Abstract
Cancer is now the second leading cause of death worldwide. It is estimated that every year, approximately 9.6 million people die of oncologic diseases. The most common origins of malignancy are the lungs, breasts, and colorectum. Even though in recent years, many new drugs and therapeutic options have been introduced, there are still no safe, effective chemopreventive agents. Cyclitols seem poised to improve this situation. There is a body of evidence that suggests that their supplementation can decrease the incidence of colorectal cancer, lower the risk of metastasis occurrence, lower the proliferation index, induce apoptosis in malignant cells, enhance natural killer (NK) cell activity, protect cells from free radical damage, and induce positive molecular changes, as well as reduce the side effects of anticancer treatments such as chemotherapy or surgery. Cyclitol supplementation appears to be both safe and well-tolerated. This review focuses on presenting, in a comprehensive way, the currently available knowledge regarding the use of cyclitols in the treatment of different malignancies, particularly in lung, breast, colorectal, and prostate cancers.
Keywords: cancer, cyclitols, IP6, inositol, chemoprevention, plant foods
1. Cyclitols
Throughout the ages, medicine has relied on natural products, most of which come from plants. However, ancient scientists did not have knowledge of which chemical particles found in plants were responsible for the positive effects of treatment. Currently, thanks to advanced technologies, we are able to extract or synthesize the chosen substances. Cyclitols are natural, widespread, and very promising. This group of compounds, from a chemical point of view, is based on a cycloalkane chain that contains at least three hydroxyl groups, each attached to a different ring carbon. What is interesting about cyclitols is that they are involved in numerous biological processes at a molecular level, so their supplementation can provide a wide range of effects. To date, their role in membrane biogenesis, signal transduction, channel physiology, osmoregulation, phosphate storage, cell wall formation, and antioxidant activity has been confirmed. The most common cyclitols in eukaryotic cells are inositols (Ins) [1,2].
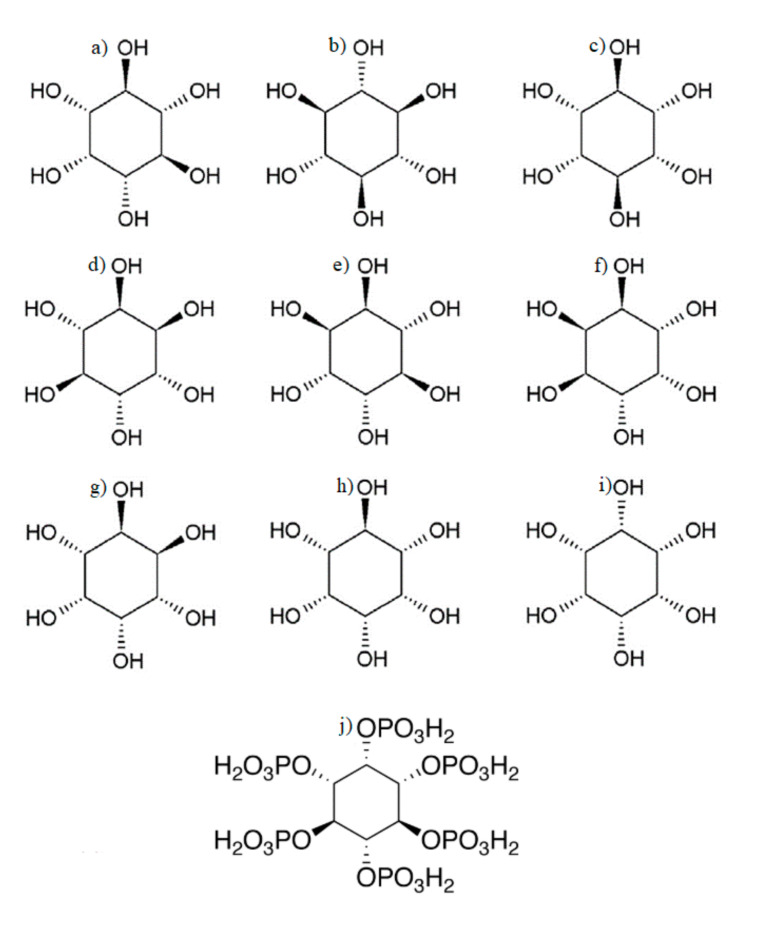
Inositols (Figure 1) are a family of compounds derived from C6 sugar alcohol, with the empirical formula C6H12O6 (1.2,3.4,5.6-cyclohexanol). There are nine known forms of inositol: myo-inositol (MI), scyllo-inositol, muco-inositol, epi-inositol, allo-inositol, cis-inositol, neo-inositol, l-chiro-inositol, and d-chiro-inositol [3]. MI is the dominant form of Ins in intracellular content [4].
Figure 1.
Structural formulas of (a) myo-inositol (MI); (b) scyllo-inositols (Ins); (c) mucco-Ins; (d) D-chiro-Ins; (e) L-chiro-Ins; (f) neo-Ins; (g) allo-Ins; (h) epi-Ins; (i) cis-Ins; and (j) phytic acid (IP6).
MI is, therefore, a widespread molecule, whose supplementation appears to have several positive effects. The most common and easiest method of supplementation of MI and other cyclitols appears to be oral. MI can be consumed by humans in three different forms: free form, inositol-containing phospholipids, and, most frequently, as a phytic acid (IP6) [5]. The largest amount of IP6 can be found in almonds (9.4% of dry weight), walnuts (6.7% of dry weight), and Brazil nuts (6.3% of dry weight) [6]. Among fruits, cantaloupe and citruses (with the exception of lemons) have a high concentration of MI [7]. A 120 g portion of grapefruit juice can provide about 470 mg of MI [8]. However, a typical 2500 kcal American diet provides only about 900 mg of MI per day, whereas a well-balanced diet can provide up to 1500 mg of MI per 1800 kcal [8].
Almost all of the free MI ingested (99.8%) is absorbed in the human gastrointestinal tract. This uptake is possible due to active transport, which occurs against the concentration gradient in a Na+-dependent manner [5,9]. Importantly, glucose or other sugars can significantly reduce MI uptake in a noncompetitive manner [3]. When it comes to the plasma levels of MI achieved by oral intake, it is currently known that supplementation with 4 g of MI powder results in a maximum plasma concentration (Cmax) of 45 µmol/L, whereas supplementation with 2 g MI powder results in a Cmax of 36.3 µmol/L [10]. In normal and healthy subjects, the plasma levels of MI were found to be approximately 30 mM, with a 22 min half-life [7]. MI is also present in serum as phytic acid, with a plasma level of approximately 0.1–0.4 mM. MI can be found in phospholipids or circulating lipoproteins [7].
MI is also synthesized endogenously—primarily in the kidneys, which produce about 4 g of MI per day [3]. Comparing the amounts of MI provided by the diet to the amounts synthesized endogenously, it may seem that supplementation of MI is futile. However, it was proven that rats fed with an MI-free diet had significantly lower concentrations of MI in every tissue, except for the brain [11]. This study found grounds for exogenous supplementation of MI. The kidneys appear to be the most important organ in MI catabolism to d-glucuronic acid through myo-inositol oxygenase [7].
Although MI is involved in numerous documented and possibly unknown processes, supplementation with MI appears to be safe and well-tolerated. The lethal dose that caused the death of 50% of a group of test animals (LD50) of orally administrated MI in mice was about 10,000 mg/kg body weight [10]. In humans, orally administrated doses are typically up to 18 g per day. The side effects of MI supplementation are rare and mild and mainly encompass gastrointestinal symptoms [12]. The safety of MI has been confirmed by its use in infant milk powders, with a generally recognized as safe (GRAS) status [13].
2. Cancers
Neoplastic tumors involve a wide group of disorders originating from different tissues. They are characterized by the uncontrollable growth of abnormal, immature cells capable of invading adjoining tissues and/or spreading to other organs of the body.
Malignancies are the second leading cause of death worldwide, with an estimated 9.6 million oncologic fatalities in 2018. The most common origins of cancer in the male population were the lungs, prostate, colorectum, stomach, and liver. In the female population, the prevailing types were breast, colorectal, lung, cervical, and thyroid cancer [14].
It is currently understood that many cancers are influenced by environmental factors such as smoking and alcohol intake, as well as inappropriate diet [14]. Eliminating these and other risk factors is one of the best ways to reduce the number of deaths caused by cancers. Although prophylactic methods, like vaccines against HPV to prevent cervical cancer [14,15], have been tested, to date, there is no safe chemopreventive agent recommended worldwide for the treatment of malignancies.
The treatment of cancer is a sophisticated, multidisciplinary, and costly field of medicine. The introduction of new drugs has increased the life span of people affected by different tumors; however, the overall cure rate remains unsatisfactory, and there is still a need for new treatment options and new medicines. An important problem is the issues caused by the toxicity of oncological drugs, in particular, neutropenia [16] and thrombocytopenia [17]. Even though new therapeutic options have been introduced to fight against the side effects of cancer treatment, the adverse effects are a critical problem in oncology. Cyclitols may play an important role, as a supplement to regular treatment, by improving the cure rate and decreasing adverse events.
3. Lung Cancer and Cyclitols
Lung cancer is the most common malignancy globally and is the leading cause of cancer-related deaths. According to WHO data, there were over 2 million new cases of lung cancer and over 1.76 million fatalities in 2018 [18]. Considering the high mortality, there have been numerous studies carried out in the search for an effective agent against lung cancer [19]. Attempts concentrating on the use of conventional X-ray scanning or cytological sputum tests did not help to reduce the morbidity of lung cancer [20]. Currently, the only effective method of decreasing the mortality of lung cancer is low-dose computed tomography (CT). The National Lung Screening Trial (NLST) showed that low-dose chest CT when compared to X-ray examination, resulted in a 20% reduction in lung cancer mortality among high-risk individuals (aged 55–74 years and with more than 30 pack-years of smoking history) [21]. Despite limited diagnostic measures and unsatisfactory treatment results, to date, no nutrient has been implemented as a lung tumor prevention agent.
MI may be a possible chemopreventive agent. The data collected by Enstensen et al. showed that mice fed with a 3% MI diet had 40% fewer lung adenomas than the control. Research has demonstrated that a diet with both 3% MI and 0.5 µg/g dexamethasone had an additive effect on the inhibition of pulmonary adenoma formation [22]. These positive effects on reducing tumor multiplicity by MI supplementation are supported by other studies [23,24,25]. A combination of MI and N-acetyl-S-(N-2-phenethylthiocarbamoyl)-l-cysteine (PEITC-NAC) may be more effective at reducing lung tumor multiplicity compared with supplementation of any of these substances alone [24]. Myo-inositol supplementation appears to reduce the activity of IL-6 related pathways, including STAT3 phosphorylation [25]. IL-6 related pathway activation is currently believed to be involved in tumor progression [26].
The results of animal studies led to studies in humans. A phase I study for lung cancer chemoprevention showed that MI supplementation should be investigated as a chemopreventive agent against lung cancer. A significantly increased rate of regression of pre-existing dysplastic lesions was observed in the MI supplementation group [12]. Researchers also found that, after MI treatment, there was a significant reduction in Akt and ERK phosphorylation in dysplastic lesions, but not in hyperplastic or metaplastic ones [27]. Unfortunately, a randomized phase IIb trial of MI used as a chemopreventive agent in smokers did not prove the effectiveness of MI. There was no statistically significant regression or progression of bronchial lesions for the MI group versus the placebo. However, a significant reduction of the IL-6 level in BAL and a significant decrease in the gene expression signature reflective of PI3K activation within the cytologically normal bronchial airway epithelium was seen among complete responders in the MI group. Such a decrease of PI3K activation was not observed among the patients with a complete response in the placebo group [28].
In summary (Table 1), despite the positive effects of MI administration in lung cancer models in animal trials and the evidence that MI reduces the levels of IL-6 and may decrease the activation of PI3K signaling pathways, the results obtained from trials conducted on humans do not support MI usage alone as a possible chemopreventive agent against lung cancer. Based on the results of animal studies, the combination of MI and PEITC-NAC or MI and dexamethasone may have a greater impact on lung cancer chemoprevention. Further studies to evaluate the possible effects of a combination of MI with other substances in lung cancer prevention are needed.
Table 1.
Antineoplastic activity of cyclitols in lung/bronchus cancer models.
| Study | Cyclitol | Human/Animal Species | Results |
|---|---|---|---|
| Enstensen et al. (1993) [22] | MI | A/J mouse |
|
| Hecht et al. (2001) [23] | MI | A/J mouse |
|
| Hecht et al. (2002) [24] | MI | A/J mouse |
|
| Lam et al. [12] (2006) | MI | Human |
|
| Han et al. [27] (2009) | MI | Human |
|
| Lam et al. [28] (2016) | MI | Human |
|
| Unver et al. (2018) [25] | MI | Mouse model (CcspCre/+; Kras LSL-G12D/+) |
|
4. Breast Cancer and Cyclitols
Breast cancer is the most common malignancy in the female population globally, and the second most common malignancy in both sexes [14]. There were over 2 million new cases of breast cancer and over 626,000 fatalities (fifth most-common cause of cancer mortality globally in both sexes) in 2018 [14]. This remarkable difference between the number of new cases of breast cancer and mortality was attributed to the introduction of screening programs (mammography) and the development of new chemotherapy regimens, which have changed the treatment of breast cancer in recent years. Now, new substances to prevent breast cancer, support its treatment and reduce side effects are needed.
Animal trials have provided evidence that IP6 and inositol may be promising chemopreventive agents. The data collected by Shivapurkar et al. showed that the administration of 2% IP6 with a low-fiber diet resulted in a reduction of breast tumor incidence in rats [29]. Similar results of IP6 efficiency in preventing breast cancer were reported in other studies [30,31]. There is also evidence that MI, either alone or in combination with IP6, may have chemopreventive properties against breast cancer [30].
The results of in vitro trials showed that treatment with IP6 was able to reduce the growth of cancer cells, suppress DNA synthesis, increase the expression of lactalbumin, which is associated with luminal cell differentiation [32], arrest cancer cells in the G0/G1 phase [33], decrease the S phase and level of KI-67 expression in cancer cell lines [33], reduce adhesion and motility [34,35], increase the expression of antiproliferative agent PKCδ, increase the activity of p27Kip1, decrease Erk1/2 and Akt activity, and reduce pRb phosphorylation [36]. IP6 may have a synergistic effect on breast cancer treatment with adriamycin and tamoxifen [37]. In vitro trials also showed that the exposure of breast cancer cell lines to inositol resulted in reduced PI3K and phosphorylated Akt activity, decreased SNAI1 expression, increased levels of E-cadherin and β-catenin, and reduced motility, invasiveness, and cytoskeleton stabilization [38].
Human trials demonstrated a potentially positive role of IP6 and MI in the treatment of breast cancer. Bačić et al. reported that supplementation of IP6 + inositol in the treatment of invasive ductal breast cancer with the FEC chemotherapy protocol resulted in fewer side effects and better quality of life as well as a better functional status of supplemented patients as compared to controls. A remarkable observation was that IP6 + inositol-treated patients had smaller decreases in their white blood cell count and platelet count after chemotherapy, while a more significant decrease was noted in the control group [39]. This is an important finding because neutropenia is a common reason to reduce or withhold chemotherapy [40]. Similar results were obtained by Proietti et al. [41].
The group found that the topical usage of 5 g of 4% IP6 as a sodium salt (200 mg of IP6), applied once a day on the breast after lumpectomy during CMF chemotherapy, resulted in fewer side effects, fewer postponed chemotherapy cycles and an improvement in the quality of life and functional status [41].
There is evidence that MI may be a possible therapeutic option in the treatment of other breast conditions, like high breast density [42], which is recognized as a risk factor of breast cancer [43]. MI appears to have a positive effect on the management of breast fibroadenomas [44], a common finding among young women, with a very low risk of cancer transformation [45]. To date, no preventive treatment has been implemented for asymptomatic fibroadenomas, although some grow and require intervention due to clinical symptoms or a bulky size [46]. In such cases, a possible conservative treatment option may be an anti-estrogen drug instead of surgical management [46]. Pasta et al. found supplementation with 400 mg MI (+Boswellia 100 mg and Betaine 350 mg) twice a day for six months resulted in a reduction of the fibroadenoma median volume. There were no side effects recorded during the study [44].
In brief, supplementation with IP6 alone and with IP6 + inositol both seem to exert positive effects in the management of breast cancer, particularly decreasing the side effects of chemotherapy such as neutropenia. Protective molecular changes in gene expression were noted in trials on animals. There is evidence that MI may be a possible therapeutic option in the management of increased breast density or fibroadenomas. The effects of inositol on managing breast conditions are summarized in Table 2.
Table 2.
Antineoplastic activity of cyclitols in breast tumors.
| Study | Clinical status | Cyclitol | Results |
|---|---|---|---|
| Shivapurkar et al. (1995) [29] | MUN-induced rat mammary cancer | IP6 |
|
| Vucenik et al. (1995) [30] | DMBA-induced rat mammary cancer | IP6; Ins; IP6 + Ins |
|
| Shamsuddin et al. (1996) [32] | Human breast cancer cell lines MDA-MB-231 and MCF-7 | IP6 |
|
| El-Sherbiny et al. (2001) [33] | Human breast cancer cell lines MDA-MB-231 and MCF-7 | IP6 |
|
| Tantivejkul et al. (2003) [34] | Human breast cancer cell line: MDA-MB-231 | IP6 |
|
| Tantivejkul et al. (2003) [35] | Human breast cancer cell line: MDA-MB-231 | IP6 |
|
| Tantivejkul et al. (2003) [37] | MCF-7, MDA-MB 231 and adriamycin-resistant MCF-7 (MCF-7/Adr) human breast cell lines | IP6 |
|
| Vucenik et al. (2005) [36] | MCF-7 human breast cancer cell line | IP6 |
|
| Bačić et al. (2010) [39] | Ductal invasive breast cancer during FEC chemotherapy protocol | IP6 |
|
| Pasta et al. (2015) [42] | Mammographic breast density (premenopausal women) | MI |
|
| Pasta et al. (2016) [44] | Breast fibroadenomas | MI |
|
| Dinicola et al. (2016) [38] | Human breast cancer cell lines MDA-MB-231 and ZR-75 | Inositol |
|
| Proietti et al. (2017) [41] | Ductal breast cancer stages II‒III postoperative (lumpectomy); during polychemotherapy CMF | IP6 |
|
5. Colorectal Cancer and Cyclitols
Colorectal cancer is the third most common malignancy globally. In 2018, there were almost 1.85 million new cases of colorectal cancer, with over 880,000 fatalities, making it the second leading cause of death among cancers. In terms of chemoprevention, aspirin treatment decreases the risk of colorectal cancer, although the side effects of its long-term use make it unsuitable for large-scale prophylactic programs [47].
IP6 and inositol appear to exhibit chemopreventive properties against colorectal cancer. The data collected by Shamsuddin et al. showed that supplementation with 1% of Na-IP6 in drinking water resulted in a significant reduction of tumor incidence and a decrease in the mitotic rate in the colonic crypts [48]. These results are supported by other studies [30,50,51,52,53,54]. Supplementation with both IP6 and green tea had a synergistic effect on reducing the multiplicity of tumors (particularly 2% IP6 and 2% green tea) [49]. There are also reports indicating that IP6 and Ins have an immunostimulating effect on NK-cells [50,51]. This is an important finding because it is currently believed that NK-cell activity is in reverse correlation with tumor incidence and that supplementation with both IP6 and Ins may have a synergistic effect on increasing NK activity [50].
Treatment with IP6 also appears to result in inhibitory changes in cancer cell lines, such as a significant restriction of growth, DNA synthesis, and proliferation [52], a decrease of the KI-67 index [53], and an increase of the apoptotic index [54]. The molecular changes after IP6 supplementation involve a reduction of the expression of PI3K, Akt and pAk, collagen IV, fibronectin, laminin, integrin β1, MMP-9, VEGF, bFGF, TGF-β [53], p21Waf1/Cip1 [54], and an increase in caspase-9 activity [53]. Again, treatment with both Ins and IP6 appears to have a synergistic effect [53]. Complementary results were obtained by Schröterová et al., who showed that exposure to IP6 significantly decreased the levels of MMP-2, MMP-9, I-Cam1, EpCam, and N-cadherin. Cell migration was significantly reduced after IP6 treatment [55].
In summary, IP6 demonstrated chemopreventive properties against colorectal cancer. Research showed that both IP6 and Ins enhanced NK-cell activity, which was inversely associated with tumor occurrence. There is evidence that IP6 and inositol supplementation may reduce the expression of proteins, enzymes, and factors that are crucial for the occurrence of metastasis, which leads to the conclusion that IP6 and Ins may reduce the number of colorectal cancer metastases. A summary of the effects of cyclitols on managing colorectal cancers is presented in Table 3.
Table 3.
Antineoplastic activity of cyclitols in colon cancer.
| Study | Clinical Status | Human/Animal Species | Cyclitol | Results |
|---|---|---|---|---|
| Shamsuddin et al. (1988) [48] | AOM-induced colon cancer | Rat | IP6 |
|
| Shamsuddin et al. (1989) [56] | AOM-induced colon cancer | Rat | IP6 |
|
| Baten et al. (1989) [50] | DMH-induced colon cancer | Mouse | IP6 |
|
| Ullah et al. (1990) [57] | AOM-induced colon cancer | Rat | IP6 |
|
| Pretlow et al. (1992) [49] | AOM-induced colon cancer | Rat | IP6 |
|
| Yang et al. (1995) [52] | HT-29 human colon carcinoma cells | IP6 |
|
|
| Shivapurkar et al. (1995) [29] | AOM-induced colon cancer | Rat | IP6 |
|
| Challa et al. (1997) [58] | AOM-induced colon cancer | Rat | IP6 |
|
| El-Sherbiny et al. (2001) [33] | HT-29 human colon carcinoma cells | IP6 |
|
|
| Zhang et al. (2005) [51] | DMH-induced colon cancer | Rat | IP6 |
|
| Liu et al. (2015) [53] | HT-29 human colon carcinoma cells | IP6 |
|
|
| Kapral et al. (2017) [54] | Colon cancer Caco-2 cells | IP6 |
|
|
| Schröterová et al. (2018) [55] | Colon cancer SW620 cells | IP6 |
|
|
6. Prostate Cancer and Cyclitols
The prevalence of prostate cancer has increased in recent years. It is now the most common malignancy in elderly men in the western world [59]. The risk of developing prostate cancer is mainly associated with age—the probability increases from 0.005% in men younger than 30 to around 50% in men older than 70 years [60,61]. The lifetime risk of developing prostate cancer is currently estimated at 16.7% (one in six men) [62]. Although only a small number of patients will experience fully invasive prostate cancer, the high prevalence of this malignancy shows the need for new drugs, therapeutic options, and efficient chemopreventive agents.
Cyclitols, in particular IP6, are a good candidate for possible chemopreventive and therapeutic options for prostate cancer (Table 4). A study conducted by Shamsuddin et al. on PC-3 human prostate cancer cells showed that treatment with IP6 might inhibit both growth and DNA synthesis. IP6 treatment increased the expression of HLA-1 class antigens and increased prostatic acid phosphatase (PAP) activity, indicating cell differentiation [63]. Research has reported that IP6 can induce G0/G1 cell cycle arrest of treated cells [64]. A study conducted by Singh et al. showed antiproliferative changes in the protein expression and activity in prostate cancer cells after treatment with IP6. In one report, mice that were injected with DU145 cells and then treated with 2% IP6 in drinking water had significantly reduced tumor weights. IP6 significantly decreased the proliferation index and increased the apoptotic rate. The microvessel density in prostate tumor xenografts was significantly reduced after IP6 treatment [65].
Table 4.
Antineoplastic activity of cyclitols in other cancers.
| Type of Cancer | Study | Clinical Status | Cyclitol | Results |
|---|---|---|---|---|
| Prostate | Shamsuddin et al. (1995) [62] | PC-3 human prostate cells | IP6 |
|
| Prostate | Sharma et al. (2003) [64] | Mouse prostate (TRAMP-C1) cells | IP6 |
|
| Prostate | Singh et al. (2004) [65] | DU145 cells injected into nude mice | IP6 |
|
| Prostate | Lin et al. (2013) [63] | PC-3 and DU145 cells | d-pinitol |
|
| Pancreas | Somasundar et al. (2004) [66] | MIAPACA and PANC1 pancreatic cancer cell lines | IP6 |
|
| Pancreas | McMillan et al. (2007) [67] | PANC1 and MIAPACA | IP6 |
|
| Liver | Lee et al. (2005) [68] | Rat treated with DEN | IP6 and Ins |
|
| Liver | Nishino (2009) [69] | Patients with chronic viral hepatitis and cirrhosis | MI |
|
| Osteosarcoma | Ren et al. (2017) [70] | In vitro (K7M2 and MG63.3 cells) and in vivo trials (mice with injected K7M2 cells) | IP6 |
|
| Melanoma | Khurana et al. (2018) [71] | Case report | IP6 + Ins |
|
Another cyclitol that appears to have a positive effect on prostate cancer treatment is d-pinitol. A study by Lin et al. showed that there was a significant decrease in cell migration and invasion after treatment with d-pinitol. These observations were supported by significantly lower integrin αvβ3 expression in the experimental group. Significant decreases in the levels of p-FAK and p-p65 and in the activity of c-Src kinase and NF-κβ luciferase after d-pinitol treatment were noted. However, in contrast to IP6, d-pinitol does not appear to increase the apoptotic rate of prostatic cancer cells [63].
In summary, IP6 demonstrates the properties of a possible chemopreventive agent against prostate cancer and may be implemented in the future. In contrast, d-pinitol appears to be a cyclitol that may find use in more advanced stages of the disease. The small number of studies conducted indicates the need for further research to evaluate the value of these cyclitols in prostate cancer treatment.
7. Cyclitols and Other Cancers
There are reports showing the positive effects of cyclitols on other types of cancers (Table 4). A study conducted by Somasundar et al. showed that treatment of pancreatic cancer cells (MIAPACA and PANC1) with IP6 resulted in significant growth inhibition and an increased apoptotic rate [66]. These results were supported by a study by McMillan et al., which showed that the IP6 effect on growth inhibition might be increased by catechin. The latter trial showed that treatment with IP6 reduced the level of VEGF, and synergism with catechin was noted. However, in this study, no increased apoptosis was noted in the IP6-treated group, but a synergism was noted between catechin and IP6 in inducing apoptosis [67]. Clinical trials are being performed to evaluate the possible benefits of the treatment of hepato-pancreato-biliary neoplasm with myo-inositoltrispyrophosphate (ITPP) [72].
Liver cancer is another type of malignancy that cyclitols appear to have positive effects on. A study conducted by Lee et al. showed that supplementation with either IP6 or In resulted in a significantly lower number of preneoplastic lesions. A synergistic effect was noted between IP6 and In. The next finding of the study was that in IP6- and/or In-treated groups, there was a significantly lower level of lipid peroxidation, a known factor enhancing cancer development [68]. A report by Nishino et al. demonstrated that supplementation with both MI and β-cryptoxanthin in mandarin orange juice and carotenoid capsules was able to reduce hepatocellular cancer incidence by 81% in patients with chronic viral hepatitis and cirrhosis. In this same experiment, supplementation with only carotenoid capsules reduced cancer incidence by only about 50% [69].
A study conducted by Ren et al. showed that treatment with IP6 might have positive effects on osteosarcoma. In this trial, it was not only shown that IP6 can inhibit the proliferation rate and cell growth in the K7M2 and MG63.3 cell lines, but also that IP6 treatment was able to increase the survival rate in a mouse model of osteosarcoma metastases [70].
Finally, a case report indicated that treatment with both IP6 and Ins resulted in the complete clinical and radiological remission of melanoma [71].
8. Cyclitols and Other Disorders
The supplementation of cyclitols appears to have positive effects on many other disorders. The effects on polycystic ovary syndrome (PCOS) involve a decrease in the BMI [73], a return of spontaneous ovary function, improved fertility [73], positive hormonal changes [74,75], and a decrease in the HOMA-IR index, as well as positive lipid profile changes [76]. Cyclitols appear to be useful in the treatment of metabolic syndrome and diabetes [2]. There is also a report suggesting that MI treatment may be a game-changing option for people diagnosed with Hashimoto’s thyroiditis [77].
9. Conclusions
Cancer is one of the most serious problems in medicine. Although many new drugs and therapies have been introduced in recent years, malignancies are still the second leading cause of death worldwide. One of the factors that may be responsible for such unsatisfactory statistics is that there is no known safe chemopreventive agent that may be used on a large scale for most cancers. Cyclitols may bridge the gap in this area. There is evidence that cyclitols may, in the future, become one of the elements of cancer treatment—both as an antitumor agent and as an agent helping to reduce the side effects of other therapies. The antineoplastic properties of cyclitols appear to be mainly due to the reduction of the activity of the IL-6- and STAT3-related pathways, stimulating NK cell activity, positive molecular changes in gene expression, and cytoskeleton stabilization. To fully recognize the potential of different cyclitols in both chemoprevention and the treatment of cancers, further and larger-scale human trials are required.
Abbreviations
| IL-6 | interleukin-6 |
| STAT | signal transducer and activator of transcription gene family |
| BALF | bronchoalveolar lavage fluid |
| Akt | RAC-alpha serine/threonine–protein kinase |
| ERK | extracellular signal-regulated kinase |
| FEC-5 | fluorouracil, epidoxorubicin and cyclophosphamide |
| CMF | cyclophosphamide methotrexate fluorouracil |
| HLA-1 | human leukocyte antigen I |
| P-FAK | phosphorylated focal adhesion kinase |
| BMI body | mass index |
| HOMA-IR | homeostatic model assessment for insulin Resistance |
Author Contributions
Conceptualization, J.W. and K.W.; methodology, K.W.; writing—original draft preparation, K.W.; writing—review and editing, J.W. and M.J.; visualization, K.W.; project administration, J.W.; funding acquisition, J.W. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by statutory grant No. 61610001–100, School of Medicine, Collegium Medicum, University of Warmia and Mazury in Olsztyn, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carlomagno G., Unfer V. Inositol safety: Clinical evidence. Eur. Rev. Med. Pharmacol. 2011;15:931–936. [PubMed] [Google Scholar]
- 2.Antonowski T., Osowski A., Lahuta L., Górecki R., Rynkiewicz A., Wojtkiewicz J. Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes. Nutrients. 2019;11:2314. doi: 10.3390/nu11102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizzarri M., Fuso A., Dinicola S., Cucina A., Bevilacqua A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin. On Drug Metab. Toxicol. 2016;12:1181–1196. doi: 10.1080/17425255.2016.1206887. [DOI] [PubMed] [Google Scholar]
- 4.Facchinetti F., Bizzarri M., Benvenga S., D’Anna R., Lanzone A., Soulage C., Renzo G.C.D., Hod M., Cavalli P., Chiu T.T., et al. Results from the International Consensus Conference on Myo-inositol and d-chiro-inositol in Obstetrics and Gynecology: The link between metabolic syndrome and PCOS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;195:72–76. doi: 10.1016/j.ejogrb.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Holub B.J. Metabolism and Function of myo-Inositol and Inositol Phospholipids. Annu. Rev. Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- 6.Schlemmer U., Frølich W., Prieto R.M., Grases F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009;53:S330–S375. doi: 10.1002/mnfr.200900099. [DOI] [PubMed] [Google Scholar]
- 7.Croze M.L., Soulage C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:1811–1827. doi: 10.1016/j.biochi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Clements R.S., Darnell B. Myo-inositol content of common foods: Development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1980;33:1954–1967. doi: 10.1093/ajcn/33.9.1954. [DOI] [PubMed] [Google Scholar]
- 9.Nahapetian A., Young V.R. Metabolism of 14C-Phytate in Rats: Effect of Low and High Dietary Calcium Intakes. J. Nutr. 1980;110:1458–1472. doi: 10.1093/jn/110.7.1458. [DOI] [PubMed] [Google Scholar]
- 10.Carlomagno G., Grazia S.D., Unfer V., Manna F. Myo-inositol in a new pharmaceutical form: A step forward to a broader clinical use. Expert Opin. Drug Deliv. 2012;9:267–271. doi: 10.1517/17425247.2012.662953. [DOI] [PubMed] [Google Scholar]
- 11.Burton L.E., Ray R.E., Bradford J.R., Orr J.P., Nickerson J.A., Wells W.W. myo-Inositol Metabolism in the Neonatal and Developing Rat Fed a myo-Inositol-free Diet. J. Nutr. 1976;106:1610–1616. doi: 10.1093/jn/106.11.1610. [DOI] [PubMed] [Google Scholar]
- 12.Lam S. A Phase I Study of myo-Inositol for Lung Cancer Chemoprevention. Cancer Epidemiol. Biomark. Prev. 2006;15:1526–1531. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 13.CFR—Code of Federal Regulations Title 21. [(accessed on 8 May 2020)]; Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1370.
- 14.Cancer. [(accessed on 8 May 2020)]; Available online: https://www.who.int/health-topics/cancer#tab=tab_2.
- 15.Mcnamara M., Batur P., Walsh J.M.E., Johnson K.M. HPV Update: Vaccination, Screening, and Associated Disease. J. Gen. Intern. Med. 2016;31:1360–1366. doi: 10.1007/s11606-016-3725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford J., Dale D.C., Lyman G.H. Chemotherapy-induced neutropenia. Cancer. 2004;100:228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 17.Mones J.V., Soff G. Thrombosis Hemostasis in Cancer. Springer; Berlin/Heidelberg, Germany: 2019. Management of Thrombocytopenia in Cancer Patients; pp. 139–150. [DOI] [PubMed] [Google Scholar]
- 18.Cancer Today. [(accessed on 8 May 2020)]; Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.
- 19.Szabo E., Mao J.T., Lam S., Reid M.E., Keith R.L. Chemoprevention of Lung Cancer. Chest. 2013;143:e40S–e60S. doi: 10.1378/chest.12-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krzakowski M., Jassem J., Antczak A., Chorostowska-Wynimko J., Dziadziuszko R., Głogowski M., Grodzki T., Kowalski D., Olszewski W., Orłowski T., et al. Cancer of the lung, pleura and mediastinum. Oncol. Clin. Pract. 2019;15:20–50. [Google Scholar]
- 21.National Lung Sceening Trial Research Team Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estensen R.D., Wattenberg L.W. Studies of chemo-preventive effects of myo-inositol on benzo(a)pyrene-induced neoplasia of the lung and forestomach of female A/J mice. Carcinogenesis. 1993;14:1975–1977. doi: 10.1093/carcin/14.9.1975. [DOI] [PubMed] [Google Scholar]
- 23.Hecht S.S., Kenney P.M., Wang M., Upadhyaya P. Dose–response study of myo-inositol as an inhibitor of lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2001;167:1–6. doi: 10.1016/S0304-3835(01)00454-2. [DOI] [PubMed] [Google Scholar]
- 24.Hecht S.S. Inhibition of lung tumorigenesis in A/J mice by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis. 2002;23:1455–1461. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- 25.Unver N., Delgado O., Zeleke K., Cumpian A., Tang X., Caetano M.S., Wang H., Katayama H., Yu H., Szabo E., et al. Reduced IL-6 levels and tumour-associated phospho-STAT3 are associated with reduced tumour development in a mouse model of lung cancer chemoprevention with myo-inositol. Int. J. Cancer. 2017;142:1405–1417. doi: 10.1002/ijc.31152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iriki T., Ohnishi K., Fujiwara Y., Horlad H., Saito Y., Pan C., Ikeda K., Mori T., Suzuki M., Ichiyasu H., et al. The cell-cell interaction between tumour-associated macrophages and small cell lung cancer cells is involved in tumour progression via STAT3 activation. Lung Cancer. 2017;106:22–32. doi: 10.1016/j.lungcan.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Han W., Gills J.J., Memmott R.M., Lam S., Dennis P.A. The Chemo-preventive Agent Myoinositol Inhibits Akt and Extracellular Signal-Regulated Kinase in Bronchial Lesions from Heavy Smokers. Cancer Prev. Res. 2009;2:370–376. doi: 10.1158/1940-6207.CAPR-08-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam S., Mandrekar S.J., Gesthalter Y., Ziegler K.L.A., Seisler D.K., Midthun D.E., Mao J.T., Aubry M.C., Mcwilliams A., Sin D.D., et al. A Randomized Phase IIb Trial of myo-Inositol in Smokers with Bronchial Dysplasia. Cancer Prev. Res. 2016;9:906–914. doi: 10.1158/1940-6207.CAPR-15-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivapurkar N., Tang Z., Frost A., Alabaster O. A rapid dual organ rat carcinogenesis bioassay for evaluating the chemoprevention of breast and colon cancer. Cancer Lett. 1996;100:169–179. doi: 10.1016/0304-3835(95)04097-8. [DOI] [PubMed] [Google Scholar]
- 30.Vucenik I., Yang G.-Y., Shamsuddin A.M. Inositol hexaphosphate and inositol inhibit DMBA-induced rat mammary cancer. Carcinogenesis. 1995;16:1055–1058. doi: 10.1093/carcin/16.5.1055. [DOI] [PubMed] [Google Scholar]
- 31.Vucenik I., Yang G.Y., Shamsuddin A.M. Comparison of pure inositol hexaphosphate and high-bran diet in the prevention of DMBA-induced rat mammary carcinogenesis. Nutr. Cancer. 1997;28:7–13. doi: 10.1080/01635589709514546. [DOI] [PubMed] [Google Scholar]
- 32.Shamsuddin A.M., Vucenik I., Cole K.E. IP6: A novel anti-cancer agent. Life Sci. 1997;61:343–354. doi: 10.1016/S0024-3205(97)00092-1. [DOI] [PubMed] [Google Scholar]
- 33.El-Sherbiny Y.M., Cox M.C., Ismail Z.A., Shamsuddin A.M., Vucenik I. G0/G1 arrest and S phase inhibition of human cancer cell lines by inositol hexaphosphate (IP6) Anticancer Res. 2001;21:2393–2403. [PubMed] [Google Scholar]
- 34.Tantivejkul K., Vucenik I., Shamsuddin A.M. Inositol hexaphosphate (IP6) inhibits key events of cancer metastasis: I. In Vitro studies of adhesion, migration and invasion of MDA-MB 231 human breast cancer cells. Anticancer Res. 2003;23:3671–3679. [PubMed] [Google Scholar]
- 35.Tantivejkul K., Vucenik I., Shamsuddin A.M. Inositol hexaphosphate (IP6) inhibits key events of cancer metastasis: II. Effects on integrins and focal adhesions. Anticancer Res. 2003;23:3681–3689. [PubMed] [Google Scholar]
- 36.Vucenik I., Ramakrishna G., Tantivejkul K., Anderson L.M., Ramljak D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCδ-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res. Treat. 2005;91:35–45. doi: 10.1007/s10549-004-6456-5. [DOI] [PubMed] [Google Scholar]
- 37.Tantivejkul K., Vucenik I., Eiseman J., Shamsuddin A.M. Inositol Hexaphosphate (IP6) Enhances the Anti-Proliferative Effects of Adriamycin and Tamoxifen in Breast Cancer. Breast Cancer Res. Treat. 2003;79:301–312. doi: 10.1023/A:1024078415339. [DOI] [PubMed] [Google Scholar]
- 38.Dinicola S., Fabrizi G., Masiello M.G., Proietti S., Palombo A., Minini M., Harrath A.H., Alwasel S.H., Ricci G., Catizone A., et al. Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp. Cell Res. 2016;345:37–50. doi: 10.1016/j.yexcr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Bacic I., Druzijanic N., Karlo R., Skific I., Jagic S. Efficacy of IP6 inositol in the treatment of breast cancer patients receiving chemotherapy: Prospective, randomized, pilot clinical study. J. Exp. Clin. Cancer Res. 2010;29:12. doi: 10.1186/1756-9966-29-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyman G.H., Abella E., Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit. Rev. Oncol. Hematol. 2014;90:190–199. doi: 10.1016/j.critrevonc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Proietti S., Pasta V., Cucina A., Aragona C., Palombi E., Vucenik I., Bizzarri M. Inositol hexaphosphate (InsP6) as an effective topical treatment for patients receiving adjuvant chemotherapy after breast surgery. Eur. Rev. Med. Pharm. Sci. 2017;21:43–50. [PubMed] [Google Scholar]
- 42.Pasta V., Gullo G., Giuliani A., Harrath A.H., Alwasel S.H., Tartaglia F., Cucina A., Bizzarri M. An association of boswellia, betaine and myo-inositol (Eumastós) in the treatment of mammographic breast density: A randomized, double-blind study. Eur. Rev. Med. Pharmacol. Sci. 2015;19:4419–4426. [PubMed] [Google Scholar]
- 43.Boyd N.F., Martin L.J., Yaffe M.J., Minkin S. Mammographic density and breast cancer risk: Current understanding and future prospects. Breast Cancer Res. 2011;13:223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasta V., Dinicola S., Giuliani A., Harrath A.H., Alwasel S.H., Tartaglia F., Cucina A., Bizzarri M. A Randomized Pilot Study of Inositol in Association with Betaine and Boswellia in the Management of Mastalgia and Benign Breast Lump in Premenopausal Women. Breast Cancer Basic Clin. Res. 2016;10:S38408. doi: 10.4137/BCBCR.S38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santen R.J., Mansel R. Benign Breast Disorders. N. Engl. J. Med. 2005;353:275–285. doi: 10.1056/NEJMra035692. [DOI] [PubMed] [Google Scholar]
- 46.Cerrato F., Labow B. Diagnosis and Management of Fibroadenomas in the Adolescent Breast. Semin. Plast. Surg. 2013;27:023–025. doi: 10.1055/s-0033-1343992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drew D.A., Cao Y., Chan A.T. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat. Rev. Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shamsuddin A.M., Elsayed A.M., Ullah A. Suppression of large intestinal cancer in F344 rats by inositol hexaphosphate. Carcinogenesis. 1988;9:577–580. doi: 10.1093/carcin/9.4.577. [DOI] [PubMed] [Google Scholar]
- 49.Pretlow T.P., Oriordan M.A., Somich G.A., Amini S.B., Pretlow T.G. Aberrant crypts correlate with tumour incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis. 1992;13:1509–1512. doi: 10.1093/carcin/13.9.1509. [DOI] [PubMed] [Google Scholar]
- 50.Baten A., Ullah A., Tomazic V.J., Shamsuddin A.M. Inositol-phosphate-induced enhancement of natural killer cell activity correlates with tumour suppression. Carcinogenesis. 1989;10:1595–1598. doi: 10.1093/carcin/10.9.1595. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z. Inositol hexaphosphate-induced enhancement of natural killer cell activity correlates with suppression of colon carcinogenesis in rats. World J. Gastroenterol. 2005;11:5044. doi: 10.3748/wjg.v11.i32.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang G.Y., Shamsuddin A.M. IP6-induced growth inhibition and differentiation of HT-29 human colon cancer cells: Involvement of intracellular inositol phosphates. Anticancer Res. 1995;15:2479–2487. [PubMed] [Google Scholar]
- 53.Liu G., Song Y., Cui L., Wen Z., Lu X. Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int. J. Clin. Exp. Pathol. 2015;8:1402–1410. [PMC free article] [PubMed] [Google Scholar]
- 54.Kapral M., Wawszczyk J., Jesse K., Paul-Samojedny M., Kuśmierz D., Węglarz L. Inositol Hexaphosphate Inhibits Proliferation and Induces Apoptosis of Colon Cancer Cells by Suppressing the AKT/mTOR Signaling Pathway. Molecules. 2017;22:1657. doi: 10.3390/molecules22101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schröterová L., Ježková A., Rudolf E., Caltová K., Králová V., Hanušová V. Inositol hexaphosphate limits the migration and the invasiveness of colorectal carcinoma cells in vitro. Int. J. Oncol. 2018;53:1625–1632. doi: 10.3892/ijo.2018.4488. [DOI] [PubMed] [Google Scholar]
- 56.Shamsuddin A.M., Ullah A. Inositol hexaphosphate inhibits large intestinal cancer in F344 rats 5 months after induction by azoxymethane. Carcinogenesis. 1989;10:625–626. doi: 10.1093/carcin/10.3.625. [DOI] [PubMed] [Google Scholar]
- 57.Ullah A., Shamsuddin A.M. Dose-dependent inhibition of large intestinal cancer by inositol hexaphosphate in F344 rats. Carcinogenesis. 1990;11:2219–2222. doi: 10.1093/carcin/11.12.2219. [DOI] [PubMed] [Google Scholar]
- 58.Challa A. Interactive suppression of aberrant crypt foci induced by azoxymethane in rat colon by phytic acid and green tea. Carcinogenesis. 1997;18:2023–2026. doi: 10.1093/carcin/18.10.2023. [DOI] [PubMed] [Google Scholar]
- 59.Crawford E. Epidemiology of prostate cancer. Urology. 2003;62:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Carter H.B., Piantadosi S., Isaacs J.T. Clinical Evidence for and Implications of the Multistep Development of Prostate Cancer. J. Urol. 1990;143:742–746. doi: 10.1016/S0022-5347(17)40078-4. [DOI] [PubMed] [Google Scholar]
- 61.Stangelberger A., Waldert M., Djavan B. Prostate cancer in elderly men. Rev. Urol. 2008;10:111–119. [PMC free article] [PubMed] [Google Scholar]
- 62.Shamsuddin A.M., Yang G.-Y. Inositol hexaphosphate inhibits growth and induces differentiation of PC-3 human prostate cancer cells. Carcinogenesis. 1995;16:1975–1979. doi: 10.1093/carcin/16.8.1975. [DOI] [PubMed] [Google Scholar]
- 63.Lin T.-H., Tan T.-W., Tsai T.-H., Chen C.-C., Hsieh T.-F., Lee S.-S., Liu H.-H., Chen W.-C., Tang C.-H. D-pinitol Inhibits Prostate Cancer Metastasis through Inhibition of αVβ3 Integrin by Modulating FAK, c-Src and NF-κB Pathways. Int. J. Mol. Sci. 2013;14:9790–9802. doi: 10.3390/ijms14059790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma G., Singh R.P., Agarwal R. Growth inhibitory and apoptotic effects of inositol hexaphosphate in transgenic adenocarcinoma of mouse prostate (TRAMP-C1) cells. Int. J. Oncol. 2003;23:1413–1418. doi: 10.3892/ijo.23.5.1413. [DOI] [PubMed] [Google Scholar]
- 65.Singh R.P. In Vivo Suppression of Hormone-Refractory Prostate Cancer Growth by Inositol Hexaphosphate: Induction of Insulin-Like Growth Factor Binding Protein-3 and Inhibition of Vascular Endothelial Growth Factor. Clin. Cancer Res. 2004;10:244–250. doi: 10.1158/1078-0432.CCR-1080-3. [DOI] [PubMed] [Google Scholar]
- 66.Somasundar P., Riggs D.R., Jackson B.J., Cunningham C., Vona-Davis L., Mcfadden D.W. Inositol Hexaphosphate (IP6): A Novel Treatment for Pancreatic Cancer. J. Surg. Res. 2005;126:199–203. doi: 10.1016/j.jss.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Mcmillan B., Riggs D.R., Jackson B.J., Cunningham C., McFadden D.W. Dietary Influence on Pancreatic Cancer Growth by Catechin and Inositol Hexaphosphate. J. Surg. Res. 2007;141:115–119. doi: 10.1016/j.jss.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 68.Lee H.-J., Lee S.-A., Choi H. Dietary administration of inositol and/or inositol-6-phosphate prevents chemically-induced rat hepatocarcinogenesis. Asian Pac. J. Cancer Prev. 2005;6:41–47. [PubMed] [Google Scholar]
- 69.Nishino H. Phytochemicals in Hepatocellular Cancer Prevention. Nutr. Cancer. 2009;61:789–791. doi: 10.1080/01635580903285031. [DOI] [PubMed] [Google Scholar]
- 70.Ren L., Hong E.S., Mendoza A., Issaq S., Hoang C.T., Lizardo M., Leblanc A., Khanna C. Metabolomics uncovers a link between inositol metabolism and osteosarcoma metastasis. Oncotarget. 2017;8:38514. doi: 10.18632/oncotarget.15872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khurana S., Baldeo C., Joseph R.W. Inositol hexaphosphate plus inositol induced complete remission in stage IV melanoma. Melanoma Res. 2019;29:322–324. doi: 10.1097/CMR.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 72.Limani P., Linecker M., Kron P., Samaras P., Pestalozzi B., Stupp R., Jetter A., Dutkowski P., Müllhaupt B., Schlegel A., et al. Development of OXY111A, a novel hypoxia-modifier as a potential antitumor agent in patients with hepato-pancreato-biliary neoplasms—Protocol of a first Ib/IIa clinical trial. BMC Cancer. 2016;16:812. doi: 10.1186/s12885-016-2855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Unfer V., Carlomagno G., Dante G., Facchinetti F. Effects of Myo-Inositol in Women with PCOS: A Systematic Review of Randomized Controlled Trials. Gynecol. Endocrinol. 2012;28:509–515. doi: 10.3109/09513590.2011.650660. [DOI] [PubMed] [Google Scholar]
- 74.Genazzani A.D., Lanzoni C., Ricchieri F., Jasonni V.M. Myo-Inositol Administration Positively Affects Hyperinsulinemia and Hormonal Parameters in Overweight Patients with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2008;24:139–144. doi: 10.1080/09513590801893232. [DOI] [PubMed] [Google Scholar]
- 75.Wojciechowska A., Osowski A., Jóźwik M., Górecki R., Rynkiewicz A., Wojtkiewicz J. Inositols’ Importance in the Improvement of the Endocrine–Metabolic Profile in PCOS. Int. J. Mol. Sci. 2019;20:5787. doi: 10.3390/ijms20225787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerli S., Papaleo E., Ferrari A., Di Renzo G.C. Randomized, double-blind placebo-controlled trial: Effects of myo-inositol on ovarian function and metabolic factors in women with PCOS. Eur. Rev. Med. Pharmacol. Sci. 2007;11:347–354. [PubMed] [Google Scholar]
- 77.Nordio M., Basciani S. Treatment with Myo-Inositol and Selenium Ensures Euthyroidism in Patients with Autoimmune Thyroiditis. Int. J. Endocrinol. 2017;2017:1–6. doi: 10.1155/2017/2549491. [DOI] [PMC free article] [PubMed] [Google Scholar]