Abstract
The objective of this study was to determine the clinical value of computed tomography (CT) image-based texture analysis in predicting microvascular invasion of primary hepatocellular carcinoma (HCC). CT images of patients with HCC from May 2017 to May 2019 confirmed by surgery and histopathology were retrospectively analyzed. Image features including tumor margin, tumor capsule, peritumoral enhancement, hypoattenuating halo, intratumoral arteries, and tumor-liver differences were assessed. All patients were divided into microvascular invasion (MVI)–negative group (n = 34) and MVI-positive group (n = 68). Preoperative CT images were further imported into MaZda software, where the regions of interest of the lesions were manually delineated. Texture features of lesions based on pre-contrast, arterial, portal, and equilibrium phase CT images were extracted. Thirty optimal texture parameters were selected from each phase by Fisher’s coefficient (Fisher), classification error probability combined with average correlation coefficient (POE+ACC), and mutual information (MI). Finally, receiver operating characteristic curve analysis was performed. The results showed that the Edmonson-Steiner grades, tumor size, tumor margin, and intratumoral artery characteristics were significantly different between the two groups (P = 0.012, < 0.001, < 0.001, = 0.003, respectively). There were 58 parameters with significant differences between the MVI-negative and MVI-positive groups (P < 0.001 for all). Among them, 12, 14, 17, and 15 parameters were derived from the pre-contrast phase, arterial phase, portal phase, and equilibrium phase respectively. According to the ROC analysis, optimal texture parameters based on the pre-contrast, arterial, portal, and equilibrium phases were 135dr_GLevNonU (AUC, 0.766; the cutoff value, 1055.00), Vertl_RLNonUni (AUC, 0.764; the cutoff value, 5974.38), 45dgr_GLevNonU (AUC, 0.762; the cutoff value, 924.34), and Vertl_RLNonUni (AUC, 0.754; the cutoff value, 4868.80), respectively. Texture analysis of preoperative CT images may be used as a non-invasive method to predict microvascular invasion in patients with primary hepatocellular carcinomas, and further to guide the treatment and evaluate prognosis. The most valuable parameters were derived from the gray-level run-length matrix.
Keywords: Texture analysis, Hepatocellular carcinoma, Computed tomography, Microvascular invasion
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common tumor in the world and the third leading cause of cancer-related death [1]. Although great strides have been made in the diagnosis and treatment of HCC and the prognosis of patients has improved, overall survival rate remains low and the high recurrence rate after treatment was still a problem [2, 3]. The main risk factor for HCC is chronic hepatitis B [1] and currently the main treatment includes surgical resection, liver transplantation, etc. [4]. Several studies have shown that vascular invasion is one of the most important prognostic factors [5–7]. Iwatsuki et al. reported that the risk of recurrence in the presence of microvascular and macrovascular invasion increased by 4.4 times and 15 times respectively in patients who had received liver transplantation [8]. Consequently, microvascular invasion was considered a significant impact on the recurrence and prognosis of patients with HCC and it is of great clinical value to actively explore and develop preoperative diagnostic methods.
In recent years, a large number of studies have been devoted to establishing the predictive indicators of microvascular invasion (MVI) in hepatocellular carcinoma including the detection of signal pathway proteins in biopsy samples and biomarkers such as alpha-fetoprotein (AFP) and des-gamma-carboxyl prothrombin (DCP) or gene mRNA, though their sensitivity and specificity remain sub-optimal [9–11], although some studies have reported that imaging features from computed tomography (CT), magnetic resonance imaging (MRI), and 18F-FDG PET/CT can be used to predict MVI such as unsmooth tumor margin, intratumoral arteries, and low-density halo. Currently, these imaging features were not able to quantitatively describe features in the image [12–14].
Texture analysis is a non-invasive method for quantitatively describing features in the medical images it is applied to [15]. By extracting the textural features of different phase images, we can obtain quantitative parameters which may reflect the underlying subtle structure and tissue heterogeneity of tumors[16]. This is theoretically achieved by parsing medical images in the image pixel or voxel grayscale distribution characteristics and inner links. At present, texture analysis has been successfully applied in the clinical practice of many diseases and experimentally in many other research projects [17–19]. Texture analysis is expected to become a more effective and non-invasive alternative for tumor evaluation, guiding clinicians to formulate individualized treatment plans and evaluate the prognosis of patients. MaZda (Institute of Electronics, Technical University of Lodz, Lodz, Poland) is a software package for 2D and 3D image texture analysis, which provides a complete pathway for the quantitative analysis of image texture, including texture feature computation, texture segmentation, and texture classification [20]. This software has been applied in relevant studies on liver fibrosis [21], differentiation of pancreatic lymphoma and pancreatic cancer [22], thyroid cancer [23], and carotid artery plaque [24].
At present, non-invasive evaluation of microvascular invasion before surgery in hepatocellular carcinoma is a research hotspot. The relationship between CT texture features and microvascular invasion in hepatocellular carcinoma has not been clarified, and the clinical value of CT texture features in predicting microvascular invasion needs to be further studied. Thus, the purpose of this study was to explore the clinical value of texture features based on multi-phase CT images in non-invasively predicting microvascular invasion in HCC before surgery.
Materials and Methods
Study Population
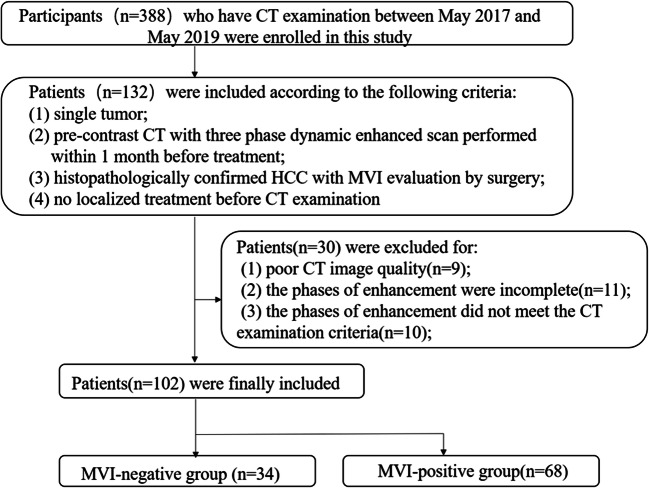
This retrospective study was approved by the institutional review board of hospital and the requirement for informed consent was waived. Participants who have CT examination between May 2017 and May 2019 were enrolled in this study according to the following inclusion criteria: (1) single tumor; (2) pre-contrast CT with three-phase dynamic-enhanced scan performed within 1 month before treatment; (3) histopathologically confirmed HCC with MVI evaluation by surgery; (4) no localized treatment such as radiofrequency ablation and transcatheter arterial chemoembolization before CT examination. The exclusion criteria included the following: (1) poor CT image quality due to severe artifacts (n = 9); (2) the phases of enhancement were incomplete (n = 11); (3) the phases of enhancement did not meet the CT examination criteria (n = 11). Finally, a total 102 patients were included in this retrospective study (Fig. 1). The relevant clinical information of the participating patients was retrieved through the electronic medical record (EMR).
Fig. 1.
Flow diagram of the inclusion and exclusion criteria for the study
Microvascular Invasion Evaluation
Pathologic characteristics were evaluated from surgical resection specimens by a team of pathologists with a minimum of 10 years of experience. MVI was defined as a tumor within a vascular space lined by endothelium that was visible only by microscopy. Moreover, they took into account the distance factor which defined three additional subgrades: M0, no MVI; M1 (the low-risk group), ≤ 5 MVI in adjacent liver tissue of tumor (≤ 1 cm); M2 (the high-risk group), > 5 MVI or MVI in adjacent liver tissue of tumor (> 1 cm) [25]. In this study, patients scored as M0 were categorized as the MVI-negative group and M1/M2 grade patients were categorized as the MVI-positive group. Other histological features such as Edmondson-Steiner grade and liver cirrhosis were also evaluated.
CT Imaging Scan
CT examination of all cases was performed in the Toshiba Aquilion One 320-slice spiral CT or the Toshiba Aquilion PRIME 80-slice spiral CT. The scanning ranges from the phrenic apex to the subhepatic angle. All patients underwent volumetric dynamic scanning and three-phase enhancement scanning. Stellakt double-barreled high-pressure syringe was used to inject the non-ionic contrast agent indophenol (370 mgI/ml) through the elbow vein, with a dose of 1.5 ml/kg and a flow rate of 3.0 ml/s, followed by an injection of 30 ml normal saline at a flow rate of 3.0 ml/s. Arterial, portal venous and equilibrium phase images were acquired at 30, 60, and 120 s after contrast material injection respectively. Scanning parameters used in this study were as follows: tube voltage and current, 120 kV and 230 mAs; rotation time, 0.35 s; slice interval, 0 mm; slice thickness, 5 mm.
CT Image Analysis
The preoperative CT images of each patient were reviewed by two radiologists (with 6 and 13 years of experience in abdominal CT images respectively) who were blind to the clinical data and pathological results. The following imaging characteristics were evaluated for each patient: (1) tumor size was measured as the maximum tumor diameter on the axial CT image; (2) smooth tumor margin was defined as nodular tumors with smooth contour in all imaging planes; (3) radiological capsule was defined as the peripheral hyperenhanced ring of tumor in the portal venous or equilibrium phase, categorized as complete, incomplete, or absent; (4) peritumoral enhancement was defined as a detectable portion enhanced in the arterial phase adjacent to the tumor border, later turning isoattenuation in the equilibrium phase; (5) hypoattenuating halo was defined as a low-density ring that was completely or partially surrounding the tumor in the portal venous phase; (6) intratumoral arteries were defined as the presence of internal arteries in the arterial phase; (7) tumor-liver difference was defined as the tumor density in the arterial phase which was significantly lower than the adjacent liver parenchyma. After independent image review, interobserver agreement was evaluated. When there was a discrepancy between the two radiologists, a common review was performed and the senior physician made the final judgment.
Texture Analysis and Feature Selection
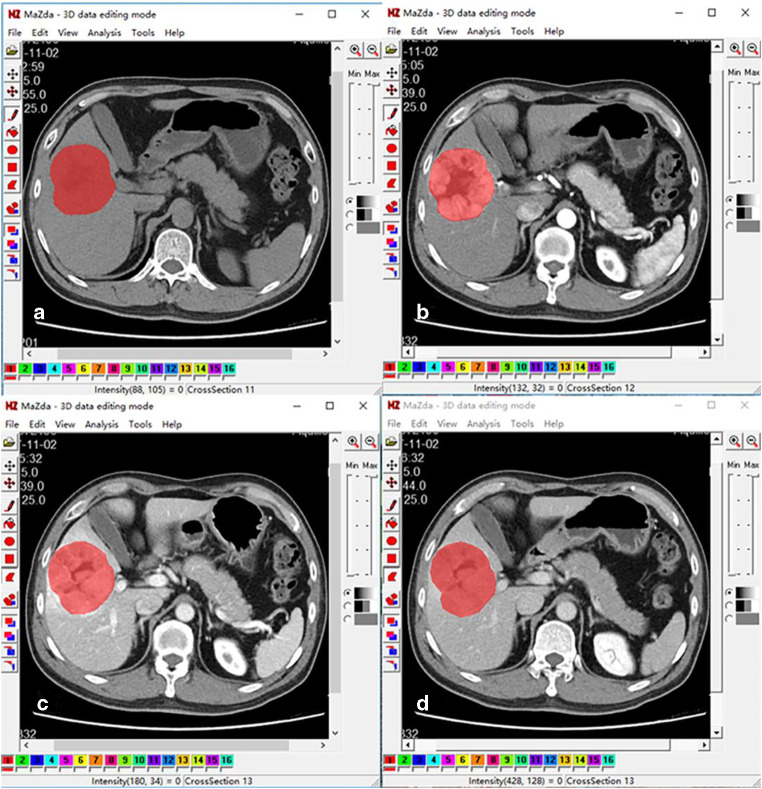
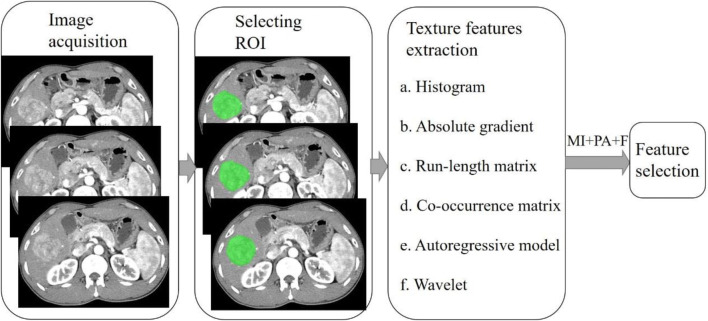
MaZda software (version 4.6, available at http://www.eletel.p.lodz.pl/mazda/) was used for texture analysis. All images were saved as BMP format for compatibility with MaZda. Using the 3D image editing function, the region of interest (ROI) was outlined by a radiologist on three cross-sections, including the largest slice of the lesion and the upper and lower slices adjacently. Subsequently, the ROI was copied onto other phase images as required. If the tumor location had changed due to the respiratory movement, fine adjustments were made to the ROI, ensuring the ROI was roughly in the same place (Fig. 2). Gray-level normalization was conducted in each ROI to minimize the influence of contrast variation and brightness using the method that normalizes image intensities in the range μ ± 3SD (μ, gray-level mean; SD, standard deviation). The extracted texture features included the following: gray-level histogram, the gray-level co-occurrence matrix (GLCM), the gray-level run-length matrix (GLRLM), the absolute gradient (GrM), the autoregressive model (ARM), and wavelet transform. More detailed texture features are listed in Table 1. Feature selection algorithms were included (Fisher coefficient [Fisher], mutual information [MI], and probability of classification error and average correlation coefficient [POE+ACC]). These were combined for the identification of 30 texture features in total, with the highest discriminative power for classification. A flowchart of image texture analysis is shown in Fig. 3.
Fig. 2.
Delineated ROIs cover the entire lesion on pre-contrast phase (a), arterial phase (b), portal phase (c), and equilibrium phase (d) of CT images
Table 1.
The list of texture features
| Main features | Detailed features |
|---|---|
| Histogram | Mean, variance, skewness, kurtosis, and 1st, 10th, 50th, 90th, and 99th percentiles |
| Absolute gradient | Mean, variance, skewness, kurtosis, and percentage of pixels with nonzero gradient |
| Run-length matrix | Run-length nonuniformity, gray-level nonuniformity, long-run emphasis, short-run emphasis, and fraction of image in runs; parameters computed for horizontal, 45°, vertical, and 135° directions |
| Co-occurrence matrix | Angular second moment, contrast, correlation, sum of squares, inverse difference moment, sum average, sum variance, sum entropy, entropy, difference variance, and difference entropy; parameters computed for 4 directions: (a, 0), (0, a), (a, a), (a, –a); and 5 distances: a = 1, 2, 3, 4, 5, between image pixels |
| Autoregressive model | Model parameter vector includes 4 parameters; SD of the driving noise |
| Wavelet | Energy of wavelet coefficients in low-frequency sub-bands, horizontal high-frequency sub-bands, vertical high-frequency sub-bands, and diagonal high-frequency sub-bands at successive scales |
Fig. 3.
Flowchart of image texture analysis. MI+PA+F are Fisher’s coefficient (Fisher), classification error probability combined with average correlation coefficient (POE+ACC), and mutual information (MI)
Statistical Analysis
Baseline characteristics and radiological findings of patients were recorded as mean and standard deviation or count and proportion. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test. Continuous variables were compared with Student’s t test or the Mann-Whitney U test if not normally distributed. Interobserver agreement was evaluated by the Cohen κ statistic: agreement was considered excellent if κ was greater than 0.80, good if κ ranged from 0.61 to 0.80, moderate if κ ranged from 0.41 to 0.60, and poor if 0.40 or less. By using receiver operating characteristic (ROC) analysis, the diagnostic accuracy of texture features to predict MVI was investigated and the area under the curve (AUC) was calculated. All statistical analyses were performed using SPSS (version 25.0, SPSS, Chicago, IL, USA). A P value less than 0.05 was considered statistically significant difference.
Results
In total, 102 patients were included in this study. Thirty-four cases were classified as MVI-negative, while the remaining 68 cases were classified as MVI-positive. The baseline characteristics of the 102 patients are summarized in Table 2. As can been seen, the higher Edmondson-Steiner grade (p = 0.012) showed statistically significant associations with microvascular invasion. However, there were no significant differences attributed to age, gender, Child-Pugh, history of liver cirrhosis, and serum alpha-fetoprotein levels between the two groups (P > 0.05).
Table 2.
Baseline characteristics of the study population
| Variables | All patients (n = 102) | MVI-negative (n = 34) | MVI-positive (n = 68) | P value |
|---|---|---|---|---|
| Age (year)* | 56 (24–81) | 59 (35–79) | 59 (24–81) | 0.062 |
| Sex | 0.556 | |||
| Male | 92 | 32 | 60 | |
| Female | 10 | 2 | 8 | |
| AFP* | 697.32 ± 1959.43 | 697.32 ± 1959.43 | 892.00 ± 2350.16 | 0.066 |
| E-S grade | 0.012 | |||
| I | 1 | 0 | 1 | |
| II | 62 | 27 | 35 | |
| III | 39 | 7 | 32 | |
| Child-Pugh class | 0.553 | |||
| A | 87 | 30 | 57 | |
| B | 15 | 4 | 11 | |
| C | 0 | 0 | 0 | |
| Liver cirrhosis | 0.187 | |||
| Present | 36 | 9 | 27 | |
| Absent | 66 | 25 | 41 |
Unless otherwise indicated, data are numbers of patients. *Data are median (range).
AFP, alpha-fetoprotein; E-S grade, Edmondson-Steiner grade
Imaging Features
The morphological characteristics between the MVI-negative and MVI-positive groups are described in Table 3. The differences in tumor size, intratumoral arteries, and tumor margin between the two groups were significantly different (P < 0.05). For other imaging features, including radiological capsule, peritumoral enhancement, hypoattenuating halo, and tumor-liver difference, there was no significant difference between the two groups (P > 0.05). And interobserver agreements were good to excellent (0.648–0.859). The tumor sizes in the MVI-positive group were significantly larger than those in the MVI-negative group. The intratumoral arteries and unsmooth tumor margins were more frequently observed in the MVI-positive group (Figs. 4 and 5).
Table 3.
CT image features
| Variables | MVI-negative (n = 34) | MVI-positive (n = 68) | P value | κ |
|---|---|---|---|---|
| Size (cm)* | 4.74 ± 2.61 | 4.74 ± 2.61 | < 0.001 | – |
| Intratumoral arteries | 0.003 | 0.717 | ||
| Present | 25 | 65 | ||
| Absent | 9 | 3 | ||
| Tumor margin | < 0.001 | 0.859 | ||
| Smooth | 22 | 10 | ||
| Non-smooth | 12 | 58 | ||
| Capsule | 0.145 | 0.656 | ||
| Absent | 6 | 11 | ||
| Present | 28 | 57 | ||
| Incomplete | 19 | 49 | ||
| Complete | 9 | 8 | ||
| Peritumoral enhancement | 0.655 | 0.648 | ||
| Present | 1 | 5 | ||
| Absent | 33 | 63 | ||
| Hypoattenuating halo | 0.534 | 0.657 | ||
| Present | 2 | 1 | ||
| Absent | 32 | 67 | ||
| Tumor-liver difference | 0.418 | 0.653 | ||
| Present | 3 | 2 | ||
| Absent | 31 | 66 |
Unless otherwise indicated, data are numbers of patients. *Data are means ± standard deviation
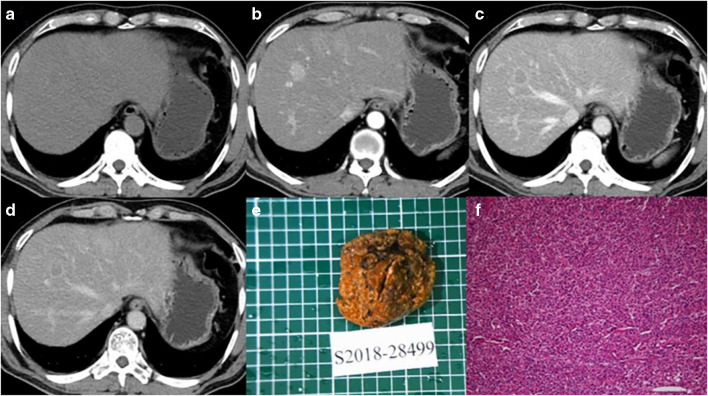
Fig. 4.
Male, 65 years old, HCC without MVI. Panels a–d correspond to the pre-contrast, arterial, portal, and equilibrium phases of CT images, respectively. The tumor with smooth margin on axial imaging. Panels e and f are gross specimens of the tumor and photomicrograph (H&E, × 100)
Fig. 5.
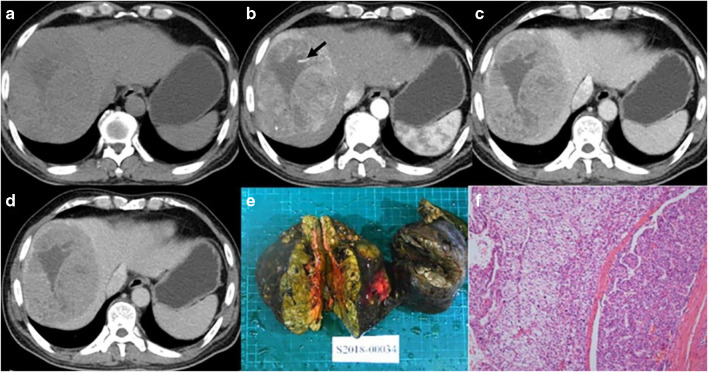
Male, 52 years old, HCC with MVI. Panels a–d correspond to the pre-contrast, arterial, portal, and equilibrium phases of CT images, respectively. The tumor with non-smooth margin on axial imaging, and intratumoral arteries can be seen on arterial phase (arrow). Panels e and f are gross specimens of tumor and photomicrograph (H&E, × 100)
Textural Features
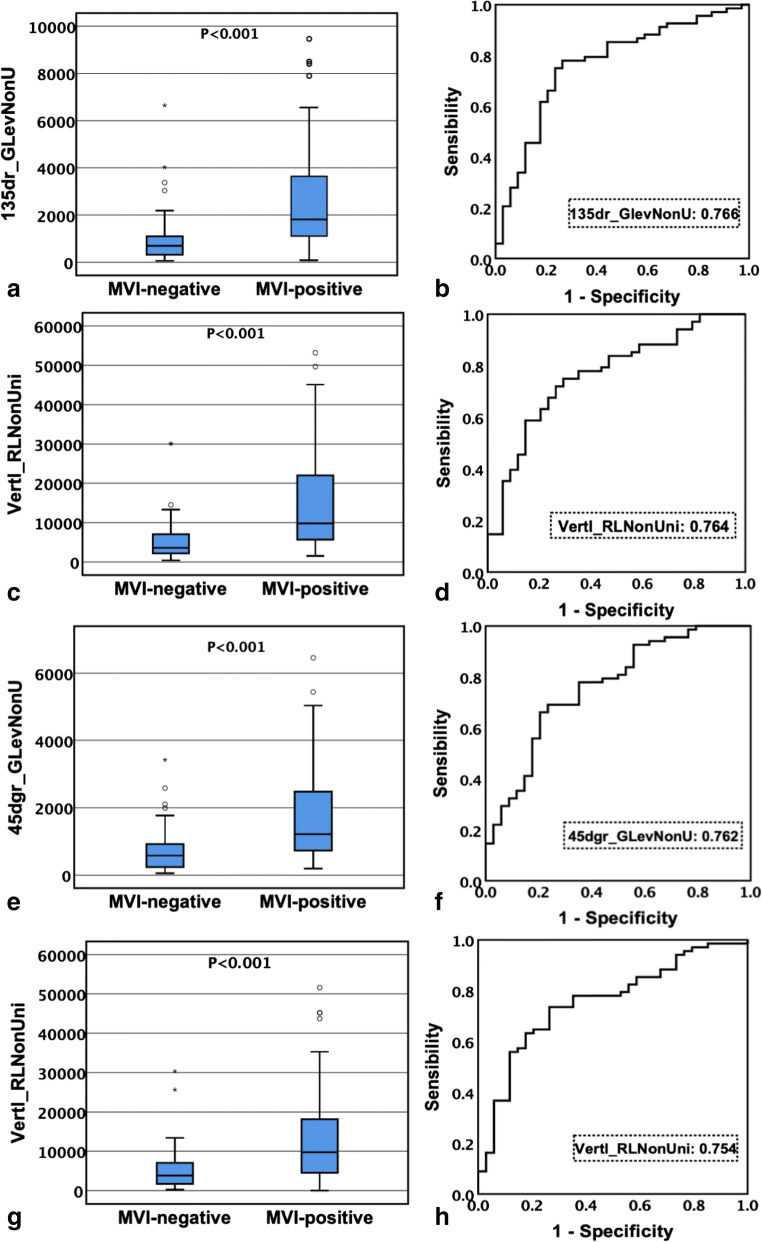
The 30 selected textural features with the highest discriminative power observed for classification were derived from pre-contrast, arterial, portal, and equilibrium phase CT images, respectively. Among them, there were 58 parameters with significant differences between the MVI-negative and MVI-positive groups (P < 0.001 for all). Moreover, 12, 14, 17, and 15 parameters were derived from the pre-contrast phase, arterial phase, portal phase, and equilibrium phase respectively. These parameters are listed in Table 4. Texture parameters generated from GLRLM in each phase image were the most frequently significant difference between the two groups. Comparing the values of gray-level run-length matrix parameters of CT images in each phase, the median value of the MVI-positive group was significantly higher than that of the MVI-negative group. Conversely, by comparing the values of gray-level co-occurrence matrix parameters of CT images in the portal phase and equilibrium phase, the median value of the MVI-positive group was significantly lower than that of the MVI-negative group. Through ROC [26, 27] curve analysis, the largest area under the curve (AUC) of each phase is shown in Fig. 6. The optimal textural parameters based on pre-contrast, arterial, portal vein, and equilibrium phase images were 135dr_GLevNonU (AUC, 0.766; the cutoff value, 1055.00), Vertl_RLNonUni (AUC, 0.764; the cutoff value, 5974.38), 45dgr_GLevNonU (AUC, 0.762; the cutoff value, 924.34), and Vertl_RLNonUni (AUC, 0.754; the cutoff value, 4868.80), respectively, which were all derived from GLRLM (Table 5).
Table 4.
Parameters with statistical significance between the MVI-negative group and the MVI-positive group in 30 best texture features
| Parameters | Pre-contrast phase | Parameters | Arterial phase | Parameters | Portal phase | Parameters | Equilibrium phase |
|---|---|---|---|---|---|---|---|
| GrSkewness | 0.025 | Skewness3D | 0.001 | Mean3D | 0.002 | Mean3D | 0.002 |
| S(0,0,1)Correlat | 0.001 | Kurtosis3D | 0.002 | S(1,0,0)SumAverg | 0.002 | S(1,0,0)SumAverg | 0.003 |
| Horzl_RLNonUni | < 0.001 | GrSkewness | 0.005 | S(0,1,0)SumAverg | 0.003 | S(0,1,0)SumAverg | 0.002 |
| Horzl_GLevNonU | < 0.001 | GrKurtosis | 0.006 | S(1,1,0)SumAverg | 0.003 | S(1,1,0)SumAverg | 0.003 |
| Vertl_RLNonUni | < 0.001 | Horzl_RLNonUni | <0.001 | S(1,-1,0)SumAverg | 0.002 | S(0,0,1)Correlat | 0.033 |
| Vertl_GLevNonU | < 0.001 | Horzl_GLevNonU | < 0.001 | S(0,0,1)Correlat | 0.007 | Horzl_RLNonUni | < 0.001 |
| 45dgr_RLNonUni | < 0.001 | Vertl_RLNonUni | < 0.001 | S(0,0,1)SumAverg | 0.003 | Horzl_GLevNonU | < 0.001 |
| 45dgr_GLevNonU | < 0.001 | Vertl_GLevNonU | < 0.001 | Horzl_RLNonUni | < 0.001 | Vertl_RLNonUni | < 0.001 |
| 135dr_RLNonUni | < 0.001 | 45dgr_RLNonUni | < 0.001 | Horzl_GLevNonU | < 0.001 | Vertl_GLevNonU | < 0.001 |
| 135dr_GLevNonU | < 0.001 | 45dgr_GLevNonU | < 0.001 | Vertl_RLNonUni | < 0.001 | 135dr_RLNonUni | < 0.001 |
| Z_RLNonUni | < 0.001 | 135dr_RLNonUni | < 0.001 | Vertl_GLevNonU | < 0.001 | 135dr_GLevNonU | < 0.001 |
| Z_GLevNonU | < 0.001 | 135dr_GLevNonU | < 0.001 | 45dgr_RLNonUni | < 0.001 | 45dgr_RLNonUni | < 0.001 |
| Z_RLNonUni | < 0.001 | 45dgr_GLevNonU | < 0.001 | 45dgr_GLevNonU | < 0.001 | ||
| Z_GLevNonU | < 0.001 | 135dr_RLNonUni | < 0.001 | Z_RLNonUni | < 0.001 | ||
| 135dr_GLevNonU | < 0.001 | Z_GLevNonU | < 0.001 | ||||
| Z_RLNonUni | < 0.001 | ||||||
| Z_GLevNonU | < 0.001 |
P < 0.05 were significant difference
Fig. 6.
Box plots and ROC curves. a, b The optimal parameter 135dr_GlevNonU based on pre-contrast phase of CT image; c, d the optimal parameter Vertl_RLNonUni based on arterial phase of CT image; e, f the optimal parameter 45dgr_RLNonUni based on portal phase of CT image; g, h the optimal parameter Vertl_RLNonUni based on equilibrium phase of CT image
Table 5.
The specificity, sensitivity, and corresponding cutoff value of optimal texture parameters based on pre-contrast, arterial, portal, and equilibrium phases of CT images
| Phase | Parameters | AUC | Specificity (%) | Sensitivity (%) | Youden Index | Cutoff value |
|---|---|---|---|---|---|---|
| Pre-contrast phase | 135dr_GLevNonU | 0.766 | 73.53 | 77.94 | 0.51 | 1055 |
| Arterial phase | Vertl_RLNonUni | 0.764 | 70.59 | 75 | 0.64 | 5974.38 |
| Portal phase | 45dgr_GLevNonU | 0.743 | 82.35 | 58.82 | 0.41 | 7445.56 |
| Equilibrium phase | Vertl_RLNonUni | 0.754 | 73.53 | 73.53 | 0.47 | 4867.8 |
AUC, area under the curve
Discussion
Microvascular invasion (MVI) has been shown to be one of the most important risk factors for postoperative recurrence in patients with hepatocellular carcinoma, in addition to affecting the overall survival of patients with HCC [28, 29]. In recent years, several studies have shown that MVI is a risk factor of early postoperative recurrence of liver cancer [2, 5]. Thus, MVI was considered to be an important biological indicator for the improvement of the postoperative survival of liver cancer patients, which has been demonstrated by a large number of clinical and basic research studies. How to predict MVI preoperatively, especially the exploration of non-invasive examination methods, has become a “hot spot” of medical research.
In this study, the results demonstrated that non-smooth tumor margins were effective in predicting microvascular invasion. Some research has previously reported that solitary masses with irregular margin are more likely to display microvascular invasion than masses with smooth margin. This study finding was consistent with prior studies [30–32]. Tumor size and histopathological grade were both significantly different between the MVI-negative and MVI-positive groups in this study. Compared with the MVI-negative group, the average diameter of the MVI-positive group was larger and the histopathological grade was more severe. These results are consistent with a study by Kim et al. which reported that tumor size was closely related to histological grade. The larger the tumor, the higher its histological grade and the greater the possibility of MVI [33]. The results of this study also showed that there was a significant difference in the intratumoral arteries to predict MVI, where the occurrence probability in the MVI-positive group was higher than that in the MVI-negative group. This result was in line with the finding of a study by Segal et al. which demonstrated that the causes may be related to angiogenesis and cell proliferation [34].
In this study, there was no significant correlation between the presence of tumor capsule and microvascular invasion. It has been reported that the tumor capsule in HCC is a favorable prognostic factor because it prevents liver cancer from invading the adjacent liver parenchyma [35–37]. However, Adachi et al. demonstrated that the vessels of the fibrous capsule are frequently invaded by cancer cells and indicated that the presence of the fibrous capsule is actually a predictor of portal infiltration [38]. The correlation between the presence or absence of tumor capsule and the occurrence of MVI is still a matter of academic controversy, requiring further study. There were no significant differences in peritumoral enhancement, hypoattenuating halo, and tumor-liver difference between the two groups. However, recent studies have indicated that peritumoral enhancement, hypoattenuating halo, and tumor-liver difference can be taken as risk factors, suggesting an increased risk of MVI [34, 39–42]. The reasons for conflicting results may be the differences in imaging modalities or the low percentage of enhancement of HCC on dynamic CT images in this study. These results remain to be confirmed by larger case studies.
In recent years, with the ongoing development and in-depth research of radiomics, extensive exploration has been carried out across various diseases through medical imaging. Textural analysis, a part of radiomics, has naturally become a research hotspot of medical imaging. It has been reported that medical research utilizing texture analysis has been increasing year after year in efforts for the differentiation of benign and malignant tumors, the prediction of tumor pathological grading and overall survival rates, etc. In general, texture analysis is a very interesting research direction in the medical imaging field [43–46].
In this study, 30 optimal textural parameters of each phase were automatically screened. A total of 58 texture parameters were significantly different between MVI-negative and MVI-positive groups. The gray-level run-length matrix displayed the most frequent textural parameters. By comparing the parameter values of gray-level run-length matrix in each phase, the values of the MVI-positive group were significantly higher than those of the MVI-negative group. And ROC curve analysis of these textural parameters which were selected from each phase revealed that the AUC of optimal textural parameters derived from pre-contrast, arterial, portal, and equilibrium phases was from 0.754 to 0.766. The AUC of 135dr_GLevNonU based on pre-contrast CT image was 0.766, the AUC of Vertl_RLNonUni based on arterial phase image was 0.764, the AUC of 45dgr_GLevNonU based on portal phase imaging was 0.762, and the AUC of Vertl_RLNonUni based on equilibrium phase imaging was 0.754, showing a discriminative performance in predicting MVI. The textural parameters of GLRLM reflect the image heterogeneity in different directions, indicating that the density of the MVI-positive group was more heterogeneous in different directions. We speculate that there are more blood vessels with abnormal hyperplasia in HCC with MVI. In addition, there may also be more necrosis due to fast tumor growth, leading to more uneven internal structure of tumor. Previous studies [47, 48] have shown that texture analysis can assist in the diagnosis of microvascular invasion of hepatocellular carcinoma. However, the extracted parameters are not the same, which may be related to the number of cases, extraction methods, and institutions.
This study has the following limitations: (1) this study was a retrospective study. Despite having selected samples according to strict inclusion and exclusion criteria, selection bias may not be understated. (2) Only three slices of lesion were chosen to evaluate MVI, which may cause loss of volumetric information. Whether this research strategy is sufficient to interpret the characteristics of tumor via medical imaging remains to be validated in future research. (3) At present, the algorithms of feature extraction/selection for texture analysis do not have a uniform standard and how to standardize feature extraction/selection is a difficult problem. Which feature extraction/selection algorithms can more accurately predict MVI requires extensive clinical research and verification.
In conclusion, the tumor size, non-smooth tumor margin, and intratumoral arteries may serve as radiological signs in the prediction of microvascular invasion for patients with HCC. The results of this study suggest that texture analysis based on multi-phase CT imaging can be used to predict microvascular invasion in HCC. This is significant because it provides a non-invasive imaging method for patients with hepatocellular carcinoma. And it has potential clinical application in preoperative treatment planning and tumor prognosis evaluation which could improve the treatment effect and life quality of those affected.
Acknowledgments
We thank the radiographers at the First Affiliated Hospital of Fujian Medical University for scanning the patients and data collections in this study.
Abbreviations
- AFP
alpha-fetoprotein
- ARM
autoregressive model
- AUC
area under curve
- CT
computed tomography
- DCP
des-gamma-carboxyl prothrombin
- EMR
electronic medical record
- GLCM
gray-level co-occurrence matrix
- GLRLM
gray-level run-length matrix
- GrM
absolute gradient
- HCC
hepatocellular carcinoma
- MIP
maximum intensity projection
- MPR
multiplanar reformat
- MI
mutual information
- MRI
magnetic resonance imaging
- MVI
microvascular invasion
- PACS
picture archiving and communication system
- POE+ACC
probability of classification error and average correlation coefficient
- ROC
receiver operating characteristic curve
- ROI
region of interest
- VR
volume rendering
Funding
This study has received funding by Joint Funds for the Innovation of Science and Technology, Fujian province (CN) (Award Number: 2019Y9125)
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Xuru Xu and Yueming Li are co-first authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yueming Li and Xuru Xu contributed equally to this work.
Contributor Information
Yueming Li, Email: fjmulym@163.com.
Xuru Xu, Email: xrxufj666@163.com.
Shuping Weng, Email: wellison@sina.com.
Chuan Yan, Email: fjyanc@163.com.
Jianwei Chen, Email: cjjwei@163.com.
Rongping Ye, Email: rongpingye222@163.com.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 2.Imamura H, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman MA, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143(2):182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 4.Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34(2):153–159. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, et al. Recurrence of hepatocellular carcinoma after laparoscopic hepatectomy: risk factors and treatment strategies. J Laparoendosc Adv Surg Tech A. 2017;27(7):676–684. doi: 10.1089/lap.2016.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu XF, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SA, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141(3):330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsuki S, et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 2000;191(4):389–394. doi: 10.1016/s1072-7515(00)00688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minguez B, et al. Gene-expression signature of vascular invasion in hepatocellular carcinoma. J Hepatol. 2011;55(6):1325–1331. doi: 10.1016/j.jhep.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, et al. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2016;143(1):17–27. doi: 10.1007/s00432-016-2256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pote N, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62(4):848–854. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SY, et al. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015;40(4):843–851. doi: 10.1007/s00261-014-0256-0. [DOI] [PubMed] [Google Scholar]
- 13.Cuccurullo V, et al. Microvascular invasion in HCC: the molecular imaging perspective. Contrast Media Mol Imaging. 2018;2018:9487938. doi: 10.1155/2018/9487938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang M, et al. Prediction of microvascular invasion in hepatocellular carcinoma: preoperative Gd-EOB-DTPA-dynamic enhanced mri and histopathological correlation. Contrast Media Mol Imaging. 2018;2018:9674565. doi: 10.1155/2018/9674565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Incoronato M, et al. Radiogenomic analysis of oncological data: a technical survey. Int J Mol Sci. 2017;18(4):805. doi: 10.3390/ijms18040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer Imaging. 2013;13:140–149. doi: 10.1102/1470-7330.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maani R, et al. Cerebral degeneration in amyotrophic lateral sclerosis revealed by 3-dimensional texture analysis. Front Neurosci. 2016;10:120. doi: 10.3389/fnins.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giganti F, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol. 2017;27(5):1831–1839. doi: 10.1007/s00330-016-4540-y. [DOI] [PubMed] [Google Scholar]
- 19.López-Gómez C, et al. ALTEA: a software tool for the evaluation of new biomarkers for Alzheimer’s disease by means of textures analysis on magnetic resonance images. Diagnostics (Basel) 2018;8(3):47. doi: 10.3390/diagnostics8030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczypinski PM, et al. MaZda--a software package for image texture analysis. Comput Methods Prog Biomed. 2009;94(1):66–76. doi: 10.1016/j.cmpb.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Bahl G, et al. Noninvasive classification of hepatic fibrosis based on texture parameters from double contrast-enhanced magnetic resonance images. J Magn Reson Imaging. 2012;36(5):1154–1161. doi: 10.1002/jmri.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Z, et al. Two-dimensional texture analysis based on CT images to differentiate pancreatic lymphoma and pancreatic adenocarcinoma: a preliminary study. Acad Radiol. 2019;26(8):e189–e195. doi: 10.1016/j.acra.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Meyer HJ, et al. MRI texture analysis reflects histopathology parameters in thyroid cancer - a first preliminary study. Transl Oncol. 2017;10(6):911–916. doi: 10.1016/j.tranon.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, et al. Classification of carotid plaque echogenicity by combining texture features and morphologic characteristics. J Ultrasound Med. 2016;35(10):2253–2261. doi: 10.7863/ultra.15.09002. [DOI] [PubMed] [Google Scholar]
- 25.Cong WM, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22(42):9279–9287. doi: 10.3748/wjg.v22.i42.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thanh DNH, et al. melanoma skin cancer detection method based on adaptive principal curvature, colour normalisation and feature extraction with the ABCD rule. J Digit Imaging. 2020;33(3):574–585. doi: 10.1007/s10278-019-00316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erkan U, Thanh DNH. Autism spectrum disorder detection with machine learning methods. Curr Psychiatry Res Rev. 2020;15(4):297–308. [Google Scholar]
- 28.Jang SY, et al. The combination of periostin overexpression and microvascular invasion is related to a poor prognosis for hepatocellular carcinoma. Gut Liver. 2016;10(6):948–954. doi: 10.5009/gnl15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao H, et al. Prognostic value and preoperative predictors of microvascular invasion in solitary hepatocellular carcinoma </= 5 cm without macrovascular invasion. Oncotarget. 2017;8(37):61203–61214. doi: 10.18632/oncotarget.18049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi KK, et al. Portal venous invasion: the single most independent risk factor for immediate postoperative recurrence of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(11):1646–1651. doi: 10.1111/j.1440-1746.2011.06780.x. [DOI] [PubMed] [Google Scholar]
- 31.Eguchi S, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34(5):1034–1038. doi: 10.1007/s00268-010-0424-5. [DOI] [PubMed] [Google Scholar]
- 32.Shimada S, et al. Clinicopathological characteristics of hepatocellular carcinoma with microscopic portal venous invasion and the role of anatomical liver resection in these cases. World J Surg. 2017;41(8):2087–2094. doi: 10.1007/s00268-017-3964-0. [DOI] [PubMed] [Google Scholar]
- 33.Kim BK, et al. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J Surg Oncol. 2008;97(3):246–252. doi: 10.1002/jso.20953. [DOI] [PubMed] [Google Scholar]
- 34.Nishie A, et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol. 2008;190(1):81–87. doi: 10.2214/AJR.07.2810. [DOI] [PubMed] [Google Scholar]
- 35.Ng IO, et al. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992;70(1):45–49. doi: 10.1002/1097-0142(19920701)70:1<45::aid-cncr2820700108>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Ishizaki M, et al. The formation of capsule and septum in human hepatocellular carcinoma. Virchows Arch. 2001;438(6):574–580. doi: 10.1007/s004280000391. [DOI] [PubMed] [Google Scholar]
- 37.Torimura T, et al. Mechanism of fibrous capsule formation surrounding hepatocellular carcinoma. Immunohistochemical study. Arch Pathol Lab Med. 1991;115(4):365–371. [PubMed] [Google Scholar]
- 38.Adachi E, et al. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77(10):2022–2031. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2022::AID-CNCR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Miyata R, et al. Accuracy of preoperative prediction of microinvasion of portal vein in hepatocellular carcinoma using superparamagnetic iron oxide-enhanced magnetic resonance imaging and computed tomography during hepatic angiography. J Gastroenterol. 2006;41(10):987–995. doi: 10.1007/s00535-006-1890-2. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, et al. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19(7):1744–1751. doi: 10.1007/s00330-009-1331-8. [DOI] [PubMed] [Google Scholar]
- 41.Peng J, et al. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24(3):121–127. doi: 10.5152/dir.2018.17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee S, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62(3):792–800. doi: 10.1002/hep.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira Junior JR, et al. Radiomics-based features for pattern recognition of lung cancer histopathology and metastases. Comput Methods Prog Biomed. 2018;159:23–30. doi: 10.1016/j.cmpb.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Yan L, et al. Angiomyolipoma with minimal fat: differentiation from clear cell renal cell carcinoma and papillary renal cell carcinoma by texture analysis on CT images. Acad Radiol. 2015;22(9):1115–1121. doi: 10.1016/j.acra.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Canellas R, et al. Characterization of portal vein thrombosis (neoplastic versus bland) on CT images using software-based texture analysis and thrombus density (Hounsfield units) AJR Am J Roentgenol. 2016;207(5):W81–W87. doi: 10.2214/AJR.15.15928. [DOI] [PubMed] [Google Scholar]
- 46.Fu S, et al. Texture analysis of intermediate-advanced hepatocellular carcinoma: prognosis and patients’ selection of transcatheter arterial chemoembolization and sorafenib. Oncotarget. 2017;8(23):37855–37865. doi: 10.18632/oncotarget.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakr S, et al. Noninvasive radiomics signature based on quantitative analysis of computed tomography images as a surrogate for microvascular invasion in hepatocellular carcinoma: a pilot study. J Med Imaging (Bellingham) 2017;4(4):041303. doi: 10.1117/1.JMI.4.4.041303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu YJ, et al. Model-based three-dimensional texture analysis of contrast-enhanced magnetic resonance imaging as a potential tool for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Oncol Lett. 2019;18(1):720–732. doi: 10.3892/ol.2019.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]