Abstract
Background:
In rare diseases such as ryanodine receptor 1-related myopathies (RYR1-RM), Health-related Quality of Life (HRQoL) measures are critically important so clinicians and researchers can better understand what symptoms are most important to participants with the ultimate goal of finding tangible solutions for them.
Objectives:
The main objective of this study was to characterize symptoms in individuals with RYR1-RM to inform future research. A secondary objective of this study was to analyze positive and negative sentiments regarding symptoms and treatment effects post N-acetylcysteine (NAC) administration in individuals with RYR1-RM.
Methods:
The study used a mixed-methods design applying methodological triangulation. Qualitative data were collected via semi-structured interviews at three visits to characterize symptoms in individuals with RYR1-RM and to analyze treatment effects. Qualitative data were then transformed into quantitative results to measure the frequency with which each symptom was mentioned by participants.
Results:
Twelve symptoms were identified as areas of interest to participants with RYR1-RM, highlighting fatigue and weakness as key symptoms. Data transformation categorized more than 1000 citations, reporting a greater number of positive comments for post-intervention interviews when compared to baseline and pre-intervention visits, and that NAC group participants stated more positive comments than placebo group regarding treatment effect.
Conclusions:
We present a comprehensive characterization of symptoms in RYR1-RM and how those symptoms influence HRQoL. Besides, the introduction of mixed-methods may be a valuable way to better understand patient-centered data in rare diseases to support affected individuals in coping with their symptoms.
Keywords: Mixed methods, Data transformation, Sentiment analysis, Neuromuscular disorders, Health-related quality of life
1. Introduction
In recent years, a burgeoning transition has taken place from traditional clinical research methods that are centered on study goals to the inclusion of affected individuals’ input in the research process. This change emphasizes a direct approach to measuring how affected individuals feel and function and highlights the value of patient-centered data, not only for developing Patient-Reported Outcome (PRO) Measures (2009) [1], but also for drug development (2018) [2].
Patient-centered data and research are becoming increasingly recognized and are informing regulatory decisions, health policies, research design, and research cost analyses [3]. Also, they provide important insights into affected individuals’ experience as it relates to their functional outcomes, responses to disease symptoms or treatment side effects, and/or an overall assessment of Quality of Life (QoL). Health-related Quality of Life (HRQoL) looks at these facets through the lens of disease and medical care.
Where treatments have not yet been discovered, HRQoL measures are particularly important so clinicians and researchers can better understand what disease effects are most important to participants with the ultimate goal of finding tangible solutions for them [4,5]. Additionally, in smaller populations such as rare diseases, PRO trial endpoints become more critical in targeting the most significant symptoms [6]. Therefore, selecting appropriate PRO measures offers a unique added value not only to understand the impact of medical conditions and treatments from the affected individuals’ perspective, but also to ensure any impact of a trial intervention would be comprehensively assessed and meaningful, supporting trial interpretations. This requires the development of specific instruments to address the effects of rare diseases in their limited population rather than using existing generic HRQoL measures. Results from our research will enhance the development of these tools for neuromuscular disorders.
Overall, PROs enrich data to improve future clinical trial methods, study design, participant selection, and biomedical outcomes [7,8]. Furthermore, PROs help enable positive research experiences that enhance future recruitment, another challenge in rare disease [9–11]. However, quantitative PRO data have known limitations in neuromuscular diseases, such as the “disability paradox” (i.e., affected individuals with neuromuscular disease self-report a high QoL on HRQoL scales, whereas their family, caregivers, or external observers report them as having a low QoL) [12–15]. It is therefore important to add qualitative analyses to studies to better understand this issue.
Mixed-methods analysis, which combines quantitative and qualitative approaches to provide a more comprehensive understanding of data, has been proposed as the best practice to address patient-centered data in rare diseases since this methodology allows the use of data from small sample sizes [16,17]. Recent guidance by the U.S. Food and Drug Administration (FDA) have also provided industry recommendations in ensuring patient perspectives are captured in clinical research [18].
Therefore, we explored the application of mixed-methods to a study of individuals affected with ryanodine receptor 1-related myopathies (RYR1-RM).
RYR1-RM are caused by pathogenic variants in the RYR1 gene, which is highly intolerant to change and encodes the major calcium channel in skeletal muscle [19–21]. RYR1-RM comprise the most common form of congenital myopathy, with a pediatric incidence of > 1:90,000 in the United States [21–23]. Common RYR1-RM clinical manifestations include proximal/axial muscle weakness, delayed motor milestones, impaired mobility, pain, and fatigue [23–29]. Although affected individuals report fatigue as one of the more pervasive symptoms [25], these symptoms vary greatly in severity. Currently there is no FDA-approved treatment for RYR1-RM [20]. For this reason, it was necessary to perform additional research to characterize symptoms, especially regarding treatment effects and symptom alleviation. The application of mixed-methods in RYR1-RM research to incorporate participants’ perspectives on their symptoms may inform future PRO measures and maximize our understanding of RYR1 symptoms. Both qualitative (exploratory primary endpoint) and quantitative (secondary endpoint) PRO methods were employed during a combined natural history study and clinical trial of N-acetylcysteine (NCT02362425) to facilitate an understanding of the affected individuals’ point of view regarding their symptoms and to analyze treatment effects.
2. Materials and methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
The RYR1 N-acetylcysteine (NAC) clinical trial consisted of two components; a prospective natural history assessment and a parallel-group, randomized, double-blind, placebo-controlled trial. The trial incorporated a comparison of quantitative and qualitative PRO data to identify symptoms and subjective improvements post NAC treatment (Harmonic®, BioAdvantex Pharma Inc., Toronto, Ontario, Canada). Given the potential therapeutic efficacy of NAC for RYR1-RM [30–32], NAC or placebo were administered orally for 6 months (adult dose 2,700 mg/d; pediatric dose 30 mg/kg/d). At the conclusion of the trial, no difference in the primary outcome measure for oxidative stress was observed. The six-minute walk test was 24 meters longer on average in the NAC arm, but did not reach statistical significance [20]. The study was conducted at the National Institutes of Health (NIH) Clinical Center, Bethesda, MD, USA between 2015 and 2017. This was the first clinical trial conducted in this patient population. The clinical trial is registered in the U.S. National Library of Medicine (NCT02362425). All procedures were approved by the NIH Combined Neuroscience Institutional Review Board (CNS IRB), and an Independent Monitoring Committee was established to oversee trial safety. All participants and parents of participants < 18 years of age provided written informed consent, according to the Declaration of Helsinki, before enrollment. Assent was also obtained for those < 18 years of age. The total study duration was 18 months and consisted of a six-month natural history assessment, followed by a six-month intervention phase, and a follow-up phone call at 18 months. Participants attended three study visits at the NIH: baseline, pre-intervention, and post-intervention. Randomization was performed at the end of the second visit. For more information regarding the clinical trial, see the following publication [20].
2.2. Study design
An exploratory sequential mixed-methods design with methodological triangulation was employed, QUAL→(quan) [33,34]. The mixed-methods approach was recently recommended by the FDA to incorporate patients’ perspectives in clinical research [18]. Qualitative data were collected via semi-structured interviews at each visit to characterize symptoms in individuals with RYR1-RM and to analyze treatment effects. Thus, individual and collective viewpoints were addressed. The implementation of this design has previously been used in HRQoL and Neuromuscular Disorders (NMD) research [35–37] as well as in symptoms research [38]. Qualitative data were used to assess the importance and depth of comments from the participants. Then data transformation was applied to obtain quantitative results. In this standard procedure of mixed-methods research, investigators take the qualitative themes or codes and counts them to form quantitative measures [34,39]. Therefore, data transformation were used to measure the frequency with which each area of interest was mentioned by participants.
2.3. Study goals
The primary goal of this study was to characterize symptoms in individuals with RYR1-RM to inform future research. As a secondary goal, this study aimed to analyze positive and negative sentiments regarding symptoms and treatment effects post NAC administration in individuals with RYR1-RM.
2.4. Research questions and hypotheses
Q1:
What are the major symptoms in individuals affected with RYR1-RM?
Q2:
How do these symptoms impact individuals affected with RYR1-RM pre- and post- NAC administration?
We hypothesized that data transformation would identify a greater number of positive comments in individuals with RYR1-RM for post-intervention interviews compared to baseline and pre-intervention interviews, and that NAC group participants would mention more positive comments than placebo group participants regarding treatment effect.
2.5. Participants
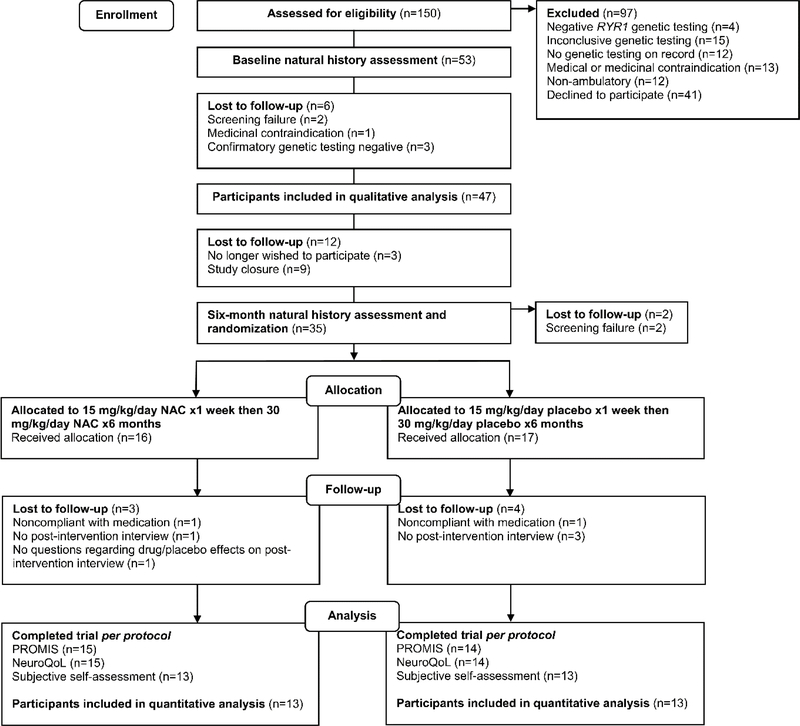
Overall, 150 individuals were screened for participation in this study, of which 53 were eligible and enrolled (Figure 1). Of these, 47 participants completed at least one interview with which we were able to perform qualitative analysis. Therefore, a criterion-i purposeful sampling approach was used [40]. Although 27 post-intervention interviews were performed, in one of those interviews the two specific questions to assess treatment effect were not asked. Therefore, the number of participants to assess treatment effect was actually 26, with 13 in each arm of the study. Demographic information is presented in Table 1.
Fig. 1.
Consort diagram of study flow
Table 1.
Participant demographic information
| Variables | Overall participants | Qualitative analysis | NAC group | Placebo group |
|---|---|---|---|---|
| N | 53 | 47 | 13 | 13 |
| Cohort % | 100% | 89% | 25% | 25% |
| Gender | ||||
| Female | 29 (55%) | 27 (57%) | 8 (62%) | 7 (54%) |
| Male | 24 (45%) | 20 (43%) | 5 (38%) | 6 (46%) |
| Age | ||||
| Total | 29.8 ± 17.6 | 29.6 ± 17.1 | 32.8 ± 16.3 | 23.8 ± 17.6 |
| Adults (>18) |
35 (66%) 39.9 ± 12.7 |
31 (66%) 39.4 ± 12.4 |
9 (69%) 42.4 ± 7.3 |
6 (46%) 39.7 ± 13.2 |
| Peds (<18) |
18 (34%) 10.2 ± 2.7 |
16 (34%) 10.5 ± 2.8 |
4 (31%) 11.0 ± 2.9 |
7 (54%) 10.1 ± 2.0 |
| Ethnicity | ||||
| White | 47 (89%) | 42 (89%) | 12 (92%) | 13 (100%) |
| Black | 6 (11%) | 5 (11%) | 1 (8%) | 0 (0%) |
| Location | ||||
| Northeast | 10 (19%) | 10 (21%) | 1 (8%) | 3 (23%) |
| Southeast | 9 (17%) | 9 (19%) | 5 (39%) | 2 (15%) |
| Midwest | 10 (19%) | 5 (11%) | 2 (15%) | 0 (0%) |
| Southwest | 4 (7 %) | 4 (9 %) | 2 (15%) | 1 (8%) |
| West | 10 (19%) | 10 (21%) | 2 (15%) | 2 (15%) |
| Abroad | 10 (19%) | 9 (19%) | 1 (8%) | 5 (39%) |
2.6. Procedures
2.6.1. Qualitative analysis
A total number of 107 semi-structured interviews were conducted using open-ended questions (supplementary materials). Two post-intervention questions were added at the final visit. Interviews were conducted between March 2015-November 2017 (baseline), September 2015-January 2017 (pre-intervention), and March 2016-June 2017 (post-intervention). Interviews had an average duration of 13 minutes. All interviews were conducted at the NIH by the principal investigator, a research nurse, or a research fellow. All interviews were recorded and transcribed verbatim to conduct content analysis [41] applying a multiphase approach, open-coding and axial-coding [42]. Content analysis usually involves converting qualitative data into a quantitative form throughout frequency counts [39]. This was implemented in our research design, and this procedure is described in the section on data transformation analysis. An initial set of five interviews was analyzed by three researchers to identify common areas of interest (domains/subdomains) and to assess agreement. After confirming agreement, the remaining interviews were distributed among the researchers for analysis. Three additional meetings were conducted to discuss the inclusion of new domains/subdomains until saturation was achieved [43]. Differences in coding were resolved through consensus and tighter definitions of subdomains [44]. Likewise, interpretation and selection of quotes for publication were also discussed to ensure agreement among researchers.
2.6.2. Data transformation and sentiment analyses
To perform data transformation, the number of times each domain/subdomain was mentioned in semi-structured interviews was counted [36]. Those counts were used to calculate the average as well as the percentage of citations for each domain/subdomain. When necessary, percentage scores were normalized to compare domains/subdomains. This procedure was conducted globally (i.e., analyzing all data), by domain (i.e., considering every identified domain independently), by visit (i.e., evaluating data for each visit separately), by treatment (i.e., analyzing treatment effect comments for NAC and Placebo groups independently), and by participant (i.e., assessing the records provided for all participants individually). Additionally, a word frequency analysis was also conducted to identify the most cited words from semi-structured interviews [45].
To conduct sentiment analyses, all comments related to the identified domains/subdomains, were labeled as either “positive” or “negative”. In this process, reports of “no change” regarding treatment effect were categorized as negative sentiments. Very few comments were considered as neutral (i.e., no positive nor negative sentiment) or dual (i.e., positive and negative sentiments at the same time). For this reason, numbers from sentiment analysis slightly differ from the original counts. In order to ensure the same approach when attributing sentiments to participants’ comments, one researcher conducted this task. After completing sentiment analysis, results were reviewed to confirm agreement among researchers.
Nvivo version 12.5 software (QSR International Pty Ltd., Doncaster, Victoria, Australia) was used to perform qualitative research, data transformation, and sentiment analyses.
3. Results
3.1. Qualitative Analysis
Two domains were identified after conducting data analysis: symptoms and post-intervention. Twelve subdomains comprised the symptoms domain: fatigue, fine motor, heat intolerance, mobility, muscle spasms, numbness, pain, respiratory difficulties, rhabdomyolysis (comments related to the destruction of striated muscle cells or elevated levels of creatine kinase not directly linked to any other symptom), scoliosis, weakness, and compounding illness-conditions (impacts of other issues that were not caused by the RYR1 mutation(s), for example, symptoms that worsen their quality of life, like a cold, cancer, etc.). Post-intervention, the identified domain that assessed treatment effect, included two subdomains: treatment benefits and side effects. Below, the most representative quotes for each of these domains/subdomains, selected by their importance and depth (i.e., the best described and the most detailed ones, respectively), are highlighted to exemplify participants’ experiences/opinions (Table 2). As stated before, these quotes were selected through consensus to ensure agreement among researchers.
Table 2.
Transcripts from the most representative domains/subdomains
|
Symptoms > Fatigue: participants reported extreme levels of fatigue, greater than what is typically defined as tiredness and impacting them all day. The most commonly reported ways to minimize this effect were reducing physical activity, increasing breaks to rest and recover, using external support tools, and asking other people for help. Fatigue not only impacted participants’ physical performance, but also restricted social interactions and required psychological adaptations to cope with this effect from the disease. The importance of this symptom highlighted the value of addressing it in order to reduce limitations and improve HRQoL. Additionally, the impact of fatigue was a top target when assessing treatment effects, reemphasizing the significance of this symptom. “Just being drained by the end of the day. Since I work full time, it’s hard to get everything done I need to do at home. Thankfully, he helps me do a lot of the things around the home... A lot of mornings, even when I wake up, I always have to have eight hours sleep or I just can’t function, but I wake up tired... <C32VA_CCP> Ref 1–2 [Dual sentiments (positive and negative)]. |
|
Symptoms > Weakness: participants also highlighted the difficulty in completing several common tasks due to a lack of strength. In addition to reducing their range of movements, this pointed out a need for external support to perform some actions. As before, this physical effect had social and psychological impacts, not only for the participants but also for their family, friends, co-workers, etc. Weakness was closely related to participants’ fatigue as well as mobility, reinforcing its identification as one of the most important symptoms for individuals with RYR1-RM. Weakness was also a top target for treatment assessment. “Not as much as the strength, I would say, because...my muscles get tired first before I get out of breath. That comes with, like, going up the stairs. Like, when I play sports or if I do exercise, like go to the gym, and usually, I can go further, because I’m just, my muscles are getting tired and getting sore rather than me being out of breath and have to catch my breath.” <C26VA_ICC> Ref 2 [Negative sentiment]. |
|
Symptoms > Pain: similarly, to previous symptoms, pain was reported by participants with RYR1-RM as a persistent effect and revealed the impact and difficulty of dealing with symptoms of pain on a regular basis. Pain was described as myalgia, affecting different muscles throughout the body and impairing motor function in daily life activities. This symptom affected not only physical performance but also mood and mental wellbeing. “It’s been a little more difficult because it seems like I’m having a little more weakness and a little more pain, which keeps me from being able to do a little bit more than what I could do before... My legs hurt really bad at movies, where my feet don’t touch the floor... then if it’s, to go shopping like we used to, I can’t walk” <C17VB_MMC> Ref 1–2 [Negative sentiments]. |
|
Post-intervention > Treatment benefits: several participants experienced positive effects after using NAC. The most important benefits were focused on fatigue and weakness. Also, some individuals affected by RYR1-RM reported reduced pain. Positive impacts were not limited to physical effects, providing social and psychological benefits as well. Despite the multiple benefits reported, there were also several participants who described no changes in response to drug treatment. Those comments were categorized as negative sentiments. “Before January, I was taking different things CoQ10 Ubiquinol, and that had seemed to work short-term, but this new thing that we’re on worked a lot better... Considerably better... now I could probably recover quicker” <C20VC_CCP> Ref 1–2 [Positive sentiments/NAC]. “Since I have been on the medicine, the pain seems to have been lessened and more tolerable, and the weakness seems to be somewhat better. But it still exists, but it seems noticeably... since I’ve been on the medicine, I do notice that my recovery has been quicker than normal” <C17VC_MMC> Ref 1–3 [Positive sentiments/NAC]. “Not at all, I think I was on the placebo. Like, I genuinely think that I didn’t notice a single difference... I think I was expecting to have more endurance or something, so like going up the stairs with my friends, but I still felt exactly as weak as before. Still just as tired from my normal life... Yeah, it didn’t do anything” <C45VC_ICC> Ref 1–4 [Negative sentiments/Placebo]. |
|
Post-intervention > Side effects: the only reported observation that was possibly related to the drug (i.e., feeling extreme fatigue), because the others were in placebo group, was described in conjunction with potential benefits, reporting higher energy and more strength in the same comment. This minimized the impact of this finding. Additionally, the remaining two side effects reported by participants were assessed as not serious (i.e., acid reflux and headaches). “I did have two really extreme points in the last six months where I had extreme fatigue and I don’t know if it’s just because it was the weather change or if it was the drug or what, and then I’ve had a shift where I’ve had less fatigue for a couple weeks, too”. <C01VC_CCP> Ref 1 [Dual sentiment (positive and negative)/NAC]. “From the medicine I had? Headaches’ <C24VC_ICC> Ref 1 [Negative sentiment/Placebo]. “Some acid reflux” <C34VC_MMC> Ref 1 [Negative sentiment/Placebo]. |
Researchers’ interpretations of participants’ interviews are labeled with domain and subdomain (e.g., Symptoms > Fatigue). To represent the voice of individuals affected with RYR1-RM, transcripts of participants’ comments are also displayed for each case. A reference code noting de-identified case number, visit, researcher initials, Nvivo transcript reference number, and sentiment assessment follows each quote. For post-intervention interviews, drug assignment it is also included at the end of the reference code. Pseudonyms were used to protect patient identity
3.2. Data transformation and sentiment analyses
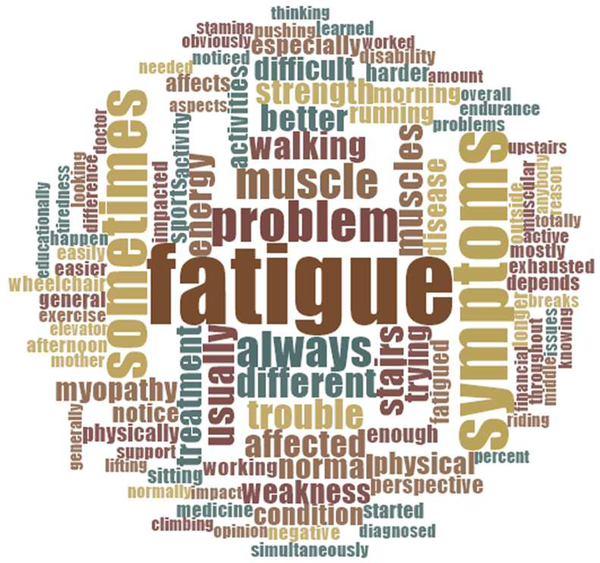
This section counts the number of comments associated with every analyzed domain/subdomain from qualitative analysis. In general, more than 1000 citations were categorized and submitted to sentiment analyses. Results for the symptoms domain are displayed in Figure 2. Results for analyses by visit are provided in Table 3. Results for the post-intervention domain with treatment analysis are displayed in Figure 3. Results for the word frequency analysis are presented through a word cloud image in Figure 4. This word cloud hightlighted the presence of several groups of terms such as “sometimes, always, usually, morning, summer”; “different, trying, difficult, harder, affected”; and “physical, activity, working, playing, sports”. Those words described in detail when, how, and which activities were more limited for participants because of their symptoms.
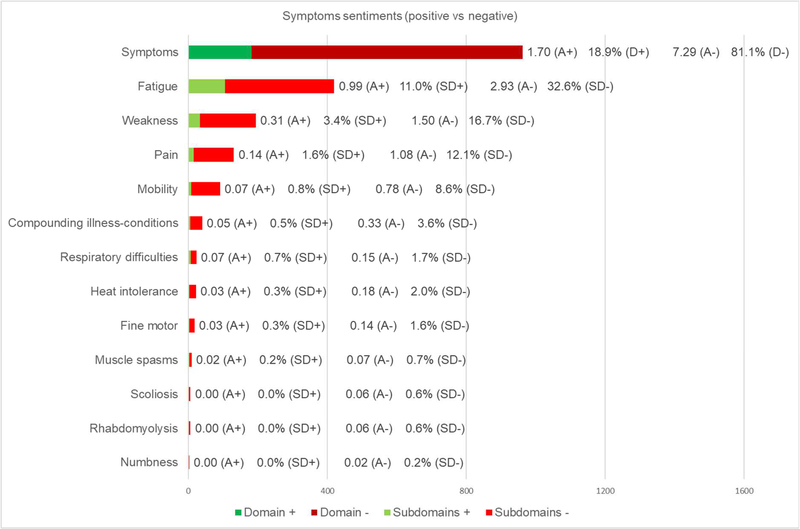
Fig. 2. Data transformation results for symptoms domain (N=107).
Total count of mentions (whole bar) with green colors reflecting positive comments and red colors reflecting negative comments, positive comments average per person (A+), overall positive comments percentage for this domain (D+), negative comments average per person (A-), negative comments percentage for this domain (D-), positive comments percentage for each subdomain (SD+), and negative comments percentage for each subdomain (SD-) are displayed
Table 3.
Results for analyses by visit (N=107)
| Baseline interviews (n=46) |
Pre-intervention interviews (n=34) |
Post-intervention interviews (n=27) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | A+ | SD+ | − | A− | SD− | + | A+ | SD+ | − | A− | SD− | + | A+ | SD+ | − | A− | SD− | |
| Fatigue | 27 | 0.59 | 6.8% | 133 | 2.89 | 33.7% | 19 | 0.56 | 7.5% | 92 | 2.71 | 36.4% | 60 | 2.22 | 19.1% | 89 | 3.30 | 28.3% |
| Weakness | 6 | 0.13 | 1.5% | 75 | 1.63 | 19.0% | 2 | 0.06 | 0.8% | 37 | 1.09 | 14.6% | 25 | 0.93 | 8.0% | 49 | 1.81 | 15.6% |
| Pain | 2 | 0.04 | 0.5% | 40 | 0.87 | 10.1% | 7 | 0.21 | 2.8% | 39 | 1.15 | 15.4% | 6 | 0.22 | 1.9% | 37 | 1.37 | 11.8% |
| Mobility | 2 | 0.04 | 0.5% | 50 | 1.09 | 12.7% | 1 | 0.03 | 0.4% | 17 | 0.50 | 6.7% | 5 | 0.19 | 1.6% | 16 | 0.59 | 5.1% |
| Compounding illness-Conditions | 1 | 0.02 | 0.3% | 19 | 0.41 | 4.8% | 0 | 0.00 | 0.0% | 9 | 0.26 | 3.6% | 4 | 0.15 | 1.3% | 7 | 0.26 | 2.2% |
| Respiratory difficulties | 2 | 0.04 | 0.5% | 5 | 0.11 | 1.3% | 2 | 0.06 | 0.8% | 8 | 0.24 | 3.2% | 3 | 0.11 | 1.0% | 3 | 0.11 | 1.0% |
| Heat intolerance | 2 | 0.04 | 0.5% | 9 | 0.20 | 2.3% | 0 | 0.00 | 0.0% | 7 | 0.21 | 2.8% | 1 | 0.04 | 0.3% | 3 | 0.11 | 1.0% |
| Fine motor | 0 | 0.00 | 0.0% | 12 | 0.26 | 3.0% | 0 | 0.00 | 0.0% | 3 | 0.09 | 1.2% | 3 | 0.11 | 1.0% | 0 | 0.00 | 0.0% |
| Muscle spasms | 0 | 0.00 | 0.0% | 3 | 0.07 | 0.8% | 0 | 0.00 | 0.0% | 4 | 0.12 | 1.6% | 2 | 0.07 | 0.6% | 0 | 0.00 | 0.0% |
| Scoliosis | 0 | 0.00 | 0.0% | 4 | 0.09 | 1.0% | 0 | 0.00 | 0.0% | 2 | 0.06 | 0.8% | 0 | 0.00 | 0.0% | 0 | 0.00 | 0.0% |
| Rhabdomyolysis | 0 | 0.00 | 0.0% | 2 | 0.04 | 0.5% | 0 | 0.00 | 0.0% | 4 | 0.12 | 1.6% | 0 | 0.00 | 0.0% | 0 | 0.00 | 0.0% |
| Numbness | 0 | 0.00 | 0.0% | 1 | 0.02 | 0.3% | 0 | 0.00 | 0.0% | 0 | 0.00 | 0.0% | 0 | 0.00 | 0.0% | 1 | 0.04 | 0.3% |
| Totals per visit | 42 | 0.91 | 10.6% | 353 | 7.67 | 89.4% | 31 | 0.9 | 12.3% | 222 | 6.53 | 87.7% | 109 | 4.0 | 34.7% | 205 | 7.59 | 65.3% |
Number of positive comments per subdomain (+), positive comments average per person in each subdomain (A+), positive comments percentage for each subdomain and visit (SD+), number of negative comments per subdomain (−), negative comments average per person in each subdomain (A−), negative comments percentage for each subdomain and visit (SD−) are displayed
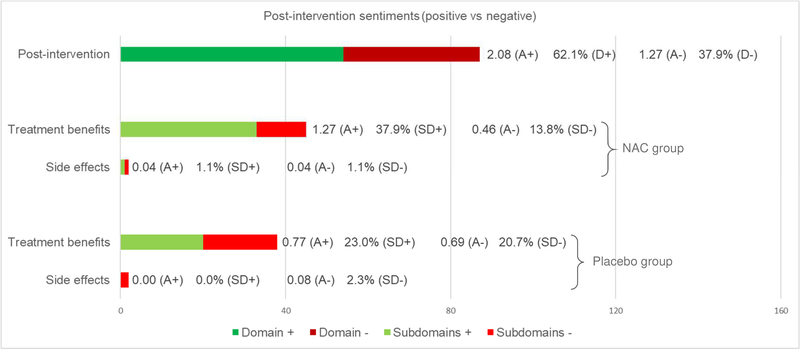
Fig. 3. Data transformation results for post-intervention domain with treatment analysis (N=26).
Total count of mentions (whole bar) with green colors reflecting positive comments and red colors reflecting negative comments, positive comments average per person (A+), overall positive comments percentage for this domain (D+), negative comments average per person (A−), overall negative comments percentage for this domain (D−), positive comments percentage for each subdomain and group (SD+), and negative comments percentage for each subdomain and group (SD-) are displayed
Fig. 4. Word cloud summarizing word frequency analysis (N=107).
Larger font sizes indicate a higher frequency of mentions; smaller font sizes a lower frequency
Examples of results for analysis by participant are available at appendix section (Figures e-1 and e-2).
4. Discussion
According to our sentiment analyses, more than 80% of the comments made by participants, while talking about their symptoms, were related to negative experiences. This shows the impact of the disease on participants regarding their symptoms was mainly negative, clearly reducing their HRQoL [46,47]. However, when considering only post-intervention domain, there was a shift in this trend, displaying 62.1% of positive comments. This trend was more noticeable in the NAC group (39.0% positive vs 14.9% negative) than in the Placebo group (23.1% positive vs 23.0% negative). The analysis by visit reinforced these results showing higher positive and lower negative percentages of comments for post-intervention interviews, compared to baseline and pre-intervention. Additionally, the analysis by participant and treatment on post-intervention domain, revealed that 7 out of 13 participants from the NAC group registered more positive than negative comments, of which 6 reported only one or zero positive comments in baseline and pre-intervention interviews. Regarding the placebo group, only 4 out of 13 participants displayed more positive than negative comments for post-intervention domain, suggesting little positive effect on this group. Those positive reports maybe due to the placebo effect, which was recently studied in NMD [48,49]. Together, qualitative and data transformation results suggested a positive trend regarding the effect of NAC in this context. However, as with the six-minute walk test, this did not reach statistical significance [20]. We should note that participants responses may be biased by positivity and/or social desirability because a study was being done in their rare disease and they felt supported. However, it is important to emphasize that positive comments tripled in number on post-intervention visit compared to baseline and pre-intervention visits.
Based on our qualitative analysis, the most important symptom for individuals with RYR1-RM was fatigue, which is in agreement with documented clinical manifestations of this disease [23,25,50]. Data transformation revealed that fatigue was the most cited symptom, representing more than 40% of the total count, which highlighted its importance and agrees with participant reports. The analysis by visit strengthened these findings, revealing consistent results compared to the rest of the data. Specifically, fatigue was the most cited symptom in all visits and displayed similar percentages of positive (approaching to 7%) and negative statements (approximately 35%) for baseline and pre-intervention interviews. On the other hand, on post-intervention visit, positive comments tripled in number, while negative ones decreased below 30%. Finally, the word frequency analysis exposed the value of terms such as “fatigue/d, stamina, endurance, active, energy, breaks, exhausted, tiredness”, reemphasizing the importance of fatigue as a symptom for individuals affected with RYR1-RM.
Regarding weakness, qualitative analysis revealed this symptom was of secondary importance to participants, which was consistent with previous RYR1-RM research [23,25,27,29,50–54]. Although weakness was mentioned half as many times as fatigue, data transformation yielded approximately 200 quotes suggesting that this too was of high importance to participants. The analysis by visit showed a balanced percentage of negative experiences for all visits (around 16.5%). However, positive statements increased from baseline and pre-intervention compared to post-intervention interviews (from 1.5% and 0.8% to 8%). Also, weakness registered 3.4% out of 18.9% of all positive comments, suggesting that NAC may have also improved strength in participants. Additionally, the word cloud revealed the importance of several words such as “muscle, pushing, strength, weakness, stairs, support, problem, trouble”, highlighting the significance of weakness for participants.
Based on qualitative analysis, pain and mobility were on a third level of importance to patients, after other symptoms. They were closely linked to fatigue and weakness as antecedents. Both symptoms produced limitations on motor function and impacted additional areas (i.e., social, psychological, etc.). As before, there is precedence for pain and impaired mobility in affected individuals with RYR1-RM [23–27]. Although pain was cited more times in the data transformation analysis (131 vs 91), mobility was also described with great detail in qualitative interviews. The analysis by visit displayed a weak trend of more positive and fewer negative comments in post-intervention interviews for mobility. Regarding pain, there was no clear pattern. This finding supported the idea that NAC had little direct effect regarding pain and mobility, which is consistent with the fact that these are not indications for NAC therapy [55]. Based on qualitative data, we considered that positive comments regarding pain and mobility, on post-intervention interviews, may be due to improvements on fatigue and weakness. Finally, the word frequency analysis displayed several terms such as “lifting, sitting, climbing walking, running, upstairs, elevator, and wheelchair” that stressed the value of addressing impaired mobility for this population.
The remaining symptoms, fine motor, heat intolerance, muscle spasms, numbness, respiratory difficulties, rhabdomyolysis, scoliosis, and compounding illness-conditions, captured minimal attention in qualitative analysis. They were described as hand dexterity problems, malignant hyperthermia incidents, non-voluntary muscular contractions, insensitivity feelings, breathing insufficiency, elevated levels of creatine kinase abnormal curvatures of the spine, and additional limitations, all of which were previously reported in RYR1-RM research [23,25,27,29,51–54,56,57]. Compared to fatigue (420), weakness (194), pain (131), and mobility (91), the remining symptoms were clearly less cited: compounding illness-conditions (40), respiratory difficulties (23), heat intolerance (22), fine motor (18), muscle spasms (9), scoliosis (6), rhabdomyolysis (6), and numbness (2). To put this in perspective, all positive and negative citations regarding these symptoms represented 13% of mentions for the whole study, suggesting very little impact regarding those symptoms. In the analysis by visit, respiratory difficulties, fine motor, muscle spasms, and compounding illness-conditions, displayed a trend of more positive and fewer negative quotes for post-intervention interviews. However, the number of comments were very low. Altogether, these findings suggest that these symptoms were linked to individual cases in very few participants.
Whilst prior studies stressed the importance of rhabdomyolysis in RYR1-RM [24,56,57], our data transformation and qualitative results yielded little impact in this symptom. However, it is probable that rhabdomyolysis involvement was also reported by participants within the weakness symptom. Also, rhabdomyolysis may be a more important symptom in the absence of having myopathy, and having myopathy was an inclusion criterion in this research.
Regarding the treatment effect assessment, our qualitative analysis describes that any treatment benefits from NAC were directly linked to reducing fatigue and increasing strength. In addition, some indirect improvements were also accounted for with pain and mobility. On the other hand, only three side effects were identified throughout the entire study.
Additionally, the analysis by visit displayed a higher number of quotes for baseline interviews. However, considering the ratio of participants-comments for each visit, post-intervention interviews provided more comments by participant, which may be due to participants’ positive expectations regarding the clinical trial [58]. The analysis by participant displayed consistent results compared to global analysis (i.e., analyzing all data), showing similar trends of results for most of the participants.
The main limitation in this study was the small sample size. Additionally, the context in which questions were asked may have shaped participants’ responses. Also, because affected individuals report fatigue as one of the more pervasive symptoms and preclinical data suggested NAC may impact fatigue, several open-ended questions requested information regarding this symptom specifically. This may have increased the number of mentions for fatigue. In addition, there may be a loss in depth of data from performing data transformation, reducing qualitative data to a binary or frequency. Another limitation may be that all enrolled participants were ambulatory and thus were not representative across the RYR1-RM population.
5. Conclusion
Our qualitative analysis provides relevant information regarding the major symptoms of individuals affected with RYR1-RM and how those symptoms influence their HRQoL. Data transformation analysis reported a greater number of positive comments for post-intervention interviews, compared to baseline and pre-intervention visits, and that NAC group participants stated more positive comments than placebo group regarding treatment effect. Our findings, especially the identification of fatigue and weakness as key symptoms, the positive effect trend on those symptoms due to NAC treatment, and the description of participants’ experiences regarding their symptoms, will inform future studies in this rare disease.
In addition, given the FDA’s guidance and prioritization on the inclusion of affected individuals’ feedback in clinical trials, PRO measures, and drug development [18], the proposed approach is an excellent design for patient involvement in a clinical trial.
Supplementary Material
Key points.
Symptoms of individuals with a rare disease, RYR1-RM, were systematically characterized from a patient perspective to inform and support affected individuals, family members, and health care providers.
A specific research design was applied to better understand patient-centered data to promote its application in future studies of RYR1-RM.
This approach meets FDA guidance on the inclusion of affected individuals’ feedback in developing patient-reported outcome measures and clinical trials for drug development.
Acknowledgements
The authors would like to thank all study participants for their sacrifice and commitment to be in this study. We also appreciate the support provided during the study by Ms. Karez Hawkins, Ms. Monique O. Shelton, and Dr. Joshua J. Todd (Neuromuscular Symptoms Unit, National Institute of Nursing Research, National Institutes of Health). We would like to thank Dr. Joan K. Austin (School of Nursing, Indiana University-Purdue University Indianapolis) for reviewing the manuscript. Also, we acknowledge the RYR1 Foundation for assistance with recruitment for this study.
Funding
This work was supported by the Intramural Programs of the National Institute of Nursing Research, National Institute of Neurological Disorders and Stroke, the National Institutes of Health Clinical Center, and Bench to Bedside Award [10-2013/Office of Rare Disease/NINR].
Footnotes
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.
References
- 1.U.S. Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Rockville, MD; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. Patient-focused drug development: collecting comprehensive and representative input. Rockville, MD; 2018. [Google Scholar]
- 3.Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landfeldt E, Edström J, Jimenez-Moreno C, van Engelen BGM, Kirschner J, Lochmüller H. Health-Related Quality of Life in patients with adult-onset Myotonic Dystrophy Type 1: a systematic review. Patient - Patient-Centered Outcomes Res. 2019;12:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon A, Pompilus F, Querbes W, Wei A, Strzok S, Penz C, et al. Patient perspective on Acute Intermittent Porphyria with frequent attacks: a disease with intermittent and chronic manifestations. Patient - Patient-Centered Outcomes Res. 2018;11:527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logviss K, Krievins D, Purvina S. Characteristics of clinical trials in rare vs. common diseases: a register-based Latvian study. Rosenkranz G, editor. PLoS One. 2018;13:e0194494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvert M, Thwaites R, Kyte D, Devlin N. Putting patient-reported outcomes on the ‘Big Data Road Map.’ J R Soc Med. 2015;108:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshpande P, Sudeepthi Bl, Rajan S, Abdul Nazir C. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slade A, Isa F, Kyte D, Pankhurst T, Kerecuk L, Ferguson J, et al. Patient reported outcome measures in rare diseases: a narrative review. Orphanet J Rare Dis. Orphanet Journal of Rare Diseases; 2018;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin K, Vernon MK, Patrick DL, Perfetto E, Nestler-Parr S, Burke L. Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an ISPOR COA emerging good practices task force report. Value Heal. 2017;20:838–55. [DOI] [PubMed] [Google Scholar]
- 11.International Rare Diseases Research Consortium (IRDiRC). Patient-centered outcome measures initiatives in the field of rare diseases [Internet]. 2016. Available from: https://www.irdirc.org/wp-content/uploads/2017/12/PCOM_Post-Workshop_Report_Final.pdf
- 12.Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 1999;48:977–88. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira CM, de Araújo APQC. Self-reported quality of life has no correlation with functional status in children and adolescents with spinal muscular atrophy. Eur J Paediatr Neurol. 2011;15:36–9. [DOI] [PubMed] [Google Scholar]
- 14.Landfeldt E, Lindgren P, Bell CF, Guglieri M, Straub V, Lochmüller H, et al. Health-related quality of life in patients with Duchenne muscular dystrophy: a multinational, cross-sectional study. Dev Med Child Neurol. 2016;58:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peric S, Vujnic M, Dobricic V, Marjanovic A, Basta I, Novakovic I, et al. Five-year study of quality of life in myotonic dystrophy. Acta Neurol Scand. 2016;134:346–51. [DOI] [PubMed] [Google Scholar]
- 16.Regnault A, Willgoss T, Barbic S. Towards the use of mixed methods inquiry as best practice in health outcomes research. J Patient-Reported Outcomes. Journal of Patient-Reported Outcomes; 2018;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morel T, Cano SJ. Measuring what matters to rare disease patients – reflections on the work by the IRDiRC taskforce on patient-centered outcome measures. Orphanet J Rare Dis. 2017;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. Patient-focused drug development: methods to identify what is important to patients. Rockville, MD; 2019. [Google Scholar]
- 19.Petrovski S, Wang Q, Heinzen EL, Allen AS, Goldstein DB. Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes. Williams SM, editor. PLoS Genet. 2013;9:e1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd JJ, Lawal TA, Witherspoon JW, Chrismer IC, Razaqyar MS, Punjabi M, et al. Randomized controlled trial of N -acetylcysteine therapy for RYR1 -related myopathies. Neurology. 2020; 10.1212/WNL.0000000000008872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amburgey K, McNamara N, Bennett LR, McCormick ME, Acsadi G, Dowling JJ. Prevalence of congenital myopathies in a representative pediatric united states population. Ann Neurol. 2011;70:662–5. [DOI] [PubMed] [Google Scholar]
- 22.Colombo I, Scoto M, Manzur AY, Robb SA, Maggi L, Gowda V, et al. Congenital myopathies: Natural history of a large pediatric cohort. Neurology. 2015;84:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todd JJ, Razaqyar MS, Witherspoon JW, Lawal TA, Mankodi A, Chrismer IC, et al. Novel variants in individuals with RYR1-related congenital myopathies: Genetic, laboratory, and clinical findings. Front Neurol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dlamini N, Voermans NC, Lillis S, Stewart K, Kamsteeg E-J, Drost G, et al. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis. Neuromuscul Disord. 2013;23:540–8. [DOI] [PubMed] [Google Scholar]
- 25.van Ruitenbeek E, Custers JAE, Verhaak C, Snoeck M, Erasmus CE, Kamsteeg EJ, et al. Functional impairments, fatigue and quality of life in RYR1-related myopathies: A questionnaire study. Neuromuscul Disord. 2019;29:30–8. [DOI] [PubMed] [Google Scholar]
- 26.Klein A, Jungbluth H, Clement E, Lillis S, Abbs S, Munot P, et al. Muscle magnetic resonance imaging in congenital myopathies due to ryanodine receptor type 1 gene mutations. Arch Neurol. 2011;68:1171–1170. [DOI] [PubMed] [Google Scholar]
- 27.Matthews E, Neuwirth C, Jaffer F, Scalco RS, Fialho D, Parton M, et al. Atypical periodic paralysis and myalgia. Neurology. 2018;90:e412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawal TA, Todd JJ, Meilleur KG. Ryanodine receptor 1-related myopathies: Diagnostic and therapeutic approaches. Neurotherapeutics. Neurotherapeutics; 2018;15:885–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Jungbluth H, Sewry CA, Feng L, Bertini E, Bushby K, et al. Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain. 2007;130:2024–36. [DOI] [PubMed] [Google Scholar]
- 30.Dowling JJ, Arbogast S, Hur J, Nelson DD, McEvoy A, Waugh T, et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain. 2012;135:1115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CS, Hanna AD, Wang H, Dagnino-Acosta A, Joshi AD, Knoblauch M, et al. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat Commun. Nature Publishing Group; 2017;8:14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. Thousand Oaks, CA: Sage Publications; 2007. [Google Scholar]
- 34.Creswell JW. Research Design. Qualitative, quantitative and mixed methods approaches. 4th ed. London, UK: Sage Publications; 2014. [Google Scholar]
- 35.Geirdal AØ, Lund-Petersen I, Heiberg A. Understanding the experience of Myotonic Dystrophy. Mixed method study. J Genet Couns. 2015;24:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson NE, Luebbe E, Eastwood E, Chin N, Moxley RT, Heatwole CR. The impact of congenital and childhood myotonic dystrophy on quality of life: a qualitative study of associated symptoms. J Child Neurol. 2014;29:983–6. [DOI] [PubMed] [Google Scholar]
- 37.Vorster N, Evans K, Murphy N, Kava M, Cairns A, Clarke D, et al. Powered standing wheelchairs promote independence, health and community involvement in adolescents with Duchenne muscular dystrophy. Neuromuscul Disord. 2019;29:221–30. [DOI] [PubMed] [Google Scholar]
- 38.Sherman DW, McGuire DB, Free D, Cheon JY. A pilot study of the experience of family caregivers of patients with advanced pancreatic cancer using a mixed methods approach. J Pain Symptom Manage. 2014;48:385–ø. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson S Women with breast cancer talking causes: comparing content, biographical and discursive analyses. Fem Psychol. 2000;10:431–60. [Google Scholar]
- 40.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Heal Ment Heal Serv Res. 2015;42:533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun V, Clarke V. Using thematic analysis in psychology Brooks J, King N, editors. Qual Res Psychol. London: Macmillan Education UK; 2006;3:77–101. [Google Scholar]
- 42.Flick U An introduction to qualitative research. 5th ed. Thousand Oaks, CA: Sage Publications; 2014. [Google Scholar]
- 43.Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaFaver K, Miyasaki JM, Keran CM, Rheaume C, Gulya L, Levin KH, et al. Age and sex differences in burnout, career satisfaction, and well-being in US neurologists. Neurology. 2018;91:e1928–41. [DOI] [PubMed] [Google Scholar]
- 45.Miyasaki JM, Rheaume C, Gulya L, Ellenstein A, Schwarz HB, Vidic TR, et al. Qualitative study of burnout, career satisfaction, and well-being among US neurologists in 2016. Neurology. 2017;89:1730–8. [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Speechley K, Campbell C. Health-related quality of life in children with Duchenne Muscular Dystrophy: a review. J Neuromuscul Dis. 2015;2:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winter Y, Schepelmann K, Spottke AE, Claus D, Grothe C, Schröder R, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol. 2010;257:1473–81. [DOI] [PubMed] [Google Scholar]
- 48.Frisaldi E, Shaibani A, Vollert J, Ferrero B, Carrino R, Ibraheem HD, et al. The placebo response in myasthenia gravis assessed by quantitative myasthenia gravis score: a meta-analysis. Muscle Nerve [Internet]. 2019;59:671–8. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mus.26469 [DOI] [PubMed] [Google Scholar]
- 49.Shaibani A, Frisaldi E, Benedetti F. Placebo response in pain, fatigue, and performance: possible implications for neuromuscular disorders. Muscle Nerve. 2017;56:358–67. [DOI] [PubMed] [Google Scholar]
- 50.Dowling JJ, Lillis S, Amburgey K, Zhou H, Al-Sarraj S, Buk SJA, et al. King–Denborough syndrome with and without mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord. 2011;21:420–7. [DOI] [PubMed] [Google Scholar]
- 51.Witting N, Werlauff U, Duno M, Vissing J. Phenotypes, genotypes, and prevalence of congenital myopathies older than 5 years in Denmark. Neurol Genet. 2017;3:e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jungbluth H, Müller CR, Halliger–Keller B, Brockington M, Brown SC, Feng L, et al. Autosomal recessive inheritance of RYR1 mutations in a congenital myopathy with cores. Neurology. 2002;59:284–7. [DOI] [PubMed] [Google Scholar]
- 53.Jungbluth H, Zhou H, Hartley L, Halliger-Keller B, Messina S, Longman C, et al. Minicore myopathy with ophthalmoplegia caused by mutations in the ryanodine receptor type 1 gene. Neurology. 2005;65:1930–5. [DOI] [PubMed] [Google Scholar]
- 54.Scacheri PC, Hoffman EP, Fratkin JD, Semino-Mora C, Senchak A, Davis MR, et al. A novel ryanodine receptor gene mutation causing both cores and rods in congenital myopathy. Neurology. 2000;55:1689–96. [DOI] [PubMed] [Google Scholar]
- 55.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–27. [PubMed] [Google Scholar]
- 56.Capacchione JF, Sambuughin N, Bina S, Mulligan LP, Lawson TD, Muldoon SM. Exertional Rhabdomyolysis and Malignant Hyperthermia in a Patient with Ryanodine Receptor Type 1 Gene, L-type Calcium Channel α−1 Subunit Gene, and Calsequestrin-1 Gene Polymorphisms. Anesthesiology. 2010;112:239–44. [DOI] [PubMed] [Google Scholar]
- 57.Davis M, Brown R, Dickson A, Horton H, James D, Laing N, et al. Malignant hyperthermia associated with exercise-induced rhabdomyolysis or congenital abnormalities and a novel RYR1 mutation in New Zealand and Australian pedigrees. Br J Anaesth. 2002;88:508–15. [DOI] [PubMed] [Google Scholar]
- 58.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid Remifentanil. Sci Transl Med. 2011;3:70ra14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.