Abstract
Objective
There are few studies evaluating the impact of Aspirin-exacerbated respiratory disease (AERD) treatment on otologic symptoms. The aim of this study is to evaluate the effects of endoscopic sinus surgery (ESS) and aspirin desensitization (AD) on otologic symptoms in subjects with AERD.
Methods
Retrospective chart review of adult patients diagnosed with AERD at our tertiary Care Academic Medical Center - Otorhinolaryngology Department. Charts of adult patients diagnosed with AERD who underwent ESS and ASA desensitization at our institution's AERD Center from 2016 to 2019 were reviewed. Sino-Nasal Outcomes Test 22-item survey (SNOT-22) scores were evaluated for patients at various time points including: pre-surgery, post-surgery/pre-aspirin desensitization, and various times post-desensitization up to >12 months. Within the SNOT-22, otologic-specific subdomain scores were evaluated at similar time points. Patients on immunomodulatory medications other than corticosteroids were excluded from analysis.
Results
SNOT-22 scores were analyzed for 121 patients. There was a significant improvement in overall SNOT scores from pre-surgery (44.62) to post surgery/pre-desensitization (23.34) (P < 0.0005). Similarly, SNOT-22 otologic-specific scores also improved after surgery prior to desensitization (3.19–2.04) (P = 0.005). Following AD, the improvement in the overall SNOT-22 continued to improve for up to 12 months (P < 0.005). While the otologic-specific SNOT-22 scores remained stable after surgery and ASA desensitization.
Conclusion
ESS and AD reduce otologic-specific SNOT-22 scores and parallel trends in overall SNOT-22 scores. The effect of treatment is durable over the course of 12 months. Future work should aim to correlate otologic SNOT-22 scores with objective otologic data.
Keywords: Aspirin exacerbated respiratory disease, Otologic symptoms, SNOT-22
Introduction
Aspirin-exacerbated respiratory disease (AERD) is a progressive upper and lower airway inflammatory disease distinguished by a number of findings that include asthma, chronic rhinosinusitis (CRS), nasal polyposis (NP), and sensitivity to aspirin and other drugs inhibiting the cyclooxygenase-1 (COX-1) pathway.1 AERD presents within 0.3%–2.5% of the general population, but its presence increases significantly to 7.15% and 14.89% in adult asthmatic patients and those with severe asthma, respectively.2 Although disease prevalence in males vs. females is debated, it is agreed upon that AERD typically develops around the third or fourth decade of life.2,3 Treatment for AERD is centered around aspirin desensitization (AD), which requires a multifaceted approach, consisting of an initial low-dose aspirin challenge with escalation to 325–650 mg over a multi-day period.4 In addition, management of nasal polyposis and chronic sinusitis often requires surgical intervention in conjunction with AD. The combined modality treatment has been shown to effectively manage AERD and its associated symptoms for prolonged durations (>12 months) and improve quality of life.1,4
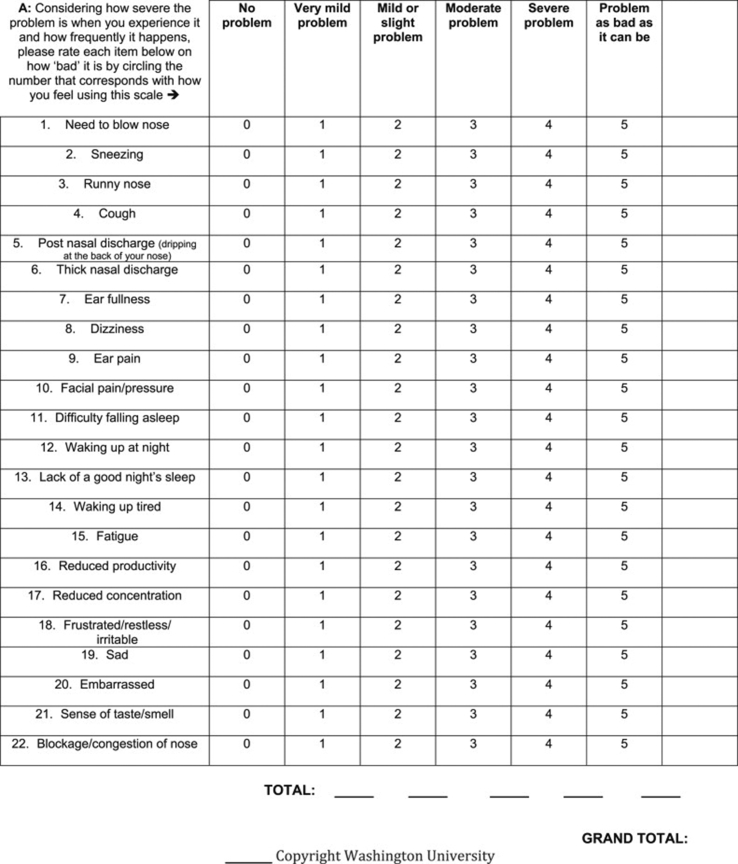
While the majority of treatment outcomes have focused on airway and nasal symptom control, there are few studies that report on the relationship between middle ear disease and AERD. Among the limited literature available, polyps of the ear have been reported in AERD.5,6 Middle ear dysfunction has also been reported in patients with CRS and CRS with NP.7 Additionally, a 2018 study assessing the otologic complications of AERD found that patients with AERD-associated nasal polyposis and middle ear symptoms were at a higher risk of hearing loss than those without AERD.8 It is intuitive that AERD and CRS can impact otologic symptoms. The mechanism of this association is not completely elucidated, but may be due to mechanical obstruction of the eustachian tube or through direct mucosal extension into the middle ear.7,9 Currently, the Sino-Nasal Outcomes Test 22-item questionnaire (SNOT-22) is used to evaluate sinonasal symptoms in patients with CRS and related sinonasal disorders. The twenty-two item Sino-Nasal Outcomes Test grades symptoms on a scale from one to five, with higher scores defining worse symptoms. Within this questionnaire, otologic symptoms are assessed through the otologic subdomain questions (Fig. 1).1,4,10
Fig. 1.
SNOT-22 Scale. Otologic subdomains include #7, 8, and 9.
Despite the known otologic associations in AERD, there is a paucity of literature that directly evaluates otologic-specific outcomes after AERD treatment. Given this, the aim of our research was to evaluate otologic symptoms before and after AERD treatment using the previously validated SNOT-22 questionnaire. This work is important to better understand this outcome because it may offer improved opportunities for counseling patients about evaluating hearing and pursuing middle ear procedures if necessary.
Materials and methods
The Institutional Review Board at the Hospital of the University of Pennsylvania approved this study (Protocol 832053).
SNOT-22
The SNOT-22 questionnaire is a 22-question survey validated for use in patients with CRS that evaluates the four distinct categories of CRS symptoms.11 There are four subdomains of this questionnaire that evaluate outcomes related to the nose (rhinologic), ear/face (otologic), sleep, and emotion. The otologic subdomain evaluates symptoms related to ear fullness, dizziness, and ear pain (Fig. 1).
Chart review
Retrospective review of patient charts from 8/2016 to 7/2019 was performed. Patients at the University of Pennsylvania's AERD Center were evaluated as a part of this study. Patients included for analysis were 18 years or older, had a challenge proven diagnosis of AERD, went through successful treatment of their AERD using AD, and had at least one pre-AD and one post-AD SNOT-22 survey completed. Those who had failed AD, had a negative ASA challenge, were on immunomodulatory medications other than corticosteroids, or did not have pre- and post-AD SNOT-22 scores available were excluded from our analysis (Table 1).
Table 1.
Demographic data.
| Item | Demographic data |
|---|---|
| Gender | |
| Male | 56 patients |
| Female | 65 patients |
| Surgery | |
| Primary | 0 patient |
| Revision | 121 patients |
| Mean # of FESS | (2.53 ± 2.1) times |
| Follow up time (Mean ± SD) | (13.95 ± 12.3) months |
Notes: Total subjects included in our analysis are displayed. All of our patients were revision ESS cases, and mean follow up time is measured as time from first appointment within the University of Pennsylvania's Otorhinolaryngology department (in months). ESS = Endoscopic sinus surgery, SD = Standard deviation.
In our study all patients underwent revision endoscopic sinus surgery (ESS) approximately 6–8 weeks prior to AD. Post-operatively, all patients were on a tapering dose of 6–8 weeks of oral corticosteroids. Patients completed SNOT-22 surveys at their initial visit (baseline), post-surgery/pre-desensitization, and post-desensitization at 0–3, 3–6, 6–12, and >12 months. The SNOT-22 surveys were provided to patients to complete in the at routine follow-up visits. Incomplete SNOT-22 surveys were excluded from analysis.
Statistical analysis
Data collected included age, sex, BMI, number of previous sinus surgeries, and SNOT-22 survey scores. The primary outcome was the change SNOT-22 scores pre-/pre-desensitization and over time at various time points of interest. The minimum value for clinically significant difference for the entire SNOT-22 is 8.9 points. Theoretically, when prorated, the statistically significant difference for each question when calculated would be 0.40 points.12 Two-tailed t-test was used to analyze SNOT-22 scores for direct comparison of time points (pre-surgery and pre-desensitization). Linear regression analysis was used to analyze SNOT-22 scores over time. The SNOT-22 scores were broken down by intervals of 0–3 months, 3–6 months, 6–12 months, and >12 months for analysis. Statistical significance was considered for P-values <0.05.
Results
One-hundred and twenty-nine patients with AERD underwent aspirin desensitization and met the inclusion criteria for the study. Eight patients were excluded from analysis because they did not complete the required SNOT-22 questionnaires. The remaining 121 patients were included in the study and were followed for a maximum of 1-year post aspirin desensitization.
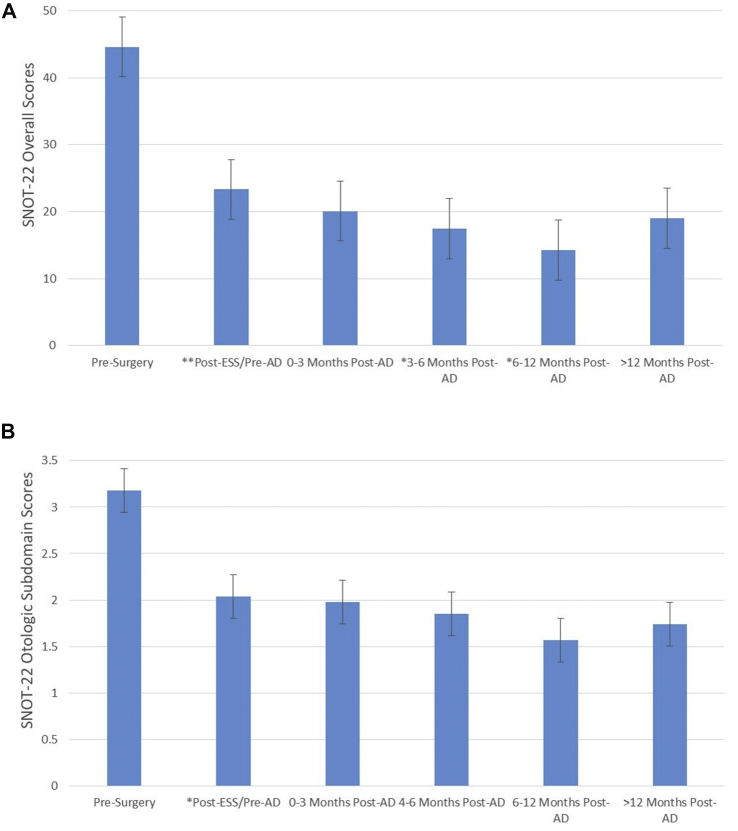
The mean pre-operative total SNOT-22 score was 44.62 ± 20.34 (Mean ± Standard Deviation). Total SNOT-22 score post-surgery/pre-aspirin desensitization showed a significant decrease to 23.34 ± 19.28 (P < 0.0005; 95% CI 15.47–27.48). After Aspirin desensitization, total SNOT-22 scores remained statistically unchanged at three months with a score of 20.08 ± 18.5 (95% CI 10.83–29.33; P = 0.238), but dropped significantly at both 3–6 and 6–12 month time periods with scores of 17.47 ± 19.2 (P = 0.042) and 14.24 ± 11.3 (P = 0.002), respectively. Total SNOT-22 scores remained statistically unchanged at >12 months with a score of 19.02 ± 15.9 (P = 0.197, Fig. 2A).
Fig. 2.
A: Total SNOT-22 scores. Total SNOT-22 scores are displayed with average score and 95% confidence intervals over the study time-points. Pre-Surgery is baseline, and Post ESS/Pre-AD is 3–6 weeks post ESS (prior to desensitization). All other subsequent time points refer to the number of months post-AD. ESS = Endoscopic sinus surgery; SNOT-22 = Sino-Nasal Outcomes Test; AD = Aspirin Desensitization. B: Otologic subdomain SNOT-22 scores. Otologic subdomain scores of the SNOT-22 survey are displayed. Average scores at all time points were used with a 95% confidence interval. Pre-Surgery is baseline, and Post ESS/Pre-AD is 3–6 weeks post ESS. All other subsequent time points refer to the number of months post-AD. ESS = Endoscopic sinus surgery; SNOT-22 = Sino-Nasal Outcomes Test; AD = Aspirin Desensitization.
The trends in the otologic-specific subdomain of the SNOT-22 scores paralleled the total SNOT-22 scores over the 12-month post-desensitization period. The mean pre-operative otologic subdomain score was 3.18 ± 3.1. Post-ESS/Pre-AD otologic subdomain SNOT-22 scores showed a significant decrease to 1.91 ± 2.9 (P = 0.005; 95% CI 0.38–2.17). Unlike the total SNOT-22 scores, the otologic subdomain scores remained statistically unchanged throughout the post-desensitization time periods, with scores of 1.98 ± 3.02 (P = 0.858), 1.85 ± 32.9 (P = 0.886), 1.57 ± 2.2 (P = 0.426), and 1.74 ± 2.6 (P = 0.740) at 0–3, 3–6, 6–12, and >12-month time points, respectively (Fig. 2B). Consistent with our statistical findings, the otologic-specific SNOT-22 scores do not achieve clinically significant declines over time after AD (ie: <0.40).
We further analyzed each of the otologic subdomain questions individually to see if there were trends specific to each question within the subdomain. The symptoms of ear fullness and ear pain had pre-op scores of 1.79 ± 1.4 and 0.73 ± 1.2, and showed significant decreases in post ESS/pre-AD SNOT-22 scores to 0.96 ± 1.3 (P = 0.000; 95% CI 0.41–1.26) and 0.37 ± 0.8 (P = 0.05; 95% CI 0.001–0.69), respectively. However, these scores remained statistically unchanged throughout the post-desensitization time periods, with scores of 0.96 ± 1.3 (P = 0.573), 0.87 ± 1.3 (P = 0.580), 0.83 ± 1.2 (P = 0.861), and 1.07 ± 1.4 (P = 0.391) for ear fullness, and 0.40 ± 0.8 (P = 0.87), 0.52 ± 1.0 (P = 0.89), 0.38 ± 0.8 (P = 0.43), and 0.35 ± 0.8 (P = 0.94) for ear pain. Unlike the other otologic subdomain questions, the pre-surgical score for dizziness symptoms was 0.64 ± 1.0 and did not show any statistically significant change in SNOT-22 scores, initially 0.46 ± 1.1 (P = 0.283) or over time (Table 2).
Table 2.
Otologic subdomain specific question scores.
| Symptoms | Pre-ESS | Post ESS/Pre-AD | 0–3 months post-AD | 3–6 months post-AD | 6–12 months post AD | >12 months post-AD |
|---|---|---|---|---|---|---|
| Ear fullness | 1.79 | 0.96∗∗ | 0.96 | 0.87 | 0.83 | 1.07 |
| Ear pain | 0.72 | 0.37∗ | 0.40 | 0.52 | 0.38 | 0.35 |
| Dizziness | 0.63 | 0.46 | 0.6 | 0.36 | 0.40 | 0.35 |
Notes: The P values at Pre-AD compare estimates to pre-ESS (baseline); P values at all other time points post-desensitization compare estimates to Pre-AD to assess for a difference in the SNOT-22 trajectory over time after aspirin desensitization. ∗P < 0.05, ∗∗P < 0.001.
Discussion
AERD is a complex disorder that mainly affects the upper airway. Treatment protocols require multi-modality therapy with ESS, medical management, and AD.1,4 While, most therapies aim to improve sinonasal and airway-related symptoms, this review suggests that otologic symptoms should be considered in part of the evaluation of these patients.
Middle ear disease presenting in the presence of AERD is difficult to manage and currently has no standard recommendations for treatment. This issue is apparent, as recent literature has demonstrated that hearing loss and otologic symptoms are common in these patients, but many patients do not routinely have audiologic evaluation.8 Our study, supports the notion that otologic symptoms are present and contribute to overall SNOT-22 scores. Our data suggest that patients who underwent AD for AERD had reductions in otologic subdomain SNOT-22 scores, similar to the trend seen in overall SNOT-22 scores.
Surgery accounted for the initial drop in otologic SNOT-22 scores, but AD likely contributed to the continued maintenance of the score reduction over time as all of our cases were revision surgeries that did not have the benefit of AD following their previous ESS. Patients within our cohort received ESS plus 6–8 weeks of oral steroids before undergoing AD. The combination led to the initial drop in SNOT-22 scores prior to AD. It is well known that AERD associated nasal polyps regrow rapidly after surgery without systemic medical therapy.13 Since all of our subjects were revision ESS, they did not have the benefit of AD after their previous surgeries, strongly suggesting that AD did play a role in maintaining decreased SNOT-22 scores post-ESS. The discontinuation of steroids at the start of AD plus the maintenance and further reduction in SNOT-22 scores suggests even more that AD helped this trend. Our findings are consistent with previous studies, which reported that ESS followed by AD successfully maintains lower SNOT-22 scores.
Within the otologic subdomain questions, ear fullness and ear pain both maintained lower SNOT-22 scores over periods of time >12 months. However, questions regarding dizziness did not reproduce this same early reduction in SNOT-22 scores. This could be due to the complexities related to various organs and systems that cause dizziness as a symptom or the low baseline scores in our cohort. We felt this distinction between specific questions within the otologic subdomain was interesting because it offered insights as to how to counsel patients in regards to their symptoms of dizziness. While ear pain and fullness may improve after in management of AERD, dizziness may not improve during treatment for AERD, While our data suggest otologic symptoms are present in many patients with AERD, it still does not answer the question about how to manage those symptoms. There is limited information available on quantifiable otologic data, like audiometric data. Thus, it is unclear if these patients experience objective hearing loss. Similarly, the symptoms present could be due to middle ear obstruction of fluid, which raises the question as to whether some patients would benefit from myringotomy if appropriate. Ultimately, this study emphasizes the concept that otologic and audiometric evaluation may be warranted in some patients with AERD. The next step in this line of research should consider getting audiometry on patients to objectify their subjective SNOT-22 complaints.
Limitations of the study are similar to shortcomings in other retrospective analysis of data. Incomplete medical records may contribute to bias in the analysis. Similarly, pre- and post-intervention with surgery and AD offer comparison of data, but there is no true control group for our cohort, however, all of our cases were revision surgeries in patients who had not had the benefit of timely AD after their previous failed ESS procedures. While the SNOT-22 scores are available at various time points before and after intervention, it is challenging to say what specific effect each intervention (ESS or AD) is having on SNOT-22 scores. Finally, our study did not account for patients who were being treated with newer biologic agents. These agents have demonstrated improvement in symptoms of patients with AERD, however their impact on otologic symptoms is unknown.14 Despite these limitations, we feel that this work takes initial steps towards asking an interesting question about the association of AERD and otologic symptoms.
Conclusion
AERD is a complex disorder of the sinonasal tract and upper airway. Treatment often requires ESS and AD, which improve overall symptoms of AERD. This work introduces the association of otologic symptoms and how they contribute to overall SNOT-22 scores in AERD. Specifically, changes in otologic-specific scores are similar to changes in overall SNOT-22 scores in AERD patients. It is important to consider counseling patients with otologic concerns about expectations during the course of their AERD treatment.
Funding
None.
Author roles
James Naples: Conceptualization, data curation, formal analysis, investigation, methodology, supervision, writing - original draft and editing, final approval.
Andrew Corr: Data curation, formal analysis, investigation, methodology, writing - original draft and editing, final approval.
Siddhant Tripathi: Data curation, formal analysis, investigation, methodology, writing - original draft and editing, final approval.
Morgan Berman: Data curation, formal analysis, investigation, methodology, writing - original draft and editing, final approval.
Jason A. Brant: Conceptualization, formal analysis, investigation, methodology, supervision, writing - editing, final approval.
Michael J. Ruckenstein: conceptualization, data curation, formal analysis, investigation, methodology, supervision, writing - editing, final approval.
John Bosso: Conceptualization, data curation, formal analysis, investigation, methodology, supervision, writing - editing, approval.
Declaration of competing interest
None of the authors involved have a conflict of interest related to production of this manuscript.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Adappa N.D., Ranasinghe V.J., Trope M. Outcomes after complete endoscopic sinus surgery and aspirin desensitization in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol. 2018;8:49–53. doi: 10.1002/alr.22036. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Jiménez J.C., Moreno-Paz F.J., Terán L.M., Guaní-Guerra E. Aspirin exacerbated respiratory disease: current topics and trends. Respir Med. 2018;135:62–75. doi: 10.1016/j.rmed.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy J.L., Stoner A.N., Borish L. Aspirin-exacerbated respiratory disease: prevalence, diagnosis, treatment, and considerations for the future. Am J Rhinol Allergy. 2016;30:407–413. doi: 10.2500/ajra.2016.30.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tajudeen B.A., Schwartz J.S., Bosso J.V. The role of aspirin desensitization in the management of aspirin-exacerbated respiratory disease. Curr Opin Otolaryngol Head Neck Surg. 2017;25:30–34. doi: 10.1097/MOO.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 5.Brobst R., Suss N., Joe S., Redleaf S. Bilateral inflammatory aural polyps: a manifestation of Samter's triad. Int J Otolaryngol. 2009;2009:464958. doi: 10.1155/2009/464958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J., Peterson M., Mafee M., Nguyen Q.T. Aural polyps in Samter's triad: case report and literature review. Otol Neurotol. 2012;33:774–778. doi: 10.1097/MAO.0b013e318259522f. [DOI] [PubMed] [Google Scholar]
- 7.Tangbumrungtham N., Patel V.S., Thamboo A. The prevalence of Eustachian tube dysfunction symptoms in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8:620–623. doi: 10.1002/alr.22056. [DOI] [PubMed] [Google Scholar]
- 8.Mullur J., Singer J., Roditi R., Cahill K.N. Hearing loss and middle ear symptoms in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2019;7:1671–1672. doi: 10.1016/j.jaip.2018.11.034. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S.N., Lee W.H., Lee S.H., Rhee C.S., Lee C.H., Kim J.W. Chronic rhinosinusitis with nasal polyps is associated with chronic otitis media in the elderly. Eur Arch Otorhinolaryngol. 2017;274:1463–1470. doi: 10.1007/s00405-016-4363-0. [DOI] [PubMed] [Google Scholar]
- 10.Cho K.S., Soudry E., Psaltis A.J. Long-term sinonasal outcomes of aspirin desensitization in aspirin exacerbated respiratory disease. Otolaryngol Head Neck Surg. 2014;151:575–581. doi: 10.1177/0194599814545750. [DOI] [PubMed] [Google Scholar]
- 11.Feng A.L., Wesely N.C., Hoehle L.P. A validated model for the 22-item Sino-Nasal Outcome Test subdomain structure in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:1140–1148. doi: 10.1002/alr.22025. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins C., Gillett S., Slack R., Lund V.J., Browne J.P. Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.E., Kountakis S.E. The prevalence of Samter's triad in patients undergoing functional endoscopic sinus surgery. Ear Nose Throat J. 2007;86:396–399. [PubMed] [Google Scholar]
- 14.Laidlaw T.M. Clinical updates in aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2019;40:4–6. doi: 10.2500/aap.2019.40.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]