Abstract
Rationale & Objective
The removal of metabolic waste by passing blood through synthetic tubing and membranes generates an immune response, even with the most biocompatible materials available. We evaluated blood levels of neutrophil activation and cell death during dialysis to devise a set of markers by which future dialysis interventions might be measured for biocompatibility.
Study Design
Observational, case control.
Setting & Participants
30 patients with end-stage kidney disease in Seattle, WA, evaluated during 30 dialysis procedures in out- and inpatient settings were compared with 27 healthy (negative) controls and 20 nondialysis patients with systemic lupus erythematosus as positive controls.
Predictor(s)
Blood levels of neutrophil activation (calprotectin and peroxidase activity) and cell death (cell-free DNA and neutrophil extracellular traps) were assayed.
Outcome(s)
Markers of neutrophil activation and cell death can be used to assess immune response during dialysis.
Analytical Approach
Descriptive analysis and group comparisons.
Results
Intradialytic levels of neutrophil activation markers are higher than prehemodialysis levels (P < 0.05), demonstrating neutrophil activation during hemodialysis. Less neutrophil activation occurs with peritoneal dialysis (P < 0.05). Immunosuppressive treatment and anticoagulant therapy did not seem to affect the capacity of neutrophils to undergo activation with hemodialysis. Finally, levels of hemodialysis-induced neutrophil activation correlated with markers of endothelial activation (r = 0.44; P = 0.01).
Limitations
Low sample size with heterogeneous patient cohort.
Conclusions
Neutrophil activation occurs during hemodialysis, potentially contributing to endothelial inflammation and damage. Neutrophil activation markers are novel and sensitive measures of biocompatibility for improving dialysis.
Index Words: Neutrophil, hemodialysis, biocompatibility, kidney, endothelium
Graphical abstract
Plain-Language Summary.
Dialysis keeps patients with kidney failure alive by removing metabolic waste and fluid through synthetic filters and tubing. The materials used for dialysis and the process itself, by their nature of being foreign to the body, activate the immune system. We study the response of the immune cells known as neutrophils to dialysis by very sensitive measures. Neutrophils become activated with each hemodialysis treatment and this in turn may lead to injury of blood vessels and cardiovascular disease. Less neutrophil activation occurs with peritoneal dialysis. Improvements in dialysis need to reduce immune activation. The neutrophil activation markers we measured can be used to assess future improvements in dialysis.
Dialysis revolutionized the care of patients with end-stage kidney disease (ESKD). Removal of metabolic waste by passing blood through synthetic tubing and membranes allowed patients with kidney failure to live. However, circulating blood through synthetic dialyzers is not a natural design. The innate immune system, poised to protect us from harm, becomes activated in current kidney replacement therapies, with evidence of monocyte, neutrophil, and platelet activation; complement system activation; and production of inflammatory cytokines, among others.1, 2, 3 Biocompatibility of materials used for dialysis has been a focus in the history of kidney replacement therapies.4, 5, 6, 7 Improvements in dialyzer membrane polymers and sterilization techniques virtually eliminated first-use syndrome immune responses.8 A continual goal of improving dialysis should be reduction in innate immune system and platelet activation. This is particularly relevant given the contribution of immune responses and platelet activation to cardiovascular disease and infections, which are major causes of death and morbidity for patients with kidney disease.9 Of note, neutrophils, through several mechanisms including activation and damage to endothelium, are thought to be main contributors to cardiovascular disease in several diseases, including systemic lupus erythematosus (SLE).10
The Advancing American Kidney Health Initiative ushers in the opportunity to make dialysis better. Improvements in dialysis can be expected in many areas. Our focus is to reduce the immune system activation that occurs with dialysis. This requires sensitive measures of immune activation for evaluating modifications to dialysis. Although we have progressed beyond the first-use reactions that occurred in the early days of dialysis, assays to measure the more subtle immune responses that are likely to cause long-term sequela for dialysis patients are needed.
In the current study, we investigated whether neutrophil activation, in particular neutrophil extracellular trap (NET) formation, could be useful when evaluating the immune-activating properties of dialyzer membranes. Reduction of neutrophil activation during the dialysis procedure is particularly attractive because of the multiple roles of these cells. Not only do neutrophils migrate to areas of infection to phagocytose microbes and release granules to kill extracellular microbes, they also extrude NETs.11 NETs are extracellular chromatin containing a variety of granular proteins. They have been implicated in vascular inflammation12 and occlusion, for example, thrombosis13,14 and sterile inflammation; and by exposing intracellular antigens, in autoimmunity.15 Hence reducing neutrophil activation with dialysis has the potential benefit of preventing both acute and chronic inflammatory conditions for patients with kidney disease.
We assessed neutrophil activation and cell death in patients undergoing peritoneal dialysis (PD) and hemodialysis (HD) by measuring plasma peroxidase activity, cell-free DNA (cfDNA), NET (myeloperoxidase [MPO]-DNA complexes), and calprotectin (proteins S100A8 and S100A9).
Methods
Patient Characteristics
Blood samples (3 mL in EDTA-containing tubes) were collected during 30 dialysis procedures (24 HD and 6 PD) in patients with ESKD and hemoglobin levels > 6 g/dL (Table 1). PD patients used either 1.25% or 2.5% glucose solutions for their dialysis. Additional patient characteristics on treatment and underlying medical conditions are summarized in Table 1. Our initial plan was to obtain blood samples immediately before and after the dialyzer at 2 and 4 hours into a dialysis treatment. However, in our initial samples, we did not find significant differences in neutrophil activation across the dialyzer, possibly due to the dialyzer absorbance or clearance of activation products. Hence, we simplified our approach to collecting blood before and during HD. For PD patients, a single blood sample was obtained when they had dialysate in their abdomen. HD was performed using polysulfone membranes. The blood was processed immediately upon blood draw with the plasma being stored at −80 °C until analyzed for neutrophil activation markers. Twenty-seven healthy controls without kidney disease served as negative controls and 20 patients with SLE served as positive controls. Among the patients with SLE, 3 (15%) had active disease (SLE Disease Activity Index score > 6). Informed consent was obtained according to Institutional Review Board standards at University of Washington, Seattle, WA (STUDY00002529). No patient refused consent.
Table 1.
Patient Characteristics
| Patient Group | HD | PD | HC | SLE |
|---|---|---|---|---|
| N | 24 | 6 | 27 | 20 |
| Female sex | 13 (54%) | 3 (50%) | 23 (85%) | 15 (75%) |
| Age, y | 58 (12) | 54 (17) | 52 (15) | 47 (17) |
| Citratea | 12 (50%) | NA | 0 (0%) | 0 (0%) |
| Warfarin | 3 (12%) | NA | 0 (0%) | 2 (10%) |
| Heparin | 16 (67%) | NA | 0 (0%) | 0 (0%) |
| Immunosuppression | 6 (25%) | 2 (33%) | 0 (0%) | 16 (80%) |
| Diabetes mellitus type 2 | 6 (25%) | 1 (17%) | 0 (0%) | 1 (5%) |
| Failed kidney transplant | 4 (17%) | 2 (33%) | 0 (0%) | 0 (0%) |
| Amyloid A amyloidosis | 3 (12%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Polycystic kidney disease | 2 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Liver transplant | 4 (17%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Glomerulonephritis | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Interstitial nephritis | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nephrectomy | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MesoAmerican nephropathy | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| IgA nephropathy | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Cardiomyopathy | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ANCA vasculitis | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| SLE | 1 (4%) | 0 (0%) | 0 (0%) | 20 (100%) |
| Childhood teratoma | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| COPD | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ischemia | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Tacrolimus toxicity | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
Note: Values expressed as number (percent) or mean (standard deviation).
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; COPD, chronic obstructive pulmonary disease; HC, healthy controls; HD, hemodialysis; IgA, immunoglobulin A; NA, not applicable; PD, peritoneal dialysis; SLE, systemic lupus erythematosus.
Note that some patients were treated with 2 or more anticoagulants.
Neutrophil and Endothelial Activation Markers
Markers of neutrophil activation and cell death were analyzed as described previously.16,17 Briefly, levels of calprotectin were analyzed using an enzyme-linked immunosorbent assay kit according to the manufacturer’s instruction (R&D Systems). The lower detection limit of the assay is 94 pg/mL. For the detection of NETs, a 96-well microtiter plate (Corning) was coated with a mouse monoclonal anti-MPO antibody (4 μg/mL; Biorad, clone 4A4) overnight at 4 °C, followed by blocking with 1% bovine serum albumin in phosphate-buffered saline for 2 hours at room temperature. After blocking, plasma samples (10%) were added and incubated overnight at 4 °C. For detection, anti–double-stranded DNA–horseradish peroxidase antibody (diluted 1/100; Roche Diagnostic) was added for 2 hours at room temperature. The reaction was developed with 3,3′,5,5′-tetramethylbenzidine (TMB; BD Biosciences), and ended by the addition of 2 N of sulfuric acid. Absorbance was measured at 450 nm by a plate reader (Synergy; BioTek). Isolated NETs were used as a standard curve with 1 U/mL equaling NETs released by 10,000 neutrophils.
Peroxidase activity was analyzed as previously described. Briefly, plasma samples (10%) were incubated with TMB at a final volume of 100 μL for 30 minutes at room temperature. The reaction was ended by the addition of 2 N of sulfuric acid. The absorbance was analyzed by a plate reader at 450 nm. Values are reported as mU/mL using horseradish peroxidase (Sigma) as standard curve. cfDNA was analyzed using the DNA-binding dye SytoxGreen (Invitrogen). Values are reported as μg/mL using isolated DNA as standard curve. Levels of soluble intercellular adhesion molecule 1 (sICAM1) were analyzed according to the manufacturer’s instruction (R&D Systems).
Statistical Analysis
For statistical analyses, Mann-Whitney U test and Wilcoxon were used for nonpaired and paired group analyses, respectively. P<0.05 was considered statistically significant. Results are presented as median with 25th to 75th percentiles.
Results
HD and Neutrophil Activation
To assess whether neutrophil activation occurs with HD, levels of neutrophil activation and cell death markers, for example, NETs, calprotectin, peroxidase activity, and cfDNA, were measured in pre-HD samples and compared with samples obtained 2 to 4 hours into HD. The average intradialytic levels of NETs (median, 2.16; 25th-75th percentiles, 1.86-4.19 vs 2.00; 25th-75th percentiles, 1.51-2.93; P = 0.02), calprotectin (median, 2.61; 25th-75th percentiles, 1.50-6.47 vs 1.96; 25th-75th percentiles, 0.53-5.89; P = 0.04), peroxidase activity (median, 5.51; 25th-75th percentiles, 3.83-6.81 vs 3.78; 25th-75th percentiles, 2.95-6.08; P < 0.001), and cfDNA (median, 5.17; 25th-75th percentiles, 4.26-7.22 vs 4.19; 25th-75th percentiles, 3.18-5.32; P = 0.007) were higher than pre-HD levels (Fig 1A-D). We did not follow up patients postdialysis to determine when this activation resolved. However, for patients for whom more than 1 HD procedure was assessed, neutrophil activation measures generally returned to lower levels before the start of subsequent treatments. The change in NET levels between pre-HD and intradialysis correlated well with the change in peroxidase activity and calprotectin (r = 0.45; P < 0.05, and r = 0.58; P = 0.02, respectively, Fig 1E and F), but not with the nonspecific marker cfDNA (r = 0.12; P = 0.61; Fig 1G).
Figure 1.
Neutrophil activation during hemodialysis (HD). Levels of neutrophil activation markers: (A) peroxidase activity, (B) cell-free DNA (cfDNA), (C) neutrophil extracellular traps (NETs), and (D) S100A8/A9 (calprotectin) were analyzed in HD patients (n = 24) before (pre-HD) and at least 2 hours into the dialysis procedure. (E-G) Delta levels of NETs, comparing pre-HD values with intradialysis values, were correlated with delta levels of (E) peroxidase activity, (F) calprotectin, and (G) cfDNA. For statistical analysis, Mann-Whitney U test and Spearman correlation test were used, with ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Abbreviation: Av, average.
Anticoagulants and Immunosuppressive Treatment
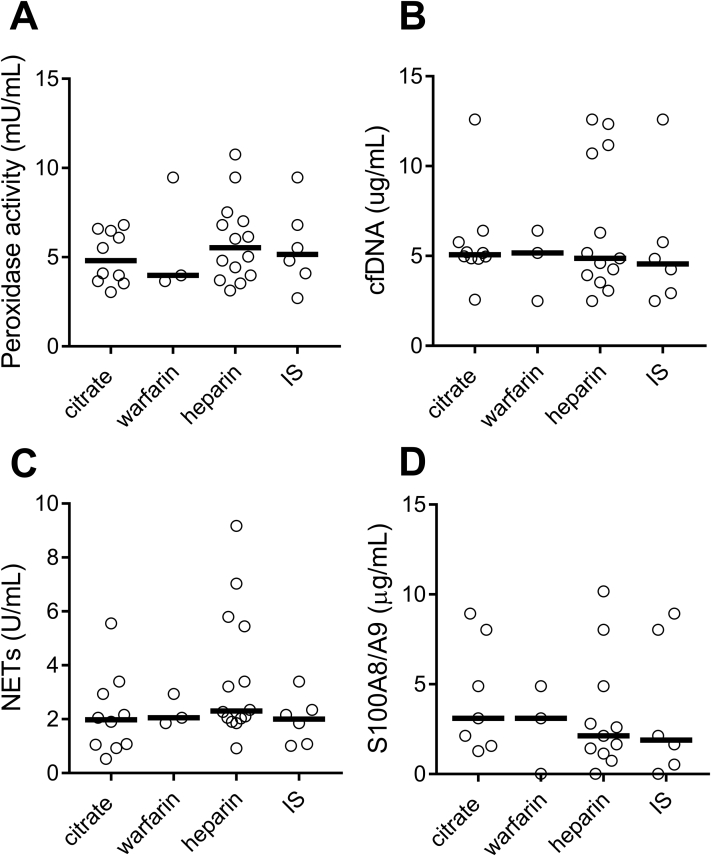
Considering the important interplay between platelets, coagulation, and neutrophil activation,18 we next asked whether the anticoagulant used affected neutrophil activation during HD. The use of Citrasate (Fresenius Medical Care) dialysate, heparin, and warfarin showed similar levels of intradialytic neutrophil activation (Fig 2), suggesting that anticoagulation did not affect neutrophil activation. Further, use of immunosuppressive treatment, due to solid-organ transplant, was not associated with reduced neutrophil activation (Fig 2).
Figure 2.
Levels of neutrophil activation markers are not affected by anticoagulant. Levels of neutrophil activation markers: (A) peroxidase activity, (B) cell-free DNA (cfDNA), (C) neutrophil extracellular traps (NETs), and (D) S100A8/A9 (calprotectin) were analyzed in hemodialysis patients (n = 24) treated with Citrasate (n = 10), warfarin (n = 3), heparin (n = 13), and immunosuppressive treatment (IS; n = 6). No statistical differences between any of the groups.
HD Compared With PD
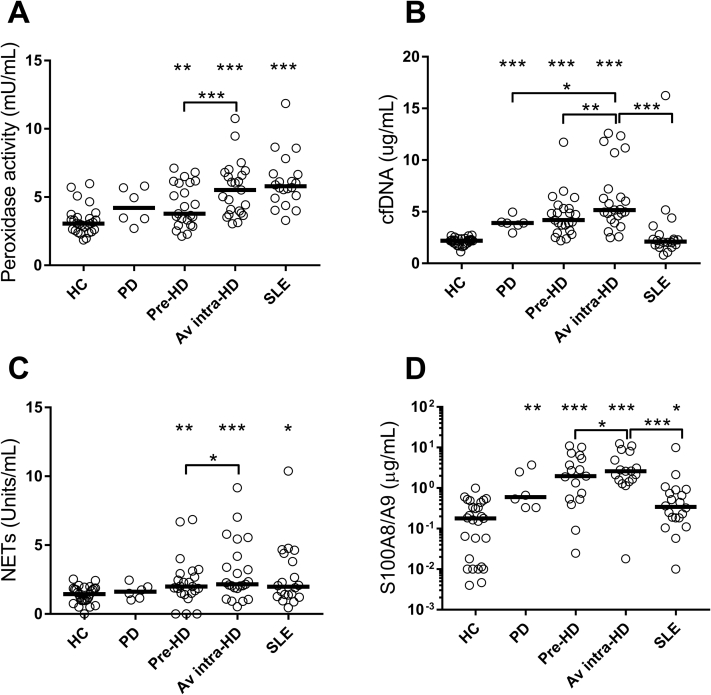
Finally, we assessed whether neutrophil activation was higher in dialysis patients compared with healthy controls and how it compared with patients with SLE, who are known to have increased levels of neutrophil activation.17,19 Consistent with prior work, our study confirmed elevated levels of NETs (median, 1.98 [25th-75th percentiles, 1.29-4.25] vs 1.45 [25th-75th percentiles, 0.98-1.86]; P = 0.03), calprotectin (median, 0.34 [25th-75th percentiles, 0.18-0.86] vs 0.18 [25th-75th percentiles, 0.01-0.44]; P = 0.02), and peroxidase activity (median, 5.80 [25th-75th percentiles, 4.96-6.69] vs 3.06 [25th-75th percentiles, 2.54-3.50]; P < 0.001), but not cfDNA (median, 2.12 [25th-75th percentiles, 1.80-2.67] vs 2.20 [25th-75th percentiles, 1.93-2.37]; P = 0.92) in patients with SLE as compared with healthy controls (Fig 3). Neutrophil activation was higher by all parameters in patients with ESKD maintained on HD compared with healthy controls (NET levels; median, 2.00 [25th-75th percentiles, 1.51-2.93] vs 1.45 [25th-75th percentiles, 0.98-1.86]; P = 0.009), calprotectin (median, 1.96 [25th-75th percentiles, 0.53-5.89] vs 0.18 [25th-75th percentiles, 0.01-0.44]; P < 0.001), peroxidase activity (median, 3.78 [25th-75th percentiles, 2.95-6.08] vs 3.06 [25th-75th percentiles, 2.54-3.50]; P = 0.007), and cfDNA (median, 4.19 [25th-75th percentiles, 3.18-5.32] vs 2.20 [25th-75th percentiles, 1.93-2.37]; P < 0.001). Neutrophil activation was less with PD compared with HD. Finally, intradialytic levels of neutrophil activation markers were similar or higher than the levels found in patients with SLE (Fig 3).
Figure 3.
Comparison of neutrophil activation markers in healthy controls (HCs), peritoneal dialysis (PD) patients, hemodialysis (HD) patients, and patients with systemic lupus erythematosus (SLE). Levels of neutrophil activation markers: (A) peroxidase activity, (B) cell-free DNA cfDNA), (C) neutrophil extracellular traps (NETs), and (D) S100A8/A9 (calprotectin) were analyzed in HCs (n = 27), PD patients (n = 6), matched pre- and intra-HD patients (n = 24), and patients with SLE (n = 20). For statistical analyses, Mann Whitney U test and Wilcoxon paired t test were used, with ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Unless noted, all analyses are compared with HCs. Abbreviation: Av, average.
Neutrophil Activation Association With Endothelial Activation
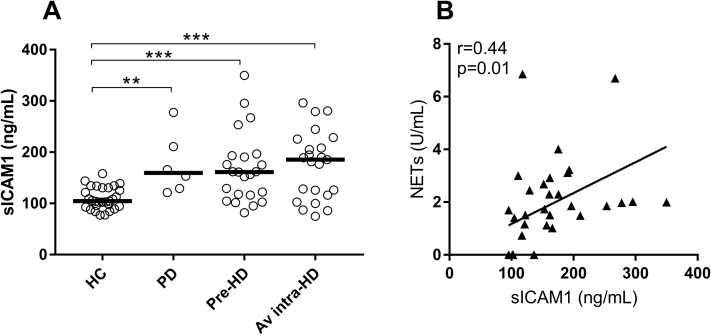
Prior work has established a causal link between neutrophil activation, in particular NET formation, and endothelial damage and activation, thereby contributing to vascular impairment, inflammation, and atherosclerosis.13,14,20,21 In patients undergoing dialysis, independent of HD or PD, elevated sICAM1 levels were found (healthy controls: median, 104.5 [25th-75th percentiles, 93.7-130.4]; PD: median, 159.5 [25th-75th percentiles, 127.2-227.5]; P = 0.001; pre-HD: median, 161 [25th-75th percentiles, 116.2-193.2]; P = 0.0002; and intradialytic HD: median, 185.5 [25th-75th percentiles, 116-225.4]; P < 0.001), indicative of endothelial activation (P < 0.001; Fig 4A). Of note, sICAM1 levels correlated with levels of neutrophil activation markers, including NETs (r = 0.44; P = 0.01; Fig 4B).
Figure 4.
Endothelial activation markers are associated with neutrophil extracellular trap (NET) formation. Levels of endothelial activation marker soluble intercellular adhesion molecule 1 (sICAM1) were (A) analyzed in healthy controls (HCs; n = 27), peritoneal dialysis (PD) patients (n = 6), and matched pre- and intra-hemodialysis (HD) patients (n = 24), and (B) intradialytic levels of sICAM1 correlated with NET levels. For statistical analyses, Mann Whitney U test and Wilcoxon paired t test were used, with ∗∗P < 0.01 and ∗∗∗P < 0.001. Abbreviation: Av, average.
Discussion
As we work to improve dialysis therapies, an area of focus should be reducing immune activation induced with the mode of kidney replacement. We show that neutrophil activation, assessed by peroxidase activity, cfDNA, NET, and calprotectin (proteins S100A8 and S100A9) levels, occurred with each HD procedure, consistent with prior findings on MPO and reactive oxygen species.22,23 Although specifics of the long-term clinical effects of this are unknown and difficult to decipher from other problems of patients with kidney disease, it is reasonable to assume that neutrophil activation is undesirable given the well-known contribution to inflammation and organ damage.19,24 Neutrophils, through release of NETs and calprotectin, contribute to vascular inflammation and cardiovascular disease.13,14,25,26
In this study, less neutrophil activation was associated with PD compared with HD. HD differs from PD in that large volumes of blood are rapidly and directly exposed to extracorporeal components of the dialysis circuit, which are foreign to the immune system. In PD, the amount of foreign material is limited to the catheter and the peritoneal fluid used for the dialysis. Although the slow exchange of fluid in and out of the abdomen may induce a localized immune response on peritoneal surfaces in direct contact with peritoneal fluids and catheter materials, the potential for a systemic inflammatory response is less (in the uninfected patient).
Although reduced neutrophil activation potentially weighs favorably toward PD, we recognize that many factors need to be considered in selecting the optimal mode of kidney replacement for an individual patient. Our study did not evaluate markers of neutrophil activation in the peritoneal space or peritoneal membrane. It is possible that neutrophil activation occurs locally in PD patients with effects on the function of the peritoneal membrane. Further study of neutrophil activation in peritoneal membranes could help determine this risk and could have an effect in the selection of more biocompatible peritoneal dialysate solutions. Additionally, in our study, there may be confounding factors at the level of patient selection among the PD population that were not controlled.
We did not find a difference in the degree of neutrophil activation based on the type of anticoagulant used for the procedure. Hence, reduction of neutrophil activation might focus on other undetermined variables such as blood and dialysate flows, temperature, dialysate composition, and tubing or dialyzer structure and composition. Of note, we did not account for the effectiveness of the anticoagulation strategies used in this study. Our investigation was not designed to evaluate the relationship between clotting and neutrophil activation.
Interestingly, the 5 patients who were receiving immunosuppression for their nonkidney solid-organ transplants and others receiving immunosuppression for nontransplant medical problems showed similar neutrophil activation to those not receiving immunosuppression. This finding is most likely because long-term immunosuppression therapy is generally focused on preventing lymphocyte activation, not neutrophil activation. Steroids, antimetabolites, and calcineurin inhibitors in this limited study do not appear to reduce neutrophil activation.
Attempts to measure calprotectin levels in patients with ESKD have been reported by others with conflicting results. Malickova et al27 found calprotectin levels elevated in patients with ESKD predialysis and unchanged after dialysis; intradialytic levels were not obtained. The focus of their study was not on neutrophil activation per se and the multiple parameters we assessed were not measured. We believe our data to be more robust and suggest that new dialysis technology innovations be evaluated for neutrophil activation, as we have done, to assess whether innovations represent an immune improvement for patients.
Article Information
Authors’ Full Names and Academic Degrees
Scott Bieber, DO, Kimberly A. Muczynski, MD, and Christian Lood, PhD.
Authors’ Contributions
Study design: KAM, SB, CL; patient recruitment: KAM, SB; experiments: CL; data analysis: CL. All authors approved the final version of the manuscript and accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Dr Muczynski is supported through the Kidney Immunology Research at the University of Washington, to which the Robert Dolsen family, Edward Kibble, and John DuBois have been major contributors. Dr Lood is supported through Lupus Research Alliance (#519414). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
Drs Bieber and Muczynski declare that they have no relevant financial interests. Dr Lood holds a patent pending (PCT/US2019/036398) related to the biomarker assays mentioned in this article.
Peer Review
Received March 19, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form June 24, 2020. The involvement of an Acting Editor-in-Chief was to comply with Kidney Medicine’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
References
- 1.Ekdahl K.N., Soveri I., Hilborn J., Fellstrom B., Nilsson B. Cardiovascular disease in haemodialysis: role of the intravascular innate immune system. Nat Rev Nephrol. 2017;13(5):285–296. doi: 10.1038/nrneph.2017.17. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen A.T., Lethias C., Zingraff J. Hemodialysis membrane-induced activation of phagocyte oxidative metabolism detected in vivo and in vitro within microamounts of whole blood. Kidney Int. 1985;28(2):158–167. doi: 10.1038/ki.1985.136. [DOI] [PubMed] [Google Scholar]
- 3.Horl W.H. Hemodialysis membranes: interleukins, biocompatibility, and middle molecules. J Am Soc Nephrol. 2002;13(suppl 1):S62–S71. [PubMed] [Google Scholar]
- 4.Hakim R.M., Fearon D.T., Lazarus J.M. Biocompatibility of dialysis membranes: effects of chronic complement activation. Kidney Int. 1984;26(2):194–200. doi: 10.1038/ki.1984.155. [DOI] [PubMed] [Google Scholar]
- 5.Himmelfarb J., Hakim R.M. The use of biocompatible dialysis membranes in acute renal failure. Adv Ren Replace Ther. 1997;4(2 suppl 1):72–80. [PubMed] [Google Scholar]
- 6.Himmelfarb J., Tolkoff Rubin N., Chandran P. A multicenter comparison of dialysis membranes in the treatment of acute renal failure requiring dialysis. J Am Soc Nephrol. 1998;9(2):257–266. doi: 10.1681/ASN.V92257. [DOI] [PubMed] [Google Scholar]
- 7.Twardowski Z.J. History of hemodialyzers’ designs. Hemodial Int. 2008;12(2):173–210. doi: 10.1111/j.1542-4758.2008.00253.x. [DOI] [PubMed] [Google Scholar]
- 8.Upadhyay A., Jaber B.L. Reuse and biocompatibility of hemodialysis membranes: clinically relevant? Semin Dial. 2017;30(2):121–124. doi: 10.1111/sdi.12574. [DOI] [PubMed] [Google Scholar]
- 9.Fox C.S., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight J.S., Kaplan M.J. Cardiovascular disease in lupus: insights and updates. Curr Opin Rheumatol. 2013;25(5):597–605. doi: 10.1097/BOR.0b013e328363eba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann V., Reichard U., Goosmann C. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber A., Rousselle A., Becker J.U. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A. 2017;114(45):E9618–E9625. doi: 10.1073/pnas.1708247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight J.S., Luo W., O’Dell A.A. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114(6):947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight J.S., Zhao W., Luo W. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123(7):2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 16.Bach M., Moon J., Moore R. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol. 2020;72(1):47–56. doi: 10.1002/art.41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore S., Juo H.H., Nielsen C.T. Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol. 2020;47(11):1652–1660. doi: 10.3899/jrheum.190875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark S.R., Ma A.C., Tavener S.A. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 19.Lood C., Blanco L.P., Purmalek M.M. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight J.S., Subramanian V., O’Dell A.A. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015;74(12):2199–2206. doi: 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan M.J. Premature vascular damage in systemic lupus erythematosus. Autoimmunity. 2009;42(7):580–586. doi: 10.1080/08916930903002479. [DOI] [PubMed] [Google Scholar]
- 22.Himmelfarb J., Lazarus J.M., Hakim R. Reactive oxygen species production by monocytes and polymorphonuclear leukocytes during dialysis. Am J Kidney Dis. 1991;17(3):271–276. doi: 10.1016/s0272-6386(12)80473-2. [DOI] [PubMed] [Google Scholar]
- 23.Himmelfarb J., McMenamin M.E., Loseto G., Heinecke J.W. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic Biol Med. 2001;31(10):1163–1169. doi: 10.1016/s0891-5849(01)00697-9. [DOI] [PubMed] [Google Scholar]
- 24.Ma L., Sun P., Zhang J.C., Zhang Q., Yao S.L. Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int J Mol Med. 2017;40(1):31–38. doi: 10.3892/ijmm.2017.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Averill M.M., Kerkhoff C., Bornfeldt K.E. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol. 2012;32(2):223–229. doi: 10.1161/ATVBAHA.111.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyden H., Lood C., Gullstrand B. Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology (Oxford) 2013;52(11):2048–2055. doi: 10.1093/rheumatology/ket263. [DOI] [PubMed] [Google Scholar]
- 27.Malickova K., Brodska H., Lachmanova J. Plasma calprotectin in chronically dialyzed end-stage renal disease patients. Inflamm Res. 2010;59(4):299–305. doi: 10.1007/s00011-009-0103-x. [DOI] [PubMed] [Google Scholar]