Abstract
Rationale & Objective
Evaluation of glomerular filtration rate (GFR) is challenging in adults undergoing bariatric surgery because creatinine and cystatin C levels are influenced by changes in muscle and fat mass. Additionally, indexing of GFR by body surface area (BSA) may by affected by decreases in BSA.
Study Design
Prospective observational study.
Setting & Participants
27 adults with body mass index (BMI) ≥ 35 kg/m2 who underwent measurement of GFR before and after bariatric surgery.
Outcomes
Indexed and nonindexed GFRs measured (mGFRs) using plasma iohexol clearance, indexed and nonindexed estimated GFR (eGFR) based on levels of creatinine, cystatin C, or both from Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations.
Analytic Approach
Bias and percent of estimates within 20% and 30% of mGFR (P20 and P30) for estimating equations were examined.
Results
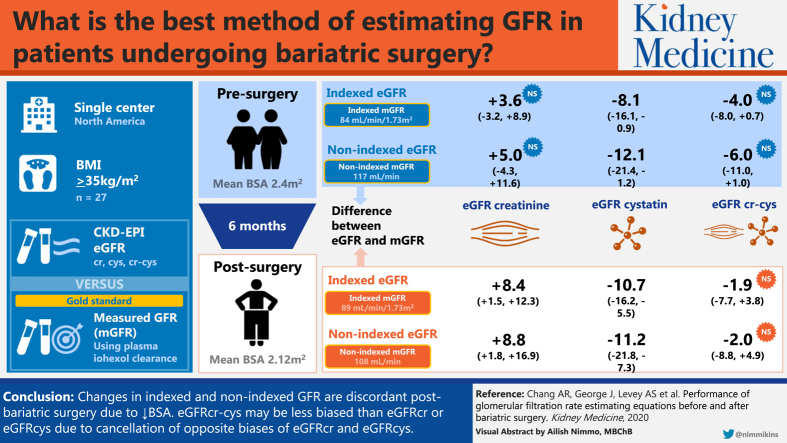
Mean presurgery BMI was 49.5 (SD, 9.4) kg/m2, BSA was 2.42 (SD, 0.27) m2, nonindexed mGFR was 117.3 (SD, 34.1) mL/min, and indexed mGFR was 84.1 (SD, 22.0) mL/min/1.73 m2. After 6 months, mean BMI changed by –13.8 (95% CI, −15.9 to −11.8) kg/m2, BSA by −0.30 (95% CI, −0.33 to −0.27) m2, and nonindexed mGFR by −9.2 (95% CI, −17.2 to −1.1) mL/min, while indexed mGFR was unchanged at 5.1 (95% CI, −0.1 to 10.4) mL/min/1.73 m2. Nonindexed eGFRcr was unbiased (median bias, 5.0 [95% CI, −4.3 to 11.6] mL/min) before surgery, but overestimated mGFR (8.8 [95% CI, 1.8 to 16.9] mL/min) after surgery. Nonindexed eGFRcys underestimated mGFR before (median bias, −12.1 [95% CI, −21.4 to −1.2] mL/min) and after surgery (−11.2 [95% CI, −21.8 to −7.3] mL/min). Nonindexed eGFRcr-cys was unbiased before (median bias, −6.0 [95% CI, −11.0 to 1.0] mL/min) and after surgery (−2.0 [95% CI, −8.8 to 4.9] mL/min). Findings were similar for indexed eGFR compared with indexed mGFR.
Limitations
Small, mostly white sample.
Conclusions
Changes in indexed and nonindexed GFRs may be discordant after bariatric surgery in adults because of decreases in BSA. Indexed and nonindexed eGFRcr-cys may be less biased than indexed or nonindexed eGFRcr or eGFRcys because of opposite biases in estimating mGFR.
Index Words: Bariatric surgery, obesity, filtration markers, glomerular filtration rate, weight loss, CKD-EPI, creatinine, cystatin C, body surface area
Graphical abstract
Plain-Language Summary.
Kidney function estimation is important for determining drug dosing and prognosis. In clinical practice, kidney function is estimated from glomerular filtration rate (GFR) using creatinine level and, less often, with cystatin C level. However, levels of these kidney markers can be influenced by changes in muscle and fat mass, which decrease substantially after bariatric surgery. In this study, we compared directly measured GFR with equations to estimate kidney function in patients with severe obesity before and after bariatric surgery. We found that equations using creatinine level overestimated kidney function after surgery, whereas an equation using both creatinine and cystatin C levels was the most accurate. These findings are important because an increasing number of patients are undergoing bariatric surgery and some drugs can have toxic effects if dosed inappropriately.
The obesity epidemic continues to expand worldwide, now affecting ∼40% of US adults.1 The prevalence of severe obesity (body mass index [BMI] ≥ 40 kg/m2) has also increased from 5.7% in 2007 to 2008 to 7.7% in 2015 to 2016 in US adults, as has the number of bariatric surgery procedures performed in the United States (∼158,000 in 2011 to ∼228,000 in 2017).2 Persons with severe obesity are at high risk for comorbid conditions, including chronic kidney disease (CKD) and end-stage kidney disease,3 resulting in high levels of health care use and drug prescriptions.4 Thus, it is highly important to evaluate glomerular filtration rate (GFR) accurately in this high-risk population, both for prognosticating risk and for safe and efficacious drug dosing.
There are several challenges to accurate GFR evaluation in patients with severe obesity and particularly in the setting of bariatric surgery.5 First, commonly used GFR estimating equations such as the CKD Epidemiology Collaboration (CKD-EPI) equation and the Modification of Diet in Renal Disease (MDRD) Study equation were created using older data from cohorts when severe obesity was much less common than now.6 Second, obesity is associated with both creatinine and cystatin C levels, independent of GFR.7 Creatinine generation is directly related to muscle mass, and skeletal muscle decreases by 20% to 25% after bariatric surgery.8 Cystatin C is associated with inflammation, which is common in patients with severe obesity. However, studies examining changes in inflammatory factors after bariatric surgery have been inconsistent, and it is unclear whether changes in cystatin C levels after bariatric surgery reflect changes in the generation or GFR.7,9
As a result, both estimated GFR based on creatinine level (eGFRcr) and eGFR based on cystatin C level (eGFRcys) may be biased after bariatric surgery. A study by Friedman et al10 found that the combined creatinine-cystatin C–based eGFR (eGFRcr-cys) using the CKD-EPI equation was more accurate than using either marker alone pre– and post–bariatric surgery. However, the reported accuracy estimates for eGFRcr and eGFRcys were much lower than what has been reported in other studies of obese patients.5,10, 11, 12, 13 Another study of patients after bariatric surgery suggested that eGFRcys may be the most useful.9
A particular concern in evaluating GFR after bariatric surgery is indexing of GFR for body surface area (BSA) because BSA changes substantially with large weight changes. Generally, measured GFR (mGFR) and eGFR are expressed indexed to 1.73 m2 of BSA, with the rationale that GFR is associated with kidney mass, which in turn varies by body mass across mammalian species,5,14,15 and indexing for BSA reduces variation in kidney function parameters among healthy individuals.16,17 Indexing to BSA results in significantly lower mGFRs and eGFR in patients with severe obesity and whether BSA indexing is appropriate for patients with severe obesity and after bariatric surgery is controversial.18 In this study, our main objective was to examine the performance of indexed and nonindexed eGFR using creatinine and/or cystatin C levels compared with indexed and nonindexed mGFRs before and after surgery.
Methods
Study Population
A total of 44 adults at least 18 years of age with BMI ≥ 35 kg/m2 undergoing evaluation for bariatric surgery at Geisinger’s Center for Nutrition and Weight Management clinic were recruited and gave written informed consent for this study. The study was approved by the Geisinger Institutional Review Board (#2014-0293).
Exclusion criteria included allergy to iodine or contrast dye, pregnancy, eGFRcr < 30 mL/min/1.73 m2, end-stage kidney disease, history of kidney transplant, current use of trimethoprim or cimetidine, multinodular goiter, Graves disease, autoimmune thyroiditis, cirrhosis, and active treatment for cancer. Of the 44 adults, 34 underwent bariatric surgery, 27 completed a 6-month follow-up visit, and 25 completed a 12-month follow-up visit. For these analyses, we included data from the 27 participants who completed at least 1 post–bariatric surgery research visit.
Measurement of GFR
Participants were instructed to eat a light breakfast the morning of their visit and then had 5 mL of iohexol (Omnipaque-300; GE Healthcare) administered intravenously over 30 seconds, followed by 10 mL of normal saline solution flush. Blood samples were drawn at approximately 10, 30, 240, and 300 minutes (only if eGFR was <60 mL/min/1.73 m2), with exact times recorded, similar to a protocol used in the Multi-Ethnic Study of Atherosclerosis (MESA) Kidney study.19 GFR was calculated using plasma iohexol clearance, using all time points in a 2-compartment model.
Laboratory Methods
Serum creatinine was measured at the Geisinger Medical Laboratory using the isotope-dilution mass spectrometry–traceable Roche enzymatic method (Roche Diagnostics) according to manufacturer specifications (interassay coefficient of variation, 1.7%). Iohexol and cystatin C were measured at the University of Minnesota, using thawed serum samples stored at −80 °C. Iohexol concentration was measured using high-performance liquid chromatography (coefficient of variation, 1.8% at 10.2 mg/dL and 2.0% at 42.6 mg/dL). Cystatin C was measured on the Roche COBAS 6000 chemistry analyzer (coefficient of variation, 4.3% at 0.75 mg/L and 3.2% at 3.83 mg/L). The assay for cystatin C is traceable to the international standard.20 Previously published CKD-EPI estimating equations were used to estimate GFR using creatinine and cystatin C levels, alone or in combination.6,21 We also estimated creatinine clearance using the Cockcroft-Gault (CG) equation, using actual body weight and “adjusted” body weight [0.4 × (actual body weight − ideal body weight) + ideal body weight].22
Other Variables of Interest
Weight, height, and waist circumference were measured during research visits using standardized methods. BSA was calculated using the DuBois equation.23 Blood pressure was measured using the Omron 907XL after a 5-minute rest period, with an averaged value of 3 readings separated by 1-minute intervals. Hypertension was defined as taking at least 1 antihypertensive medication or having a study systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg. Diabetes was defined as taking at least 1 glucose-lowering agent, most recent hemoglobin A1c level ≥ 6.5%, or having a fasting glucose level ≥ 126 mg/dL; diabetes was considered to be resolved if no longer meeting these criteria for diabetes diagnosis.24 Electronic health record data were supplemented by patient interview and chart review to ascertain comorbid conditions and medications at each time point. During study visits, a timed 6-hour urine collection was performed, and 24-hour values for urinary sodium, albumin, urea nitrogen, and creatinine were estimated by multiplying values by 24/collection time.
Statistical Analysis
Characteristics were summarized at each time point and differences between the postsurgery and baseline visits were evaluated using generalized estimating equations (clustered by individuals) for continuous variables and exact McNemar test for categorical variables. Performance of estimating equations was assessed pre- and postsurgery at 6- and 12-month visits. Bias was calculated as the median difference between eGFR and mGFR. Precision was reported as the interquartile range (IQR) of the bias. Accuracy was assessed by the percentage of values within 20% (P20) and 30% (P30) of mGFR. For analyses with nonindexed GFR as the reference, we “de-indexed” eGFR values by multiplying by current BSA/1.73 m2. To better understand the influence of weight change on the performance of GFR estimating equations, we additionally conducted stratified analyses, above and below median weight change at 6 months. STATA/MP 15.1 (StataCorp LLC) was used for analyses. CIs were calculated using bootstrapping (2,000 replications) for bias, precision, P20, and P30. The significance of the differences between equations compared with eGFRcr was evaluated using signed rank test for bias and precision and exact McNemar test for P20 and P30.
Results
A total of 27 participants underwent research visits presurgery and approximately 6 months postsurgery, with 25 returning for a research visit approximately 12 months postsurgery. Median number of days from the time of bariatric surgery were −140 (IQR, −180 to −80), 193 (IQR, 182 to 217), and 376 (IQR, 351 to 384) for the baseline, 6-month, and 12-month visits. At the presurgery visit, mean age was 46.2 (standard deviation [SD], 10.8) years, mean BMI was 49.5 (SD, 9.4) kg/m2, mean BSA was 2.42 (SD, 0.27) m2, mean nonindexed mGFR was 117.3 (SD, 34.1) mL/min, and mean indexed mGFR was 84.1 (SD, 22.0) mL/min/1.73 m2. Two-thirds were women, 59% had hypertension, 41% had diabetes, 15% had coronary artery disease, 11% had mGFRs < 60 mL/min/1.73 m2, and 48% had albuminuria with albumin excretion ≥ 30 mg/d (Table 1). The most common bariatric surgery was Roux-en-Y gastric bypass (74%), followed by biliopancreatic diversion with duodenal switch (15%) and then laparoscopic sleeve gastrectomy (11%).
Table 1.
Characteristics Presurgery and Approximately 6 Months Postsurgery Among 27 Patients Who Had Bariatric Surgery
| Presurgery | ∼6 mo Postsurgery | |
|---|---|---|
| Age, y | 46.2 (10.8) | 47.1 (10.8) |
| Female sex | 18 (66.7%) | 18 (66.7%) |
| Black race | 1 (3.7%) | 1 (3.7%) |
| Hypertension | 16 (59.3%) | 10 (37.0%)a |
| Diabetes | 11 (40.7%) | 3 (11.1%)a |
| Coronary artery disease | 4 (14.8%) | 4 (14.8%) |
| SBP, mm Hg | 115.4 (15.5) | 114.9 (15.1) |
| DBP, mm Hg | 74.6 (9.5) | 69.0 (9.2)a |
| Weight, kg | 140.8 (28.4) | 103.1 (21.9)a |
| BMI, kg/m2 | 49.5 (9.4) | 35.6 (6.6)a |
| Waist circumference, cm | 138.8 (15.9) | 114.4 (15.0)a |
| BSA, m2 | 2.42 (0.27) | 2.12 (0.25)a |
| mGFR, mL/min | 117.3 (34.1) | 108.2 (24.2)a |
| mGFR, mL/min/1.73 m2 | 84.1 (22.0) | 89.2 (19.9) |
| mGFR < 60 mL/min/1.73 m2 | 3 (11.1%) | 2 (7.4%) |
| Creatinine, mg/dL | 0.89 (0.23) | 0.78 (0.19)a |
| Cystatin C, mg/L | 1.08 (0.32) | 1.03 (0.25) |
| Glucose, mg/dL | 124.7 (75.1) | 94.6 (26.2)a |
| No. of BP medications | 1.2 (1.6) | 0.6 (1.0)a |
| ACEi or ARB | 11 (41%) | 5 (19%)a |
| Diuretic | 7 (26%) | 2 (7%) |
| Metformin | 9 (33%) | 2 (7%)a |
| Insulin | 5 (19%) | 1 (4%) |
| NSAIDs | 3 (11%) | 0 (0%) |
Note: Values are reported as number (percent) for categorical variables and mean (standard deviation) for continuous variables. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; glucose in mg/dL to mmol/L, ×0.05551.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; BSA, body surface area; DBP, diastolic blood pressure mGFR, measured glomerular filtration rate; NSAID, nonsteroidal anti-inflammatory drug; SBP, systolic blood pressure.
P < 0.05 when compared with presurgery value.
Changes After Bariatric Surgery
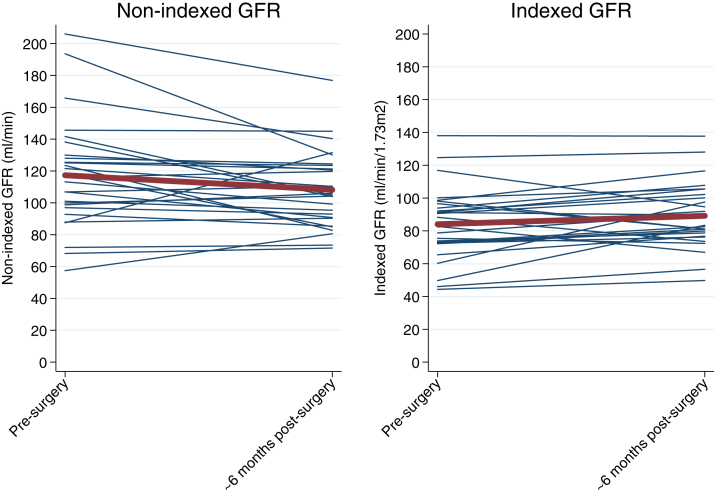
After 6 months, mean BMI decreased by −13.8 (95% CI, −15.9 to −11.8) kg/m2, mean BSA decreased by −0.30 (95% CI, −0.33 to −0.27) m2, nonindexed mGFR decreased by −9.2 (95% CI, −17.2 to −1.1) mL/min, and indexed GFR tended to increase by 5.1 (95% CI, −0.1 to 10.4) mL/min/1.73 m2 (Fig 1). The proportion of patients with diabetes decreased from 44% to 11% at 6 months after surgery (P = 0.002) along with the proportion of patients with hypertension (59% to 37%; P = 0.008). There were also decreases in study visit glucose levels, diastolic blood pressures, numbers of antihypertensive medications, metformin use, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use (Table 1). Serum cystatin C level was unchanged, whereas serum creatinine level decreased by 0.11 (95% CI, −0.15 to −0.06) mg/dL at 6 months. Estimated urinary creatinine excretion (−329; 95% CI, −461 to −197) mg/d decreased, but the decline in urinary albumin excretion was not significant (−38.1%; 95% CI, −64.4% to 7.6%). These changes remained consistent at the 12-month visit (Table S1).
Figure 1.
Changes in glomerular filtration rate (GFR) from presurgery to approximately 6 months postsurgery. The red line shows mean GFR presurgery and approximately 6 months postsurgery. Blue lines depict each individual’s GFR values.
Estimating Equation Performance Presurgery
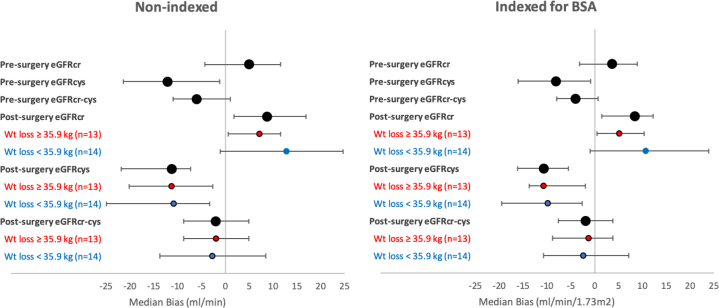
Presurgery, median bias significantly differed between nonindexed eGFRcr, eGFRcys, and eGFRcr-cys (P < 0.05 for all comparisons; Table 2; Fig 2). There was significant underestimation of mGFR with nonindexed eGFRcys by a median of −12.1 (95% CI, −21.4 to −1.2) mL/min, whereas nonindexed eGFRcr and nonindexed eGFRcr-cys were unbiased (5.0; 95% CI, −4.3 to 11.6; and −6.0; 95% CI, −11.0 to 1.0 mL/min, respectively). Nonindexed eGFRcr-cys was the most precise (IQR of bias, 21.9 mL/min), followed by nonindexed eGFRcr (IQR of bias, 25.9 mL/min) and nonindexed eGFRcys (IQR of bias, 31.2 mL/min; Table 2). Results for indexed eGFR compared with indexed mGFR were qualitatively the same (Table 2; Fig 2). Point estimates for P20 were numerically highest with eGFRcr-cys (85%), followed by eGFRcr (78%) and then eGFRcys (59%), though P20 for eGFRcr-cys and eGFRcys were not significantly different from P20 for eGFRcr. Point estimates for P30 were eGFRcr-cys (93%), eGFRcys (78%), and eGFRcr (85%). CG estimated creatinine clearance using actual body weight performed poorly presurgery (median bias, 72.9; 95% CI, 51.3-81.2 mL/min/1.73 m2).
Table 2.
Performance of eGFR and Estimated Creatinine Clearance Nonindexed and Indexed for BSA at Baseline and 6 Months After Surgery
| Nonindexed GFR |
Indexed GFR |
Nonindexed and Indexed GFR |
||||
|---|---|---|---|---|---|---|
| Median Bias, mL/min | IQR of Bias, mL/min | Median Bias, mL/min/1.73 m2 | IQR of Bias, mL/min/1.73 m2 | P20, % | P30, % | |
| Presurgery | ||||||
| eGFRcr (reference) | 5.0 (−4.3 to 11.6) | 25.9 (12.5 to 39.1) | 3.6 (−3.2 to 8.9) | 20.6 (8.8 to 30.2) | 78% (63% to 93%) | 85% (70% to 96%) |
| eGFRcys | −12.1 (−21.4 to −1.2)a | 31.2 (19.3 to 47.8)a | −8.1 (−16.1 to −0.9)a | 21.8 (13.7 to 35.8)a | 59% (41% to 78%) | 78% (59% to 93%) |
| eGFRcr-cys | −6.0 (−11.0 to 1.0)a | 21.9 (10.4 to 29.6)a | −4.0 (−8.0 to 0.7)a | 16.2 (8.2 to 21.9)a | 85% (70% to 96%) | 93% (81% to 100%) |
| eCLcr | 72.9 (51.3 to 81.2)a | 43.5 (23.5 to 89.5)a | 52.9 (42.7 to 66.4)a | 40.4 (19.7 to 57.7)a | 7% (0% to 19%)a | 19% (4% to 33%)a |
| eCLcr, adjusted for IBW | 8.9 (1.2 to 20.9)a | 33.4 (14.6 to 50.6)a | 6.5 (0.8 to 15.0)a | 27.0 (11.0 to 37.5)a | 67% (48% to 82%) | 81% (67% to 96%) |
| ∼6 mo Postsurgery | ||||||
| eGFRcr (reference) | 8.8 (1.8 to 16.9) | 26.3 (12.2 to 32.5) | 8.4 (1.5 to 12.3) | 21.6 (9.4 to 28.0) | 70% (52% to 89%) | 85% (70% to 96%) |
| eGFRcys | −11.2 (−21.8 to −7.3)a | 22.2 (11.2 to 32.0)a | −10.7 (−16.2 to −5.5)a | 16.8 (10.2 to 26.5)a | 59% (41% to 78%) | 93% (81% to 100%) |
| eGFRcr-cys | −2.0 (−8.8 to 4.9)a | 21.2 (10.6 to 27.5)a | −1.9 (−7.6 to 3.8)a | 16.4 (8.6 to 22.0)a | 85% (70% to 96%) | 93% (81% to 100%) |
| eCLcr | 44.7 (29.2 to 55.8)a | 41.9 (23.6 to 65.6)a | 37.8 (24.6 to 44.4)a | 38.1 (18.1 to 47.6)a | 22% (7% to 41%)a | 37% (19% to 59%)a |
| eCLcr, adjusted for IBW | 12.1 (0.3 to 22.4)a | 31.3 (18.0 to 48.7)a | 9.4 (0.3 to 16.6)a | 25.3 (15.3 to 40.3)a | 67% (48% to 85%) | 81% (67% to 96%) |
Note: Bias calculated as eGFR − measured GFR. Accuracy calculated as P20 or P30; results are the same for both indexed and nonindexed GFRs. We calculated eCLcr, indexed to 1.73 m2 using the Cockcroft-Gault equation, with actual body weight and “adjusted” body weight [0.4 × (actual body weight − IBW) + IBW].22
Abbreviations: BSA, body surface area; cr, creatinine; cys, cystatin C; eCLcr, estimated creatinine clearance; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; IBW, ideal body weight; IQR, interquartile range; P20(30), percent of values within 20% (30%) of measured glomerular filtration rate.
P < 0.05 for comparison to eGFRcr.
Figure 2.
Median bias of estimating equations pre- and postsurgery. Median bias (estimated glomerular filtration rate [eGFR] − measured GFR) and 95% CIs are shown presurgery overall and then approximately 6 months postsurgery, overall and then stratified by weight (Wt) loss (above and below median 6-month weight loss). Abbreviations: BSA, body surface area; eGFRcr, eGFR based on creatinine level; eGFRcr-cys, eGFR based on creatinine and cystatin C levels; eGFRcys, eGFR based on cystatin C level.
Estimating Equation Performance Postsurgery
At the 6-month postsurgery visit, nonindexed eGFRcr-cys was unbiased (−2.0; 95% CI, −8.8 to 4.9 mL/min), whereas nonindexed eGFRcr overestimated mGFR (median bias, 8.8; 95% CI, 1.8 to 16.9 mL/min) and nonindexed eGFRcys underestimated mGFR (median bias, −11.2; 95% CI, −21.8 to −7.3 mL/min; Table 2; Fig 2). Nonindexed eGFRcr-cys was the most precise (IQR of bias, 21.2 mL/min), followed by eGFRcys (IQR of bias, 22.2 mL/min) and eGFRcr (IQR of bias, 26.3 mL/min). Results for indexed eGFR compared with indexed mGFR were qualitatively the same (Table 2; Fig 2). Point estimates for P20 were numerically highest for eGFRcr-cys (85%), followed by eGFRcr (70%) and eGFRcys (59%), though P20 for eGFRcr-cys and eGFRcys were not significantly different from P20 for eGFRcr. Point estimates for P30 were eGFRcr-cys (93%), eGFRcys (93%), and eGFRcr (85%). CG estimated creatinine clearance using actual body weight performed poorly postsurgery (median bias, 44.7; 95% CI, 29.2-55.8 mL/min).
Results at the 12-month postsurgery visit for the 25 patients with available data were largely consistent with the 6-month postsurgery visit. Again, nonindexed eGFRcr-cys was unbiased (0.4; 95% CI, −9.3 to 6.0 mL/min), whereas nonindexed eGFRcr overestimated mGFR (7.6; 95% CI, 2.2 to 19.6 mL/min) and nonindexed eGFRcys underestimated mGFR (−11.5; 95% CI, −17.0 to −2.1 mL/min; Table S2). Interestingly, the equation at the approximately 12-month postsurgery visit with the best precision was nonindexed eGFRcys (IQR of bias, 17.2 mL/min), followed by nonindexed eGFRcr (IQR of bias, 18.9 mL/min) and nonindexed eGFRcr-cys (IQR of bias, 22.9 mL/min). Despite the lower precision, eGFRcr-cys had the numerically highest P20 (88%), followed by eGFRcys (72%) and eGFRcr (60%).
Estimating Equation Performance 6-Months Postsurgery by Weight Loss Groups (above and below median weight loss)
Estimating equations performed fairly similarly for the lesser weight loss subgroup and the greater weight loss subgroup (Fig 2; Table S3). At the approximately 6-month postsurgery visit, nonindexed eGFRcr-cys was unbiased for both the lesser weight loss subgroup (−1.9; 95% CI, −8.8 to 4.9 mL/min) and the greater weight loss subgroup (−2.7; 95% CI, −13.7 to 8.5 mL/min), whereas nonindexed eGFRcr overestimated mGFR and nonindexed eGFRcys underestimated mGFR for both weight loss subgroups.
Discussion
In this study of 27 patients with a wide range of baseline GFRs, mean nonindexed mGFR declined but there was a trend for mean indexed mGFR to increase because of the decrease in BSA. Performance of indexed and nonindexed CKD-EPI equations for eGFRcr-cys was better than that of eGFRcr and eGFRcys before and after surgery. Before surgery, both eGFRcr and eGFRcr-cys were unbiased, whereas eGFRcys tended to underestimate mGFR. After surgery, eGFRcr-cys was unbiased, whereas eGFRcr significantly overestimated mGFR and eGFRcys underestimated mGFR. eGFRcr-cys was most precise both before and approximately 6 months after bariatric surgery, and point estimates for P20 and P30 were numerically higher for eGFRcr-cys than for eGFRcr and eGFRcys. These findings are important because the prevalence of severe obesity continues to increase worldwide and inaccurate GFR evaluation in the severely obese could result in errors in drug dosing.
Ongoing debate exists over the level of accuracy needed for estimating GFR in clinical care.25, 26, 27 Cost, availability, and convenience must be considered when considering whether to use eGFRcr, eGFRcr-cys, or direct measurement of GFR. Our study suggests that the current clinical standard, eGFRcr using the CKD-EPI equation, is a reasonable option for estimating GFR in severely obese patients who have not undergone bariatric surgery. However, in the setting of bariatric surgery, clinicians should consider using eGFRcr-cys or direct measurement of GFR when more accurate estimation of GFR is required (ie, drugs excreted by the kidney with narrow therapeutic windows).26,28,29 Results from other literature also suggest that eGFRcr-cys may be preferable in severely obese patients undergoing bariatric surgery, though we found overall higher accuracy,10,30 which may reflect the rigor of our methods of measuring GFR in a research setting at a single site. Other studies of severely obese (non–bariatric surgery) individuals have shown either overestimation, underestimation, or minimal bias when using creatinine-based estimating equations.10,11,13,31, 32, 33 Postsurgery, weight loss results in a decrease in creatinine production due to loss of muscle mass accompanying loss of fat mass. It is interesting that cystatin C levels tended to underestimate GFR both before and after bariatric surgery. Reasons for this underestimation both pre- and postsurgery are unclear but suggest a relative increase in cystatin C production after bariatric surgery, although other inflammatory markers such as serum C-reactive protein, interleukin 6, and tumor necrosis factor α have been shown to decrease it.34
In addition to bias related to GFR-independent changes in levels of filtration markers, GFR evaluation in severely obese individuals is challenging due to substantial changes in BSA accompanying surgical weight loss. Although indexing to BSA reduces variation in GFR in healthy individuals,16,17 there is controversy whether indexing GFR to BSA for individuals with severe obesity undergoing bariatric surgery is appropriate.18 Use of nonindexed GFR might be preferable for patients who have bariatric surgery because a decline in BSA leads to changes in opposite directions of indexed versus nonindexed mGFR and eGFR and could mask a decline in nonindexed mGFR. A recent study of 3,506 participants from 9 cohorts found that nonindexed eGFRcr, eGFRcys, eGFRcr-cys performed reasonably well, and using nonindexed eGFR should be considered when appropriate.35 Using indexed GFR in patients with severe obesity could result in errors in drug dosing.5 For example, indexed mGFR in our cohort was 28% lower before surgery and 17% lower 6 months after surgery than unindexed mGFR at these times.
Because many clinicians still use the CG equation for drug dosing, we also examined its performance, both unadjusted and using the “adjusted” body weight that is recommended for individuals for whom actual body weight exceeds ideal body weight by >30%.36 The rationale for using CG in drug dosing is that most older drugs were studied using CG estimates and it is not indexed for BSA. However, there are several reasons to avoid using the CG equation. First, use of the unadjusted CG equation results in exceptionally large bias in severe obesity because weight is in the equation, and this bias persists even after marked weight loss after bariatric surgery. Second, the CG equation lacks face validity in this setting because it was derived from a study of 249 white men in 1973 when average BMI was much lower,22 and it has not been re-expressed for use with serum creatinine values that are standardized to international reference values.37 Third, several studies have shown that use of nonindexed eGFRcr equations results in better accuracy than the CG equation.29,38, 39, 40
There were several strengths of our study. We examined estimating equations using creatinine and cystatin C levels before and after bariatric surgery, compared to a gold standard reference. We also compared eGFR with indexed mGFR and nonindexed eGFR with mGFR because nonindexed eGFR should be used for assessing drug dosing in severely obese individuals.
The main limitation is that our research cohort consisted of a relatively small sample of mostly white individuals at a single institution, and only 3 participants had baseline mGFRs < 60 mL/min/1.73 m2, which may have limited statistical power and generalizability of our findings. However, research participants in this study were fairly similar to the general bariatric surgery population at Geisinger with the exception of slightly higher BMI and lower eGFR (Table S4).41 Larger studies including severely obese individuals pre– and post–bariatric surgery with mGFR could be helpful but may not be completed due to the burdensome nature of measuring GFR. Additional research is needed assessing the utility of other filtration markers unaffected by muscle or fat for improving GFR evaluation in this population.
In conclusion, changes in indexed and nonindexed GFR may be discordant after bariatric surgery in adults because of decreases in BSA. Indexed and nonindexed eGFRcr-cys may be less biased than indexed or nonindexed eGFRcr or eGFRcys because of opposite biases in estimating mGFR.
Article Information
Authors’ Full Names and Academic Degrees
Alex R. Chang, MD, MS, Jason George, MD, Andrew S. Levey, MD, Josef Coresh, MD, PhD, Morgan E Grams, MD, PHD, and Lesley A. Inker, MD, MS.
Authors’ Contributions
Research idea: ARC; study design: ARC, ASL, JC, MEG, LAI; data acquisition: JG; data analysis/statistical analysis: ARC, MEG; interpretation: all authors; supervision or mentorship: MEG, LAI. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Research was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants K23 DK106515-01 and R01DK097020. The funders had no role in study design; data collection, analysis, or reporting; or the decision to submit for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Prior Presentation
An abstract of this research was presented at ASN Kidney Week 2019, November 9, 2019, Washington D.C.
Data Sharing Statement
Deidentified data may be available on reasonable request.
Peer Review
Received March 5, 2020. Evaluated by 2 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Editorial Board Member Vianda S. Stel, PhD). Accepted in revised form August 21, 2020. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with Kidney Medicine’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
Table S1: Characteristics at 12 month among 25 patients who completed all visits
Table S2: Performance of eGFR and eCLcr indexed and nonindexed for BSA at 12 months after surgery
Table S3: Performance of eGFR indexed and nonindexed for BSA at 6 months, by weight loss ≥ 35.9 and <35.9 kg
Table S4: Characteristics compared with general bariatric surgery population at Geisinger
Supplementary Material
Tables S1-S4.
References
- 1.NCHS Fact Sheet, December 2017. https://www.cdc.gov/nchs/data/factsheets/factsheet_nhanes.htm Updated 2/25/2019. Accessed.
- 2.Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers Updated 6/18/2018. Accessed.
- 3.Chang A.R., Grams M.E., Ballew S.H. Adiposity and risk of decline in glomerular filtration rate: meta-analysis of individual participant data in a global consortium. BMJ. 2019;364:k5301. doi: 10.1136/bmj.k5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchini M. Use of healthcare services and expenditure in the US in 2025: the effect of obesity and morbid obesity. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0206703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang A.R., Zafar W., Grams M.E. Kidney function in obesity-challenges in indexing and estimation. Adv Chronic Kidney Dis. 2018;25(1):31–40. doi: 10.1053/j.ackd.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens L.A., Schmid C.H., Greene T. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson L.E., Yu W., Goodpaster B.H. Fat-free mass and skeletal muscle mass five years after bariatric surgery. Obesity (Silver Spring) 2018;26(7):1130–1136. doi: 10.1002/oby.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Scholten B.J., Persson F., Svane M.S., Hansen T.W., Madsbad S., Rossing P. Effect of large weight reductions on measured and estimated kidney function. BMC Nephrol. 2017;18(1):52. doi: 10.1186/s12882-017-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman A.N., Moe S., Fadel W.F. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol. 2014;39(1):8–15. doi: 10.1159/000357231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouquegneau A., Vidal-Petiot E., Moranne O. Creatinine-based equations for the adjustment of drug dosage in an obese population. Br J Clin Pharmacol. 2016;81(2):349–361. doi: 10.1111/bcp.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyman U., Grubb A., Sterner G., Bjork J. The CKD-EPI and MDRD equations to estimate GFR. Validation in the Swedish Lund-Malmo Study cohort. Scand J Clin Lab Invest. 2011;71(2):129–138. doi: 10.3109/00365513.2010.543143. [DOI] [PubMed] [Google Scholar]
- 13.Lemoine S., Guebre-Egziabher F., Sens F. Accuracy of GFR estimation in obese patients. Clin J Am Soc Nephrol. 2014;9(4):720–727. doi: 10.2215/CJN.03610413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt J.P., Rhode E.A. Similarity of renal glomerular hemodynamics in mammals. Am Heart J. 1976;92(4):465–472. doi: 10.1016/s0002-8703(76)80046-4. [DOI] [PubMed] [Google Scholar]
- 15.Singer M.A. Of mice and men and elephants: metabolic rate sets glomerular filtration rate. Am J Kidney Dis. 2001;37(1):164–178. doi: 10.1016/s0272-6386(01)80073-1. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh J.F., Moller E., Van Slyke D.D. Studies of urea excretion. III: the influence of body size on urea output. J Clin Invest. 1928;6(3):467–483. doi: 10.1172/JCI100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor F.B., Drury D.R., Addis T. The regulation of renal activity. Am J Physiol Legacy Content. 1923;65(1):55–61. [Google Scholar]
- 18.Delanaye P., Radermecker R.P., Rorive M., Depas G., Krzesinski J.M. Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example. Nephrol Dial Transplant. 2005;20(10):2024–2028. doi: 10.1093/ndt/gfh983. [DOI] [PubMed] [Google Scholar]
- 19.Inker L.A., Shafi T., Okparavero A. Effects of race and sex on measured GFR: the Multi-Ethnic Study of Atherosclerosis. Am J Kidney Dis. 2016;68(5):743–751. doi: 10.1053/j.ajkd.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Grubb A., Blirup-Jensen S., Lindstrom V., Schmidt C., Althaus H., Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 21.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 23.Du Bois D., Du Bois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [PubMed] [Google Scholar]
- 24.Ramos-Levi A.M., Cabrerizo L., Matía P., Sánchez-Pernaute A., Torres A.J., Rubio M.A. Which criteria should be used to define type 2 diabetes remission after bariatric surgery? BMC Surg. 2013;13:8. doi: 10.1186/1471-2482-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porrini E., Ruggenenti P., Luis-Lima S. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol. 2019;15(3):177–190. doi: 10.1038/s41581-018-0080-9. [DOI] [PubMed] [Google Scholar]
- 26.Levey A.S., Coresh J., Tighiouart H., Greene T., Inker L.A. Strengths and limitations of estimated and measured GFR [letter] Nat Rev Nephrol. 2019;15(12):784. doi: 10.1038/s41581-019-0213-9. [DOI] [PubMed] [Google Scholar]
- 27.Levey A.S., Coresh J., Tighiouart H., Greene T., Inker L.A. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16(1):51–64. doi: 10.1038/s41581-019-0191-y. [DOI] [PubMed] [Google Scholar]
- 28.Wang E., Paulus J.K., Hackenyos D., Inker L.A., Levey A.S., Mathew P. Imprecise kidney function thresholds in cancer clinical trials and the potential for harm. JNCI Cancer Spectr. 2018;2(4):pky060. doi: 10.1093/jncics/pky060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matzke G.R., Aronoff G.R., Atkinson A.J., Jr. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80(11):1122–1137. doi: 10.1038/ki.2011.322. [DOI] [PubMed] [Google Scholar]
- 30.Bouquegneau A., Vidal-Petiot E., Vrtovsnik F. Modification of Diet in Renal Disease versus Chronic Kidney Disease Epidemiology Collaboration equation to estimate glomerular filtration rate in obese patients. Nephrol Dial Transplant. 2013;28(suppl 4):iv122–iv130. doi: 10.1093/ndt/gft329. [DOI] [PubMed] [Google Scholar]
- 31.Lieske J.C., Collazo-Clavell M.L., Sarr M.G., Rule A.D., Bergstralh E.J., Kumar R. Gastric bypass surgery and measured and estimated GFR in women. Am J Kidney Dis. 2014;64(4):663–665. doi: 10.1053/j.ajkd.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navaneethan S.D., Malin S.K., Arrigain S., Kashyap S.R., Kirwan J.P., Schauer P.R. Bariatric surgery, kidney function, insulin resistance, and adipokines in patients with decreased GFR: a cohort study. Am J Kidney Dis. 2015;65(2):345–347. doi: 10.1053/j.ajkd.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fotheringham J., Weatherley N., Kawar B., Fogarty D.G., Ellam T. The body composition and excretory burden of lean, obese, and severely obese individuals has implications for the assessment of chronic kidney disease. Kidney Int. 2014;86(6):1221–1228. doi: 10.1038/ki.2014.112. [DOI] [PubMed] [Google Scholar]
- 34.Askarpour M., Khani D., Sheikhi A., Ghaedi E., Alizadeh S. Effect of bariatric surgery on serum inflammatory factors of obese patients: a systematic review and meta-analysis. Obes Surg. 2019;29(8):2631–2647. doi: 10.1007/s11695-019-03926-0. [DOI] [PubMed] [Google Scholar]
- 35.Titan S., Miao S., Tighiouart H. Performance of indexed and nonindexed estimated GFR. Am J Kidney Dis. 2020;76(3):446–449. doi: 10.1053/j.ajkd.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Spinler S.A., Nawarskas J.J., Boyce E.G., Connors J.E., Charland S.L., Goldfarb S. Predictive performance of ten equations for estimating creatinine clearance in cardiac patients. Iohexol Cooperative Study Group. Ann Pharmacother. 1998;32(12):1275–1283. doi: 10.1345/aph.18122. [DOI] [PubMed] [Google Scholar]
- 37.Levey A.S., Inker L.A. Assessment of glomerular filtration rate in health and disease: a state of the art review. Clin Pharmacol Ther. 2017;102(3):405–419. doi: 10.1002/cpt.729. [DOI] [PubMed] [Google Scholar]
- 38.Stevens L.A., Nolin T.D., Richardson M.M. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33–42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beumer J.H., Inker L.A., Levey A.S. Improving carboplatin dosing based on estimated GFR. Am J Kidney Dis. 2018;71(2):163–165. doi: 10.1053/j.ajkd.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janowitz T., Williams E.H., Marshall A. New model for estimating glomerular filtration rate in patients with cancer. J Clin Oncol. 2017;35(24):2798–2805. doi: 10.1200/JCO.2017.72.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang A.R., Chen Y., Still C. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90(1):164–171. doi: 10.1016/j.kint.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4.