Abstract
Objective
To review and evaluate outcomes of patients with aspirin-exacerbated respiratory disease (AERD) following endoscopic sinus surgery and subsequent aspirin desensitization.
Methods
Electronic searches of OVID MEDLINE (1948 to September 10, 2019), EMBASE (1980 to September 10, 2019), and PubMed were performed on September 10, 2019. A systematic review of the literature was performed using the 2009 PRISMA guidelines. Studies with both preoperative and postoperative data for patients with AERD who underwent sinus surgery and aspirin desensitization were considered appropriate for inclusion. Publications were written in English and included patients aged 18 years or older.
Results
Six studies met inclusion criteria for this systematic review. The primary outcome measure was change in symptom profile measured by patient-reported quality of life scores. The results demonstrate statistically significant improvement in symptoms following endoscopic sinus surgery, with sustained improvement following aspirin desensitization. Revision surgery rates were significantly lower in patients maintained on aspirin therapy.
Conclusion
This review suggests that surgery followed by aspirin desensitization results in improvement in both subjective and objective outcome measures. The adjunctive use of aspirin desensitization allows for long-term stability in symptom scores. Recurrence of polyps and worsening symptoms requiring revision surgery occurs when aspirin maintenance therapy is interrupted.
Keywords: AERD, Aspirin-exacerbated respiratory disease, Aspirin desensitization, Endoscopic sinus surgery, Nasal polyps, Chronic sinusitis with nasal polyps
Abbreviations
- AERD
Aspirin-Exacerbated Respiratory Disease
- COX-1
Cyclooxygenase-1
- 5-LO
5-Lipoxygenase
- CysLT
Cysteinyl Leukotrienes
- PGD2
Prostaglandin D2
- ESS
Endoscopic Sinus Surgery
- AD
Aspirin Desensitization
- AIA
Aspirin Intolerant Asthma
- NSAID
Nonsteroidal Anti-Inflammatory Disease
- NERD
NSAID exacerbated respiratory disease
- SNOT
Sino-Nasal Outcome Test
- RSDI
Rhinosinusitis Disability Index
- CSS
Chronic Sinusitis Survey
- RQLQ
Rhinoconjunctivitis Quality of Life Questionnaire
- VAS
Visual Analog Scale
- CT
Computed Tomography
- QoL
Quality of Life
- PRISMA
Preferred Reporting of Items for Systematic Reviews and Meta-Analyses
- ND
Nondesensitized
- ACT
Asthma Control Test
- FEV1
First Second Forced Expiratory Volume
Introduction
Aspirin-exacerbated respiratory disease (AERD) is a distinctly challenging adult onset syndrome characterized by severe eosinophilic chronic rhinosinusitis with recurrent nasal polyposis, asthma, and a pseudoallergic (non-IgE mediated) reaction to aspirin and all other inhibitors of the Cyclooxygenase-1 (COX-1) pathway. All three components of AERD are typically severe and difficult to treat.1 The pathophysiology is not fully elucidated, however, current studies demonstrate that this syndrome is driven by dysregulation of mast cell function and arachidonic acid metabolism.2 Through inflammatory processes mediated by infiltration of eosinophils, basophils, and mast cells into the respiratory and sinonasal mucosa, AERD patients endogenously synthesize and secrete increased levels of cysteinyl leukotrienes (CysLT). This leukotriene response is augmented by prostaglandin D2 (PGD2) and histamine released from mast cells.3 Patients display intense (three-to four-fold) upregulation of the enzymes 5-lipoxygenase (5-LO) and LTC4 synthase, both of which are involved in CysLT synthesis.4 In AERD, increased synthesis of CysLT combined with upregulation of CysLT receptors creates a proinflammatory milieu. Cyclooxygenase-1 (COX-1) inhibitors such as aspirin exacerbate this effect by disinhibiting CysLT production. This ultimately leads to augmented inflammation through cellular recruitment, increased mucus production via sinonasal epithelial cells, and mucosal edema caused by increased permeability and vasodilation of nasal vasculature.5
Notably, AERD results in significant chronic sinusitis with nasal polyps that are difficult to control with both medical and surgical therapy. Recent studies have shown that patients with AERD benefit from sinus surgery as much as the chronic rhinosinusitis population.6,7 A multifaceted approach with endoscopic sinus surgery (ESS) and adjunct medications has been shown to be of most benefit to AERD patients.
In this systematic review, we evaluate outcomes in AERD patients that have undergone endoscopic sinus surgery followed by aspirin desensitization (AD). This is the first systematic review for outcomes of ESS followed by AD.
Materials and methods
Eligibility criteria
Eligibility criteria for this review required published and unpublished studies conducted between 1946 and 2019 of adult patients (aged ≥18 years old) who underwent sinus surgery for AERD and were followed postoperatively with an adjunct protocol of aspirin desensitization. AERD was defined as nasal polyposis in the context of chronic eosinophilic rhinosinusitis, asthma, and insensitivity to aspirin. The authors of this article chose to define Aspirin-intolerant Asthma (AIA), Aspirin-Exacerbated Respiratory Disease (AERD), and Non-steroidal Anti-inflammatory drug (NSAID) exacerbated respiratory disease (NERD) patients synonymously. In all studies, endoscopic sinus surgery had to be a primary intervention, followed by adjunctive aspirin desensitization postoperatively. Included studies had to have a published series of at least 5 patients and a clear description of outcome measures. The primary outcomes were patient-reported subjective measures: Sino-Nasal Outcome Test (SNOT), Rhinosinusitis Disability Index (RSDI), Chronic Sinusitis Survey (CSS), rhinoconjunctivitis quality of life questionnaire (RQLQ), and symptom score scale [i.e., visual analog scale(VAS) ]. Secondary outcomes included objective measures: endoscopy score, computed tomography (CT) grading, peripheral eosinophil count, serum IgE levels, revision surgery rates. We did not exclude patients who received concomitant therapies, such as oral or inhaled steroids or leukotriene modifiers. Preoperative and postoperative care was not specified in the eligibility criteria because of the diversity across the literature. Articles in any language were considered as long as a translated English version was attainable.
Information sources
Electronic searches of OVID MEDLINE (1948 to September 10, 2019), EMBASE (1980 to September 10, 2019), and PubMed were performed on September 10, 2019. The following search strategy was used: (Sinusitis OR Nasal Polyps) AND (AERD OR Samter's Triad OR Aspirin-Exacerbated Respiratory Disease OR ASA Triad OR Aspirin Triad OR Aspirin Intolerant Asthma OR NSAID Pseudoallergy) AND (Surgery OR Sinus Surgery OR Endoscopic Sinus Surgery OR Nasalization OR Polypectomy OR Turbinectomy OR Antrostomy) AND (Aspirin desensitization OR NSAID challenge OR Drug challenge OR Desensitization). Abstracts and conference proceedings published between 2005 and 2018 were searched using the Web of Science. Reference lists of retrieved articles were screened to identify other potential studies for inclusion. Only human studies were included in the study.
Study selection
Two independent reviewers (L.R. and D.T.S.) screened the titles and abstracts of studies retrieved from the systematic search. After collaboration, relevant abstracts were selected for full-article review. Each independent reviewer selected articles based on previously defined eligibility criteria to be included in this systematic review. Relevant data were also extracted independently using a data collection form. Any disagreement regarding the inclusion or exclusion of a study was resolved through discussion and consensus agreement. If disagreement persisted, an arbitrator (A.P.) was consulted for a final decision.
Methodological assessment and data synthesis
The Preferred Reporting of Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were utilized in this study. Each reviewer used the Cochrane Handbook of Systematic Reviews (Version 6.0) to determine the methodological assessment. Each study was assigned a level of evidence based on guidelines published by the Oxford Center for Evidence-Based Medicine-Levels of Evidence. Articles were also graded using the National Institute for Health (NIH) and Clinical Excellence scoring scale for case series, which has been previously applied in other systematic reviews in otolaryngology.
Results
Search results
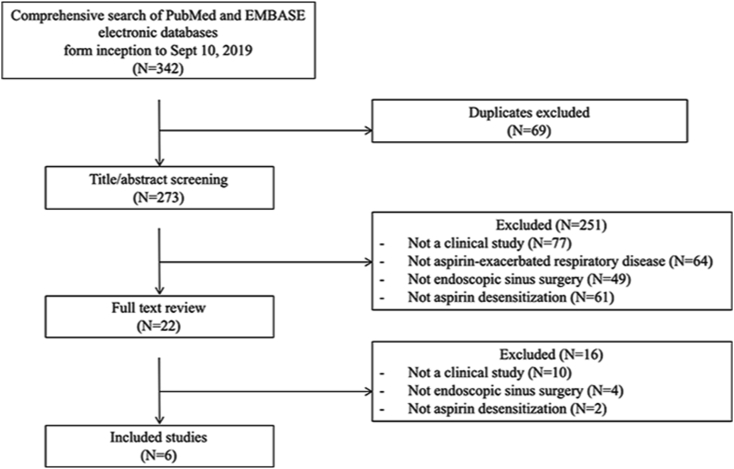
The systematic search of PubMed, EMBASE, and Web of Science databases led to a total of 342 potential abstracts (citations) for review (Fig. 1). After removal of the duplicates, 273 abstracts remained. During the screening of abstracts, 22 studies were selected for full text review. After the examination of full text, 16 studies were excluded, because they did not meet the inclusion criteria. There were 6 studies identified that met inclusion criteria.
Fig. 1.
Systematic review search results.
Methodological quality of included studies
The level of evidence for each study is shown in Table 1.
Table 1.
Study design.
| Source | Study Design | Study population | Objective | Outcomes Measured | Surgical Technique | Aspirin desensitization protocol | Follow up (months) | Level of Evidence |
|---|---|---|---|---|---|---|---|---|
| Adappa et al. | Retrospective chart review | 32 AERD patients who underwent AD following ESS | Compare AERD cohort pre-AD versus post-AD | QoL (SNOT-22) | Complete ESS, polypectomy, wide bilateral maxillary antrostomy, total sphenoethmoidectomy, frontal sinusotomy | Prior to 2012, aspirin desensitization described by Stevenson. After 2012, modified intranasal ketorolac and aspirin challenge protocol described by Lee et al AD beginning 4–6 weeks after surgery |
30 | 3b |
| Cho et al. | Retrospective chart review | 21 AERD patients who underwent AD following ESS | Assess sinonasal outcomes after AD following ESS. | QoL (SNOT-22), endoscopic polyp score | ESS, extent of surgery determined by extent of disease. Goal was removal of all polyps and drainage of all CT thickened sinuses | Aspirin desensitization beginning with 20.25 mg increasing to 40.5, 81, 162, 325, 650. Maintenance dose 650 qAM plus 325 qhs, or 325 BID daily AD beginning 4–6 weeks after surgery |
30 | 3b |
| Fruth et al. | Prospective double-blind randomized placebo-controlled trial | 31 AERD patients with recurrent nasal polyposis and >2 prior ESS. 18 in AD group, 13 in ND group | Compare outcomes between ESS AD versus ESS ND groups. | QoL (RSDI), symptom score (nonvalidated), olfactory function (Sniffin sticks), and endoscopy scores postoperatively | No comment on extent of surgery | Aspirin desensitization beginning with 180 mg increasing to 800 mg. Maintenance dose 100 mg daily AD beginning 6 weeks after surgery |
36 | 3b |
| Havel et al. | Retrospective chart review | 89 AERD patients who underwent ESS. 56 in AD group, 33 patients in ND group | Compare outcomes between ESS AD versus ESS ND groups. | QoL (VAS), symptom score (nonvalidated), endoscopy score (Rasp polyp grading) and revision surgery rates | ESS, polypectomy, uncinectomy, anterior ethmoidectomy, exploration of posterior ethmoids according to criteria of Messerklinger technique, septoplasty when necessary | Aspirin desensitization beginning with 25 mg increasing to 50, 100, 150, 200, 300, 400 and 500 mg. Maintenance dose 500 mg daily AD beginning 4–6 weeks after surgery |
18 | 3b |
| McMains and Kountakis | Retrospective chart review | 15 AERD patients who underwent ESS. 5 in AD group, 10 in ND group | Compare outcomes between ESS AD versus ESS ND groups. | QoL (SNOT), endoscopy scores based on rhinosinusitis task force methodology, revision surgery rates | No comment on extent of surgery | Aspirin desensitization described by Stevenson. Did not specify timing of initiation of aspirin desensitization |
24 | 3b |
| Shah et al. | Prospective Cohort Study | 24 AERD patients who underwent ESS and AD who failed previous medical therapy and AD | Assess whether recent ESS improved aspirin treatment outcomes in AERD patients who initially failed AD | Rhinoconjunctivitis Quality of Life | Unilateral or bilateral maxillary antrostomy, total ethmoidectomy, sphenoidotomy, frontal sinus procedures | Aspirin desensitization beginning with 650 mg BID. After 4 weeks increased to 975 mg daily for 8 weeks. Maintenance dose of 325 mg BID AD preoperatively and beginning 3–4 weeks after surgery |
6 | 2b |
Subjective outcomes
For our primary research question, we aimed to determine whether aspirin desensitization following sinus surgery would change the subjective quality of life (QoL) and symptom profile of patients with AERD.
Quality of life
Sino-Nasal Outcomes Test (SNOT) is a corroborated, symptom-based questionnaire that evaluates nasal, sinus, behavioral and psychological metrics on a 0 (absent problem) to 5 (severe problem) survey response scale.8 Larger SNOT scores represent increased health burden affecting quality of life in the context of rhinosinusitis-related disease. Three studies weighed the impact of post-ESS aspirin desensitization on SNOT scores: two utilizing the SNOT-22 survey, and one using the SNOT-20 survey.
In a study by McMains and Kountakis,9 prospectively collected data were evaluated on patients undergoing revision ESS between 1999 and 2001. They identified fifteen patients that met criteria for AERD and were included in the study. Five patients underwent aspirin desensitization (AD) following endoscopic sinus surgery and ten patients underwent revision surgery alone (ND). Aspirin desensitization was performed according to the protocol described by Stevenson.10 However, the timing for initiation of aspirin desensitization was not specified. SNOT-20 symptom scores were recorded prior to surgery and following aspirin desensitization. Preoperative CT scans were graded according to Lund–Mackay grading scale,11 and endoscopy exams were scored according to the Rhinosinusitis Task Force methodology both pre- and postoperatively. The patients were followed for a minimum of twenty-four months. The results of the study are outlined in Table 2. In the aspirin desensitized group, SNOT-20 scores improved from 32 ± 3.6 preoperatively to 7.3 ± 1.7 postoperatively. The SNOT-20 scores for the surgery alone group improved from 31.8 ± 3.9 preoperatively to 8.8 ± 1.7 postoperatively. This study demonstrated similar improvement in postoperative SNOT scores with the aspirin desensitized group and the surgery alone group; however, the results were not statistically significant.
Table 2.
Study outcomes.
| Source | N= (# of AERD patients that completed the study) | Subjective Outcomes | Objective Outcomes | Follow up (months) |
|---|---|---|---|---|
| Adappa et al | 30 | SNOT-22 Scores mean (95% CI;p-value) | Revision Surgery Rate: | 30 |
| Preop: 47.0 (39.0–55.1) | 3/32 (9.4%) | |||
| 4 weeks postop: 15.2 (7.3–23.1; p < 0.001 comparing preop to postop SNOT-22) | ||||
| 1 month post-DS: 20.7 (12.1–29.3; p = 0.241 comparing preop to postop 1 month) | ||||
| 6 months post-DS: 21.0 (12–30; p = 0.245) | ||||
| 12 months post-DS: 17.2 (8.8–25.6; p = 0.669) | ||||
| 18 months post-DS: 26.2 (16.7–35.7; p = 0.037) | ||||
| 24 months post-DS: 22.3 (12.8–31.0; p = 0.188) | ||||
| 30 months post-DS: 22.6 (11.6–33.7; p = 0.218) | ||||
| Cho et al. | 21 | SNOT-22 Scores: mean (standard deviation;p-value) | Endoscopic polyp Score: Mean (SD) | 30 |
| Preop: 53.4 (12.4) | Preop: 5.6 (1.2) | |||
| 1 week postop: 14.5 (4.5; p = 0.042 preop-postop) | 1 week postop: 0.3 (0.5) (p < 0.001) preop-postop) | |||
| 4 weeks postop: 11.6 (2.5; p = 0.046 comparing preop to 4 weeks postop) | 4 weeks postop: 0.6 (0.8) (p = 0.001 comparing preop to 4 weeks postop) | |||
| 1 month post-DS: 11.0 (2.3) | 1 month post-DS: 0.5 (0.9) | |||
| 6 months post-DS: 9.2 (2.1) | 6 months post-DS: 0.3 (0.7) | |||
| 12 months post-DS: 8.9 (1.7) | 12 months post-DS: 0.6 (0.7) | |||
| 18 months post-DS: 8.6 (1.8) | 18 months post-DS: 0.8 (0.5) | |||
| 24 months post-DS: 8.7 (1.6) | 24 months post-DS: 0.7 (0.5) | |||
| 30 months post-DS: 8.9 (1.7) | 30 months post-DS: 0.6 (0.4) | |||
| Fruth et al | 31 | Quality of life questionnaire: median | Nasal polyposis Score: Median | 36 |
| (18/31 aspirin group, | Aspirin group: 46.0 | Aspirin group: 0 | ||
| 13/31 placebo group) | Placebo group: 68.6 | Placebo group: 2 | ||
| P = 0.0324 | P = 0.0702 | |||
| Overall nasal and paranasal complaints: mean | Recurrence of polyps | |||
| Aspirin: 4.3 | Aspirin group: 5/18 (28%) | |||
| Placebo: 2.4 | Placebo group: 8/13 (62%) | |||
| P = 0.0019 | P = 0.0785 | |||
| Quality of life impairment by nasal and sinus complaints: mean | Revision Surgery Rate | |||
| Aspirin: 4.0 | Aspirin group: 2/18 | |||
| Placebo:2.4 | Placebo group: 6/13 | |||
| P = 0.0083 | ||||
| General health condition: mean | ||||
| Aspirin:2.2 | ||||
| Placebo: 3.3 | ||||
| P = 0.029 | ||||
| Havel et al | 89 (56 aspirin desensitized, 33 surgery alone) |
QoL scores(18 months AD; 18 month ND) Nasal obstruction 1.77; 2.58 Rhinorrhea 2; 2.35 Post nasal drip 2; 2.58 Sneezing 1.96; 1.93 Sense of smell 2.94; 3.55 Snoring 2; 2.03 Dry Mouth 1.56; 2.48 Hoarse throat 1.32; 1.53 Facial pain 1.54; 2.21 Short breath 1.87; 1.97 Chest tightness 1.64; 1.69 Dyspnea 1.5; 1.45 Cough 1.57; 1.86 Sleep disturbance 1.6; 2.43 Frustrated 1.73; 2.4 Irritable 1.67; 2.3 Sad 1.43; 2.13 Tissue use 2.16; 2.87 |
Endoscopy Score Preop: 5.6 ± 1.2 Postop: 0.6 ± 0.4 Surgery alone group Postop: 1.0 ± 0.18 |
18 |
| McMains and Kountakis | 15 | SNOT-20 Scores | Nasal endoscopy scores: | 24 |
| (5 aspirin desensitized, 10 surgery alone) | AD group | AD Group: | ||
| Preop: 32.0 ± 3.6 | Preop: 7.6 ± 1.3 | |||
| Postop: 7.3 ± 1.7 | Postop: 1.1 ± 0.4 | |||
| Surgery alone group | Surgery alone group | |||
| Preop: 31.8 ± 3.9 | Preop: 7.6 ± 1.2 | |||
| Postop: 8.8 ± 1.7 | Postop: 2.0 ± 0.4 | |||
| Revision Surgery Rate | ||||
| AD Group: | ||||
| 0/5 | ||||
| Surgery alone group | ||||
| 8/10∗ (p = 0.003) | ||||
| Shah et al | 24 |
Rhinoconjunctivitis QoL score (preop; postop;pvalue) 2.5; 0.7; p < 0.001 Asthma Control Test (preop; postop;pvalue) 22; 19.5; p = 0.1 |
Nasal Peak Flow (preop; postop;pvalue) 140; 100; p = 0.01 FeNO (preop; postop;pvalue) 45; 38; p < 0.05 |
6 |
Adappa et al12 performed a retrospective review of thirty-two AERD patients that underwent complete endoscopic sinus surgery followed by aspirin desensitization. The patients underwent aspirin desensitization four to six weeks following ESS. SNOT-22 scores were recorded preoperatively, four weeks postoperatively, then at 1, 6 12, 18, 24, and 30 months post-desensitization. The need for revision surgery within the thirty months was recorded. Results are outlined in Table 2. SNOT-22 scores showed a significant decrease from 47.0 (95% CI 39.0–55.1) preoperatively to 15.2 (7.3–23.1; p < 0.001) four weeks postoperatively. The SNOT-22 scores remained statistically unchanged from the initial postoperative score throughout the follow-up period.
A retrospective study by Cho et al13 included twenty-one AERD patients who underwent endoscopic sinus surgery followed by aspirin desensitization. SNOT-22 scores were recorded preoperatively, postoperatively prior to aspirin desensitization and at 1, 6, 12, 18, 24, and 30 months following aspirin desensitization. The mean preoperative SNOT-22 scores were 53.4 ± 12.4). After endoscopic sinus surgery but prior to desensitization, the SNOT-22 scores were significantly reduced to 14.5 ± 4.5 (p = 0.042) and 11.6 ± 2.5 (p = 0.046) at 1 and 4 weeks, respectively. This improvement in symptom scores remained stable throughout the follow-up period.
Fruth et al14 conducted a placebo-controlled double-blinded study allocating AERD patients following endoscopic sinus surgery to either aspirin desensitization group or placebo group (surgery alone). Subjective outcomes measured were the rhinosinusitis disability index (RDSI) questionnaire and non-validated questions regarding “overall nasal and paranasal sinus complaints”, “quality of life impairment by nasal and paranasal complaints”, and “general health condition” during aspirin desensitization. Thirty-one AERD patients were included in the study. Eighteen patients were allocated to the aspirin desensitization group and thirteen in the placebo group. Following surgery and aspirin desensitization, the aspirin group had a significant improvement in quality of life scores compared to the placebo cohort (46.0 versus 68.6; p = 0.0324). Nasal and paranasal complaint score was 2.4, reduced from 4.3 (p = 0.0019). General health scores decreased from 3.3 to 2.2 (p = 0.029), and quality of life impairment by nasal and sinus complaints improved from 4 to 2.4 (p = 0.0083). Subjectively, this study was in strong favor of aspirin desensitization versus endoscopic sinus surgery alone.
In a retrospective chart review of 89 patients, Havel et al compared 56 patients who underwent sinus surgery followed by aspirin desensitization to 33 patients who underwent sinus surgery alone.15 They utilized a modified, pre-validated quality-of-life questionnaire and visual analog scale (VAS) for overall nasal and asthma conditions to compare aspirin desensitization cohort against non-desensitized patients. The following symptoms showed significant differences in their rating of symptoms, ultimately favoring AD: use of tissue paper, frustration, irritability, sadness, sleep disturbance, facial pain, dry mouth, sense of smell, post-nasal drip, and nasal obstruction (all p < 0.05). Of note, none of the pulmonary symptoms (shortness of breath, chest tightness, dyspnea, cough) demonstrated significant difference in ratings between the two groups. Sinonasal symptom scores dropped an average of 1.144 points below baseline, indicating symptomatic improvement. Ratings in the general categories dropped an average of 0.966 points.
Shah et al16 performed a prospective cohort study evaluating 24 patients that previously failed aspirin desensitization. These patients subsequently underwent endoscopic sinus surgery (ESS) and postoperative aspirin desensitization. There was a significant improvement in the rhinoconjunctivitis quality of life questionnaire immediately following surgery compared to baseline (0.7 vs. 2.5 p < 0.001). This improvement was sustained at all time points following aspirin sensitization. The asthma control test (ACT) score showed no significant improvement following ESS from baseline (22 vs. 19.5 p = 0.1). This remained stable at all time points following aspirin desensitization.
Objective outcomes
In the placebo-controlled double-blind study by Fruth et al used an endoscopic nasal assessment to quantify nasal polyp recurrence was used. As previously described, the two groups in this study included an aspirin desensitized patient group following surgery and a placebo group with surgery alone. In the placebo group, 62% (8/13) of patients had recurrence of polyps versus 28% (5/18) of the aspirin desensitization group. Nasal polyp score in the aspirin group showed a median of 0, and a median of 2 in the placebo group. While the aspirin desensitization group trended toward improvement in polyp recurrence rate and nasal polyp score (these results were not statistically significant with p value of 0.0785 and p = 0.0702, respectively).
In the retrospective study by Cho et al, endoscopic polyp scores following surgery, but prior to aspirin desensitization showed statistically significant improvement from 5.6 ± 1.2 to 0.3 ± 0.5 (p < 0.001) and 0.6 ± 0.8 (p = 0.001) at 1 and 4 weeks postoperatively, respectively. The endoscopic polyp scores remained improved at all time points post-desensitization.
Havel et al analyzed aspirin desensitization outcomes using the Rasp polyp grading system. The aspirin desensitization group showed significant improvement in postoperative rasp polyp scores compared with preoperative scores (1.47 ± 0.186 versus 3.47 ± 0.11, p < 0.001). The surgery alone group had significantly higher rate of polyp recurrence compared to the aspirin desensitization group (p < 0.001). None of the fifty-six patients who completed aspirin desensitization required surgical revision at 18-month follow up. By comparison, seven of thirty-three (21%) of the non-desensitized patients necessitated revision.
In the study by McMains and Kountakis,9 the endoscopy scores in the aspirin desensitization group were 7.6 ± 1.3 preoperatively and 1.1 ± 0.4 postoperatively. In the surgery alone group, preoperative and postoperative endoscopy scores were 7.6 ± 1.2 and 2.0 ± 0.4. There was no difference in postoperative endoscopy scores between the aspirin desensitized group and surgery alone. Additional revision surgery was required in eight of ten patients that underwent surgery alone. None of the five patients that underwent aspirin desensitization following surgery required revision surgery in the follow up period (p = 0.003).
The study by Adappa et al demonstrated similar reduction in revision surgery rates. Of the thirty-two patients that underwent aspirin desensitization, only three (9.4%) required additional surgery. Notably, one of these revisions was required one year after discontinuation of aspirin therapy.
Shah et al analyzed nasal peak flow, FEV1, serum eosinophil count, and serum IgE at baseline and all time points following aspirin desensitization.16 Nasal peak flow was significantly improved immediately postoperatively (140 vs. 100 L/min, p = 0.01) and remained stable at all time points. There was no significant improvement in FEV1 following ESS (78% vs. 81%, p = 0.8) or following AD. Serum eosinophil counts decreased initially following ESS (0.6 vs 0.3, p < 0.01). They then increased from post-ESS to 4 weeks following aspirin therapy (0.3 vs 0.5, p < 0.05). Serum IgE levels remained unchanged from baseline to any point post-endoscopic sinus surgery. FeNO levels decreased significantly from pre-to post-ESS (45 vs 28 ppb, p < 0.01). However, FeNO levels increased significantly from post-ESS to 4 weeks after initiation of aspirin therapy (28 vs. 38 ppb, p < 0.05), but then remained stable at 12 and 24 weeks after aspirin therapy.
Discussion
AERD represents a significant challenge in management of its disease process. It is characterized by a triad of adult-onset asthma, chronic sinusitis with nasal polyposis, and respiratory reactions to COX-1-inhibiting medications. A multi-pronged strategy with endoscopic sinus surgery, medical therapy, and aspirin desensitization are often needed to yield quality outcomes. Aspirin desensitization has proven to be a safe and effective method for treatment of AERD. A systematic review by Xu et al demonstrated significant improvement in health-related quality of life, improved olfaction, decreased rates of revision sinus surgery and decreased systemic corticosteroid use in patients that underwent aspirin desensitization.17 Surgical therapy for treatment of AERD has also shown significant improvement in objective sinonasal symptom scores, improved quality of life, and improved nasal endoscopy scores postoperatively.18
Advances in endoscopic sinus surgery have led to consistent improvements of symptom profile in this population. However, long-term management is needed as AERD patients can require repeated surgical revisions, as polyp recurrence and worsening rhinosinusitis manifest. These patients often benefit from a multifaceted approach that encompasses surgery and parallel preoperative and postoperative medical management. To date, there are no systematic reviews analyzing subjective and objective outcomes in patients that have undergone aspirin desensitization following endoscopic sinus surgery. This is the first systematic review study to evaluate outcomes of ESS followed by AD. Several studies included in this review show statistical improvement in SNOT-20 and SNOT-22 scores at the 4 week postoperative period,9,12,13 and these symptom scores remain stable following aspirin desensitization and maintenance therapy of up to 30 months.
While there was significant heterogeneity in the evaluation and reporting of subjection outcomes, the studies showed universal improvement in symptom scores following surgery and aspirin desensitization. The study by Havel et al compared aspirin desensitized group versus a control group, and their results demonstrated significant improvement in several sinus-related symptoms and overall sinonasal symptoms by visual analog scale when compared to the control group. Revision surgery rates are significantly lower in groups that underwent aspirin desensitization following endoscopic sinus surgery versus sinus surgery alone.9 In the study by Adappa et al, three of the thirty-two patients required revision surgery after aspirin desensitization, one of which had interrupted the aspirin maintenance therapy. This suggests that postoperative aspirin desensitization and maintenance therapy reduces long term need for revision sinus surgery. The study by Shah et al, suggests that endoscopic sinus surgery may actually improve responsiveness for aspirin desensitization in those patients that previously failed aspirin desensitization.16
This article is limited by the biases inherent to a systematic review. There was no standardized approach or reporting of the extent of endoscopic sinus surgery. Thus, it is difficult to determine the optimal extent of surgery. There was also heterogeneity in the protocol for aspirin desensitization. Tapers and maintenance doses differed from study to study, with follow up times not demonstrating a uniform pattern. High quality randomized controlled trials will help elucidate which aspirin dosing regimens are best and will serve to guide timing of drug delivery postoperatively. This systematic review indicates that endoscopic sinus surgery and aspirin desensitization plays an important role and should be considered a mainstay in the treatment of this condition. Future studies evaluating new biologic agents will likely have an effect on the long-term management of this disease process as well.
Conclusions
AERD continues to present a challenge for effective long-term management. Symptomatic improvement through ESS can be further enhanced with adjunct postoperative therapies. This study validates the benefit of postoperative aspirin desensitization for this patient population and also demonstrates that patients who previously failed aspirin desensitization achieve better results with aspirin desensitization following ESS. Specifically, these results display progress in AERD symptomatology scores as well as measures of surgical revision and response to aspirin treatment. This systematic review, the first examining outcomes of ESS followed by AD, demonstrates that endoscopic sinus surgery followed by aspirin desensitization produces lasting subjective and objective results for disease control and quality of life in patients with AERD. Future studies are needed to further elucidate optimal regimens for permanent symptomatic control.
Declaration of competing interest
None.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Mascia K., Borish L., Patrie J., Hunt J., Phillips C.D., Steinke J.W. Chronic hyperplastic eosinophilic sinusitis as a predictor of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;94:652–657. doi: 10.1016/S1081-1206(10)61323-3. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J.A. Aspirin sensitivity: lessons in the regulation (and dysregulation) of mast cell function. J Allergy Clin Immunol. 2019;144:875–881. doi: 10.1016/j.jaci.2019.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinke J.W., Bradley D., Arango P. Cysteinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: importance to eosinophilia and asthma. J Allergy Clin Immunol. 2003;111:342–349. doi: 10.1067/mai.2003.67. [DOI] [PubMed] [Google Scholar]
- 4.Adamjee J., Suh Y.J., Park H.S. Expression of 5-lipoxygenase and cyclooxygenase pathway enzymes in nasal polyps of patients with aspirin-intolerant asthma. J Pathol. 2006;209:392–399. doi: 10.1002/path.1979. [DOI] [PubMed] [Google Scholar]
- 5.Li K.L., Lee A.Y., Abuzeid W.M. Aspirin exacerbated respiratory disease: epidemiology, pathophysiology, and management. Med Sci (Basel) 2019;7:45. doi: 10.3390/medsci7030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad O.G., Lee J.H., Fasano M.B., Graham S.M. Sinonasal outcomes after endoscopic sinus surgery in asthmatic patients with nasal polyps: a difference between aspirin-tolerant and aspirin-induced asthma. Laryngoscope. 2008;118:1282–1286. doi: 10.1097/MLG.0b013e318170af1e. [DOI] [PubMed] [Google Scholar]
- 7.Jang D.W., Comer B.T., Lachanas V.A., Kountakis S.E. Aspirin sensitivity does not compromise quality-of-life outcomes in patients with Samter's triad. Laryngoscope. 2014;124:34–37. doi: 10.1002/lary.24220. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins C., Gillett S., Slack R., Lund V.J., Browne J.P. Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 9.McMains K.C., Kountakis S.E. Medical and surgical considerations in patients with Samter's triad. Am J Rhinol. 2006;20:573–576. doi: 10.2500/ajr.2006.20.2913. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson D.D. Aspirin desensitization. N Engl Reg Allergy Proc. 1986;7:101–104. doi: 10.2500/108854186779047708. [DOI] [PubMed] [Google Scholar]
- 11.Lund V.J., Mackay I.S. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 12.Adappa N.D., Ranasinghe V.J., Trope M. Outcomes after complete endoscopic sinus surgery and aspirin desensitization in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol. 2018;8:49–53. doi: 10.1002/alr.22036. [DOI] [PubMed] [Google Scholar]
- 13.Cho K.S., Soudry E., Psaltis A.J. Long-term sinonasal outcomes of aspirin desensitization in aspirin exacerbated respiratory disease. Otolaryngol Head Neck Surg. 2014;151:575–581. doi: 10.1177/0194599814545750. [DOI] [PubMed] [Google Scholar]
- 14.Fruth K., Pogorzelski B., Schmidtmann I. Low-dose aspirin desensitization in individuals with aspirin-exacerbated respiratory disease. Allergy. 2013;68:659–665. doi: 10.1111/all.12131. [DOI] [PubMed] [Google Scholar]
- 15.Havel M., Ertl L., Braunschweig F. Sinonasal outcome under aspirin desensitization following functional endoscopic sinus surgery in patients with aspirin triad. Eur Arch Otorhinolaryngol. 2013;270:571–578. doi: 10.1007/s00405-012-2048-x. [DOI] [PubMed] [Google Scholar]
- 16.Shah S.J., Abuzeid W.M., Ponduri A. Endoscopic sinus surgery improves aspirin treatment response in aspirin-exacerbated respiratory disease patients. Int Forum Allergy Rhinol. 2019;9:1401–1408. doi: 10.1002/alr.22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J.J., Sowerby L., Rotenberg B.W. Aspirin desensitization for aspirin-exacerbated respiratory disease (Samter's Triad): a systematic review of the literature. Int Forum Allergy Rhinol. 2013;3:915–920. doi: 10.1002/alr.21202. [DOI] [PubMed] [Google Scholar]
- 18.Adelman J., McLean C., Shaigany K., Krouse J.H. The role of surgery in management of samter's triad: a systematic review. Otolaryngol Head Neck Surg. 2016;155:220–237. doi: 10.1177/0194599816640723. [DOI] [PubMed] [Google Scholar]