Abstract
Background
There is inadequate evidence of long-term benefit from opioid medications for chronic pain and substantial evidence of potential harms. For patients, dose reduction may be beneficial when implemented voluntarily and supported by a multidisciplinary team but experts have advised against involuntary opioid reduction.
Objectives
To assess the prevalence of self-reported involuntary opioid reduction and to examine whether involuntary opioid reduction is associated with changes in pain severity.
Design
Prospective observational cohort study.
Participants
Primary care patients treated with long-term opioid therapy in the Veterans Health Administration (N = 290).
Main Measures
The primary exposure was self-reported past year involuntary opioid reduction. The primary outcome was the three-item PEG scale, which measures past-week average pain intensity and interference with enjoyment of life and general activity.
Key Results
Past year opioid reduction or discontinuation was reported by 63% (184/290). Similar numbers reported involuntary (88/290) and voluntary (96/290) opioid reduction. At baseline, there were no significant differences in pain severity between the groups (mean PEG, 7.08 vs. 6.73 vs. 7.07 for past year involuntary opioid reduction, past year voluntary opioid reduction, and no past year opioid reduction, respectively; P = 0.32). For the primary outcome of change in pain severity from baseline to 18 months, there were no significant differences between groups (mean PEG change, − 0.05 vs. − 0.44 vs. − 0.23 for past year involuntary opioid reduction, past year voluntary opioid reduction, and no past year opioid reduction, respectively; P = 0.28).
Conclusions
Self-reported past year involuntary opioid reduction was common among a national sample of veterans treated with long-term opioid therapy. Opioid dose reduction, whether involuntary or voluntary, was not associated with change in pain severity. Future studies should examine involuntary opioid reduction in different populations and trends over time and explore further patient- and provider-level factors that may impact patient experience and outcomes during opioid reduction.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-06294-z) contains supplementary material, which is available to authorized users.
KEY WORDS: opioid analgesics, pain management, Veterans health, medication therapy management, health services research
BACKGROUND
There is inadequate evidence of long-term benefit from opioid medications for chronic pain and substantial evidence of potential harms.1 Expert guidelines recommend individualized assessment of risks and benefits and advise dose reduction or discontinuation when benefits of long-term opioid therapy (LTOT) no longer outweigh risks.2 Opioid reduction may improve pain and function when implemented voluntarily by patients and supported by a multidisciplinary team.3 There is also concern that opioid reduction may worsen pain and increase risk of adverse outcomes for some patients. Experts have expressed concern over involuntary opioid dose reduction or discontinuation, and the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration have recently cautioned against abrupt opioid dose reduction of LTOT.4–6
Expert guidelines recommend that providers work collaboratively with patients during opioid reduction and employ strategies to increase patient engagement.7,8 However, patients and providers may disagree on whether and how to implement opioid reduction, and involuntary opioid reduction may at times be clinically appropriate, especially when risk is high (e.g., following an overdose event). Changes to long-term opioid treatment plans can be difficult and anxiety-provoking for patients, and ambivalence is common.9,10 For providers, these conversations can be challenging.11 While involuntary opioid reduction may not be avoidable in all cases, little is known about the prevalence or outcomes of involuntary opioid reduction.
The Effects of Prescription Opioid Changes for Veterans (EPOCH) study is a nationwide prospective population-based observational study of US VA primary care patients treated with LTOT. Among a random sample of EPOCH participants, we conducted a structured follow-up survey to assess the prevalence of self-reported past year involuntary opioid reduction and to examine whether involuntary opioid reduction is associated with changes in pain severity compared with voluntary reduction or no reduction in the past year.
METHODS
Study Setting and Population
We conducted a prospective cohort study from June 2016 to July 2018 of Veterans Health Administration (VA) patients receiving LTOT, nested within an ongoing nationwide prospective cohort study, the Evaluating Opioid Prescription Changes in Veterans (EPOCH) study. The study design, survey response, and baseline characteristics of the EPOCH study have been published.12 Patients were eligible for the EPOCH study if they had current LTOT at baseline and at least one primary care clinic visit within 12 months before the most recent opioid dispensing date. Current LTOT at baseline was defined as a qualifying opioid analgesic dispensed within the prior 30 days and ≥ 150 days’ supply in the 180 days before the most recent dispensing date with no between-fill gaps > 40 days. Qualifying opioid analgesics were on the VA formulary and indicated for pain, not including tramadol or buprenorphine. Patients were excluded if they received past year treatment for opioid use disorder, cancer, dementia, or end-of-life care. The EPOCH study enrolled 9,253 participants who completed a baseline paper or telephone survey and has subsequently invited participants to complete annual follow-up surveys.
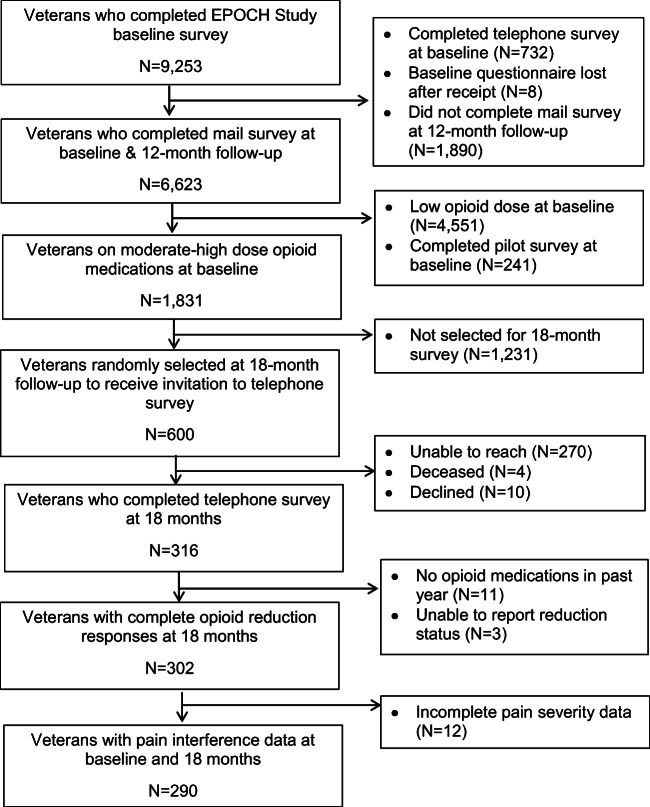
For this study, we randomly sampled 600 EPOCH participants who met two criteria: (1) prescribed moderate-high-dose opioids at EPOCH study baseline, defined as ≥ 50 mg morphine-equivalent (ME) daily dose using CDC-recommended conversion factors;13 and (2) completed mail survey at baseline and 12-month follow-up (Fig. 1). We invited these participants to complete a structured telephone survey approximately 18 months after baseline survey completion. The Minneapolis VA Health Care System Institutional Review Board and the Colorado Multiple Institutional Review Board approved the study.
Fig. 1.
Follow-up survey flow diagram. EPOCH, Evaluating Opioid Prescription Changes in Veterans (EPOCH) study.
Recruitment and Data Collection
For the overall EPOCH study, a multiple contact, mail and telephone approach was used for recruitment and data collection. The baseline survey was fielded in six survey waves conducted at monthly intervals beginning in June 2016. Patients who did not return the mailed survey were contacted with a second round of mailed materials and then by telephone. Patients reached by telephone were offered telephone surveys, and 7.9% (732/9,253) completed the baseline survey by phone.
For the present study, eligible patients were randomly selected in monthly batches of 100 over a 6-month period from January to July 2018, scheduled to align with the 18-month time point since their baseline survey completion. For example, participants who completed the EPOCH baseline survey in July 2016 were invited to participate in the follow-up survey in January 2018. Participants were mailed a letter with an invitation to participate in a telephone survey and were contacted by phone up to three times approximately 1 week after the invitation letter. Participants who agreed to participate were scheduled for a telephone survey within 2 weeks. Verbal consent was obtained prior to each survey. Telephone surveys were administered by five research staff members who were trained in survey administration and supervised by the study coordinator. Data were entered directly into a secure database system.
Study Measures
The primary exposure variable is self-reported past year involuntary opioid reduction. At the 18-month telephone survey, we first assessed past year opioid reduction by asking “In the past year, have you cut down the amount of opioid medicines you use?”. This question was adapted from a validated questionnaire assessing problems and concerns with opioid medications.14 We defined past year opioid reduction as an affirmative response to this question. Among participants who reported past year opioid reduction, we defined involuntary opioid reduction as an affirmative response to the following question: “In the past 12 months, has any doctor, dentist, nurse, or other health professional cut down or stopped your opioid medicines without your consent or against your wishes?”
The primary outcome variable is change in self-reported pain severity from baseline to 18 months. The 3-item PEG is a brief composite measure that assesses average past-week pain intensity, past-week pain interference with enjoyment of life, and past-week pain interference with general activity.15 Response options range from 0 to 10 with higher scores representing greater pain intensity/interference; the overall PEG score is the average of 3 responses. We selected the PEG scale as it has been shown to be sensitive to change and is recommended by the CDC and other groups as a brief, validated pain measure.2,16,17 As a secondary outcome, we assessed health-related quality of life with a single question from the Veterans RAND 12-item Health Survey (VR-12).18 Participants were asked “In general, would you say your health is…” (excellent, very good, good, fair, and poor).
Baseline data on demographic characteristics, comorbid diagnoses, and outpatient pharmacy dispensing were extracted from national VA databases for the year prior to the baseline date. Pain and mental health diagnosis categories were created based on work of other VA researchers and have been published elsewhere.12 We calculated baseline morphine-equivalent (ME) opioid daily dose by calculating the average dose over the 6-month period prior to each participants’ baseline date.13 For a post hoc exploratory analysis, we obtained outpatient pharmacy dispensing data for qualifying opioid medications from baseline survey completion through 18-month follow-up. For each participant, opioid daily dose in ME was calculated for each follow-up month by calculating the average opioid dose over the prior 60 days.
Data Analysis
For each participant, we calculated the change from baseline survey to 18-month follow-up for the 3-item PEG scale as well as for secondary outcomes of the individual PEG questions and the single-question health-related quality of life measure. We excluded participants who did not have complete data at both time points (Fig. 1). Estimating appropriate variance parameters of a sub-population (or domain) in a survey (such as Veterans reporting involuntary opioid reduction) requires implementing an appropriate domain analysis using information about the survey design.19 Accordingly, we implemented a domain analysis assuming a finite population correction of 0.01.20 We calculated t tests and chi-square tests adjusted for design-based standard errors to assess differences in baseline characteristics by self-reported opioid reduction status and to assess the association between self-reported opioid reduction status and change in patient-reported outcomes from baseline to 18-month follow-up survey. Because groups were well-balanced at baseline, we present unadjusted effect estimates. In a sensitivity analysis adjusting for race/ethnicity, findings were not qualitatively different.
For post hoc analyses of opioid dose, we assessed past year opioid medication dispensing using three approaches. First, we calculated mean opioid dose for each group at 6-month intervals during EPOCH study follow-up. Second, we created mutually exclusive categories based on change in opioid medication dose in average ME daily dose from 6 months to 18 months, approximating the past year time period assessed by self-report. We defined past year opioid dose reduction as a > 10% decrease from 6 months to 18 months and designated four subcategories of dose reduction (10–25%, 25–50%, 50–99%, discontinuation) to assess the magnitude of past year opioid dose reduction. We defined past year dose increase as a > 10% increase from 6 months to 18 months. Stable past year dose was defined as a dose change of ≤ 10% from 6 months to 18 months. Third, we identified participants with any monthly dose change of > 10% compared with the 6-month dose to identify short-term dose changes that were not maintained at 18-month follow-up. All analyses were conducted in R software.20
RESULTS
We conducted structured follow-up surveys with 316 participants, a 58% response rate, and obtained complete exposure and primary outcome data for 290 participants (Fig. 1). The only statistically significant differences between responders and non-responders were the prevalence of neck pain (26% of responders vs. 18% of non-responders) (Appendix Table 1). Among responders, 54% were treated with opioid doses from 50 to 89 mg ME daily dose while 9% were treated with > 200 mg ME daily dose. At baseline, the study sample was predominantly of older age, male, and White (Table 1). The most common pain-related diagnoses were back pain and other joint pain. The most common comorbid mental health diagnoses were depressive disorders and post-traumatic stress disorder (PTSD).
Table 1.
Baseline Characteristics According to Self-Reported Past Year Opioid Reduction Status (N = 290)
| Involuntary opioid reduction (N = 88) | Voluntary opioid reduction (N = 96) | No opioid reduction (N = 106) | P | |
|---|---|---|---|---|
| Age, mean (95% CI) | 63.4 (61.8-65.0) | 66.0 (64.1-67.9) | 64.7 (63.2-66.3) | 0.11 |
| Male sex, N (%) | 83 (94%) | 89 (93%) | 94 (89%) | 0.33 |
| Race, N (%) | ||||
| White | 69 (78%) | 86 (90%) | 98 (93%) | 0.01 |
| Black | 13 (15%) | 6 (6%) | 5 (5%) | 0.03 |
| Other race | 2 (2%) | 3 (3%) | 2 (2%) | 0.84 |
| Not reported | 4 (5%) | 2 (2%) | 2 (2%) | 0.47 |
| Hispanic ethnicity, N (%) | 3 (3%) | 4 (4%) | 6 (6%) | 0.74 |
| Urban residence, N (%) | 47 (53%) | 54 (56%) | 63 (59%) | 0.70 |
| Pain diagnoses, N (%) | ||||
| Back pain | 72 (82%) | 73 (76%) | 78 (74%) | 0.39 |
| Neck pain | 17 (19%) | 32 (33%) | 33 (31%) | 0.08 |
| Other joint pain | 41 (47%) | 42 (44%) | 49 (46%) | 0.91 |
| Neuropathic pain | 24 (27%) | 30 (31%) | 25 (24%) | 0.47 |
| Comorbid diagnoses, N (%) | ||||
| Depression | 29 (33%) | 27 (28%) | 42 (40%) | 0.22 |
| PTSD | 26 (30%) | 19 (20%) | 31 (29%) | 0.22 |
| Anxiety | 16 (18%) | 21 (22%) | 22 (21%) | 0.82 |
| Alcohol use disorder | 2 (2%) | 9 (9%) | 8 (8%) | 0.13 |
| Opioid use disorder | 8 (9%) | 9 (9%) | 7 (7%) | 0.73 |
| Opioid dose, N (%) | ||||
| 50–89 mg ME | 41 (47%) | 52 (54%) | 65 (61%) | 0.33 |
| 90–200 mg ME | 37 (42%) | 34 (35%) | 34 (32%) | |
| > 200 mg ME | 10 (11%) | 10 (10%) | 7 (7%) | |
| Concurrent benzodiazepine | 25 (27%) | 23 (23%) | 26 (24%) | 0.81 |
ME, morphine equivalents; PTSD, post-traumatic stress disorder; SD, standard deviation
Past year opioid dose reduction or discontinuation was reported by 63% (184/290). Similar numbers reported involuntary (88/290) and voluntary (96/290) opioid reduction while 37% (106/290) reported no past year opioid reduction. Black participants were more likely to report involuntary opioid reduction compared with non-Black participants, and White participants were less likely to report involuntary opioid reduction compared with non-White participants. Pain diagnoses, comorbid diagnoses, baseline opioid dose, and concurrent benzodiazepine prescription were not associated with self-reported opioid reduction status.
At baseline, there were no significant group differences in pain severity (mean PEG, 7.08 vs. 6.73 vs. 7.07 for past year involuntary opioid reduction, past year voluntary opioid reduction, and no past year opioid reduction, respectively; P = 0.32) (Table 2). For the primary outcome of change in pain severity from baseline to 18-month follow-up, there were no significant group differences (mean PEG change, − 0.05 vs. − 0.44 vs. − 0.23 for the involuntary opioid reduction, voluntary opioid reduction, and no opioid reduction groups, respectively; P = 0.28). Similarly, there were no significant group differences in the secondary outcomes.
Table 2.
Association between Self-Reported Past Year Opioid Reduction and Change in Patient-Reported Outcomes from Baseline to 18-Month Follow-up
| Involuntary opioid reduction | Voluntary opioid reduction | No opioid reduction | P | |
|---|---|---|---|---|
| Primary outcome | ||||
| Pain severity (PEG), mean (95% CI)* | ||||
| Baseline | 7.1 (6.7, 7.5) | 6.7 (6.4, 7.1) | 7.1 (6.7, 7.4) | 0.32 |
| 12 months | 7.3 (6.9, 7.6) | 6.7 (6.3, 7.1) | 7.0 (6.6, 7.3) | --- |
| 18 months | 7.0 (6.6, 7.46) | 6.3 (5.88, 6.71) | 6.8 (6.4, 7.2) | --- |
| Change from baseline | − 0.05 (− 0.38, 0.28) | − 0.44 (− 0.78, − 0.09) | − 0.23 (− 0.57, 0.11) | 0.28 |
| Secondary outcomes | ||||
| Pain intensity, mean (95% CI)* | ||||
| Baseline | 6.9 (6.5, 7.2) | 6.6 (6.3, 6.9) | 6.9 (6.6, 7.2) | 0.35 |
| 12 months | 7.1 (6.8, 7.5) | 6.7 (6.4, 7.0) | 6.9 (6.5, 7.2) | --- |
| 18 months | 6.8 (6.4, 7.2) | 6.1 (5.7, 6.5) | 6.5 (6.1, 6.8) | --- |
| Change from baseline | − 0.12 (− 0.41, 0.16) | − 0.50 (− 0.82, − 0.18) | − 0.41 (− 0.7, − 0.11) | 0.20 |
| Interference with enjoyment, mean (95% CI)* | ||||
| Baseline | 7.2 (6.7, 7.7) | 6.7 (6.2, 7.3) | 7.3 (6.8, 7.7) | 0.32 |
| 12 months | 7.1 (6.6, 7.7) | 6.7 (6.2, 7.3) | 7.0 (6.5, 7.5) | --- |
| 18 months | 7.2 (6.7, 7.7) | 6.3 (5.8, 6.8) | 6.9 (6.4, 7.5) | --- |
| Change from baseline | 0.03 (− 0.46, 0.52) | − 0.41 (− 0.9, 0.09) | − 0.31 (− 0.86, 0.23) | 0.43 |
| Interference with general activity, mean (95% CI)* | ||||
| Baseline | 7.2 (6.8, 7.6) | 6.9 (6.5, 7.3) | 7.1 (6.7, 7.5) | 0.55 |
| 12 months | 7.5 (7.1, 8.0) | 6.7 (6.2, 7.2) | 7.1 (6.7, 7.5) | --- |
| 18 months | 7.1 (6.6, 7.6) | 6.5 (5.3, 7.0) | 7.1 (6.6, 7.6) | --- |
| Change from baseline | − 0.06 (− 0.47, 0.36) | − 0.41 (− 0.91, 0.1) | 0.03 (− 0.4, 0.45) | 0.41 |
| Health-related quality of life, mean (95% CI)† | ||||
| Baseline | 3.9 (3.8, 4.1) | 3.7 (3.5, 3.9) | 4.0 (3.8, 4.1) | 0.11 |
| 12 months | 4.0 (3.8, 4.1) | 3.7 (3.5, 3.9) | 3.9 (3.8, 4.1) | --- |
| 18 months | 3.9 (3.7, 4.1) | 3.6 (3.4, 3.8) | 4.0 (3.8, 4.1) | --- |
| Change from baseline | − 0.02 (− 0.19, 0.14) | − 0.12 (− 0.3, 0.06) | 0 (− 0.13, 0.13) | 0.56 |
CI, confidence interval; PEG, Pain, Enjoyment and General activity scale
*Pain intensity/interference assessed with three-item PEG scale (N = 290)
†Health status assessed with a single question from the Veterans RAND 12-item Health Survey (VR-12) (N = 280). Response options were excellent (1), very good (2), good (3), fair (4), and poor (5)
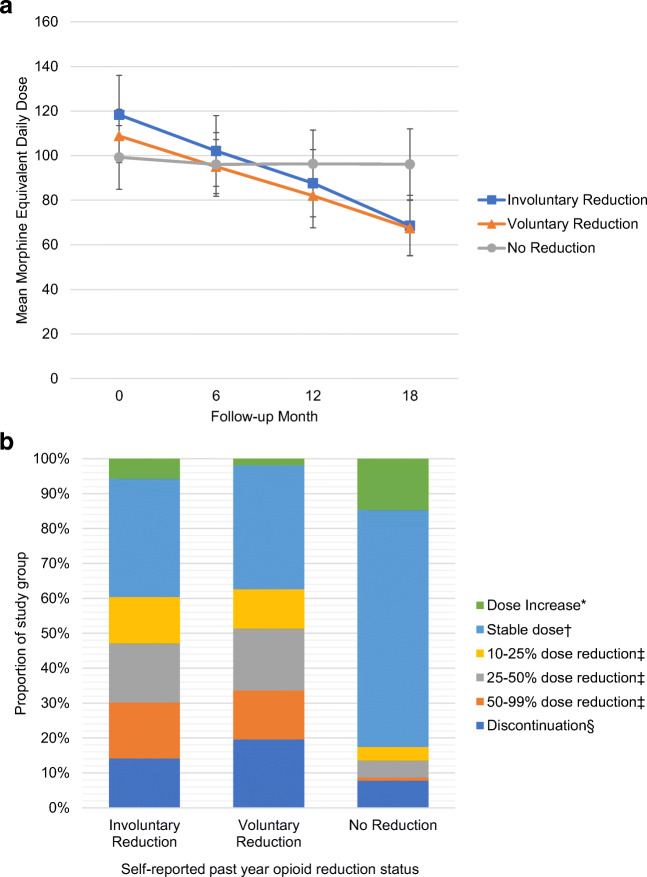
In post hoc analyses of VA pharmacy data, there were distinct opioid dose patterns according to self-reported opioid reduction status (Fig. 2a). Pharmacy dispensing data demonstrated past year opioid dose reduction among 58% and 60% of participants in the self-reported involuntary reduction group and the voluntary reduction group, respectively, compared with 15% in the no past year opioid reduction group (Table 3; Fig. 2b).
Fig. 2.
a Opioid medication dose from VA pharmacy data according self-reported past year opioid reduction status. Mean opioid dose and standard deviation for each self-reported opioid reduction group at 6-month intervals during EPOCH study follow-up. b Magnitude of opioid reduction from VA pharmacy data from 6 months to 18 months according to self-reported opioid reduction status. * Overall past year opioid dose increase defined as > 10% dose increase from 6 months to 18 months. † Overall past year stable dose defined as dose change of ≤ 10% from 6 months to 18 months. ‡ Overall past year opioid dose reduction grouped into three categories based on percent dose decrease from 6 months to 18 months: 10–25%, 25–50%, 50–99%. § Discontinuation defined as no opioid medications dispensed by VA pharmacy over the prior 60 days at 18-month time point.
Table 3.
Pharmacy Medication Data According to Self-Reported Past Year Opioid Reduction Status (N = 290)
| Involuntary opioid reduction | Voluntary opioid reduction | No opioid reduction | |
|---|---|---|---|
| Opioid dose in milligrams ME, mean (SD) | |||
| 0 months | 118.3 (101–136) | 108.9 (97–121) | 99.3 (85–114) |
| 6 months | 102.1 (86–118) | 95.1 (83–107) | 96.1 (82–110) |
| 12 months | 87.7 (73–103) | 82.0 (68–96) | 96.3 (81–111) |
| 18 months | 68.6 (55–82) | 67.5 (55–80) | 96.1 (80–112) |
| Past year change at 18 months | − 33.5 (− 43.1; − 23.8) | − 27.6 (− 36.4; − 18.8) | 0.06 (− 6.0; 6.18) |
| Past year dose change at 18 month follow-up, N (%) | |||
| Opioid dose increase* | 5 (6%) | 2 (2%) | 16 (15%) |
| Stable dose† | 32 (36%) | 36 (38%) | 74 (70%) |
| Opioid dose reduction‡ | 51 (58%) | 58 (60%) | 16 (15%) |
| Any past year opioid dose increase§ | 26 (30%) | 18 (19%) | 34 (32%) |
| Any past year opioid dose reduction║ | 57 (65%) | 67 (70%) | 32 (30%) |
ME, morphine equivalents; SD, standard deviation
*Overall past year opioid dose increase defined as > 10% dose increase from 6 months to 18 months
†Overall past year stable dose defined as dose change of ≤ 10% from 6 months to 18 months
‡Overall past year opioid dose reduction defined as > 10% dose decrease from 6 months to 18 months
§Any past year opioid dose increase defined as any month with dose with > 10% increase compared with dose at 6-month time point
║Any past year opioid dose reduction defined as any month with dose with > 10% decrease compared with dose at 6-month time point
DISCUSSION
Among a sample of 290 VA primary care patients treated with LTOT, two-thirds reported past year opioid reduction, and roughly half of this group reported opioid reduction “without your consent or against your wishes,” which we describe as “involuntary.” To our knowledge, this is the first study to quantify the prevalence of self-reported involuntary opioid reduction. Involuntary opioid reduction will at times be clinically appropriate when medication risks outweigh benefits, but the optimal prevalence of involuntary reduction to balance population-level benefit and risk is not known. As rates of opioid prescribing decline,21 patients on LTOT are increasingly likely to receive a recommendation to reduce opioid medications, both those who agree with this change and those for whom reduction would be involuntary. Future studies should examine this phenomenon in different populations and examine trends over time. Clinical trials involving opioid reduction should consider specifically assessing patient-reported experience with involuntary opioid reduction.
In this sample, self-reported past year opioid reduction status was not associated with a change in pain severity. Several potential explanations for this finding warrant discussion. First, opioid reduction may not, on average, impact pain severity and pain-related function. A prior VA study of opioid discontinuation did not find a significant change in single-item pain numeric rating scores collected in routine clinical care.22 Similarly, a prior systematic review found low-quality evidence that pain and function may improve with voluntary opioid reduction supported by a multidisciplinary team.3 Our study adds to this literature by assessing pain severity with the 3-item PEG scale, which includes both pain intensity and interference items, and by assessing patient-reported involuntary reduction. Alternatively, it is possible that opioid reduction, whether involuntary or voluntary, may have effects that could not be measured in this sample. Our definition of self-reported past year opioid reduction did not include a detailed assessment of the magnitude or speed of dose reduction, which are likely important factors in patients’ experience during opioid reduction. Also, this study was not powered to detect small changes in pain severity or infrequent but important adverse events such as overdose or suicide.
Importantly, pain was severe at baseline for all groups. A majority of participants in all groups reported health-related quality of life as fair or poor. No group, on average, experienced a clinically important improvement in pain severity during the study period. This finding is consistent with findings of prior prospective observational studies of patients with chronic pain treated with LTOT. In a 2-year prospective study of 517 VA and non-VA patients, the Chronic Pain Grade pain intensity and pain disability scores were 62.5 and 50.7 (out of 100), respectively; there was a small but not clinically important improvement in pain intensity (about 1 point per year on a scale of 0–100) and no change in pain disability during follow-up.23 The Australian Pain and Opioids In Treatment (POINT) study observed more than 1200 participants over 4 years; for this cohort, the mean Brief Pain Inventory pain interference score was 5.7 (out of 10) at baseline and remained relatively stable (5.4–5.7) at annual follow-up over 4 years.24 Together these findings highlight the long-term impact of chronic pain for many patients prescribed LTOT.25
This study found that 54% (13/24) of Black participants and 27% (69/253) of White participants reported involuntary opioid reduction. Racial differences in self-reported involuntary opioid reduction were not a pre-specified objective of this study and should be considered exploratory. Racial differences have been previously demonstrated in rates of opioid discontinuation26,27 as well as in perceived quality of care and satisfaction with patient-provider communication.28 These findings warrant further examination in larger and more diverse samples and in other healthcare systems.
In exploratory post hoc analyses, VA pharmacy dispensing data demonstrated more dose reduction in patients who reported dose reduction than in those who did not and similar dose reduction patterns among the two opioid reduction groups. Among participants in the two opioid reduction groups, 65–70% demonstrated at least one opioid reduction of > 10% during the prior year in VA pharmacy data. Potential explanations for discordance between self-report and VA pharmacy data include inaccurate recall about the timing or nature of opioid medication change as well as reduction of opioid medications that were prescribed outside the VA healthcare system. Approximately 80% of patients enrolled in VA have other types of public or private health insurance coverage.29 In one study of Veterans with dementia ages 68 and older in 2010, 25% of individuals who received an opioid medication obtained at least one opioid prescription from both VA and Medicare Part D.30
Study findings should be interpreted in the context of the study’s limitations. First, as this was an observational study and the primary exposure was not randomly assigned, we cannot exclude unmeasured confounding. However, as it is challenging to randomize patients to opioid reduction, observational studies will remain an important resource in efforts to improve knowledge on this topic. Second, participants’ recall of past year medication changes may be incomplete or inaccurate. It was beyond the scope of this study to assess actual past year opioid medication use or to examine opioid medication receipt from multiple healthcare system. Third, this study did not evaluate differences associated with the magnitude or speed of opioid dose change. Future analyses of the full EPOCH study cohort will address these questions. Fourth, we cannot assess the clinical appropriateness of opioid reduction from the standpoint of the prescriber. Future studies should examine clinician decision-making in instances of involuntary opioid reduction, including interviews and chart review. Finally, the study’s primary outcome measure, the PEG scale, assesses past week symptoms, and data points at baseline and 18-month follow-up may not fully capture the dynamic nature of chronic pain over time. The PEG scale has been shown to be responsive over time compared with other validated measures.16
In conclusion, although we did not find adverse effects of involuntary opioid reduction on pain severity, we do not conclude that involuntary opioid reduction is harmless. Our outcome assessment was not comprehensive and did not evaluate potential harms of involuntary opioid reduction such as emotional distress or disruption of patient-clinician relationships. Furthermore, this study did not evaluate rare but serious harms such as hospitalization and suicide. Consistent with expert guidelines, we believe patient-provider discussions of opioid reduction should be collaborative, whenever possible, and should be individualized to address patients’ individual goals and concerns. These findings have important implications for clinicians and patients. For clinicians, study findings build on prior studies showing that, on average, pain severity does not appear to change following opioid reduction. Study findings may be reassuring to patients with chronic pain who fear worsening of pain and function if opioid medications are reduced.
Electronic supplementary material
(DOCX 16 kb)
Acknowledgments
The authors would like to acknowledge the important contributions of Indy Rutks and Agnes Jensen at the Minneapolis VA Health Care System and Lexus Ujano-De Motta, Lindsay Miller and Amy Ladebue at the VA Eastern Colorado Health Care System.
Authors’ Contribution
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Funding
This work was supported by the Department of Veterans Affairs Health Services Research & Development (IIR 14-295). Dr. Frank was supported by VA Health Services Research & Development Career Development Award (HSR&D CDA 15-059). The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US Government.
Compliance with Ethical Standards
Conflict of Interest
The authors have no conflicts of interest to disclose.
Footnotes
Prior Presentations
A version of these results was presented at the Society of General Internal Medicine Annual Meeting in Washington, DC, in May 2019.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krebs EE, Gravely A, Nugent S, et al. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA. 2018;319(9):872–882. doi: 10.1001/jama.2018.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank JW, Lovejoy TI, Becker WC, et al. Patient Outcomes in Dose Reduction or Discontinuation of Long-Term Opioid Therapy: A Systematic Review. Ann Intern Med. 2017;167(3):181–191. doi: 10.7326/M17-0598. [DOI] [PubMed] [Google Scholar]
- 4.Darnall BD, Juurlink D, Kerns RD, et al. International Stakeholder Community of Pain Experts and Leaders Call for an Urgent Action on Forced Opioid Tapering. Pain Med. 2019;20(3):429–433. doi: 10.1093/pm/pny228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich T, Chou R. No Shortcuts to Safer Opioid Prescribing. N Engl J Med. 2019;380(24):2285–2287. doi: 10.1056/NEJMp1904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. FDA Drug Safety Communication Web site. https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes. Published 2019. Accessed July 4, 2020.
- 7.Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20(4):724–735. doi: 10.1093/pm/pny307. [DOI] [PubMed] [Google Scholar]
- 8.Dowell D, Haegerich TM. Changing the Conversation About Opioid Tapering. Ann Intern Med. 2017;167(3):208–209. doi: 10.7326/M17-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank JW, Levy C, Matlock DD, et al. Patients’ Perspectives on Tapering of Chronic Opioid Therapy: A Qualitative Study. Pain Med. 2016;17(10):1838–1847. doi: 10.1093/pm/pnw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howe CQ, Sullivan MD, Saunders KW, et al. Depression and ambivalence toward chronic opioid therapy for chronic noncancer pain. Clin J Pain. 2012;28(7):561–566. doi: 10.1097/AJP.0b013e31823ade59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy LC, Binswanger IA, Mueller SR, et al. “Those Conversations in My Experience Don’t Go Well”: A Qualitative Study of Primary Care Provider Experiences Tapering Long-term Opioid Medications. Pain Med. 2018;19(11):2201–2211. doi: 10.1093/pm/pnx276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs EE, Clothier B, Nugent S, et al. The evaluating prescription opioid changes in veterans (EPOCH) study: Design, survey response, and baseline characteristics. PLoS One. 2020;15(4):e0230751. doi: 10.1371/journal.pone.0230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Center for Injury Prevention and Control. CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2018 version. https://www.cdc.gov/drugoverdose/resources/data.html. Accessed July 4, 2020.
- 14.Banta-Green CJ, Von Korff M, Sullivan MD, Merrill JO, Doyle SR, Saunders K. The prescribed opioids difficulties scale: a patient-centered assessment of problems and concerns. Clin J Pain. 2010;26(6):489–497. doi: 10.1097/AJP.0b013e3181e103d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kean J, Monahan PO, Kroenke K, et al. Comparative Responsiveness of the PROMIS Pain Interference Short Forms, Brief Pain Inventory, PEG, and SF-36 Bodily Pain Subscale. Med Care. 2016;54(4):414–421. doi: 10.1097/MLR.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Korff M, Scher AI, Helmick C, et al. United States National Pain Strategy for Population Research: Concepts, Definitions, and Pilot Data. J Pain. 2016;17(10):1068–1080. doi: 10.1016/j.jpain.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 18.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006;21(3):267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy PS, Lemeshow S. Sampling of Population, Methods and Application. 3. New York: John Wiley & Sons; 1999. [Google Scholar]
- 20.Lumley T. Survey analysis in R: Analysis of Complex Survey Samples. http://r-survey.r-forge.r-project.org/survey/. Accessed July 4, 2020.
- 21.Hadlandsmyth K, Mosher H, Vander Weg MW, Lund BC. Decline in Prescription Opioids Attributable to Decreases in Long-Term Use: A Retrospective Study in the Veterans Health Administration 2010-2016. J Gen Intern Med. 2018;33(6):818–824. doi: 10.1007/s11606-017-4283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPherson S, Lederhos Smith C, Dobscha SK, et al. Changes in pain intensity after discontinuation of long-term opioid therapy for chronic noncancer pain. Pain. 2018;159(10):2097–2104. doi: 10.1097/j.pain.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morasco BJ, Smith N, Dobscha SK, Deyo RA, Hyde S, Yarborough BJH. Outcomes of prescription opioid dose escalation for chronic pain: results from a prospective cohort study. Pain. 2020;161(6):1332–1340. doi: 10.1097/j.pain.0000000000001817. [DOI] [PubMed] [Google Scholar]
- 24.Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health. 2018;3(7):e341–e350. doi: 10.1016/S2468-2667(18)30110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC Prevalence of chronic pain and high-impact chronic pain among adults—United States. MMWR Morb Mortal Wkly Rep. 2016;2018(67):1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371–376. doi: 10.1016/j.drugalcdep.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker WC, Starrels JL, Heo M, Li X, Weiner MG, Turner BJ. Racial differences in primary care opioid risk reduction strategies. Ann Fam Med. 2011;9(3):219–225. doi: 10.1370/afm.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DR, Lawrence JA, Davis BA, Vu C. Understanding how discrimination can affect health. Health Serv Res. 2019;54(Suppl 2):1374–1388. doi: 10.1111/1475-6773.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang G, Kim S, Muz B, Gasper J. 2017 Survey of Veteran Enrollees’ Health and Use of Health Care. 2018. www.va.gov/HEALTHPOLICYPLANNING/SoE2017/VA_Enrollees_Report_Data_Findings_Report2.pdf. Accessed August 2, 2020.
- 30.Schleiden LJ, Thorpe CT, Cashy JP, et al. Characteristics of dual drug benefit use among veterans with dementia enrolled in the Veterans Health Administration and Medicare Part D. Res Social Adm Pharm. 2019;15(6):701–709. doi: 10.1016/j.sapharm.2018.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)