Abstract
Background
Proton pump inhibitors (PPIs) are recommended by the latest guidelines to reduce the risk of bleeding in acute myocardial infarction (AMI) patients treated with dual antiplatelet therapy (DAPT). However, previous pharmacodynamic and clinical studies have reported controversial results on the interaction between PPI and the P2Y12 inhibitor clopidogrel. We investigated the impact of PPIs use on in-hospital outcomes in AMI patients, aiming to provide a new insight on the value of PPIs.
Methods
A total of 23, 380 consecutive AMI patients who received clopidogrel with or without PPIs in the China Acute Myocardial Infarction (CAMI) registry were analyzed. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE) defined as a composite of in-hospital cardiac death, re-infarction and stroke. Propensity score matching (PSM) was used to control potential baseline confounders. Multivariate logistic regression analysis was performed to evaluate the effect of PPIs use on MACCE and gastrointestinal bleeding (GIB).
Results
Among the whole AMI population, a large majority received DAPT and 67.5% were co-medicated with PPIs. PPIs use was associated with a decreased risk of MACCE (Before PSM OR: 0.857, 95% CI: 0.742-0.990, P = 0.0359; after PSM OR: 0.862, 95% CI: 0.768-0.949, P = 0.0245) after multivariate adjustment. Patients receiving PPIs also had a lower risk of cardiac death but a higher risk of complicating with stroke. When GIB occurred, an alleviating trend of GIB severity was observed in PPIs group.
Conclusions
Our study is the first nation-wide large-scale study to show evidence on PPIs use in AMI patients treated with DAPT. We found that PPIs in combination with clopidogrel was associated with decreased risk for MACCE in AMI patients, and it might have a trend to mitigate GIB severity. Therefore, PPIs could become an available choice for AMI patients during hospitalization.
Keywords: Acute myocardial infarction, Clopidogrel, Drug interaction, Propensity score matching, Proton-pump inhibitors
1. Introduction
Patients who suffered acute myocardial infarction (AMI) usually had a much worse baseline clinical characteristic, including higher Killip class, unstable hemodynamics and stress condition, which could facilitate gastrointestinal bleeding (GIB).[1] Moreover, GIB was associated with increased mortality and morbidity despite optimal treatment and successful revascularization after an AMI.[2, 3] Therefore, the latest guideline have recommended the co-medication of proton pump inhibitors (PPIs) and dual antiplatelet therapy (DAPT) to minimize the bleeding risk.[4] However, the value of PPIs use in patients receiving clopidogrel has been questioned for years. Pharmacodynamic studies reported that the potential interaction between clopidogrel and PPIs would attenuate the antiplatelet function of clopidogrel.[5] Meanwhile, clinical research showed conflicting results of this interaction on cardiovascular outcomes.[6] To date, few existing data have well characterized the current status of PPIs use in a large Chinese population with AMI. In previous observational studies, there might be baseline confounders which cannot be adjusted by statistic model and this would affect the evaluation on PPIs co-medication. Hence, we performed this propensity score matched (PSM) analysis using a national administrative database and explored the effect of PPIs on in-hospital outcomes when co-administered with clopidogrel in AMI patients.
2. Methods
The China Acute Myocardial Infarction (CAMI) registry served as a national hospital-based registry and surveillance program for AMI to timely obtain real-world information about clinical characteristics, medical care and outcomes of Chinese patients with AMI across different provinces, prefectures and counties.[7] It was organized and conducted by Fuwai Hospital, National Center for Cardiovascular Diseases of China (NCCD). The final inclusion criteria met the third Universal Definition for myocardial infarction (2012).[8] This study was registered with ClinicalTrials.gov (NCT01874691) and was approved by the institutional review board of all participating hospitals. The Data Management and Statistics Teams are managed by Medical Research and Biostatistics Center, NCCD and all data were protected at all time. Other detailed description about CAMI registry can be found in the trial design article published previously.[7]
All 26, 660 consecutive patients from 108 hospitals in CAMI registry who suffered AMI between January 2013 and September 2014 were enrolled. The following patients were excluded due to: (1) less than 20 and more than 100 years old (n = 370); (2) missing data of the gastrointestinal prophylaxis (n = 1436); (3) not receiving clopidogrel (n= 853); (4) using H2 receptor antagonists (H2RAs) instead of PPIs (n = 621). PPIs use was determined at the physician's discretion and was recorded at the time of admission. The specific kind of PPIs was not reported. Finally, a total of 23, 380 patients were analyzed (Figure 1).
Figure 1.
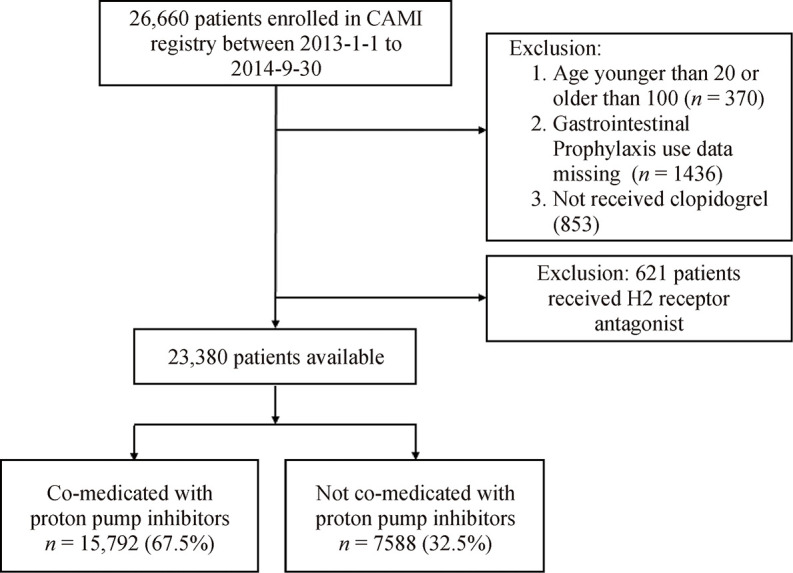
Patient flowchart for the study cohort.
Demographic characters, past history, admission feature, in-hospital medication and procedure were collected. The CRUSADE (Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA Guidelines) bleeding score was calculated since admission as previously described.[9] The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE) defined as a composite of in-hospital cardiac death, re-infarction, and stroke. Secondary endpoints included each component of the primary endpoint and GIB. Re-infarction was defined as an acute MI that occurred within 28 days of initial MI with evidence of recurred ischemic symptoms, ECG changes and elevated cardiac troponin.[8] GIB was defined as clinically evident bleeding (gross hematemesis, heme positive coffee ground emesis, heme positive melena) from alimentary canal.
Continuous variables were expressed as mean ± SD or median with 25th and 75th percentiles. Categorical variables were described as a number (n) with percentage (%). Differences of baseline characteristics and outcomes between patients with or without PPIs use were assessed using the Chi-square test or Fisher's exact test for categorical variables and the analysis of variance test or Wilcoxon rank test for continuous variables. The impact of PPIs on in-hospital outcomes was assessed using multivariate logistic regression analysis. Potential relevant risk factors for ischemic and hemorrhagic events were enrolled in the multivariate model, including age, history of hypertension, diabetes, congestive heart failure, stroke, peripheral arterial disease, peptic ulcer disease/helicobacter pylori infection, prior GIB, malignancy, presence of STEMI, hemoglobin, use of aspirin, GPIIb/Ⅲa receptor inhibitor, oral anticoagulants, heparin/LMWH, β-blockers, ACEI/ARB, and treatment with primary PCI, emergent CABG and/or thrombolysis. Odds ratio (OR) were presented with the 95% confidence intervals (CIs) and a two- tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4.
In order to control the effect of confounding factors caused by baseline characteristics differences between patients with and without PPIs use, we preformed propensity score matching (PSM) for the entire AMI population. A propensity score was estimated for each patient using a logistic regression model. Patients were matched on estimated propensity scores, with replacement, using a nearest neighbor approach. The detailed information about propensity score model can be found in Supplementary Table 1.
3. Results
3.1. Baseline characteristics
As shown in Table 1, among 23, 380 analyzed patients with AMI, 15, 972 (67.5%) were co-medicated with PPIs. PPIs users were older and inclined to be female with higher Killip class and hematocrit. They tended to have higher frequent presence of NSTEMI and the history of hypertension, diabetes mellitus, stroke, peptic ulcer disease and GIB. They also had more chance to receive GPIIb/Ⅲa receptor inhibitor, heparin/LMWH, and primary percutaneous coronary intervention (PCI). After PSM, 7169 patients had an estimated propensity score that matched to 7169 patients without PPIs use.
Table 1.
Baseline characteristics among all patients according to PPIs use before and after PSM.
| Parameters | Before PSM | After PSM | |||||
|
PPIs (n = 15, 792) |
No PPIs (n = 7588) |
P |
PPIs
(n = 7169) |
No PPIs
(n = 7169) |
P | ||
| Data are presented as mean ± SD or n (%). ACEI/ARB: angiotensin converting enzyme inhibitor/angiotensin receptor blocker; BP: blood pressure; CABG: coronary artery bypass grafting; CCr: creatinine clearance rate; CRUSADE: can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines; GIB: gastrointestinal bleeding; GPIIb/Ⅲa: glycoprotein IIb/Ⅲa; Hp: Helicobacter pylori; LMWH: low molecular weight heparin; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; PPIs: proton pump inhibitors; PSM: propensity score matching; PUD: peptic ulcer disease; STEMI: ST-elevation myocardial infarction. | |||||||
| Demographics | |||||||
| Age, yrs | 62.65 ± 13.37 | 61.62 ± 13.62 | < 0.0001 | 61.83 ± 13.24 | 61.79 ± 13.46 | 0.5941 | |
| Gender (female) | 4048 (25.6%) | 1858 (24.5%) | < 0.0001 | 1814 (25.3%) | 1785 (24.9%) | 0.5584 | |
| Medical history | |||||||
| Hypertension | 8096 (51.3%) | 3647 (48.2%) | < 0.0001 | 3613 (50.5%) | 3451 (48.2%) | 0.0063 | |
| Dyslipidemia | 1110 (7.0%) | 467 (6.2%) | 0.0050 | 526 (7.4%) | 439 (6.1%) | 0.0036 | |
| Diabetes mellitus | 3142 (20.0%) | 1342 (17.7%) | 0.0001 | 1413 (19.8%) | 1266 (17.7%) | 0.0014 | |
| Prior MI | 1136 (7.2%) | 528 (7.0%) | 0.5374 | 478 (6.7%) | 502 (7.0%) | 0.3898 | |
| Prior PCI | 761 (4.8%) | 339 (4.5%) | 0.2845 | 318 (4.5%) | 321 (4.5%) | 0.9026 | |
| Prior CABG | 63 (0.4%) | 32 (0.4%) | 0.7699 | 27 (0.4%) | 31 (0.4%) | 0.5994 | |
| Congestive heart failure | 390 (2.5%) | 158 (2.1%) | 0.0499 | 162 (2.3%) | 147 (2.1%) | 0.3726 | |
| Prior stroke | 1537 (9.8%) | 590 (7.8%) | < 0.0001 | 637 (8.9%) | 560 (7.8%) | 0.0220 | |
| Peripheral arterial disease | 108 (0.7%) | 27 (0.4%) | 0.0008 | 45 (0.6%) | 25 (0.4%) | 0.0138 | |
| Chronic kidney disease | 199 (1.3%) | 92 (1.2%) | 0.6126 | 85 (1.2%) | 84 (1.2%) | 0.8744 | |
| PUD/Hp infection | 579 (3.7%) | 81 (1.1%) | < 0.0001 | 54 (0.8%) | 54 (0.8%) | 0.4651 | |
| Prior GIB | 338 (2.1%) | 67 (0.9%) | < 0.0001 | 36 (0.5%) | 36 (0.5%) | 0.5213 | |
| Malignancy | 216 (1.4%) | 79 (1.0%) | 0.0416 | 90 (1.3%) | 69 (1.0%) | 0.0916 | |
| Admission features | |||||||
| NSTEMI | 3774 (23.9%) | 2039 (26.9%) | < 0.0001 | 1845 (25.7%) | 1935 (27.0%) | 0.0506 | |
| STEMI | 12018 (76.1%) | 5549 (73.1%) | < 0.0001 | 5342 (74.3%) | 5234 (73.0%) | 0.0506 | |
| Heart rate, beats/min | 78.0 ± 18.8 | 78.1 ± 19.0 | 0.6344 | 77.8 ± 18.4 | 78.0 ± 18.3 | 0.5465 | |
| Systolic BP, mmHg | 128.6 ± 25.3 | 129.6 ± 26.1 | 0.0043 | 129.5 ± 25.3 | 129.8 ± 25.9 | 0.3548 | |
| Killip class ≥ 2 | 4223 (26.7%) | 1643 (21.6%) | < 0.0001 | 1453 (20.4%) | 1454 (20.4%) | 0.3173 | |
| CCr ≤ 30 mL/min per 1.73 m2 | 634 (4.0%) | 409 (5.4%) | < 0.0001 | 96 (1.3%) | 96 (1.3%) | 0.9986 | |
| Hemoglobin, g/L | 135.52 ± 21.15 | 136.62 ± 22.38 | 0.0009 | 136.21 ± 21.01 | 136.51 ± 22.15 | 0.4657 | |
| Hematocrit | 40.41% ± 50.09% | 38.92% ± 14.57% | 0.0006 | 40.52% ± 53.17% | 38.93% ± 14.70% | 0.0172 | |
| LVEF | 53.40% ± 11.12% | 53.60% ± 10.50% | 0.2072 | 53.85% ± 11.16% | 53.85% ± 10.46% | 0.1878 | |
| CRUSADE score | 19.96 ± 15.23 | 20.03 ± 15.36 | 0.8690 | 19.28 ± 14.82 | 19.30 ± 14.89 | 0.8701 | |
| In-hospital medication | |||||||
| Aspirin | 15268 (96.8%) | 7303 (96.4%) | < 0.0001 | 6848 (95.6%) | 6915 (96.6%) | < 0.0001 | |
| GPIIb/Ⅲa receptor inhibitor | 5251 (34.2%) | 1773 (24.5%) | < 0.0001 | 1736 (25.0%) | 1699 (24.7%) | < 0.0001 | |
| Oral anticoagulants | 154 (1.0%) | 213 (2.9%) | < 0.0001 | 73 (1.0%) | 192 (2.7%) | < 0.0001 | |
| Heparin/LMWH | 14301 (90.6%) | 6437 (86.7%) | < 0.0001 | 6285 (89.8%) | 6234 (88.7%) | 0.0006 | |
| β-blockers | 11338 (71.9%) | 5098 (67.3%) | < 0.0001 | 5073 (71.0%) | 4766 (66.6%) | < 0.0001 | |
| ACEI/ARB | 9647 (61.3%) | 4442 (58.6%) | < 0.0001 | 4283 (59.9%) | 4168 (58.3%) | < 0.0001 | |
| Revascularization | |||||||
| Primary PCI | 5835 (37.0%) | 2673 (35.2%) | < 0.0001 | 2447 (34.1%) | 2446 (34.1%) | 0.5637 | |
| Emergent CABG | 15 (0.1%) | 22 (0.3%) | 0.0066 | 4 (0.1%) | 4 (0.1%) | 0.6819 | |
| Thrombolysis | 1190 (7.5%) | 528 (7.0%) | 0.0532 | 482 (6.7%) | 479 (6.7%) | 0.4386 | |
3.2. Clinical outcomes
The occurrence of in-hospital MACCE in PPIs population was significantly lower than that in non-PPIs group before (4.1% vs. 4.9%, P = 0.0056) and after PSM (4.0% vs. 4.7%, P = 0.0025) (Table 2). At multivariate logistic regression analysis (Table 3), PPIs use was strongly associated with the decreased risks of MACCE (OR = 0.862, 95% CI: 0.768-0.949, P = 0.0245) and cardiac death (OR = 0.813, 95% CI: 0.709-0.936, P = 0.0145) after PSM, while an increased risk of stroke was observed in PPIs group.
Table 2.
In-hospital outcomes among all patients according to PPIs use before and after PSM.
| Clinical endpoint | Before PSM | After PSM | |||||
|
PPIs
(n = 15, 792) |
No PPIs
(n = 7588) |
P |
PPIs
(n = 7169) |
No PPIs
(n = 7169) |
P | ||
| Data are presented as n (%). PSM: propensity score matching; PPIs: proton pump inhibitors; MACCE: major adverse cardiovascular and cerebrovascular events; GIB: gastrointestinal bleeding. | |||||||
| Primary endpoint | |||||||
| MACCE | 647 (4.1%) | 372 (4.9%) | 0.0056 | 286 (4.0%) | 337 (4.7%) | 0.0025 | |
| Secondary endpoint | |||||||
| Cardiac death | 569 (3.6%) | 349 (4.6%) | 0.0004 | 251 (3.5%) | 315 (4.4%) | 0.0008 | |
| Re-infarction | 111 (0.7%) | 30 (0.4%) | 0.0094 | 43 (0.6%) | 28 (0.4%) | 0.0098 | |
| Stroke | 142 (0.9%) | 38 (0.5%) | 0.0055 | 65 (0.9%) | 28 (0.4%) | 0.0031 | |
| GIB | 252 (1.6%) | 23 (0.3%) | < 0.0001 | 108 (1.5%) | 20 (0.3%) | < 0.0001 | |
Table 3.
Multivariate logistic regression analysis among all patients according to PPIs use before and after PSM.
| Clinical endpoint | Before PSM | After PSM | |||||
| Adjusted OR | 95% CI | P | Adjusted OR | 95% CI | P | ||
| Odds ratio (OR) of each endpoint in PPIs group was calculated as compared with no PPIs group. GIB: gastrointestinal bleeding; MACCE: major adverse cardiovascular and cerebrovascular events; PPIs: proton pump inhibitors; PSM: propensity score matching. | |||||||
| Primary endpoint | |||||||
| MACCE | 0.857 | 0.742-0.990 | 0.0359 | 0.862 | 0.768-0.949 | 0.0245 | |
| Secondary endpoint | |||||||
| Cardiac death | 0.799 | 0.687-0.929 | 0.0035 | 0.813 | 0.709-0.936 | 0.0145 | |
| Re-infarction | 1.409 | 0.929-2.136 | 0.1067 | 1.378 | 0.979-2.156 | 0.2386 | |
| Stroke | 1.621 | 1.127-2.331 | 0.0091 | 1.635 | 1.341-2.517 | 0.0024 | |
| GIB | 5.847 | 3.376-9.302 | < 0.0001 | 5.471 | 3.127-8.796 | < 0.0001 | |
We did not find protective effectiveness of PPIs against GIB before and after PSM (Table 2 and Table 3). However, PPIs co-medication among patients who suffered GIB after AMI showed a nonsignificant trend to alleviate hemoglobin reduction, reduce the chance of using hemostatics and blood transfusion, and decrease the incidence of death caused by GIB (Table 4).
Table 4.
GIB severity among patients suffering GIB according to PPIs use before and after PSM.
| GIB Severity | Before PSM | After PSM | |||||
|
PPIs
(n = 261) |
No PPIs
(n = 20) |
P |
PPIs
(n = 107) |
No PPIs
(n = 20) |
P | ||
| Data are presented as n (%). GIB: gastrointestinal bleeding; Hb: hemoglobin; NA: not assessed; PSM: propensity score matching; PPIs: proton pump inhibitors. | |||||||
| Hb reduction | 0.7941 | 0.6455 | |||||
| No | 86 (33.0%) | 5 (25.0%) | 40 (37.4%) | 5 (25%) | |||
| < 3 g/L | 105 (40.2%) | 8 (40.0%) | 40 (37.4%) | 8 (40.0%) | |||
| 3-5 g/L | 33 (12.6%) | 3 (15.0%) | 13 (12.1%) | 3 (15.0%) | |||
| > 5 g/L | 37 (14.2%) | 4 (20.0%) | 14 (13.1%) | 4 (20.0%) | |||
| Treatment | 0.6190 | 0.6513 | |||||
| None | 78 (29.8%) | 4 (20.0%) | 31 (28.7%) | 4 (20.0%) | |||
| Endoscopic hemostasis | 2 (0.8%) | 0 (0%) | NA | NA | |||
| Pharmacologic hemostasis | 182 (69.5%) | 16 (80%) | 76 (70.4%) | 16 (80.0%) | |||
| Blood Transfusion | 64 (24.5%) | 7 (35.0%) | 0.3146 | 24 (22.4%) | 7 (35.0%) | 0.2602 | |
| Death caused by GIB | 4 (1.5%) | 1 (5.0%) | 0.3096 | 0 (0.0%) | 1 (5.0%) | 0.1562 | |
4. Discussion
Clopidogrel is a prodrug needed to be metabolized by cytochrome P450 (CYP) with isoenzyme CYP2C19, and this also played a major role in generating active metabolite of PPIs. Therefore, potential drug-interaction may inhibit the conversion of clopidogrel to its active metabolite and further attenuate its antiplatelet properties[10]. A randomized trial revealed that pantoprazole significantly increased platelet aggregation in patients treated with DAPT even after correction for the bias of CYP2C19 polymorphism.[11] Recently, a meta-analysis indicated that the observational studies tended to yield higher rate of adverse events in patients using PPIs, while randomized controlled trials (RCTs) assessing omeprazole compared with placebo showed no difference in ischemic outcomes. This discrepancy may partially due to the selection bias in observational studies. Thus, we made a PSM analysis to eliminate some of these baseline differences between patients who received PPIs and those who did not.
We found that PPIs use was associated with decreased MACCE before and after PSM, which was mainly driven by the reduced risk of cardiac death. However, this finding was different from the recent clinical studies. Of these, TRANSLATE-ACS study 12] and PRODIGY trial[13] showed that the concomitant use of PPIs in patients receiving clopidogrel did not significantly affect clinical outcomes, while results from ADAPT-DES study[14] and PARIS study[15] indicated that PPIs in combination with clopidogrel was associated with high platelet reactivity and a greater rate of 2-year adverse events. In this study, we focused on in-hospital outcomes while the other studies evaluated the long- term value of PPIs use. The potential effect of PPIs on clopidogrel might not be obvious in the short run. Moreover, we speculated that PPIs use would improve patients' compliance to a high-dose of DAPT and intensive anticoagulation therapy, and this may contribute to a lower incidence of cardiac death.
As for other key endpoints, we found that PPIs had no impact on re-infarction risk. Another US national registry[16] also confirmed that there were no increase in AMI risk among adults prescribed PPIs as compared with H2RAs, indicating that patients should not avoid starting a PPI because of concerns related to MI risk. Unexpectedly, our data showed that patients using PPIs had a higher risk of developing stroke. We supposed that this might due to the higher presence rate of prior stroke and risk profiles in PPIs group even after PSM. Recently, a meta-analysis[17] proved that co-prescription of PPIs and thienopyridines increased the risk of incident ischemic strokes. However, another study[18] revealed that concomitant use of PPIs and clopidogrel was not associated with adverse outcomes after ischemic stroke in Chinese population. These conflicting results underscore the need for future RCTs to assess the safety of PPIs in patients with stroke. As stroke remains one of the leading cause of death in China and the incidence of stroke is higher in Chinese than that in other ethnic population, [19, 20] our study may indicate that physicians should avoid prescribing PPIs to high risk patients for stroke.
Consistent with another study in Chinese population, we did not find protectiveness of PPIs against GIB.[21] Yet, another PSM analysis showed the effectiveness of PPIs in reducing the rate of GIB in Japanese population treated with clopidogrel after coronary stenting.[22] This inconsistency might come from study design and the timing when patients received PPIs. Physicians in China tend to prescribe PPIs to AMI patients after emergency procedure, but GIB would have occurred before PPIs were used among AMI patients, especially in those with unstable hemodynamics and high stress state. Even though, our analysis still showed the importance of PPIs use as a remedial method when GIB had occurred. Among 281 patients who suffered GIB in our study (127 after PSM), those treated with PPIs had less hemoglobin reduction and received less drug hemostasis and transfusion. The rate of death caused by GIB was also lower in PPIs group. Although the differences were not statistically significant and the multivariate logistic regressions were not performed because of small sample, these results indicated that PPIs might play a critical role in recovering the injured gastrointestinal mucosa.
4.1. Strengths and limitations
The CAMI registry represents a well-supported registry-base clinical investigation, which not only has large sample size but also serves as a resource to educate physicians and administrative personnel. Our study provided new evidence on the benefit/risk of PPIs use in Chinese hospitalized AMI patients. However, several limitations should be acknowledged. First, the impact of long-term PPIs use on clinical outcomes in patients receiving clopidogrel was not evaluated in our study, and further analysis should assess the long-term prognosis of co-medication. Second, the number of incident GIB was small and multivariate logistic regression could not be performed in Table 4. Finally, although PSM has been used to control baseline confounders, our results may still be subject to selection bias related to this type of observational research. Future large observational studies and RCTs are warranted to further address the potential benefit/risk of PPIs use in AMI patients taking clopidogrel.
4.2. Conclusions
We noticed that our research is the first large-scale study providing evidence on PPIs and clopidogrel co-medication in Chinese AMI population. The co-medication of PPIs and clopidogrel was associated with decreased risk of in-hospital MACCE in AMI patients. When GIB occurred, using PPIs may have a trend to alleviate GIB severity. Our results indicated that PPIs could be an available choice for physician to reduce MACCE and alleviate GIB severity in AMI patients.
Acknowledgement
We are grateful to the CAMI Study Group for their contributions in the design, conduct, and data analyses for our manuscript. We also appreciate all participating hospitals for their active engagement in enrolling patients, collecting submitting data on patients' characteristics. This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-009), Twelfth Five-Year Planning Project of the Scientific and Technological Department of China (2011BAI11B02), and National Natural Science Foundation of China (No 81670415).
Contributor Information
YU Meng-Yue, Email: yumy73@163.com.
YANG Yue-Jin, Email: China.yangyjfw@126.com.
References
- 1.Nikolsky E, Stone GW, Kirtane AJ, et al. Gastrointestinal bleeding in patients with acute coronary syndromes: incidence, predictors, and clinical implications: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009:1293–1302. doi: 10.1016/j.jacc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Costa F, Lokhnygina Y, et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. 2017;38:804–810. doi: 10.1093/eurheartj/ehw525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steg PG, Huber K, Andreotti F, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32:1854–1864. doi: 10.1093/eurheartj/ehr204. [DOI] [PubMed] [Google Scholar]
- 4.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 5.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 6.Melloni C, Washam JB, Jones WS, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcome. 2015;8:47–55. doi: 10.1161/CIRCOUTCOMES.114.001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu HY, Li W, Yang JG, et al. The China Acute Myocardial Infarction (CAMI) Registry: A national long-term registry- research-education integrated platform for exploring acute myocardial infarction in China. Am Heart J. 2016;175:193–201. doi: 10.1016/j.ahj.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. doi: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KA, Park PW, Hong SJ, et al. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: a possible mechanism for clopidogrel resistance. Clin Pharmacol Ther. 2008;84:236–242. doi: 10.1038/clpt.2008.20. [DOI] [PubMed] [Google Scholar]
- 11.Parri MS, Gianetti J, Dushpanova A, et al. Pantoprazole significantly interferes with antiplatelet effect of clopidogrel: results of a pilot randomized trial. Int J Cardiol. 2013;167:2177–2181. doi: 10.1016/j.ijcard.2012.05.080. [DOI] [PubMed] [Google Scholar]
- 12.Jackson LR, Peterson ED, McCoy LA, et al. Impact of Proton Pump Inhibitor Use on the Comparative Effectiveness and Safety of Prasugrel Versus Clopidogrel: Insights From the Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome (TRANSLATE-ACS) Study. J Am Heart Assoc. 2016;5:e003824. doi: 10.1161/JAHA.116.003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: Insights from the PROlonging Dual-antiplatelet treatment after Grading stent- induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95–102. doi: 10.1016/j.ahj.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Weisz G, Smilowitz NR, Kirtane AJ, et al. Proton Pump Inhibitors, Platelet Reactivity, and Cardiovascular Outcomes After Drug-Eluting Stents in Clopidogrel-Treated Patients: The ADAPT-DES Study. Circ Cardiovasc Interv. 2015;8:e001952. doi: 10.1161/CIRCINTERVENTIONS.114.001952. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekhar J, Bansilal S, Baber U, et al. Mehran, Impact of proton pump inhibitors and dual antiplatelet therapy cessation on outcomes following percutaneous coronary intervention: Results From the PARIS Registry. Catheter Cardiovasc Interv. 2017;89:E217–E225. doi: 10.1002/ccd.26716. [DOI] [PubMed] [Google Scholar]
- 16.Landi SN, Sandler RS, Pate V, et al. No increase in risk of acute myocardial infarction in privately insured adults prescribed proton pump inhibitors vs. histamine-2 receptor antagonists (2002-2014) Gastroenterology. 2018;154:861–873. doi: 10.1053/j.gastro.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra K, Katsanos AH, Bilal M, et al. Cerebrovascular outcomes with proton pump inhibitors and thienopyridines: a systematic review and meta-analysis. Stroke. 2018;49:312–318. doi: 10.1161/STROKEAHA.117.019166. [DOI] [PubMed] [Google Scholar]
- 18.Yi XY, Zhou Q, Wang C, et al. Concomitant use of proton pump inhibitors and clopidogrel is not associated with adverse outcomes after ischemic stroke in chinese population. J Stroke Cerebrovasc Dis. 2016;25:2859–2867. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Yang GH, Wang Y, Zeng YX, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu S, Cui BJ, Mlynash M, et al. Stroke epidemiology and stroke policies in China from 1980 to 2017: A systematic review and meta-analysis. Int J Stroke. 2020;15:18–28. doi: 10.1177/1747493019873562. [DOI] [PubMed] [Google Scholar]
- 21.Zhu P, Gao Z, Tang XF, et al. Impact of proton-pump inhibitors on the pharmacodynamic effect and clinical outcomes in patients receiving dual antiplatelet therapy after percutaneous coronary intervention: a propensity score analysis. Chin Med J (Engl) 2017;130:2899–2905. doi: 10.4103/0366-6999.220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aihara H, Sato A, Takeyasu N, et al. Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: results from the Ibaraki Cardiac Assessment Study Registry. Catheter Cardiovasc Interv. 2012;80:556–563. doi: 10.1002/ccd.23327. [DOI] [PubMed] [Google Scholar]


