Abstract
Mechanical ventilation is a lifesaving intervention in critically ill preterm and term neonates. However, it has the potential to cause significant damage to the lungs resulting in long-term complications. Understanding the pathophysiological process and having a good grasp of the basic concepts of conventional and high-frequency ventilation is essential for any medical or allied healthcare practitioner involved in the neonates’ respiratory management. This review aims to describe the various types and modes of ventilation usually available in neonatal units. It also describes recommendations of an individualized disease-based approach to mechanical ventilation strategies implemented in the authors’ institutions.
Keywords: Mechanical ventilation, Pulmonary mechanics, Volume targeted ventilation, High frequency ventilation, Lung injury
1. Introduction
Mechanical ventilation aims to achieve adequate gas exchange. There is a growing body of evidence to avoid invasive mechanical ventilation via the endotracheal tube whenever feasible. The indications for intubation and invasive mechanical ventilation are severe respiratory failure, as evidenced by severely impaired oxygenation and alveolar ventilation, reduced respiratory effort, and circulatory failure in certain instances [1]. Once the decision for invasive mechanical ventilation is taken, steps to minimize ventilation-induced lung injury (VILI) should be considered by choosing the appropriate mode and modality of ventilation and appropriate settings. The choice of conventional versus high-frequency ventilation is guided by the pathophysiology of the underlying disease and institutional practice. Depending on the neonatal respiratory disease static and dynamic compliance of the lungs, airway resistance, alveolar surface tension, work of breathing, and time constant may be very different [2]. This review article is focused on discussing the different modes and modalities of ventilation commonly used in neonatology. It provides (patho)physiology-based guidance for selecting appropriate invasive ventilatory supports in some common neonatal respiratory pathologies. For the purpose of this review a breath/inflation is defined as the inspiratory part of the respiratory cycle where a breath is a spontaneous breath and an inflation is defined as a ventilator-generated breath.
2. Conventional ventilation
The modalities of conventional ventilation are pressure targeted, volume targeted, and hybrid ventilation. In pressure-controlled (PC) ventilation, the ventilator delivers a flow of gas until the pressure set by the operator is delivered to the baby. Similarly, in the volume-controlled (VC) ventilation, the ventilator delivers the volume set by the operator [3,4].
2.1. Pressure targeted ventilation
Pressure targeted ventilation for respiratory failure in neonates has been the most commonly used method for several decades. The gas is driven to achieve the peak pressure set to overcome the resistance of airways and the lung parenchyma, and the elastic forces to open the lung and to deliver the volume of gas (tidal volume). The flow waveform in PC ventilation is a sine wave type. However, one has to monitor the changing lung compliance to avoid under- or over-ventilation due to atelectasis or hyperinflation. In premature infants with respiratory distress syndrome (RDS), improving lung compliance after surfactant replacement may rapidly expand the previously atelectatic lung. Hence, with every inflation, a larger tidal volume will be delivered. This can result in over-inflation unless the tidal volumes generated are closely monitored, and the operator decreases the set peak pressure. Similarly, if the lung compliance decreases with disease progression, the dialed-in peak pressure might not deliver the desired tidal volume and result in progressive atelectasis. Both, under- or over-inflation might not be picked up unless monitored by regular blood gases or careful review of pulmonary graphics (including tidal volume monitoring) on modern ventilators.
2.2. Volume targeted ventilation
Advances in microprocessor technology have resulted in the ability to measure and deliver small volumes, and hence the desired volume can be targeted for ventilation in small premature infants. It is suggested that the range of neonatal tidal volume in a normal-sized lung is probably 4–6 ml/kg. Larger tidal volumes would increase VILI risk by overdistension, whereas a lower tidal volume may result in lung collapse and VILI by atelectotrauma. In volume-targeted ventilation, the ventilator will either increase or decrease the peak pressure to deliver the volume set by the operator based on the compliance of the lung and resistance of the pulmonary unit. The flow waveform in VC ventilation is typical of square wave type. Volume targeted ventilation is now the preferred mode of invasive ventilation in newborn infants to limit VILI. It has been shown to reduce bronchopulmonary dysplasia (BPD), severe intraventricular hemorrhage, duration of ventilation, pneumothorax, and mortality in preterm infants [5].
2.3. Hybrid ventilation
The conventional hybrid ventilation modes combine the benefits of delivering the desired tidal volume using pressure-limited ventilation. The principle advantage is that this allows to correct for losses of inspired volume secondary to leak. Hybrid ventilation is achieved by breath to breath adjustment of pressure based on the expired tidal volume of the preceding breaths by either increasing or decreasing peak pressure to deliver set volume. VG (volume guarantee), TTV (targeted tidal volume), PRVC (pressure regulated volume control), VAPS (volume assured pressure support) are hybrid modes available on various ventilators, and these modalities adjust by using different software algorithms [6,7].
3. Important features to understand in mechanical ventilation
To understand the mechanical ventilation, one must understand three crucial parameters which control the phases of mechanical inflation.
3.1. Trigger and synchronization
Triggering controls the initiation of inspiration in synchronized modes of ventilation. Flow sensors between the endotracheal tube and the Y-piece of the respiratory circuit can detect a small movement of gas initiated by the patient’s spontaneous inspiratory breath and may be used to trigger the ventilator to generate inflation support. In essence, triggering tries to synchronize the baby’s initiation of breath with the beginning of the ventilator inflation, delivering the preset pressure or volume. Synchronization is one of the few advances in ventilation technology that has shown in studies to improve at least short-term outcomes [[8], [9], [10]]. Most ventilators use flow triggering, i.e., a small negative flow to the lungs generated by the baby triggers the ventilator (once the negative flow reaches the set trigger level). Ideally, the trigger should be set appropriately and optimally for different babies of different weights. The trigger threshold should be above artifacts generated by humidity, secretions, etc. If the baby is breathing at a high rate, one of the mechanisms to address this could be to adjust the trigger sensitivity to prevent high rates of triggered inflations. However, this would increase the work of breathing, which may not be in the desired effect. Therefore, before increasing the threshold in this setting, one needs to rule out auto triggering due to condensation of moisture in the circuit and their movements leading to inappropriate triggering.
3.2. Limiting the inflation
Once the inspiratory valve opens after inspiratory synchronization, the ventilator will limit the delivery of gas based on the modality of ventilation – pressure or volume. In volume ventilation, the inspiratory gas flow is controlled, whereas, in pressure ventilation, the set peak pressure is altered to limit the flow of gas along the pressure gradient. After a set interval, depending on the mode of ventilation, which can either be controlled by time or flow, the ventilator opens the expiratory valve allowing cycling to expiration.
3.3. Cycling
Cycling controls the initiation of expiration. Ventilators can be set to cycle by time or flow. Therefore, in a time cycled ventilator mode, the inspiration ends at the end of preset inspiratory time (e.g., 0.3 s), when the expiration will start. In time cycled ventilation, there might be an ‘inspiratory hold,’ i.e., the baby is ready to exhale, but the expiratory valve will only open after the set inspiratory time is reached. In comparison, once the set volume of gas is delivered during flow cycling, the inspiratory flow will slow down, and the ventilator ends the inspiration (e.g. at a certain percentage of peak flow).
4. Modes of ventilation
In pressure controlled or targeted ventilation, a peak inspiratory pressure (PIP) is set by the operator. The ventilator delivers the flow required to deliver the pressure during the preset inspiratory time. The volume delivered in this type of ventilation varies depending on the compliance of the lung/respiratory system if the inspiratory time is sufficient to obtain pressure equilibrium between the ventilator circuit and the alveoli. Positive end-expiratory pressure (PEEP) is the constant pressure present during expiration. It helps to keep the lung distended at the optimum functional residual capacity.
4.1. Synchronized intermittent mandatory ventilation (SIMV) mode
In SIMV mode, the ventilator will provide a preset number of mandatory inflations. However, it synchronizes the mandatory inflations with the baby’s spontaneous breath if the baby has any spontaneous breathing effort within the triggering window and if the effort is strong enough to trigger the ventilator. Patient efforts beyond the set ventilator rate are only supported with PEEP. By definition, there may be uneven tidal volumes, especially in the acute phase of lung disease and increased work of breathing during weaning due to high airway resistance. Reducing the set ventilator rate in this mode will result in more unsupported spontaneous breaths through the relatively high resistance of the endotracheal tube, which may result in weaning failure once the operator has reduced the rate of mandatory inflations. In this mode, the operator controls the pressures (PIP and PEEP), the inspiratory time (i-time), trigger sensitivity, and the rate of mechanical inflations. Taking a spontaneous breath during early opening the triggering window will result in a somewhat higher rate of mandatory breaths than if the baby is apneic, leading to some variation of the mandatory ventilator rate depending on respiratory effort/rate of the baby.
4.2. Assist control (AC) or synchronized intermittent positive pressure ventilation (SIPPV)
In AC mode, the ventilator will provide support for every spontaneous breath. Usually, a mandatory ventilator rate is provided as a backup, if the baby has a limited respiratory drive. This mode is also called as “SIPPV”. AC provides more consistent tidal volume delivery and decreases the work of breathing compared to modes like SIMV, which supports only a proportion of the spontaneous breaths. In this mode, all breaths are synchronized with the ventilator during inspiration and result in mechanical inflations, unless the spontaneous rate is very high, and the next spontaneous breaths occurs within the mandatory expiratory time given by the ventilator. In AC mode, during the weaning period, one has to wean the PIP as the rate is usually controlled primarily by the neonate. In this mode, the operator controls the pressure (PIP and PEEP) and the inspiratory time. The backup ventilator rate is essential in extremely preterm infants with an irregular respiratory drive to avoid hypoventilation during phases of low respiratory effort/apnea. However, a backup rate set above the infant’s spontaneous rate may result in the ventilator to take over full respiratory support by inhibiting the spontaneous respiratory drive.
4.3. Flow cycled pressure-controlled ventilation
In this mode of ventilation, cycling is controlled by the infant’s inspiratory flow and not the preset inspiratory time (unless the backup is initiated due to low spontaneous respiratory effort). As described previously, during flow cycling, the inspiration will end when the inspiratory flow decreases below a certain proportion of the peak inspiratory flow, which can be adjusted in many ventilators. The ventilator detects this as the end of inspiration as driven by the baby. It means the infant controls the inspiratory time and actually may vary with every infant breath, as well as the initiation of expiration may vary in each respiratory cycle. It is called expiratory flow cycling, and some ventilators have previously labeled this as termination sensitivity. Leaks may result in airflow during the inspiratory phase towards the infant even after the inspiration of the lungs is complete; in this case, flow cycling may be affected, and the duration of inspiration will be limited by the preset inspiratory time as chosen by the operator.
4.4. Pressure support ventilation (PSV)
PSV is a flow cycled mode. Flow cycling means that inspiration is terminated when inspiratory flow declines to a preset threshold, usually default settings of 10–15% of peak flow. The main advantage of flow cycling is, it eliminates the inspiratory hold, described previously, and provides more optimal synchrony. Thus, PSV automatically adjusts the inspiratory time to be appropriate to the breathing pattern of the baby. The leak around the endotracheal tube can affect flow cycling, as mentioned before. While changing from time cycled AC ventilation to flow cycled PSV, one should remember that mean airway pressure will drop because the inspiratory time in PSV mode is controlled by the neonate, which is usually shorter. Hence, adequate PEEP should be used to maintain mean airway pressure during this shift, especially if there is significant alveolar lung disease (RDS, Pneumonia). Similar to AC mode, PSV is usually used with a mandatory ventilator rate as backup control if the baby goes into apnoea and will support every breath the baby takes, which are triggered. Therefore, in this mode, the baby controls the inspiratory time, the rate, and the operator only sets the pressure support. Hence this mode is considered more physiological in comparison to SIMV and SIPPV. Some ventilators allow using PSV to support spontaneous breaths between (usually larger) SIMV inflations. This application helps to wean from SIMV. With this application, PSV with relatively low-pressure results in additional support to the increasing number of spontaneous breaths, which mitigates against the increased resistive work of breathing through the endotracheal tube when the rate of SIMV inflations is decreased during weaning [11]. It also suggests that the baby needs to be reasonably well and able to have adequate respiratory effort. One sets the maximum inspiratory time to allow for fluctuation of inspiratory time required to complete the required flow or if there is a large leak preventing automated cycling off. This mode is useful when the baby has sufficient respiratory drive. Otherwise, the ventilator will default to the preset back up respiratory rate with maximum inspiratory time, which may not be lung protective. This mode is very good in assessing the baby’s respiratory drive before extubation with a backup respiratory rate set to a minimum. If the baby develops apnea on this ventilator mode, this implies insufficient respiratory drive, and the infant may not be ready for extubation.
5. Types of volume controlled (VC) ventilation
Preclinical studies suggest that volutrauma causes more VILI than barotrauma (high pressure without large tidal volume). In volume ventilation, the ventilator delivers the preset volume, using pressure over the inspiratory time that has been set. The pressure needed to deliver the volume will vary depending on the compliance. As the compliance improves with the recovery of the lungs, the pressure needed reduces and the baby ‘auto weans.’
5.1. Volume controlled ventilation
In volume-controlled (VC) ventilation, cycling is controlled by delivery of set tidal volume, and inspiratory pressure rises depending on the mechanical characteristics of the respiratory system. In VC ventilation, one regulates the volume of gas delivered into the proximal end of the ventilator circuit, not the volume of gas entering the lungs. It is affected by the compliance of ventilator tubing, the compressible volume of the circuit and humidifier, and the significance of leak around the uncuffed endotracheal tube. In modern ventilators, this loss of volume can be overcome using a separate flow sensor at the airway opening to monitor the exhaled tidal volume. However, the leak around the endotracheal tube is still an issue in modern ventilators that need sophisticated monitoring, which may be affected with extremely large and especially with variable leaks.
5.2. Tidal volume targeted ventilation
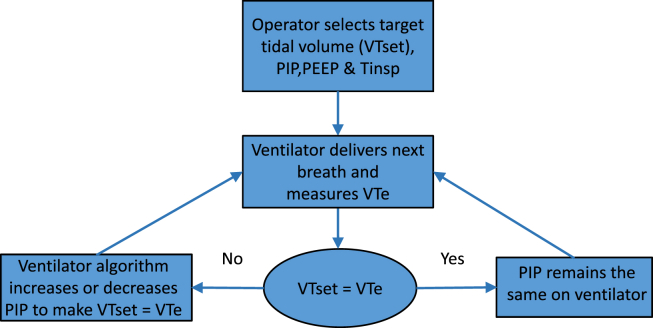
Tidal volume targeted ventilation is designed to deliver the targeted tidal volume by adjusting inspiratory pressure. For every breath, delivered exhaled tidal volume (VTe) is compared with the set tidal volume. The PIP for the next breath is then adjusted up or down to match the set tidal volume. The leak around the endotracheal tube is usually more significant during inspiration, and the measured VTe is usually accurate. If the leak is vast and present during expiration (at PEEP level), then expired air might be lost through the leak and VTe may underestimate accurate tidal volume, but would still regulate peak pressure for the next cycle based on the measured tidal volume during the previous ventilator cycle (Fig. 1). Modern ventilators have features to compensate for leaks, at least for inspiratory leaks. As an additional safety mechanism, inspiration is terminated if inspired tidal volume exceeds 130% of set tidal volume (ventilator-specific).
Fig. 1.
Algorithm for tidal volume targeted ventilation. PIP = peak inspiratory pressure, PEEP = positive end expiratory pressure, VTe = exhaled tidal volume, VTset = target tidal volume.
6. Hybrid modes of ventilation
Hybrid modes try to combine the advantages of various modes to make ventilation more physiological and gentler on the lungs [12].
6.1. SIMV/SIPPV + VG
It is a hybrid mode of ventilation where one can combine volume targeted ventilation with time cycled, a pressure-controlled form of ventilation. In SIMV + VG mode, the set number of breaths will be given with the targeted tidal volume by adjusting PIP based on the previous exhaled tidal volume. As mentioned before, in SIPPV + VG mode, every spontaneous breath triggered by the baby is supported to deliver the targeted tidal volume, whereas, during SIMV + VG, this is only the case for the preset number of mechanical inflations (SIMV rate). Weaning of PIP occurs usually automatically when respiratory function improves and there is sufficient respiratory drive. The maximum PIP becomes smaller and smaller and may not increase significantly above PEEP level, indicating more and more work of breathing being done by the baby.
6.2. PSV + VG
In this mode, VG combines flow-cycled pressure support ventilation to support every ventilator inflation triggered by the baby’s breaths. The advantage is that the baby receives the targeted tidal volume with the required pressure support but can regulate its own inspiratory time and rate. It gains more liberty to increase or decrease minute ventilation when being on flow cycled ventilation. It is important not to keep a high back up rate and to wean rate to a minimum before extubation to assess the respiratory drive’s adequacy. Auto weaning of PIP is similar as during SIPPV + VG.
6.3. SIMV + PS
This mode combines the time cycled ventilation (SIMV) with flow cycled ventilation (PS). The SIMV breaths are to ensure set breaths where adequate pressure (PIP) is delivered during the chosen inspiratory time, which may help recruit recurrent atelectasis. Simultaneously, the spontaneous breaths are supported using PS, which partially compensates for airway resistance and elastance and allows the baby to breathe in a more ‘physiological’ way rather than breathing through a “straw” with high inspiratory resistance (endotracheal tube). The PS level can be adjusted to allow full support to (almost) match the SIMV breaths or minimal support that just overcomes the ET tube resistance. Some experts in the field use this approach for weaning of VLBWI. This may increase minute ventilation, reduce tachypnea, and support the tidal volume of spontaneous breaths, promoting weaning and decreasing the time on mechanical ventilation as suggested in a randomized trial..11 During weaning, one has to reduce the SIMV rate and the pressure support level. During SIMV + PS mode, the PS component does not have any mandatory ventilator rate as backup control if the baby goes into apnoea. The SIMV rate needs to be set to provide adequate backup.
7. High-frequency ventilation (HFV)
High-frequency ventilation is a non-tidal form of ventilation that uses small tidal volumes (lower than the anatomic dead space) and very rapid ventilator rates. Proclaimed benefits of high-frequency ventilation over and above conventional mechanical ventilation modes are the use of lower peak airway pressure, ability to adequately and independently manage oxygenation and ventilation while using low tidal volumes and the preservation of lung architecture even when using high mean airway pressures [13,14]. Furthermore, HFV is usually an extremely efficient way for CO2 elimination due to the active expiratory phase in certain types of ventilators. Primary gas transport mechanisms in HFV include convective gas flow, convection and diffusion, diffusion flow, pendelluft, laminar flow with Taylor dispersion, turbulent flow, cardiogenic mixing, and perialveolar collateral ventilation.
The variables set by the operator for HFV are the frequency (Hz), Mean Airway Pressure (MAP), amplitude, inspiratory time (as a percentage of the respiratory cycle), and FiO2.
It has been recommended to increase MAP on HFV by 1–3 cm of H2O above the mean airway pressure required during conventional ventilation when transferring a neonate with severe lung disease to HFV. Subsequent adjustments are made based on oxygen requirements and optimizing lung expansion based on diaphragm position on chest x-rays to approximately 8–9 posterior ribs. Overdistension of the lung may affect hemodynamics, and functional echocardiography may be useful in cases to assess hemodynamics. Amplitude is initially titrated to achieve adequate chest ‘wiggle’ and maintain CO2 levels in the desired range based on transcutaneous and blood gas measurements. Setting the appropriate frequency based on the underlying disease process is another important step in HFV (Table 1) [15].
Table 1.
HFOV parameter adjustments.
| Condition |
||||
|---|---|---|---|---|
| Poor oxygenation | Over oxygenation | Under ventilation | Over ventilation | |
| 1st choice | ↑↑ FiO2 | ↓↓ FiO2 | ↑↑ Amplitude | ↓↓ Amplitude |
| 2nd choice | ↑↑ MAP (1–2 cmH20) if CXR shows high diaphragm position |
↓↓ MAP (1–2 cmH20) if CXR shows low diaphragm position |
↓↓ Frequency (1–2 Hz) if Amplitude maximal, or if Air Leak present |
↑↑ Frequency (1–2 Hz) if Amplitude minimal |
There are three types of high-frequency ventilation modes currently in use in NICUs.
7.1. High-frequency oscillation ventilation (HFOV)
It is produced by a device that moves air back and forth at the airway opening and provides a limited bulk flow. Both, inspiration and expiration are active. A Cochrane review including randomized controlled trials comparing HFOV and CV in preterm or low birth weight infants with pulmonary dysfunction, mainly due to RDS, who required assisted ventilation revealed no evidence of an effect on mortality at 28–30 days or approximately term equivalent age. However, there may be a small reduction in the rate of CLD with HFOV use, but the evidence is weakened by the inconsistency of this effect across trials and the overall borderline significance [16,17].
7.2. High-frequency jet ventilation (HFJV)
It is produced by ventilators that deliver a high-velocity jet of gas directly into the airway and have passive exhalation. In 2016, a Cochrane review found no evidence to support the superiority of HFJV or HFOV as elective or rescue therapy [18].
7.3. High-frequency flow interruption (HFFI)
It generates pulses of fresh gas and also uses passive exhalation. After the Infant Star has been withdrawn from the market, there is currently no neonatal ventilator available, which is capable of provide HFFI.
7.4. High frequency with volume guarantee ventilation
The volume guarantee in HFV mode is different from VG in CMV. The Dräger Babylog VN500 ventilator offers volume-guaranteed HFOV (HFOV-VG) mode when the delivered high-frequency tidal volume (VThf) can be set. The high-frequency tidal volume is much smaller (1–3 ml/kg), i.e., with tidal volumes usually smaller than dead space. HFO + VG mode can be used to maintain a stable tidal volume in HFV by matching the required amplitude to lung compliance changes. Belteki G et al. reported that during HFOV-VG, the tidal volume of oscillations varies in the short term but is maintained very close to the target over the longer term [19]. It may provide advantages for blood gas targeting, but this mode of ventilation is currently considered investigational. Operator should be aware of effects of change of frequency in this mode of ventilation (HFOV+VG) as effects are opposite of pure HFOV ventilation. In pure HFOV ventilation decreasing frequency will eliminate more CO2 and vice versa where as effects are opposite once VG is added to HFOV.
8. Neurally adjusted ventilatory assist (NAVA)
NAVA involves using the electrical signal from the baby’s diaphragm to synchronize the mandatory ventilator assisted inflation with the spontaneous breath. The delivered ventilator pressure is proportional to the baby’s respiratory effort within each point in time of each spontaneous breath. Thus, the ventilator pressure is actually “tailored” to the individual needs during the respiratory cycle.
NAVA has been proven to provide respiratory support in clinical studies looking at short-term variables [20,21]. NAVA may be particularly useful for non-invasive ventilation as it uses a sensor technique independent of airflow. Because of the approach to “tailor” ventilator pressure to spontaneous airflow, the baby requires less pressure from the ventilator, which may cause less damage to the lungs, better tidal volume, and less need for sedation medications. A Cochrane review 2017 reported no significant difference in outcomes of interest between NAVA and patient triggered time cycled pressure limited ventilation in neonatal respiratory support [22]. More controlled studies are warranted before this technique can be applied in routine clinical practice.
9. Suggested guidelines for the initial approach to mechanical ventilation by lung condition and ventilatory mode
The most common lung conditions that present with respiratory distress are discussed below with different types of assisted ventilation modes and ventilatory settings [[23], [24], [25], [26], [27], [28]].
9.1. Respiratory distress syndrome (RDS)
Surfactant and respiratory support (if needed) are the main components of treatment for RDS. It is recommended to start with PC-AC/SIPPV + VG as one is not sure about the initial severity of the disease, and once pathology improves, wean on PSV + VG or to SIMV + PS + VG (personal preference).
9.1.1. Pathophysiological basis of recommendations in RDS
RDS lungs have low compliance and low resistance and thus a short time constant. Low compliance needs an adequate volume of air/oxygen to open the lungs (driven usually by flow and pressure with adequate inspiratory time. Low resistance and short time constant results in quick emptying and collapse of the lung in expiration. This is managed by counter-acting with adequate PEEP. Because of the short time constant, a higher ventilator rate can be used, which may help to prevent atelectasis during the expiratory phase and to limit PIP.
9.1.2. Conventional mechanical ventilation (CMV)
We use volume targeted ventilation with AC or SIMV + PS, or PSV mode of ventilation in CMV. Recommended CMV settings are as follows;
-
•
Targeted tidal volume (VT) 4–6 ml/kg
-
•
Backup rate between 30 and 60 inflations per minute
-
•
Inspiratory Time (Ti) of 0.30–0.35 s
-
•
Positive End Expiratory Pressure (PEEP) of 5–8 cm H2O
-
•
Pressure support (PS) to achieve 50–75% of set VT during SIMV/backup breaths
9.1.3. High-frequency ventilation
Recommended HFOV settings are as follows:
-
•
Mean airway pressure between 10 and 16 cm of H2O, usually 1–2 cmH2O higher compared to previous CMV settings when switched over. Subsequent adjustments are made based on oxygen requirements and optimizing lung expansion based on diaphragm position on CXR to approximately 8–9 posterior ribs.
-
•
Amplitude – roughly twice as mean airway pressure – adjust to vibrate chest/abdomen and aim for an early blood gas.
-
•
Frequency 8–15 Hz (lower end for mature infants, higher end with very premature infants; most clinical reports used 10 Hz). Especially in ELBWI and PCO2 values on the low side while already on low amplitudes, tidal volume control and thus PCO2 may be improved by increasing frequency to the higher range.
Recommended HFJV settings are as follows;
-
•
Rate between 360 and 420 bpm (breaths per minute)
-
•
PEEP as needed to optimize lung ventilation (usually 7–10 cmH2O)
-
•
Minimal or no backup rate
9.2. Meconium aspiration syndrome (MAS)
We would consider surfactant therapy and inhaled nitric oxide based on additional assessment and as needed to maintain/improve gas exchange. We recommend starting with PC-AC/SIPPV + VG initially and wean on PSV + VG or SIMV + PS + VG once pathology improves (personal preference). Details see below.
9.2.1. Pathophysiological basis of recommendations in MAS
The pathophysiology of MAS depends on the distribution of meconium within the lung. In cases of homogeneous alveolar lung disease, restrictive lung disease dominates. In cases causing mainly inflammation and obstruction of airways, there may be areas of atelectasis next to overinflated areas. In these overinflated areas, there is often a low to normal compliance and high resistance resulting in a longer time constant. Thus, adequate PEEP without overdistension, lower respiratory rates allowing time for exhalation to occur are the usually recommended strategies. Pressure support for spontaneous breaths when the mandatory ventilator breaths are kept low ensures that the baby does not have excess work of breathing and gets exhausted. In cases with severe respiratory failure HFOV may be helpful, especially if there is more restrictive rather than obstructive lung disease.
9.2.2. Conventional mechanical ventilation
We can use volume targeted ventilation with AC or SIMV + PS or PSV mode of ventilation in CMV.
Recommended CMV settings are as follows;
-
•
Targeted tidal volume (VT) 5–6 ml/kg
-
•
Backup rate between less than 30 per minute
-
•
Inspiratory Time (Ti) of 0.35–0.50 s (enough to deliver the tidal volume)
-
•
Positive End Expiratory Pressure (PEEP) of 4–7 cm H2O based on lung inflation
-
•
Pressure support (PS) to achieve 50–75% of set VT
-
•
Ventilator rate: observe expiratory airflow to avoid air trapping
9.2.3. High-frequency ventilation
Recommended HFOV settings are as follows;
-
•
Frequency between 6 and 9 Hz
-
•
Mean airway pressure as needed to aim for adequate lung inflation as judged based on oxygen requirements and lung expansion based on diaphragm position on CXR to approximately 8–9 posterior ribs.
-
•
Amplitude as needed to vibrate chest/abdomen and adjust based on PCO2
Recommended HFJV settings are as follows;
-
•
Rate between 240 and 360 breaths/min
-
•
Increase Ti as needed
-
•
PEEP as needed to optimize lung ventilation and minimal or no backup rate
9.3. Lung hypoplasia and congenital diaphragmatic hernia (CDH)
9.3.1. Pathophysiological basis of recommendations in CDH
The main concern is pulmonary hypoplasia and the current concept of minimizing lung injury to the functioning lung from excessive aggressive ventilator strategies to achieve oxygenation. The guiding principle is to ventilate with adequate PEEP and lowest possible PIP (i.e., a smaller than usual tidal volume) to achieve oxygenation.
9.3.2. Conventional mechanical ventilation
We can use the PC-AC mode of ventilation in CMV.
Recommended CMV settings are as follows;
-
•
Peak Inspiratory Pressure (PIP) less than 25 cm H2O
-
•
Backup rate between 40 and 60 per minute
-
•
Inspiratory Time (Ti) of 0.25–0.40 s
-
•
Positive End Expiratory Pressure (PEEP) of 3–5 cm H2O based on lung inflation (chest X-ray)
9.3.3. High-frequency ventilation
Recommended HFOV settings are as follows:
-
•
The frequency is usually set at 10 Hz
-
•
Mean airway pressure between 10 and 13 cm of H2O initially adjusted based on the diaphragm position on CXR.
-
•
Amplitude – twice as mean airway pressure – adjust to vibrate chest/abdomen
Recommended HFJV settings are as follows:
-
•
Rate between 360 and 420 breaths/min
-
•
PEEP of 5–8 cm H2O as needed to optimize lung ventilation
-
•
Minimal or no backup rate
9.4. Bronchopulmonary dysplasia (BPD) or chronic lung disease (CLD)
In established chronic lung disease, wean on SIMV + PS with or without VG. For intermittent exacerbation, one can go back to AC or AC + VG. As lung compliance is often not decreased and large airways dilate with chronic ventilation, larger (8–12 ml/kg) tidal volumes to overcome dead space may be needed.
9.4.1. Pathophysiological basis of recommendations in BPD/CLD
CLD lungs have (almost) normal compliance and high resistance resulting in a long time constant. Highly compliant lungs are often better managed with larger tidal volumes. Lower ventilator rates may help to avoid air trapping. Bronchoconstriction and/or excessive secretions result in high expiratory resistance and the long-time constant. To allow complete exhalation, low rates and longer expiratory times are needed. Spontaneous breaths may be supported additionally to reduce the work of breathing and reduce energy expenditure. Respiratory infections (i.e., RSV) may cause severe pneumonia or an ARDS-like picture with more restrictive lung disease with a short time constant, which may respond to higher ventilator rates or HFOV.
9.4.2. Conventional mechanical ventilation
We can use volume targeted ventilation with AC or SIMV + PS or PSV mode of ventilation in CMV.
Recommended CMV settings are as follows;
-
•
Targeted tidal volume (VT) 6–12 ml/kg owing to increased dead space
-
•
Backup rate between 20 and 30 per minute – slower to allow adequate lung emptying
-
•
Inspiratory Time (Ti) of 0.50–0.70 s to match the usually longer than the usual time constant (to provide the desired tidal volume and to overcome the airway resistance).
-
•
Positive End Expiratory Pressure (PEEP) of 8–12 cm H2O to stent the airway open (particularly helpful in cases of tracheo/bronchomalacia).
9.5. Pulmonary interstitial emphysema (PIE)
9.5.1. Pathophysiological basis of recommendation in PIE
PIE is commonly seen in ventilated preterm infants, secondary to terminal bronchioles’ tendency to dilate and rupture in the face of atelectasis downstream, especially in inhomogeneous alveolar lung disease. Furthermore, preterm infants have more interstitial space to accommodate extra-alveolar air. Usually, overdistended lung areas (with a longer time constant) tend to rupture. Treatment principles prevent further air leak into the connective tissue sheaths and facilitate the resorption of gas from emphysematous areas while maintaining the adequate expansion of the distal airspaces and ventilation.
9.5.2. Conventional mechanical ventilation
-
•
Minimize airway trauma using short inspiratory times, low inflation pressures, and small tidal volumes. Limiting Ti may result in redirection of air away from overdistended lung areas (with long time constants) to other areas with a short time constants (more homogeneous aeration).
-
•
Expiratory time as long as possible to allow reabsorption of interstitial gas.
-
•
Lateral positioning with diseased lung nursed in dependant position or selective main bronchial intubation in predominantly unilateral disease.
9.5.3. High-frequency ventilation
HFV is widely used in the treatment of PIE. It offers the advantage of achieving adequate gas exchange at a lower mean airway pressure than on conventional ventilation, limiting damage to the distal small airway. The strategy used in PIE is usually a low lung volume rather than a high lung volume strategy (RDS).
-
a.High-Frequency Jet Ventilation (HFJV)
-
•Ideally suited for reabsorption of trapped gas in the emphysematous areas with its short jet pulses and prolonged expiratory phase.
-
•The lack of widespread availability and familiarity with this specialized modality in many institutions limits its use.
-
•
-
b.HFOV settings & strategy
-
•Frequency of 12–15 Hz for the prevention of PIE or early mild changes to minimize shear forces on the epithelium of the distal airspaces.
-
•Frequency of 5–6 Hz in established PIE with poor oxygenation and hypercarbia [28].
-
•Important to wean amplitude appropriately to prevent exposure to the high tidal volume at the distal airway and carbon dioxide washout when switching to the low-frequency strategy for established PIE.
-
•Muscle relaxation (helps prevent larger spontaneous breaths) and the gradual reduction in mean airway pressure over 24–48 h, followed by gentle recruitment once the interstitial air has disappeared.
-
•
10. Conclusions
A good understanding of different types of assisted mechanical ventilation modes with underlying pathophysiology of lung condition will provide optimal respiratory support in critically ill newborn infants. There is limited evidence to actively support one mode or approach over another for many neonatal lung conditions. Thus, a physiology-based approach seems to be reasonable in clinical practice. It is essential to recognize that most of the neonates present with respiratory distress are at different stages of lung development and may be more susceptible to ventilator-induced lung injury. Each NICU should develop local guidelines based on a consensus and on best evidence to limit the variability of approaches often chosen by individual clinicians and thus to facilitate safe and effective ventilator management of sick neonates requiring invasive mechanical ventilation.
Author’s contributions
Dr Aravanan Anbu Chakkarapani contributed to the conception, design of the manuscript, drafted and edited the article.
Dr Roshan Adappa revised the article critically.
Dr Sanoj Karayil Mohammad Ali reviewed the manuscript.
Dr Samir Gupta reviewed the manuscript.
Dr Naharmal B Soni reviewed the manuscript.
Dr Louis Chicoine reviewed the manuscript.
Dr Helmut D Hummler reviewed, edited and supervised the article.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Name of department and institution to which the work should be credited: Division of Neonatology, Department of Pediatrics, Sidra Medicine, Doha, Qatar.
Ethical statement
The authors declare that they have no known ethical issues identified in construction of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Carlo W.A., Martin R.J. Principles of neonatal assisted ventilation. Pediatr Clin. 1986 doi: 10.1016/S0031-3955(16)34977-X. [DOI] [PubMed] [Google Scholar]
- 2.Chakkarapani A.A., Adappa R., Mohammad Ali S.K., Gupta S., Soni N.B., Chicoine L. “Current concepts of mechanical ventilation in neonates” – Part 1: Basics. Int J Pediatr Adolesc Med. 2020 doi: 10.1016/j.ijpam.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keszler M., Mammel M.C. Assist. Vent. Neonate an evidence-based approach to newborn respir. Care. sixth ed. 2017. Basic modes of synchronized ventilation. [DOI] [Google Scholar]
- 4.Keszler M., Chatburn R.L. Assist. Vent. Neonate an evidence-based approach to newborn respir. Care. sixth ed. 2017. Overview of assisted ventilation. [DOI] [Google Scholar]
- 5.Klingenberg C., Wheeler K.I., McCallion N., Morley C.J., Davis P.G. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 2017;10(10):CD003666. doi: 10.1002/14651858.CD003666.pub4. Published 2017 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donn S.M., Sinha S.K. Newer modes of mechanical ventilation for the neonate. Curr Opin Pediatr. 2001 doi: 10.1097/00008480-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Donn S.M., Boon W. Mechanical ventilation of the neonate: should we target volume or pressure? Respir Care. 2009 [PubMed] [Google Scholar]
- 8.Cleary J.P., Bernstein G., Mannino F.L., Heldt G.P. Improved oxygenation during synchronized intermittent mandatory ventilation in neonates with respiratory distress syndrome: a randomized, crossover study. J Pediatr. 1995 doi: 10.1016/S0022-3476(95)70460-4. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein G., Mannino F.L., Heldt G.P., Callahan J.D., Bull D.H., Sola A. Randomized multicenter trial comparing synchronized and conventional intermittent mandatory ventilation in neonates. J Pediatr. 1996 doi: 10.1016/S0022-3476(96)70354-2. [DOI] [PubMed] [Google Scholar]
- 10.Chen J.Y., Ling U.P., Chen J.H. Comparison of synchronized and conventional intermittent mandatory ventilation in neonates. Pediatr Int. 1997 doi: 10.1111/j.1442-200X.1997.tb03644.x. [DOI] [PubMed] [Google Scholar]
- 11.Reyes Z.C., Claure N., Tauscher M.K., D’Ugard C., Vanbuskirk S., Bancalari E. Randomized, controlled trial comparing synchronized intermittent mandatory ventilation and synchronized intermittent mandatory ventilation plus pressure support in preterm infants. Pediatrics. 2006;118(4):1409–1417. doi: 10.1542/peds.2005-2923. [DOI] [PubMed] [Google Scholar]
- 12.Dargaville P.A., Tingay D.G. Lung protective ventilation in extremely preterm infants. J Paediatr Child Health. 2012 doi: 10.1111/j.1440-1754.2012.02532.x. [DOI] [PubMed] [Google Scholar]
- 13.Thome U.H., Carlo W.A. High-frequency ventilation in neonates. Am J Perinatol. 2000 doi: 10.1055/s-2000-7297. [DOI] [PubMed] [Google Scholar]
- 14.Alper C.A. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. N Engl J Med. 1989 doi: 10.1056/NEJM198901123200204. [DOI] [PubMed] [Google Scholar]
- 15.Rimensberger P.C. 2015. Pediatric and neonatal mechanical ventilation: from basics to clinical practice. [DOI] [Google Scholar]
- 16.Cools F., Henderson-Smart D.J., Offringa M., Askie L.M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.cd000104.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Cools F., Henderson-Smart D.J., Offringa M., Askie L.M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD000104.pub4. [DOI] [PubMed] [Google Scholar]
- 18.Ethawi Y.H., Abou Mehrem A., Minski J., Ruth C.A., Davis P.G. High frequency jet ventilation versus high frequency oscillatory ventilation for pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD010548.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belteki G., Morley C.J. High-frequency oscillatory ventilation with volume guarantee: a single-centre experience. Arch Dis Child Fetal Neonatal. 2019 doi: 10.1136/archdischild-2018-315490. [DOI] [PubMed] [Google Scholar]
- 20.Gibu C.K., Cheng P.Y., Ward R.J., Castro B., Heldt G.P. Feasibility and physiological effects of noninvasive neurally adjusted ventilatory assist in preterm infants. Pediatr Res. 2017 doi: 10.1038/pr.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty S., Hunt K., Peacock J., Ali K., Greenough A. Crossover study of assist control ventilation and neurally adjusted ventilatory assist. Eur J Pediatr. 2017 doi: 10.1007/s00431-017-2866-3. [DOI] [PubMed] [Google Scholar]
- 22.Rossor T.E., Hunt K.A., Shetty S., Greenough A. Neurally adjusted ventilatory assist compared to other forms of triggered ventilation for neonatal respiratory support. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD012251.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweet D.G., Carnielli V., Greisen G., Hallman M., Ozek E., Te Pas A. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. 2019 doi: 10.1159/000499361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chettri S., Bhat B.V., Adhisivam B. Current concepts in the management of meconium aspiration syndrome. Indian J Pediatr. 2016 doi: 10.1007/s12098-016-2128-9. [DOI] [PubMed] [Google Scholar]
- 25.Logan J.W., Cotten C.M., Goldberg R.N., Clark R.H. Mechanical ventilation strategies in the management of congenital diaphragmatic hernia. Semin Pediatr Surg. 2007 doi: 10.1053/j.sempedsurg.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Yoder B.A. Assist. Vent. Neonate an evidence-based approach to newborn respir. Care. sixth ed. 2016. Mechanical ventilation: disease-specific strategies. [DOI] [Google Scholar]
- 27.Ambalavanan N., Carlo W.A. Ventilatory strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006 doi: 10.1053/j.semperi.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Squires K.A.G., De Paoli A.G., Williams C., Dargaville P.A. High-frequency oscillatory ventilation with low oscillatory frequency in pulmonary interstitial emphysema. Neonatology. 2013 doi: 10.1159/000353376. [DOI] [PubMed] [Google Scholar]