Abstract
Introduction
Methicillin-resistant Staphylococcus aureus infections have been increasingly reported in patients with cystic fibrosis (CF) who have progressive deterioration in their pulmonary function.
Objectives
To determine the prevalence of MRSA infections in CF in a tertiary care center in Saudi Arabia.
Methodology
This is a retrospective chart review conducted as part of the CF registry data from 1 January 2002 to 1 June 2016. All patients with confirmed CF of all age groups who had a respiratory culture positive for MRSA were included in the study.
Results
Among 385 patients with CF who had respiratory samples, 43 (11%) were positive for MRSA at a mean age of 10.4 ± 7.2 years. Twenty-two patients out of the 43 (51%) were treated with different regimens: nasal Bactroban in 13/22 (59%); a combination of nasal Bactroban, oral vancomycin, and rifampicin for 2 weeks in 5 patients (23%); Bactroban and linezolid in one patient (5%); and oral vancomycin and rifampicin in 3 patients (14%). Eight out of the 22 treated patients (36%) achieved MRSA eradication. Six out of the 22 treated (27%) had experienced MRSA recurrence within 3–6 months, and another 5/22 (23%) continued to have MRSA colonization up to 2–4 years of follow-up despite using a proper eradication protocol. Twelve out of the 43 (28%) patients with MRSA infection died.
Conclusion
MRSA infection in our population with CF is common. Therefore, an eradication protocol should be instituted at an early stage to prevent chronic colonization. Children with persistent MRSA colonization have high morbidity and mortality rate.
Abbreviation list
- MRSA
Methicillin-Resistant Staphylococcus aureus
- CF
Cystic Fibrosis
- BAL
Bronchoalveolar lavage
- NPA
Nasopharyngeal aspirates
- CFTR
Cystic fibrosis transmembrane conductance regulator
- MSSA
Methicillin-Sensitive Staphylococcus aureus
1. Introduction
Cystic fibrosis (CF) is an inherited recessive disorder of chloride transport characterized by recurrent and persistent pulmonary infections due to resistant organisms, resulting in lung function deterioration and early mortality [1]. CF is due to mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [2]. It is the most common life-threatening autosomal recessive disease among Caucasian populations, with a frequency of 1 in 2000–3000 live births [3]. Deletion of phenylalanine in amino acid position 508 (deltaF508) on chromosome 7 is considered the most common mutation in North America and western Europe [4]. By contrast, in Saudi Arabia, the most common mutations described were c.1418delG (p.G473EfsX54), Exon 11 (Legacy name: 1548delG), Exon 10 and c.3700A > G (p.I1234V), Exon 22 (Legacy name: I1234V), Exon 19. [5,6].
Methicillin-resistant Staphylococcus aureus (MRSA) strains are the major causative agents of numerous hospital- and community-acquired infections [7].
MRSA infections have been increasingly reported among populations with CF worldwide [8]. There has been a hypothesis that MRSA infection of the airways in CF could be associated with more severe disease than that seen with methicillin-sensitive Staphylococcus aureus (MSSA) [9]. Patients with MRSA infection were described to have frequent exacerbations and poorer lung function; thus, infection control is important, and patients should be adequately monitored [10]. A chromogenic selective medium for MRSA detection may improve its surveillance in patients with CF [11].
Eradication of MRSA is advised to be applied in most CF treatment centers [12]. Effective microbiological eradication of MRSA in patients with CF can be achieved, but its effect is not always clear-cut in terms of spirometric indices [13]. Intravenous vancomycin, a time-dependent antibiotic that is extensively eliminated by the kidneys, is frequently used to treat MRSA infections. Its therapeutic levels are difficult to achieve in patients with CF because of increased renal clearance [14].
Yurdakul et al. reported the prevalence of MRSA infection among 604 patients with CF in Turkey between October 2003 and January 2010, and the percentage of MRSA-positive patients was 3.9% [7].
Several proposed eradication protocols have been reported thus far (Table 1) [[12], [13], [14], [15], [16]]. Lo, D. et al. found that patients who clear MRSA within one year have the same risk of death as those who never had a positive culture for MRSA, which leads us to the importance of having clear guidelines on how to eradicate MRSA infection in patients with CF [1].
Table 1.
Literature Review on Cystic Fibrosis and MRSA management.
| Author | No. Pts. | Age (Yrs) | Treatment protocol | Eradication success (%) |
|---|---|---|---|---|
| Vanderhelst et al. 2013 [12] | 11 | Range 1-43 | Six-month course:
|
90% Within 6-month period The 10% failure was due to lack of adherence of the treatment |
| Vanderhelst et al. 2013 [13] | 6 | Median 21.4 | Six-month course:
|
83% |
| Fung, L 2012 [14] | 3 | 3,17, and 15 | continuous infusion of Vanco (50/40/31) mg/kg/day respectively every 8 h | Eradication is unknown No Nephrotoxicity |
| Burdge 1995 [15] | 2 | 28 and 31 | Two-month course:
|
100% At 8-month follow up |
| Kiefer A. 2018 [16] | 7 | Range 4-30 | 7-day course:
|
86% |
| Banjar 2019 | 22 | Mean 10 ± 7.2 | 2-weeks course divided:
|
36% |
MRSA: Methicillin Resistant Staphylococcus Aureus. Pts. = Patients, Yrs = Years, NaCl = Sodium Chloride. Vanco = Vancomycin, Rifa = Rfampin, Bac = Bactroban, Clin = Clindamycin. n. = Nasal, o. = Oral, t. = Topical, IV = Intravenous. bid = twice-daily, max. = maximum, FA = Fusidic Acid, No. = number of.
Chmiel J. et al. reviewed lung infections in patients with CF in a two-part series study. They reported that the prevalence of MRSA infection is increasing in the USA, 25% as compared to that of 3–11% in Canada and Europe, and chronic MRSA infection is associated with a high decline rate of lung function, failure to recover lung function after a pulmonary exacerbation, and decreased survival. They found that the most successful treatment regimens included two oral antibiotics (one of which was rifampicin) and nebulized vancomycin, and these drugs were found to be safe and well tolerated [17]. On the other hand, monotherapy developed resistance. They also suggested the use of the fosfomycin tobramycin inhalation (FTI) solution, as it has activity against anaerobic, gram-negative, and gram-positive organisms including MRSA. They also found that the most difficult-to-treat patients with MRSA infection are those with chronic infection and who do not present enough symptoms to start the administration of IV antibiotics but have persistent respiratory symptoms. They suggested the administration of 250 mg of IV formulation of vancomycin in 5 ml of sterile water through nebulization mist treatment, twice daily for 28 days. Albuterol is often inhaled before the administration of the antibiotic [17].
1.1. Objective
This retrospective study is the first of its kind conducted in Saudi Arabia, with an aim to identify, recognize, and determine the prevalence of MRSA infection in patients with CF and to further study the methods and protocols of treatment and prevention.
1.2. Methodology
After obtaining ethical approval, we retrospectively reviewed the charts of 385 patients with confirmed CF of all age groups who had respiratory samples with positive culture for MRSA on regular follow-up or during respiratory exacerbation from January 2002 to June 2016.
1.3. Definitions
Patients with CF are defined as those who have typical pulmonary manifestations and/or typical gastrointestinal manifestations (GI) and/or a history of CF in the immediate family in addition to a sweat chloride concentration of 60 mmol/L or if they have the pathologic CFTR mutations on both chromosomes [18].
1.4. Inclusion criteria
We included all patients with confirmed CF of all age groups who had MRSA infection during their follow-up period in CF clinic from 1 January 2002 to 31 June 2016.
1.5. Types of samples
Nasopharyngeal aspirates (NPA) were collected from patients who were unable to expectorate below the age of 4 years. Induced sputum samples were obtained from patients above 4 years of age. Bronchoalveolar lavage (BAL) samples were collected from patients with severe CF pulmonary disease. MRSA cultures were repeated every 3–6 months until complete clearance of MRSA infection or death.
2. Method of sample collection
Bronchoalveolar lavage and NPA samples were collected for bacterial cultures and processed according to standard methodology [19]. Samples were collected following standard hospital precautions.
2.1. Statistical method
For continuous variables, mean, standard deviation, and median were calculated using the Student t-test. Chi-square was calculated for all nominal variables. Results were presented at a level of significance of P < .05. All values were expressed as mean, standard deviation (SD).
2.2. Method used to assess lung capacity
Pulmonary function test (PFT) was performed according to standard procedure. FEV1 less than 35% predicted for age is considered very severe, 35–49% is severe, 50–59% is moderately severe, 60–69% is moderate, and more than 70% is considered mild [20].
2.3. Eradication protocol
Nasal Bactroban is routinely prescribed to all patients with a positive MRSA culture 4 times for 7 days [15,16]. Recently, oral vancomycin and rifampicin were used for 2 weeks [[12], [13], [14], [15], [16]].
3. Results
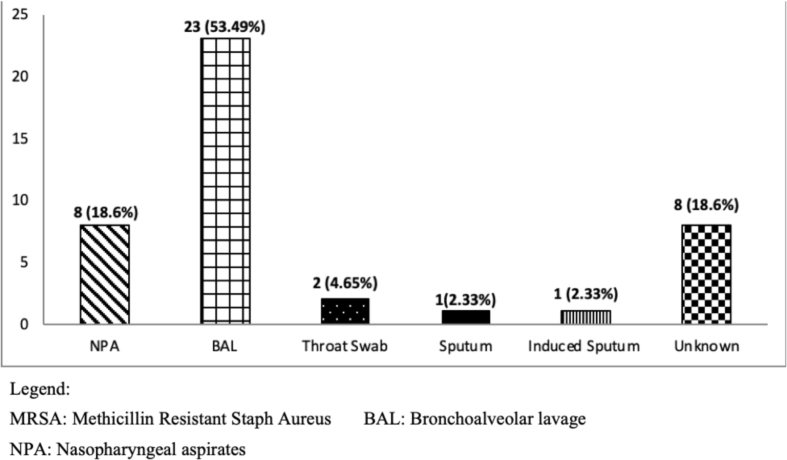
We examined (NPA/sputum and BAL) samples from 385 patients with CF at respiratory exacerbation or during the follow-up period. Out of 385 patients, 43 (11%) tested MRSA-positive from respiratory samples (Fig. 1), at a mean age of 10.4 ± 7.2 years. The source of the cultures was BAL in 23/43 patients (53%) and 8 samples (19%) from NPA. Twenty-two patients out of 43 (51%) were males as compared to 21 (49%) females.
Fig. 1.
Source of respiratory culture of MRSA (total of 43).
The number of MRSA-positive infections was studied during the periods 2002–2009 and 2010–2016. Eleven out of 43 (26%) patients were reported to be MRSA positive during 2002–2009 when compared with 32 (74%) patients during 2010–2016.
Of the total 43 patients with MRSA infection, 22 (51%) received medical treatment, whereas 21 (49%) did not receive medical treatment.
These 22 (51%) patients with MRSA infection were treated with the following different regimens: 13 (59%) were treated with nasal Bactroban, 5 (23%) were treated with a combination of nasal Bactroban and oral vancomycin + rifampicin, 1 (0.5%) received a combination of nasal Bactroban and linezolid, and 3 (14%) were treated with oral vancomycin and rifampicin. Microbiological eradication was achieved in 8 of the 22 (36%) patients within 3–6 months of applying the eradication protocol. One out of these 8 (13%) patients received the vancomycin–rifampicin combination, 3 (38%) received a combination of nasal Bactroban and oral vancomycin + rifampicin, 1 (13%) received a combination of nasal Bactroban and linezolid, and 3 (38%) received nasal Bactroban alone. In 6 of the 22 patients (27%), MRSA infection recurred within the 6-month follow-up period: 1 (17%) who received vancomycin and rifampicin, 2 (33%) who received the combination of nasal Bactroban and vancomycin + rifampicin, one (17%) who received linezolid and Bactroban, and 2 (33%) who received nasal Bactroban only. Five out of these patients (83%) remained colonized with the MRSA up to 2–4 years. Two out of the 22 (9%) died (1 from the group treated with vancomycin, rifampicin, and Bactroban and 1 from the group treated with Bactroban).
Twenty-one (49%) out of 43 patients did not receive medication, and five of the nontreated (24%) achieved spontaneous MRSA eradication. MRSA recurred in 3 out of 21 (14%). Ten (48%) of those 21 died.
3.1. Pulmonary function test results
Twenty (47%) out of 43 patients underwent PFT analysis for the detection and determination of disease severity. Twelve (60%) out of the 20 had normal PFT, 3 (15%) mild, 2 (10%) moderate, 2 (10%) moderately severe, and 1 (5%) severe.
3.2. CFTR analysis
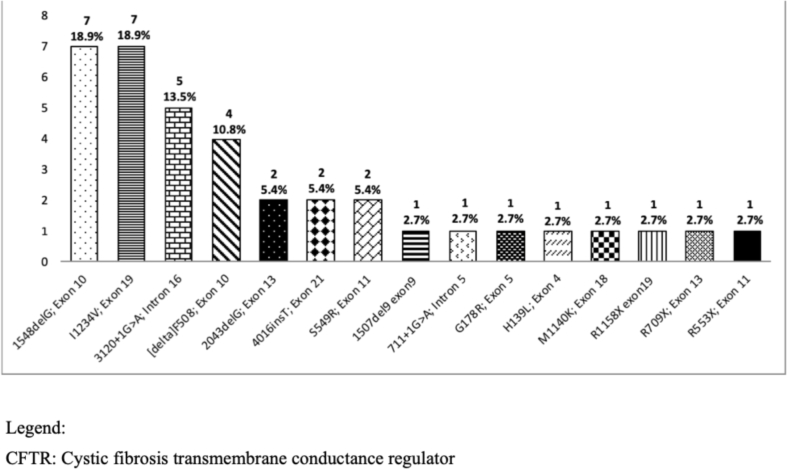
Analysis of the most common CFTR mutation in 38 out of 43 (88%) patients who had a culture positive for MRSA showed that the most common CFTR mutations are c.3700A > G (p.I1234V), Exon 22 (Legacy name: I1234V; Exon 19, c.3700A > G) that accounted for 7 (18%) patients, followed by c.1418delG (p.G473EfsX54), Exon 11 (Legacy name: 1548delG; Exon 10), and c.2988 + 1G > A (IVS18 + 1G > A), Intron 18 (Legacy name: 3120 + 1G > A; Intron 16), with 5 (13%) patients for each mutation type (Fig. 2).
Fig. 2.
CFTR mutations in MRSA population (total of 37).
4. Discussion
Our study showed that there is an increasing number of MRSA infections from 11 (26%) to 32 (79%) among patients with CF between the 2 periods (2002–2009 and 2010–2016). This is consistent with Cafiso V et al. who reported an increase in the prevalence of MRSA-positive cases from 0.1% in 1995 to 18.9% in 2010 [8].
MRSA eradication is of utmost importance. Lo, D. et al. found that patients who clear MRSA infection within one year have the same risk of death as those who never had a positive culture for MRSA, which leads us to the importance of having clear guidelines on how to eradicate MRSA in patients with CF [1]. In our study, we found that patients who were able to eradicate MRSA had less mortality rates than those who did not eradicate it.
Different studies used different eradication protocols [[12], [13], [14], [15], [16]], e.g., Vanderhelst, E. et al. [12] used 6-month period of rifampicin and fusidic acid for 6 months with topical decolonization including mupirocin-containing nasal ointment 3 times daily for 5 days and chlorhexidine hair and body wash once a day for 5 days. This method achieved 90% eradication of the disease after finishing the 6-month protocol with 9% recurrence of MRSA infection [12]. They also reported improvement in median forced expiratory volume in 1 s (FEV1) in 6 patients [13], whereas Burdge et al. [15] who used a 2-month course of rifampicin and clindamycin and achieved 100% eradication in an 8-month follow-up. On the other hand, Kiefer et al. [16] used oral rifampicin and fusidic acid for 7 days in addition to the inhalation of vancomycin and achieved 86% eradication.
Our protocol eradicated only 36% of those with MRSA who received treatment, which could be due to the short period of antibiotic use, and we may need to prolong the period up to 6 months to achieve 86–100%, like other studies (Table 1).
These results will help us in concluding that the prevalence of MRSA in the population with CF is increasing. Thus, new recommendations for eradication protocol and prevention should be observed and followed to reduce the morbidity and mortality associated with MRSA in CF.
5. Conclusion
MRSA is common in our CF population. Eradication protocol should be instituted early before it becomes a chronic colonization. Children with persistent MRSA colonization had high morbidity and mortality rate.
Acknowledgment
(1)- Sara AlKaf and Dhefaf AlAbdaly from Biostatistics, Epidemiology, and Scientific Computing Department (KFSHRC), Riyadh.
(2)- Manal Sheikh, Department of Pediatrics Research Unit (KFSHRC).
(3)- Mohammad Chaballout, College of Medicine, Alfaisal University, Riyadh.
(4)- BIO Pharma, Middle East & Africa, P.O. Box 214989, Dubai, United Arab Emirates. Tel: +971 (4) 3692828, Fax: +971 (4) 3697391, www.biopharma-mea.com.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpam.2019.10.005.
Contributor Information
Hanaa Banjar, Email: hanaa@kfshrc.edu.sa.
Hend Al-Qahtani, Email: ahend@kfshrc.edu.sa.
Waseem Yasin, Email: wyasin@alfaisal.edu.
Waad Al-wgait, Email: waalwgait@kfshrc.edu.sa.
Hanan Al-Amer, Email: haalamer@kfshrc.edu.sa.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lo DKH, Hurley MN, Muhlebach MS, Smyth AR. Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst Rev. 2015;7 doi: 10.1002/14651858.CD009650.pub4. Matched ISSN : 1469-493X. [DOI] [PubMed] [Google Scholar]
- 2.Zielenski J, Rozmahel R, Bozon D, Kerem B sheva, Grzelczak Z, Riordan JR. Genomic DNA sequence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics. 1991;10(1):214–228. doi: 10.1016/0888-7543(91)90503-7. Matched ISSN : 0888-7543. [DOI] [PubMed] [Google Scholar]
- 3.Anon . Cyst Fibros Found; 2014. Cystic fibrosis foundation patient registry 2013 annual data report to the center directors. [Google Scholar]
- 4.Rana M., Munns C.F., Selvadurai H., Donaghue K.C., Craig M.E. Cystic fibrosis-related diabetes in children—gaps in the evidence? Nat Rev Endocrinol. 2010 May 25;6:371. doi: 10.1038/nrendo.2010.85. Available from: https://doi.org/(View via CrossRef). Matched ISSN : 1759-5029. [DOI] [PubMed] [Google Scholar]
- 5.Banjar H. Geographic distribution of cystic fibrosis transmembrane regulator gene mutations in Saudi arabia. East Mediterr Health J. 1999;5(6) [PubMed] [Google Scholar]
- 6.Banjar H. Morbidity and mortality data of cystic fibrosis patients. Saudi Med J. 2003;7 Matched ISSN : 0379-5284. [PubMed] [Google Scholar]
- 7.Yurdakul P, Ocal HY, Gulmez D, Yalcin E, Dogru D, Cinel G. Predominance of hospital-associated MRSA among cystic fibrosis patients in a Turkish reference cystic fibrosis centre. J Chemother. 2012;4:195–200. doi: 10.1179/1973947812Y.0000000024. Matched ISSN : 1120-009X. [DOI] [PubMed] [Google Scholar]
- 8.Cafiso V, Bertuccio T, Spina D, Campanile F, Bongiorno D, Santagati M. Methicillin resistance and vancomycin heteroresistance in Staphylococcus aureus in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 2010;29(10):1277–1285. doi: 10.1007/s10096-010-1000-5. Matched ISSN : 0934-9723. [DOI] [PubMed] [Google Scholar]
- 9.Ren C.L., Morgan W.J., Konstan M.W., Schechter M.S., Wagener J.S., Fisher K.A. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol. 2007 doi: 10.1002/ppul.20604. Matched ISSN : 8755-6863. [DOI] [PubMed] [Google Scholar]
- 10.Girón RM, Buendía B, Pinedo C, Casanova Á, Hoyos N, Ancochea J. Staphylococcus aureus resistente a meticilina en pacientes adultos con fibrosis quística Methicillin-resistant Staphylococcus aureus in patients with cystic fibrosis. J Enfermedades Infecciosas Y Microbiología Clínica. 2009;27(2) doi: 10.1016/j.eimc.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Perez LRR, Antunes ALS, Bonfanti JW, Pinto JB, Roesch EW, Rodrigues D. Detection of methicillin-resistant Staphylococcus aureus in clinical specimens from cystic fibrosis patients by use of chromogenic selective agar. J Clin Microbiol. 2012;50(7):2506–2508. doi: 10.1128/JCM.00549-12. Matched ISSN : 0095-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderhelst E, De Wachter E, Willekens J, Piérard D, Vincken W, Malfroot A. Eradication of chronic methicillin-resistant Staphylococcus aureus infection in cystic fibrosis patients. An observational prospective cohort study of 11 patients. J Cyst Fibros. 2013;12(6) doi: 10.1016/j.jcf.2013.04.009. Matched ISSN : 1569-1993. [DOI] [PubMed] [Google Scholar]
- 13.Vanderhelst E, De Wachter E, Willekens J, Schuermans D, Vincken W, Malfroot A. Increase in ventilated air spaces after eradication of chronic methicillin-resistant Staphylococcus aureus infection in cystic fibrosis patients. Acta Clin Belg. 2014;70(1):30–33. doi: 10.1179/2295333714Y.0000000079. Matched ISSN : 1784-3286. [DOI] [PubMed] [Google Scholar]
- 14.Fung L. Continuous infusion vancomycin for treatment of methicillin-resistant staphylococcus aureus in cystic fibrosis patients. Ann Pharmacother. 2012;46(10) doi: 10.1345/aph.1R272. Matched ISSN : 1060-0280. [DOI] [PubMed] [Google Scholar]
- 15.Burdge DR, Nakielna EM, Noble MA. Eradication of methicillin-resistant Staphylococcus aureus from the lower respiratory tract of patients with cystic fibrosis. Can J Infect Dis. 1995;6(2):97–101. doi: 10.1155/1995/176396. Matched ISSN : 1180-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiefer A., Bogdan C., Melichar V.O. Successful eradication of newly acquired MRSA in six of seven patients with cystic fibrosis applying a short-term local and systemic antibiotic scheme. BMC Pulm Med. 2018;18(1):1–5. doi: 10.1186/s12890-018-0588-6. Matched ISSN : 1471-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chmiel JF, Aksamit TR, Chotirmall SH, Dasenbrook EC, Elborn JS, LiPuma JJ. Antibiotic management of lung infections in cystic fibrosis: I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann Am Thorac Soc. 2014;11(7) doi: 10.1513/AnnalsATS.201402-050AS. Matched ISSN : 2329-6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut. 2007;56(8) doi: 10.1136/gut.2004.062786. Matched ISSN : 0017-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banjar H. Microbiological Data of cystic fibrosis patients in a tertiary care center in Saudi Arabia.pdf. Kuwait Med J. 2004;6(3):179–181. Matched ISSN : 1607-8047. [Google Scholar]
- 20.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [aphylococcus aureus from the lower respiratory tract of patients with cystic fibrosis] [DOI] [PubMed] [Google Scholar]