Abstract
Increasing evidence indicates that mRNAs are often subject to posttranscriptional modifications. Among them, N6-methyladenosine (m6A), which has been shown to play key roles in RNA splicing, stability, nuclear export, and translation, is the most abundant modification of RNA. Extensive studies of m6A modification of mRNAs have been carried out, while little is known about m6A modification of long non-coding RNAs (lncRNAs). Recently, several studies reported m6A modification of lncRNAs. In this review, we focus on these m6A-modified lncRNAs and discuss possible functions of m6A modification.
Keywords: Erasers, LncRNA, N6-methyladenosine, Readers, Writers
Introduction
Long non-coding RNAs (lncRNAs) are non-protein coding transcripts of over 200 nucleotides in length and are found in both nuclear and cytosolic fractions. Increasing evidence indicates that lncRNAs play key roles in gene regulation and disease processes.1, 2, 3, 4, 5, 6 It has been demonstrated that lncRNAs mediate gene regulation through binding with DNA, RNA, or proteins.7, 8, 9, 10 For example, lncRNAs may act as signals or decoys of transcription, as protein scaffolds, or as epigenetic regulators.11 Thus, lncRNAs may regulate gene expression at transcriptional and/or posttranscriptional levels and participate in disease processes.6
N6-methyladenosine (m6A), which is the most abundant modification in eukaryotic mRNAs, was discovered by Ronald et al in 1974.12 M6A modification exerts multiple functions on mRNA and lncRNA, as it regulates mRNA splicing, stability, nuclear export, and translation. The m6A system includes “writers” (methyltransferases), “erasers” (demethylases), and “readers”.13 The writers add the methyl group to the m6A modification sites, whereas the erasers remove the methyl group from the m6A-modified sites.14 The readers recognize the m6A-modified RNAs and regulate various functional biological processes.14 M6A modification of mRNA is well demonstrated. Methylated RNA immunoprecipitation and sequencing (MeRIP-Seq) analysis has suggested that lncRNAs are also subject to such modification, although the number is much lower.15 Here, we summarize some m6A-modified lncRNAs and their functions.
Methyltransferases (writers)
The m6A methyltransferase complex consists of several key components, such as METTL3, METTL14, WTAP, IRMA, and RBM15.16 METTL3 is the first discovered m6A methyltransferase and is highly conserved.17 METTL14 is another m6A methyltransferase. METTL14 and METTL3 form a stable heterodimer complex and catalyze m6A methylation.18 METTL3 acts as the catalytic core, whereas METTL14 serves as an RNA binding platform.19 WTAP (Wilms' tumor 1-associated protein) is another component of the m6A methyltransferase complex. Because WTAP lacks a catalytic m6A methylation domain, WTAP may just act as a platform for interacting with METTL3 and METTL14.18 WTAP can also bind with BCLAF1/THRAP3 and MALAT1.20 METTL16 is a putative methyltransferase that catalyzes U6 snRNA and non-coding RNA m6A methylation.21 In addition, RBM15, RBM15B, ZC3H13, and VIRMA have been reported to be components of the m6A methyltransferase complex.22, 23, 24, 25 It has been shown that m6A methyltransferases regulate lncRNA functions. For example, METTL3 could regulate LINC00958 expression by m6A modification.26 Also, METTL3 increased the abundance of m6A modification and the stability of MALAT1,27 and METTL16 interacted with the 3′-terminal triple helix of MALAT1.28
Demethylases (erasers)
M6A methylation of RNA is a reversible chemical modification. Fat mass and obesity-associated protein (FTO) serves as an m6A demethylase.29 ALKBH5 (α-ketoglutarate-dependent dioxygenase homolog 5) is another m6A demethylase that impacts RNA metabolism and mouse fertility.30 FTO and ALKBH5 both belong to the α-ketoglutarate-dependent dioxygenase family and clear m6A methylation in an Fe2+- and α-ketoglutarate-dependent manner.31 ALKBH3 is a recently identified m6A demethylase and mediates tRNA demethylation.32 ALKBH5 suppresses pancreatic motility via demethylating the lncRNA KCNK15-AS1.33 Chen et al have demonstrated ALKBH5 mediates lncRNA PVT1 m6A demethylation and participates in osteosarcoma progression.34 Moreover, it has been reported that overexpression of ALKBH5 suppresses lncRNA RP11expression.35 These findings suggest that the demethylases of m6A are involved in the regulation of lncRNAs expression.
Readers
Once the m6A information is recognized by different “readers,” this will lead to different downstream effects. Members of the YT521-B homology (YTH) domain family (YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2) have been identified as direct m6A readers.36 YTHDF2 mediates the degradation of m6A containing RNAs through recruiting the CCR4-NOT complex.37 YTHDF1 is another m6A reader that can increase protein synthesis by interacting with the translation machinery.38 Interestingly, YTHDF3 can interact with YTHDF1 and YTHDF2 in such a way that it not only helps YTHDF1 promote the translation of methylated mRNAs but also increases YTHDF2-mediated mRNA degradation.39 The heterogeneous nuclear ribonucleoprotein (HNRNP) family, which includes HNRNPA2B1, HNRNPC, and HNRNPG, can also play a role as m6A readers.40, 41, 42 Recently, insulin-like growth factor 2 mRNA binding proteins (IGF2BPs, including IGF2BP1/2/3) have also been found to recognize m6A modifications, acting as a distinct family of m6A readers.43 YTHDC1 acts as an m6A reader of XIST, regulating XIST-mediated transcription repression.23 It has been demonstrated that YTHDF3 facilitates the degradation of the m6A-modified lncRNA GAS5, serving as an m6A reader.44 Recently, IGF2BP2 has been found to regulate DANCR by acting as an m6A reader.45 These observations indicate m6A readers play important roles in the regulation of lncRNAs.
M6A modification of lncRNAs
Given the important role of m6A modification in gene regulation, large numbers of mRNAs have been shown to be substrates for m6A modification. Recent reports have indicated that lncRNAs can also be modified by m6A. Here, we provide an overview of the recent studies of m6A modification of lncRNAs.
MALAT1
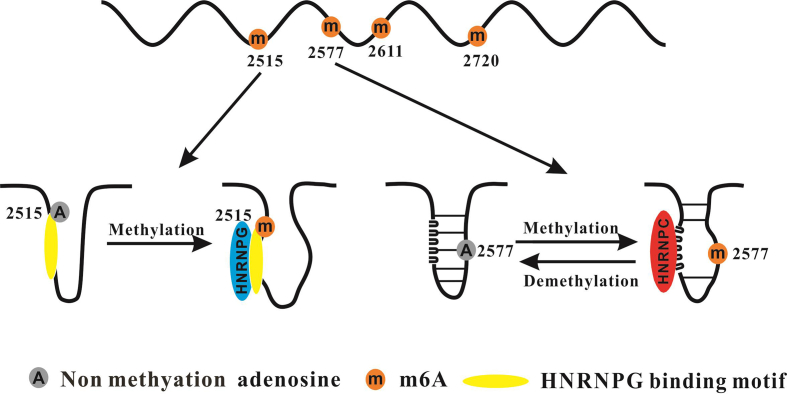
MALAT1 is located at chromosome 11q13 and encodes a 7.9 kb transcript. Several studies have shown that MALAT1 is an oncogenic lncRNA.46, 47, 48, 49 However, Kim et al showed that MALAT1 suppresses breast cancer metastasis.50 MALAT1 is a highly m6A-modified lncRNA, carrying multiple m6A modification sites.15,51, 52, 53 By SCARLET, it has been verified that there are four m6A motifs in MALAT1 (A2515, A2577, A2611, and A2720)52. The MALAT1 RNA hairpin contains an m6A modification site (A2577) and a poly-U HNRNPC binding site. When A2577 is unmethylated, the HNRNPC binding domain is partially inaccessible. However, once A2577 is modified with m6A, such modification destabilizes the RNA hairpin, releasing the poly-U tract and increasing the binding with HNRNPC (Fig. 1). Moreover, HNRNPG has recently been reported to be an m6A reader, which interacts with m6A modifications in low-complexity regions. HNRNPG can bind the m6A-modified hairpin of MALAT1 (A2515)42. However, HNRNPG does not directly bind the m6A site; MALAT1 m6A modification facilitates the binding with HNRNPG (Fig. 1). METTL16, which adds a methyl group to U6 small nuclear RNAs (snRNAs) and regulates MAT2A splicing, also functions as an m6A methyltransferase.54 METTL16 regulates the methylation of MAT2A and is essential for mouse embryonic development.55 METTL16 can bind to the 3’ triple helix region of MALAT1, and the complex of METTL16-MALAT1 may participate in MALAT1-mediated tumorigenesis.28 However, whether METTL16 mediates the methylation of MALAT1 and the underlying mechanisms remain to be elucidated. M6A modification of MALAT1 affects its structure and regulates the interaction with HNRNPC/HNRNPG, which suggests that m6A modification may play a role in switching between lncRNAs structures and contribute to the binding of proteins.
Figure 1.
N6-methyladenosine modification of MALAT1.
XIST
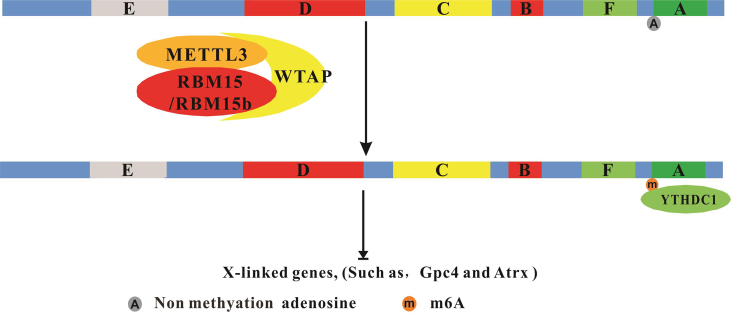
XIST is a 17.5 kb capped nucleotide transcript transcribed from the XIST gene. Its function is to mediate the silencing of the X chromosome.56, 57, 58 XIST is modified by m6A23, 51, 59. It carries three major m6A modification sites in mouse embryonic stem cells, and multiple potential m6A modification sites have been identified in human.23,51,59, 60, 61 XIST RNA contains six repetitive elements, named the A, F, B, C, D, and E repeats, and these repeats can bind to protein factors and regulate silencing of downstream genes.53,62,63 One major m6A modification site, which is located after the A-repeats, is a key XIST silencing factor.59,63,64 RBM15, which has been reported to be a component of the m6A methyltransferase complex, may interact with m6A sites of the A-repeats of XIST.23,65 WTAP is a protein that binds to the A-repeats of XIST.62,66,67 RBM15 and WTAP are required for XIST-mediated silencing, are co-localized, and potentially interact with XIST RNA.67 RBM15 and its paralogue RBM15B bind to the m6A methylation complex and recruit it to RNA-specific sites.23 YTHDC1 is the m6A reader of XIST and is required for XIST function. Thus, m6A modification is required for XIST-mediated transcriptional repression of X-linked genes, such as Gpc4 and Atrx23 (Fig. 2).
Figure 2.
N6-methyladenosine modification of XIST.
LncRNA RP11
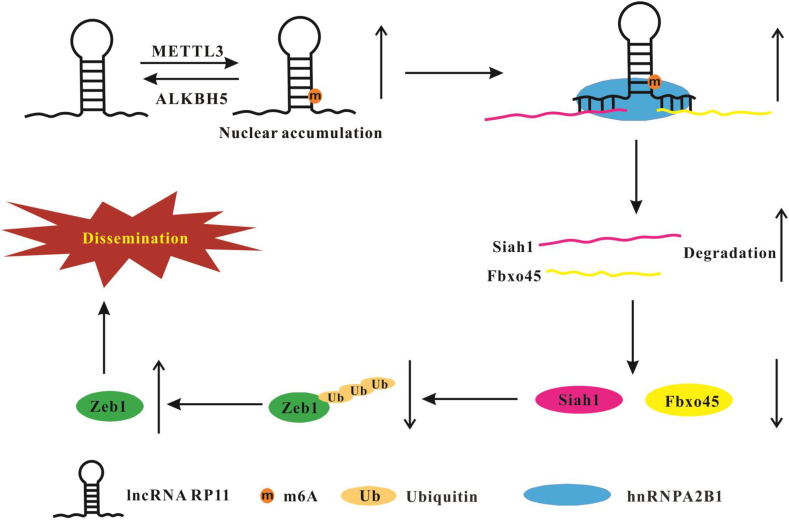
LncRNA RP11 is a recently identified lncRNA that is upregulated in colorectal cancer (CRC) tissues and promotes migration and invasion via the epithelial–mesenchymal transition.35 RP11 interacts with hnRNPA2B1, downregulating the mRNA expression of Siah1 and Fbxo45. Finally, RP11 stimulates Zeb1 expression. M6A modification plays a role in the upregulation of RP11 in CRC cells. M6A antibody could enrich RP11 in CRC cells (HCT15 and HCT8), while the level of enrichment of CRCs is higher than NCM460 cells. Overexpression of METTL3 upregulates RP11 expression in CRC cells. Overexpression of ALKBH5 downregulates RP11 expression. It has been demonstrated that m6A methylation promotes nuclear export of mRNAs,30,68 so m6A modification may affect the cellular distribution of mRNAs. However, it remains unclear whether the localization of lncRNAs is regulated by m6A modification. The authors have shown that METTL3 overexpression can dramatically increase RP11 nuclear localization,35 suggesting that m6A modification exerts different effects on the distribution of mRNAs and lncRNAs. Moreover, METTL3 overexpression enhances the interaction of RP11 with hnRNPA2B1. It has been shown that hnRNPA2B1 can interact with RBPs and induce mRNA degradation.69 RP11 can also directly bind with the coding sequence of Siah1 and the 3′-UTR of Fbxo45, which both are E3 ligases. Both Siah1 and Fbxo45 can promote Zeb1 degradation in a ubiquitin–proteasome-dependent way.70,71 RP11 promotes the degradation of Siah1 and Fbxo45 mRNA and decreases Siah1 and Fbxo45 expression. Thus, the m6A-mediated nuclear accumulation of RP11 may inhibit the ubiquitination of Zeb1 and induce Zeb1 to trigger the dissemination of CRC35 (Fig. 3).
Figure 3.
N6-methyladenosine modification of lncRNA RP11.
FAM225A
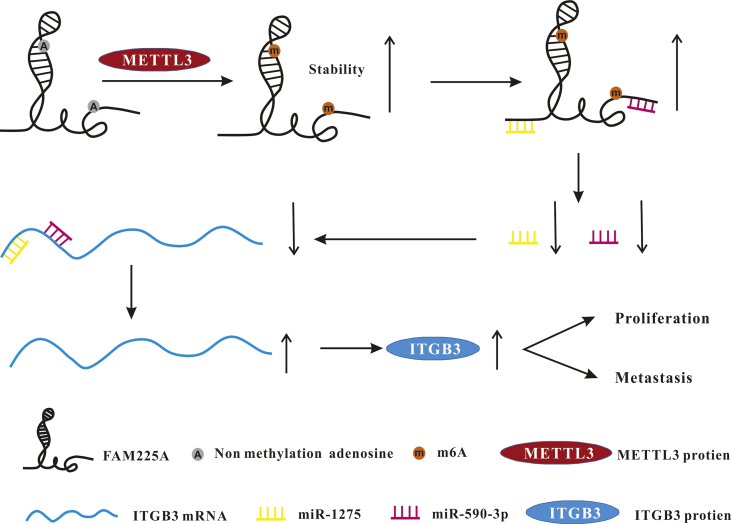
Zheng and colleagues have reported that FAM225A is a novel and one of the most upregulated lncRNAs in nasopharyngeal carcinogenesis (NPC), and FAM225A is associated with poor clinical outcomes.72 There are two RRACU m6A motifs (2808 and 5460) in FAM225A, as predicted with m6AVar. Me-RIP assays have shown the m6A levels of HONE-1 and SUNE-1 are higher than those of NP69 and N2Tert. Silencing METTL3 decreases m6A levels of total RNA and FAM225A. Knockdown of METTL3 downregulates the expression of FAM225A. Silencing METTL3 decreases FAM225A RNA stability, which indicates that m6A modification may affect FAM225A stability. Accumulating evidence has demonstrated that lncRNAs may act as competing endogenous RNAs (ceRNAs) and regulate miRNAs through competitively binding miRNA targets.73,74 FAM225A serves as the ceRNA for sponging both miR-590-3p and miR-1275, increasing the levels of their target integrin β3 (ITGB3), finally stimulating FAK/PI3K/Akt signaling. MiR-590-3p has been reported as a tumor suppressor in cholangiocarcinoma and hepatocellular carcinoma.75,76 It has been reported that miR-1275 can inhibit NPC cell growth and suppress hepatocellular carcinoma cell proliferation.77,78 In conclusion, m6A modification of FAM225A could improve its stability, upregulate FAM225A levels, and promote NPC proliferation and invasion35 (Fig. 4).
Figure 4.
N6-methyladenosine modification of FAM225A.
GAS5
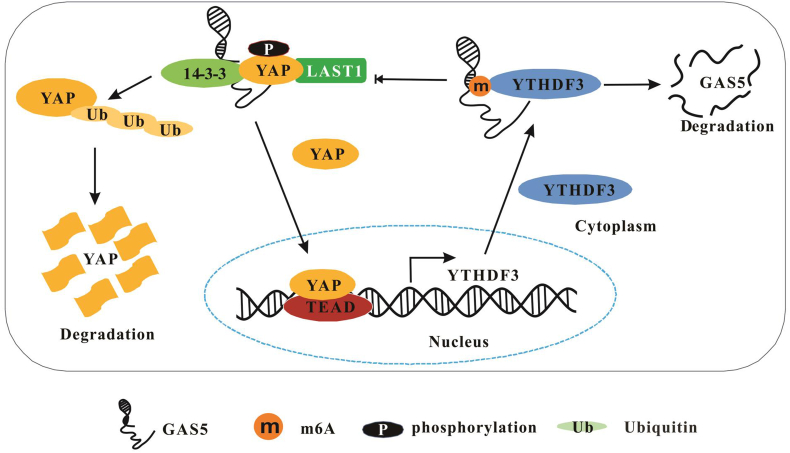
It has been shown that GAS5 is downregulated in cancers, and it acts as a tumor suppresser.79, 80, 81 Recently, it has been reported that GAS5, which may inhibit the progression of colorectal cancer, is m6A-modified.44 Mechanically, GAS5 directly interacts with YAP to promote YAP phosphorylation and ubiquitin-mediated degradation. Moreover, YAP mediates transcription of YTHDF3, which serves as an m6A reader and binds with m6A-modified GAS5 to trigger GAS5 degradation (Fig. 5). It has been demonstrated that YTHDF3 can facilitate protein translation and degrade m6A-modified mRNA,39 indicating that YTHDF3 may degrade both mRNA and lncRNA in an m6A-dependent way. Thus, the m6A-mediated degradation of GAS5 through YAP signaling in CRC may suggest that targeting of m6A modification could be a new approach for CRC treatment.
Figure 5.
N6-methyladenosine modification of GAS5.
Future directions
The information available in the literature suggests that m6A modification of lncRNAs plays a role in gene regulation through various mechanisms. First, m6A modification of lncRNAs may change the structure of lncRNAs and affect the interaction with proteins.42,52 Second, m6A modification of lncRNAs could mediate gene transcription repression.23 Third, m6A modification of lncRNAs possibly alters its subcellular distribution.35 Fourth, m6A modification of lncRNAs regulates lncRNAs stability.44,72 However, irrespective of the mechanism involved, m6A modification of lncRNAs regulates lncRNAs stability and/or localization through interactions among lncRNAs, proteins, miRNAs, and mRNAs. However, overall, studies on m6A modification of lncRNAs are still low in number. There is a need for further characterization of components required for lncRNAs m6A modification and recognition. Moreover, the underlying mechanisms by which m6A modifications contribute to gene regulation and whether and how m6A modification of mRNAs differs from lncRNAs m6A modification remain to be elucidated. An improved understanding of lncRNAs m6A modification will expand our knowledge of m6A modification and gene regulation.
Conflict of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of China (81773165), Hunan Province Science Fund for Distinguished Young Scholars (2018JJ1021), the Key R&D Program of Hunan Province (2017SK2172), and the Science and Technology Foundation of Chenzhou (jsyf2017023).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Rong-Zhang He, Email: rongzhang412@163.com.
Di-Xian Luo, Email: luodixian_2@163.com.
References
- 1.Li T., Hu P.S., Zuo Z. Mettl3 facilitates tumor progression via an m(6)a-igf2bp2-dependent mechanism in colorectal carcinoma. Mol Canc. 2019;18:112. doi: 10.1186/s12943-019-1038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu C.L., Sang B., Liu G.Z. Senebloc, a long non-coding rna suppresses senescence via p53-dependent and independent mechanisms. Nucleic Acids Res. 2020 doi: 10.1093/nar/gkaa063. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keihani S., Kluever V., Mandad S. The long noncoding rna neurolnc regulates presynaptic activity by interacting with the neurodegeneration-associated protein tdp-43. Sci Adv. 2019;5 doi: 10.1126/sciadv.aay2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y.P., Jin Y.P., Wu X.S. Lncrna-hgbc stabilized by hur promotes gallbladder cancer progression by regulating mir-502-3p/set/akt axis. Mol Canc. 2019;18:167. doi: 10.1186/s12943-019-1097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang J., Yan T., Bao Y. Lncrna glcc1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-myc. Nat Commun. 2019;10:3499. doi: 10.1038/s41467-019-11447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He R.Z., Luo D.X., Mo Y.Y. Emerging roles of lncrnas in the post-transcriptional regulation in cancer. Genes Dis. 2019;6:6–15. doi: 10.1016/j.gendis.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta R.A., Shah N., Wang K.C. Long non-coding rna hotair reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariel F., Lucero L., Christ A. R-loop mediated trans action of the apolo long noncoding rna. Mol Cell. 2020;77(5):1055–1065. doi: 10.1016/j.molcel.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Pitchiaya S., Cieslik M. Analysis of the androgen receptor-regulated lncrna landscape identifies a role for arlnc1 in prostate cancer progression. Nat Genet. 2018;50:814–824. doi: 10.1038/s41588-018-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K., Han X., Zhang Z. The liver-enriched lnc-lfar1 promotes liver fibrosis by activating tgfbeta and notch pathways. Nat Commun. 2017;8:144. doi: 10.1038/s41467-017-00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng W.X., Koirala P., Mo Y.Y. Lncrna-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger rna from novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan Q., Liu P.Y., Haase J., Bell J.L., Huttelmaier S., Liu T. The critical role of rna m(6)a methylation in cancer. Canc Res. 2019;79:1285–1292. doi: 10.1158/0008-5472.CAN-18-2965. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Hsu P.J., Chen Y.S., Yang Y.G. Dynamic transcriptomic m(6)a decoration: writers, erasers, readers and functions in rna metabolism. Cell Res. 2018;28:616–624. doi: 10.1038/s41422-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mrna methylation reveals enrichment in 3' utrs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng X., Su R., Weng H., Huang H., Li Z., Chen J. Rna n(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bokar J.A., Shambaugh M.E., Polayes D., Matera A.G., Rottman F.M. Purification and cdna cloning of the adomet-binding subunit of the human mrna (n6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Yue Y., Han D. A mettl3-mettl14 complex mediates mammalian nuclear rna n6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Feng J., Xue Y. Structural basis of n(6)-adenosine methylation by the mettl3-mettl14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi K., Kawamura T., Iwanari H. Identification of wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288:33292–33302. doi: 10.1074/jbc.M113.500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warda A.S., Kretschmer J., Hackert P. Human mettl16 is a n(6)-methyladenosine (m(6)a) methyltransferase that targets pre-mrnas and various non-coding rnas. EMBO Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz S., Mumbach M.R., Jovanovic M. Perturbation of m6a writers reveals two distinct classes of mrna methylation at internal and 5' sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil D.P., Chen C.K., Pickering B.F. M(6)a rna methylation promotes xist-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen J., Lv R., Ma H. Zc3h13 regulates nuclear rna m(6)a methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69 doi: 10.1016/j.molcel.2018.02.015. 1028-1038 e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knuckles P., Lence T., Haussmann I.U. Zc3h13/flacc is required for adenosine methylation by bridging the mrna-binding factor rbm15/spenito to the m(6)a machinery component wtap/fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo X., Chen Z., Gao W. M6a-mediated upregulation of linc00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol. 2020;13:5. doi: 10.1186/s13045-019-0839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin D., Guo J., Wu Y. M(6)a mrna methylation initiated by mettl3 directly promotes yap translation and increases yap activity by regulating the malat1-mir-1914-3p-yap axis to induce nsclc drug resistance and metastasis. J Hematol Oncol. 2019;12:135. doi: 10.1186/s13045-019-0830-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Brown J.A., Kinzig C.G., DeGregorio S.J., Steitz J.A. Methyltransferase-like protein 16 binds the 3'-terminal triple helix of malat1 long noncoding rna. Proc Natl Acad Sci USA. 2016;113:14013–14018. doi: 10.1073/pnas.1614759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia G., Fu Y., Zhao X. N6-methyladenosine in nuclear rna is a major substrate of the obesity-associated fto. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng G., Dahl J.A., Niu Y. Alkbh5 is a mammalian rna demethylase that impacts rna metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun T., Wu R., Ming L. The role of m6a rna methylation in cancer. Biomed Pharmacother. 2019;112:108613. doi: 10.1016/j.biopha.2019.108613. Biomedecine & pharmacotherapie. [DOI] [PubMed] [Google Scholar]
- 32.Ueda Y., Ooshio I., Fusamae Y. Alkb homolog 3-mediated trna demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7:42271. doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Y., Hu H., Wang Y. Alkbh5 inhibits pancreatic cancer motility by decreasing long non-coding rna kcnk15-as1 methylation. Cell Physiol Biochem : Int J Experim Cell Physiol Biochem Pharmacol. 2018;48:838–846. doi: 10.1159/000491915. [DOI] [PubMed] [Google Scholar]
- 34.Chen S., Zhou L., Wang Y. Alkbh5-mediated m(6)a demethylation of lncrna pvt1 plays an oncogenic role in osteosarcoma. Canc Cell Int. 2020;20:34. doi: 10.1186/s12935-020-1105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y., Yang X., Chen Z. M(6)a-induced lncrna rp11 triggers the dissemination of colorectal cancer cells via upregulation of zeb1. Mol Canc. 2019;18:87. doi: 10.1186/s12943-019-1014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Lu Z., Gomez A. N6-methyladenosine-dependent regulation of messenger rna stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du H., Zhao Y., He J. Ythdf2 destabilizes m(6)a-containing rna through direct recruitment of the ccr4-not deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Zhao B.S., Roundtree I.A. N(6)-methyladenosine modulates messenger rna translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi H., Wang X., Lu Z. Ythdf3 facilitates translation and decay of n(6)-methyladenosine-modified rna. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alarcon C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. Hnrnpa2b1 is a mediator of m(6)a-dependent nuclear rna processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. N(6)-methyladenosine-dependent rna structural switches regulate rna-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu N., Zhou K.I., Parisien M., Dai Q., Diatchenko L., Pan T. N6-methyladenosine alters rna structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang H., Weng H., Sun W. Recognition of rna n(6)-methyladenosine by igf2bp proteins enhances mrna stability and translation. Nat Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni W., Yao S., Zhou Y. Long noncoding rna gas5 inhibits progression of colorectal cancer by interacting with and triggering yap phosphorylation and degradation and is negatively regulated by the m(6)a reader ythdf3. Mol Canc. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu X., Peng W.X., Zhou H. Igf2bp2 regulates dancr by serving as an n6-methyladenosine reader. Cell Death Differ. 2019 doi: 10.1038/s41418-019-0461-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Feng T., Lian Y., Zhang G., Garen A., Song X. Role of human noncoding rnas in the control of tumorigenesis. Proc Natl Acad Sci USA. 2009;106:12956–12961. doi: 10.1073/pnas.0906005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji Q., Zhang L., Liu X. Long non-coding rna malat1 promotes tumour growth and metastasis in colorectal cancer through binding to sfpq and releasing oncogene ptbp2 from sfpq/ptbp2 complex. Br J Canc. 2014;111:736–748. doi: 10.1038/bjc.2014.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latorre E., Carelli S., Raimondi I. The ribonucleic complex hur-malat1 represses cd133 expression and suppresses epithelial-mesenchymal transition in breast cancer. Canc Res. 2016;76:2626–2636. doi: 10.1158/0008-5472.CAN-15-2018. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X., Hamblin M.H., Yin K.J. The long noncoding rna malat1: its physiological and pathophysiological functions. RNA Biol. 2017;14:1705–1714. doi: 10.1080/15476286.2017.1358347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J., Piao H.L., Kim B.J. Long noncoding rna malat1 suppresses breast cancer metastasis. Nat Genet. 2018;50:1705–1715. doi: 10.1038/s41588-018-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominissini D., Moshitch-Moshkovitz S., Schwartz S. Topology of the human and mouse m6a rna methylomes revealed by m6a-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 52.Liu N., Parisien M., Dai Q., Zheng G., He C., Pan T. Probing n6-methyladenosine rna modification status at single nucleotide resolution in mrna and long noncoding RNA. RNA. 2013;19:1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coker H., Wei G., Brockdorff N. M6a modification of non-coding rna and the control of mammalian gene expression. Biochimi Biophys Acta. Gene Regul Mech. 2019;1862:310–318. doi: 10.1016/j.bbagrm.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Pendleton K.E., Chen B., Liu K. The u6 snrna m(6)a methyltransferase mettl16 regulates sam synthetase intron retention. Cell. 2017;169 doi: 10.1016/j.cell.2017.05.003. 824-835 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendel M., Chen K.M., Homolka D. Methylation of structured rna by the m(6)a writer mettl16 is essential for mouse embryonic development. Mol Cell. 2018;71 doi: 10.1016/j.molcel.2018.08.004. 986-1000 e1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown C.J., Ballabio A., Rupert J.L. A gene from the region of the human x inactivation centre is expressed exclusively from the inactive x chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 57.Brown C.J., Hendrich B.D., Rupert J.L. The human xist gene: analysis of a 17 kb inactive x-specific rna that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- 58.Clemson C.M., McNeil J.A., Willard H.F., Lawrence J.B. Xist rna paints the inactive x chromosome at interphase: evidence for a novel rna involved in nuclear/chromosome structure. J Cell Biol. 1996;132:259–275. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nesterova T.B., Wei G., Coker H. Systematic allelic analysis defines the interplay of key pathways in x chromosome inactivation. Nat Commun. 2019;10:3129. doi: 10.1038/s41467-019-11171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ke S., Alemu E.A., Mertens C. A majority of m6a residues are in the last exons, allowing the potential for 3' utr regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linder B., Grozhik A.V., Olarerin-George A.O., Meydan C., Mason C.E., Jaffrey S.R. Single-nucleotide-resolution mapping of m6a and m6am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brockdorff N. X-chromosome inactivation: closing in on proteins that bind xist rna. Trends Genet. 2002;18:352–358. doi: 10.1016/s0168-9525(02)02717-8. [DOI] [PubMed] [Google Scholar]
- 63.Wutz A., Rasmussen T.P., Jaenisch R. Chromosomal silencing and localization are mediated by different domains of xist rna. Nat Genet. 2002;30:167–174. doi: 10.1038/ng820. [DOI] [PubMed] [Google Scholar]
- 64.Pintacuda G., Wei G., Roustan C. Hnrnpk recruits pcgf3/5-prc1 to the xist rna b-repeat to establish polycomb-mediated chromosomal silencing. Mol Cell. 2017;68:955–969 e910. doi: 10.1016/j.molcel.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cirillo D., Blanco M., Armaos A. Quantitative predictions of protein interactions with long noncoding rnas. Nat Methods. 2016;14:5–6. doi: 10.1038/nmeth.4100. [DOI] [PubMed] [Google Scholar]
- 66.Chu C., Zhang Q.C., da Rocha S.T. Systematic discovery of xist rna binding proteins. Cell. 2015;161:404–416. doi: 10.1016/j.cell.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moindrot B., Cerase A., Coker H. A pooled shrna screen identifies rbm15, spen, and wtap as factors required for xist rna-mediated silencing. Cell Rep. 2015;12:562–572. doi: 10.1016/j.celrep.2015.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fustin J.M., Doi M., Yamaguchi Y. Rna-methylation-dependent rna processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 69.Lan X., Yan J., Ren J. A novel long noncoding rna lnc-hc binds hnrnpa2b1 to regulate expressions of cyp7a1 and abca1 in hepatocytic cholesterol metabolism. Hepatology. 2016;64:58–72. doi: 10.1002/hep.28391. [DOI] [PubMed] [Google Scholar]
- 70.Xu M., Zhu C., Zhao X. Atypical ubiquitin e3 ligase complex skp1-pam-fbxo45 controls the core epithelial-to-mesenchymal transition-inducing transcription factors. Oncotarget. 2015;6:979–994. doi: 10.18632/oncotarget.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen A., Wong C.S., Liu M.C. The ubiquitin ligase siah is a novel regulator of zeb1 in breast cancer. Oncotarget. 2015;6:862–873. doi: 10.18632/oncotarget.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Z.Q., Li Z.X., Zhou G.Q. Long noncoding rna fam225a promotes nasopharyngeal carcinoma tumorigenesis and metastasis by acting as cerna to sponge mir-590-3p/mir-1275 and upregulate itgb3. Canc Res. 2019;79:4612–4626. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- 73.Wang C.J., Zhu C.C., Xu J. The lncrna uca1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor mirnas. Mol Canc. 2019;18:115. doi: 10.1186/s12943-019-1032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu P., Cai J., Chen Q. Lnc-talc promotes o(6)-methylguanine-DNA methyltransferase expression via regulating the c-met pathway by competitively binding with mir-20b-3p. Nat Commun. 2019;10:2045. doi: 10.1038/s41467-019-10025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge X., Gong L. Mir-590-3p suppresses hepatocellular carcinoma growth by targeting tead1. Tum Biol : J Int Soci Oncodevelopmental Biol Med. 2017;39 doi: 10.1177/1010428317695947. 1010428317695947. [DOI] [PubMed] [Google Scholar]
- 76.Zu C., Liu S., Cao W. Mir-590-3p suppresses epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma by inhibiting sip1 expression. Oncotarget. 2017;8:34698–34708. doi: 10.18632/oncotarget.16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gu L., Lu L.S., Zhou D.L., Liu Z.C. Uca1 promotes cell proliferation and invasion of gastric cancer by targeting creb1 sponging to mir-590-3p. Cancer medicine. 2018;7:1253–1263. doi: 10.1002/cam4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun K.Y., Peng T., Chen Z., Huang J., Zhou X.H. Microrna-1275 suppresses cell growth, and retards g1/s transition in human nasopharyngeal carcinoma by down-regulation of hoxb5. J Cell Commun Sign. 2016;10:305–314. doi: 10.1007/s12079-016-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pickard M.R., Williams G.T. Molecular and cellular mechanisms of action of tumour suppressor gas5 lncrna. Genes. 2015;6:484–499. doi: 10.3390/genes6030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pickard M.R., Williams G.T. Regulation of apoptosis by long non-coding rna gas5 in breast cancer cells: implications for chemotherapy. Breast Canc Res Treat. 2014;145:359–370. doi: 10.1007/s10549-014-2974-y. [DOI] [PubMed] [Google Scholar]
- 81.Arshi A., Sharifi F.S., Khorramian Ghahfarokhi M. Expression analysis of malat1, gas5, sra, and neat1 lncrnas in breast cancer tissues from young women and women over 45 years of age. Molecular therapy. Nucleic acids. 2018;12:751–757. doi: 10.1016/j.omtn.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]