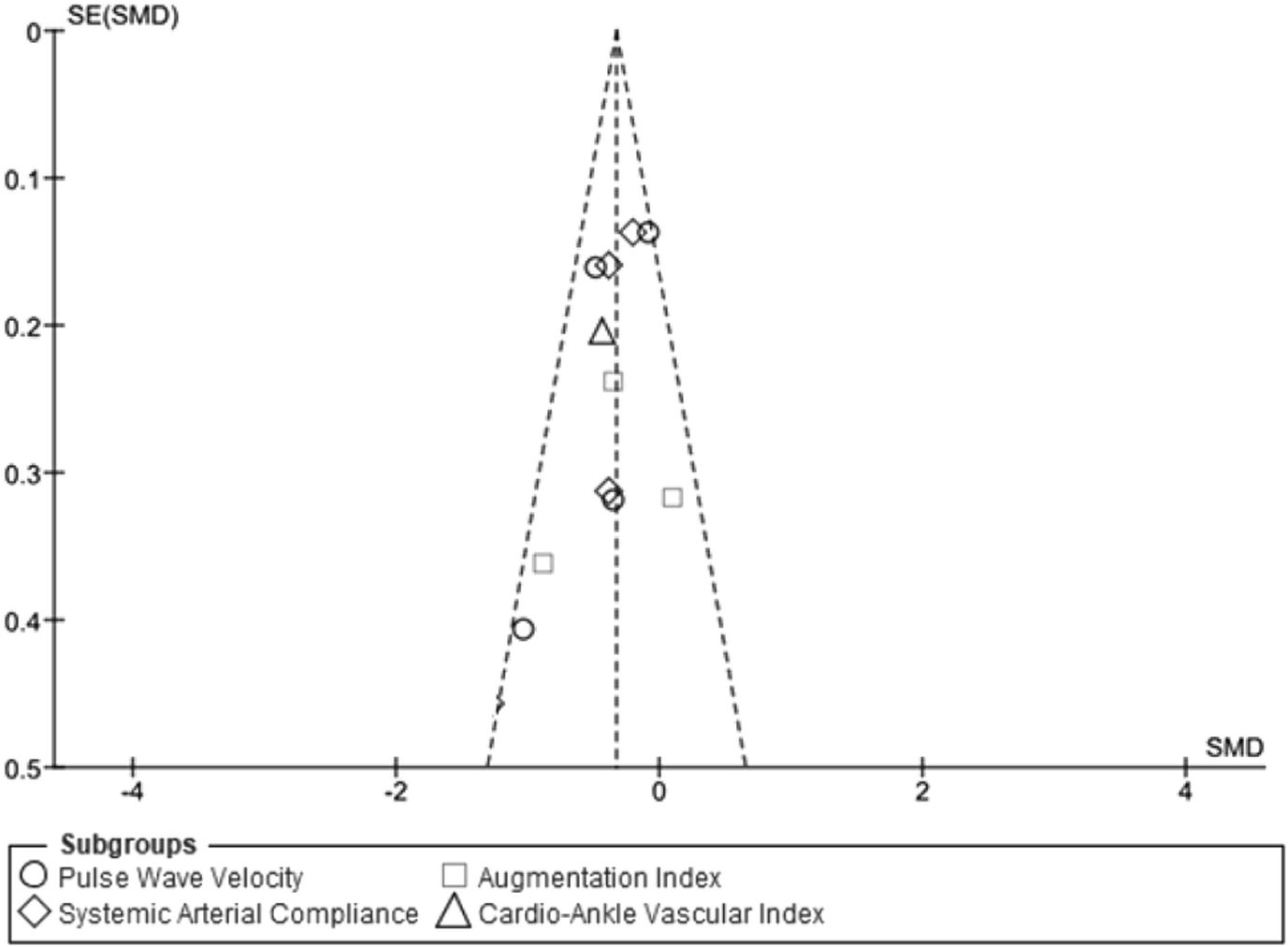
Funnel plot of each of the four subgroup measurements of outcomes (pulse wave velocity, arterial compliance, augmentation index, cardio-ankle vascular index score) to identify potential publication bias.
Fig 3.
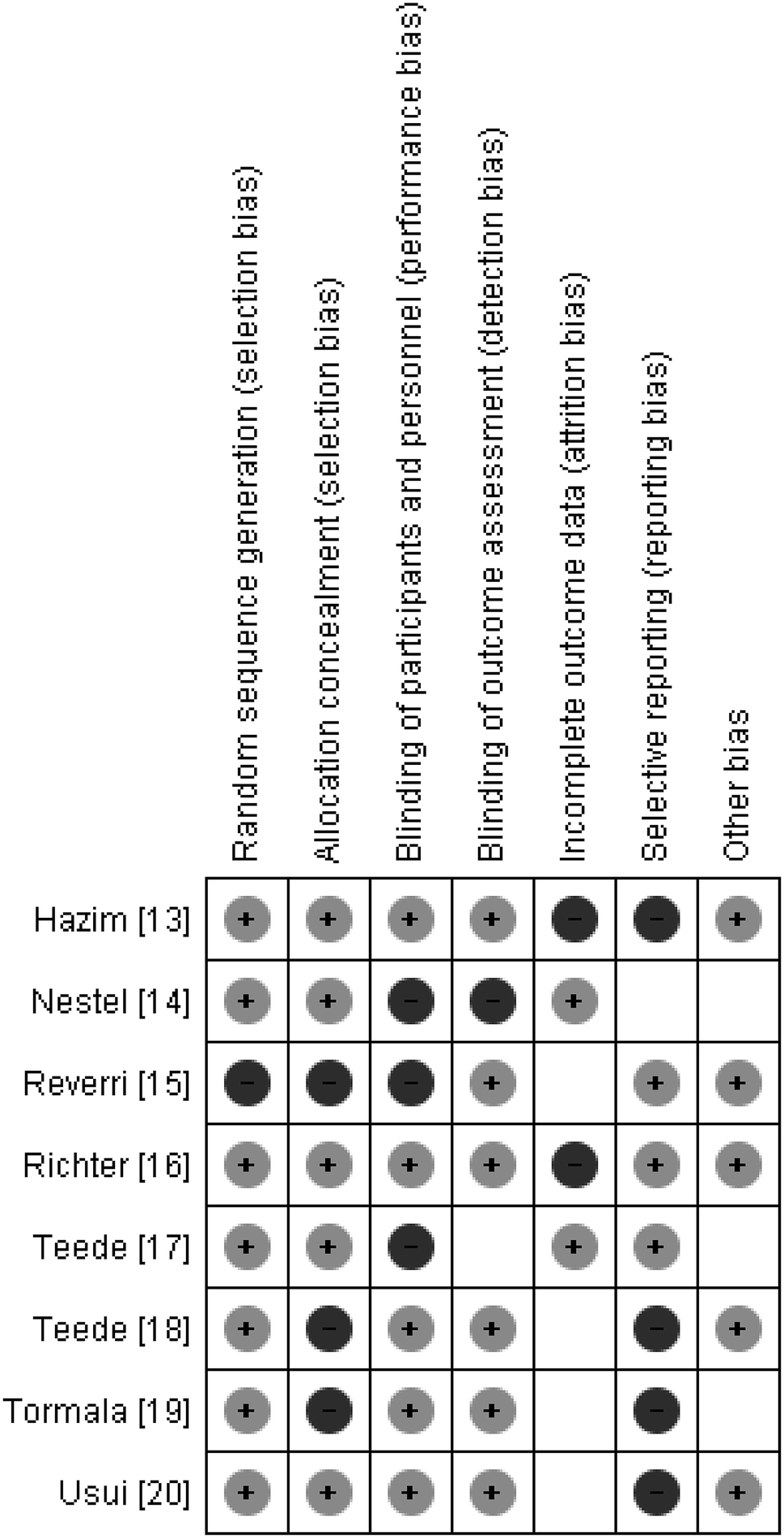
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study
Overall, the results showed a positive effect of soy isoflavones on arterial stiffness (Fig. 4). The overall SMD was −0.33 (95% CI: −0.47, −0.19). A statistically effect was observed for each of PWV (SMD: −0.38, 95% CI: −0.71, −0.05), SAC (SMD: −0.29, 95% CI: −0.49, −0.10), and CAVI (SMD: −0.43, 95% CI: −0.83, −0.02), and a marginal effect was observed for AI (SMD: −0.36, 95% CI: −0.85, 0.13). The overall I2 was 19%.
Fig 4.
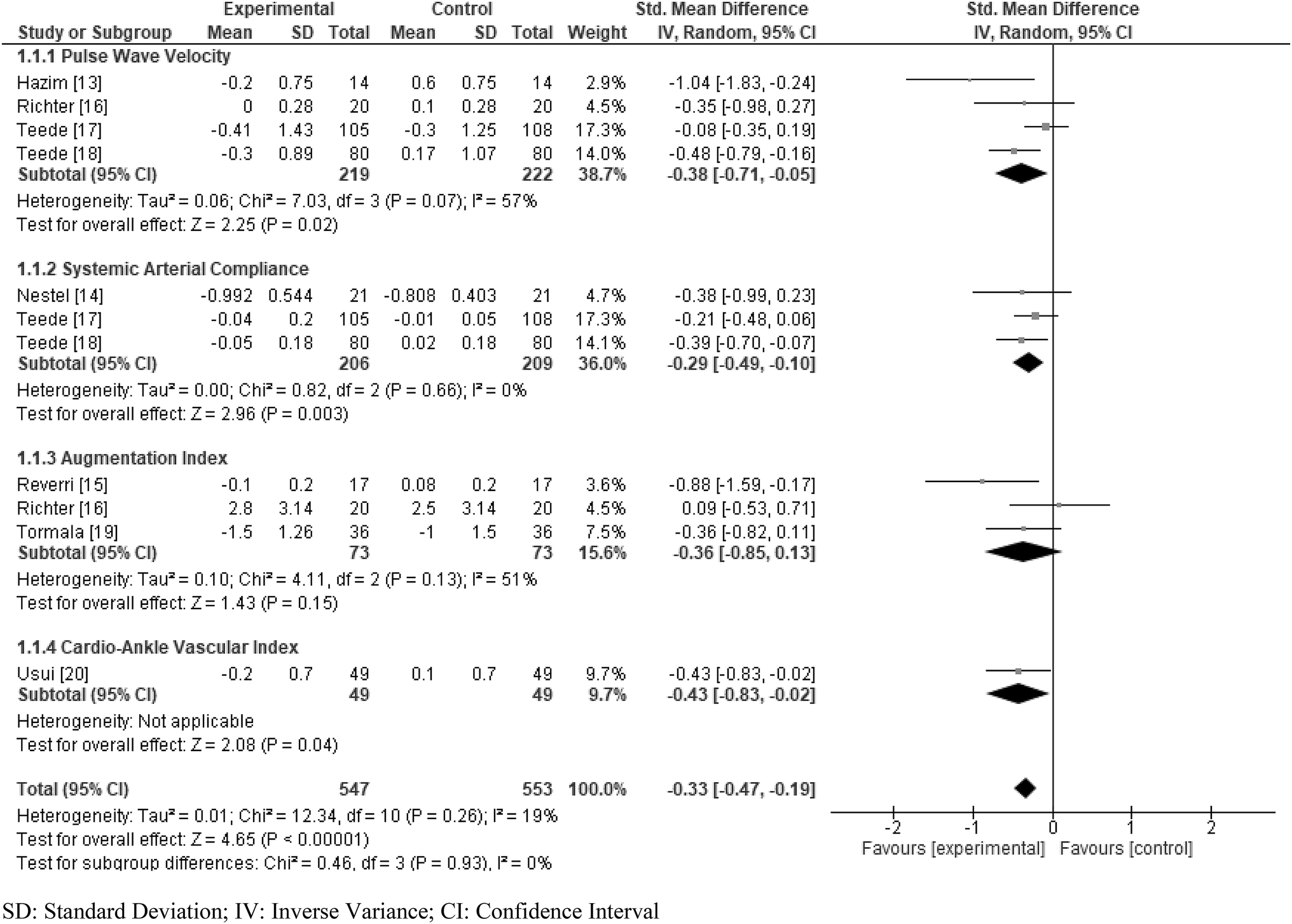
Standardized mean difference (SMD) in arterial stiffness was presented for each study, displayed by four measurements of outcomes (pulse wave velocity, arterial compliance, augmentation index, cardio-ankle vascular index score). The summary SMD was calculated by a random effects model.
The subgroup analysis showed no difference in treatment effects of soy isoflavones on arterial stiffness by intervention duration (<6 weeks and ≥6 weeks) and gender (women only studies, combined gender studies, and the men only study) (Fig. 5). Additionally, the sensitivity analysis showed no difference in overall SMD value between PWV, CAVI, AI, and SAC. (Online Resource Figure 1a through 1f)
Fig 5.
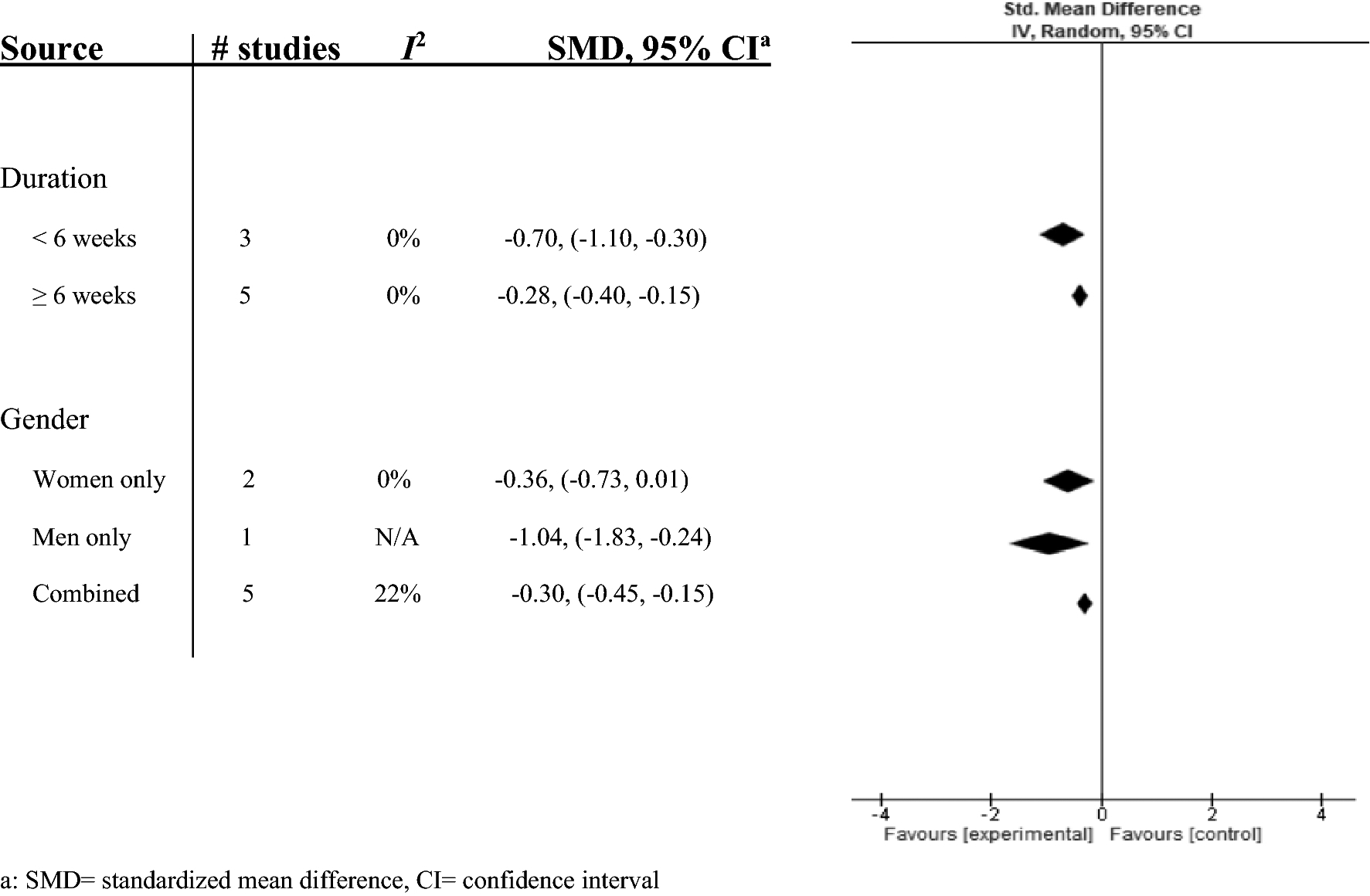
Subgroup analysis by intervention duration and gender was calculated with the SMD and 95% CI and displayed by a forest plot.
Discussion
This systematic review and meta-analysis of RCTs found that supplementation of soy isoflavones reduced arterial stiffness. The subgroup analysis showed the effect was not different by intervention duration or gender. The sensitivity analysis also showed no difference in the effect among the four outcome measures, leading to the same conclusion of a beneficial effect of soy isoflavones on arterial stiffness. This study is the first systematic review and meta-analysis to assess the effect of soy isoflavones on arterial stiffness.
The potential risk of bias among included RCTs was minimal as a majority of criteria were marked as low risk of bias. However, possible explanations for some studies marked as high risk of bias are warranted. A few studies experienced participant withdrawals which could have led to a higher risk of attrition bias [13, 16]. In the Hazim et al. study [13], incomplete data could have led to a higher risk of reporting bias. Additionally, one study was funded by a pharmaceutical company [20]. Limitations potentially leading to publication bias include the restriction to studies written in English as well as unpublished data was not sought out.
The rise of noninvasive methods has seen an increased usage of arterial stiffness measurements [3, 11]. PWV is the rate at which pressure waves, generated by the systolic contraction of the heart, moves along the arterial tree [24]. PWV is also known to predict cardiovascular end points independent of blood pressure and other risk factors such as blood lipid profile, diabetes, and smoking [12]. CAVI is a measurement that reflects the stiffness of the ascending aorta to the ankle arteries, largely independent of blood pressure [25]. SAC is measured by ultrasound as the relationship between pressure (carotid artery) and volume (outflow into aorta) [26]. AI is a measurement derived from the ascending aortic pressure waveform [27]. Each of the four measurement of outcomes pointed towards a positive effect of soy isoflavones on the reduction of arterial stiffness. The effect of soy isoflavones was observed in PWV, CAVI, and SAC. Although the effect in AI did not reach statistical significance, it showed a positive effect and the I2 value for heterogeneity did not differ between each outcome (Fig. 4).
The biological mechanisms by which soy isoflavones reduce arterial stiffness are currently not fully understood. However, preclinical studies indicate three possible explanations for this association: anti-hypertensive, anti-oxidant, and anti-inflammatory effects. Isoflavones display an anti-hypertensive effect by increasing nitric oxide (NO) production in endothelial cells [28]. In addition, isoflavones can bind to estrogen receptor beta and mimic the effect of estrogen [29, 30]. This effect leads to increased endothelial NO synthesis [31]. Isoflavones also confer anti-hypertensive effects by direct inhibition of the angiotensin-converting enzyme (ACE) [28]. ACE helps to produce vasoconstrictive angiotensin (Ang) II from Ang I in the reninangiotensin-aldosterone system. Isoflavones exert an anti-oxidative effect by direct scavenging reactive oxygen species and reactive nitrogen species, as well as reacting with the peroxidation products of macromolecules [32]. Isoflavones are able to induce a response by activating the nuclear factor erythroid 2-related factor 2/antioxidant response element protein (ARE) pathway, which regulates ARE-mediated transcriptions of various genes encoding antioxidant enzymes [31, 32]. Finally, isoflavones directly inhibit nuclear factor kappa-light-chain-enhancer of activated B cells, which is a transcription factor that simulates the encoding of several inflammatory genes [31, 32]. Isoflavones also stimulate peroxisome proliferator-activated receptor gamma or Sirtuin-1 mediated signaling, and interfere with transforming growth factor alpha-induced mitogen-activated protein kinases signaling transductions, resulting in the repression of inflammation [32]. Some RCTs of soy isoflavones in human reported that soy isoflavones have anti-hypertensive [33], anti-oxidant [34, 35], and inflammatory effects [36–39]. Future research is needed to understand the mechanisms behind the potential anti-hypertensive, anti-oxidant, and anti-inflammatory effects of soy isoflavones and its further effect on reducing arterial stiffness.
Currently, arterial stiffness is recognized as an important factor in the pathophysiology of adverse cardiovascular outcomes such as CHD and stroke as well as all-cause mortality independent of cardiovascular risk factors [1, 3, 12]. Moreover, epidemiological evidence is accumulating of the association of arterial stiffness with impaired cognitive function [1]. We have reported cross-sectional and longitudinal associations of arterial stiffness with deposition of cerebral β -amyloid in the brain by Pittsburgh Compound B [40, 41]. We have also reported that among nondemented elderly, arterial stiffness has a positive association with incident dementia from the Cardiovascular Health Study [2]. Furthermore, our recent systematic review and meta-analysis showed that supplementation of soy isoflavones improved cognition [42]. However, it remains unknown whether reduction in arterial stiffness is translated to the reduction in these outcomes.
The current study results should be interpreted in light of several key strengths and limitations. Strengths include that the review was performed with a systematic methodology and the quantitative analysis standardized the results across each study. Previous systematic reviews in the same field have also provided a firm basis for our research [21]. Limitations include small sample sizes, short intervention duration, varied intervention dose, characteristics between study population (gender, weight, equol producing), and modality of intervention (tablet, drink, nuts). These factors may have affected standardization when combining individual study results, but we accounted for this using a random effects model. Of note, two studies (Nestel 1997 and Hazim 2016) had very short durations of intervention (5 days and 24 hours). However, when excluding these two studies, the effect of soy isoflavones on arterial stiffness remained (data not shown). Generalizability could not be applied to other areas including intervention duration, sample size and gender ratios, and washout periods. Additional limitations include differences in measurements of outcomes used in each study and therefore, the reflection of different arterial mechanisms. Furthermore, some outcome measurements (e.g., AI) may not be the ideal measure of arterial stiffness. Though some of these study characteristics vastly differed, the I2 measurement for heterogeneity was consistently low to moderate.
This systematic review and meta-analysis revealed that supplementation of soy isoflavones had a positive effect towards reducing the stiffness of the arteries compared with the control group. However, short-duration of intervention and small number of participants were major limitations. Because arterial stiffness has associations with cardiovascular disease, dementia and all-cause mortality independent of its risk factors, trials with large number and longer intervention duration are important to investigate whether supplementation of soy isoflavones actually improves these outcomes.
Supplementary Material
Online Resource Figure 1a-f. Standardized mean difference (SMD) in arterial stiffness was presented for each study, displayed by four measurements of outcomes. Studies with multiple measurements of outcomes were removed individually to determine their effect on overall results. The summary SMD was calculated by a random effects model.
Acknowledgements
The present systematic review and meta-analysis is funded in part by the National Institutes of Health (NIH) grants (NIEHS) R21 ES029734 and (NIA) RF1 AG051615.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
The authors declare no conflicts of interest in relation to this research.
References
- 1.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. (2015) Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension (Dallas, Tex: 1979). 66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui C, Sekikawa A, Kuller LH, Lopez OL, Newman AB, Kuipers AL, et al. (2018) Aortic Stiffness is Associated with Increased Risk of Incident Dementia in Older Adults. Journal of Alzheimer’s disease: JAD. 66(1):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology. 55(13):1318–27. [DOI] [PubMed] [Google Scholar]
- 4.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. (2006) Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 113(5):657–63. [DOI] [PubMed] [Google Scholar]
- 5.Messina M (2016) Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients. 8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Ge X, Tian X, Zhang Y, Zhang J, Zhang P (2013) Soy isoflavone: The multipurpose phytochemical (Review). Biomedical reports. 1(5):697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S (2007) Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. The American journal of clinical nutrition. 85(4):1148–56. [DOI] [PubMed] [Google Scholar]
- 8.Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, et al. (2012) Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutrition, metabolism, and cardiovascular diseases: NMCD. 22(6):463–70. [DOI] [PubMed] [Google Scholar]
- 9.Zhuo XG, Melby MK, Watanabe S (2004) Soy isoflavone intake lowers serum LDL cholesterol: a meta-analysis of 8 randomized controlled trials in humans. The Journal of nutrition. 134(9):2395–400. [DOI] [PubMed] [Google Scholar]
- 10.Uemura H, Katsuura-Kamano S, Nakamoto M, Yamaguchi M, Fujioka M, Iwasaki Y, et al. (2018) Inverse association between soy food consumption, especially fermented soy products intake and soy isoflavone, and arterial stiffness in Japanese men. Scientific reports. 8(1):9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. (2017) Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension (Dallas, Tex: 1979). 69(6):1045–52. [DOI] [PubMed] [Google Scholar]
- 12.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C (2012) Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension (Dallas, Tex: 1979). 60(2):556–62. [DOI] [PubMed] [Google Scholar]
- 13.Hazim S, Curtis PJ, Schär MY, Ostertag LM, Kay CD, Minihane AM, et al. (2016) Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. American Journal of Clinical Nutrition. 103(3):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nestel PJ, Yamashita T, Sasahara T, Pomeroy S, Dart A, Komesaroff P, et al. (1997) Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arteriosclerosis, thrombosis, and vascular biology. 17(12):3392–8. [DOI] [PubMed] [Google Scholar]
- 15.Reverri EJ, LaSalle CD, Franke AA, Steinberg FM. (2015) Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Molecular nutrition & food research. 59(2):323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter CK, Skulas-Ray AC, Fleming JA, Link CJ, Mukherjea R, Krul ES, et al. (2017) Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: a dose-response randomised controlled trial. The British journal of nutrition. 117(10):1403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP (2001) Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 86(7):3053–60. [DOI] [PubMed] [Google Scholar]
- 18.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ (2003) Isoflavones reduce arterial stiffness: A placebo-controlled study in men and postmenopausal women. Arteriosclerosis, thrombosis, and vascular biology. 23(6):1066–71. [DOI] [PubMed] [Google Scholar]
- 19.Tormala R, Appt S, Clarkson TB, Groop PH, Ronnback M, Ylikorkala O, et al. (2008) Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis. 198(1):174–8. [DOI] [PubMed] [Google Scholar]
- 20.Usui T, Tochiya M, Sasaki Y, Muranaka K, Yamakage H, Himeno A, et al. (2013) Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clinical endocrinology. 78(3):365–72. [DOI] [PubMed] [Google Scholar]
- 21.Pase MP, Grima NA, Sarris J (2011) Pulse Wave Velocity Measurement. Journal of medical and biological engineering. 35(5):555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2015. [Available from: http://www.prisma-statement.org/.
- 23.Collaboration TC. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 In: Higgins JPTGS, editor. 2011. p. http://handbook.cochrane.org. [Google Scholar]
- 24.Pereira T, Correia C, Cardoso J (2015) Novel Methods for Pulse Wave Velocity Measurement. Journal of medical and biological engineering. 35(5):555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushita K, Ding N, Kim ED, Budoff M, Chirinos JA, Fernhall B, et al. (2019) Cardio-ankle vascular index and cardiovascular disease: Systematic review and meta-analysis of prospective and cross-sectional studies. Journal of clinical hypertension (Greenwich, Conn). 21(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laskey WK, Parker HG, Ferrari VA, Kussmaul WG, Noordergraaf A (1990) Estimation of total systemic arterial compliance in humans. Journal of applied physiology (Bethesda, Md: 1985). 69(1):112–9. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ (2000) The influence of heart rate on augmentation index and central arterial pressure in humans. The Journal of physiology. 525 Pt 1:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilamand M, Kelaiditi E, Guyonnet S, Antonelli Incalzi R, Raynaud-Simon A, Vellas B, et al. (2014) Flavonoids and arterial stiffness: promising perspectives. Nutrition, metabolism, and cardiovascular diseases: NMCD. 24(7):698–704. [DOI] [PubMed] [Google Scholar]
- 29.Morito K, Aomori T, Hirose T, Kinjo J, Hasegawa J, Ogawa S, et al. (2002) Interaction of phytoestrogens with estrogen receptors alpha and beta (II). Biological & pharmaceutical bulletin. 25(1):48–52. [DOI] [PubMed] [Google Scholar]
- 30.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. (2001) Interaction of phytoestrogens with estrogen receptors alpha and beta. Biological & pharmaceutical bulletin. 24(4):351–6. [DOI] [PubMed] [Google Scholar]
- 31.Goszcz K, Duthie GG, Stewart D, Leslie SJ, Megson IL (2017) Bioactive polyphenols and cardiovascular disease: chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br J Pharmacol. 174(11):1209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Tsao R (2016) Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Current Opinion in Food Science. 8:33–42. [Google Scholar]
- 33.Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, et al. (2010) Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. Journal of hypertension. 28(10):1971–82. [DOI] [PubMed] [Google Scholar]
- 34.Wiseman H, O’Reilly JD, Adlercreutz H, Mallet AI, Bowey EA, Rowland IR, et al. (2000) Isoflavone phytoestrogens consumed in soy decrease F2-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 72(2):395–400. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhang H (2017) Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food & function. 8(8):2935–44. [DOI] [PubMed] [Google Scholar]
- 36.Charles C, Yuskavage J, Carlson O, John M, Tagalicud AS, Maggio M, et al. (2009) Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause (New York, NY). 16(2):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson JF, Ryan MF, Gibney ER, Brennan L, Roche HM, Reilly MP (2014) Dietary isoflavone intake is associated with evoked responses to inflammatory cardiometabolic stimuli and improved glucose homeostasis in healthy volunteers. Nutrition, metabolism, and cardiovascular diseases: NMCD. 24(9):996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins DJ, Kendall CW, Connelly PW, Jackson CJ, Parker T, Faulkner D, et al. (2002) Effects of high- and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism: clinical and experimental. 51(7):919–24. [DOI] [PubMed] [Google Scholar]
- 39.Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA (2006) Soy isoflavones modulate immune function in healthy postmenopausal women. The American journal of clinical nutrition. 83(5):1118–25. [DOI] [PubMed] [Google Scholar]
- 40.Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, et al. (2013) Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 81(19):1711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, et al. (2014) Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA neurology. 71(5):562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui C, Birru RL, Snitza BE, Ihara M, Kakuta C, et al. (2020) Effects of soy isoflavones on cognitive function: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource Figure 1a-f. Standardized mean difference (SMD) in arterial stiffness was presented for each study, displayed by four measurements of outcomes. Studies with multiple measurements of outcomes were removed individually to determine their effect on overall results. The summary SMD was calculated by a random effects model.


