Abstract
Aseptic loosening of total joint replacements is driven by a macrophage-mediated inflammatory reaction to implant-derived wear particles. Phagocytosis of implant debris has been suggested to activate the NLRP3 inflammasome leading to secretion of interleukin (IL)-1 β. However, factors and molecular mechanisms driving the particle-induced inflammasome activation are yet to be fully elucidated. In this study, we investigated the inflammasome response of human primary macrophages to titanium, chromium, and molybdenum particles in vitro. We observed that particles alone were not sufficient to induce IL-1 β secretion, but an additional priming signal—such as bacterial lipopolysaccharide (LPS)—was required to license the inflammasome activation. By using specific inhibitors against the inflammasome signaling pathway, we demonstrate that the particle-induced IL-1 β secretion depended upon activation of the NLRP3 inflammasome. We further hypothesized that tumor necrosis factor (TNF) could substitute for LPS as a priming signal, and found that particle stimulation together with preceding TNF treatment resulted in inflammasome-dependent IL-1 β production as well. Our results show that the NLRP3 inflammasome mediates wear particle responses in human primary macrophages, and its activation does not necessarily require the presence of bacterial components, but can be induced under aseptic conditions by TNF priming.
Keywords: Macrophage, Wear particle, Inflammasome, IL-1 β, TNF
1. Introduction
Aseptic loosening remains a major long-term complication of total joint replacements and is expected to affect an increasing number of patients [1]. This process, primarily driven by implant-derived wear particles, results in a slowly progressive inflammatory osteolysis, jeopardizing the stability of the prosthesis [2]. Due to ongoing wear between implant components, particles are continuously generated and dispersed in the periprosthetic tissues. Consequently, accumulating particulate debris activates macrophages of the innate immune system and the debris becomes engulfed by these phagocytes [3]. To eliminate any potentially dangerous substances and recruit further immune cellsto the insult site, macrophages secrete multiple inflammatory mediators [4,5]. Despite an intense immune response, wear particles en-dure the enzymatic degradation of macrophages and sustain the inflammatory stimulus [6]. As a result, macrophage-mediated inflammation develops into a chronic foreign body reaction invading the bone-implant interface [7]. Eventually, this condition increases local bone resorption over bone formation and leads to loosening of the implant.
Although the established model of aseptic loosening is widely accepted, the exact mechanisms of wear particle-induced macrophage activation remain obscure. Some foreign body particulate materials, such as asbestos and silica, have been shown to activate the NLRP3 (NLR family pyrin domain containing 3) inflammasome—a large intracellular machinery mediating the activation of interleukin (IL) −1 β [8,9]. Indeed, IL-1 β represents one of the most potent pro-inflammatory cytokines and has been identified as a product of wear particle-stimulated macrophages [5,10]. Since IL-1 β promotes osteoclast function as well, IL-1 β is considered a key cytokine in the pathogenetic cascade of aseptic loosening [11]. However, few studies have characterized the underlying mechanisms of NLRP3 inflammasome activation leading to wear particle-induced IL-1 β secretion [12–15]. Furthermore, these studies have been conducted primarily with murine macrophages or cell lines.
IL-1 β is first synthesized as a precursor protein (pro-IL-1 β) with the production of biologically active IL-1 β strictly regulated by inflammasomes [16]. Among these, the NLRP3 inflammasome is the most versatile [16]. The cytosolic NLRP3 protein belongs to the NLR (nucleotide-binding oligomerization domain-like receptor or NOD) family of pattern recognition receptors capable of sensing various intracellular aberrations such as ion flows, mitochondrial dysfunction, or phagosome rupture [17]. These physiological alterations may result from a diverse array of endo- or exogenous stressors—reportedly also from phagocytosed biomaterial wear particles [12–15]. Upon activation, NLRP3 triggers the assembly of the multimeric inflammasome complex. Subsequent interactions between recruited adaptor proteins ASC (apoptosis-associated speck-like protein containing a caspase-recruitment domain) and pro-caspase-1 lead to autocleavage and formation of active caspase-1. Ultimately, this proteolytic enzyme cleaves precursor protein pro-IL-1 β into the mature secreted form.
General consensus agrees that activation of the inflammasome requires two distinct signals [18].Inadditiontotheac-tual inflammasome-activating signal detected by NLRP3, a nuclear factor-ĸB (NF-ĸB) activating priming signal is needed. This NF-?B-mediated priming licenses the NLRP3 inflammasome by initiat-ing the synthesis of pro-IL-1 β and NLRP3 itself. Only together can these two signals activate the inflammasome assembly and induce IL-1 β secretion from macrophages. To date, it remains uncertain whether wear particles alone can provide both of these signals, or whether an additional NF-ĸB activating priming signal is required. Bacterial lipopolysaccharide (LPS) has been suggested to work in tandem with particles to activate the NF-ĸB signaling thus licensing the inflammasome, but the amount of LPS in the aseptic interface remains controversial [19–21]. Moreover, the inflammasome-activating potential of different prosthesis materials remains unexamined in a uniform study setting.
In the present study, we investigated the ability of titanium (Ti), chromium (Cr), and molybdenum (Mo) particles to activate the NLRP3 inflammasome in human primary macrophages. The inflammasome activation was assessed in vitro by using qRT-PCR and Western blot analyses, and by measuring the production of IL-1 β from culture media with ELISA. We hypothesized that IL-1 β secretion would depend upon a co-stimulatory priming signal and different events related to activation of the NLRP3 inflammasome. We further asked whether tumor necrosis factor (TNF) could replace LPS as a priming signal and license macrophages for the particle-inducedinflammasomeactivation.
2. Materials & methods
2.1. Particle sterilization
Commercially available particles of common implant materials were purchased from Alfa Aesar (Titanium powder, Product No. 00681; Chromium powder, Product No. 41797; Molybdenum powder, Product No. 10030; Alfa Aesar, Ward Hill, MA). Titanium particles were sterilized with five alternating treatments of 0.1 N NaOH in 95% ethanol and 25% nitric acid as introduced by Ragab et al. [22]. Chromium and molybdenum particles were cleaned with three overnight washes in 70% ethanol followed by heat sterilization in 175 °C oven for 3 h. Particles were suspended in sterile water, and their endotoxin decontamination was verified using EndoLISA (detection limit 0.05 endotoxin units (EU)/mL; ELISA-based Endotoxin Detection Assay, Hyglos, Bernried am Starnberger See, Germany), a limulus amebocyte lysate (LAL) assay kit (detection limit 0.1 EU/mL; Pierce LAL Chromogenic Endotoxin Quanti-tation Kit, Waltham, MA) and HEK-Blue hTLR4 Cells (InvivoGen, San Diego, CA). These Toll-like receptor (TLR) 4 reporter cells were cultured in DMEM medium (Gibco, Waltham, MA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% Penicillin-Streptomycin antibiotic solution (Gibco), and exposed to particles for 24 h. Proportional to TLR4 activation, production of secreted embryonic alkaline phosphatase (SEAP)was assessed from the culture medium with QUANTI-Blue detection reagent (InvivoGen)fol-lowing the manufacturers instructions.
For endotoxin detection, particles were analyzed at concentrations corresponding to doses used in cell culture: 2.3 mg/mL for Ti, 3.6 mg/mL for Cr, and 5.1 mg/mL for Mo. LPS levels of the particle solutions remained below the detection limits of EndoLISA and LAL assays, and particle-challenged HEK-Blue cells indicated an SEAP activity comparable to untreated controls. Using the LAL assay, particles were also measured with LPS (from Escherichia coli O111:B4, Sigma, Saint Louis, MO) spikes resulting in recovery rates of 100% for Ti, 27% for Cr, and 16% for Mo. Proper function of TLR4 reporter cells was verified using ultrapure LPS (logarithmic standard curve starting from 0.1 EU/mL; from E. coli O111:B4, InvivoGen) and TLR4 inhibitor CLI-095 (1 μg/mL, InvivoGen), and as studied with particles, recovery of LPS (InvivoGen) spikes was 99% for Ti, 73% forCr, and 86% for Mo.
2.2. Transmission electron microscopy (TEM)
Particle size and shape were assessed by TEM. 5-μl droplets of the metal particle dispersions were pipetted on formvar/carbon 200 mesh grids (FCF200-CU, Electron Microscopy Sciences, Hatfield, PA), and dried by removing excess water using a filter paper (Whatman, Little Chalfont, UK). TEM imaging was performed using a transmission electron microscope (JEM-1400, JEOL, Tokyo, Japan) operated at 80 keV acceleration voltage in bright-field mode.
2.3. Monocyte isolation and macrophage differentiation
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy volunteers, who had signed an informed consent. Buffy coats were by-products of blood preparation intended for clinical use, and their allocation for scientific purposes was approved by the Finnish Red Cross Blood Service (Helsinki, Finland). Blood donors comprised of 30 women and 15 men with a mean age of 33 years (range 18–64) for females and 42 years (range 21–67) for males. As qualified for blood donation, donors had no major illnesses or medications.
According to a previously introduced protocol [23],buffycoat blood was diluted in Ca/Mg-free phosphate-buffered saline (PBS), added on top of Ficoll-Paque PLUS (GE Healthcare, Chicago, IL) density gradient medium, and centrifuged to form a PBMC layer. Mononuclear cells were collected and repeatedly washed with PBS for four times. After the final wash, freshly isolated monocytes were suspended in DMEM medium (Sigma) supplemented with 1% Penicillin-Streptomycin antibiotic solution (Gibco). Cells were counted using a TC20 automated cell counter (Bio-Rad, Hercules, CA) and plated onto 24-well culture plates (Greiner CELL-STAR, Kremsmünster, Austria) at a concentration of 1.5 × 106 cells/well. Cells were allowed to attach for one hour in a humidified + 37 °C incubator at 5% CO2, until rinsed twice with PBS to remove non-adherent cells. Attached monocytes were differentiated into macrophages during a seven-day culture in macrophage serum-free medium (macrophage-SFM, Gibco) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF, Mil-tenyi Biotec, Bergisch Gladbach, Germany).
2.4. Macrophage stimulation
Inflammasome priming was induced by culturing macrophages for one hour in the presence of either 1 μg/mL LPS (Sigma) or 100 ng/mL TNF (R&D Systems, Minneapolis, MN). Where appropriate, medium of LPS- and TNF-primed macrophages was supplemented for another hour with established inhibitors against NLRP3 inflammasome components or its recognized upstream activating events: leakage of cathepsin B from lysosomes, formation of mitochondrial reactive oxygen species (mtROS), and potassium (K+) efflux. Two small-molecule inhibitors CY-09 (30 μM, Glixx Laboratories Inc, Southborough, MA) and MCC950 (1 μM, Avistron, Bude, UK) were used to directly inhibit NLRP3 [24,25], whereas Z-YVAD-FMK (25 μM, Santa Cruz Biotechnology, Dallas, TX) was employed to block the function of caspase-1. Intracellular perturbations sensed by NLRP3 were inhibited using 25 μM pan-cathepsin inhibitor CA-074-Me (Calbiochem, San Diego, CA) primarily for cathepsin B, 200 μM MitoTEMPO (Sigma) for mtROS, and 130 mM KCl for K+ efflux. For the latter, the effect of increased osmolarity was assessed by including a control group treated with 260 mM sorbitol. Working concentrations of LPS and TNF were determined according to previous reports [23,26], whereas the inhibitor concentrations were optimized in our laboratory.
Following the priming step and addition of a specific inhibitor, macrophages were challenged for eight hours with an equal volume of Ti, Cr, or Mo particles. All experiments were conducted in duplicate wells maintaining the presence of priming and inhibiting agents in the medium with the particles. After particle exposure, culture supernatants were collected, and macrophages were disrupted by adding RLT Plus lysis buffer (Qiagen, Valencia, CA). Cell viability was immediately measured from the media using a lactate dehydrogenase (LDH) detection kit (Roche Diagnostics, Mannheim, Germany). Remaining media and cell lysates were stored at - 75°C for later use.
An optimal particle concentration for macrophage stimulation was determined in preliminary experiments, in which we assessed particle doses ranging from 0.1 to 0.5 mm3/well and an exposure time up to 24 h. Taking into account the dose-dependent inflammatory responses and cytotoxicity, an eight-hour stimulation time with a particle load of 0.25 mm3/well was chosen for further experiments. In all conditions, phagocytosis of particles was verified by microscopy (Supplementary Video 1).
2.5. qRT-PCR
Total RNA (ribonucleic acid) was extracted and purified from cell lysates by using RNeasy Mini Kit (Qiagen) according to the manufacturers instructions. The amount of RNA was measured with NanoDrop 1000 spectrophotometer (Thermo scientific, Wilmington, DE) and 1 μg of RNA from each sample was reverse transcribed into complimentary DNA (cDNA) using iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR was performed in LightCycler (Roche) instrument from a reaction mix containing 5 ng of sample cDNA, a pair of human forward and reverse primers (Supplementary Table 1), and HOT FIREPol EvaGreen qPCR Su-perMix reagent (Solis BioDyne, Tartu, Estonia). Results were normalized to geometric mean of housekeeping genes ribosomal protein lateral stalk subunit P0 (RPLP0), hypoxanthine phosphoribosyltransferase 1 (HPRT1), and beta-2-microglobulin (B2M), and obtained using the comparative Ct method.
2.6. Enzyme-Linked immunosorbent assay (ELISA)
The concentration of cytokines IL-1 β, TNF, IL-6, IL-1Ra, and IL-18 was measured from the culture supernatants using ELISA DuoSet kits (R&D) and high protein binding affinity 96-well microplates (SpectraPlate-96 HB, Perkin Elmer, Waltham, MA) following the manufacturers protocol.
2.7. Western blotting
For immunoblot analyses, PBMCs were plated onto 6-well culture plates at a concentration of 6 × 106 cells/well and differentiated into macrophages as described above. Mature macrophages were primed with 1 μg/mL LPS and challenged with metal particles for eight hours at a concentration of 1.0 mm3/well. The experiments were run in triplicate wells accompanied by appropriate control groups. Culture media were removed, and adherent macrophages were treated with Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA) containing protease and phosphatase inhibitors (cOmplete Protease Inhibitor Cocktail, Roche; Phosphatase Inhibitor Mini Tablets, Pierce Biotechnology, Waltham, MA). While keeping the plates on ice, cells were scraped, and lysates from triplicate wells were combined. Samples were stored at - 75 °C for later use.
Cell lysates were thawed, and their protein concentration was measured using a BCA protein assay kit (Pierce) as per the manufacturers instructions. An equal amount of protein from each sample (40 μg) was loaded on gels prepared using TGX FastCast Acrylamide Solutions (Bio-Rad). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerrica, MA). The blotted PVDF membranes were blocked with tris-buffered saline containing 0.05% Tween 20 (TBST) and 5% milk for one hour at room temperature, after which membranes were incubated overnight at + 4 °C with antibodies against human IL-1 β (1:400, Santa Cruz Biotechnology), NLRP3 (1:10 0 0, AdipoGen, San Diego, CA), and caspase-1 (1:10 0 0, AdipoGen). HRP-conjugated goat-anti-mouse or goat-anti-rabbit immunoglobulins were used as secondary antibodies (1:2000, Dako, Glostrup, Denmark). The band intensities were quantified by Bio-Rad ChemiDoc MP imaging system and normalized to the intensity of total protein staining on PVDF membrane per lane using stain free technology. This method utilizes polyacrylamide gels containing compounds that modify tryptophan residues in the loaded samples enabling highly sensitive visualization of the protein loading and transfer onto the PVDF membrane [27].
2.8. Caspase-Glo 1 assay
To detect active caspase-1 released in the culture medium, a bioluminescent assay (Caspase-Glo 1 Inflammasome Assay, Promega, Madison, WI) was performed following the manufacturers protocol. In principle, supernatants were mixed with assay reagent 1:1 and incubated for one hour at room temperature. All conditions were run in duplicates, and the luminescence was measured using a plate-reading luminometer (Tecan, Männedorf, Switzerland).
2.9. Statistical analyses
Statistical comparison between the experimental groups was conducted with repeated measures one-way analysis of variance (ANOVA) and Holm-Sidaks post hoc tests using GraphPad Prism version 8 (GraphPad Software, La Jolla, CA). Post hoc analyses were performed separately for each particle material with experimental conditions comprising of at least four independent donors. Paired t-test was used to study statistical significances for TNF-induced pro-IL-1 β expression, and for the particle-related secretion of TNF, IL-6, IL-1Ra, and IL-18. P-value of 0.05 was selected as the threshold for significance. Results are presented as mean ± standard error of mean (SEM).
3. Results
3.1. Characterization of metal particles
TEM imaging confirmed the particle shape roundish yet irregularly angular (Fig. 1a–c). Analysis of 600 particles revealed an aver-age particle size of 1.6 ± 1.1 μm (range 0.1–7.5 μm, median 1.3 μm) for Ti particles, and a diameter of 1.4 ± 0.9 μm (range 0.2–5.6 μm, median 1.0 μm) for Mo particles. Measurement of 400 Cr particles indicated a mean diameter of 1.7 ± 1.0 μm (range 0.3–7.2 μm, median 1.5 μm). Particles of this size range are phagocytable for macrophages and have been used in previous in vitro studies [2]. Similar particles have also been identified in periprosthetic tissues around aseptically loosened total joint replacements [28,29].
Fig. 1.
Transmission electron microscopy (TEM) of metal particles. The appearance and size-distribution of (a) Ti, (b) Cr, and (c) Mo particles were assessed by TEM imaging. Scale bars 5 μm.
3.2. Inflammasome priming was required for particle-induced IL-1 β production
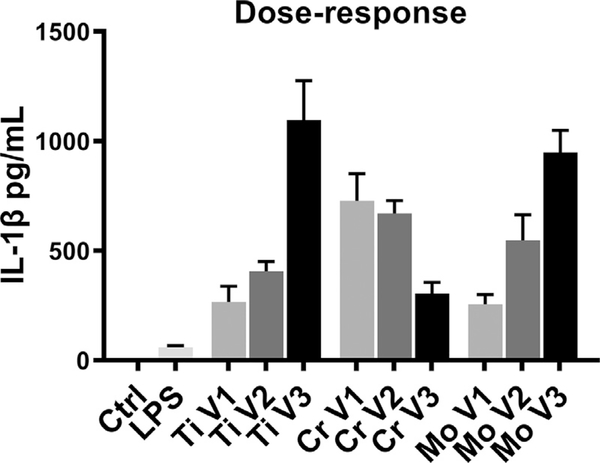
Using ELISA, we detected no IL-1 β secretion from macrophages challenged with wear particles alone. Instead, particle stimulation together with preceding LPS priming led to significant IL-1 β production in a dose-dependent manner (Fig. 2). Increasing concentrations of Ti and Mo particles enhanced the amount of IL-1 β secreted, whereas a high dose of Cr particles diminished the cytokine release. A particle volume of 0.25 mm3 per well was therefore selected for subsequent experiments. LPS stimulation alone induced small and consistent IL-1 β production as well.
Fig. 2.
Dose-response of particle-challenged macrophages. Human primary macrophages (n = 6) were primed with LPS and stimulated for eight hours with increasing concentrations of Ti, Cr, and Mo particles. IL-1 β secretion induced by particle doses of (V1) 0.1, (V2) 0.25, and (V3) 0.5 mm3/well was determined from culture supernatants by ELISA.
3.3. LPS priming upregulated the expression of pro-IL-1 β and NLRP3
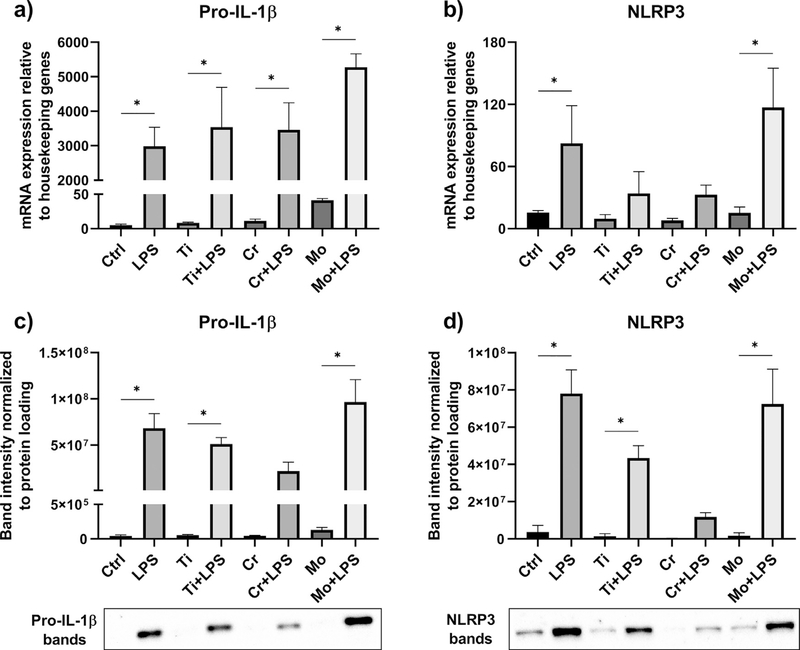
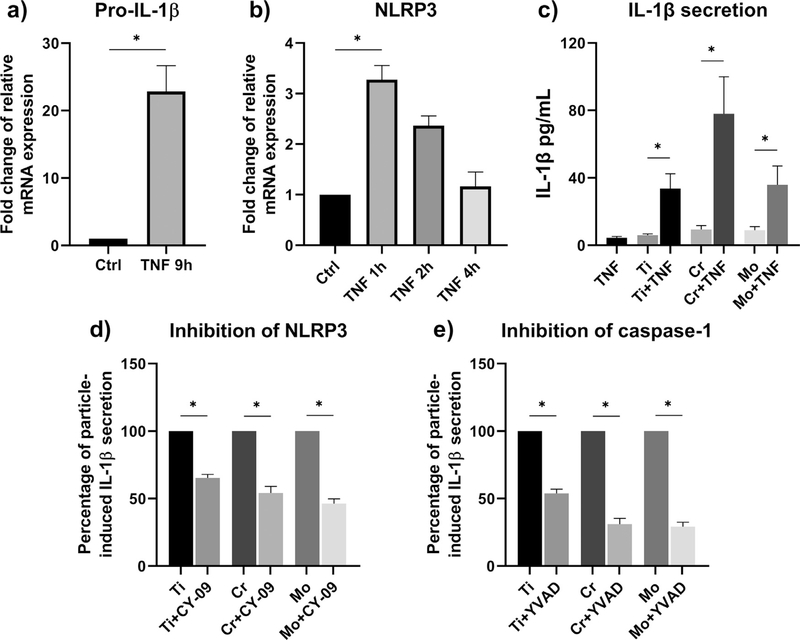
As studied by qRT-PCR, we found that particles alone initiated neither pro-IL-1 β nor NLRP3 expression from macrophages. In contrast, LPS-primed cells increased the messenger RNA (mRNA) expression of these markers with or without the addition of metal particles (Fig. 3a–b). This phenomenon was further verified by Western blot analysis: While LPS priming resulted in elevated amounts of pro-IL-1 β and NLRP3 proteins, these components could not be detected in macrophages challenged with particles alone (Fig. 3c–d). Interestingly, Mo particles in conjunction with LPS induced higher mRNA expression and protein levels of both pro-IL-1 β and NLRP3 when compared to Ti or Cr particles.
Fig. 3.
The expression of pro-IL-1 β and NLRP3. Priming effects of LPS and particles alone on macrophages (n = 4) were analyzed by qRT-PCR and Western blotting. The relative mRNA expression of (a) pro-IL-1 β and (b) NLRP3, and the production of intracellular (c) pro-IL-1 β and (d) NLRP3 proteins were determined after an eight-hour stimulation with Ti, Cr, and Mo particles. Representative Western blot images are included with * illustrating statistically significant difference between indicated conditions (p < 0.05).
3.4. IL-1 β secretion depended upon activation of the NLRP3 inflammasome
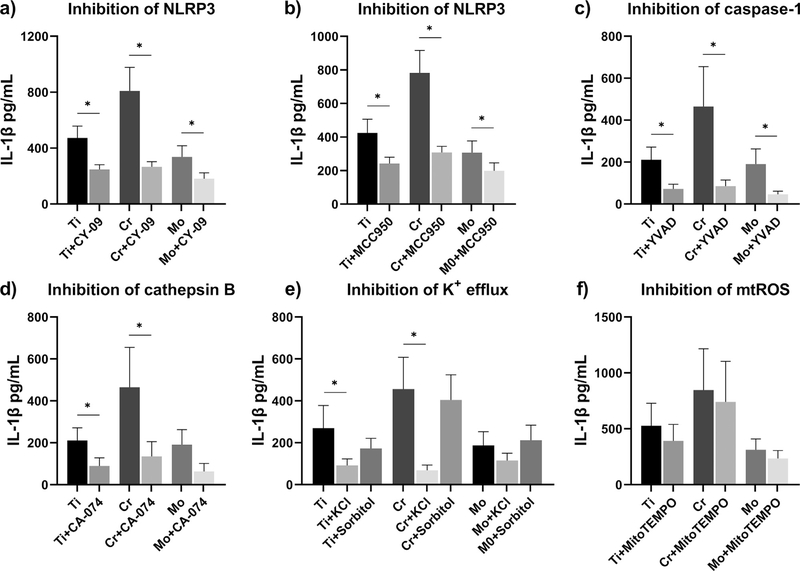
To study the role of inflammasome activation in particle-induced IL-1 β secretion, we treated LPS-primed macrophages with different inhibitors of NLRP3 inflammasome pathway. Using ELISA assay, we observed that direct inhibition of NLRP3 by either CY-09 or MCC950 significantly diminished the IL-1 β release (Fig. 4a–b). With Cr particles intensifying IL-1 β secretion to a higher degree, also the inhibitory effect appeared stronger on Cr-challenged macrophages. A similar response profile was seen when inhibiting caspase-1 or cathepsin B by Z-YVAD-FMK or CA-074-Me, respectively; these inhibitors reduced the amount of IL-1 β secreted in an equally effective manner (Fig. 4c–d). Inhibition of K+ efflux by high extracellular K+ concentration also suppressed the particle-induced IL-1 β production (Fig. 4e). The potential effect of increased osmolarity was evaluated by inclusion of sorbitol control groups; particle-stimulated macrophages maintained higher IL-1 β levels in otherwise highly osmotic medium (Fig. 4e). Neu-tralization of mtROS with mitochondria-targeting anti-oxidant MitoTEMPO only slightly mitigated the amount of IL-1 β secreted with no statistical significance observable (Fig. 4f). Macrophages treated with inhibitors alone secreted IL-1 β comparable to the level of corresponding control cells (Supplementary Fig. 1). Based on LDH release, cytotoxicity induced by particles, priming stimuli, or inhibitors was considered non-significant (Supplementary Fig. 2).
Fig. 4.
Inhibition of NLRP3 inflammasome activation. LPS-primed macrophages were treated with inhibitors of NLRP3 inflammasome pathway, and challenged with Ti, Cr, and Mo particles for eight hours. Inhibition of inflammasome components (a-b) NLRP3 and (c) caspase-1, and upstream inflammasome activating signals (d) cathepsin B, (e) K+ efflux, and (f) mtROS were assessed by studying the IL-1 β secretion from culture media with ELISA. A control group of sorbitol-treated macrophages was included to take into account the effect of increased osmolarity. In graphs (b) and (e) n = 5, whereas other experiments were performed with n = 4. * indicates statistical significance between the conditions (p < 0.05).
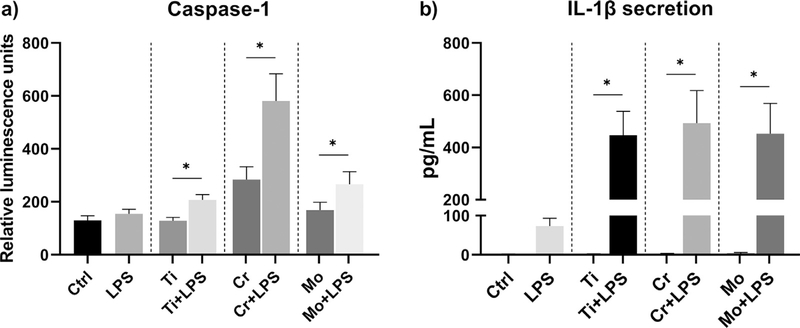
3.5. LPS-primed macrophages increased caspase-1 release after particle stimulation
Inflammasome activation was further confirmed by studying the release of active caspase-1 from cell culture supernatants. Using a luminescence assay, we observed that LPS-primed macrophages showed an enhanced release of active caspase-1 in response to metal particles when compared to cells without a priming signal (Fig. 5a). Surprisingly, Cr particles alone induced some degree of caspase-1 secretion as well. Without LPS priming, however, IL-1 β secretion from particle-challenged macrophages remained undetectable (Fig. 5b).Using gel electrophoresis, we observed that neither LPS priming nor particle treatment affected the protein expression of pro-caspase-1. Regardless, no active caspase-1 could be detected from the cell lysates (data not shown).
Fig. 5.
Activation of caspase-1. Human primary macrophages (n = 5) were primed with LPS and challenged with Ti, Cr, and Mo particles for eight hours. (a) The release of active caspase-1 was measured from culture supernatants using a luminescence assay, and (b) the corresponding IL-1 β secretion was determined by ELISA. * represents statistical significance between indicated conditions (p < 0.05).
3.6. TNF priming enabled particle-induced NLRP3 activation and IL-1 β secretion
In search of a sterile agent that could replace LPS as a priming signal for particle-induced IL-1 β production, we found that TNF priming resulted in enhanced pro-IL-1ß and NLRP3 expressions at mRNA level (Fig. 6a–b). Whereas pro-IL-1β expression was elevated still at nine hours after the initiation of TNF priming, NLRP3 expression proved to be transient and attenuated already by the four-hour time point. Together with particle stimulation, an elevated IL-1 β secretion became evident (Fig. 6c). This finding oc-curred for all materials studied with Cr particles again inducing the highest levels of IL-1 β release. Inhibition of NLRP3 and caspase-1 reduced the particle-related IL-1 β secretion also from TNF-primed macrophages (Fig. 6d–e).
Fig. 6.
The effect of TNF priming. Human primary macrophages were cultured in the presence of TNF up to nine hours, after which the relative mRNA expression of (a) pro-IL-1 β (n = 3) and (b) NLRP3 (n = 4) was assessed by qRT-PCR analysis. (c) TNF-primed macrophages (n = 5) were exposed to an eight-hour stimulation with Ti, Cr, and Mo particles, and the IL-1 β secretion was assessed by ELISA. (d-e) Particle-induced IL-1 β secretion from TNF-primed cells (n = 4) was further modified by inhibition of NLRP3 and caspase-1. Data in graphs (a) and (b) are presented as fold change compared to untreated control, whereas results from experiments (d) and (e) are shownas percentage of particle-induced IL-1 β secretion. * indicates statistically significant differences between groups (p < 0.05).
3.7. Metal particles alone elicited secretion of other inflammatory cytokines
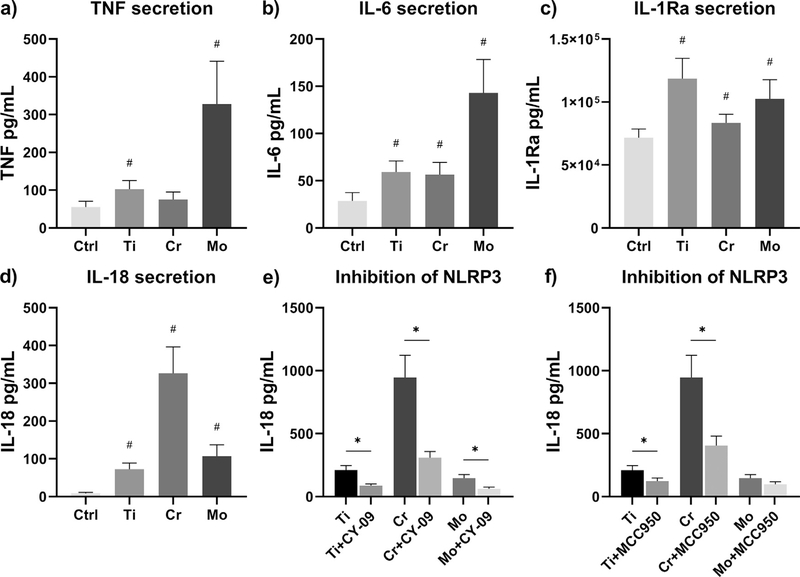
As analyzed by ELISA, we discovered that metal particles were able to initiate the secretion of TNF, IL-6, IL-1Ra, and IL-18 without the need of an additional priming signal (Fig. 7a–d). Interestingly, Mo particles markedly increased the release of TNF and IL-6, whereas IL-18 production was enhanced to the highest degree by Cr particles. LPS priming further intensified the secretion of IL-18, which proved to be inflammasome-mediated as inhibition of NLRP3 decreased the secretion of this pro-inflammatory cytokine (Fig. 7e–f).
Fig. 7.
Particle-induced secretion of inflammatory cytokines. Unprimed human primary macrophages were challenged with Ti, Cr, and Mo particles for eight hours, and the secretion of (a) TNF, (b) IL-6, (c) IL-1Ra, and (d) IL-18 was analyzed from culture supernatants using ELISA assays. LPS-primed macrophages were treated with NLRP3 inhibitors and exposed to an eight-hour particle stimulation as well; the inhibitory effects of (e) CY-09 and (f) MCC950 on particle-induced IL-18 secretion were assessedby ELISA. In graphs (a-c) n = 8, whereas experiments illustrated in graphs (d-f) were performed with n = 4. # illustrates statistical significance for particle stimulation, and* represents significant difference between indicated conditions (p < 0.05).
4. Discussion
Excessive and prolonged IL-1 β production has been associated with many chronic inflammatory conditions causing progressive tissue damage [30,31]. This scenario seems to prevail in aseptic loosening as well, due to wear debris activation of the innate immune system. Indeed, IL-1 β is considered an essential pro-inflammatory mediator driving osteolysis at the bone-implant interface [5]. However, the underlying cellular mechanisms leading to wear particle-induced IL-1 β production remain incompletely understood. Therefore, we challenged human primary macrophages with particles from common implant materials, and characterized their effects on priming and activation of the NLRP3 inflammasome pathway—an established regulator of IL-1 β secretion.
We observed that macrophages stimulated with metal particles alone elicited no observable IL-1 β secretion. While the production of pro-IL-1 β and NLRP3 also remained absent, these findings indicate that none of the particles was able to induce NLRP3 inflammasome priming. These results are in line with two recent studies, in which sterile Ti particles induced neither NF-ĸB activation nor IL-1 β secretion from murine macrophages [32,33]. At the same time, our results are different from previous reports, in which Ti and CrMo alloy particles, without an additional priming signal, induced macrophage-mediated IL-1 β secretion [10,12,15]. While these in vitro studies were performed under different experimental conditions likely contributing to priming of cells, traces of endotoxin contamination on particle surfaces could also be one explanation for this discrepancy. Indeed, commonly used endotoxin detection assays are sensitive to inhibition by test sample, and may be interfered with implant particles. This can lead to false negative results and misinterpretation of wear particle responses. Rather poor recovery of LPS spikes with Cr and Mo particles in our LAL assay further demonstrates this issue.
Despite these concerns, our results obtained with human primary macrophages and TLR4 reporting HEK-Blue cells imply that no biologically significant amount of endotoxin remained on particles used in the current study. On the contrary, pretreatment with LPS dramatically enhanced secretion of IL-1 β from macrophages suggesting a co-stimulatory priming step necessary for particle-induced inflammasome activation. Increased expressions of pro-IL-1 β and NLRP3 at both mRNA and protein levels verify inflammasome priming by LPS treatment. In fact, bacterial LPS stands as a well-documented TLR4 ligand that primes the inflammasome through activation of transcrip-tion factor NF-ĸB [34], and it is commonly used as a priming agent in the field of inflammasome research. To examine the particle-induced inflammasome activation in more de-tail, further experiments were performed using LPS as a priming signal.
Whereas macrophage responses to Ti and Mo particles proved tobe dose-dependent, the highest concentration of Cr particles reduced the amount of IL-1 β released. While this result was not due to increased cytotoxicity, it is possible that a negative feedback response restricted the effect of an overwhelming particle load. Given that the particle shape and size were similar between different materials, this discrepancy may also originate from unidentified, chromium-specific physico-chemical factors. Conversely, with the selected dose of 0.25 mm3/well, Cr particles actually induced the most vigorous IL-1 β secretion throughout the experiments. Hence, it seems that apart from the particle dose, also the material itself affects the magnitude of inflammasome activation. Regardless, particles of every material substantially increased secretion of IL-1 β from LPS-primed macrophages suggesting their potential to provide the actual inflammasome-activating signal.
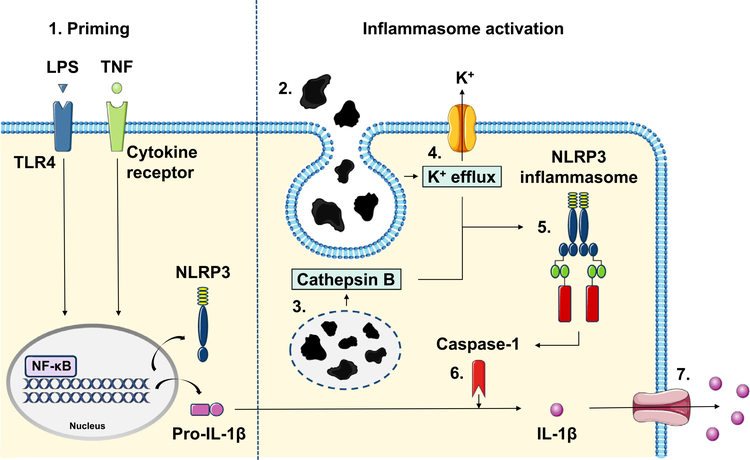
In subsequent mechanistic studies, we demonstrate that the particle-related IL-1 β secretion depended upon activation of the NLRP3 inflammasome. Since NLRP3 inflammasome is activated by structurally and chemically divergent triggers, it has emerged that instead of detecting each particular activator separately, NLRP3 receptor in fact responds to common cellular stress-signals caused by these stimuli [17]. NLRP3 activation has been closely linked at least with production of mtROS, K+ efflux, and lysosomal destabilization followed by cathepsin B release [17,35]. The latter occurs especially from phagocytosis of particulate matter and has been suggested as a means for implant wear to activate the inflammasome [15,36]. As pan-cathepsin inhibition reduced the amount of IL-1 β released, this proposal is further supported by our results. In addition, the current study highlights the importance of NLRP3 itself, K+ efflux, and caspase-1 in particle-induced IL-1 β secretion; all of these components were needed for a pronounced activation of the NLRP3 inflammasome cascade (Fig. 8). WhilealsomtROS have been suggested to participate in particle-related inflammatory reaction, their inhibition had no significant effect on IL-1 β secretion. These products might be more pertinent regarding non-phagocytable particles larger than 20 μm in size [37].
Fig. 8.
Schematic view of the wear particle-induced inflammasome activation. 1. An NF-ĸB activating priming signal such as LPS or TNF is required to synthesize NLRP3 and pro-IL-1 β thus licensing macrophages for the NLRP3 inflammasome activation. 2. Recognition of foreign body particulate material results in particle phagocytosis and formation of phagolysosomes. Subsequent intracellular aberrations including 3. leakage of lysosomal protease cathepsin B and 4. outflow of potassium () are sensed by the NLRP3 receptor. 5. Upon activation, NLRP3 triggers the assembly of the inflammasome complex by recruiting specific adaptor proteins. 6. NLRP3 inflammasome eventually generates active caspase-1, which cleaves precursor protein pro-IL-1 β into the secreted form. 7. Mature IL-1 β is released from the cell through membrane pores formed after the inflammasome activation.
To verify complete inflammasome activation, active caspase-1 was further shown from culture supernatants of particle-challenged macrophages. Indeed, inflammasome activation results in formation of membrane pores that allow not only the secretion of IL-1 β, but also the release of the entire NLRP3 inflammasome complex including active caspase-1 [38,39]. This phenomenon was consistent with the absence of active intracellular caspase-1, and with low levels of cytosolic NLRP3 protein in Cr-challenged macrophages, which eventually induced the strongest inflammasome activation. Compared to stimulation with particles alone, addition of LPS increased formation of active caspase-1 emphasizing once again the relevance of a separate priming signal. After all, inhibition of caspase-1 effectively decreased IL-1 β secretion indicating activation and involvement of this enzyme in particle-induced inflammasome activation.
Even though LPS intensifies macrophage responses to wear particles, its role in the pathogenesis of aseptic loosening remains de-bated. On the one hand, endotoxins may be released into circula-tion from infection foci or due to increased intestinal permeability [40]. In this case, LPS tends to localize onto foreign surfaces such as joint replacement components or wear particles, and there by likely contributes to implant loosening [41,42]. On the other hand, evidence for the presence of LPS remains somewhat inconclusive and it is also possible that endotoxins are not required for the development of peri–implant osteolysis. Indeed, although a recent study found endotoxin in periprosthetic tissues, endotoxin levels remained undetectable in majority of the samples collected around aseptically loosened joint replacements [43]. Therefore, we studied whether TNF as an alternative sterile agent could provide an adequate priming signal to license the particle-induced inflammasome activation.
Previous studies have identified TNF capable of priming NLRP3 inflammasome by activating the NF-ĸB via cytokine receptor-mediated signaling pathway [44,45]. This pro-inflammatory cytokine is also produced by particle-challenged macrophages and, importantly, its presence in the aseptic interface tissue has been widely recognized [46–48]. Together with TNF priming, we found that particles were able to induce increased IL-1 β secretion from human macrophages in an environment devoid of any bacterial constituents. This TNF-related IL-1 β production proved to be NLRP3 inflammasome-dependent as well. In comparison with LPS, TNF led to weaker priming and activation of the inflammasome, which is in line with previous studies and fits the clinical picture of a low-grade peri–implant inflammation slowly leading to osteolysis [26]. These results suggest that LPS may not be necessary for wear particle-mediated inflammasome activation, but also TNF can provide the priming signal required for IL-1 β secretion (Fig. 8). Of note, along with inflammasome components NLRP3, ASC, and caspase-1, these two cytokines have been identified from the aseptic interface together—correlating with each other and to the severity of osteolysis [46–50]. These findings further underline ther ela-tionship between TNF and IL-1 β in wear particle-mediated inflammation.
We acknowledge selected doses of LPS and TNF higher than physiologically relevant. Nevertheless, such doses have been previously used to enable inflammasome priming, and the need to overload certain stimulants is generally admitted as a shortcoming of in vitro studies. Particle concentrations used might also be higher than found in periprosthetic tissues, but these doses were verified non-cytotoxic and optimal for this cell culture model. Moreover, inhibition of NLRP3 inflammasome components and its upstream activating events did not entirely block the particle-induced IL-1 β or IL-18 secretion. As complete inhibition is difficult to achieve, these results reflect the compromise between inhibitor efficiency and cytotoxicity due to adverse effects of exaggerated inhibitor concentrations. Since several upstream activating events may simultaneously contribute to inflammasome activation, inhibition of one event at a time can result in incomplete inhibition as well. While both Cr and Mo particles activated the NLRP3 inflammasome, no direct conclusions can be made for the inflammasome activating potential of CrMo alloy particles. The proposed stimulatory effects of Co2+ and other metal ions, as well as cobalt based implant debris also remain speculative [51,52].
To validate our cell culture model, macrophage responses to metal wear particles were further characterized by analyzing the secretion of inflammatory mediators other than IL-1 β. In line with previous reports, cytokines of both pro- and anti-inflammatory nature were detected from culture supernatants [4,5]. Even though production of pro-inflammatory cytokine IL-18 is associated with inflammasome activation as well [18], this cytokine was observed in culture media already without a co-stimulatory priming signal. Particle-induced secretion of mature IL-18 was likely allowed by ready-madepro-IL-18 mRNA, which unlike IL-1 β precursor, is con-stitutively expressed and does not need a priming signal [53]. Particles also induced secretion of TNF, yet by eight-hour time point, its concentrations remained considerably lower than required for inflammasome priming in our cell culture model.
While TNF secretion would likely be increased over time, a multiplicity of additional macrophage stimulating factors can contribute to TNF production and its effects in vivo. Besides accumulating wear particles, complex tissue signaling at the aseptic interface could further enhance TNF secretion from macrophages and increase their activation in response to TNF. In addition to macrophages, TNF may also originate from several other cell types including monocytes, endothelial cells, lymphocytes, and fibroblasts. It is noteworthy that LPS is an extremely potent activator of TNF production as well. Thus, its involvement in both TNF secretion and direct inflammasome priming cannot be ruled out. Indeed, although neither TNF nor particles themselves seem to stimulate TLRs, these receptors have been proposed to play a role in the pathogenesis of aseptic loosening [54]. Thus, besides TNF, LPS or other danger signals might still contribute to inflammasome priming through TLR signaling. Since all these environmental signals could not be modeled in our experimental set up, future studies on this subject are warranted in vivo.
Taken together, this study shows that the NLRP3 inflammasome mediates metal wear particle responses in human primary macrophages. Metal particles themselves, however, remained insufficient to prime the inflammasome, and an additional priming signal was therefore needed. Importantly, the presence of LPS was not necessarily required, but inflammasome activation could be induced under truly aseptic conditions by TNF priming. These results highlight the role of NLRP3 inflammasome in metal particle-induced inflammation, and provide a concept of TNF-related IL-1 β secretion in this context. Regardless of the priming signal, inhibition of the NLRP3 inflammasome activation appears a promising means to prevent development of aseptic loosening.
Supplementary Material
Statement of Significance.
This study was conducted to elucidate the molecular mechanisms of metal particle-induced IL-1 β secretion in human primary macrophages. Production of this pro-inflammatory mediator from wear particle-activated macrophages has been associated with increased bone loss around total joint replacements—a condition eventually requiring revision surgery. Our results confirm that together with a co-stimulatory priming signal, particles of common implant metals elicit macrophage-mediated IL-1 β secretion through activation of the NLRP3 inflammasome pathway. We also present a concept of TNF priming in this context, demonstrating that the particle-related IL-1 β secretion can take place in a truly sterile environment. Thus, inhibition of inflammasome signaling appears a means to prevent wear particle-induced inflammation and development of peri–prosthetic osteolysis.
Acknowledgments
This work was supported by grants from the Finnish Cultural Foundation, ORTON Orthopaedic Hospital of the ORTON Foundation, Alfred Kordelin Foundation, Finnish Medical Foundation, Päivikki and Sakari Sohlberg Foundation, Orion Research Foundation, and Finnish Research Foundation of Orthopaedics and Traumatology. Templates from Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License, were used in graphical abstract and Fig. 8; https://smart.servier.com. University of Helsinki Biostatistics unit is acknowledged for consulting with statistical analyses.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosures
Authors have no conflicts of interest to disclose.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.actbio.2020.03.017.
References
- [1].Karachalios T, Komnos G, Koutalos A, Total hip arthroplasty: survival and modes of failure, EFORT Open Rev. 3 (5) (2018) 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abu-Amer Y, Darwech I, Clohisy JC, Aseptic loosening of total joint replace-ments: mechanisms underlying osteolysis and potential therapies, Arthritis Res. Therapy 9 (2007) S6 Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nich C, Goodman SB, Role of macrophages in the biological reaction to wear debris from joint replacements, J. Long Term Eff. Med. Implants 24 (4) (2014) 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, Konttinen YT, Goodman SB, Gallo J, Macrophages-Key cells in the response to wear debris from joint replacements, J. Biomed. Mater. Res. A 101 (10) (2013) 3033–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Landgraeber S, Jager M, Jacobs JJ, Hallab NJ, The pathology of orthopedic implant failure is mediated by innate immune system cytokines, Mediat. In-flamm. 2014 (2014) 185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wooley PH, Schwarz EM, Aseptic loosening, Gene Ther. 11 (4)(2004) 402–407. [DOI] [PubMed] [Google Scholar]
- [7].Gallo J, Goodman SB, Konttinen YT, Raska M, Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty, Innate Immun. 19 (2) (2013) 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J, Innate immune activation through nalp3 inflammasome sensing of asbestos and silica, Science 320 (5876) (2008) 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jo EK, Kim JK, Shin DM, Sasakawa C, Molecular mechanisms regulating NLRP3 inflammasome activation, Cell. Mol. Immunol. 13 (2) (2016) 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maloney WJ, James RE, Smith RL, Human macrophage response to retrieved titanium alloy particles in vitro, Clin. Orthop. Relat. Res. (322) (1996) 268–278. [PubMed] [Google Scholar]
- [11].Shiratori T, Kyumoto-Nakamura Y, Kukita A, Uehara N, Zhang J, Koda K, Kamiya M, Badawy T, Tomoda E, Xu X, Yamaza T, Urano Y, Koyano K, Kukita T, IL-1beta induces pathologically activated osteoclasts bearing extremely high levels of resorbing activity: a possible pathological subpopulation of osteoclasts, accompanied by suppressed expression of kindlin-3 and talin-1, J. Immunol. (Baltimore, Md.: 1950) 200 (1) (2018) 218–228. [DOI] [PubMed] [Google Scholar]
- [12].Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ, Solu-ble and particulate co-cr-mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity, J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 27 (7) (2009) 847–854. [DOI] [PubMed] [Google Scholar]
- [13].St Pierre CA, Chan M, Iwakura Y, Ayers DC, Kurt-Jones EA, Finberg RW, Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles, J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 28 (11) (2010) 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burton L, Paget D, Binder NB, Bohnert K, Nestor BJ, Sculco TP, Santambrogio L, Ross FP, Goldring SR, Purdue PE, Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation, J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 31 (1) (2013) 73–80. [DOI] [PubMed] [Google Scholar]
- [15].Caicedo MS, Samelko L, McAllister K, Jacobs JJ, Hallab NJ, Increasing both cocrmo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms, J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 31 (10) (2013) 1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sutterwala FS, Haasken S, Cassel SL, Mechanism of NLRP3 inflammasome activation, Ann. N. Y. Acad. Sci. 1319 (2014) 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Abderrazak A, Syrovets T, Couchie D, El Hadri K, Friguet B, Simmet T, Rouis M, N.L.R.P.3. inflammasome, from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases, Redox Biol. 4 (2015) 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He Y, Hara H, Nunez G, Mechanism and regulation of NLRP3 inflammasome activation, Trends Biochem. Sci. 41 (12) (2016) 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Daniels AU, Barnes FH, Charlebois SJ, Smith RA, Macrophage cytokine response to particles and lipopolysaccharide in vitro, J. Biomed. Mater. Res. 49 (4) (2000) 469–478. [DOI] [PubMed] [Google Scholar]
- [20].Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM, Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation, J. Bone Min. Res.: Off. J. Am. Soc. Bone Min. Res. 16 (11) (2001) 2082–2091. [DOI] [PubMed] [Google Scholar]
- [21].Hoenders CS, Harmsen MC, van Luyn MJ, The local inflammatory environment and microorganisms in “aseptic” loosening of hip prostheses, J. Biomed. Mater. Res. B Appl. Biomater. 86 (1) (2008) 291–301. [DOI] [PubMed] [Google Scholar]
- [22].Ragab AA, Van De Motter R, Lavish SA, Goldberg VM, Ninomiya JT, Carlin CR, Greenfield EM, Measurement and removal of adherent endotoxin from titanium particles and implant surfaces, J. Orthop. Res.: Off. Publ. Orthop. Res. Soc. 17 (6) (1999) 803–809. [DOI] [PubMed] [Google Scholar]
- [23].Nurmi K, Kareinen I, Virkanen J, Rajamaki K, Kouri VP, Vaali K, Levonen AL, Fyhrquist N, Matikainen S, Kovanen PT, Eklund KK, Hemin and cobalt pro-toporphyrin inhibit NLRP3 inflammasome activation by enhancing autophagy: a novel mechanism of inflammasome regulation, J. Innate Immun. 9 (1) (2017) 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, In-serra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Mas-ters SL, Schroder K, Cooper MA, O'Neill LA, A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases, Nat. Med. 21 (3) (2015) 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R, Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders, J. Exp. Med. 214 (11) (2017) 3219–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bezbradica JS, Coll RC, Schroder K, Sterile signals generate weaker and de-layed macrophage NLRP3 inflammasome responses relative to microbial signals, Cell. Amp; Mol. Immunol. 14 (2016) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moritz CP, Tubulin or not tubulin: heading toward total protein staining as loading control in western blots, ProteomicsProteomics 17 (20) (2017). [DOI] [PubMed] [Google Scholar]
- [28].Grosse S, Haugland HK, Lilleng P, Ellison P, Hallan G, Høl PJ, Wear particles and ions from cemented and uncemented titanium-based hip prostheses-a histological and chemical analysis of retrieval material, J. Biomed. Mater. Res. B Appl. Biomater. 103 (3) (2015) 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang F, Tang J, Dai K, Huang Y, Metallic wear debris collected from patients induces apoptosis in rat primary osteoblasts via reactive oxygen speciesmedi-ated mitochondrial dysfunction and endoplasmic reticulum stress, Mol. Med. Rep. 19 (3) (2019) 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dinarello CA, A clinical perspective of IL-1beta as the gatekeeper of inflammation, Eur. J. Immunol. 41 (5) (2011) 1203–1217. [DOI] [PubMed] [Google Scholar]
- [31].Lukens JR, Gross JM, Kanneganti T-D, IL-1 family cytokines trigger sterile inflammatory disease, Front. Immunol. 3 (2012) 315 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Manzano GW, Fort BP, Dubyak GR, Green field EM, Wear particle-induced priming of the NLRP3 inflammasome depends on adherent pathogen-associated molecular patterns and their cognate toll-like receptors: an in vitro study, Clin. Orthop. Relat. Res. 476 (12) (2018) 2442–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jamsen E, Pajarinen J, Lin TH, Lo CW, Nabeshima A, Lu L, Nathan K, Eklund KK, Yao Z, Goodman SB, Effect of aging on the macrophage response to titanium particles, J. Orthop. Res.: Off. Publ. Orthop. Res. Soc 38 (2) (2020) 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lu YC, Yeh WC, Ohashi PS, LPS/TLR4 signal transduction pathway, CytokineCytokine 42 (2) (2008) 145–151. [DOI] [PubMed] [Google Scholar]
- [35].Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G, K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter, ImmunityImmunity 38 (6) (2013) 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E, Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization, Nat. Immunol. 9 (8) (2008) 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cobelli N, Scharf B, Crisi GM, Hardin J, Santambrogio L, Mediators of the inflammatory response to joint replacement devices, Nat. Rev. Rheumatol. 7 (10) (2011) 600–608. [DOI] [PubMed] [Google Scholar]
- [38].Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Ini-esta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, Bu-jan S, Couillin I, Brough D, Arostegui JI, Pelegrin P, The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response, Nat. Immunol. 15 (8) (2014) 738–748. [DOI] [PubMed] [Google Scholar]
- [39].Tapia VS, Daniels MJD, Palazón-Riquelme P, Dewhurst M, Luheshi NM, Rivers-Auty J, Green J, Redondo-Castro E, Kaldis P, Lopez-Castejon G, Brough D, The three cytokines IL-1 β, IL-18, and IL-1α share related but distinct secretory routes, J. Biol. Chem. 294 (21) (2019) 8325–8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fukui H, Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm. Intest. Dis. 1 (3) (2016) 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM, Does endotoxin contribute to aseptic loosening of orthopedic implants? J. Biomed. Mater. Res. B Appl. Biomater. 72 (1) (2005) 179–185. [DOI] [PubMed] [Google Scholar]
- [42].Nalepka JL, Lee MJ, Kraay MJ, Marcus RE, Goldberg VM, Chen X, Greenfield EM, Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis, Clin. Orthop. Relat. Res. 451 (2006) 229–235. [DOI] [PubMed] [Google Scholar]
- [43].Hartmann ES, Kohler MI, Huber F, Redeker JI, Schmitt B, Schmitt-Sody M, Summer B, Fottner A, Jansson V, Mayer-Wagner S, Factors regulating bone remodeling processes in aseptic implant loosening, J. Orthop. Res.: Off. Publ. Orthop. Res. Soc 35 (2) (2017) 248–257. [DOI] [PubMed] [Google Scholar]
- [44].Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E, Cutting edge: nF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression, J. Immunol. (Baltimore, Md.: 1950) 183 (2) (2009) 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Franchi L, Eigenbrod T, Núñez G, Cutting edge, TNF-alpha mediates sensitiza-tion to atp and silica via the NLRP3 inflammasome in the absence of microbial stimulation, J. Immunol. (Baltimore, Md.: 1950) 183 (2) (2009) 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chiba J, Rubash HE, Kim KJ, Iwaki Y, The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis, Clin. Orthop. Relat. Res. (300) (1994) 304–312. [PubMed] [Google Scholar]
- [47].Jones LC, Frondoza C, Hungerford DS, Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components, J. Biomed. Mater. Res. 48 (6) (1999) 889–898. [DOI] [PubMed] [Google Scholar]
- [48].Naganuma Y, Takakubo Y, Hirayama T, Tamaki Y, Pajarinen J, Sasaki K, Goodman SB, Takagi M, Lipoteichoic acid modulates inflammatory response in macrophages after phagocytosis of titanium particles through toll-like receptor 2 cascade and inflammasomes, J. Biomed. Mater. Res. A 104 (2) (2016) 435–444. [DOI] [PubMed] [Google Scholar]
- [49].Stea S, Visentin M, Granchi D, Ciapetti G, Donati ME, Sudanese A, Zanotti C, Toni A, Cytokines and osteolysis around total hip prostheses, CytokineCytokine 12 (10) (2000) 1575–1579. [DOI] [PubMed] [Google Scholar]
- [50].Jamsen E, Kouri VP, Ainola M, Goodman SB, Nordstrom DC, Eklund KK, Pajarinen J, Correlations between macrophage polarizing cytokines, inflammatory mediators, osteoclast activity, and toll-like receptors intissues around aseptically loosened hip implants, J. Biomed. Mater. Res. A 105 (2) (2017) 454–463. [DOI] [PubMed] [Google Scholar]
- [51].Samelko L, Landgraeber S, McAllister K, Jacobs J, Hallab NJ, Cobalt alloy implant debris induces inflammation and bone loss primarily through danger sig-naling, not TLR4 activation: implications for DAMP-ening implant related inflammation, PLoS One 11 (7) (2016) e0160141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ferko M-A, Catelas I, Effects of metal ions on caspase-1 activation and interleukin-1 β release in murine bone marrow-derived macrophages, PLoS One 13 (8) (2018) e0199936 e0199936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Puren AJ, Fantuzzi G, Dinarello CA, Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells, Proc. Natl. Acad. Sci. U. S. A. 96 (5) (1999) 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Takagi M, Takakubo Y, Pajarinen J, Naganuma Y, Oki H, Maruyama M, Goodman SB, Danger of frustrated sensors: role of toll-like receptors and NOD-like receptors in aseptic and septic inflammations around total hip replacements, J. Orthop. Translat. 10 (2017) 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.