Abstract
There are few case reports of concomitant chronic inflammatory demyelinating polyneuropathy (CIDP) and focal segmental glomerulosclerosis. A rare autoantibody to a neuronal and podocyte structural component, neurofascin, may be contributory. A Black man in his 40s presented with worsening polyneuropathy requiring mechanical ventilation and initially acute inflammatory demyelinating polyneuropathy was diagnosed. After a poor response to intravenous immunoglobulin, plasmapheresis was initiated. The patient also had concomitant new-onset nephrotic-range proteinuria. A limited kidney biopsy was interpreted as minimal change disease and was treated with prednisone. After some improvement, the patient was extubated; however, he later re-presented with worsening symptoms requiring mechanical ventilation and was re-treated with plasmapheresis. Due to the protracted course and poor response to intravenous immunoglobulin, acute-onset CIDP was diagnosed and a neuromuscular antibody workup returned positive for neurofascin, supporting the diagnosis of seropositive acute-onset CIDP. A repeat kidney biopsy demonstrated focal segmental glomerulosclerosis and acute tubular damage. The patient was treated with steroids and tacrolimus and later transitioned to rituximab. Neurofascin enzyme-linked immunosorbent assay then tested negative with concomitant resolution of both neuropathy and proteinuria. Further studies will help validate these findings and the treatment strategy.
Index Words: FSGS, anti-neurofascin, nephrotic syndrome, proteinuria, CIDP, demyelinating polyneuropathy
Introduction
There is limited knowledge regarding the pathogenesis of focal segmental glomerulosclerosis (FSGS) in the setting of chronic inflammatory demyelinating polyneuropathy (CIDP). Previous studies have listed complex immune dysregulation as a cause of neuron and podocyte injury; however, a definite cause remains elusive. We believe that a rare autoantibody to an intrinsic part of both neurons and podocytes may be contributing to both pathologic states. We present the first case of anti-neurofascin–seropositive CIDP with concurrent FSGS and provide evidence of the pathogenicity of this autoantibody for both pathologic states. Our case also illustrates a potential treatment strategy. As more cases are described and the pathophysiology is further elucidated, optimal treatment strategies can be instituted earlier in hopes of improving outcomes.
Case Report
A Black man in his 40s presented to the hospital with 2 weeks of lower extremity paresthesias and progressive weakness. Neurologic examination was notable for an inability to lift his arms against gravity, inability to walk unassisted, distal predominant decrease in vibration sensation, and diffuse areflexia. A lumbar puncture was performed, which noted an albuminocytologic gradient with white blood cell count of 11,000 μL and protein level of 127 mg/dL. An electromyogram and nerve conduction study demonstrated sural nerve-sparing sensorimotor neuropathy with prolonged and absent F waves, findings associated with a diagnosis of acute inflammatory demyelinating polyneuropathy. Also, conduction block and markedly reduced recruitment of normal-appearing motor unit potentials was noted, consistent with a diagnosis of acute inflammatory demyelinating polyneuropathy (Fig S1). The patient was treated with 2 g/kg of intravenous immunoglobulin (IVIG) over 5 days.
Concomitantly, the patient was noted to have worsening bilateral lower extremity edema and new-onset proteinuria with protein excretion of 10 g on spot urinary albumin-creatinine ratio. A limited kidney biopsy with 3 glomeruli showed no glomerular abnormalities and tubulointerstitial damage on light microscopy and severe podocyte foot-process effacement on electron microscopy and was interpreted as minimal change disease. The patient was subsequently started on treatment with 1.0 mg/kg of prednisone daily.
The neuromuscular symptoms progressed and the patient subsequently developed respiratory failure requiring mechanical ventilation. It was then decided to treat with plasmapheresis for acute inflammatory demyelinating polyneuropathy progression. The patient underwent 7 sessions of plasmapheresis, after which he was able to be extubated with minimal motor improvement and was discharged to a skilled nursing facility. The patient re-presented within 1 week of discharge with worsening neurologic symptoms including quadriplegia, a complete lack of sensation throughout trunk and limbs, new-onset bulbar weakness, and autonomic dysfunction, including urinary retention and constipation. The patient was retreated with IVIG; however, the symptoms progressed, requiring reintubation and mechanical ventilation. After another 5 sessions of plasmapheresis, the patient was able to be extubated.
Given the acuity of onset, persistence of symptoms, and refractory course, there was concern for an atypical acute-onset seropositive CIDP, and blood testing for neuromuscular antibodies including neurofascin 155 (NF155), NF140, and contactin-1 antibodies was sent.
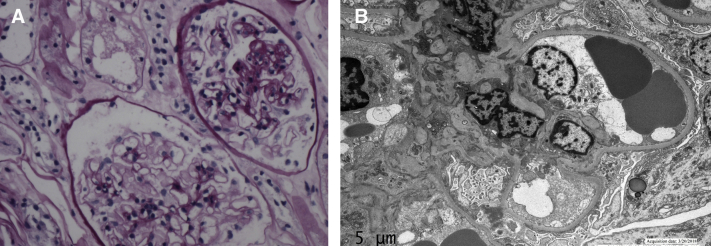
Concurrently, proteinuria had also worsened to protein excretion of 24 g on spot urinary albumin-creatinine ratio despite continued prednisone therapy. A repeat kidney biopsy showed 1 of 12 glomeruli with segmental sclerosis, and acute tubular damage on light microscopy, no deposits on immunofluorescence, and severe podocyte foot-process effacement on electron microscopy (Fig 1). Given these findings, FSGS was diagnosed and tacrolimus therapy was initiated. In addition, the patient was started on weekly treatment with pulse methylprednisolone, 1,000 mg, intravenously. One week after the first treatment, the patient was able to move his distal extremities in the plane of the bed. Given this rapid improvement, the patient was discharged to a rehabilitation facility and, on follow-up in the neuromuscular clinic 5 weeks after discharge, walked into the clinic unassisted with only minimal weakness at hip flexors bilaterally (4/5). The demyelinating antibody workup returned positive for autoantibodies against NF140 and NF155, which have been reported with IVIG-refractory acute-onset seropositive CIDP.
Figure 1.
(A) Light microscopy: glomerulus with segmental sclerosis. (B) Electron microscopy: severe visceral podocyte effacement.
High-dose steroid and tacrolimus treatments were continued for 6 months and on follow-up, complete remission of proteinuria had been achieved in association with resolution of the neuropathy. The patient was then transitioned to rituximab therapy and was tested again for the neurofascin antibody after a single dose of 1 g. An enzyme-linked immunosorbent assay was negative for the neurofascin antibody and the patient has no neurologic or kidney disease recurrence at the 1-year follow-up.
Discussion
This case makes 3 important points that may affect future care. First, this case provides a rare phenotype of acute-onset anti-neurofascin CIDP in association with FSGS. Second, this case supports the role of anti-neurofascin antibodies in seropositive CIDP. Finally, this case demonstrates the importance of clarifying disease phenotypes and antibodies for early optimal treatment.
Neurofascin is a cell adhesion molecule located in the paranodal region of the node of Ranvier and axonal initial segment, which is responsible for the initial formation of the action potential.1 It is critical to saltatory conduction, axonal subcellular targeting, and synapse formation during neural development. It is also integral for cell-to-cell contact between axon and myelin loops from Schwann cells. There are 3 recognized isoforms of neurofascin termed NF140, NF155, and NF186.2 NF140 and NF186 are produced by neuronal cells and are located at nodal and axonal initial segments. NF155 is produced by glial cells and is located within the paranodal junction.1, 2, 3, 4
The most common autoantibodies to neurofascin belong to the immunoglobulin G4 (IgG4) subclass.2 Autoantibodies against neurofascin have been found in 2% to 7% of patients with CIDP. This subset of CIDP has been increasingly recognized as acute-onset seropositive CIDP.5 Moreover, the pathology caused by these antibodies is referred to as a nodopathy/paranodopathy. Paranodopathies are characterized by dissection of myelin loops from the axon at the paranode and subsequent axonal degeneration.6 Notably, neurofascin is also a component of the podocyte cytoskeleton and has been reportedly involved in glomerulogenesis and podocyte maturation. Studies have shown that neurofascin is closely associated with vimentin, an integral part of podocyte major processes that are made of bundles of microtubules and intermediate filaments. This close association suggests their role in cell-cell contact and microtubule cytoskeletal support. Further studies are needed to better elucidate the physiologic role of neurofascin in podocytes.7
CIDP is traditionally seronegative with an incidence reported between 0.8 and 0.9 per 100,000 patients. Seronegative CIDP is characterized by symmetric proximal and distal weakness with paresthesias that typically progress over 8 weeks. Treatment consists of IVIG, corticosteroids, and plasma exchange, and overall, 80% to 90% of patients improve with one of these therapies.8 Acute-onset seropositive CIDP generally manifests in a younger patient demographic with a subacute and more severe presentation, distal dominant weakness, sensory ataxia, tremor, and, importantly, refractory to IVIG.5,6
There have been a few case reports of seronegative CIDP associated with proteinuria. However, most of these cases have been reported with membranous nephropathy.9 The pathogenesis of concomitant CIDP and FSGS has been poorly understood to date. To our knowledge, there have been only a few known cases of CIDP and concomitant FSGS.10, 11, 12 Previous cases have proposed a common antigenic target on neuronal and renal cells, host immunologic factors affected by dysregulated T- and B-cell responses,10 and immunologic mechanisms13,14 as potential underlying pathologies. One of the case reports suggested inverted formin 2 expression on Schwann cells and podocytes to be the target of antibodies.15 Two case reports discussed dysregulation of cellular and humoral responses resulting in the formation of antibodies to monosialoganglioside GM1 of the IgG1 subclass, along with deposition of a circulating immune complex in the setting of increased interleukin 2 and vascular endothelial growth factor levels and increased capillary permeability.10,11 A decreased erythropoietin level in the setting of nephropathy and its role as a neurotrophic factor, vital to neuronal growth and myelin integrity, was also deemed responsible for neuropathy in another report.9
The previous cases of seropositive CIDP have reported a poor or suboptimal response to the use of IVIG. In a study of 533 patients with CIDP, neurofascin antibodies were identified in 38 patients and 80% of these patients had a poor response to IVIG.5 After a transient improvement with steroids and plasma exchange, a rebound was noted after 2 to 3 weeks. A benefit has been reported with steroids and other immunotherapies including cyclophosphamide and rituximab.16,17 Rituximab has been used successfully in other IgG4-mediated diseases and is known to reduce IgG4 titers.18
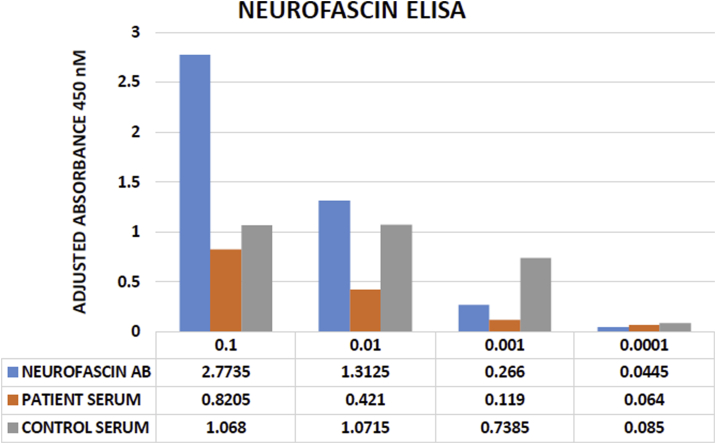
There are only a few case reports in which anti-neurofascin antibodies have been isolated in concomitant seropositive CIDP and FSGS. The pathogenesis of anti-neurofascin antibodies and their production and prognosis remain undefined. In our patient, neurofascin antibody was initially isolated and then was negative on enzyme-linked immunosorbent assay testing after treatment (Fig 2). These results support the need to further study the role of neurofascin autoantibody in patients with concomitant CIDP and FSGS.
Figure 2.
Neurofascin sandwich enzyme-linked immunosorbent assay (ELISA) results demonstrate the lack of neurofascin-specific binding in patient serum. This inference is based on comparison of values between patient serum versus control serum and positive control values. Abbreviation: AB, antibody.
Because there are no guidelines for an optimal treatment regimen for such patients, we propose that in patients suspected of seropositive CIDP with or without proteinuria, anti-neurofascin antibodies should be checked. Furthermore, these patients should initially be offered plasma exchange and/or steroid therapy along with immunomodulation with either calcineurin inhibitors or rituximab. Further studies are needed to validate these findings.
Article Information
Authors’ Full Names and Academic Degrees
Syed Bukhari, MD, Margaret Bettin, MD, Helen P. Cathro, MBChB, MPH, Kelly Gwathmey, MD, Jitendra Gautam, PhD, and Brendan Bowman, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank Mark Okusa, MD, and Kambiz Kalantari, MD, of the University of Virginia for providing valuable input and support for this project.
Prior Presentation
Abstract accepted as a poster at The ISN World Congress of Nephrology on April 12-15, 2019 at Melbourne, Australia.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Peer Review
Received March 23, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form June 28, 2020.
Footnotes
Complete author and article information provided before references.
Figure S1: Nerve conduction study (NCS): the image on the left demonstrates conduction block in the right peroneal nerve. The image on the right demonstrates the presence of an F wave in the right tibial nerve.
Supplementary Material
Figure S1.
References
- 1.Zonta B., Desmazieres A., Brophy P.-J. A critical role for neurofascin in regulating action potential initiation through maintenance of the axon initial segment. Neuron. 2011;69(5):945–956. doi: 10.1016/j.neuron.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delmont E., Manso C., Devaux J.-J. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain. 2017;140(7):1851–1858. doi: 10.1093/brain/awx124. [DOI] [PubMed] [Google Scholar]
- 3.Vallat J.-M., Yuki N., Devaux J.J. Paranodal lesions in chronic inflammatory demyelinating polyneuropathy associated with anti-neurofascin 155 antibodies. Neuromusc Disord. 2017;27(3):290–293. doi: 10.1016/j.nmd.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Zhang A., Desmazieres A., Brophy P.-J. Neurofascin 140 is an embryonic neuronal neurofascin isoform that promotes the assembly of the node of Ranvier. J Neurosci. 2015;35(5):2246–2254. doi: 10.1523/JNEUROSCI.3552-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devaux J.J., Miura Y., Yuki N. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology. 2016;86(9):800–807. doi: 10.1212/WNL.0000000000002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vural A., Doppler K., Meinl E. Autoantibodies against the node of Ranvier in seropositive chronic inflammatory demyelinating polyneuropathy: diagnostic, pathogenic, and therapeutic relevance. Front Immunol. 2018;9:1029. doi: 10.3389/fimmu.2018.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sistani L., Rodriguez P.-Q., Patrakka J. Neuronal proteins are novel components of podocyte major processes and their expression in glomerular crescents supports their role in crescent formation. Kidney Int. 2013;83(1):63–71. doi: 10.1038/ki.2012.321. [DOI] [PubMed] [Google Scholar]
- 8.Cocito D., Paolasso I., Nobile-Orazio E. A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2010;17(2):289–294. doi: 10.1111/j.1468-1331.2009.02802.x. [DOI] [PubMed] [Google Scholar]
- 9.Smyth S., Menkes D.L. Coincident membranous glomerulonephritis and chronic inflammatory demyelinating polyradiculoneuropathy: questioning the autoimmunity hypothesis. Muscle Nerve. 2008;37(1):130–135. doi: 10.1002/mus.20841. [DOI] [PubMed] [Google Scholar]
- 10.Girolami F., Galassi G., Cappelli G. Coincident chronic inflammatory demyelinating polyneuropathy and focal segmental glomerulosclerosis: a common autoimmunity? Clin Exp Nephrol. 2010;14(3):294–295. doi: 10.1007/s10157-009-0259-2. [DOI] [PubMed] [Google Scholar]
- 11.Olbricht C.J., Stark E., Koch K.-M. Glomerulonephritis associated with inflammatory demyelinating polyradiculoneuropathy: a case report and review of the literature. Nephron. 1993;64(1):139–141. doi: 10.1159/000187294. [DOI] [PubMed] [Google Scholar]
- 12.Souayah N., Cros D., Chong P.S.T. Relapsing Guillain Barré syndrome and nephrotic syndrome secondary to focal segmental glomerulosclerosis. J Neurol Sci. 2008;270(1-2):184–188. doi: 10.1016/j.jns.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Kohli A., Tandon P., Kher V. Chronic inflammatory demyelinating polyradiculoneuropathy with membranous glomerulonephritis: report of one case. Clin Neurol Neurosurg. 1992;94(1):31–33. doi: 10.1016/0303-8467(92)90115-j. [DOI] [PubMed] [Google Scholar]
- 14.Wu A.D., Russell J.A., Bouthout B.A. Chronic inflammatory demyelinating polyneuropathy and membranous glomerulonephropathy: report of two cases. J Clin Neuromusc Dis. 2001;3(2):70–74. doi: 10.1097/00131402-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Quek A.M.L., Soon D., Yuki N. Acute-onset chronic inflammatory demyelinating polyneuropathy with focal segmental glomerulosclerosis. J Neurol Sci. 2014;41(1-2):139–143. doi: 10.1016/j.jns.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Chen K.-H., Chang C.-T., Hung C.-C. Glomerulonephritis associated with chronic inflammatory demyelinating polyneuropathy. Ren Fail. 2006;28(3):255–259. doi: 10.1080/08860220600580415. [DOI] [PubMed] [Google Scholar]
- 17.Querol L., Rojas-García R., Illa I. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm. 2015;2(5):e149. doi: 10.1212/NXI.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quattrocchio G., Barreca A., Roccatello D. IgG4-related kidney disease: the effects of a rituximab-based immunosuppressive therapy. Oncotarget. 2018;9:21337–21347. doi: 10.18632/oncotarget.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.