HPV16-positive cancers have a better clinical outcome that their non-HPV anatomical counterparts. Furthermore, the presence of HPV16 E2 RNA predicts a better outcome for HPV16-positive tumors; the reasons for this are not known.
KEYWORDS: human papillomavirus, E2, TWIST1, cervical cancer, head and neck cancer, epithelial to mesenchymal transition, EMT, repression, HPV, cancer outcomes, transcription
ABSTRACT
Human papillomaviruses (HPVs) are causative agents in around 5% of all cancers, including cervical and oropharyngeal. A feature of HPV cancers is their better clinical outcome compared with non-HPV anatomical counterparts. In turn, the presence of E2 predicts a better clinical outcome in HPV-positive cancers; the reason(s) for the better outcome of E2-positive patients is not fully understood. Previously, we demonstrated that HPV16 E2 regulates host gene transcription that is relevant to the HPV16 life cycle in N/Tert-1 cells. One of the genes repressed by E2 and the entire HPV16 genome in N/Tert-1 cells is TWIST1. Here, we demonstrate that TWIST1 RNA levels are reduced in HPV-positive versus HPV-negative head and neck cancer and that E2 and HPV16 downregulate both TWIST1 RNA and protein in our N/Tert-1 model; E6/E7 cannot repress TWIST1. E2 represses the TWIST1 promoter in transient assays and is localized to the TWIST1 promoter; E2 also induces repressive epigenetic changes on the TWIST1 promoter. TWIST1 is a master transcriptional regulator of the epithelial to mesenchymal transition (EMT), and a high level of TWIST1 is a prognostic marker indicative of poor cancer outcomes. We demonstrate that TWIST1 target genes are also downregulated in E2-positive N/Tert-1 cells and that E2 promotes a failure in wound healing, a phenotype of low TWIST1 levels. We propose that the presence of E2 in HPV-positive tumors leads to TWIST1 repression and that this plays a role in the better clinical response of E2-positive HPV tumors.
IMPORTANCE HPV16-positive cancers have a better clinical outcome that their non-HPV anatomical counterparts. Furthermore, the presence of HPV16 E2 RNA predicts a better outcome for HPV16-positive tumors; the reasons for this are not known. Here, we demonstrate that E2 represses expression of the TWIST1 gene; an elevated level of this gene is a marker of poor prognosis for a variety of cancers. We demonstrate that E2 directly binds to the TWIST1 promoter and actively represses transcription. TWIST1 is a master regulator promoting EMT, and here, we demonstrate that the presence of E2 reduces the ability of N/Tert-1 cells to wound heal. Overall, we propose that the E2 repression of TWIST1 may contribute to the better clinical outcome of E2-positive HPV16-positive tumors.
INTRODUCTION
Human papillomaviruses (HPVs) are causative agents in around 5% of all human cancers (1). HPV16 is the most prevalent high-risk (those that cause cancer) type of HPV, responsible for around 50% of cervical cancers and 90% of HPV-positive oropharyngeal cancers (HPV16 plus oligodendrocyte precursor cells [OPC]). The latter has reached an epidemic proportion in the past generation (2–5). The HPV genome is circular and around 8 kbp in size.
HPV infects basal epithelial cells, and upon nuclear entry, a host of cellular factors activate transcription from the viral long control region (LCR) (6). The resultant viral transcript is processed into individual gene RNAs that are then translated. The viral oncoproteins E6 and E7 target several cellular proteins and disrupt their functions, including the tumor suppressors p53 and pRb, respectively (7, 8). Both p53 and pRb are transcription factors; therefore, the presence of E6 and E7 in cells results in a disruption of host gene transcription that contributes to the oncogenic properties of HPV. HPV uses two proteins to regulate replication of the viral genome. The E2 protein forms homodimers via a carboxyl terminus domain and binds to four 12-bp palindromic sequences within the viral LCR. Three of these surround the viral origin of replication (9), and via the amino terminus of the E2 protein, the viral helicase E1 is recruited to the A/T-rich viral origin of replication (10). E1 forms a dihexameric helicase that then replicates the viral genome in association with host polymerases (11–14). Following infection, the virus replicates to around 50 copies per cell to establish the infection. This copy number is maintained as the infected cell migrates through the epithelium before amplifying in the upper layers; the L1/L2 structural proteins are then expressed and the viral genomes encapsulated, resulting in viral particles that egress from the upper layers of the epithelium (15).
As well as acting as a replication factor, the E2 protein can regulate transcription. Where E2 sites are present upstream from a heterologous promoter such as herpes simplex virus 1 thymidine kinase (tk) promoter, E2 can activate transcription (16, 17). In addition, overexpression of E2 can repress transcription from the viral LCR (18–20). Given the ability of E2 to act as a transcription factor, the ability of E2 to regulate host gene transcription has been studied. E2 can regulate transcription via AP1 (21–25), nuclear receptors (26), and C/EBP (27). Transient E2 overexpression identified global gene changes induced by E2 (28–30). To gain a greater understanding of E2 regulation of host gene transcription by physiologically tolerated levels of E2, we generated stable cell lines expressing E2 and identified E2-induced host gene expression changes. This was originally done in U2OS cells (31, 32). However, we wished to develop a more physiologically relevant model, and to do this, we used N/Tert-1 cells, foreskin keratinocytes immortalized by telomerase. We generated N/Tert-1 cell lines stably expressing HPV16 E2. For comparison, we also prepared N/Tert-1 cells that contained the entire HPV16 genome and have previously demonstrated that N/Tert-1 cells support late stages of the HPV16 life cycle, making it an appropriate model for the study of HPV16 (33). Transcriptome sequence (RNA-seq) analysis demonstrated a significant overlap between genes regulated by E2 and those regulated by the entire HPV16 genome (34). Many innate immune genes were repressed by HPV16 E2 and the entire genome, and these genes are also repressed by E6 and E7 expression. We recently demonstrated that one of these genes, SAMHD1, is a restriction factor for HPV16, as it controls the viral life cycle in the differentiating epithelium (35). However, we wished to identify a gene that was only regulated by E2, not by E6/E7. Our RNA-seq analysis predicted that TWIST1 was transcriptionally repressed by E2 and the entire HPV16 genome in N/Tert-1 cells; in addition, TWIST1 was also downregulated in HPV16-positive versus HPV16-negative head and neck cancer (34).
TWIST1 is a basic helix-loop-helix transcription factor critical for promoting epithelial-to-mesenchymal transition (EMT) and embryogenesis (36, 37). EMT is a critical process in cancer, as this trait is associated with high-grade malignancy and resistance to chemotherapeutic agents (38–41). EMT is an epigenetic process that proceeds independently from DNA mutations (42). In addition, EMT promotes immune escape of cancer cells (43).
The potential repression of TWIST1 expression by HPV16 E2 is intriguing, as it could play a role in dictating therapeutic outcomes. Several reports have demonstrated that the expression of E2 in HPV16-positive tumors predicts improved survival, and repression of TWIST1 would correlate with this improved survival (44–46). Here, we demonstrate that TWIST1 is transcriptionally repressed by E2 in N/Tert-1 cells and that TWIST1 is downregulated in HPV-positive head and neck cancer versus HPV negative. The mechanism of E2 repression is not due to DNA methylation but involves direct binding of E2 to the TWIST1 promoter, repressing transcription, and inducing repressive histone markers. We demonstrate that TWIST1 target genes are also downregulated in E2-expressing cells and that wound healing is compromised. As EMT is a process that occurs during wound healing, the observed change in wound healing suggests that E2 contributes to EMT suppression in N/Tert-1 cells (47). Two HPV16-positive head and neck cancer cell lines were also studied, one that has episomal viral genomes and therefore expresses E2, and the other with an integrated HPV16 genome that has lost E2 expression. In the episomal genome-containing cell line, the presence of E2 resulted in decreased levels of TWIST1 compared with the integrated non-E2-expressing cell line. Finally, neither E6 nor E7 is able to regulate the expression of TWIST1. Overall, our results support the idea that E2 represses TWIST1 expression during the HPV16 life cycle and that this downregulation persists into HPV16-positive tumors. TWIST1 repression would promote a better patient outcome, a hallmark of E2 expression (44–46).
RESULTS
TWIST1 is transcriptionally repressed by E2, but not E6/E7.
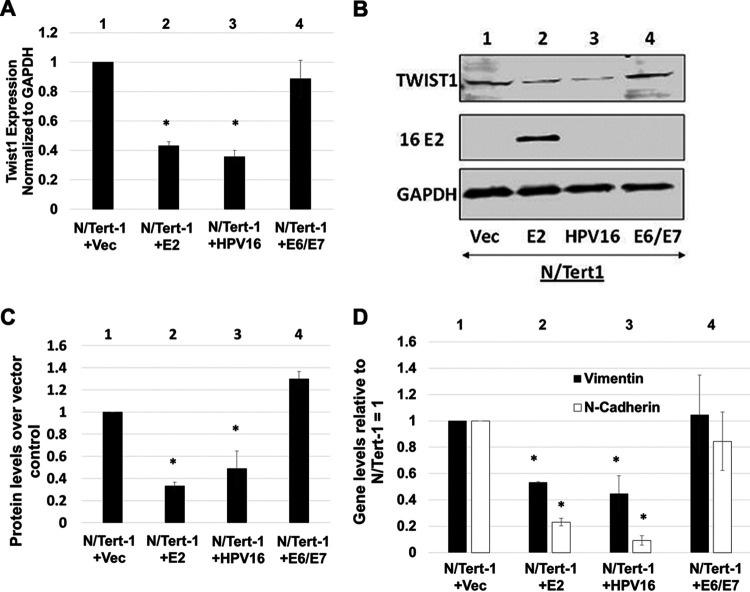
Our RNA-seq analysis of N/Tert-1+E2 (expressing HPV16 E2 only) and N/Tert-1+HPV16 (containing the entire HPV16 genome) demonstrated that there was a highly statistically significant overlap between the genes regulated by E2 and the entire HPV16 genome (34). In addition, using our analysis of The Cancer Genome Atlas (TCGA) data, we observed a significant downregulation of TWIST1 in HPV16-positive versus negative head and neck cancers (HNSCCs) (33, 34). Figure 1A summarizes the expression of TWIST1 in both episomal and integrated HPV16-positive versus HPV16-negative HNSCCs. Because integration disrupts E2 gene expression, integration and episomal groups were determined depending on their E2 expression (46, 48). TWIST1 mRNA expression data were obtained from 528 TCGA tumors using the cBio Cancer Genomic Portal (49, 50). We have previously characterized HPV16 status and viral integration in these samples (46). TWIST1 mRNA expression was then compiled and reported by HPV16 status (Fig. 1A). We found that TWIST1 mRNA is expressed at statistically significantly lower levels in episomal HPV16-positive tumors than those that were either integrated or HPV16 negative. There was no statistical difference in TWIST1 expression between integrated and HPV-negative tumors. Figure 1B summarizes our previous data from our RNA-seq analysis in N/Tert-1 cells. TWIST1 was found to be significantly downregulated in N/Tert-1 cells expressing HPV16 E2 or the entire viral episome compared to parental control cells (33, 34).
FIG 1.
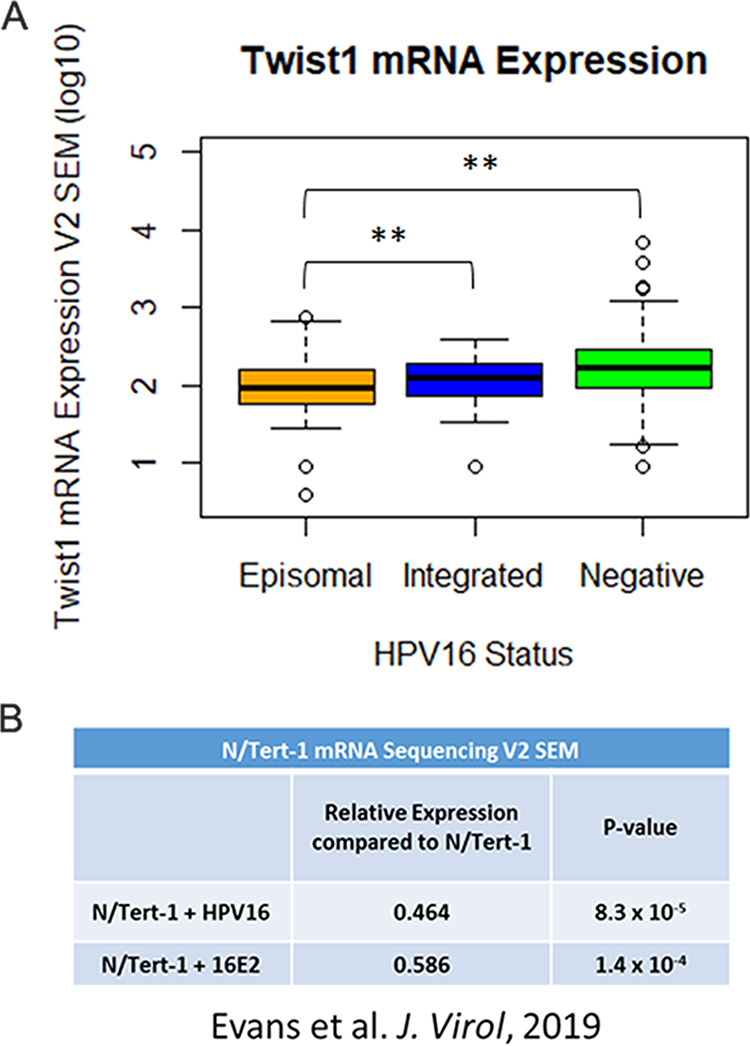
TWIST1 expression is downregulated by HPV16 E2. (A) Five hundred twenty-eight samples were previously evaluated for HPV genome status utilizing the E2:E7 ratio of mRNA sequencing reads (46, 48). Four hundred sixty-six samples were HPV negative. Forty samples retained HPV16 episome status, and 17 samples had integrated HPV16 genomes. Firehose Legacy TWIST1 mRNA expression data were obtained using cBioPortal. TWIST1 mRNA between HPV16 status was then compared using two-tailed Student’s t test. Vertical axis in log10. (B) N/Tert-1 RNA-sequencing results adapted from reference 34. TWIST1 expression was compared to parental N/Tert-1 cells with no HPV16 or HPV16 E2. **, P < 0.05 using Bonferroni correction when applicable.
Figure 2A validates the downregulation of TWIST1 RNA expression in N/Tert-1 cells expressing E2 or the entire HPV16 genome (compare lanes 2 and 3, respectively, with lane 1). We have also previously reported the overexpression of HPV16 E6 and E7 in the same cell background, and these oncogenes did not alter the expression of TWIST1 (lane 4). This is in contrast with our prior study of innate immune response gene regulation by HPV16, which demonstrated that E2, E6, and E7 can all repress these genes (34). Therefore, TWIST1 is the first gene we have determined to be likely exclusively regulated by E2 during the HPV16 life cycle. To confirm that the RNA expression was reflected at the functional protein level, Western blotting was carried out for TWIST1 (Fig. 2B). There is a clear downregulation of TWIST1 protein expression in cells expressing E2 or HPV16 (compare lanes 2 and 3, respectively, with lane 1) but not in cells expressing E6/E7 (compare lane 4 with lane 1). This was repeated another two times and the results quantified (Fig. 2C); both E2 and HPV16 induce a significant reduction in TWIST1 protein levels, while E6/E7 do not. To confirm that TWIST1 function is also downregulated in the presence of E2, we monitored expression of TWIST1 target genes vimentin and N-cadherin in N/Tert-1+Vec, N/Tert-1+E2, N/Tert-1+HPV16, and N/Tert-1+E6/E7 cells (Fig. 2D). The expression levels of these TWIST1 target genes are reflective of the TWIST1 levels in the cell. This demonstrates that there is a functional loss of TWIST1 in E2 and HPV16 expressing N/Tert-1.
FIG 2.
TWIST1 RNA and protein levels are downregulated by E2 and the HPV16 genome in N/Tert-1 cells. (A) qRT-PCR of N/Tert-1 lines with E2 (lane 2), HPV16 (lane 3), E6 and E7 (lane 4), or empty pCDNA3.1 vector (lane 1). Results are expressed as fold change from that observed in the vector control N/Tert-1 cells (lane 1). (B) Western blot analysis was carried out on protein extracted from N/Tert-1 cells with empty vector (lane 1) or those with E2, HPV16, or E6 and E7 (lanes 2 to 4). GAPDH is shown as an internal control. Western blotting was visualized using a Li-Cor system. (C) Western blotting was quantitated, and TWIST1 protein expression was calculated relative to vector using ImageJ. (D) Repression of TWIST1 by E2 and HPV16 leads to reduction in EMT marker expression. N/Tert-1 cells were harvested for RNA and processed for cDNA. qRT-PCR was performed for TWIST1 target genes CDH2 and VIM, which encode the proteins N-cadherin and vimentin, respectively. Results are expressed as fold change from that observed in the vector control N/Tert-1 cells (lane 1). Data in panels A, C, and D represent the averages of at least 3 independent experiments, and error bars indicate standard error of the mean. *, P < 0.05.
E2 binds to the TWIST1 promoter region and directly represses transcription.
Our previous work demonstrated that E2 represses the transcription of innate immune genes by regulating the DNA methylation of the corresponding promoters (34). To determine whether E2 is also regulating TWIST1 expression via DNA methylation of the promoter, we treated the cells with 1 μM decitabine, a DNA methylase inhibitor that relieves E2-mediated repression of innate immune genes (Fig. 3). Figure 3A demonstrates that the drug has worked, as there is an increase in both MX1 and IFIT1 when decitabine is added, as we observed previously (34). The increase occurs in the vector control cells (compare lanes 3 and 4 versus 1 and 2), but the relief of repression is greater in the presence of E2 (compare lanes 7 and 8 versus 5 and 6) and HPV16 (compare lanes 9 and 10 versus 11 and 12); these results duplicate those we observed previously (34). However, the TWIST1 levels were not altered in any of the cell lines following treatment with decitabine. Therefore, TWIST1 is regulated by E2 differently from innate immune response genes and is independent of DNA methylation.
FIG 3.
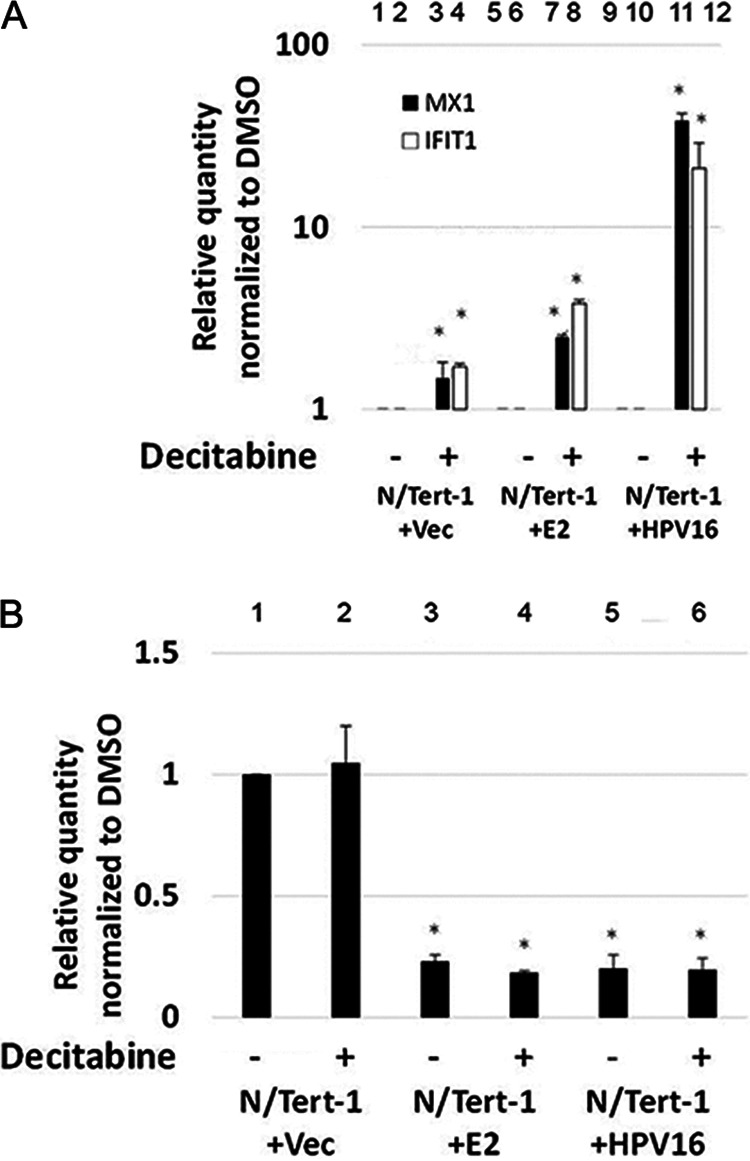
TWIST1 expression is not repressed by E2 via methylation of the gene promoter. N/Tert-1 cells were treated with 1 μM decitabine (5-aza-cytidine) for 72 h. Afterward, cells were harvested and processed for RNA, which was reverse transcribed into cDNA. (A) qRT-PCR on N/Tert-1 cells for innate immune genes MX1 and IFIT1 illustrates robust restoration of expression following decitabine treatment. Results are expressed as fold change from that observed in the untreated vector control N/Tert-1 cells. (B) The same cDNA was then analyzed for TWIST1 expression. Unlike the innate immune genes, decitabine does not restore TWIST1 repression. Data in panels A and B represent the mean of two independent experiments, and error bars indicate standard error of the mean. *, P < 0.05.
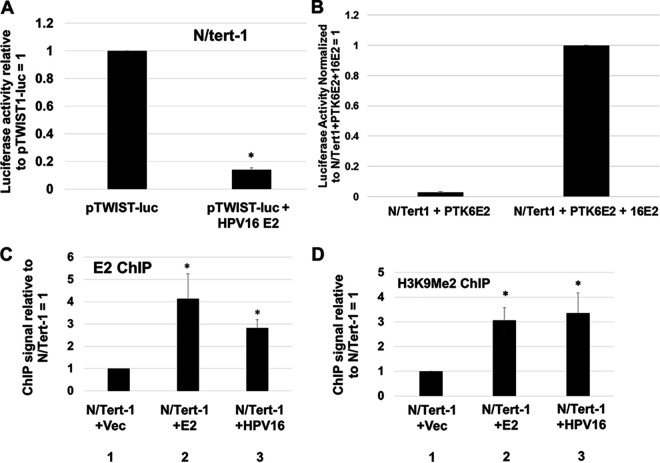
We next investigated whether E2 could directly repress transcription from the TWIST1 promoter. A construct containing the TWIST1 promoter upstream from the luciferase gene (pTWIST-luc) was cotransfected with E2 into N/Tert-1 cells to determine whether E2 can repress expression directly from the TWIST1 promoter (Fig. 4A). The expression of E2 resulted in a ∼10-fold reduction in luciferase activity, demonstrating that E2 directly represses this promoter. To confirm that this was not due to E2 toxicity in the transiently transfected cells, we included transcriptional activation studies on ptk6E2-luc, a plasmid we have shown is responsive to E2. Figure 4B demonstrates that cotransfection with E2 increases transcription from ptk6E2-luc, demonstrating that there is no overall toxicity induced by E2 in the N/Tert-1 cells. To determine whether E2 can bind directly to the TWIST1 promoter, we carried out a chromatin immunoprecipitation assay (ChIP) using N/Tert-1+Vec, N/Tert-1+E2, and N/Tert-1+HPV16 cells using a sheep E2 antibody as previously described (51, 52) (Fig. 4C). There was a significant increase in signal in N/Tert-1+E2 and N/Tert-1+HPV16 compared with the signal obtained with N/Tert-1+Vec (compare lanes 2 and 3, respectively, with lane 1), demonstrating that E2 binds to the TWIST1 promoter region when overexpressed but also in the context of the entire HPV16 genome. We next investigated whether the levels of repressive chromatin markers implicated in TWIST1 regulation are changed in the presence of E2. H3K9me2 is a repressive chromatin marker involved in the regulation of TWIST1 expression (53, 54), and the levels of this marker on the TWIST1 promoter are increased in N/Tert-1+E2 and N/Tert-1+HPV16 compared with N/Tert-1+Vec (Fig. 4D; compare lanes 2 and 3, respectively, with lane 1). These results suggest that E2 interacts with the TWIST1 promoter directly and modifies the local epigenetic environment around the promoter, leading to a reduced level of transcription.
FIG 4.
E2 binds to the TWIST1 promoter and actively represses transcription. (A) N/Tert-1 cells were transfected with 1 μg of pTWIST1-luc alone or with 1 μg HPV16 E2. Forty-eight hours after transfection, a luciferase-based assay was utilized to monitor levels of TWIST1 promoter activation. Data were obtained as relative fluorescence units (RFU), normalized to total protein concentration as monitored by a standard bovine serum albumin (BSA) assay, and presented as relative to pTWIST1-luc-only transfection. (B) Cells were transfected with ptk6E2-luc, which E2 activates. Results are normalized to 1 for the experiments with E2 cotransfected. Data were quantitated as described in panel A. This demonstrates that E2 is not killing the cells upon transfection, as there is functional transcriptional activation by E2. (C) Chromatin immunoprecipitation of E2 onto the TWIST1 promoter. In both E2 (lane 2) and HPV16 cells (lane 3), E2 was observed directly binding to the promoter region of TWIST1. (D) E2 binding at the TWIST1 promoter leads to accumulation of repressive histone marker H3K9me2. Once again, this repressive marker was upregulated in both E2- (lane 2) and HPV16-expressing (lane 3) N/Tert-1 cells. Results were normalized to input DNA and expressed as fold change over vector control N/Tert-1. Data represent the mean of at least three independent experiments, and error bars depict standard error of the mean. *, P < 0.05.
E2 expression reduces the rate of wound healing in N/Tert-1 cells.
The repression of TWIST1 expression by E2 suggested suppression of the EMT phenotype in N/Tert-1 cells. EMT is not a defined status but is a spectrum of phenotypes that range from epithelial through to fully mesenchymal (47). The N/Tert-1+E2 cells do not look appreciably different from N/Tert-1 control cells; therefore, we propose that there is a subtle influence of E2 on the EMT status of these cells. During wound healing, there is an EMT transition of the wounded epithelia cells that promotes migration and eventual wound closure (55). Our previous studies on U2OS cells demonstrated that the expression of E2 slowed the ability of these cells to close wounds in monolayer cells (32); therefore, we carried out “scratch” assays with our N/Tert-1 cells in order to investigate wound healing. Figure 5A shows images of the results from the wound-healing experiment. Twenty hours following wound induction, the wound is almost completely healed in N/Tert-1+Vec cells (top 3 panels). However, with N/Tert-1+E2 and N/Tert-1+HPV16, there is a failure to close the wound after 20 h (second and third from top panels, respectively). To determine whether the oncogenes E6/E7 play a role in regulating wound healing, we also determined wound closure in N/Tert-1+E6/E7 cells (characterized and described in reference 34). The presence of the viral oncogenes made no difference to the wound healing (compare the bottom three panels with the top three). Therefore, the failure to close the wound is reflective of TWIST1 levels and E2 expression. This assay was repeated several times and the data quantified (Fig. 5B). There is a significant delay in wound healing 12 and 20 h after the initial “scratch” in both the N/Tert-1+E2 and N/Tert-1+HPV16 cell lines compared with N/Tert-1+Vec cells.
FIG 5.
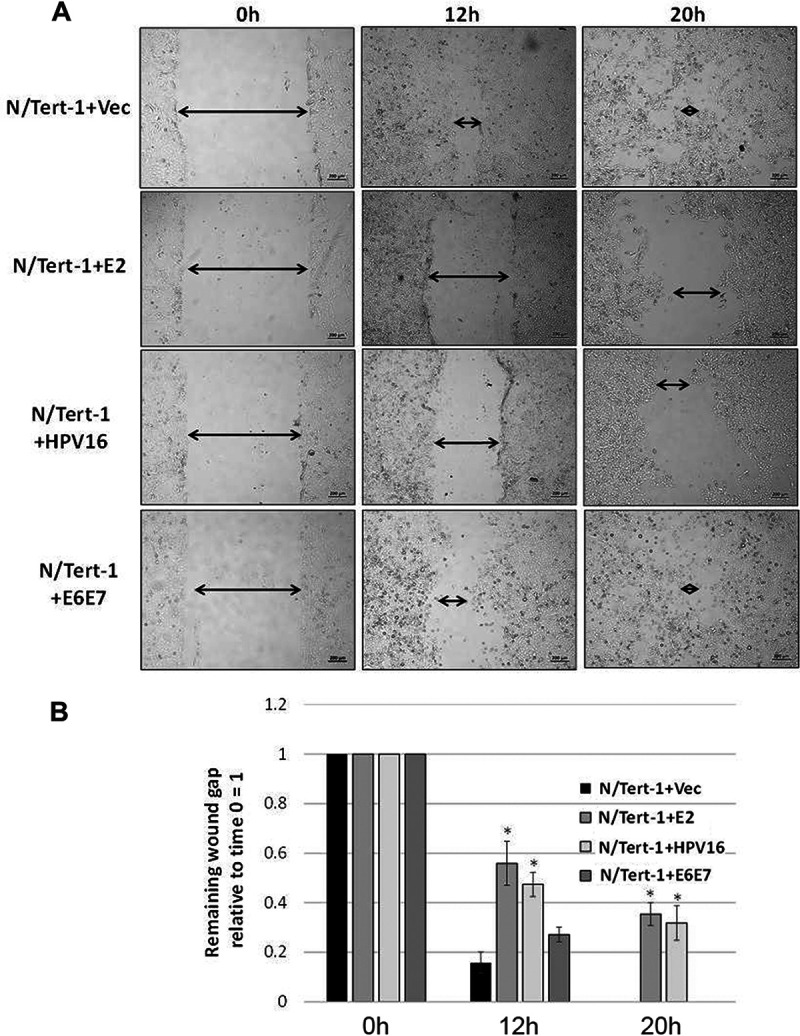
E2 expression correlates with attenuated ability to wound heal. (A) N/Tert-1 cells were plated and allowed to grow to near confluence. Afterward, a ∼1-mm scratch was made in the cell monolayer using a pipette tip. The wound gap was imaged and measured at the same field at 0, 12, and 20 h. Arrows have been added for clarity. Size bars have been added at the bottom right of each image to illustrate a length of 200 μm. (B) The remaining wound gap was calculated relative to time zero for each field. By 20 h, the vector and E6 and E7 cells have completely closed wound gaps, while the E2 and HPV16 cells retain considerable gaps. Results are expressed as fold change from that observed in the vector control N/Tert-1 cells (lane 1). Data represent the mean of at least three independent experiments, and error bars depict standard error of the mean. *, P < 0.05.
E2 and TWIST1 levels inversely correlate in HPV16-positive head and neck cancer cell lines.
Figure 1 demonstrates that there is, on average, less expression of TWIST1 in HPV-positive head and neck cancers than in HPV-negative head and neck cancers. Our results suggest that the expression of E2 is influential on the expression of TWIST1 within the HPV-positive group. Previous studies suggested that UMSCC104 contained episomal HPV16 genomes (and therefore retained E2 expression), while UMSCC47 had integrated viral genomes (and therefore had lost E2 expression). We confirmed that UMSCC104 expressed E2 and that UMSCC47 did not (Fig. 6A). We next investigated the TWIST1 levels in these cell lines. TWIST1 RNA expression was significantly downregulated in UMSCC104 compared with UMSCC47 (Fig. 6B), and the levels of RNA expression were reflected in a downregulation of TWIST1 protein levels (Fig. 6C). Western blotting was repeated several times and quantitated; there is significantly less TWIST1 protein expressed in UMSCC47 than in UMSCC104. The results in these two HPV16-positive head and neck cancer cell lines support the model of E2 repressing TWIST1 gene expression.
FIG 6.
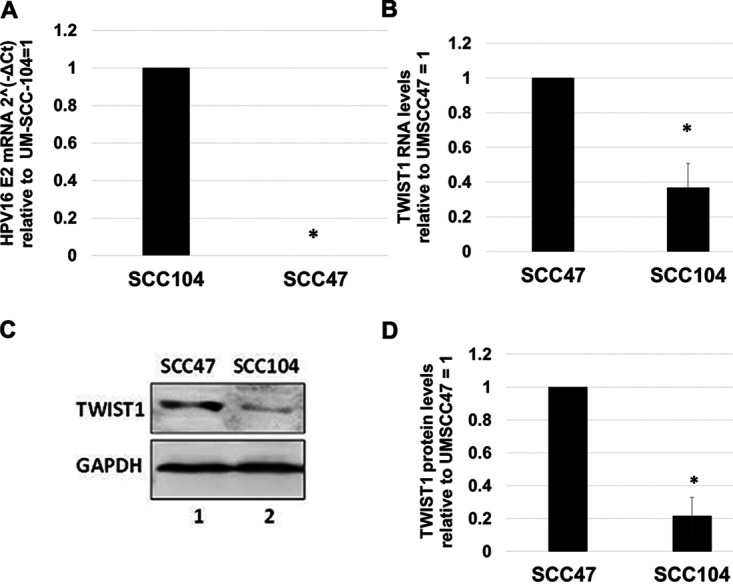
Expression of TWIST1 inversely correlates with E2 expression in head and neck cancer cell lines. (A) qRT-PCR for HPV16 E2 expression in UM-SCC-104 and UM-SCC-47 cell lines. Results are expressed as threshold cycle (2−ΔΔCT) using GAPDH as internal control. Integrated UM-SCC-47 exhibited no 2−ΔΔCT change over negative experimental control, illustrating no presence of E2 mRNA. (B) The same cDNA in panel A was analyzed for TWIST1 expression. Episomal UM-SCC-104 has lower TWIST1 transcription levels than UM-SCC-47. Results are expressed as relative fold change from that observed in UM-SCC-47 cells. (C) Western blot analysis was carried out on protein extracted from the cells in panels A and B. Decreased TWIST1 transcription leads to reduced expression on the protein level in UM-SCC-104 compared to UM-SCC-47 cells. GAPDH is shown as internal control. (D) Western blotting was repeated and quantitated, and TWIST1 protein expression was calculated relative to UM-SCC-47 protein levels using ImageJ. Data represent the averages of at least 3 independent experiments, and error bars indicate standard error of the mean. *, P < 0.05.
DISCUSSION
The HPV E2 protein plays multiple crucial roles during the viral life cycle. It is essential for replication of the viral genome, can regulate the segregation of the viral genome into daughter cells, and has transcriptional properties with the potential to regulate transcription from the viral and human genomes (9, 56). Regulation of host gene expression by E2, and its importance in the viral life cycle, is relatively understudied compared with its role in viral replication and segregation. Our recent work demonstrates that E2 regulates host gene transcription that is relevant during the viral life cycle (34). That report demonstrated an overlap in the ability of E2 and E6/E7 to repress the expression of innate immune genes. In this current report, we extend our observations on the transcriptional regulatory functions of E2. We demonstrate that E2 can repress TWIST1 gene expression and that this repression is via a distinct mechanism compared to innate immune gene repression. E6 and E7 did not affect the levels of TWIST1 RNA and protein; therefore, it is likely that TWIST1 expression is regulated by E2 during the HPV16 life cycle. For innate immune genes, methylation of the gene promoters played a significant role in repressing expression, but for TWIST1, DNA methylation plays no role. Rather, E2 binds to the TWIST1 promoter and actively represses transcription from this region. E2 also increases H3K9me2 at the promoter, a hallmark of transcriptionally repressed genes previously implicated in regulation of TWIST1 transcription (53). To our knowledge, this is the first time E2 has been demonstrated to directly bind to a host gene promoter and induce repressive epigenetic markers. The repression of TWIST1 by E2 resulted in downregulation of TWIST1 target genes vimentin and N-cadherin. During wound healing, there is an EMT-like transition that promotes wound closure, and wound healing in E2-expressing N/Tert-1 is compromised compared to parental cells (55).
Repression of TWIST1 expression by E2 may play a role in HPV cancer outcomes. Overexpression of TWIST1 is associated with poorer overall survival in head and neck cancer (57), while E2 expression is associated with improved outcomes in HPV-positive head and neck cancer (44–46). We demonstrate here that TWIST1 is downregulated in HPV16-positive tumors that retain E2 expression compared with HPV16 tumors that have no E2 expression or are HPV negative. We therefore highlight this correlation between E2 expression and TWIST1 repression, which may contribute to the better clinical outcomes of HPV16-positive tumors that retain E2 expression. We demonstrate, in two HPV16-positive head and neck cancer cell lines (one that is E2 positive, one that is negative), that E2 expression inversely correlates with TWIST1 expression, which correlates with our observations in N/Tert-1 cells and TCGA data sets. While these lines are not isogenic, they provide a model for studying the repression of TWIST1 in cancer cell lines that is potentially mediated by expression of the E2 protein. Not all E2-positive head and neck tumors have downregulation of TWIST1 compared with E2-negative tumors as evidenced by TCGA data, but there is a significant trend for downregulation. Not only has elevated TWIST1 been associated with poorer survival in several cancers, including head and neck, but TWIST1 protein attenuates the response to chemotherapeutic drugs, which provides a rationale for the worse clinical outcomes in high TWIST1 expression patients (47, 55).
The mechanism of E2 repression of TWIST1 expression remains to be fully elucidated. The TWIST1 promoter is methylated in cancer, although this did not correlate with low TWIST1 RNA or protein levels (58). Methylation does not play a role in the mechanism that E2 uses to repress TWIST1 levels, as treatment of cells with decitabine did not relieve TWIST1 repression; therefore, E2 uses multiple mechanisms to regulate host gene transcription. We demonstrate here for the first time that E2 binds to and represses transcription from the TWIST1 promoter and modifies chromatin around the promoter start site by inducing elevated levels of H3K9me2, a repressive marker. The transcription factor SP1 is involved in the basal transcription levels from the TWIST1 promoter, and displacement of this factor from the promoter repressed TWIST1 transcription (59). The E2 protein has been shown to displace SP1 from HPV LCRs, resulting in transcriptional repression, and may act similarly on the TWIST1 promoter (60, 61). This active repression may be required to block TWIST1 expression, as STAT3 is activated by HPV in cervical cancer cells (62), and active STAT3 is an activator of TWIST1 expression, promoting EMT (63). We demonstrate here that there is an increase in the level of the repressive marker H3K9me2 at the TWIST1 promoter in the presence of E2. The chromatin repressor NuRD complex can be recruited to the TWIST1 promoter via DOC1, resulting in transcriptional repression via induction of a nucleosome on the TWIST1 promoter. It is feasible that E2 recruits a repressor complex to the TWIST1 promoter in order to regulate transcriptional repression via nucleosome assembly (64). E2 functionally interacts with BRD4, and this interaction is involved in regulating E2 repression of the viral LCR. Again, such a mechanism may be used by E2 to contribute to the repression of the TWIST1 promoter (65). Future studies will characterize the mechanism(s) that E2 uses to repress the TWIST1 promoter. Enhancing our understanding of this mechanism is important, as E2 offers a model system for repressing TWIST1 transcription. For example, if H3K9me2 methylation of the TWIST1 promoter is a major contributor to repression by E2, then induction of this modification via drug treatment could be an opportunity to promote a better response of high TWIST1-expressing tumors to chemotherapeutic agents. In addition, if TWIST1 repression is an important mechanism promoting the HPV16 life cycle in epithelial cells, then reversing this repression offers an opportunity to disrupt this process and alleviate HPV16 infections and disease.
Another question is, why does HPV16 repress the expression of TWIST1? While TWIST1 expression is important in mouse embryogenesis, demonstrating an important role in cellular proliferation and differentiation, there is less known about the effect of TWIST1 on epithelial cell differentiation (37). HPV manipulates epithelial differentiation in order to create an environment that supports viral replication (66). While TWIST1 is clearly a promoter of EMT, the effect of this protein on the epithelial differentiation pathway is less clear and is worthy of future study. Perhaps the repression of TWIST1 plays a role in promoting an environment that allows HPV16 replication in the infected epithelium. TWIST1 and HPV16 E2 interact with the NF-κB pathway, and downregulation of TWIST1 may promote the ability of E2 to regulate NF-κB, which could be important for the HPV16 life cycle (67, 68). TWIST1 has been shown to downregulate expression of C/EBPα, a protein that E2 functionally interacts with to regulate transcription (27, 69). Therefore, downregulation of TWIST1 expression may prevent disruption of E2’s ability to regulate host gene transcription via C/EBPα during the viral life cycle. Recently, it has been demonstrated that C/EBPα is a crucial factor for epithelial maintenance and prevents EMT; thus, E2 downregulation of TWIST1 may enhance the ability of C/EBPα to carry out this function (70). This would also promote the enhanced epithelial status of the E2-positive cells.
In conclusion, this report demonstrates that HPV16 E2 downregulates the expression of TWIST1. This occurs in cells that contain the entire HPV16 genome and also occurs in HPV16-positive cancers that are E2 positive. Given the important role that TWIST1 plays in EMT, cancer outcomes, and response to chemotherapeutic agents, a fuller understanding of the interaction of HPV16 with TWIST1 is warranted.
MATERIALS AND METHODS
Differential expression in TCGA.
Head and neck squamous cell carcinoma (HNSCC) TWIST1 mRNA expression data were obtained from The Cancer Genome Atlas using cBio Cancer Genomics Portal (49, 50). HNSCC tumor samples (Firehose Legacy) were analyzed for HPV status and viral genome integration as previously described utilizing the HPV16 E2:E7 ratio of mRNA sequencing reads (46, 71). Samples without available TWIST1 mRNA expression data were omitted. Data for 528 HNSC samples were available. Of these, 522 were identified with available TWIST1 mRNA expression and HPV status, which was correlated and reported using R. Statistical analysis was performed using two-way Student's t test with Bonferroni correction for two comparisons.
Cell culture.
Low-passage N/Tert-1 with stably expressing HPV16, HPV16 E2, and HPV16 E6+E7 cells were generated as previously described and characterized in previous studies (31–35). These cells were cultured alongside empty vector, drug-selected pcDNA 3 with 111 μg/ml G418 sulfate (Genticin) (Thermo Fisher Scientific). All N/Tert-1 cells were grown in keratinocyte serum-free medium (K-SFM; Invitrogen) with a 1% (vol/vol) penicillin-streptomycin mixture (Thermo Fisher Scientific) containing 4 μg/ml hygromycin B (MilliporeSigma) at 37°C in 5% CO2 and passaged every 3 to 4 days. UMSCC47 and UMSCC104 cell lines were obtained from MilliporeSigma (catalog nos. SCC071 and SCC072, respectively). UMSCC47 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Invitrogen). UMSCC104 cells were grown in Eagle’s minimum essential medium (EMEM) (Invitrogen) supplemented with nonessential amino acids (NEAA) (Gibco) and 10% (vol/vol) FBS. All cells were routinely checked for mycoplasma contamination. For protein and RNA analyses, 1 × 106 cells were plated onto 100-mm2 plates, trypsinized, and washed twice with 1× phosphate-buffered saline (1× PBS).
SYBR green reverse transcription-quantitative PCR.
RNA was isolated using the SV Total RNA isolation system (Promega) following the manufacturer’s instructions. We reverse transcribed 2 μg of RNA into cDNA using the high-capacity reverse transcription kit (Applied Biosystems). cDNA and relevant primers were added to PowerUp SYBR green master mix (Applied Biosystems), and real-time PCR was performed using 7500 Fast real-time PCR system. The primer sequences utilized were as follows: Twist1 forward, 5′-GTCCGCAGTCTTACGAGGAG-3′; Twist1 reverse, 5′-GCTTGAGGGTCTGAATCTTGCT-3′; HPV16 E2 forward, 5′-ATGGAGACTCTTTGCCAACG-3′; HPV16 E2 reverse, 5′-TCATATAGACATAAATCCAG-3′, VIM (vimentin) forward, 5′-GACGCCATCAACACCGAGTT-3′; VIM reverse, 5′-CTTTGTCGTTGGTTAGCTGGT-3′, CDH2 (N-Cadherin) forward, 5′-AGCCAACCTTAACTGAGGAGT-3′; and CDH2 reverse, 5′-GGCAAGTTGATTGGAGGGATG-3′.
Decitabine treatment.
N/Tert-1 cells were plated at a density of 1.5 × 105 in 6-well plates (60 mm2/well). The following day, cells were treated with 1 μM decitabine or 1 μM DMSO for 72 h as previously described (34). Afterward, the cells were harvested and processed for reverse transcription-quantitative PCR (qRT-PCR) as described above.
Immunoblotting.
N/Tert-1, UMSCC47, and UMSCC104 cells were trypsinized, washed with 1× phosphate-buffered saline (PBS), and resuspended in 2× pellet volume protein lysis buffer (0.5% Nonidet P-40, 50 mM Tris [pH 7.8], and150 mM NaCl) supplemented with protease inhibitor (Roche Molecular Biochemicals) and phosphatase inhibitor cocktail (Sigma). The cell suspension was lysed for 30 min on ice and then centrifuged for 20 min at 184,000 relative centrifugal force (rcf) at 4°C. Protein concentration was determined using the Bio-Rad protein estimation assay. We boiled 25-μg protein samples in equal volume 2× Laemmli sample buffer (Bio-Rad). Samples were run down a Novex 4 to 12% Tris-glycine gel (Invitrogen) and transferred onto a nitrocellulose membrane (Bio-Rad) at 30 V overnight using the wet blot method. Membranes were blocked with Odyssey (PBS) blocking buffer (diluted 1:1 with 1× PBS) at room temperature for 1 h and probed with indicated primary antibody diluted in Odyssey blocking buffer. Membranes were then washed with PBS supplemented with 0.1% Tween (PBS-Tween) and probed with the indicated Odyssey secondary antibody (goat anti-mouse IRdye 800CW or goat anti-rabbit IRdye 680CW) diluted in Odyssey blocking buffer at 1:10,000. Membranes were washed and underwent infrared scanning using the Odyssey CLx Li-Cor imaging system. Immunoblots were quantified using ImageJ. The following primary antibodies were used for immunoblotting: HPV16 E2 (TVG 261) at 1:1,000 dilution from Abcam; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (catalog no. sc-47724) and p53 (catalog no. sc-47698) at 1:1,000 dilution from Santa Cruz Biotechnology, and TWIST1 (catalog no. 25465-1-AP) at 1:300 from Proteintech.
ChIP assay.
N/Tert-1 cells were plated at a density of 2 × 106 in 150-mm2 plates. The following day, the cells were harvested via scraping and processed for chromatin as previously described (72). Chromatin concentration was determined with a NanoDrop spectrophotometer. Approximately 100 μg of chromatin was used per antibody experiment. The following antibodies were used for ChIP: 2 μl sheep anti-HPV16 E2 (amino acids 1 to 201) prepared and purified by Dundee Cell Products, United Kingdom; 2 μg rabbit anti-histone H3K9me2 (Abcam; catalog no. ab1220). Chromatin was then processed for qPCR. ChIP DNA primers include Twist1 forward, 5′-TCAGGCCAATGACACTGCT-3′, and Twist1 reverse, 5′-GACGGTGTGGATGGCCCCGA-3′.
Transcription assay.
We plated out 5 × 105 N/Tert-1 cells on 100-mm2 plates, and they were transfected 24 h later with either 1 μg HPV16 E2 plasmid and 1 μg pTWIST1-Luc/ptk6E2-luc, or 1 μg pTWIST1-Luc/ptk6E2-luc alone using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions as previously described (73). pTWIST1-luc contains the human promoter and has been described previously (74, 75). ptk6E2-luc has been described (16, 17). Briefly, the cells were harvested 72 h posttransfection utilizing the Promega reporter lysis buffer and analyzed for luciferase using the Promega luciferase assay system. Concentrations were normalized to protein levels, as measured by the Bio-Rad protein estimation assay mentioned above. Relative fluorescence units were measured using the BioTek Synergy H1 hybrid reader.
Wound healing assay.
We plated 2.5 × 105 N/Tert-1 cells at the center of 6-well plates (60 mm2/well). The cells were left to grow to confluence. Afterward, the monolayer was scratched using a 1,000-μl pipette tip, creating an ∼1-mm wound. Wounds were imaged at 0-, 12-, and 20-h intervals (Zeiss; Axiovert 200 M microscope). Multiple images were taken randomly along the wounds, and measurements were taken from the leading cell edge. Wounds were measured using ImageJ software, and wound healing was calculated as a ratio of the 0-h time point.
Statistical analyses.
The standard error was calculated from no less than three independent experiments. The only exception was in the TWIST1-Luc transcription assay where statistical power was achieved after 2 replicates. Significance was determined using two-tailed Student's t test. Bonferroni correction for significance was utilized when indicated.
ACKNOWLEDGMENTS
This work was supported by VCU Philips Institute for Oral Health Research and the National Cancer Institute Designated Massey Cancer Center grant P30 CA016059 (to I.M.M.), and by NCI 5R01CA210911 (to A.A.) and 5R01CA240484 (to A.A.). We thank Lu-Hai Wang for pTWIST1-luc.
REFERENCES
- 1.Zur Hausen H. 2009. Papillomaviruses in the causation of human cancers - a brief historical account. Virology 384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Marur S, D'Souza G, Westra WH, Forastiere AA. 2010. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. 2000. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tã¢N PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. 2010. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML. 2004. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol 31:744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Thierry F. 2009. Transcriptional regulation of the papillomavirus oncogenes by cellular and viral transcription factors in cervical carcinoma. Virology 384:375–379. doi: 10.1016/j.virol.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. 2018. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol 26:158–168. doi: 10.1016/j.tim.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Songock WK, Kim SM, Bodily JM. 2017. The human papillomavirus E7 oncoprotein as a regulator of transcription. Virus Res 231:56–75. doi: 10.1016/j.virusres.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBride AA. 2013. The papillomavirus E2 proteins. Virology 445:57–79. doi: 10.1016/j.virol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson JD, Howley PM. 1995. Amino-terminal domains of the bovine papillomavirus type 1 E1 and E2 proteins participate in complex formation. J Virol 69:4364–4372. doi: 10.1128/JVI.69.7.4364-4372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergvall M, Melendy T, Archambault J. 2013. The E1 proteins. Virology 445:35–56. doi: 10.1016/j.virol.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loo YM, Melendy T. 2004. Recruitment of replication protein A by the papillomavirus E1 protein and modulation by single-stranded DNA. J Virol 78:1605–1615. doi: 10.1128/jvi.78.4.1605-1615.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, Loo YM, Militello KT, Melendy T. 1999. Interactions of the papovavirus DNA replication initiator proteins, bovine papillomavirus type 1 E1 and simian virus 40 large T antigen, with human replication protein A. J Virol 73:4899–4907. doi: 10.1128/JVI.73.6.4899-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melendy T, Sedman J, Stenlund A. 1995. Cellular factors required for papillomavirus DNA replication. J Virol 69:7857–7867. doi: 10.1128/JVI.69.12.7857-7867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. 2012. The biology and life-cycle of human papillomaviruses. Vaccine 30(Suppl 5):F55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 16.Vance KW, Campo MS, Morgan IM. 2001. A novel silencer element in the bovine papillomavirus type 4 promoter represses the transcriptional response to papillomavirus E2 protein. J Virol 75:2829–2838. doi: 10.1128/JVI.75.6.2829-2838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vance KW, Campo MS, Morgan IM. 1999. An enhanced epithelial response of a papillomavirus promoter to transcriptional activators. J Biol Chem 274:27839–27844. doi: 10.1074/jbc.274.39.27839. [DOI] [PubMed] [Google Scholar]
- 18.Bouvard V, Storey A, Pim D, Banks L. 1994. Characterization of the human papillomavirus E2 protein: evidence of trans-activation and trans-repression in cervical keratinocytes. EMBO J 13:5451–5459. doi: 10.1002/j.1460-2075.1994.tb06880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demeret C, Desaintes C, Yaniv M, Thierry F. 1997. Different mechanisms contribute to the E2-mediated transcriptional repression of human papillomavirus type 18 viral oncogenes. J Virol 71:9343–9349. doi: 10.1128/JVI.71.12.9343-9349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanczuk H, Thierry F, Howley PM. 1990. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J Virol 64:2849–2859. doi: 10.1128/JVI.64.6.2849-2859.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldak M, Maksym RB, Sperling T, Yaniv M, Smola H, Pfister HJ, Malejczyk J, Smola S. 2010. Human papillomavirus type 8 E2 protein unravels JunB/Fra-1 as an activator of the beta4-integrin gene in human keratinocytes. J Virol 84:1376–1386. doi: 10.1128/JVI.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delcuratolo M, Fertey J, Schneider M, Schuetz J, Leiprecht N, Hudjetz B, Brodbeck S, Corall S, Dreer M, Schwab RM, Grimm M, Wu SY, Stubenrauch F, Chiang CM, Iftner T. 2016. Papillomavirus-associated tumor formation critically depends on c-Fos expression induced by viral protein E2 and bromodomain protein Brd4. PLoS Pathog 12:e1005366. doi: 10.1371/journal.ppat.1005366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlen S, Behren A, Iftner T, Simon C. 2010. Influence of HPV16 E2 and its localisation on the expression of matrix metalloproteinase-9. Int J Oncol 37:337–345. doi: 10.3892/ijo_00000682. [DOI] [PubMed] [Google Scholar]
- 24.Muhlen S, Behren A, Iftner T, Plinkert PK, Simon C. 2009. AP-1 and ERK1 but not p38 nor JNK is required for CRPV early protein 2-dependent MMP-9 promoter activation in rabbit epithelial cells. Virus Res 139:100–105. doi: 10.1016/j.virusres.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Behren A, Simon C, Schwab RM, Loetzsch E, Brodbeck S, Huber E, Stubenrauch F, Zenner HP, Iftner T. 2005. Papillomavirus E2 protein induces expression of the matrix metalloproteinase-9 via the extracellular signal-regulated kinase/activator protein-1 signaling pathway. Cancer Res 65:11613–11621. doi: 10.1158/0008-5472.CAN-05-2672. [DOI] [PubMed] [Google Scholar]
- 26.Wu MH, Chan JY, Liu PY, Liu ST, Huang SM. 2007. Human papillomavirus E2 protein associates with nuclear receptors to stimulate nuclear receptor- and E2-dependent transcriptional activations in human cervical carcinoma cells. Int J Biochem Cell Biol 39:413–425. doi: 10.1016/j.biocel.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Hadaschik D, Hinterkeuser K, Oldak M, Pfister HJ, Smola-Hess S. 2003. The papillomavirus E2 protein binds to and synergizes with C/EBP factors involved in keratinocyte differentiation. J Virol 77:5253–5265. doi: 10.1128/jvi.77.9.5253-5265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunthamala N, Pang CL, Thierry F, Teissier S, Pientong C, Ekalaksananan T. 2014. Genome-wide analysis of high risk human papillomavirus E2 proteins in human primary keratinocytes. Genom Data 2:147–149. doi: 10.1016/j.gdata.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunthamala N, Thierry F, Teissier S, Pientong C, Kongyingyoes B, Tangsiriwatthana T, Sangkomkamhang U, Ekalaksananan T. 2014. E2 proteins of high risk human papillomaviruses down-modulate STING and IFN-kappa transcription in keratinocytes. PLoS One 9:e91473. doi: 10.1371/journal.pone.0091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thierry F, Benotmane MA, Demeret C, Carcinogenesis P, Desaintes C. 2004. A genomic approach reveals a novel mitotic pathway in papillomavirus carcinogenesis. 64:895–903. doi: 10.1158/0008-5472.CAN-03-2349. [DOI] [PubMed] [Google Scholar]
- 31.Gauson EJ, Wang X, Dornan ES, Herzyk P, Bristol M, Morgan IM. 2016. Failure to interact with Brd4 alters the ability of HPV16 E2 to regulate host genome expression and cellular movement. Virus Res 211:1–8. doi: 10.1016/j.virusres.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauson EJ, Windle B, Donaldson MM, Caffarel MM, Dornan ES, Coleman N, Herzyk P, Henderson SC, Wang X, Morgan IM. 2014. Regulation of human genome expression and RNA splicing by human papillomavirus 16 E2 protein. Virology 468–470:10–18. doi: 10.1016/j.virol.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans MR, James CD, Loughran O, Nulton TJ, Wang X, Bristol ML, Windle B, Morgan IM. 2017. A keratinocyte life cycle model identifies novel host genome regulation by human papillomavirus 16 relevant to HPV positive head and neck cancer. Oncotarget 8:81892–81909. doi: 10.18632/oncotarget.18328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans MR, James CD, Bristol ML, Nulton TJ, Wang X, Kaur N, White EA, Windle B, Morgan IM. 2019. Human papillomavirus 16 E2 regulates keratinocyte gene expression relevant to cancer and the viral life cycle. J Virol 93:e01941-18. doi: 10.1128/JVI.01941-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James CD, Prabhakar AT, Otoa R, Evans MR, Wang X, Bristol ML, Zhang K, Li R, Morgan IM. 2019. SAMHD1 regulates human papillomavirus 16-induced cell proliferation and viral replication during differentiation of keratinocytes. mSphere 4:e00448-19. doi: 10.1128/mSphere.00448-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SM, Coljee VW, Pignolo RJ, Rotenberg MO, Cristofalo VJ, Sierra F. 1997. Cloning of the human twist gene: its expression is retained in adult mesodermally-derived tissues. Gene 187:83–92. doi: 10.1016/S0378-1119(96)00727-5. [DOI] [PubMed] [Google Scholar]
- 37.Chen ZF, Behringer RR. 1995. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev 9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 38.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. 2008. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. 2012. EMT and dissemination precede pancreatic tumor formation. Cell 148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A, Settleman J. 2010. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. 2015. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam WL, Weinberg RA. 2013. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, Chouaib S. 2017. New insights into the role of EMT in tumor immune escape. Mol Oncol 11:824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anayannis NV, Schlecht NF, Ben-Dayan M, Smith RV, Belbin TJ, Ow TJ, Blakaj DM, Burk RD, Leonard SM, Woodman CB, Parish JL, Prystowsky MB. 2018. Association of an intact E2 gene with higher HPV viral load, higher viral oncogene expression, and improved clinical outcome in HPV16 positive head and neck squamous cell carcinoma. PLoS One 13:e0191581. doi: 10.1371/journal.pone.0191581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramqvist T, Mints M, Tertipis N, Nasman A, Romanitan M, Dalianis T. 2015. Studies on human papillomavirus (HPV) 16 E2, E5 and E7 mRNA in HPV-positive tonsillar and base of tongue cancer in relation to clinical outcome and immunological parameters. Oral Oncology 51:1126–1131. doi: 10.1016/j.oraloncology.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Nulton TJ, Kim N-K, DiNardo LJ, Morgan IM, Windle B. 2018. Patients with integrated HPV16 in head and neck cancer show poor survival. Oral Oncol 80:52–55. doi: 10.1016/j.oraloncology.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dongre A, Weinberg RA. 2019. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 48.Nulton TJ, Olex AL, Dozmorov M, Morgan IM, Windle B. 2017. Analysis of the cancer genome atlas sequencing data reveals novel properties of the human papillomavirus 16 genome in head and neck squamous cell carcinoma. Oncotarget 8:17684–17699. doi: 10.18632/oncotarget.15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das D, Bristol ML, Smith NW, James CD, Wang X, Pichierri P, Morgan IM. 2019. Werner helicase control of human papillomavirus 16 E1-E2 DNA replication is regulated by SIRT1 deacetylation. mBio 10:e00263-19. doi: 10.1128/mBio.00263-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das D, Smith N, Wang X, Morgan IM. 2017. The deacetylase SIRT1 regulates the replication properties of human papillomavirus 16 E1 and E2. J Virol 91:e00102-17. doi: 10.1128/JVI.00102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higashihori N, Lehnertz B, Sampaio A, Underhill TM, Rossi F, Richman JM. 2017. Methyltransferase G9A regulates osteogenesis via Twist gene repression. J Dent Res 96:1136–1144. doi: 10.1177/0022034517716438. [DOI] [PubMed] [Google Scholar]
- 54.Park SY, Park JW, Chun YS. 2016. Jumonji histone demethylases as emerging therapeutic targets. Pharmacol Res 105:146–151. doi: 10.1016/j.phrs.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 55.Derynck R, Weinberg RA. 2019. EMT and cancer: more than meets the eye. Dev Cell 49:313–316. doi: 10.1016/j.devcel.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McBride AA, Sakakibara N, Stepp WH, Jang MK. 2012. Hitchhiking on host chromatin: how papillomaviruses persist. Biochim Biophys Acta 1819:820–825. doi: 10.1016/j.bbagrm.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wan Y, Liu H, Zhang M, Huang Z, Zhou H, Zhu Y, Tao Y, Xie N, Liu X, Hou J, Wang C. 2020. Prognostic value of epithelial-mesenchymal transition-inducing transcription factors in head and neck squamous cell carcinoma: a meta-analysis. Head Neck 42:1067–1076. doi: 10.1002/hed.26104. [DOI] [PubMed] [Google Scholar]
- 58.Gort EH, Suijkerbuijk KP, Roothaan SM, Raman V, Vooijs M, van der Wall E, van Diest PJ. 2008. Methylation of the TWIST1 promoter, TWIST1 mRNA levels, and immunohistochemical expression of TWIST1 in breast cancer. Cancer Epidemiol Biomarkers Prev 17:3325–3330. doi: 10.1158/1055-9965.EPI-08-0472. [DOI] [PubMed] [Google Scholar]
- 59.Asanoma K, Liu G, Yamane T, Miyanari Y, Takao T, Yagi H, Ohgami T, Ichinoe A, Sonoda K, Wake N, Kato K. 2015. Regulation of the mechanism of TWIST1 transcription by BHLHE40 and BHLHE41 in cancer cells. Mol Cell Biol 35:4096–4109. doi: 10.1128/MCB.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan SH, Gloss B, Bernard HU. 1992. During negative regulation of the human papillomavirus-16 E6 promoter, the viral E2 protein can displace Sp1 from a proximal promoter element. Nucleic Acids Res 20:251–256. doi: 10.1093/nar/20.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan SH, Leong LE, Walker PA, Bernard HU. 1994. The human papillomavirus type 16 E2 transcription factor binds with low cooperativity to two flanking sites and represses the E6 promoter through displacement of Sp1 and TFIID. J Virol 68:6411–6420. doi: 10.1128/JVI.68.10.6411-6420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan EL, Wasson CW, Hanson L, Kealy D, Pentland I, McGuire V, Scarpini C, Coleman N, Arthur JSC, Parish JL, Roberts S, Macdonald A. 2018. STAT3 activation by E6 is essential for the differentiation-dependent HPV18 life cycle. PLoS Pathog 14:e1006975. doi: 10.1371/journal.ppat.1006975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim MS, Lee HS, Kim YJ, Lee DY, Kang SG, Jin W. 2019. MEST induces Twist-1-mediated EMT through STAT3 activation in breast cancers. Cell Death Differ 26:2594–2606. doi: 10.1038/s41418-019-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohd-Sarip A, Teeuwssen M, Bot AG, De Herdt MJ, Willems SM, Baatenburg de Jong RJ, Looijenga LHJ, Zatreanu D, Bezstarosti K, van Riet J, Oole E, van Ijcken WFJ, van de Werken HJG, Demmers JA, Fodde R, Verrijzer CP. 2017. DOC1-dependent recruitment of NURD reveals antagonism with SWI/SNF during epithelial-mesenchymal transition in oral cancer cells. Cell Rep 20:61–75. doi: 10.1016/j.celrep.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 65.Smith JA, White EA, Sowa ME, Powell ML, Ottinger M, Harper JW, Howley PM. 2010. Genome-wide siRNA screen identifies SMCX, EP400, and Brd4 as E2-dependent regulators of human papillomavirus oncogene expression. Proc Natl Acad Sci U S A 107:3752–3757. doi: 10.1073/pnas.0914818107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatterschide J, Bohidar AE, Grace M, Nulton TJ, Kim HW, Windle B, Morgan IM, Munger K, White EA. 2019. PTPN14 degradation by high-risk human papillomavirus E7 limits keratinocyte differentiation and contributes to HPV-mediated oncogenesis. Proc Natl Acad Sci U S A 116:7033–7042. doi: 10.1073/pnas.1819534116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li S, Kendall SE, Raices R, Finlay J, Covarrubias M, Liu Z, Lowe G, Lin YH, Teh YH, Leigh V, Dhillon S, Flanagan S, Aboody KS, Glackin CA. 2012. TWIST1 associates with NF-κB subunit RELA via carboxyl-terminal WR domain to promote cell autonomous invasion through IL8 production. BMC Biol 10:73. doi: 10.1186/1741-7007-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prabhavathy D, Vijayalakshmi R, Kanchana MP, Karunagaran D. 2014. HPV16 E2 enhances the expression of NF-κB and STAT3 target genes and potentiates NF-κB activation by inflammatory mediators. Cell Immunol 292:70–77. doi: 10.1016/j.cellimm.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Zheng S, Hedl M, Abraham C. 2015. Twist1 and Twist2 contribute to cytokine downregulation following chronic NOD2 stimulation of human macrophages through the coordinated regulation of transcriptional repressors and activators. J Immunol 195:217–226. doi: 10.4049/jimmunol.1402808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lourenço AR, Roukens MG, Seinstra D, Frederiks CL, Pals CE, Vervoort SJ, Margarido AS, van Rheenen J, Coffer PJ. 2020. C/EBPɑ is crucial determinant of epithelial maintenance by preventing epithelial-to-mesenchymal transition. Nat Commun 11:785. doi: 10.1038/s41467-020-14556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Facompre ND, Rajagopalan P, Sahu V, Pearson AT, Montone KT, James CD, Gleber-Netto FO, Weinstein GS, Jalaly J, Lin A, Rustgi AK, Nakagawa H, Califano JA, Pickering CR, White EA, Windle BE, Morgan IM, Cohen RB, Gimotty PA, Basu D. 2020. Identifying predictors of HPV-related head and neck squamous cell carcinoma progression and survival through patient-derived models. Int J Cancer 147:3236–3249. doi: 10.1002/ijc.33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gauson EJ, Donaldson MM, Dornan ES, Wang X, Bristol M, Bodily JM, Morgan IM. 2015. Evidence supporting a role for TopBP1 and Brd4 in the initiation but not continuation of human papillomavirus 16 E1/E2-mediated DNA replication. J Virol 89:4980–4991. doi: 10.1128/JVI.00335-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bristol ML, James CD, Wang X, Fontan CT, Morgan IM. 2020. Estrogen attenuates the growth of human papillomavirus-positive epithelial cells. mSphere 5:e00049-20. doi: 10.1128/mSphere.00049-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, Xu LM, Costanzo C, Cheng JQ, Wang LH. 2008. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem 283:14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parajuli P, Singh P, Wang Z, Li L, Eragamreddi S, Ozkan S, Ferrigno O, Prunier C, Razzaque MS, Xu K, Atfi A. 2019. TGIF1 functions as a tumor suppressor in pancreatic ductal adenocarcinoma. EMBO J 38:e101067. doi: 10.15252/embj.2018101067. [DOI] [PMC free article] [PubMed] [Google Scholar]