Abstract
Background
Immune checkpoint inhibitors (ICIs) are used widely for treating metastatic urothelial carcinoma (mUC). In practical settings, evidence is lacking on the efficacy of ICIs in some difficult-to-treat patients, such as those with end-stage renal disease (ESRD). Herein, we evaluate the safety and efficacy of ICIs for patients with mUC and ESRD.
Methods
For this retrospective study, patients with mUC who were given ICIs at Kaohsiung Chang Gang Memorial Hospital and Linkou Chang Gung Memorial Hospital between April 2016 and November 2019 were consecutively enrolled. All clinicopathologic data, treatment responses, and adverse events were recorded. The immune-related adverse events (AEs), objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) were compared between ESRD and non-ESRD groups.
Results
In total, 129 patients with mUC were enrolled, with 11 patients categorized as the ESRD group. Among these patients with ESRD receiving ICIs, 7 of 11 (63.6%) had high-grade (grade ≥3) AEs, chiefly hematologic toxicity. Some rarely encountered AEs were noted, including toxic epidermal necrolysis, tuberculosis reactivation, ascites, and cytokine release syndrome. Patients in the ESRD group had numerically higher ORR (54.5% vs. 28.8%, p = 0.09), PFS (7.1 vs. 3.5 months, p = 0.42), and OS (not reached vs. 15.4 months) than the non-ESRD group. A multivariate Cox regression model demonstrated that leukocytosis (hazard ratio [HR]: 2.63; 95% confidence interval [CI]: 1.23–5.63; p = 0.01) and neutrophil-to-lymphocyte ratio (HR 2.91; 95% CI: 1.30–6.53; p = 0.01) were independent prognostic factors.
Conclusion
Administration of ICIs in patients with mUC and ESRD demonstrated a modest antitumor activity, and should be used with caution for increasing risk of hematologic toxicity.
Keywords: immune checkpoint inhibitor, end-stage renal disease, metastatic urothelial carcinoma, safety, survival
Introduction
Urothelial carcinoma (UC) is a common cancer worldwide, with approximately 500,000 new cases diagnosed annually and an estimated 150,000 cancer-related deaths (1). Early-stage UC can be cured through radical surgery, including cystectomy for bladder cancer and nephroureterectomy for upper tract urothelial carcinoma (UTUC). Nevertheless, approximately 10–30% of these patients experience local recurrence or distant metastasis, leading to mortality from such diseases (2). Cisplatin-based chemotherapy has been the gold standard therapy since 1990, with an objective response rate (ORR) of 40–50% and an overall survival (OS) of 14–15 months (3). As the recent breakthrough of immune checkpoint inhibitors (ICIs) has been widely studied for various cancer types, the paradigm of treatment has shifted to ICIs for patients failing to respond to platinum-based chemotherapy and those who are ineligible for cisplatin (4–8). In the pivotal phase 3 KEYNOTE-045 study, compared with conventional chemotherapy, pembrolizumab conferred a significant survival benefit on patients with metastatic UC (mUC) whose conditions were refractory to first-line platinum-based chemotherapy, regardless of the patients’ PD-L1 expression (4). At this time, five ICIs have been approved by the U.S. Food and Drug Administration (FDA) for mUC treatment.
The efficacy of cisplatin-based chemotherapy in patients with mUC is generally limited by poor Eastern Cooperative Oncology Group (ECOG) performance status or chronic kidney disease. In general, the proportion of patients for whom cisplatin is unsuitable may be 30–50% of the population with stage IV mUC (9). Given their more favorable toxicity profile, ICIs have been investigated as first-line treatments for cisplatin-ineligible patients with mUC. The promising OS results from the IMVigor 210 trial demonstrated that atezolizumab monotherapy provided an excellent OS of 15.8 months, prompting the FDA to grant accelerated approval for ICIs as first-line treatment for cisplatin-ineligible patients with mUC (10). However, many patients have been excluded from prospective trials owing to poor ECOG performance status or having coexisting autoimmune disease or end-stage renal disease (ESRD) requiring hemodialysis. Treatment options for patients with such rare conditions remain uncertain, and related evidence is lacking.
ESRD is a common comorbidity in patients with mUC. UTUC and urothelial carcinoma of the bladder (UCB) independently increase the risk of ESRD, with hazard ratios (HRs) for ESRD up to 7.75 and 3.12 in patients with UTUC and UCB, respectively (11). Patients with ESRD, especially women aged 50 to 60 years, also have a high risk of developing UC (12). As ICIs are eliminated through the reticuloendothelial system and are not excreted through renal filtration, their use in patients receiving dialysis provides an alternative therapeutic choice to avoid cumulative toxicity from conventional chemotherapy (13). Only small case series have provided evidence of the safety and efficacy of ICIs in patients with ESRD, and most of such studies have been on melanoma, lung cancer, and renal cell carcinoma (14, 15). To assist such difficult-to-treat patients, data on the safety and efficacy of ICIs are urgently required. The aim of this retrospective study was to evaluate the safety and efficacy of immune ICIs in patients with mUC and ESRD.
Methods
Patients
We retrospectively reviewed patients with mUC who received ICIs between April 2016 and November 2019 at Kaohsiung Chang Gung Memorial Hospital and Linkou Chang Gung Memorial Hospital in Taiwan. All clinicopathologic data were collected from electrical medical recording systems by physicians and trained assistants. Database variables included age, sex, ECOG performance status, primary tumor site, visceral or lymph node metastasis, PD-L1 expression by tumor proportion score, ICI type, regimen of combination treatment or previous systemic treatment, laboratory data, treatment response, and adverse events (AEs). The study was approved by the Institutional Review Board of Chang Gung Medical Foundation.
Treatment
All patients received an anti-PD-1 (nivolumab, pembrolizumab) or anti-PD-L1 (atezolizumab, durvalumab, or avelumab) medication. The regimen, treatment sequence, and combined treatment regimen were at the discretion of the physician. The regimen of combined treatment included chemotherapy, a cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitor, and a poly ADP-ribose polymerase (PARP) inhibitor.
Response Evaluation and Endpoints
All patients had attended scheduled appointments during treatment until disease progression, treatment intolerance, or death. The follow-up visit procedures included physical examinations, laboratory tests, and imaging studies. Patients were subjected to computed tomography scans of the chest or abdomen for tumor response assessments using the Response Evaluation Criteria in Solid Tumors (version 1.1).
The primary endpoint was treatment-related AEs in patients with ESRD. The observed AEs during any round of ICIs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 ( Supplementary Table 1 ). All patients who received at least one cycle of immunotherapy were included in the analysis. The secondary endpoints of the study were treatment response, OS, and progression-free survival (PFS). OS was defined as the time interval from the date of ICIs commencement (any cycle) to the date of death or final patient contact.
Statistical Analysis
All statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA), and survival curves were plotted using GraphPad Prism version 6.04 (GraphPad Software, La Jolla California, USA). The differences between the ESRD subgroup and patients without ESRD were examined using chi-squared (χ2) and t tests for categorical and continuous variables, respectively. We constructed OS and PFS curves using the Kaplan–Meier method. Univariate and multivariate analyses were performed using the Cox proportional hazards regression analysis. A p value <0.05 was considered statistically significant.
Results
Patient Characteristics
In total, 129 patients were included in this study, including 11 patients (8.5%) with ESRD who were on maintenance hemodialysis; they were categorized into the ESRD group. Basic patient characteristics are shown in Table 1 . According to group comparison, the ESRD group had a significantly higher proportion of patients with an ECOG scale score of ≥2 (45.5 vs. 16.1%, p = 0.05), UTUC (72.7% vs. 59.3%, p = 0.05), and anemia (90.0 vs. 35.1%, p = 0.001). No significant difference was noted in age, gender, site of visceral metastasis, tumor proportion score, regimen and sequence of ICIs, white blood cell count, and neutrophil to lymphocyte ratio (NLR) between the two groups. Two-thirds of patients (65.1%) were given anti-PD-1 therapy, and the majority of ICIs were used as monotherapy (64.3%) and as a first-line treatment (75.2%). The individual details of the ESRD group are listed in Table 2 .
Table 1.
Patients demographics and baseline characteristics.
| N (%) | ESRD (%) | Non-ESRD (%) | p value | |
|---|---|---|---|---|
| N | 129 | 11 | 118 | |
| Age (median, years) | 66 | 64 | 66 | 0.55 |
| Male | 76 (58.9) | 4 (36.4) | 72 (61.0) | 0.2 |
| Tumor location | 0.05 | |||
| UCB | 49 (38.0) | 2 (18.2) | 47 (39.8) | |
| UTUC | 78 (60.5) | 8 (72.7) | 70 (59.3) | |
| Multifocal | 2 (1.5) | 1 (9.1) | 1 (0.8) | |
| ECOG | 0.05 | |||
| 0-1 | 102 (79.1) | 6 (54.5) | 96 (81.4) | |
| ≧2 | 24 (18.6) | 5 (45.5) | 19 (16.1) | |
| Missing | 3 (2.3) | 0 | 3 (2.5) | |
| ICI sequence | 0.25 | |||
| 1st line | 97 (75.2) | 6 (54.5) | 91 (77.1) | |
| 2nd line | 19 (14.7) | 3 (27.3) | 16 (13.6) | |
| 3rd line or later | 13 (10.1) | 2 (18.2) | 11 (9.3) | |
| ICI type | 0.75 | |||
| Anti-PD-1 | 84 (65.1) | 8 (72.7) | 76 (64.4) | |
| Anti-PD-L1 | 45 (34.9) | 3 (27.3) | 42 (35.6) | |
| Treatment partner | 0.63 | |||
| Monotherapy | 83 (64.3) | 7 (63.6) | 76 (64.4) | |
| Chemotherapy | 38 (29.5) | 4 (36.4) | 34 (28.8) | |
| Anti-CTLA-4 | 8 (6.2) | 0 | 8 (6.8) | |
| PD-L1 testing* | 71 (55.0) | 7 (63.6) | 64 (54.2) | 0.75 |
| PD-L1 result¶ | ||||
| ≧1 | 37 (52.1) | 4 (57.1) | 33 (51.6) | 0.78 |
| ≧10 | 27 (38.0) | 2 (28.6) | 25 (39.1) | 0.59 |
| Visceral metastasis | 70 (54.3) | 4 (36.4) | 66 (55.9) | 0.34 |
| Liver | 25 (19.4) | 2 (18.2) | 23 (19.5) | 0.99 |
| Lung | 46 (35.7) | 1 (9.1) | 45 (38.1) | 0.10 |
| Bone | 25 (19.4) | 2 (18.2) | 23 (19.5) | 0.99 |
| Laboratory tests | ||||
| WBC ≧10,000/μl | 102 (79.1) | 6 (54.5) | 96 (81.4) | 0.70 |
| Hgb <10 g/dl | 49 (39.5) | 9 (90.0) | 40 (35.1) | 0.001 |
| NLR ≧5 | 49 (41.2) | 5 (50.0) | 44 (40.4) | 0.74 |
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; ESRD, end-stage renal disease; Hgb, hemoglobin; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; UCB, urothelial cancer of the bladder; UTUC, upper tract urothelial carcinoma; NLR, neutrophil to lymphocyte ratio; WBC, while blood cell count.
*PD-L1 immunohistochemistry testing used Dako 22C3 antibody.
¶Scoring by tumor proportion score (TPS) criteria.
Table 2.
Patient profiles, treatment, response, and adverse events of ESRD group.
| Patient | Age | Primary site | Therapy | Combination | Line | Response | OS (months) | Status | Hematologic AE | Other AE |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | Right renal pelvis | Atezolizumab | Paclitaxel | 3 | PD | 8.05 | AWD | Gr.4 neutropenia Gr.3 anemia Gr.1 thrombocytopenia |
Gr.1 hepatitis Gr.1 anorexia Gr.1 fatigue |
| 2 | 79 | Left ureter | Pembrolizumab | – | 1 | PR | 4.80 | AWD | Gr.2 anemia | Gr.2 ascites |
| 3 | 82 | Left renal pelvis and ureter | Pembrolizumab | Gemcitabine | 1 | PD | 0.72 | DOD | Gr.3 neutropenia Gr.3 anemia Gr.3 thrombocytopenia |
– |
| 4 | 69 | Left renal pelvis | Pembrolizumab | – | 1 | PD | 5.85 | DOD | Gr.2 anemia | Gr.1 hepatitis Gr.3 anorexia |
| 5 | 68 | Right renal pelvis | Nivolumab | Gemcitabine | 1 | PR | 12.16 | AWD | Gr.3 neutropenia Gr.4 anemia Gr.2 thrombocytopenia |
Gr.1 hepatitis Gr.3 ascites |
| 6 | 63 | Left renal pelvis | Nivolumab | – | 3 | PD | 0.23 | DOD | Gr.2 anemia Gr.1 thrombocytopenia |
Gr.4 CRS |
| 7 | 45 | Right renal pelvis and bladder | Atezolizumab | Paclitaxel | 2 | SD | 19.68 | AWD | Gr.4 anemia | – |
| 8 | 65 | Right renal pelvis | Pembrolizumab | – | 2 | PR | 27.17 | AWD | Gr.2 neutropenia Gr.2 anemia Gr.1 thrombocytopenia |
Gr.2 eczema |
| 9 | 66 | Right renal pelvis | Atezolizumab | – | 1 | PR | 15.54 | AWD | Gr.4 neutropenia Gr.4 anemia Gr.1 thrombocytopenia |
Gr.3 TB peritonitis Gr.4 TEN |
| 10 | 74 | Bladder | Pembrolizumab | – | 2 | PR | 14.26 | AWD | Gr.3 anemia Gr.1 thrombocytopenia Gr.2 anorexia |
Gr.2 fatigue |
| 11 | 35 | Bladder | Pembrolizumab | – | 1 | PR | 4.63 | AWD | Gr.2 neutropenia Gr.2 anemia Gr.1 thrombocytopenia |
– |
OS, overall survival; AE, adverse event; PD, progressive disease; PR, partial response; SD, stable disease; AWD, alive with disease; DOD, dead of disease; Gr, grade; CRS, cytokine release syndrome; TB, tuberculosis; TEN, toxic epidermal necrolysis.
Treatment-Related AEs
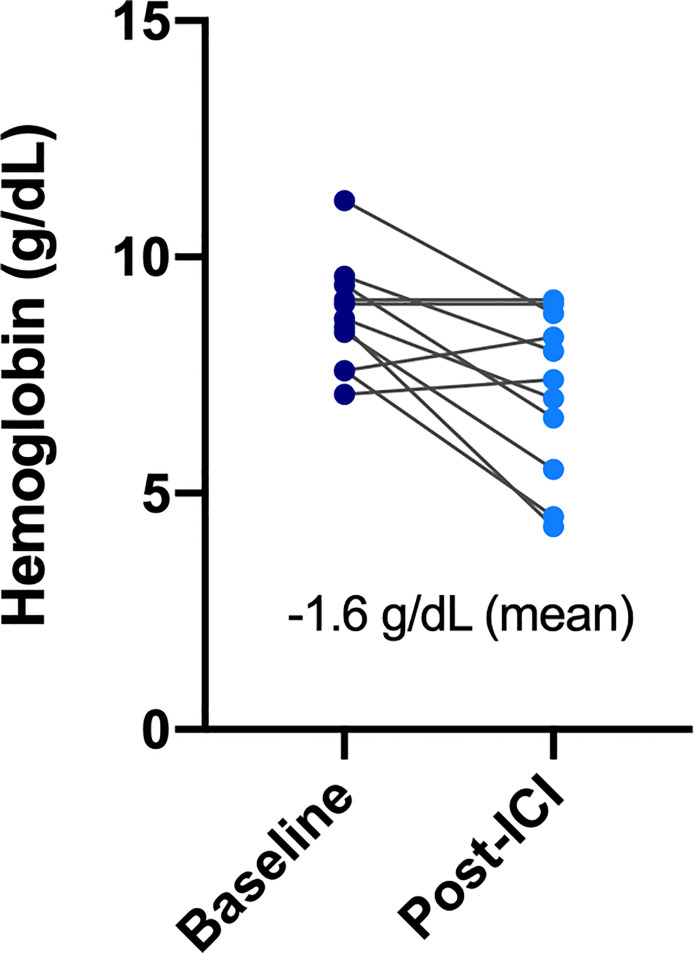
All patients in the ESRD group experienced at least one treatment-related AE during the treatment period, and seven of them (63.6%) had high-grade (grade ≥3) AEs ( Table 3 ). AEs of all grades included hematologic toxicity (neutropenia 54.5%; anemia 100%; and thrombocytopenia 72%), hepatitis (27.3%), fatigue (18.2%), anorexia (27.3%), and dermatologic toxicity (18.2%). Regarding hematologic toxicity, four patients (36.4%) had grade 3 neutropenia or higher, six (54.5%) had grade 3 anemia or higher, and one (9.1%) had grade 3 thrombocytopenia or higher. However, given the nature of defective function on hematopoiesis for patients with ESRD, the median baseline hemoglobin (Hb) of ESRD group was 8.75 g/dl. The low level of baseline Hb in ESRD group can actually be categorized in CTCAE grade 2 anemia, indicating that any decline of Hb will classified into grade 3 anemia. Although a considerable number of grade 3–4 anemia were observed in the ESRD group, the decrease in mean Hb between baseline and post-ICI administration was 1.6 g/dl, which was not substantially significant ( Figure 1 ). For one who developed toxic epidermal necrolysis (TEN), a grade 4 dermatologic AE was recorded. Two patients presented with refractory ascites after receiving a PD-1 inhibitor. The ascites subsided after ICI usage was discontinued and recurred again after the re-administration of ICIs for disease relapse. One patient had disseminated tuberculosis reactivation. A cytokine release syndrome (CRS)-like syndrome was observed in one patient who presented with intermittent spiking fever and respiratory failure after receiving a PD-1 inhibitor.
Table 3.
Adverse events in ESRD and non-ESRD group.
| Adverse events | ESRD (%) | Non-ESRD (%) | p value |
|---|---|---|---|
| Any grade | 11 (100) | 84 (71.2) | 0.04 |
| Grade 3/4 | 7 (63.6) | 42 (35.6) | 0.07 |
| Neutropenia | 6 (54.5) | 27 (22.9) | 0.02 |
| Grade 3/4 | 4 (36.4) | 10 (8.5) | 0.004 |
| Anemia | 11 (100) | 54 (45.8) | 0.001 |
| Grade 3/4 | 6 (54.5) | 32 (27.1) | 0.07 |
| Thrombocytopenia | 8 (72.0) | 43 (36.4) | 0.02 |
| Grade 3/4 | 1 (9.1) | 16 (13.6) | 0.68 |
| Hepatitis | 3 (27.3) | 34 (28.8) | 0.91 |
| Fatigue | 2 (18.2) | ||
| Anorexia | 3 (27.3) | ||
| Skin* | 2 (18.2) | ||
| AE of specific interest | |||
| Ascites | 2 (18.2) | ||
| TB reactivation | 1 (9.1) | ||
| TENS | 1 (9.1) | ||
| CRS-like syndrome | 1 (9.1) |
AE, adverse event; TB, tuberculosis; TENS, toxic epidermal necrolysis; CRS, cytokine release syndrome; ESRD, end-stage renal disease.
*One TENS classified as grade 4 dermatologic toxicity.
Figure 1.
Change of hemoglobin before and after ICIs administration in the ESRD group.
We also compared the incidence of all grade AE and hematologic AE between ESRD and non-ESRD groups. As shown in Table 3 , patients with ESRD on ICIs treatment had a higher incidence of all grade of neutropenia (54.5 vs. 22.9%, p = 0.02), anemia (100 vs. 45.8%, p = 0.001) and thrombocytopenia (72.0 vs. 36.4%, p = 0.02) than non-ESRD patients. Except for hematologic toxicity, there was no new additional safety concerns emerged from this comparative study between ESRD and non-ESRD group.
Treatment Responses
The objective response rate (ORR) was significantly higher in the ESRD group than in the non-ESRD group (54.5 vs. 28.8%, p = 0.09). In terms of the disease control rate (DCR), the ESRD group benefited more (63.6%) than the non-ESRD group did (50.0%); in the ESRD group, six patients achieved partial response (54.5%), and one patient achieved stable disease status (9.1%). All details are provided in Table 4 .
Table 4.
Treatment response.
| ESRD (%) | Non-ESRD (%) | p value | |
|---|---|---|---|
| Complete response (CR) | 0 | 15 (12.7) | |
| Partial response (PR) | 6 (54.5) | 19 (16.1) | |
| Stable disease (SD) | 1 (9.1) | 25 (21.2) | |
| Progressive disease (PD) | 4 (36.4) | 59 (50.0) | |
| Overall response rate (ORR) | 6 (54.5) | 34 (28.8) | 0.09 |
| Disease control rate (DCR) | 7 (63.6) | 59 (50.0) | 0.53 |
ESRD, end-stage renal disease.
Survival Outcomes
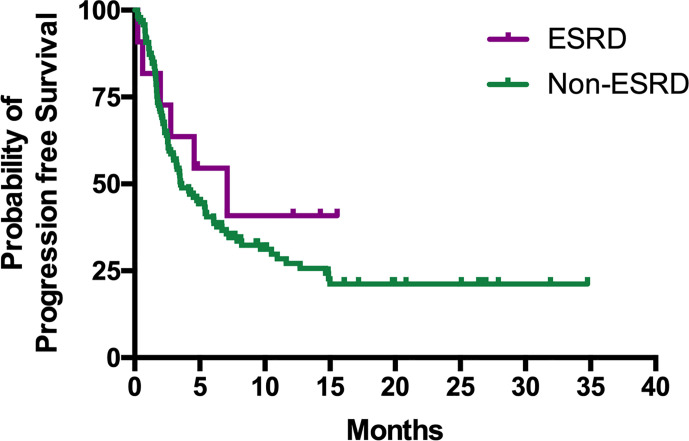
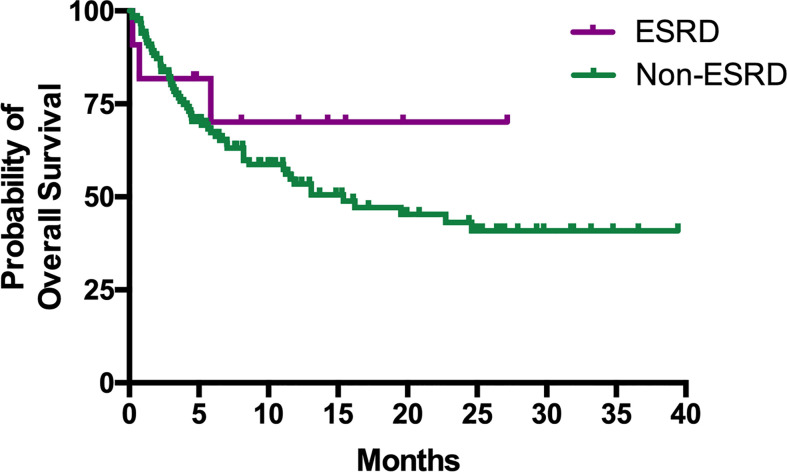
The median PFS of patients in the ESRD and non-ESRD groups was 7.1 and 3.5 months, respectively (p = 0.42; the PFS curve is plotted in Figure 2 ). The median OS of patients in the ESRD group was not reached and was 15.4 months in the non-ESRD group (the OS curve is plotted in Figure 3 ). In the univariate analysis of OS, the prognostic factors included ECOG (≥2 vs. <1; HR: 1.96; 95% CI: 1.06–3.65; p <0.03), leukocytosis (≥10,000/μl vs. <10,000/μL; HR: 3.80; 95% CI: 2.22–6.51; p <0.001), anemia (<10 g/dl vs. ≥10 g/dl; HR: 2.41; 95% CI: 1.43–4.04; p = 0.001) and NLR (≥5 vs. <5; HR: 3.93; 95% CI: 2.29–6.77; p <0.001). In the univariate analysis, a trend of survival benefits was observed for patients without liver metastasis (HR: 1.65; 95% CI: 0.92–2.98; p = 0.09) and without lung metastasis (HR: 1.59; 95% CI: 0.95–2.66; p = 0.08). After adjustments were made for all potential prognostic factors in the multivariate analysis, the only independent factor was leukocytosis (HR: 2.63; 95% CI: 1.23–5.63; p = 0.01) and NLR (HR: 2.91; 95% CI: 1.30–6.53; p = 0.01). All details are presented in Table 5 .
Figure 2.
Kaplan–Meier curves of PFS for mUC patients with or without ESRD receiving ICIs.
Figure 3.
Kaplan–Meier curves of OS for mUC patients with or without ESRD receiving ICIs.
Table 5.
Univariate and multivariate analysis of overall survival.
| Characteristics | Median OS | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| (month) | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (year) | 0.50 | 0.19 | |||
| <65 | 13.1 | 1 | 1 | ||
| ≧65 | 22.7 | 0.84 (0.50–1.40) | 0.65 (0.35–1.23) | ||
| Gender | 0.59 | 0.52 | |||
| Female | 15.4 | 1 | 1 | ||
| Male | 19.5 | 0.87 (0.52–1.45) | 1.26 (0.62–2.55) | ||
| Primary tumor | 0.21 | 0.28 | |||
| UCB | 22.7 | 1 | 1 | ||
| UTUC | 11.9 | 1.42 (0.82–2.47) | 1.45 (0.74–2.85) | ||
| ECOG | 0.03 | 0.35 | |||
| 0–1 | 16.2 | 1 | 1 | ||
| ≧2 | 4.4 | 1.96 (1.06–3.65) | 1.47 (0.66–3.29) | ||
| ICI sequence | |||||
| 1st line | 19.5 | 1 | 0.37 | 1 | 0.14 |
| 2nd line | 16.2 | 0.95 (0.46–1.95) | 0.89 | 2.22 (0.93–5.28) | 0.07 |
| 3rd line or later | 5.2 | 1.69 (0.79–3.61) | 0.18 | 0.83 (0.28–2.43) | 0.73 |
| ICI type | 0.68 | 0.78 | |||
| Anti-PD-1 | 15.4 | 1 | 1 | ||
| Anti-PD-L1 | 19.5 | 0.89 (0.52–1.54) | 0.91 (0.45–1.83) | ||
| Treatment partner | |||||
| Monotherapy | 13.1 | 1 | 0.83 | 1 | 0.64 |
| Chemotherapy | NR | 0.84 (0.47–1.52) | 0.57 | 0.85 (0.43–1.69) | 0.64 |
| Anti-CTLA-4 | 13.1 | 1.05 (0.41–2.68) | 0.92 | 0.57 (0.15–2.12) | 0.40 |
| Visceral metastasis | 0.29 | 0.91 | |||
| No | 16.2 | 1 | 1 | ||
| Yes | 13.4 | 1.33 (0.81–2.29) | 0.94 (0.29–3.05) | ||
| Liver metastasis | 0.09 | 0.30 | |||
| No | 22.7 | 1 | 1 | ||
| Yes | 8.2 | 1.65 (0.92–2.98) | 1.53 (0.68–3.42) | ||
| Lung metastasis | 0.08 | 0.17 | |||
| No | NR | 1 | 1 | ||
| Yes | 8.6 | 1.59 (0.95–2.66) | 1.95 (0.75–5.07) | ||
| Bone metastasis | 0.49 | 0.42 | |||
| No | 15.4 | 1 | 1 | ||
| Yes | 8.6 | 1.24 (0.67–2.31) | 0.72 (0.33–1.60) | ||
| Leukocytosis | <0.001 | 0.01 | |||
| WBC <10,000/μl | 24.6 | 1 | 1 | ||
| WBC ≧10,000/μl | 3.9 | 3.80 (2.22–6.51) | 2.63 (1.23–5.63) | ||
| Anemia | 0.001 | 0.38 | |||
| Hgb ≧10 g/dl | 22.7 | 1 | 1 | ||
| Hgb <10 g/dl | 4.4 | 2.41 (1.43–4.04) | 1.45 (0.64–3.28) | ||
| Neutrophil to lymphocyte ratio | <0.001 | 0.01 | |||
| NLR <5 | NR | 1 | 1 | ||
| NLR ≧5 | 4.1 | 3.93 (2.29–6.77) | 2.91(1.30–6.53) | ||
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; ESRD, end-stage renal disease; Hgb, hemoglobin; ICI, immune checkpoint inhibitor; OS, overall survival; PD-1, programmed cell death protein 1; NLR, neutrophil to lymphocyte ratio; NR, non-reach; UCB, urothelial cancer of the bladder; UTUC, upper tract urothelial carcinoma; WBC, while blood cell count.
Discussion
The present study reports the treatment experience of 11 consecutive patients with ESRD who received ICIs for mUC. Although some unexpected AEs occurred, generally, in patients with ESRD, the ICIs were well tolerated without additional toxicity. Furthermore, the major efficacy endpoints of ORR, PFS, and OS suggested benefits of ICI use in patients with ESRD. To our knowledge, this is the largest case series on the safety and efficacy of ICIs for patients with cancer who require maintenance hemodialysis. Our real-world data indicate that the administration of ICIs may be beneficial in such difficult treatment scenarios.
A few case reports and case series had examined the efficacy and safety of administrating ICIs in patients with ESRD on dialysis. In reviewing literature, only 41 patients had been reported; most of them were metastatic melanoma, NSCLC and renal cell carcinoma (RCC), only five cases were mUC (13–31) ( Table 6 ) Vitale et al. reported eight ESRD patients with metastatic RCC who received dialysis (seven on hemodialysis, one on peritoneal dialysis) and nivolumab as cancer treatment. Only two patients (25%) experienced grade 3 AEs (diarrhea, asthenia, and anorexia), and five patients (62.5%) had grade 1–2 AEs, including cutaneous toxicities, anorexia, diarrhea, nausea, vomiting, arthralgia, and hematologic toxicities. These irAEs were appropriately managed with systemic corticosteroid and symptomatic treatment (15). Strohbehn et al. presented a brief report of treatment response and side effects in 19 ESRD patients received ICI therapy. However, the study population were quite heterogeneous in cancer types (six genitourinary cancer, three melanoma, three merkel cell carcinoma, three head and neck cancer), ICI regimen (90% anti-PD-1/PD-L1, 5% anti-CTLA-4 and 5% combined anti-PD-1/CTLA-4), and dialysis modality (79% hemodialysis, 21% peritoneal dialysis), which limited to achieve a definite conclusion (32). Compared with previous reports, our study revealed more hematologic AEs, 36.4% of which were grade 3–4 neutropenia. However, a standard chemotherapy regimen, either of gemcitabine plus cisplatin or MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin), caused more than 70% of patients to experience grade 3–4 neutropenia (3). Given concerns related to neutropenia and risk of infection, ICI is a safe treatment for patients with mUC and ESRD.
Table 6.
Summary of 41 published cases of the use of immune checkpoint inhibitors in dialysis patients.
| Reference | n | Age | Dialysis | Cancer | ICI | Response | Toxicity |
|---|---|---|---|---|---|---|---|
| Cavalcante et al. (14) | 2 | 56,69 | HD | Melanoma | Ipilimumab | CR (1),PR (1) | G2 fatigue, G1-2 pruritus, G3 pemphigoid rash |
| Boils et al. (16) | 1 | 74 | HD* | NSCLC-SCC | Nivolumab | NA | Renal allograft rejection (3 doses) |
| Ong et al. (17) | 1 | 76 | HD* | Melanoma | Nivolumab | PR | Renal allograft rejection (8 days) |
| Carlo et al. (18) | 1 | 77 | HD | mRCC | Nivolumab | PR | Pseudo-progression with respiratory failure |
| Chang et al. (19) | 1 | 63 | HD | Melanoma | Pembrolizumab | CR | G1 fatigue |
| Lipson et al. (20) | 1 | 57 | HD* | Cutaneous SCC | Pembrolizumab | PR (85% reduction) | Renal allograft rejection (2 months) |
| Spain et al. (21) | 1 | 48 | HD* | Melanoma | Ipilimumab (1) Nivolumab (2) |
PR | Renal allograft rejection (8 days of nivolumab) |
| Alhamad et al. (22) | 1 | 68 | HD* | Melanoma | Ipilimumab (1) Pembrolizumab (2) |
Progression (1) NA (Pembrolizumab) |
Renal allograft rejection (3 weeks of pembrolizumab) |
| Jose et al. (23) | 1 | 40 | HD/PD* | Melanoma† | Ipilimumab | Progression | Renal allograft rejection (after two cycles) |
| Tabei et al. (24) | 1 | 49 | HD | RCC | Nivolumab | PR | No AEs |
| Boyle et al. (25) | 1 | 57 | HD | Melanoma‡ | Nivolumab | PR | No AEs |
| Park and Daniels (26) | 4 | 66–71 | HD (3) PD (1) |
RCC (2) Cutaneous SCC (2) |
Nivolumab (2) Pembrolizumab (2) |
SD (1), PR (3) | G2 rash, G2 fatigue G3 pneumonitis, G4 encephalitis¶ |
| Ishizuka et al. (27) | 1 | 66 | HD | NSCLC-SCC | Pembrolizumab | PR | G1 rash |
| Ansari et al. (28) | 1 | 72 | HD | RCC | Nivolumab | PR | No G2-4 AEs |
| Cheun et al. (13) | 3 | 64–68 | HD | RCC (2) Renal pelvic UC (1) |
Nivolumab (2) Atezolizumab (1) |
PR (1), SD (1), Progression (1) |
G2 pneumonitis |
| Vitale et al. (15) | 8 | 51–77 | HD (7) PD (1) |
RCC (8) | Nivolumab | PR (1), SD (5), Progression (2) |
G2 Nausea, G1 Vomiting, G2-3 Diarrhea G2-3 Anorexia, G1-3 Asthenia, G1 Arthralgia G1-2 Cutaneous, G1-2 Hematologic |
| Parisi et al. (29) | 1 | NA | HD | UC§ | Atezolizumab | PR | G1 itching, G1 asthenia G1 nausea, G1 dysgeusia, G1 constipation |
| Osmán-García et al. (30) | 3 | 60–77 | HD (2) PD (1) |
RCC | Nivolumab | PR (2), PD (1) | No G2-4 AEs |
| Hirsch et al. (31) | 8 | 35–83 | HD (7) PD (1) |
UC (3), HCC (1), CCA (1), HL (1), NET (1), RCC (1) |
Pembrolizumab (4) Nivolumab (3)|| Iipilimumab (1)|| Atezolizumab (1) |
SD (3) Progression (5) |
Dermatitis (1) Renal allograft rejection (1) |
| Current study | 11 | 35–82 | HD | UC (11) | Pembrolizumab (6) Nivolumab (2) Atezolizumab (3) |
PR (6), SD (1) Progression (4) |
G1-4 cytopenia, G1 hepatitis, G2-3 ascites G4 CRS, G3 TB peritonitis, G4 TEN G1-3 anorexia, G1-2 fatigue, G2 eczema |
n, case number; NA, not available; HD, hemodialysis; PD, peritoneal dialysis; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma; RCC, renal cell carcinoma; UC, urothelial carcinoma; HCC, hepatocellular carcinoma; CCA, cholangiocarcinoma; HL, Hodgkin lymphoma; NET, neuroendocrine tumor; ICI, immune checkpoint inhibitor; CR, complete response; PR, partial response; SD; stable disease; G, grade; AE, adverse event; CRS, cytokine release syndrome; TB, tuberculosis; TEN, toxic epidermal necrolysis.
*Dialysis dependence after renal graft rejection.
†Choroid melanoma.
‡Donor derived melanoma.
§Bladder sarcomatoid carcinoma.
||One patient received both nivolumab and ipilimumab.
¶In this case report, one patient died from possible treatment-related causes.
We also reported some notable irAEs in this study. A 65-year-old woman had disseminated tuberculosis reactivation and TEN after anti-PD-L1 administration. The patient fully recovered from TEN after systemic steroid administration and intensive skin care, and her tuberculosis was appropriately controlled by anti-tuberculosis agents. It is worthwhile to highlight the relationship between ICI use and TB reactivation. Barber et al. hypothesized that ICIs may boost TH1 function and increase the level of interferon γ-producing Mycobacterium tuberculosis-specific CD4 T-cells in the blood (33). The pathogenesis of TEN is also related to cell-mediated cytotoxic reactions and the clonal expansion of drug-specific T-cells with cytotoxicity against keratinocytes directly and indirectly through the recruitment of other cells (34). Cavalcante et al. reported that a patient with ESRD developed a grade 3 pemphigoid rash and bullous lesion after ipilimumab administration, achieving a complete response (14). Further studies are required to clarify the incidence of severe dermatologic irAEs in patients with ESRD and to elucidate the relationship between the intensity of cell-mediated cytotoxic reactions and the durable response rate.
One patient in our study presented with daily spiking fever, hypotension, altered mental status, hypoxia, and respiratory failure after administration of the first cycle of anti-PD-1 treatment. The clinical manifestation was thought to be severe sepsis but also resembled an unusual form of CRS, an inflammatory systemic disorder resulting from an overwhelming elevation of cytokine levels and T-cell engagement and proliferation. CRS severity can range from mild symptoms to a fulminant disease with multiple organ failure and death. CRS has been observed to be triggered by several monoclonal antibodies, systemic interleukin-2, and more recently, the CD19-CD3 chimeric antigen receptor T-cell therapy (35). A few case reports have detailed life-threatening CRS in patients after the administration of ICIs, with occurrences ranging from cycles 1 to 17 (36–39). The culprit medications were anti-PD-1 and anti-LAG-3. Alexander et al. reported the case of a patient with stage IV melanoma who received nivolumab on cycle 17 and had a CRS episode; it was controlled by tocilizumab initially, but the patient died 6 weeks later because of another CRS episode (39). Seth et al. also reported a patient with alveolar soft part sarcoma who received nivolumab and had a CRS event that was resolved by tocilizumab and corticosteroids (38). Although CRS is an uncommon complication associated with ICIs, early recognition and prompt management of CRS is crucial owing to its high mortality risk.
Among patients with ESRD in this report, ICIs conferred a significantly higher ORR and better DCR on patients with ERSD than those without. The response rate benefits reflect the trends of better PFS and median OS. Our results showed that the efficacy of ICIs for patients with ESRD was not inferior to that for patients without ESRD. A possible explanation of the superior antitumor efficacy of ICIs may be related to pharmacokinetics. Renal failure or hemodialysis seems to have no effect on the pharmacokinetics of ICIs, possibly because the clearance of ICIs is governed by numerous physiological mechanisms; this clearance predominantly occurs through nonspecific degradation within plasma and tissues. This nonspecific route of degradation reduces the influence of age, hepatic impairment, and renal failure on clearance (40). Considering the large molecular weights of ICIs (nivolumab: 146 kDa; ipilimumab: 148 kDa; pembrolizumab: 149 kDa; atezolizumab: 145 kDa), which cannot penetrate dialysis pores, drug removal and elimination through hemodialysis are unlikely (13). The pharmacokinetic characteristics of ICIs, which are unaffected by renal failure and hemodialysis, were also demonstrated by a similar incidence of AEs among patients in the ESRD and non-ESRD groups.
This study had some inevitable limitations owing to its retrospective nature; furthermore, it was limited by the relatively small sample size of the ESRD group. However, it is difficult to conduct a prospective clinical trial through recruiting patients with advanced UC or mUC to receive ICIs. The difficulty is not simply due to sample size; additionally, ESRD may develop during the treatment period among such patients with UC. Finally, the study had unpreventable bias in terms of the choice of ICIs being governed by physicians’ decisions, patients’ financial considerations, and the instructions of the National Health Insurance system in Taiwan. However, our results demonstrated that the administration of ICIs in patients with ESRD resulted in them having a better survival trend than did patients without ESRD, and no notable safety concerns arose.
In conclusion, our study revealed that administration of ICIs in patients with mUC and ESRD demonstrated a modest antitumor activity, and should be used with caution for increasing risk of hematologic toxicity. Further confirmatory studies are required to validate our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Chang Gung Medical Foundation. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
M-CK analyzed and interpreted data, prepared the tables, and wrote the original manuscript. Y-LS designed the conceptualization and methodology, prepared the figures, and reviewed and edited the manuscript. P-JS, C-CH, H-LL, T-JC, S-HL, C-CW, T-TL, Y-TC, and C-HK contributed the resources. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the multidisciplinary team of the genitourinary cancer at our hospital for their generous assistance and cooperation. The study was supported in part by a grant from Chang Gung Memorial Hospital, Kaohsiung, Taiwan (CMRPG8H1381, CMRPG8G1432). This manuscript was edited by Wallace Academic Editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.584834/full#supplementary-material
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2014) 136:E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. Mari A, Campi R, Tellini R, Gandaglia G, Albisinni S, Abufaraj M, et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: a comprehensive review of the literature. World J Urol (2018) 36:157–70. 10.1007/s00345-017-2115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J Clin Oncol (2000) 17(17):3068–77. 10.1200/JCO.2000.18.17.3068 [DOI] [PubMed] [Google Scholar]
- 4. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18(3):312–22. 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 7. Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma [abstract]. J Clin Oncol (2018) 35. 10.1200/JCO.2017.35.6_suppl.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol (2017) 35(19):2117–24. 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gómez De Liaño A, Duran I. The continuing role of chemotherapy in the management of advanced urothelial cancer. Ther Adv Urol (2018) 10(12):455–80. 10.1177/1756287218814100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (2017) 389:67–76. 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung PH, Tsai HB, Hung KY, Muo CH, Chung MC, Chang CH, et al. Patients with urothelial carcinoma have poor renal outcome regardless of whether they receive nephrouretectomy. Oncotarget (2016) 7(38):61679–89. 10.18632/oncotarget.11223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang SM, Lai MN, Chen PC, Wang JD. Increased risk of urothelial cancer in young and middle aged patients with end-stage renal disease. J Formos Med Assoc (2015) 114:52–7. 10.1016/j.jfma.2013.10.022 [DOI] [PubMed] [Google Scholar]
- 13. Cheun H, Kim M, Lee H, Oh KH, Keam B. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: a retrospective case series and literature review. Invest New Drugs (2019) 37(3):579–83. 10.1007/s10637-018-0673-y [DOI] [PubMed] [Google Scholar]
- 14. Cavalcante L, Amin A, Lutzky J. Ipilimumab was safe and effective in two patients with metastatic melanoma and end-stage renal disease. Cancer Manage Res (2015) 7:47–50. 10.2147/CMAR.S73389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vitale MG, Baldessari C, Milella M, Buti S, Militello AM, Di Girolamo S, et al. Immunotherapy in Dialysis-Dependent Cancer Patients: Our Experience in Patients With Metastatic Renal Cell Carcinoma and a Review of the Literature. Clin Genitourin Cancer (2019) 17(5):E903–8. 10.1016/j.clgc.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 16. Postow MA, Callahan MK, Wolchok JD. Use of the PD-1 Pathway Inhibitor Nivolumab in a Renal Transplant Patient With Malignancy. Am J Transplant (2016) 16:2496–7. 10.1111/ajt.13786 [DOI] [PubMed] [Google Scholar]
- 17. Ong M, Ibrahim AM, Bourassa-Blanchette S, Canil C, Fairhead T, Knoll G, et al. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer (2016) 4:64. 10.1186/s40425-016-0171-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carlo MI, Feldman DR. Response to Nivolumab in a Patient With Metastatic Clear Cell Renal Cell Carcinoma and End-stage Renal Disease on Dialysis. Eur Urol (2016) 70(6):1082–3. 10.1016/j.eururo.2016.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang R, Shirai K. Safety and efficacy of pembrolizumab in a patient with advanced melanoma on haemodialysis. BMJ Case Rep (2016) 2016:bcr2016216426. 10.1136/bcr-2016-216426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lipson EJ, Bagnasco SM, Moore J, Jr, Jang S, Patel MJ, Zachary AA, et al. Tumor Regression and Allograft Rejection after Administration of Anti-PD-1. N Engl J Med (2016) 374(9):896–8. 10.1056/NEJMc1509268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spain L, Higgins R, Gopalakrishnan K, Turajlic S, Gore M, Larkin J. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol (2016) 27(6):1135–7. 10.1093/annonc/mdw130 [DOI] [PubMed] [Google Scholar]
- 22. Alhamad T, Venkatachalam K, Linette GP, Brennan DC. Checkpoint Inhibitors in Kidney Transplant Recipients and the Potential Risk of Rejection. Am J Transplant (2016) 16:1332–3. 10.1111/ajt.13711 [DOI] [PubMed] [Google Scholar]
- 23. Jose A, Yiannoullou P, Bhutani S, Denley H, Morton M, Picton M, et al. Renal Allograft Failure After Ipilimumab Therapy for Metastatic Melanoma: A Case Report and Review of the Literature. Transplant Proc (2016) 48(9):3137–41. 10.1016/j.transproceed.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 24. Tabei T, Natsume I, Kobayashi K. Successful treatment of metastatic clear cell carcinoma with nivolumab in a patient receiving dialysis treatment. Int J Urol (2017) 24(9):708–10. 10.1111/iju.13420 [DOI] [PubMed] [Google Scholar]
- 25. Boyle SM, Ali N, Olszanski AJ, Park DJ, Xiao G, Guy S, et al. Donor-Derived Metastatic Melanoma and Checkpoint Inhibition. Transplant Proc (2017) 49(7):1551–4. 10.1016/j.transproceed.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 26. Park S, Daniels GA. Anti-PD-1 therapy in patients with end-stage renal disease on dialysis: A single-center case series. J Clin Oncol (2017) 35(15_suppl):e14553–3. 10.1200/JCO.2017.35.15_suppl.e14553 [DOI] [Google Scholar]
- 27. Ishizuka S, Sakata S, Yoshida C, Takaki A, Saeki S, Nakamura K, et al. Successful treatment by pembrolizumab in a patient with end-stage renal disease with advanced non-small cell lung cancer and high PD-L1 expression. Respir Investig (2018) 56(4):361–4. 10.1016/j.resinv.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 28. Ansari J, Ali M, Farrag A, Ali AM, Alhamad A. Efficacy of Nivolumab in a Patient with Metastatic Renal Cell Carcinoma and End-Stage Renal Disease on Dialysis: Case Report and Literature Review. Case Rep Immunol (2018) 2018:1623957. 10.1155/2018/1623957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parisi A, Cortellini A, Cannita K, Bersanelli M, Ficorella C. Safe Administration of anti-PD-L1 Atezolizumab in a Patient with Metastatic Urothelial Cell Carcinoma and End-Stage Renal Disease on Dialysis. Case Rep Oncol Med (2019) 2019:3452762. 10.1155/2019/3452762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osmán-García I, Congregado-Ruiz CB, Lendínez-Cano G, Baena-Villamarin C, Conde-Sanchez JM, Medina-López RA. Outcomes and Safety of Biweekly and Monthly Nivolumab in Patients with Metastatic Renal Cell Carcinoma and Dialysis: Three Case Reports and Literature Review. Urol Int (2020) 104(3-4):323–6. 10.1159/000504515 [DOI] [PubMed] [Google Scholar]
- 31. Hirsch JS, Wanchoo R, Ng JH, Khanin Y, Jhaveri KD. Use of Immune Checkpoint Inhibitors in End Stage Kidney Disease Patients, Single Center Experience and Review of the Literature. Kidney360 (2020) 1(5):399–402. 10.34067/KID.0000422020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strohbehn IA, Lee M, Seethapathy H, Chute D, Rahma O, Guidon A, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients on Dialysis: A Retrospective Case Series. Am J Kidney Dis (2020) 76(2):299–302. 10.1053/j.ajkd.2020.02.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barber DL, Sakai S, Kudchadkar RR, Fling SP, Day TA, Vergara JA, et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med (2019) 11(475):eaat2702. 10.1126/scitranslmed.aat2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko TM, Chung WH, Wei CY, Shih HY, Chen JK, Lin CH, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol (2011) 128(6):1266–76.e11. 10.1016/j.jaci.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 35. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J ImmunoTher Cancer (2018) 6:56. 10.1186/s40425-018-0343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dimitriou F, Matter AV, Mangana J, Urosevic-Maiwald M, Micaletto S, Braun RP, et al. Cytokine Release Syndrome During Sequential Treatment With Immune Checkpoint Inhibitors and Kinase Inhibitors for Metastatic Melanoma. J Immunother (2019) 42:29–32. 10.1097/CJI.0000000000000236 [DOI] [PubMed] [Google Scholar]
- 37. Oda H, Ishihara M, Miyahara Y, Nakamura J, Kozuka Y, Iwasa M, et al. First Case of Cytokine Release Syndrome after Nivolumab for Gastric Cancer. Case Rep Oncol (2019) 12:147–56. 10.1159/000496933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rotz SJ, Leino D, Szabo S, Mangino JL, Turpin BK, Pressey JG. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer (2017) e26642. 10.1002/pbc.26642 [DOI] [PubMed] [Google Scholar]
- 39. Slota A, Khan R, Rahman A, Warner EA. Cytokine Release Syndrome As a Rare Complication of Nivolumab: A Case Report. Blood (2019) 134(Supplement_1):5630. 10.1182/blood-2019-127586 [DOI] [Google Scholar]
- 40. Centanni M, Moes DJAR, Trocóniz I, Ciccolini J, van Hasselt JGC. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin Pharmacokinet (2019) 58:835–57. 10.1007/s40262-019-00748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.