Abstract
Objectives
Numerous studies show increased prevalence of MDR bacteria amongst asylum seekers, but data on the molecular profiles of such strains are limited. We aimed to evaluate the molecular profiles of ESBL-producing Escherichia coli (ESBL-E. coli) strains isolated from asylum seekers and investigate their phylogenetic relatedness.
Methods
WGS data of ESBL-E. coli isolates from asylum seekers, retrieved from 1 January to 31 December 2016, were analysed to assess MLST STs, fim types, phylogroups and resistance genes. Fifty-two ESBL-E. coli isolates from the Dutch–German border region were used for genome comparison purposes as a control group.
Results
Among 112 ESBL-E. coli isolates from asylum seekers, originating mostly from Syria (n = 40) and Iraq (n = 15), the majority belonged to ST131 (21.4%) and ST10 (17.0%). The predominant gene for β-lactam resistance was blaCTX-M-15 (67.9%), followed by the often co-detected blaTEM-1B (39.3%). No mcr or carbapenemase genes were detected. The majority of the strains belonged to phylogroups B2 (38.4%) and A (32.1%), carrying fimH27 (25%) and fimH30 (19.6%). A core genome MLST minimum spanning tree did not reveal clusters containing strains from the asylum seekers and the control group. Five clusters were formed within the asylum seeker group, by strains isolated from people originating from different countries.
Conclusions
The most frequently isolated clones in this study were isolated on a regular basis within the Dutch population before the increase in the asylum seeker population. No mcr- or carbapenemase-producing clones were detected among the asylum seeker population. Minor clustering was observed amongst the asylum seeker strains.
Introduction
Increased numbers of refugees and asylum seekers have entered Europe during the last decade. At the end of 2018, the United Nations High Commissioner for Refugees reported nearly 6.5 million refugees and migrants residing in Europe.1 According to the Immigration and Naturalization Service of the Ministry of Justice and Security, 196 519 asylum seekers entered the Netherlands from January 2013 to December 2018. The main countries of origin include the Syrian Arab Republic, Afghanistan, Iraq, Iran and Eritrea.2
This increase in displaced populations has public health implications. The health needs of refugees and asylum seekers require coordinated efforts from public health institutions. Medical care of refugees and asylum seekers can be challenging for public health systems and hospitals.3 Although data on the epidemiology of MDR organisms (MDROs) in most of the countries of origin are limited, studies have shown an increased MDRO prevalence amongst refugees and asylum seekers. A recently published systematic review and meta-analysis on antimicrobial resistance among migrants in Europe showed a pooled prevalence of any detected antimicrobial resistance carriage or infection of 25.4% (95% CI 19.1%–31.8%). The pooled prevalence of MDR Gram-negative bacteria was 27.2% (95% CI 17.6%–36.8%).4
The need for adequate and timely MDRO detection and, subsequently, the implementation of infection prevention measures regarding refugee inpatients is imperative. WGS has proved to be a valuable tool for microbial analysis on a molecular level and for MDRO outbreak investigation. During the last decade, WGS has been increasingly integrated in microbiology laboratories as part of the daily routine diagnostics, as it became easier, faster and cheaper to use.5 This technique combined with bioinformatics tools can provide, in less than 48 h, an abundance of valuable information regarding MDROs, including detection, identification, genetic resistance profile, genotype and epidemiological typing. Further analysis of the data can also determine genetic and phylogenetic relatedness amongst strains, revealing clustering and aiding outbreak investigation.6,7
The main mechanism of resistance to β-lactams in Escherichia coli strains is production of ESBLs, a group of enzymes mainly encoded by CTX-M, TEM and SHV variants.8 During the past decade, blaCTX-M genes have been increasingly detected in Gram-negative bacteria, including E. coli, worldwide, leading to a ‘CTX-M pandemic’ situation.9 This rapid spread of certain CTX-M-producing E. coli lineages carries with it difficulties in typing. Conventional typing methods, such as PFGE and MLST, do not have the discriminatory power to identify clusters of dissemination. Even next-generation sequencing (‘NGS’)-based typing, which has higher discriminatory power, does not always provide conclusive proof of dissemination and should always be interpreted in combination with epidemiological data. Additional methods, such as typing of fimH genes, are useful to subtype certain lineages.10 In addition, sequences of epidemiologically unrelated isolates, so-called context isolates, should be added to the analysis to provide insight into the genetic background of the bacterial population.11
In this study, we evaluated the molecular profiles, including STs, fim types, phylogroups and resistomes, of ESBL-producing E. coli (ESBL-E. coli) strains isolated from hospitalized asylum seekers in 2016. We compared the molecular epidemiology of the isolates from the refugees with that of a collection of ESBL-E. coli strains from the Dutch–German border region, from hospitalized patients and a community population in 2012, before the number of refugees started to increase.
Materials and methods
Study design
When entering the Netherlands, all asylum seekers are appointed to an asylum seeker centre (ASC) and are registered under the ASC’s address. Asylum seekers included in the study were identified by the ASC address at which they resided. Data on patient characteristics were retrospectively collected from the Certe laboratory system. Study material included screening samples for MDRO carriage before admission (throat, rectum and nose) and clinical samples (e.g. blood, wounds and urogenital) from asylum seekers. All of these samples were obtained as part of standard care.
Study population
We included asylum seekers, hospitalized in the northern part of the Netherlands from 1 January to 31 December 2016, who tested positive for ESBL-E. coli strains. Demographic data, such as age, sex and country of origin, were collected from the laboratory system and the healthcare system for asylum seekers. All ESBL-E. coli strains isolated from the study population were included in the group of asylum seeker strains. Duplicate strains with the same molecular, phenotypic and genotypic profile that were from the same asylum seeker were excluded.
Bacterial identification and antimicrobial resistance mechanism detection
ESBL-E. coli strains from asylum seekers were obtained in the Certe laboratory. This laboratory performs routine microbiological analyses for primary and secondary medical care in the north-east of the Netherlands, including the ASC population in this part of the country. Screening and clinical samples were cultured in a variety of selective (solid) media used for MDRO detection, including MacConkey agar with 0.5 mg/L ciprofloxacin and 2 mg/L gentamicin (Mediaproducts BV, Groningen, The Netherlands), ChromID ESBL agar and ChromID Carbapenemase agar (both from bioMérieux, Marcy-l’Étoile, France). The presence of ESBL was confirmed with cefotaxime/clavulanate, ceftazidime/clavulanate and cefepime/clavulanate Etests (bioMérieux). Possible carbapenemase-producing Enterobacterales (CPE) were confirmed by CIM test and PCR (Check-Direct CPE assay, Check-Points, Wageningen, The Netherlands) and typed by the national reference network for CPE at the RIVM (National Institute for Public Health), as part of standard care.
Antimicrobial phenotype detection
Susceptibility to amikacin, amoxicillin/clavulanic acid, ampicillin/sulbactam, cefepime, cefotaxime, ceftazidime, ciprofloxacin, colistin, ertapenem, fosfomycin, gentamicin, imipenem, levofloxacin, meropenem, nitrofurantoin, piperacillin/tazobactam, tigecycline and trimethoprim/sulfamethoxazole was determined using Vitek 2 (bioMérieux). EUCAST guidelines were used for interpretation of MICs.
Control group isolates
The control isolate collection consisted of 41 ESBL-E. coli from hospitalized patients and people in the community in the Netherlands and 11 ESBL-E. coli strains from hospitalized patients in Germany.12 The isolates were collected in 2012 and were used as context isolates in the genomic comparisons. All strains included in the control group were analysed using the same workflow as the asylum seeker group strains.
DNA isolation
A total of 112 frozen ESBL-E. coli strains isolated from unique asylum seekers were recultured and incubated for 24 h at 37°C. DNA was extracted using the DNeasy UltraClean Microbial Kit (MoBio Laboratories, Carlsbad, CA, USA), according to the manufacturer’s instructions. A 5 μL aliquot of each isolate was suspended with 300 μL of PowerBead solution. DNA purity was measured using a NanoDrop 2000 C spectrophotometer (Thermo Fisher, Waltham, MA, USA). DNA concentration was measured with a Qubit 2.0 fluorometer, using the double-stranded DNA BR Assay Kit (Life Technologies, Carlsbad, CA, USA).
WGS
Prior to library preparation, isolated DNA was diluted to a concentration of 0.2 ng/μL. DNA library preparation was performed with the Nextera XT v.01 (Illumina Inc., San Diego, CA, USA) kit using 5 μL of diluted DNA according to the manufacturer’s instructions. Libraries were sequenced on an MiSeq sequencer (Illumina Inc.) aiming to generate 250 bp paired-end reads.
Quality check and WGS data analysis
Trimming and de novo assembly was performed using CLC Genomics Workbench v.10.1.2 (QIAGEN, Hilden, Germany). A minimum Phred score (Qscore) of 30 was used. Six parameters were checked for assembly quality: number of contigs <1000, N50 > 15 000, maximum contig length >50 000, percentage of reads used for the assembly >90%, coverage >30× and percentage of the expected genome size >90% to <115%.
The assembled genomes were uploaded to SeqSphere v.5.5.1 (Ridom GmbH, Münster, Germany) for investigation of phylogenetic relatedness. A minimum spanning tree based on allelic mismatch between the isolates was designed. A maximum of 10 allelic differences was considered as clonal clustering.
SNP-based Neighbor–Joining (NJ) trees were constructed based on the genome sequences using Ridom SeqSphere+ version 5.1.0 (Münster, Germany) with default settings. The genomes were analysed using an ad hoc E. coli scheme based on 2764 targets, including 242 851 bp.
Assembled genomes were uploaded to the web tools ResFinder 3.1 to identify acquired resistance genes13 and FimTyper 1.0 to determine fim type.10 Phylogroups were determined via the EzClermont web app and command-line tool.14,15
Sequences are publicly available at the ENA database (study accession number PRJEB36686).
Statistical analysis
Data were collected in and analysed with SPSS (version 2.23). Descriptive statistics were used for the general characteristics of the study population.
Ethics
This study was evaluated by the Ethics Committee and approval was waived in accordance with Dutch legislation owing to its retrospective nature (University Medical Centre Groningen, METc number 2016/516). No written informed consent was obtained from patients for the use of retrospective data. Patient information was anonymized and de-identified prior to analysis.
Results
General characteristics of the study population
We evaluated single ESBL-E. coli isolates from 112 asylum seekers. General characteristics of the study population and the included samples are described in Table 1.
Table 1.
General characteristics of the study population and the included samples; N = 112
| Female, n (%) | 75 (67) |
| Age (years), median (IQR) | 28.0 (20.4–36.1) |
| Number of days in the Netherlands, median (IQR) | 192 (77–347) |
| Country of origin, n (%) | |
| Syria | 40 (35.7) |
| Iraq | 15 (13.4) |
| Iran | 12 (10.7) |
| Afghanistan | 9 (8.0) |
| Eritrea | 6 (5.4) |
| other from Europe | 6 (5.4) |
| other from Eastern Europe/Russia | 4 (3.6) |
| other from Asia | 8 (7.1) |
| other from Africa | 9 (8.0) |
| Samples, n (%) | |
| rectal | 101 (90.2) |
| urine | 6 (5.4) |
| skin | 2 (1.8) |
| sputum | 1 (0.9) |
| nasal | 1 (0.9) |
| stool | 1 (0.9) |
Routinely measured resistance
Antimicrobial susceptibility of the strains to different antibiotic agents, routinely tested in the Certe laboratory, is described in Table S1 (available as Supplementary data at JAC Online).
All of the isolates were resistant to penicillins, cephalosporins and combinations of penicillins and β-lactamase inhibitors. Also, 56.3% of the isolates were resistant to trimethoprim/sulfamethoxazole. Resistance to ciprofloxacin was observed in 31.3% of the isolates. Regarding aminoglycosides, 27.7% and 33.0% of the isolates were resistant to gentamicin and tobramycin, respectively. No isolate was resistant to meropenem or imipenem. All isolates were susceptible to fosfomycin and colistin.
ST and genotypic profile
The most frequent ST of the asylum seeker isolates was ST131 (21.4%), followed by ST10 (17.0%), ST38 (8.0%) and ST69 (8.9%). Among the control group isolates, the most frequent ST was ST38 (15.4%), followed by ST10 and ST131 (both 11.5%) and ST58 (7.7%). Table 2 shows the STs of the asylum seeker isolates and the blaCTX-M resistance genes carried by the isolates for each ST.
Table 2.
STs of the asylum seeker isolates and the blaCTX-M resistance genes carried by the isolates for each ST
| ST | Total, N (%) |
bla
CTX-M resistance gene, n (%) |
||||
|---|---|---|---|---|---|---|
| bla CTX-M-15 | bla CTX-M-27 | bla CTX-M-3 | bla CTX-M-14 | bla CTX-M-14b | ||
| ST131 | 24 (21.4) | 12 (50.0) | 7 (29.2) | 1 (4.2) | 0 (0) | 0 (0) |
| ST10 | 19 (17.0) | 16 (84.2) | 0 (0) | 2 (10.5) | 1 (5.3) | 0 (0) |
| ST69 | 10 (8.9) | 8 (80) | 0 (0) | 1 (10) | 0 (0) | 0 (0) |
| ST38 | 9 (8.0) | 1 (11.1) | 1 (11.1) | 0 (0) | 1 (11.1) | 3 (33.3) |
| ST12 | 7 (6.3) | 7 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST120 | 4 (3.6) | 3 (75.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST93 | 4 (3.6) | 4 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST1193 | 3 (2.7) | 2 (66.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST73 | 3 (2.7) | 2 (66.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST648 | 2 (1.8) | 2 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST3877 | 2 (1.8) | 2 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST58 | 2 (1.8) | 2 (100.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 23 (20.5) | 14 (60.9) | 1 (4.3) | 1 (4.3) | 2 (8.7) | 0 (0) |
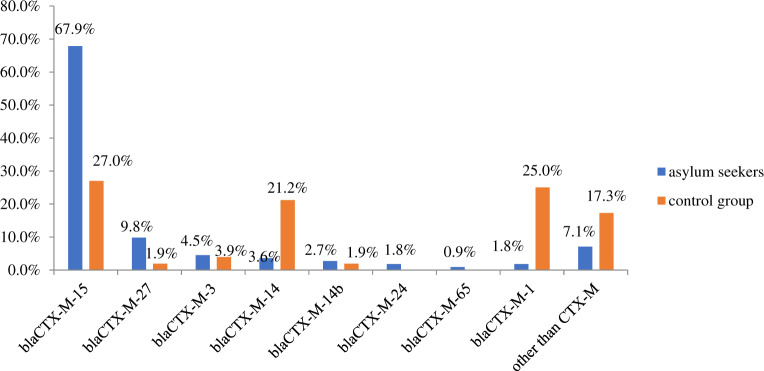
The distribution of blaCTX-M resistance genes harboured by the isolates from asylum seekers and the control group can be seen in Figure 1.
Figure 1.
Distribution of the most frequently detected blaCTX-M genes among the asylum seeker and control group isolates. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The most frequently observed CTX-M gene for β-lactam resistance was blaCTX-M-15 for both groups, followed by blaCTX-M-27 for the asylum seekers and blaCTX-M-1 for the control group. Other, non-CTX-M β-lactam resistance genes detected in the asylum seeker isolates were blaTEM-1B (n = 44, 39.3%), blaOXA-1 (n = 11, 9.8%), blaSHV-12 (n = 2), blaTEM-33 (n = 2), blaDHA-1 (n = 2), blaTEM-1C (n = 1) and blaCMY-60 (n = 1).
All of the asylum seeker isolates carried resistance genes related to more than one antibiotic group, including aminoglycosides, fluoroquinolones, sulphonamides and trimethoprim. Regarding aminoglycoside resistance, 44 isolates harboured strA, 43 harboured strB, 42 harboured addA5 and 21 harboured acc(3)-IId. The main genes carried by the isolates that encoded quinolone resistance were qnrS1 (n = 26) and aac(6′)Ib-cr (n = 10). For sulphonamide resistance, 48 isolates carried sul1 and 40 isolates carried sul2. Lastly, the main trimethoprim resistance genes detected were dfrA17 (n = 44) and dfrA14 (n = 14). Of note, no mcr and carbapenemase genes were detected.
Phylogroup and fim type
Asylum seeker isolates belonged primarily to phylogroups B2 (n = 43, 38.4%) and A (n = 36, 32.1%). The remaining isolates belonged to phylogroups D (n = 24, 21.4%), B1 (n = 6, 5.4%) and F (n = 3, 2.7%).
Subtyping of fimH alleles of isolates from asylum seekers showed that fimH27 was the most frequent type (n = 28, 25%), followed by fimH30 (n = 22, 19.6%) and fimH5 (n = 6, 5.4%). Thirteen isolates (11.6%) did not carry any fim gene. The remaining isolates carried a wide variety of different fim genes. Of note, 19 out of the 24 isolates that belonged to ST131 carried fimH30 and 8 out of the 19 isolates that belonged to ST10 carried fimH27.
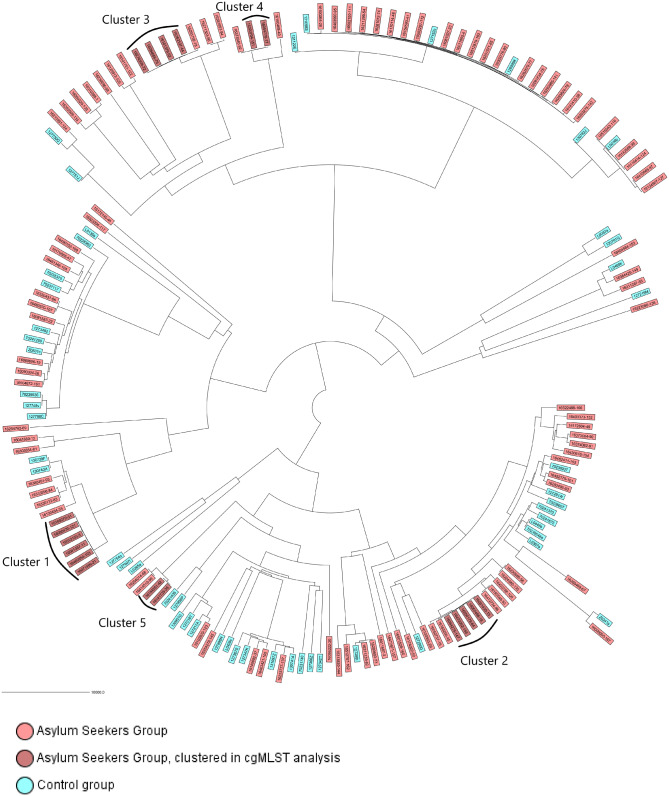
Analysis of the core genome MLST (cgMLST) NJ tree, including asylum seeker and control group isolates, is shown in Figure 2. Furthermore, the cgMLST NJ tree is shown in Figure S1 in rectangular form, including metadata, such as country of origin of the asylum seekers, isolate ST and isolate phylogroup (given in the columns next to the tree).
Figure 2.
cgMLST Neighbor–Joining tree, including asylum seeker and control group isolates. Genomes were analysed using an ad hoc E. coli scheme based on 2764 targets, including 24 2851 bp. Control group isolates were from health institutions near the Dutch–German border region. Isolates that formed clusters in further phylogenetic analysis are indicated in dark pink. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Phylogenetic relatedness and cluster analysis
A minimum spanning tree of the asylum seeker and control group isolates can be seen in Figure S2. The observed allelic distance ranged from 0 to 2371 alleles.
Cluster analysis revealed five clusters within the asylum seeker group isolates (Figure S2). Cluster 1 consisted of six isolates belonging to ST69, phylogroup D, subtype fimH27 and carrying blaCTX-M-15 (Figure 2). Two of the isolates were from asylum seekers originating from Syria and the remaining four were from asylum seekers originating from Palestine, Afghanistan, Iraq and Yugoslavia. The isolates from the Syrian and Iraqi asylum seekers were from rectal and skin samples, cultured a day apart, respectively, and the isolates from the Palestinian and Afghan asylum seekers came from sputum and urine samples, respectively, cultured 17 days apart. Cluster 2 was formed by five isolates belonging to ST10, phylogroup A, subtype fimH27 and carrying blaCTX-M-15 (Figure 2). All isolates were obtained from asylum seekers originating from different countries, namely Syria, Iraq, Eritrea, Turkey and Benin. The isolates from the Syrian and Iraqi asylum seekers were from rectal samples, cultured 4 days apart. Cluster 3 consisted of four isolates belonging to ST12, phylogroup B2, no fim subtype and carrying blaCTX-M-15 (Figure 2). Two of the strains were isolated from Syrian asylum seekers, one from a Mongolian asylum seeker and one from an Afghan asylum seeker. One of the isolates that was from a Syrian asylum seeker and the isolate that was from an Afghan asylum seeker were from rectal samples, cultured a week apart. Cluster 4 included two isolates belonging to ST1193, phylogroup B2, subtype fimH64 and carrying blaCTX-M-15 (Figure 2). One was isolated from a Syrian asylum seeker and the other from an Iranian; they were isolated from urine and rectal samples, respectively, cultured 7 months apart. Cluster 5 consisted of two isolates belonging to ST120, phylogroup A, subtype fimH237 and carrying blaCTX-M-15 (Figure 2). The two isolates were from a Syrian asylum seeker and an Eritrean asylum seeker; they were isolated from rectal samples, cultured 10 days apart. No clusters contained isolates belonging to both asylum seekers and control group isolates.
Discussion
A total of 112 ESBL-E. coli strains isolated from asylum seekers were analysed using WGS in order to investigate their phylogenetic relatedness and possible transmission within the asylum seeker population in the northern part of the Netherlands. The asylum seeker study population originated mainly from Syria and had been residing in the Netherlands for a median of 192 days.
All isolates were phenotypically resistant to β-lactams and exhibited various resistance profiles, with all of them being resistant to at least one more antibiotic group, such as aminoglycosides, quinolones and sulphonamides. The genes that encode resistance to these antibiotic agents are often located on plasmids that can co-harbour different resistance genes and can be horizontally transferred amongst Enterobacterales, such as E. coli, rendering the strains MDR.16
The majority of the isolates belonged to ST131 and ST10, and harboured a blaCTX-M-15 gene. This is in accordance with the epidemiological profile of the high-risk ST131 blaCTX-M-15 clone. ST131 blaCTX-M-15 is currently globally disseminated and is identified as the most widespread CTX-M ESBL enzyme worldwide.17,18 The Netherlands has also been affected by the ST131 blaCTX-M-15 clone. In a recently published study conducted in Dutch hospitals, between 2014 and 2016, the dominant clone found among ESBL-E. coli blood isolates was ST131 carrying blaCTX-M-15.19 Furthermore, in a study conducted in the Netherlands in 2016, the clone was isolated among community-associated and hospitalized patients,20 indicating that the clone existed in both the community and hospitals in the Netherlands before the number of refugees started to increase in 2015 and 2016.
The majority of the study isolates harboured blaCTX-M-15, regardless of the ST to which they belonged. Even though strains carrying blaCTX-M-15 have been reported all over Europe, strains carrying this gene are isolated at a higher rate in Middle Eastern, Asian and African regions.21 Furthermore, epidemiological data on the distribution of such strains indicate that African and Asian regions could serve as a reservoir and facilitate global dissemination.22 A German study that investigated the antibiotic resistomes of refugees reported high prevalence rates for β-lactamase genes: mainly blaTEM, blaCTX-M group 1 and blaSHV.23 Another German study reported high detection of blaCTX-M group 1 genes, followed by blaTEM and blaSHV, among ESBL-producing Enterobacteriaceae isolates from Libyan and Syrian patients.24 Furthermore, a study performed in Saudi Arabia showed a prevalence of blaCTX-M-15 or blaCTX-M-14 of 60% among ST131 uropathogenic E. coli strains.25 In addition, an Iranian study, published in 2017, demonstrated a high prevalence of ST131 blaCTX-M-15 amongst clinical E. coli strains.26 A high prevalence of strains carrying these genes amongst asylum seekers from Iran, Syria and Afghanistan was also documented in our study.
Despite the fact that some clustering among the isolates from the asylum seekers was observed, no clear pattern of transmission was documented. Isolates that exhibited close phylogenetic relatedness formed five clusters. As expected, isolates within each cluster exhibited identical genetic characteristics, such as ST, phylogroup and fim type. However, isolates included within each cluster did not show a clear epidemiological link, since they were isolated from asylum seekers mostly originating from different countries. Furthermore, even though certain isolates in clusters 1, 2, 3 and 5 were isolated within 10 days or less, clear epidemiological links cannot be hypothesized without additional information, such as department and institution of hospitalization, ASC of residence and countries they have travelled through before entering the Netherlands. Due to limited clustering and wide dispersion of the origin of the asylum seekers carrying the isolates within each cluster, no conclusion can be drawn regarding the geographical epidemiology and origin of the isolates.
A limited number of studies have previously sequenced MDROs in a refugee/asylum seeker patient population.27,28 To our knowledge, this is the first study to investigate ESBL-E. coli strains isolated from asylum seekers using WGS on a large scale documenting various genetic characteristics, such as STs, genotypic resistance profiles and phylogenetic relatedness. This information is still scarce in the related literature and can help to optimize treatment, hospital hygiene strategies and infection control measures. Furthermore, our study population exhibited a large variation in age, number of days in the Netherlands and country of origin, reflecting the main countries from which migrants originate, namely Syria, Afghanistan and Iraq.1
Due to the retrospective nature of this study, we did not have access to important information, such as travelling and antibiotic consumption history. In addition, information regarding asylum seeker hospitalization, such as reason for admission, department of admission, duration of hospitalization and treatment given, was not available. Furthermore, we had no access to data regarding the specific ASCs where our study population resided after their arrival in the Netherlands. Close contact within a facility can lead to transmission of MDROs. In our study, no clear pattern of transmission was observed.
Conclusions
The most frequently isolated clones in the study are already detected on a regular basis within the Dutch population. No mcr- or carbapenemase-producing clones were detected among the asylum seeker population. No clustering between asylum seekers and control group strains was observed.
Even though no assumptions can be made on whether transmission within the asylum seeker population occurs or not, small clustering within the asylum seeker strains could be an indication of this. Based on the results of this study, there is no clear evidence as to whether asylum seekers obtained their MDROs in their country of origin, during their journey to the Netherlands or after their arrival in the Netherlands. Asylum seekers originating from the same country showed a large variability in resistance and phylogenetic relatedness.
Further research on the genetic characteristics of MDRO isolates carried by asylum seekers could reveal important information on transmission and cluster formation.
Supplementary Material
Acknowledgements
Part of the results of this study was presented at the Twenty-Ninth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 2019 (Abstract 3783).
Funding
This work was supported by Marie Sklodowska-Curie Actions (grant agreement number: 713660—PRONKJEWAIL—H2020—MSCA-COFUND-2015). The study was partly supported by the INTERREG V A-funded project EurHealth-1Health (202085), which is part of a Dutch–German cross-border network supported by the European Union, the Dutch Ministry of Health, Welfare and Sport (VWS), the Ministry of Economy, Innovation, Digitalization and Energy of the German Federal State of North Rhine-Westphalia and the German Federal State of Lower Saxony. This study was partly funded by the J. K. de Cock stichting (number 2017-59) and the Gratama stichting (number 661232).
Transparency declarations
J.W.R. is currently an employee of IDbyDNA. IDbyDNA did not have any influence on the interpretation of the reviewed data and conclusions drawn, or on the drafting of the manuscript, and did not (financially) support the study. All other authors: none to declare.
Supplementary data
Table S1 and Figures S1 and S2 are available as Supplementary data at JAC Online.
References
- 1.UNHCR. Global Displacement Report 2018. https://www.unhcr.org/5d08d7ee7.pdf.
- 2.Immigration and Naturalization Services. https://ind.nl/en/Documents/Asylum%20Trends%20(Hoofdrapport)%20December%202018.pdf.
- 3. Gushulak B, Weekers J, Macpherson D.. Migrants and emerging public health issues in a globalized world: threats, risks and challenges, an evidence-based framework. Emerg Health Threats J 2010; 2: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nellums LB, Thompson H, Holmes A. et al. Antimicrobial resistance among migrants in Europe: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18: 796–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Didelot X, Bowden R, Wilson DJ. et al. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet 2012; 13: 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Köser CU, Ellington MJ, Cartwright EJP. et al. Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. PLoS Pathog 2012; 8: e1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deurenberg RH, Bathoorn E, Chlebowicz MA. et al. Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol 2017; 243: 16–24. [DOI] [PubMed] [Google Scholar]
- 8. Coque TM, Baquero F, Canton R.. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill 2008; 13: pii=19044. [PubMed] [Google Scholar]
- 9. Cantón R, Coque TM.. The CTX-M β-lactamase pandemic. Curr Opin Microbiol 2006; 9: 466–75. [DOI] [PubMed] [Google Scholar]
- 10. Roer L, Tchesnokova V, Allesøe R. et al. Development of a web tool for Escherichia coli subtyping based on fimH alleles. J Clin Microbiol 2017; 55: 2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosch T, Lutgens SPM, Hermans MHA. et al. Outbreak of NDM-1-producing Klebsiella pneumoniae in a Dutch hospital, with interspecies transfer of the resistance plasmid and unexpected occurrence in unrelated health care centers. J Clin Microbiol 2017; 55: 2380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou X, Garcia-Cobos S, Ruijs GJHM. et al. Epidemiology of extended-spectrum β-lactamase-producing E. coli and vancomycin-resistant enterococci in the Northern Dutch–German cross-border region. Front Microbiol 2017; 8: 1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carattoli A, Zankari E, García-Fernández A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 2014; 58: 3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clermont O, Christenson JK, Denamur E. et al. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 2013; 5: 58–65. [DOI] [PubMed] [Google Scholar]
- 15. Waters NR, Abram F, Brennan F. et al. Easily phylotyping E. coli via the EzClermont web app and command-line tool. bioRxiv May 2018; 10.1101/317610. [DOI] [PMC free article] [PubMed]
- 16. Rozwandowicz M, Brouwer MSM, Fischer J. et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother 2018; 73: 1121–37. [DOI] [PubMed] [Google Scholar]
- 17. Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V. et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 2008; 61: 273–81. [DOI] [PubMed] [Google Scholar]
- 18. Totsika M, Beatson SA, Sarkar S. et al. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS One 2011; 6: e26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Hout D, Verschuuren TD, Bruijning-Verhagen PCJ. et al. Extended-spectrum β-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli isolates causing bacteremia in the Netherlands (2014–2016) differ in clonal distribution, antimicrobial resistance gene and virulence gene content. PLoS One 2020; 15: e0227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. den Reijer PM, van Burgh S, Burggraaf A. et al. The widespread presence of a multidrug-resistant Escherichia coli ST131 clade among community-associated and hospitalized patients. PLoS One 2016; 11: e0150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bevan ER, Jones AM, Hawkey PM.. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 2017; 72: 2145–55. [DOI] [PubMed] [Google Scholar]
- 22. Shaikh S, Fatima J, Shakil S. et al. Antibiotic resistance and extended spectrum β-lactamases: types, epidemiology and treatment. Saudi J Biol Sci 2015; 22: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Häsler R, Kautz C, Rehman A. et al. The antibiotic resistome and microbiota landscape of refugees from Syria, Iraq and Afghanistan in Germany. Microbiome 2018; 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frickmann H, Köller T, Hagen RM. et al. Molecular epidemiology of multidrug-resistant bacteria isolated from Libyan and Syrian patients with war injuries in two Bundeswehr hospitals in Germany. Eur J Microbiol Immunol (Bp) 2018; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alghoribi MF, Gibreel TM, Farnham G. et al. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J Antimicrob Chemother 2015; 70: 2757–62. [DOI] [PubMed] [Google Scholar]
- 26. Namaei MH, Yousefi M, Ziaee M. et al. First report of prevalence of CTX-M-15-producing Escherichia coli O25b/ST131 from Iran. Microb Drug Resist 2017; 23: 879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kossow A, Stühmer B, Schaumburg F. et al. High prevalence of MRSA and multi-resistant Gram-negative bacteria in refugees admitted to the hospital—but no hint of transmission. PLoS One 2018; 13: e0198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salloum T, Arabaghian H, Alousi S. et al. Genome sequencing and comparative analysis of an NDM-1-producing Klebsiella pneumoniae ST15 isolated from a refugee patient. Pathog Glob Health 2017; 111: 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.