Abstract
Drug discovery efforts largely depend on access to structural diversity. Multicomponent reactions allow for time-efficient chemical transformations and provide advanced intermediates with three or four points of diversification for further expansion to a structural variety of organic molecules. This review is aimed at solid-phase syntheses of small molecules involving isocyanide-based multicomponent reactions. The majority of all reported syntheses employ the Ugi four-component reaction. The review also covers the Passerini and Groebke-Blackburn-Bienaymé reactions. To date, the main advantages of the solid-phase approach are the ability to prepare chemical libraries intended for biological screening and elimination of the isocyanide odor. However, the potential of multicomponent reactions has not been fully exploited. The unexplored avenues of these reactions, including chiral frameworks, DNA-encoded libraries, eco-friendly synthesis, and chiral auxiliary reactions, are briefly outlined.
Keywords: convertible isocyanides, drug discovery, multicomponent reactions, post-Ugi transformations, solid-phase synthesis, Ugi four-component reaction
1. Introduction
Multicomponent reactions (MCRs) are one-pot highly efficient transformations utilizing three or more components capable of creating highly diverse compounds. They are also atom-efficient, incorporate a substantial portion of components, and do not require any additional reagents. MCRs are ideal tools for the construction of vast arrays of biologically relevant molecules [1].
One of the most explored and the most frequently described MCRs is the Ugi four-component reaction (U-4CR), often named Ugi four-component condensation (U-4CC). The U-4CC enables the formation of a functionally complex organic molecule with a single synthetic transformation in comparison to numerous steps applied in conventional methodologies [2]. An illustrative example to generate structurally complex compounds resulted from Schreiber’s laboratory [3]. In addition, MCRs are also attractive for automated parallel synthesis [4].
A large number of MCRs performed in solution phase have been reported, while solid-phase applications appear less frequently. In this review, we include publications using isocyanide as one of the reaction components (so-called isocyanide-based multicomponent reactions, IMCRs). The majority of the presented syntheses utilized the Ugi reaction either as a one-step process resulting in the target products (not considering the additional step of cleavage from the resin) or as one of the steps in a several-step synthesis, which involves a transformation of the primary product. We also included Ugi four-center three-component reactions (U-4C-3CRs) as well as nontraditional Ugi reactions, e.g., Ugi-azide four-component reactions [5,6], or transformations unrelated to the Ugi reaction [7,8,9]. To the best of our knowledge, there are only two examples of Passerini reactions leading to small molecules via a solid-phase reaction [10,11].
Initially employed for the formation of dipeptides, the Ugi reaction has been applied for the synthesis of complex structures [3] or natural products [12], and currently, it plays an important role for finding biologically relevant molecules [13]. Numerous research groups worldwide have contributed to this ground-breaking one-pot reaction. MCRs are very attractive due to their potential and interdisciplinary applications. MCRs are advanced tools for sustainable organic synthesis [14] due to their good compatibility with green solvents [15], high atom economy, efficiency, and mild reaction conditions. At present, the solid-phase Ugi reaction is also essential in the synthesis of macropeptides, glycopeptides, lipoproteins, and in the field of ligation and bioconjugation [16,17]. Furthermore, IMCRs are attractive for their compatibility with DNA-encoded combinatorial synthesis [18]. In addition, chiral auxiliary has already been shown to enable direct enantio-enriched compounds [19,20]. MCRs also have the potential to provide a basis for large-scale molecular data storage [21].
This review is focused on the synthesis of small molecules on solid supports and demonstrates the various methodologies and strategies used in IMCRs. Herein, the presented approaches are classified according to the type of IMCRs and taking into account whether the IMCR was the only chemical transformation leading to the target products or whether it was one of several steps in the synthetic sequence. In certain sections, the reactions are classified according to the functionality bound to the solid support.
2. Isocyanide-Based Multicomponent Reactions (IMCRs)
The discovery and course of IMCRs is explained in detail and comprehensively illustrated by Ugi and Dömling [22,23]. Briefly, isocyanides were discovered in 1859 by Lieke, who prepared them by the random reaction of allyl iodide and silver cyanide [24]. The term “isonitrile” was introduced by Gautier and simultaneously by Hoffmann, who prepared these molecules by reacting primary amines with chloroform and alkali [25]. Note that we used the term “isocyanide” within this text because it is preferred by IUPAC (International Union of Pure and Applied Chemistry).
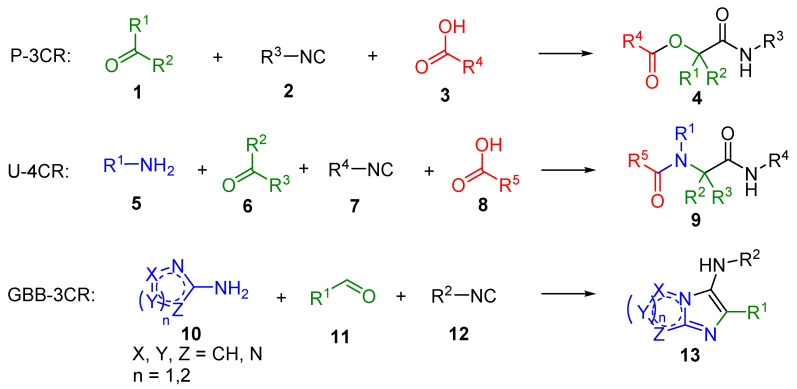
Approximately a half-century later, in 1921, Passerini introduced the first MCRs of isocyanides. Passerini three-component reaction (P-3CR) of a carbonyl compound, isocyanide, and carboxylic acid afforded α-acyloxy carboxamide 4 (Scheme 1). Since the 1950s, attempts to prepare isocyanides by a more convenient method have appeared in the literature [26]. Finally, the beginning of U-4CR dates to 1959, when Ivar Karl Ugi (1930–2005) performed a one-pot reaction of amine, carbonyl compound, isocyanide, and acid, leading to α-acylamido carboxamide 9 [7,27,28]. Since 1962, the reaction is referred to as the Ugi reaction (U-4CR). Approximately twenty years later, the transformation was also performed on a solid phase [29]. The first stereoselective Ugi reaction on the solid phase was accomplished in 2000 by Kunz et al. [19]. Finally, the newest MCR is the Groebke-Blackburn-Bienaymé three-component reaction (GBB-3CR, product 13) [30,31,32].
Scheme 1.
Types of isocyanide-based multicomponent reactions (IMCRs) described in this review.
2.1. Practical Assessments
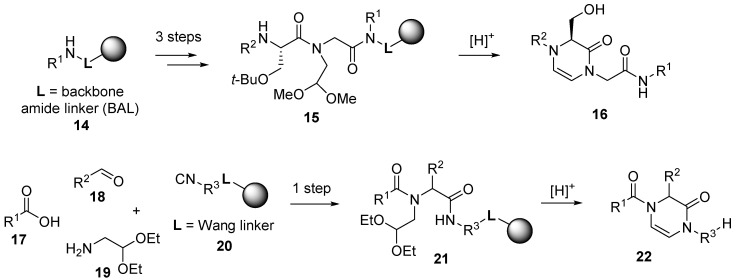
From a practical point of view, a single synthetic transformation instead of traditional follow-up synthesis is one of the advantages of IMCRs, which is reflected in the large interest in MCRs. The feasibility of the synthesis involving the Ugi reaction in comparison to the standard stepwise synthetic protocol is illustrated in the following example (Scheme 2).
Scheme 2.
Comparison of classical synthesis and Ugi four-component reaction (U-4CR).
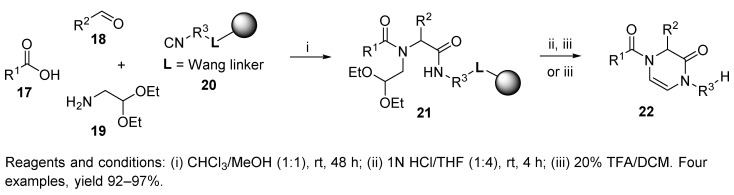
While the conventional synthesis of linear precursor 15 involved 3 steps [33], analogous intermediate 21 was formed by U-4CR in only one step [34]. Acidic treatment initiated a cleavage of the protecting groups as well as simultaneous cleavage of the intermediate from the resin and cyclization to the target 3,4-dihydropyrazin-2(1H)-one 16 and 22.
A reduction in the synthetic steps is one of several advantages of IMCRs. Other advantages are high atom-efficiency (when only one molecule of water is lost) and usually high yields of product. The yields of the products are primarily sensitive to the nature of the aldehyde [35]. The aliphatic aldehydes and aromatic aldehydes bearing electron-donating groups (EDGs) afforded good yields in comparison to the aldehydes containing electron-withdrawing groups (EWGs, e.g., References [35,36,37,38]). Even larger excess reagents or repeated addition of fresh reagents often did not improve the yield [35,38], although the opposite observation was also described [39]. In addition to the poor reactivity of aromatic aldehydes in the Ugi reaction, aromatic isocyanides were also reported to be less reactive [40].
Since a limited number of commercially available isocyanides greatly reduces the structural diversity of a library [35], convertible isocyanides play an important role in the achievement of diverse products. Another important aspect of IMCRs is the unpleasant odor of most isocyanides. This odor can be circumvented by immobilization of isocyanides onto a polymer support, which is an advantage of solid-phase synthesis (SPS) over solution-phase synthesis. The other option to avoid the odor is the preparation of the isocyanide in situ, which has been applied so far only in the solution phase [41,42].
The scope of the reaction components is not limited to amine, aldehyde, carboxylic acid, and isocyanide—alternative reagents were successfully examined. For example, sulfonamide was used as an amine reagent [43], imine was directly bound to the Rink resin [44], or ketones were applied to replace the aldehydes despite their lower reactivity [45,46,47,48,49]. Alternatively, glyoxal was used as the aldehyde component in U-4CR, and the second carbonyl functionality was employed for the imidazole ring formation [50]. Alcohol was used as an acid component [39], or application of thiocarboxylic [51,52], cyanic [53,54], thiocyanic, or hydrazoic acid [54] was reported in the literature. Alternatively, one starting component can bear two functional groups, e.g., ketoacid, thus serving as both ketone and carboxylic acid [1,54]. A list of resin-bound starting materials of IMCRs was shown by Dömling [55].
Regarding the solvents, the Ugi reaction is typically performed in methanol (MeOH) in the case of solution-phase synthesis. In solid-phase chemistry, it is essential to consider the swelling properties of the respective resin. Typical SPS is carried out using hydrophobic polystyrene (PS)-based resin, which shrinks in MeOH. Therefore, mixtures of solvents are used, including MeOH/tetrahydrofuran (THF) and MeOH/dichloromethane (DCM) in various ratios (e.g., References [3,35,56,57,58]), while the usage of MeOH/CHCl3 [36,59], MeOH/CHCl3/H2O [53,54,60], or DCM/DMF [37] was reported much less frequently. In addition, polar TentaGel resin, composed of PEG-grafted PS, was successfully used in MCR [37,39,46,60]. U-4CR was even successful when H2O was used (in the case of filter paper used as solid support) [38,61,62], and 2-methyltetrahydrofuran (2-MeTHF) [15] was described recently as a green solvent in the Ugi reaction. All practical aspects of U-4CC, performed in solution phase, were discussed in detail [63].
To be objective, the Ugi transformation suffers from a few shortcomings, which, however, cannot outperform the overall MCR benefits. The first shortcoming is the long reaction times needed (up to several days); however, the reaction time can be significantly shortened (to ≤5 min) by using microwave-irradiation [37]. The longer reaction times are also connected with the nature of the solid support, and PS resin suffers from slow reaction kinetics [64]. Furthermore, the stereochemical outcome is highly dependent on the chirality of the reagents. It is known that chiral aldehydes have the greatest effect on the diastereoselectivity due to the proximity to the newly formed stereocenter [57]. Amino-carbohydrates were used as an immobilized chiral auxiliary [19,20]. Asymmetric MCRs were reviewed by Ramón and Yus [65].
Concerning the solid-phase arrangement, this approach offers great advantages over the solution-phase chemistry. The most apparent benefits are facilitated washing of the reagents and isolating of the target products, and the ability to carry out parallel syntheses to produce large libraries containing thousands of compounds intended for biological screening. The practical aspects of combinatorial solid-phase organic synthesis were summarized [66].
2.2. Conventions
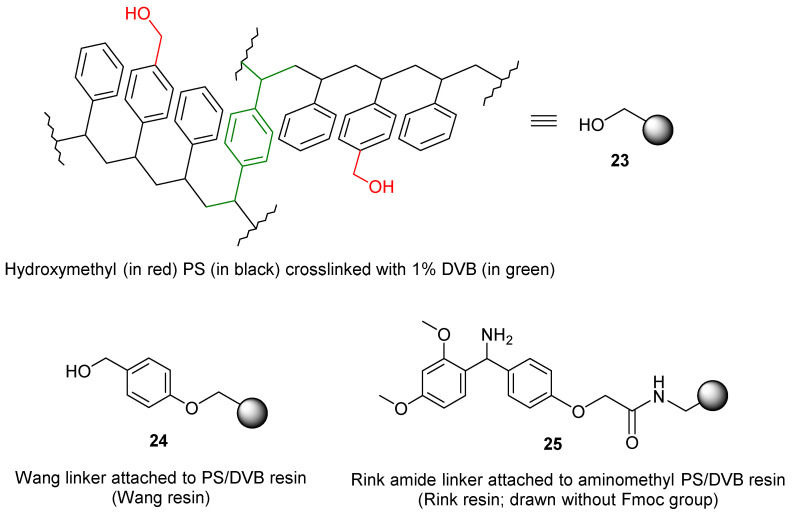
The most frequently used resin for SPS is PS crosslinked with 1% divinylbenzene (DVB, 23, Figure 1). This support is depicted in all schemes and figures by a solid ball. A chemical entity, including linkers, is drawn from the ball and is assumed to be attached predominantly to the para-position of the aromatic ring. A linker, abbreviated with the letter “L”, is always specified. The structures of the two most common linkers, Wang (24) [67], and Rink amide linkers (25) [68], are shown in Figure 1. The Rink linker can be attached to the resin via amide (shown) or ester bonds.
Figure 1.
Solid-phase supports most frequently used in the review.
3. Resins
To carry out the U-4CC on a solid phase, one component needs to be attached to the insoluble resin support. The resin contains a linker allowing for immobilization of the selected component and enabling the release of the product once the SPS is accomplished. The solid-supported component can be assembled in solution and then attached to a resin, or it can be assembled on the resin. Any of the four components, amine, aldehyde, carboxylic acid, or isocyanide, can be immobilized for U-4CC; however, the most frequently used components are amine and isocyanide.
3.1. Amine Resin
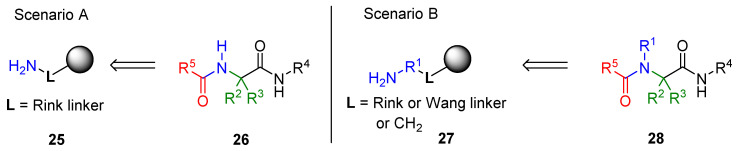
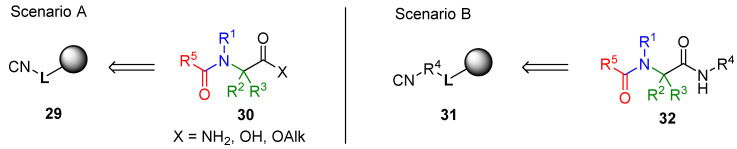
The immobilization of the amino component offers two different synthetic scenarios (Scheme 3). The ammonia equivalent is attached directly to a linker; in this scenario, the Ugi product, after cleavage from the resin, does not have a substituent on the amide nitrogen (26, Scenario A). The other option is to attach the amino group to the resin via a moiety R1 (27, Scenario B). In the latter case, the product has four diversity positions, and the amide is substituted by the R1 group.
Scheme 3.
Immobilization of the amino component.
In general, the amino group containing resin (Rink resin, solid supports modified by amino acids or any other components bearing primary amino groups) serves as an ammonia equivalent. The use of ammonia in the solution phase often leads to the formation of a significant number of unidentified products [69]. The solid-phase approach offers a solution to this issue.
Typical resin for Scenario A (Scheme 3) is commercially available Rink amide resin 25, which provides products bearing secondary amides [15,35,37,57,58,69,70,71]. There is no structural limit on resin-bound amino derivatives for carrying out the synthesis according to Scenario B. Wang and hydroxymethyl resins esterified with amino acids were frequently used [4,36,46,62], as well as Rink and aminomethyl resins acylated with amino acids [45]. Other examples include heterocycles, nucleosides [72], and carbohydrates [19,20].
3.2. Isocyanide Resin
Unlike amines, carboxylic acids, and aldehydes, structurally varied isocyanides are not commercially available. The synthesis of isocyanides suffers from several drawbacks, including multistep synthesis and unpleasant odor. However, the polymer-supported isocyanides are completely odor-free, which is an advantage of the IMCRs performed on the solid phase. Two different strategies for the preparation of isocyanide resins were developed: (i) the isocyanide-containing reagent was prepared in solution and then attached to the resin, or (ii) the isocyanide was formed on the resin, typically from a resin-bound amine by formylation followed by dehydration [8,29,50]. Analogous to the amine resins, the isocyanide was either directly linked to a resin-bound linker (typically Rink amide linker, 29, Scenario A, Scheme 4) or a diversity element R4 was introduced between the isocyanide and the linker (31, Scenario B) [73]. Accordingly, three or four diversity position products were formed.
Scheme 4.
Formation of the resin-bound isocyanides.
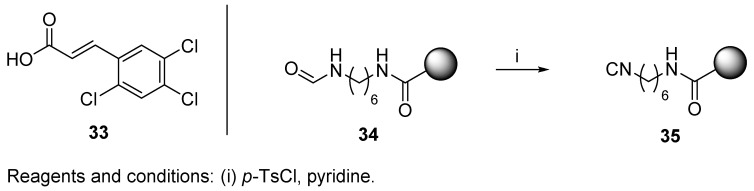
First, we discuss the most common route to polymer-supported isocyanide by dehydration of formyl amides. In 1981, isocyanide resin in U-4CC was reported for the first time by Arshady and Ugi [29]. Their original work [29] focused on peptide synthesis by ligation of a C-terminal fragment with an N-terminal fragment. Dedicated PS and polyacrylamide polymers crosslinked with bisacrylamide were prepared and evaluated. Copolymerization included 2,4,5-trichlorophenylacrylate 33 (Scheme 5), which introduced a functional group for subsequent reaction with diamine and formylation (resins 34). Both formamide polymers were subsequently dehydrated with an excess of p-TsCl in pyridine (resins 35) and afforded comparable results. The authors selected the polar polyacrylamide polymer to carry out the scope and limitation experiments described in Section 4.1.2.
Scheme 5.
Resin-bound isocyanide on a dedicated polymer support.
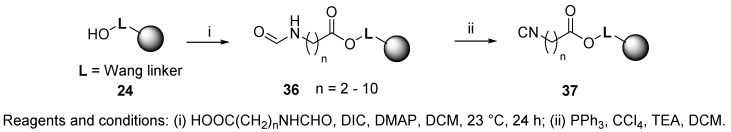
On-resin dehydration was used very frequently. Mjalli et al. reported the preparation of Wang resin-bound isocyanide 37 (Scheme 6) and its usage for the synthesis of imidazoles (cf. Scheme 57) [50]. It was further utilized for syntheses of other heterocycles, such as 2-thiohydantoin 4-imides, hydantoin 4-imides, tetrazoles, and lactams (cf. Section 8.2 and Section 9.2.2) [53,54]. The synthetic strategy was based on formylation and dehydration. Formylated resin-bound amino acids, prepared by reaction with HCOOEt or HCOOH in the presence of Ac2O, were attached to Wang resin 24, and precursor 36 was subjected to dehydration with PPh3 and CCl4 in DCM in the presence of triethylamine (TEA) to produce target resin 37. The resin was reportedly stable for up to 6 months at 23 °C, when kept in a vacuum desiccator. However, the usage of carcinogenic CCl4 makes this method less desirable.
Scheme 6.
Preparation of Wang resin-bound isocyanide.
The synthesis of isocyanides was also evaluated on various resins, including aminomethyl PS, TentaGel-NH2, polydimethylacrylamide, copolystyrene-acrylate, copolystyrene-acrylamide, and copolydimethylacrylamide-acrylate, via formylation with HCOOH/Ac2O and dehydration with TsCl in pyridine [74].
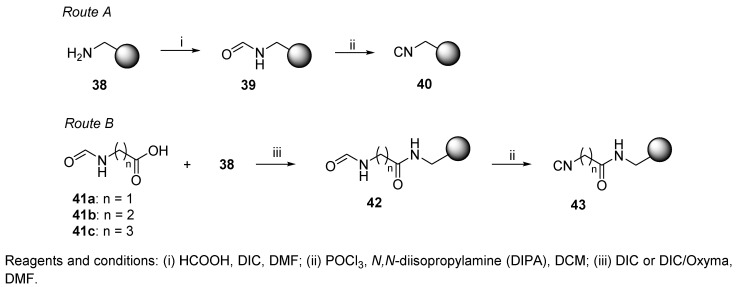
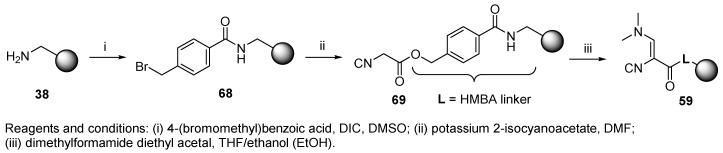
Less hazardous dehydration conditions were applied by another research group [49]. Inexpensive aminomethyl PS resin was functionalized with an isocyano group, which was either directly generated on the resin (40, Scheme 7) or attached via a spacer (43). The spacer unit (Gly, β-Ala, γ-aminobutyric acid (GABA)) decreased the steric hindrance at the isocyanide functional group and thereby facilitated the Ugi reaction. The preparation of both isocyanide resins is outlined in Scheme 7 (Route A, Route B). Oxyma (ethyl cyano(hydroxyimino)acetate) was added to facilitate the coupling reaction of formylated β-Ala 41b and GABA 41c with aminomethyl resin 38. Formylated amino acids 41a–41c were prepared by reaction of amino acid with HCOOH in the presence of acetic anhydride. While the reaction worked well for Gly and β-Ala (41a and 41b), the formylation of GABA afforded a lower yield of 41c due to internal cyclization and formation of pyrrolidin-2-one. Regarding the stability of the resins, the authors reported that formylated resins 42 were stable for months; in contrast, isocyanide resins 43 decomposed in a few weeks. The resins were used for the synthesis of α,α-dialkylglycines as highly hindered C-tetrasubstituted amino acids (cf. Scheme 27).
Scheme 7.
Preparation of aminomethyl resin-bound isocyanide.
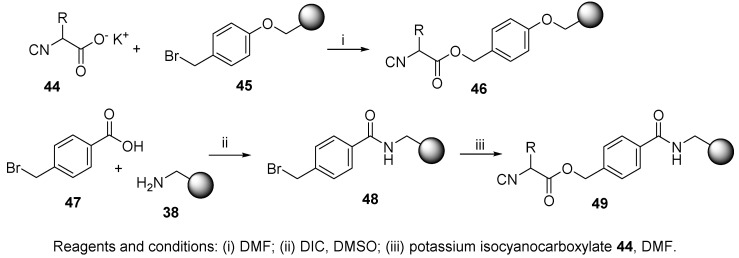
The next example shows the immobilization of isocyanide prepared in solution. This new method for the synthesis of resin-bound isocyanocarboxylic acids was described by Henkel and coworkers (Scheme 8) [73]. They developed a direct coupling method of four different potassium isocyanocarboxylates 44 (prepared from the corresponding acids by ethanolic KOH) onto two brominated resins 45 and 48. The ester bond cleavage of the Ugi product with 3-methylbutylamine yielded amides, and treatment with LiOH provided acids (cf. Scheme 33).
Scheme 8.
Preparation of isocyanide resins from brominated resins and potassium isocyanocarboxylates.
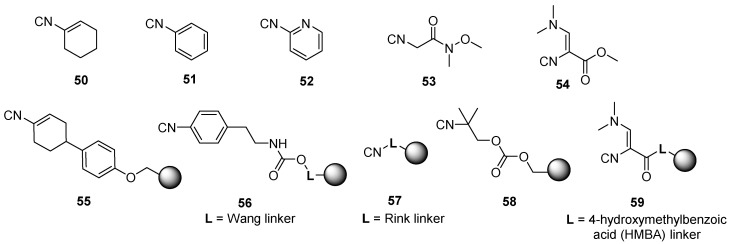
The lack of commercially available isocyanides prompted the design and synthesis of dedicated linkers for the attachment of isocyanide to the resin. These resins are referred to in the literature as universal (also named versatile or convertible) isocyanide resins and enable post-Ugi conversion of the amide to other functional groups. They allow for the release of the Ugi products from the resin through cleavage of the secondary amide bond and yielding the final products as amides, acids, or esters. The first reported convertible isocyanide was 1-isocyanocyclohexene 50 (Figure 2), which was discovered by Ugi and Rosendahl in 1963 [75], and referred to as Armstrong isocyanide [76,77]. Other convertible isocyanides are isocyanobenzene 51, 2-isocyanopyridine 52, Weinreb-type amide 53, and 3-N,N-(dimethylamino)-2-isocyanoacrylate 54. Regarding the resin-bound, cyclohexenyl isocyanide resin (Armstrong-type isocyanide) 55, safety-catch linker isocyanide resin 56, universal Rink isocyanide resin 57, carbonate convertible isocyanide (CCI) resin 58, and 3-N,N-(dimethylamino)-2-isocyanoacrylate resin 59 were developed for MCRs. The application of those isocyanides is covered in Section 6 and Section 9.2.1.
Figure 2.
Solution-phase and resin-bound convertible isocyanides.
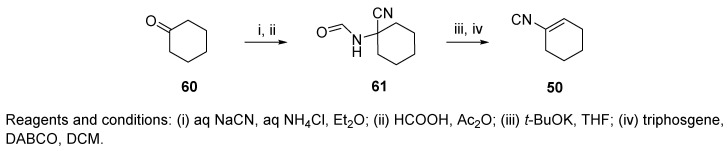
The Armstrong cyclohexenyl isocyanide 50 was originally prepared from N-(1-cyanocyclohexyl)formamide 61 by dehydration with phosphorus oxychloride by Rosendahl and Ugi [75]. Subsequently, the yields were improved by using a different dehydrating agent, triphosgene, and by purifying 61 prior to dehydration (Scheme 9) [78]. The Armstrong-type isocyanide was also prepared on the solid phase by Piscopio and coworkers (resin 55, Figure 2) [79].
Scheme 9.
Preparation of Armstrong isocyanide.
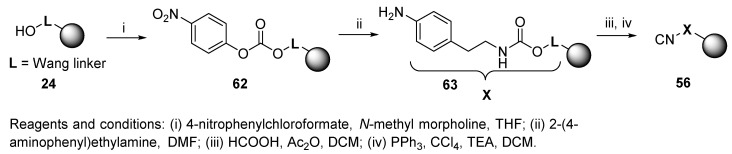
Convertible safety-catch linker 56 was prepared from resin-bound aniline 63 via formylation and subsequent dehydration (Scheme 10) [80]. After the U-4CC, the secondary amide linkage was stable and needed to be activated for nucleophile cleavage by N-Bocylation (safety-catch principle, cf. Scheme 49). Typical nucleophilic reagents included LiOH and NaOMe. The isocyanide resin 56 was stable for more than six months at low temperature.
Scheme 10.
Synthesis of safety-catch linker.
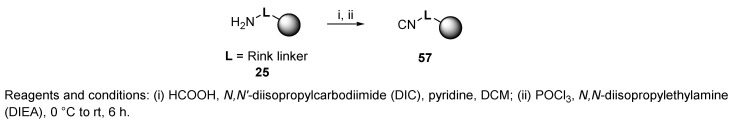
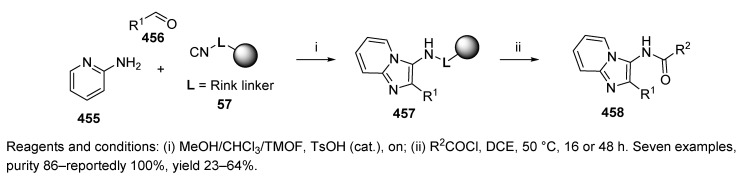
In 2001, Chen et al. first reported the preparation of convertible Rink-isocyanide resin and its usage for the traceless synthesis of 3-acylamido imidazo[1,2-a]pyridines [8]. Rink-isocyanide resin 57 (Scheme 11) was prepared via two steps from commercially available Rink resin. The primary amino group of the Rink linker underwent formylation with HCOOH via activation with DIC in the presence of pyridine in DCM, which was followed by dehydration with POCl3 in the presence of N,N-diisopropylethylamine (DIEA) under ice cooling and in an argon atmosphere. The progress of the reaction was monitored by infrared (IR) spectroscopy. The advantage of this kind of resin is its stability (the resin was reportedly stable for over 12 months when stored in a refrigerator) and easy preparation. Moreover, this isocyanide resin is odor-free.
Scheme 11.
Preparation of convertible Rink-isocyanide resin.
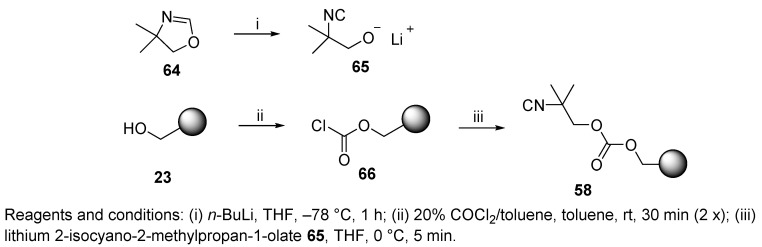
Another convertible isocyanide used in IMCRs is CCI resin, reported in 2002 by Kennedy and coworkers [81]. Lithium alkoxide 65 (Scheme 12) was prepared from 4,4-dimethyl-4,5-dihydrooxazole 64 and transferred to resin-bound chloroformate 66, prepared from hydroxymethyl resin 23 and phosgene, to yield the desired isocyanide resin 58. Treatment of the Ugi product by t-BuOK released N-acyloxazolidone, which was further reacted with NaOMe in MeOH and yielded the product as an ester (cf. Scheme 48). Polymer-supported carbonate convertible isocyanide 58 could be used to prepare a 4000-membered library.
Scheme 12.
Preparation of the CCI resin.
The last polymer-supported convertible isocyanide discussed in this review is 3-N,N-(dimethylamino)-2-isocyanoacrylate resin 59 (Scheme 13) [52], which was applied for the synthesis of thiazoles (cf. Scheme 68).
Scheme 13.
Preparation of 3-N,N-(dimethylamino)-2-isocyanoacrylate resin.
3.3. Aldehyde Resin
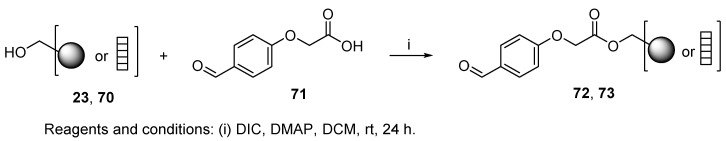
Only one example of aldehyde resin used for U-4CR was reported [82]. The authors attached 2-(4-formylphenoxy)acetic acid 71 (Scheme 14) to hydroxymethyl resin 23 or Lanterns 70 (represented by a “ladder”). The 2-oxo-1,2-ethylenedioxy group served as a linker cleavable either by Yb(OTf)3 or TMSCHN2 in MeOH or by various amines (cf. Scheme 35).
Scheme 14.
Preparation of aldehyde resin with 2-oxo-1,2-ethylenedioxy linker.
3.4. Carboxylic Acid Resin
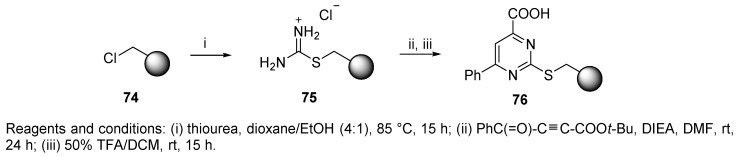
Carboxylate resin has also not been frequently used, there are only two examples of immobilized carboxylate participating in the U-4CC [40,83]. Thiourea resin 75 (Scheme 15) underwent a cycloaddition reaction with tert-butyl-4-oxo-4-phenylbut-2-ynoate, and t-Bu-protected acid was liberated by TFA [40]. The carboxylate resin 76 was further used for the synthesis of various pyrimidine derivatives achieved by multidirectional cleavage (cf. Scheme 36).
Scheme 15.
Preparation of immobilized heteroaromatic carboxylic acid.
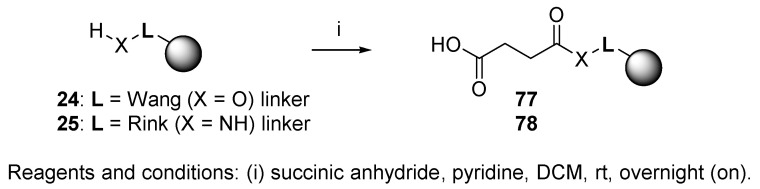
Alternatively, Wang and Rink resins were used to immobilize succinic acid via its anhydride (Scheme 16) [83]. Resin 77 and 78 served as polymer-supported acid inputs in the chemistry of münchnones (cf. Scheme 51).
Scheme 16.
Preparation of immobilized carboxylic acid.
4. Ugi Four-Component Reaction (U-4CR)
In this section, we review the products of classical solid-phase U-4CRs, which are subsequently cleaved from the resin without any other transformation to produce acyclic α-acylamido carboxamides. The formation of two new peptide bonds is the key feature in achieving Ugi products. Therefore, this reaction plays an important role in the synthesis of peptides, including the synthesis of sterically hindered peptides containing α,α-disubstituted glycines (cf. Section 4.1.2) [44]. However, the peptides are not the only compound which can be prepared by U-4CR, as shown in this review.
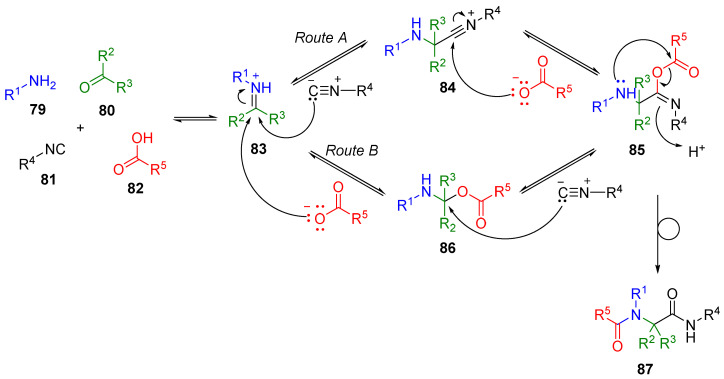
Two possible reaction mechanisms of U-4CR are reported in the literature (e.g., References [84,85]). The originally designed ionic mechanism involved the formation of nitrilium 84 formed in polar protic solvents (typically in MeOH; Route A, Scheme 17). A plausible alternative mechanism considered hemiaminal 86 as the key intermediate (Route B). Resulting imidate 85 subsequently underwent Muum rearrangement, the only irreversible step in this sequence, which shifted the equilibrium to the product side and afforded α-acylamido carboxamide 87. In the case of phenols being used as an acid input, the reaction sequence is accomplished by the Smiles rearrangement.
Scheme 17.
Two plausible mechanisms of the Ugi reaction.
4.1. α-Acylamido Carboxamides with Three Diversity Positions
4.1.1. Amine Resin
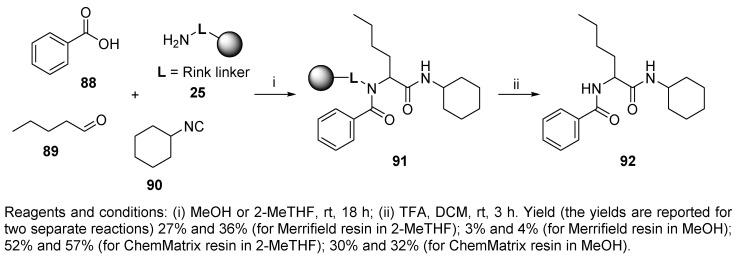
Recently, green solvents with good swelling properties essential for SPS were applied in the Ugi reaction (Scheme 18) [15]. Merrifield and ChemMatrix resins equipped with a Rink amide linker (loadings 1.0 mmol/g and 0.49 mmol/g, respectively) and two green solvents (MeOH and 2-MeTHF) were selected in this study. As expected, 2-MeTHF was a better solvent for the Merrifield resin-supported Ugi reaction, while the reaction carried out on the ChemMatrix resin was effective in both solvents. This fact is explained by the poor swelling properties of Merrifield resin in MeOH, whereas the polar ChemMatrix resin swells well in both solvents. Reactions with all four combinations of resins and solvents were carried out in duplicate to ensure that the results were reproducible. The Ugi reaction was performed in the solution phase first (the overall yield was 38% in MeOH and 50% in 2-MeTHF). Notably, this extensive study described the swelling properties of nine resins in twenty-five green solvents.
Scheme 18.
Ugi reaction performed in green solvents.
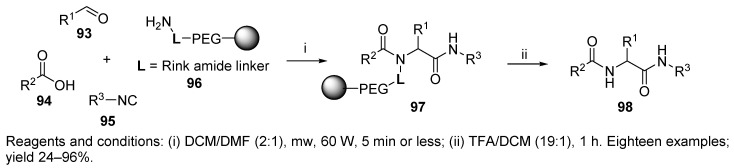
The second example in this section is the synthesis of diamide 98 (Scheme 19) on TentaGel S RAM resin 96 under microwave irradiation [37]. An eighteen-membered library was prepared by using three aldehydes, three different carboxylic acids, and two isocyanides.
Scheme 19.
U-4CR of diamide under microwave irradiation.
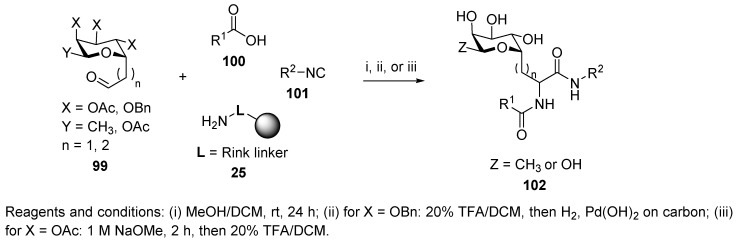
Armstrong et al. [57] were interested in the synthesis of C-glycoside peptide ligands for cell surface carbohydrate receptors. Prior to synthesizing a library, they carried out a proof-of-concept study focused on a protection strategy (OAc and OBn) of carbohydrates, in which they tested U-4CC with nine C-glycoside aldehydes 99, nine acids, including three C-glycoside acids, nine isocyanides, and Rink resin 25 as an amino-component (Scheme 20). Debenzylation of the cleaved product was carried out by H2 with Pd(OH)2 on carbon, on resin deacetylation with 1 M NaOMe. Chiral acids had practically no effect on the diastereoselectivity, whereas chiral aldehydes showed the biggest influence due to their proximity to the newly formed stereocenter. The diastereomeric ratio was up to 80:20.
Scheme 20.
Evaluation of C-glycoside aldehydes in U-4CC.
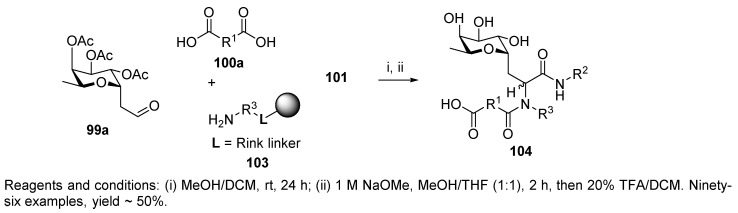
Then, a library of 96 compounds was prepared using Rink resin and five Rink-resin-supported amino acids 103 serving as the amino components, two isocyanides, eight dicarboxylic acids, and one C-glycoside aldehyde (99a, Scheme 21). A second library of 192 compounds was prepared using six resin-bound amines, eight diacids, two isocyanides, and one C-fucose as the aldehyde.
Scheme 21.
Generation of C-glycoside peptide ligands via U-4CC.
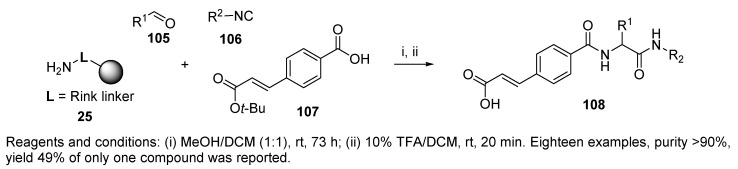
The Ugi reaction also served for the synthesis of peptides used as inhibitors of hematopoietic protein tyrosine phosphatase [69]. The starting compound, carboxylic acid 107 (Scheme 22), was prepared from 4-bromobenzoic acid by reaction with tert-butyl acrylate in the presence of Pd(OAc)2 in a yield greater than 90%. The Ugi reaction was followed by cleavage with 10% TFA in DCM. The purity of the liberated products was above 90%. Derivative 108, where R1 = Ph and R2 = Bn, was the most active tyrosine phosphatase inhibitor, with ahalf maximal inhibitory concentration (IC50) = 3.9 μM.
Scheme 22.
Synthesis of inhibitors of tyrosine phosphatase by U-4CC.
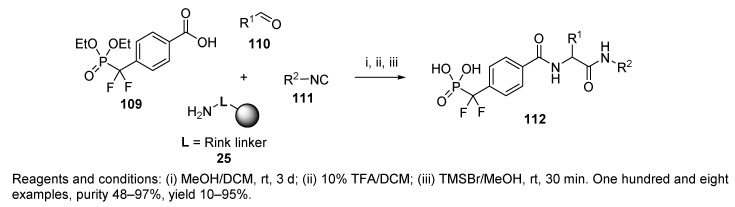
Another study aimed at tyrosine phosphatase inhibitors was dedicated to producing α,α-difluoromethylenephosphonic acids 112 (Scheme 23) [71]. Upon cleavage of the Ugi product from the polymer, the hydrolysis of phosphonate esters with bromotrimethylsilane (TMSBr) was accomplished in the solution phase. Performing the reactions in reverse order was shown to be problematic due to the sensitivity of the Rink resin to acidic conditions. Starting acid 109 was prepared in three steps. Three of the nine tested isocyanides, 1H-benzotriazol-1-ylmethyl, 2-morpholinoethyl, and toluene-4-sulfonylmethyl isocyanides, did not result in good yields. The yields of the products fluctuated considerably.
Scheme 23.
Synthesis of inhibitors of tyrosine phosphatase by U-4CC.
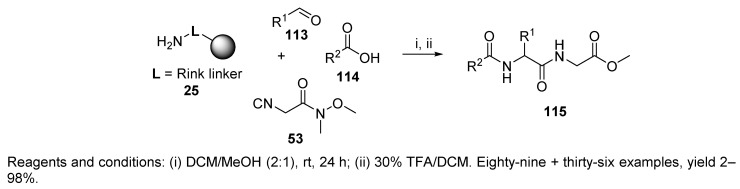
A library of peptides prepared in 96-well microtiter plates was reported by Armstrong and coworkers [35]. Rink resin 25, eight aldehydes, twelve carboxylic acids, and one isocyanide were combined in the U-4CC (Scheme 24). The yields of product 115 were most sensitive to the nature of the aldehyde component. Aliphatic aldehydes and aldehydes bearing EDG resulted in good yields in comparison to the aldehydes with EWG. Carboxylic acids with phenolic moieties also resulted in poor yields.
Scheme 24.
Synthesis of the dipeptide library by the Ugi reaction.
The authors further prepared isocyanides, otherwise not commercially available, by quenching α-lithiated isocyanides with alkyl halides. This route opened access to other isocyanides and improved the diversity of the 36-membered library, and the compounds were obtained in 10–55% yield.
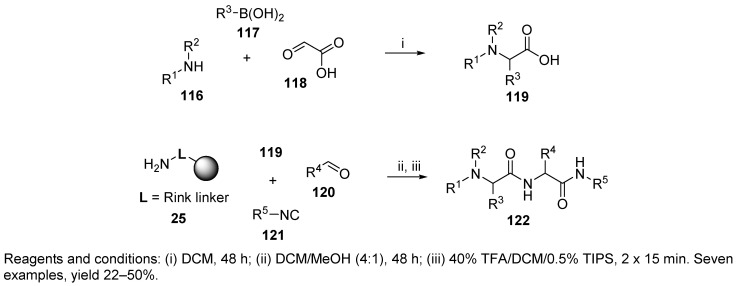
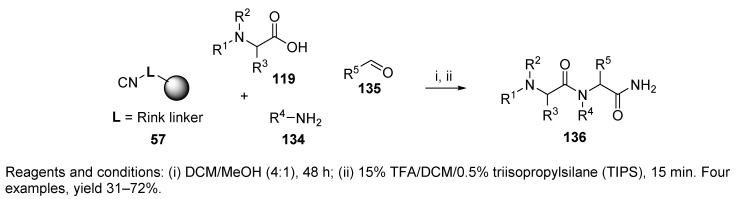
A dipeptide was prepared by tandem Petasis/Ugi reactions [58]. Combination of MCRs was first reported in 1993 by Dömling and Ugi [86]. The Petasis reaction, performed in solution, produced α-amino acids 119, which were subjected to the Ugi reaction with polymer-supported amine 25 (Scheme 25). The Petasis reaction of secondary amine 116, boronic acid 117, and glyoxylic acid 118 afforded α-amino acid 119 containing a tertiary amino group. Upon simple removal of DCM, the amino acid could be used for the solid-phase Ugi reaction.
Scheme 25.
Tandem Petasis/Ugi MCRs leading to highly substituted dipeptides.
The advantage of this concept is the ability to prepare highly substituted peptides, even on the amine terminus. The authors also applied this strategy with dipeptides and used the Rink-isocyanide resin instead of the Rink resin (cf. Scheme 28).
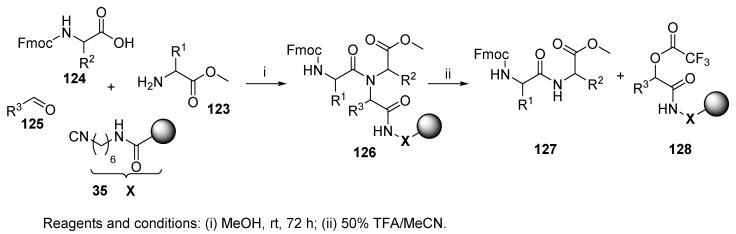
4.1.2. Isocyanide Resin
The first synthesis was carried out using acrylamide-containing resins with various chemical structures, crosslinks, and topographies (cf. Section 2.2) with the aim to synthesize peptides through ligation of a C-terminal fragment with an N-terminal fragment (Scheme 26) [29]. C-protected amino acid/peptide 123 (as an amine component) and N-protected amino acid/peptide 124 (as an acid component) were coupled in the presence of an aldehyde and resin-bound isocyanide at room temperature for 72 h. The use of H-Leu-OEt, Boc-Gly-Ala-OH, and 1-methyl-3-formylindole as amine, acid, and aldehyde components afforded tripeptides in approximately 0.5 mmol/g yield. More hindered amino acids, namely, Val-OMe and Fmoc-Leu-OH, yielded dipeptides in amounts of 0.24 mmol/g. Reactions using dipeptides and tripeptides Ala-Val-Leu-OEt with either Cbz-Gly-Phe-OH, Cbz-Gly-Ile-OH, or Boc-Leu-Ala-Gly-OH instead of protected amino acids proceeded less satisfactorily. The yields of the resulting peptides in those cases were approximately 0.05–0.07 mmol/g. The reaction was monitored by IR spectroscopy (by means of comparing the disappearance of the isocyano absorption (~2170 cm−1) and the emergence of the ester carbonyl absorption (~1700–1750 cm−1) with the intensity of the amide carbonyl moiety absorption (~1650 cm−1)). This use of U-4CC for chemical ligation has only recently attracted much attention [16].
Scheme 26.
Peptide formation on an isocyanide polymer support by U-4CC.
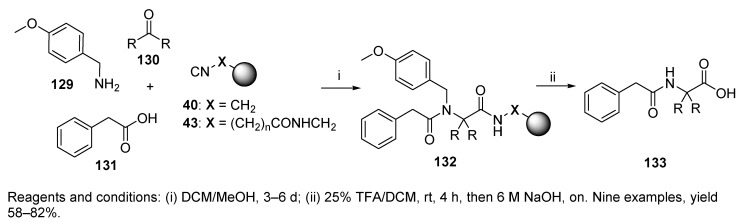
α,α-Dialkylglycine, a highly hindered C-tetrasubstituted amino acid, was produced by U-4CR [49]. The reaction utilized isocyanide resin either without a linker or equipped with a linker (cf. Scheme 7). The Ugi reaction was carried out with p-methoxybenzylamine 129 (Scheme 27), three different ketones 130, and phenylacetic acid 131 by using isocyanide resins 40 and 43. The experiment utilizing the resin without any spacer failed to release the target product. In contrast, the Ugi reaction with isocyanide resin equipped with the spacer was successful with all the used ketones (130, R = CH2CH3, (CH2)2CH3, and -(CH2)5-). The presence of the spacer seemed to be required.
Scheme 27.
U-4CR in the synthesis of α,α-dialkylglycines.
In addition to the synthesis on dipeptides by using Rink resin (cf. Scheme 25), the paper also studied the tandem Petasis/Ugi reaction utilizing universal Rink isocyanide resin 57 (Scheme 28) [58]. The Petasis reactions were carried out in the solution phase and afforded trisubstituted α-amino acid 119, which was subjected to U-4CC.
Scheme 28.
U-4CR involving Petasis product leading to highly substituted dipeptides.
4.2. α-Acylamido Carboxamides with Four Diversity Positions
4.2.1. Amine Resin
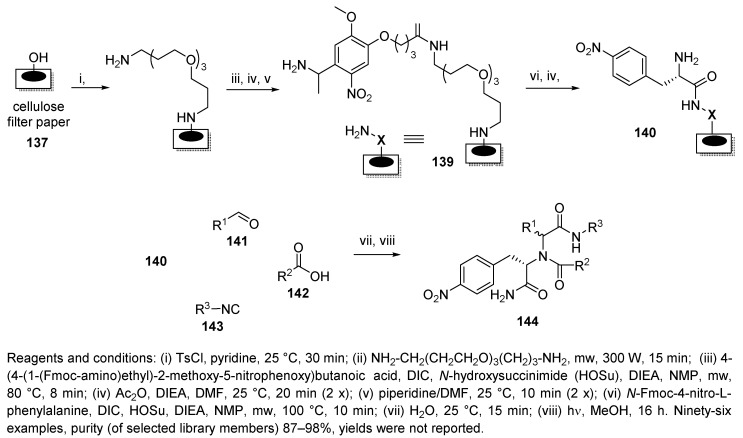
Whatman filter paper equipped with a photocleavable linker served as a planar cellulose support for the synthesis of small molecule macro-arrays [38]. First, cellulose filter paper was modified with a diamine spacer to enhance its reactivity (138, Scheme 29), followed by attachment of the photolabile linker 4-(4-(1-(Fmoc-amino)ethyl)-2-methoxy-5-nitrophenoxy)butanoic acid (139). Modified cellulose 139 was then acylated with N-Fmoc-4-nitro-L-phenylalanine, which, upon cleavage of the Fmoc group (140), could be used for U-4CR. It is important to note that the conversion at room temperature was poor, and neither a longer reaction time, multiple applications of reagents, nor microwave irradiation enhanced the yields. Finally, the rate of the Ugi reaction was successfully accelerated by water [87].
Scheme 29.
U-4CR using photolabile-linker-modified cellulose.
For the construction of a 96-membered macro-array, four aldehydes, six different carboxylic acids, and four isocyanides were chosen. The transformation was most sensitive to the nature of the aldehyde. While aliphatic aldehydes afforded the purest products, aromatic aldehydes were less effective. However, in all cases, the products were obtained in excellent purity. The authors speculated that the photocleavage efficiency was higher with the planar support than with PS beads.
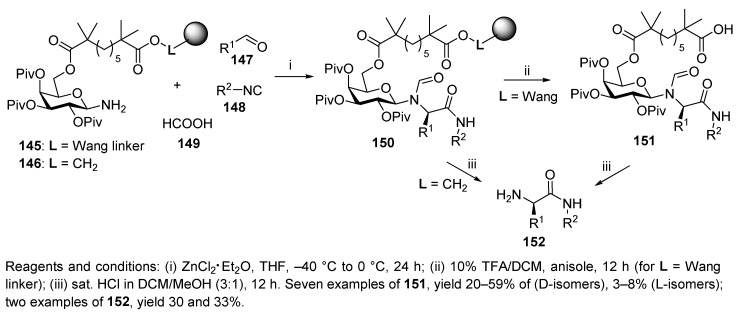
Kunz et al. developed the first stereoselective Ugi reaction on the solid phase using carbohydrate as a chiral auxiliary (Scheme 30) [19]. The synthesis sequence started with the laborious six-step preparation of the O-pivaloylated polymer-supported galactosylamine 145 and 146. The Ugi reaction was performed at −40 °C in the presence of zinc chloride etherate. The target products 151 were liberated with 10% TFA in DCM in the presence of anisole and were obtained in good yields and stereoselectivity. The diastereomeric ratios (D:L) of the products 151 ranged from 15:1 to 6:1, and both L- and D-isomers were isolated.
Scheme 30.
The first diastereoselective Ugi reaction on the solid phase.
The synthesis was also carried out on Merrifield hydroxymethyl resin. In this case, the resin-bound product was treated with DCM saturated with gaseous HCl in MeOH (3:1) to cleave the N-formyl and N-glycosidic bonds, releasing the amino acid amide 152. The same amino acid amide 152 was also obtained from released product 151 upon treatment with HCl.
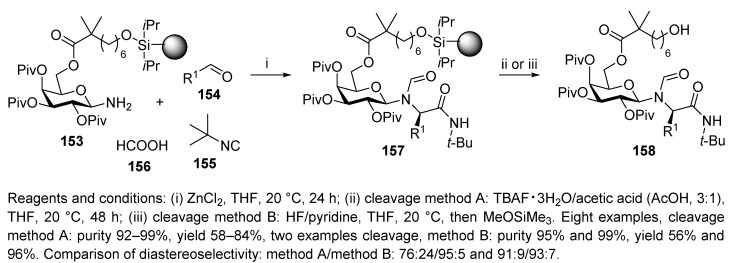
Three years later, the same research group applied galactopyranosylamine as a chiral auxiliary in the diastereoselective U-4CR synthesis of N-glycosylated N-acylated α-amino acid amides (Scheme 31) [20]. U-4CC was carried out with resin-bound galactosylamine 153, aldehyde, tert-butyl isocyanide, and formic acid in the presence of ZnCl2. Similar to a previous report [19], the preparation of galactosylamine 153 was laborious. Importantly, the U-4CC proceeded at ambient temperature (in comparison to the previously reported synthesis (Scheme 30)) [19] without any decrease in diastereoselectivity.
Scheme 31.
Diastereoselective U-4CR.
The final cleavage was accomplished with TBAF in THF buffered with acetic acid (method A). In the case of intermediates 157 composed of EWG-containing benzaldehyde only, the products were obtained by treatment with a non-basic HF-pyridine complex (method B). The products were obtained in high yield, purity, and diastereoselectivity (diastereomeric ratio (dr) was from 96:4 to 74:26), and the TBAF cleavage caused partial epimerization. Resin-bound galactosylamine 153 was further employed for the synthesis of piperidinones as a core structure in natural products.
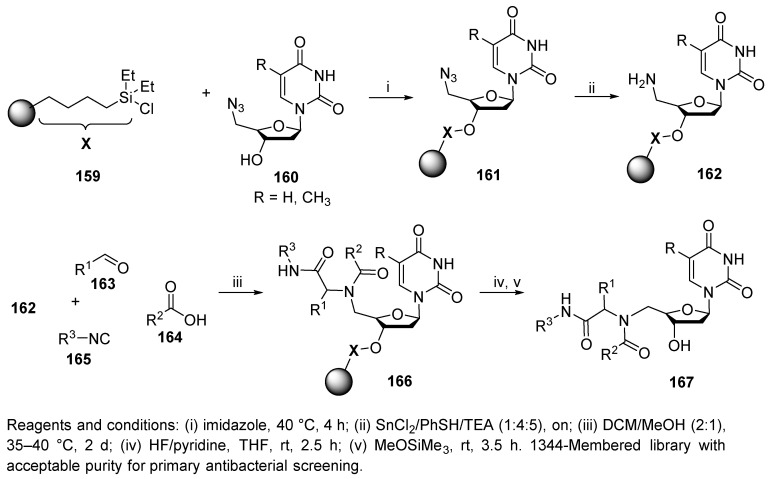
A library comprised of 1344 compounds of thymidinyl and 2′-deoxyuridinyl nucleoside-peptides was prepared by the Ugi reaction [72]. The library for antibacterial screening was prepared in fourteen 96-well Flexchem filter plates. The synthesis sequence started with the attachment of 5′-azadinucleoside to commercial PS/DVB-immobilized butyl diethylsilane 159 (Scheme 32). The resulting polymer-supported nucleoside azide 161 was reduced to amine 162, which was subjected to U-4CR. Product 167 was liberated with HF in pyridine, which avoided any degradation of the nucleoside bond. Methoxytrimethylsilane was used to quench the excess HF.
Scheme 32.
U-4CC in the synthesis of thymidinyl and 2′-deoxyuridinyl nucleoside-peptide library.
Notably, an elevated temperature in the Ugi reaction (40 °C) led to higher yields. Additionally, the influences of solvents and molar ratios of the building blocks (BBs) were studied in detail, and the best mixture of solvents was shown to be DCM/MeOH (2:1). The reaction outcomes (reactivity and purity) were most dependent on the nature of the aldehyde (which was consistent with previously reported syntheses, e.g., References [35,36,37]). Aliphatic aldehydes had the highest reactivity, while heterocyclic aldehydes were the least reactive. The Ugi reaction was optimized in a 96-well plate with eight aldehydes, twelve carboxylic acids, one isocyanide, and the resin-bound nucleoside amine.
4.2.2. Isocyanide Resin
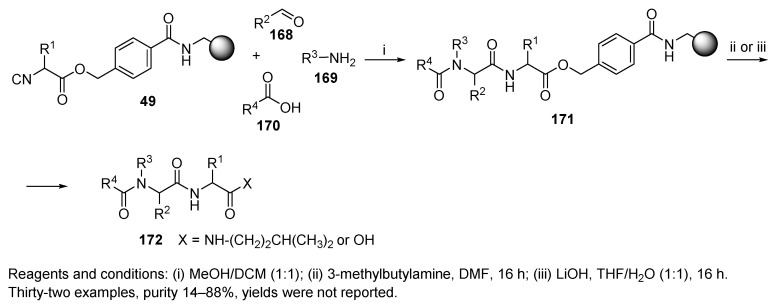
A new method for the synthesis of resin-bound isocyanocarboxylic acids 49 (cf. Scheme 8) was described by Henkel and coworkers (Scheme 33) [73]. The Ugi reaction was performed with four resin-bound isocyanides, aldehydes, amines, and acids in a mixture of MeOH and DCM (1:1). After the synthesis, each batch was divided into two parts, and cleaving was performed with two different reagents: 3-methylbutylamine or LiOH. This cleavage procedure liberated amides or carboxylic acids, introducing a fifth element of diversity.
Scheme 33.
U-4CR involving resin-bound isocyanocarboxylic acids.
Another example of U-4CC with the Wang isocyanide resin 37 (cf. Scheme 6) utilized aryl glyoxal 173, amine, and carboxylic acid as starting BBs (Scheme 34) [50]. The main aim of this work was to achieve the cyclization of polymer-supported Ugi product 176 to imidazoles 337 (cf. Scheme 57).
Scheme 34.
Preparation of α-(N-acyl-N-alkylamino)-β-keto amides via U-4CC.
4.2.3. Aldehyde Resin
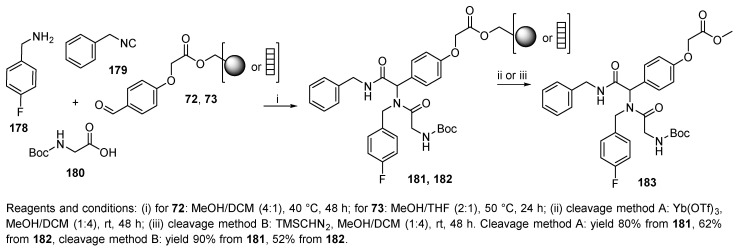
The following work was aimed at the discovery of drug-like small molecules, potent agonists for growth hormone secretagogue (Scheme 35) [82]. The Ugi reaction was performed in the solution phase as well as on three different supports, namely, soluble methoxy poly(ethylene glycol), and two solid supports (hydroxymethyl resin 23 and SynPhase® Lanterns 70, cf. Scheme 14). A crosslinked polymer with a 2-oxo-1,2-ethylenedioxy linker (72 and 73) was used for the Ugi reaction with N-Boc-glycine, 4-fluorobenzylamine, and benzylisocyanide. Polymer-supported α-(acylamino)carboxylic acid esters 181 and 182 were subsequently exposed to two different cleavage agents: Yb(OTf)3 (method A) or TMSCHN2 (method B). Although the cleavage conditions were mild, trimethylsilyl diazomethane is highly toxic.
Scheme 35.
Ugi reaction in the synthesis of drug-like small molecules.
The solid-supported reactions required heating (40–50 °C). The Ugi reaction on hydroxymethyl resin-bound aldehyde was completed within 24–48 h, while the reaction with Lantern-immobilized aldehyde was terminated after 24 h and prolonging the reaction time did not lead to better yields. The yield of the products was improved by the addition of a catalytic amount of perchloric acid. Both solid supports were quantitatively recovered after the cleavage of the product. This approach was then applied to the synthesis of a potent agonist for growth hormone secretagogue.
4.2.4. Carboxylic Acid Resin
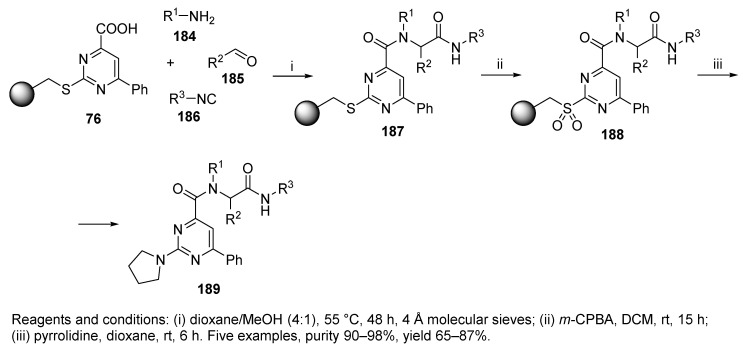
The next application of U-4CR utilizing polymer-supported carboxylic acid 76, which was prepared from the Merrifield resin via three steps (cf. Section 3.4), yielded resin-bound pyrimidines, which were liberated by oxidation with m-CPBA followed by nucleophilic displacement with pyrrolidine (product 189, Scheme 36) or with other N-nucleophiles to expand the diversity [40]. This method of cleavage is known as multifunctional (multidirectional) cleavage [88] and utilizes a safety-catch linker [89]. A description of this methodology is included in a recently published review [90].
Scheme 36.
U-4CR utilizing polymer-supported carboxylic acid.
5. U-4CR Followed by Cyclative Cleavage
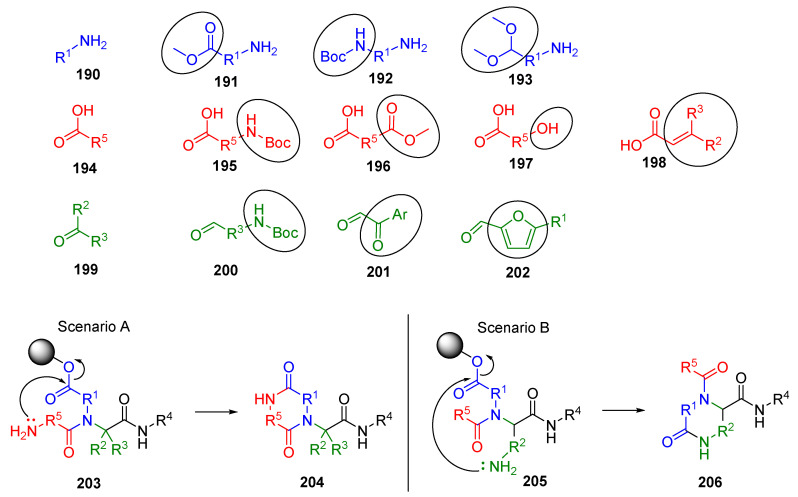
Cyclative cleavage is considered the most effective method for releasing the products from the insoluble support. Typically, an internal nucleophile (nitrogen or oxygen) attacks an ester-type bond of an acyclic U-4CC product with the resin, which results in the release of cyclic products. The reaction conditions are usually mild, and only the cyclic product is released; thus, the crude purity is generally high. This methodology opens access to six- and seven-membered cycles. The following illustration shows the bifunctional BBs (the functional group involved in the post-Ugi transformation is circled) and an example of two scenarios of cyclization (Figure 3).
Figure 3.
Examples of bifunctional BBs and ring closures.
5.1. Diketopiperazines
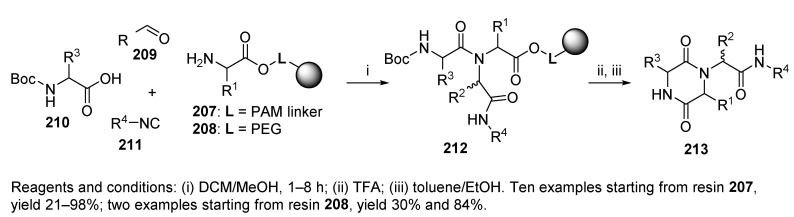
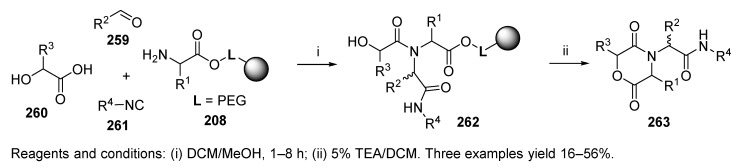
Diketopiperazines were synthesized via the Ugi reaction either on classical solid-phase support (resin beads) [6,45,46], or on cellulose filter paper [62]. Two different approaches leading to diketopiperazines were reported by Szardenings et al. [46]. The first one involved a five-step synthetic sequence (not included in this review), while the second one utilized U-4CC followed by cyclative cleavage (product 213, Scheme 37) and afforded products in higher yields. Two solid supports were tested, namely, 4-(hydroxymethyl)phenylacetamidomethyl (PAM) 207 and TentaGel S-OH 208 resins. The latter polymer afforded the products in higher yields, while the reaction on PAM resin required DCE as a cosolvent. The yields were mostly dependent on the aldehyde. While the aliphatic aldehydes reacted within minutes, aromatic aldehydes and ketones (not shown in the scheme) required a reaction time of several hours. It is important to note that the Ugi reaction tolerated hindered amino acids, such as valine, in contrast to the classical approach consisting of a five-step synthetic sequence. The authors also synthesized diketomorpholines (cf. Scheme 45).
Scheme 37.
Synthesis of diketopiperazines.
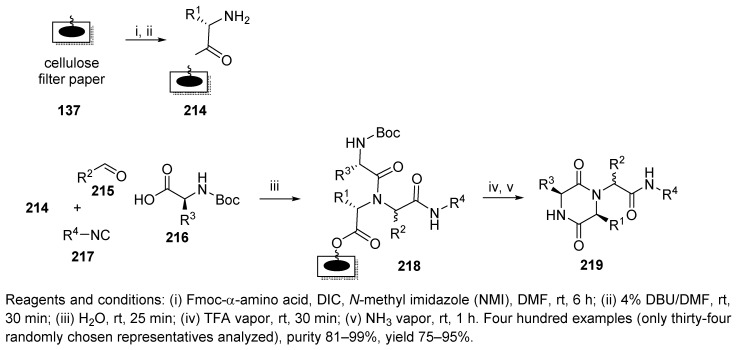
The Ugi/deBoc/cyclization (UDC) strategy, first reported by Hulme and coworkers [91], was applied for the synthesis of a 400-membered library of diketopiperazines intended as luminescence inhibitors of the bacterial symbiont Vibrio fischeri [62]. Cellulose membrane (Whatman chromatography paper) 137 was used as a solid support, and neither a spacer nor a linker was required to attach the first BB, Fmoc-α-amino acid (Scheme 38). Deprotection of the amino group (214) was followed by U-4CR with five different Boc-α-amino acids, four aldehydes, and four isocyanides. Linear Ugi-product 218 triggered acid-mediated Boc cleavage, which was followed by spontaneous cyclative cleavage to produce target diketopiperazines 219. Gaseous ammonia was used to deprotonate the amino group.
Scheme 38.
SPS of diketopiperazines via the UDC strategy.
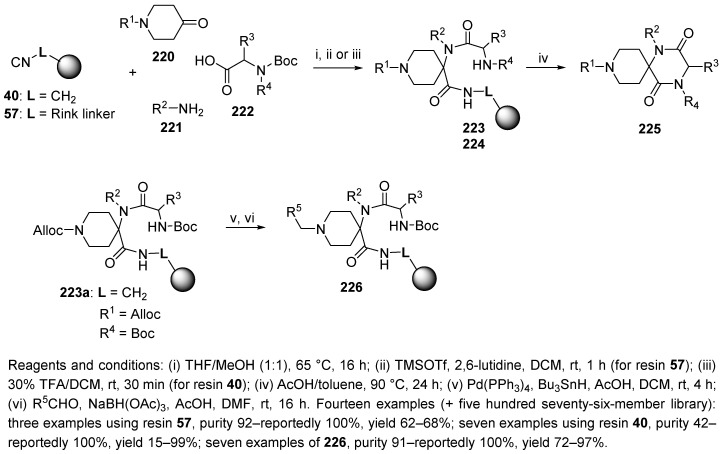
A 576-member library of spirodiketopiperazines as potent, selective CCR5 antagonists, was readily prepared through U-4CR [45]. Two different isocyanide resins (40 and 57, Scheme 39) were based on aminomethyl and Rink amide resins, respectively. In the Ugi reaction, cyclative cleavage of monocyclic precursors 223 and 224 was induced at elevated temperature in the presence of acetic acid. To expand the diversity at the R1 position, an Alloc-protected piperidinone was incorporated (resin 223a). The Alloc-protecting group was removed, the secondary amine was reductively alkylated with an aldehyde (resin 226), and the product was released upon Boc removal. For hit identification, the Ugi reaction was performed with eight piperidinones, nine amines, and eight amino acids to prepare a 576-member library. The synthesis was also studied in the solution phase.
Scheme 39.
Synthesis of spirodiketopiperazines as potent, selective CCR5 antagonists.
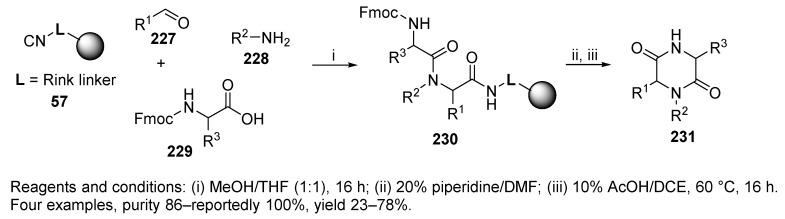
Universal Rink-isocyanide resin 57 (Scheme 40) was utilized for U-4CR, leading to diketopiperazines 231 [6]. U-4CR yielded linear resin-bound α-acylamino carboxamides 230, which was deprotected. Cyclative cleavage in 10% AcOH in DCE at elevated temperature led to the desired diketopiperazines 231 with excellent purity. The formation of benzodiazepines 258 and 5-substituted 1H-tetrazoles 433 was also investigated (cf. Schemes 44 and 73, respectively).
Scheme 40.
Diketopiperazines prepared on universal Rink-isocyanide resin.
5.2. Ketopiperazines
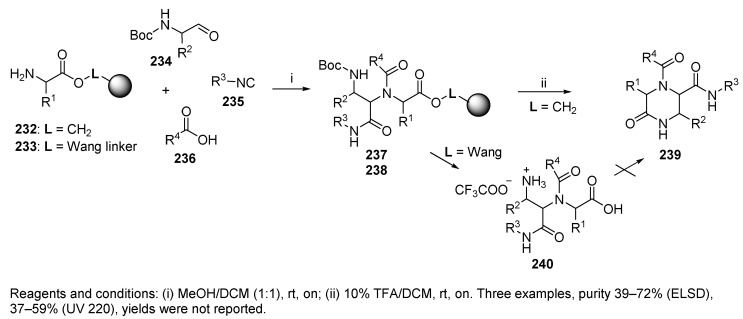
Hulme et al. applied the UDC strategy to synthesize ketopiperazines 239 (Scheme 41) and 1,4-benzodiazepine-2,5-diones 252 (cf. Scheme 43) as potential fibrinogen receptor antagonists and anticonvulsant and hypocholesteremic agents [4]. The synthesis of ketopiperazines 239 was performed on an acid-stable hydroxymethyl resin because the ester bond was not cleaved by TFA, and the release and formation of target ketopiperazines 239 occurred via a cyclative cleavage mechanism. The application of the Wang resin led to cleavage of acyclic derivative 240, which did not cyclize in solution. Interestingly, the synthesis of benzodiazepines 252 showed the opposite effect (cf. Section 5.4). The purity was judged by two methods: evaporative light scattering detection (ELSD) and UV detection at 220 nm.
Scheme 41.
Synthesis of ketopiperazines via the UDC strategy.
5.3. Bicyclic Diketopiperazines
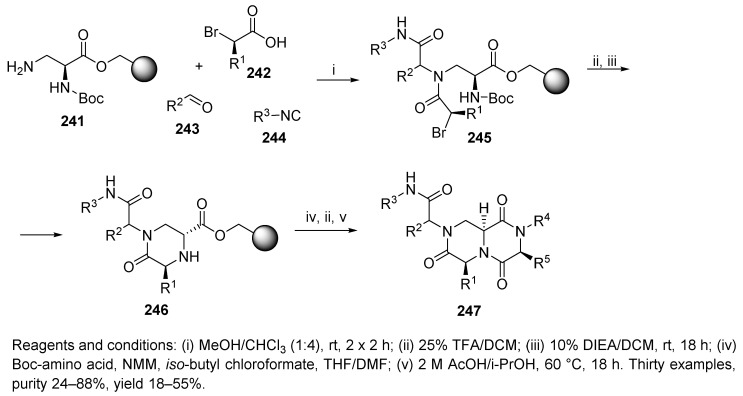
On the basis of a previously published strategy yielding peptide β-turn mimetics [59], Golebiowski et al. synthesized a library of bicyclic diketopiperazines as putative β-turn peptidomimetics [36]. The key step was the Ugi 4-CC reaction of α-N-Boc-diaminopropionic acid ester, which was prepared via the Mitsunobu reaction and attached to a Merrifield-type hydroxymethyl resin (241) with α-bromoacid 242, aldehyde, and isocyanide (Scheme 42). The poor diastereoselectivity of the Ugi 4-CC reaction resulted in a mixture of diastereomers (1:1 to 1:2) at the R2 stereocenter; however, this approach did allow for the control of all remaining chiral centers. Cyclative cleavage occurred at elevated temperatures in the presence of acetic acid.
Scheme 42.
Ugi reaction as the key step in the synthesis of β-turn peptidomimetics.
5.4. Benzodiazepines
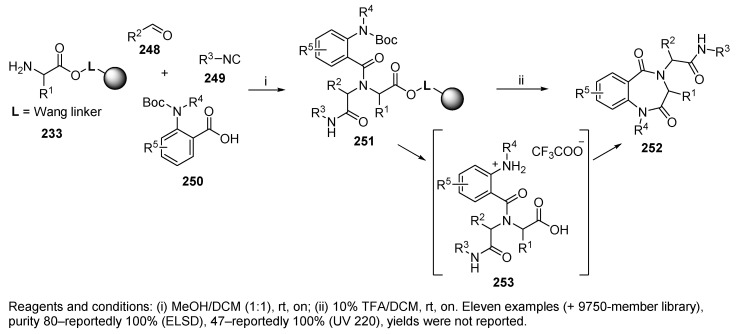
In addition to ketopiperazines 239 (cf. Scheme 41), a synthetic route to produce benzodiazepines 252 (Scheme 43) was developed by Hulme and coworkers [4]. 4-CR of the amino acid attached to the Wang resin via carboxylate 233, aldehyde, isocyanide, and N-substituted-N-Boc-anthranilic acid 250 resulted in the polymer-supported Ugi product 251, which, upon treatment with 10% TFA in DCM and evaporation at room temperature in a SAVANTTM evaporator (otherwise isocyanide-derived amide bond was hydrolyzed to carboxylic acid), resulted in target benzodiazepines 252 in good to excellent yield. In contrast to the case of ketopiperazines (cf. Scheme 41), acyclic intermediate 253 was not detected, probably due to rapid cyclization to the target compounds. A cyclocleavage mechanism was also considered.
Scheme 43.
Synthesis of 1,4-benzodiazepine-2,5-diones via the UDC strategy.
Notably, the synthesis of benzodiazepines was also performed in the solution phase; however, the solid-phase approach was preferable due to the tendency of methyl esters to form ketopiperazines in solution. The synthesis was applied to produce a 9750-member library by using eight amino acids, eight N-Boc-anthranilic acids, ten isocyanides, and fifteen aldehydes.
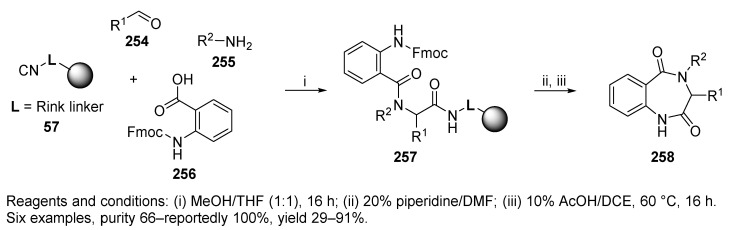
In addition to the previously mentioned diketopiperazines 231 (cf. Scheme 40), universal Rink-isocyanide resin 57 was utilized for U-4CR with Fmoc-anthranilic acid 256, leading to the acyclic Ugi products 257 (Scheme 44) [6]. Acid-mediated cyclative cleavage resulted in the formation of target benzodiazepines 258 in variable yields.
Scheme 44.
Benzodiazepines prepared on universal Rink-isocyanide resin.
5.5. Diketomorpholines
Diketomorpholine 263 (Scheme 45) [46] was produced by the Ugi reaction with α-hydroxycarboxylic acid 260 in place of N-Boc-protected amino acids, which afforded the previously mentioned diketopiperazines (cf. Scheme 37). The solid-phase Ugi reaction was performed with TentaGel S-OH resin because it afforded products in higher purity in comparison with the Wang or PAM resins. Cyclative cleavage of 262 induced by an O-nucleophile was accomplished with 5% TEA in DCM, and 95% TFA in H2O [92] resulted in numerous side products.
Scheme 45.
Synthesis of diketomorpholines.
6. Post-Ugi Transformations of Convertible Isocyanides
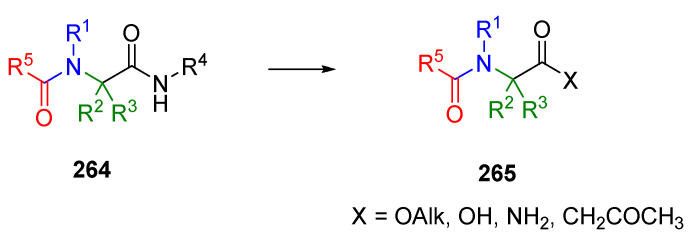
The unavailability of diverse isocyanides from commercial sources and the need to synthesize unpleasant odorous (although not particularly toxic) starting synthons triggered the development of universal isocyanides. The term universal refers to the ability to modify the secondary amide incorporated by isocyanide after completing U-4CC. This approach aimed to make the amide bond susceptible to cleavage under mild conditions while not affecting the other amide. The R4 amide (Scheme 46) was transformed into esters [1,61,80,81,83], carboxylic acids [80,83,93], and ketones [2]. The post-Ugi transformation strategy incorporates a fourth diversity element without the need to prepare a diverse set of isocyanides. The transformed product can be used for further modification (e.g., heterocycle formation). The convertible isocyanide can be either attached to the resin or used as one of the components in the solution.
Scheme 46.
Transformations of convertible isocyanide.
6.1. Polymer-Supported Convertible Isocyanides
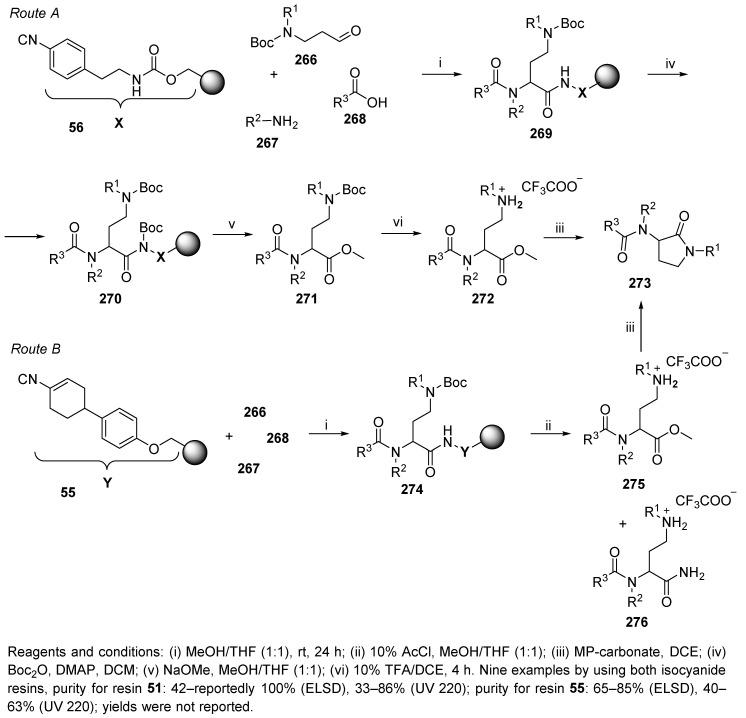
Hulme et al. applied convertible isocyanides to synthesize γ-lactam analogs [1]. The synthesis utilized a UDC strategy and was performed in solution as well as on the solid phase. U-4CR was carried out on versatile safety-catch linker 56 [80] (Route A, Scheme 47) and on resin-bound cyclohexenyl isocyanide 55 (Route B), with N-Boc-β-amino-aldehydes 266 (easily prepared from the carboxylic acid via the Weinreb amide formation and subsequent reduction with LiAlH4) [94], amines, and carboxylic acids.
Scheme 47.
Synthesis of γ-lactams via the UDC strategy.
Route A involves the reaction of polymer-supported Ugi product 269 with Boc2O (resin 270) to activate the isocyanide-derived carboxamide for a nucleophilic attack by NaOMe. Liberated methyl esters 271 were treated with 10% TFA in DCE (linear intermediate 272) and yielded the target lactams 273. The cyclization was promoted by solvent evaporation at 65 °C in a SAVANTTM evaporator. Alternatively, the Ugi product bound to the cyclohexenyl resin (intermediate 274) was converted to methyl ester 275 and subsequently cyclized to product 273 (Route B). The partial cyclization was completed by a proton scavenger. This route suffered from the formation of a side product, carboxamide 276. The purities were analyzed by ELSD and UV absorption analysis at 220 nm.
Both isocyanide resins were stable at –20 °C and odorless. In contrast, the stability of the N-Boc-β-amino-aldehydes was limited (it is necessary to use freshly prepared batches). In this paper, the synthesis of bicyclic γ-lactams was carried out (cf. Scheme 64).
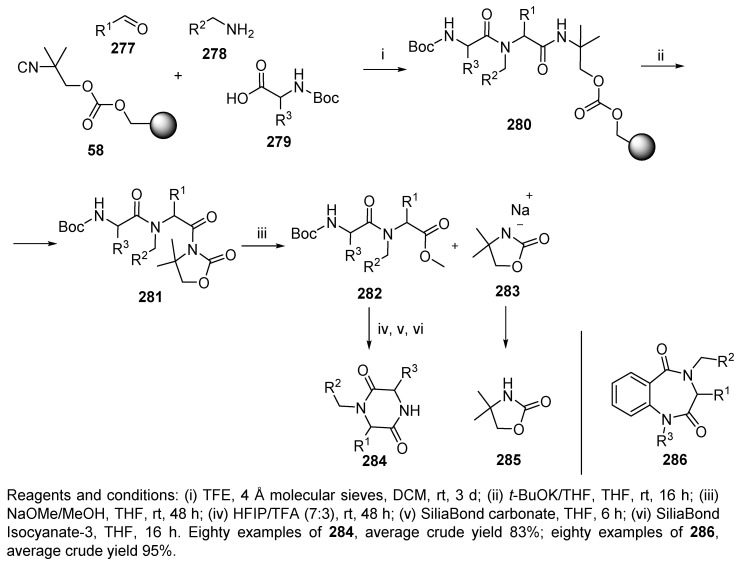
In 2002, Kennedy et al. discovered a novel CCI resin 58 (cf. Scheme 12) and demonstrated its applicability to the synthesis of 2,5-diketopiperazines 284 and 1,4-benzodiazepine-2,5-diones 286 (Scheme 48) [81]. The synthesis sequence comprised U-4CC followed by a two-step, one-pot procedure (including base-activation of the resin-bound Ugi product 280 resulting in cleavage via formation of a N-acyloxazolidone 281, which was then converted to methyl ester 282). Final post-cleavage cyclization in a mixture of HFIP (1,1,1,3,3,3-hexafluoropropan-2-ol) and TFA led to the desired 2,5-diketopiperazines 284. The crude product was treated with silica-based scavengers to remove impurities. Similarly, 1,4-benzodiazepine-2,5-diones 286 were prepared (in the Ugi reaction, N-R3-anthranilic acids were used).
Scheme 48.
Synthesis of diketopiperazines and benzodiazepines by using universal isocyanide resin.
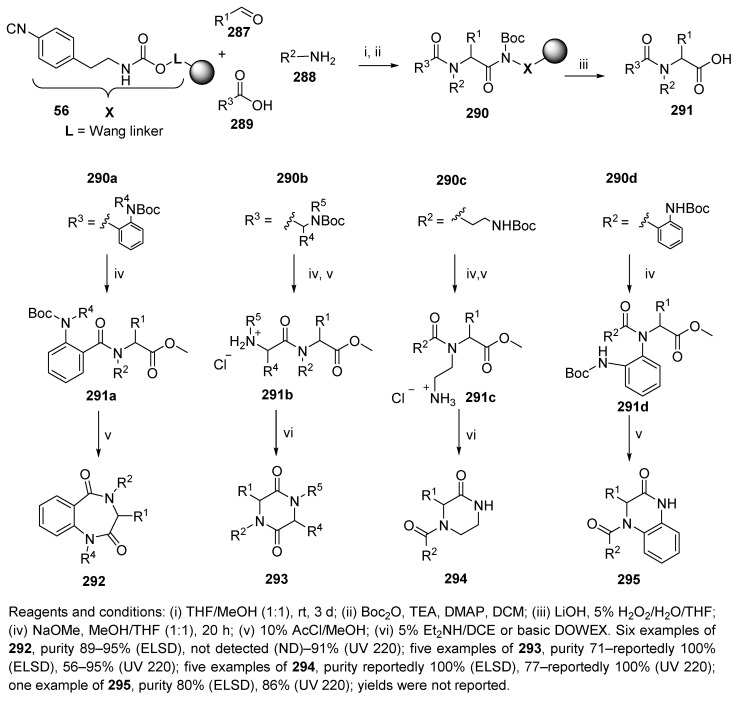
The use of safety-catch linker 56 (cf. Scheme 10) allows for cleavage of the product upon Boc activation with a nucleophile (methoxide or hydroxide) and was reported by Hulme and coworkers [80]. The UDC strategy was applied to the preparation of carboxylic acids 291, 1,4-benzodiazepine-2,5-diones 292, diketopiperazines 293, ketopiperazines 294, and dihydroquinoxalinones 295 (Scheme 49). The Ugi reaction of the Wang resin-bound isocyanide 56, which was prepared via four steps (cf. Scheme 10), aldehyde, amine, and carboxylic acid yielded α-acylamido carboxamides, which needed to be activated by Boc2O prior to cleavage (resin 290). Four different internal N-nucleophiles were tested, namely, N-Boc anthranilic acid, N-Boc α-amino acids, and two N-Boc diamines. Cleavage of the Boc group with AcCl in MeOH and evaporation at 65 °C in a SAVANTTM evaporator afforded the target bicycles 292 and 295. Partial cyclization to 293 and 294 was facilitated by a base. The purities were analyzed by ELSD and UV absorption analysis at 220 nm.
Scheme 49.
Synthesis of nitrogen-containing heterocycles on Wang resin-bound isocyanide.
6.2. Convertible Isocyanides in Solution
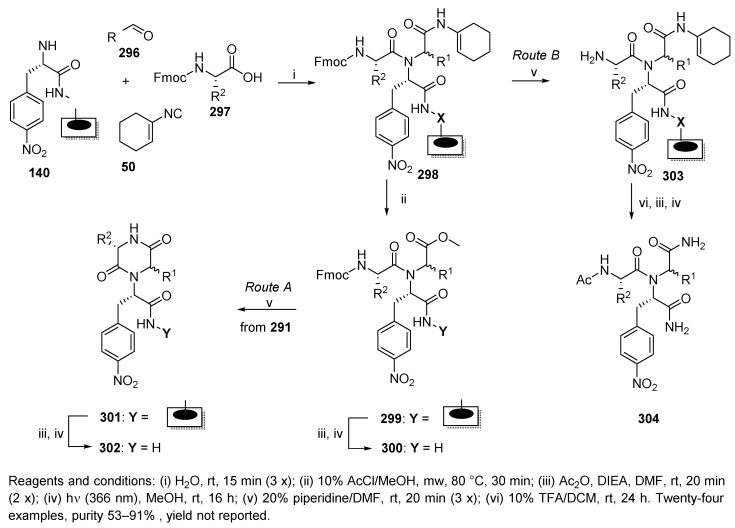
The planar cellulose support, previously described for the synthesis of peptides (cf. Scheme 29) [38], was also utilized for the preparation of diketopiperazine macro-arrays [61]. This synthesis was inspired by reports on syntheses carried out in solution [77], and on the solid phase (cf. Scheme 37) [46]. Solid-supported Ugi product 298 (Scheme 50) was converted into methyl ester 299 [78], which, upon cleavage of the Fmoc group, underwent spontaneous cyclization to immobilized diketopiperazines 301 (Route A). Subsequent treatment with Ac2O in the presence of DIEA and photocleavage afforded the target diketopiperazines 302. When intermediate 298 was directly subjected (after cleavage of the Fmoc group) to cyclization with 10% TFA in DCM, as reported by Hulme [77], the anticipated product was not obtained (Route B). Only primary amide hydrolysis product 304 and other by-products were formed. The Ugi reaction was performed with three aldehydes and eight amino acids.
Scheme 50.
Synthesis of diketopiperazines via Ugi 4-CR and subsequent cyclization.
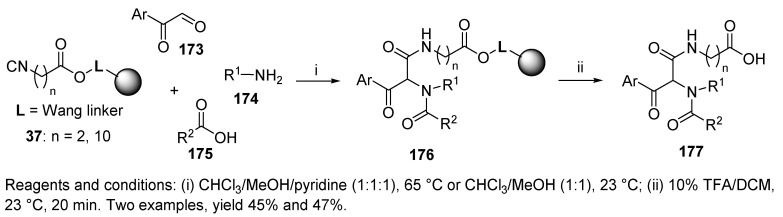
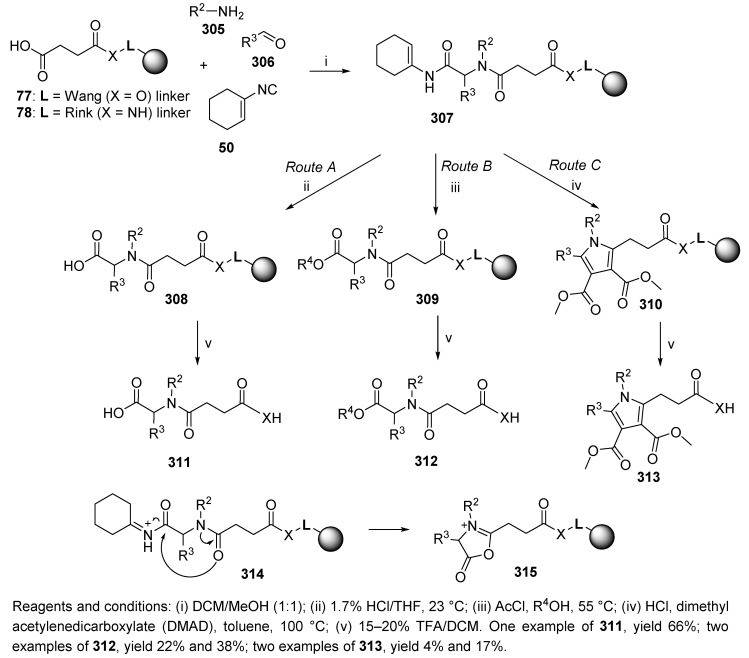
The U-4CC of resin-bound carboxylic acids 77 and 78 (cf. Scheme 16), amine, aldehyde, and 1-isocyanocyclohexene, resulted in cyclohexenamides 307 (Scheme 51) [83]. They were subsequently transformed into carboxylic acids 308, esters 309, or pyrroles 310, which, upon acidic cleavage, resulted in the desired products 311–313. The synthetic strategy was based on 1-isocyanocyclohexene as a convertible isocyanide which afforded unrelated products.
Scheme 51.
Conversion of the Ugi product to carboxylic acids, esters, and pyrroles via münchnones.
It is important to note that the intermediate formed in each route was münchnone 315, which was formed by cycloelimination of protonated cyclohexenamide 314. The münchnone then underwent a nucleophilic attack by water or alcohol, leading to carboxylic acid 311 or ester 312 (Route A and Route B), or proton cleavage, causing the formation of a 1,3-dipole, which is capable of cycloaddition with acetylene (product 313, Route C).
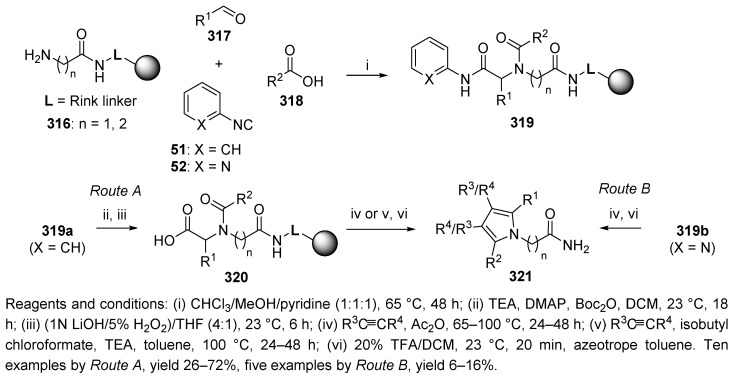
The transformation of münchnones with respect to the formation of carboxylic acids and pyrroles was also described by another research group [93]. Mjalli et al. carried out U-4CC of polymer-supported amine 316, aldehyde, carboxylic acid, and isocyanides 51 and 52, which afforded N-acyl-N-alkyl-α-amino amides 319 (Scheme 52). The reaction was carried out in a mixture of CHCl3/MeOH/pyridine (1:1:1), in which the pyridine buffered the reaction mixture and stabilized the isocyanide.
Scheme 52.
Conversion of the Ugi product to pyrroles via münchnones.
Following hydrolysis of Ugi product 319a (X = CH) to the corresponding acids, 320 proceeded in two steps (Route A). The target pyrroles 321 were achieved by a 1,3-dipolar cycloaddition of the alkynes with the resin-bound münchnones. Alternatively, polymer-supported amide 319b (X = N, Route B) was cyclized directly to münchnone, which was trapped in situ with different alkynes to yield 321. Route B produced the pyrroles in lower yields; on the other hand, it proceeded via a single step.
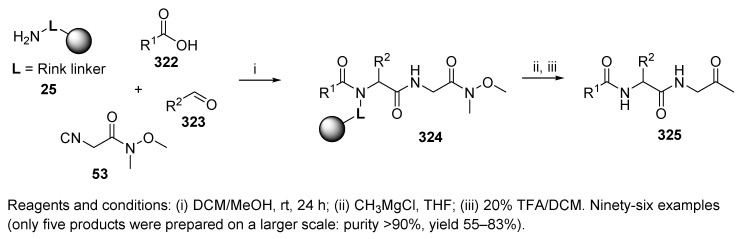
Armstrong et al. reported the Ugi four-component synthesis of diamide backbone 324 followed by derivatization through displacement of a Weinreb amide [95] with a Grignard reagent (Scheme 53) [2]. Acidic cleavage led to the target α-oxodipeptides 325. A 96-membered library was prepared by using twelve carboxylic acids and eight aldehydes. The effect of these components on the yields of the target products was studied. Aliphatic aldehydes or aldehydes bearing EDG afforded the products in good yields in comparison to those derived from EWG-containing aldehydes. While carboxylic acids with phenolic moieties resulted in low yields, the others led to products in good yields. The larger scale did not significantly influence the yields. The Weinreb amide was prepared from glycine via six steps in solution.
Scheme 53.
U-4CC with a Weinreb-type amide followed by the Grignard reaction.
7. Other Post-Ugi Transformations
In this section, we include transformations utilizing bifunctional components for the synthesis of Ugi products and exploiting the appended functional group for ring formation. These chemistries include cyclic iminium formation [34], ring-closing metathesis [56], Diels-Alder reaction [3,96], and imidazole synthesis from β-keto amides [50].
7.1. Spontaneous Transformations
Spontaneous transformations proceed simultaneously with the cleavage of the acyclic Ugi products from the resin. The first example is the formation of ketopiperazines by tandem U-4CC followed by N-acyliminium ion cyclization [34]. Wang resin-bound isocyanide (20, R3 = p-CH2-C6H4-O, Scheme 54) entered the Ugi reaction with a carboxylic acid, aldehyde, and aminoaldehyde diethyl acetal. Acidic treatment of polymer-supported Ugi product 21 cleaved the acetal, led to the formation of N-acyliminium ion, followed by the loss of proton upon neutralization, and resulted in the target unsaturated heterocyclic product 22. Note, HCl treatment prior to TFA cleavage was not necessary. The synthesis was reported in the solution phase as well.
Scheme 54.
Synthesis of ketopiperazines via N-acyliminium ion formation.
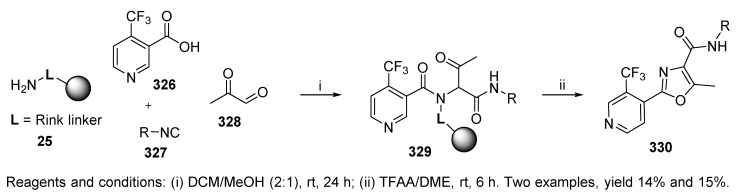
2,4,5-Trisubstituted oxazoles, which are known pesticides, were synthesized via α-acylamino ketones 329 subjected to the Robinson-Gabriel intramolecular cyclization by using TFAA in DME, where TFAA served as both a cleavage and cyclodehydration agent (Scheme 55) [70]. In addition to affording α-acylamino ketones by U-4CC, five more synthetic strategies were investigated. Despite low yields (39% and 40% crude, 14%, and 15% after passage through a silica gel plug), the Ugi reaction was beneficial due to the single step-formation of α-acylamino ketones. Only two examples were given.
Scheme 55.
U-4CC followed by Robinson-Gabriel synthesis of oxazoles.
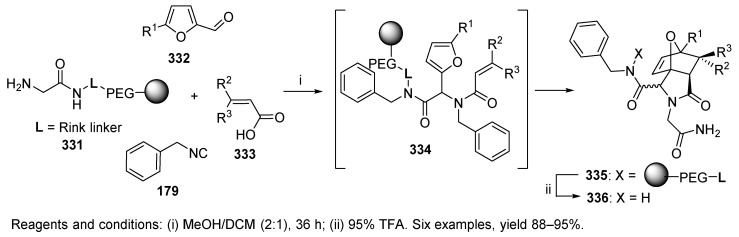
The Diels-Alder reaction is another example of a post-Ugi transformation. Resin-bound amine 331, which was prepared via two steps from ArgoGel Rink resin, furalaldehyde 332, benzylisocyanide 179, and activated dienophile acids 333 (such as maleic and fumaric acid derivatives), led to the formation of triene 334, which immediately underwent an acid-mediated intramolecular Diels-Alder reaction to afford highly functionalized rigid tricyclic lactams 335 (Scheme 56) [96].
Scheme 56.
Tandem 4-CC/intramolecular Diels-Alder reaction to rigid tricyclic lactams.
The influence of electronic and steric effects was investigated. While condensation of unsubstituted furaldehyde (332, R1 = H) led to products in high yields and good diastereoselectivities (the ratio of the two isomers ranged from 91:9 to 88:12), 5-methylfuraldehyde (R1 = CH3) afforded products with moderate diastereoselectivity (the ratio of the two isomers ranged from 88:12 to 77:23). The synthesis was initially carried out in the solution phase.
7.2. Mediated Transformations
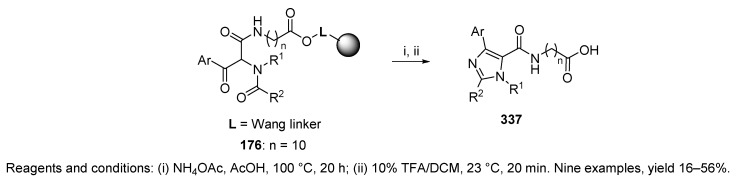
This section discusses syntheses involving additional step(s) following the Ugi product formation prior to the final cleavage from the resin. The first example in this section utilized a Wang resin-bound isocyanide (cf. Scheme 6) in the Ugi reaction leading to α-(N-acyl-N-alkylamino)-β-keto amides 177 (cf. Scheme 34) [50]. Immobilized Ugi products 176 underwent cyclization to imidazoles in NH4OAc and AcOH at 100 °C, and simultaneous release from the resin with 10% TFA in DCM led to the target tetrasubstituted imidazoles 337 (Scheme 57). The effects of the BBs on the reaction outcomes were studied. While aniline resulted in low yield (16%), isobutylamine, benzylamine, and even 4-methoxyaniline resulted in moderate yields (35–56%). Aryl glyoxals were tolerated (both unsubstituted and 4-methoxy-substituted) as well as carboxylic acids (aliphatic and aromatic). Finally, the length of the isocyanide linker had no effect on the yield of the imidazoles. Notably, some products were transformed to their methyl esters prior to purification. Direct exposure of Ugi product 176 to 10% TFA in DCM led to the corresponding amides 177 (cf. Scheme 34).
Scheme 57.
Synthesis of tetrasubstituted imidazoles.
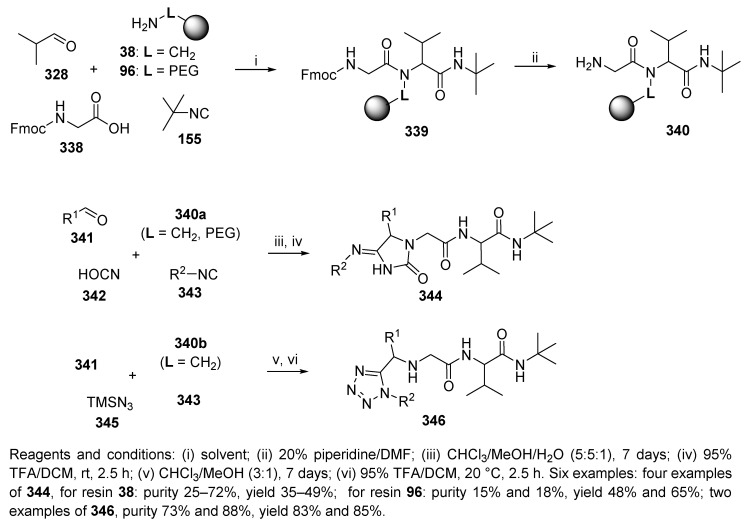
Ugi and his coworker prepared hydantoinimide 344 and tetrazole 346 derivatives by repetitive Ugi reactions using aminomethyl PS resin 38 (Scheme 58) [60], and the hydantoinimides were also synthesized on TentaGel resin 96. A sequence of two different Ugi 4-CRs was employed. The first Ugi reaction was performed with α-amino acid (Gly), and the subsequent Ugi reaction was carried out with cyanic acid and trimethylsilyl azide as alternative acid inputs (more details about the alternative acid reagents can be found in Section 9.2).
Scheme 58.
Repetitive Ugi reaction.
Hydantoinimides 344 were obtained in low purities (20–40%) when aromatic aldehyde (benzaldehyde) was used. In contrast, the purity of tetrazole derivative 346 was 88% when benzaldehyde was used. The synthesis sequence was also performed in solution.
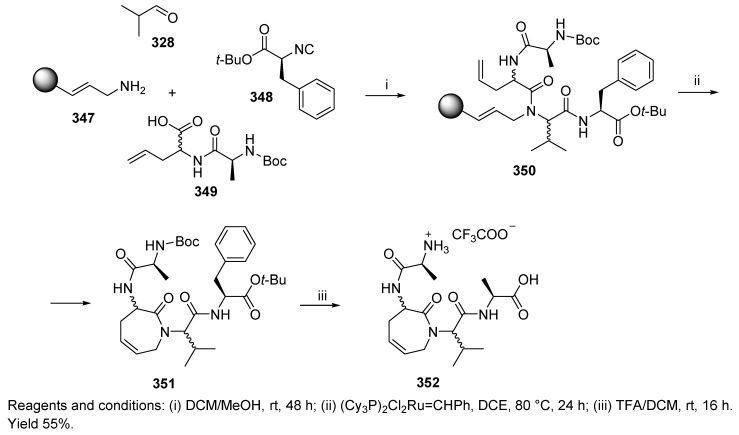
Synthesis of β-turn mimetics of the Freidinger lactam type was also achieved with U-4CC (Scheme 59) [56]. Acyclic tetrapeptide 350 was then subjected to ring-closing metathesis, including carbon–carbon bond formation and concomitant cyclative cleavage, and yielded lactam ring-containing product 351. Acid labile protecting groups were cleaved upon TFA treatment (352).
Scheme 59.
Tandem Ugi 4-CR followed by ring-closing metathesis leading to β-turn mimetics.
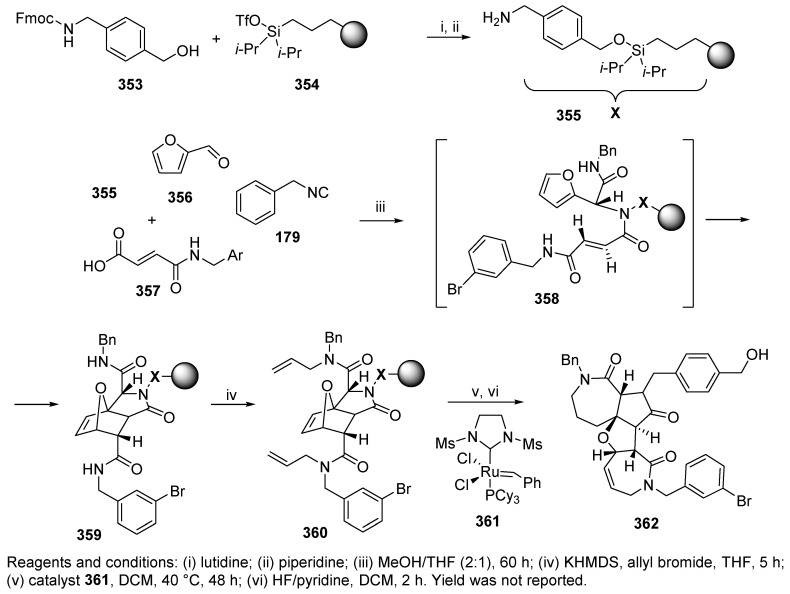
An illustrative example of the application of the Ugi reaction to generate structural complexity in synthetic products was demonstrated by Schreiber et al. [3]. Complex structures were generated via a minimal number of steps: Ugi 4-CR, intramolecular Diels-Alder reaction, and ring-opening-closing olefin metathesis, and all these steps were performed on PS modified with carbon-and-silicon-only extender 354 (Scheme 60). The Ugi reaction with immobilized amine 355, furfural 356, benzyl isocyanide 179, and fumaric acid derivative 357 was carried out in a mixture of MeOH/THF at a ratio of 2:1. Resin-bound Ugi product 358 immediately underwent an intramolecular Diels-Alder reaction, and intermediate 359 was subsequently treated with potassium hexamethyldisilazide (KHMDS) and allyl bromide in THF (360). Finally, the metathesis (20 mol% of catalyst 361, DCM, 40 °C) was followed by the release of product 362 with HF/pyridine in DCM. The synthesis sequence was initially performed in the solution phase, with a yield of 69%.
Scheme 60.
Formation of a 7-5-5-7 polycyclic ring including U-4CC.
8. Ugi Four-Center Three-Component Reaction (U-4C-3CR)
This strategy is desirable from the cyclization point of view: the product of this reaction is a heterocycle. The cyclization is achieved by a single input compound containing two functional groups required for the Ugi reaction. To date, only two types of bifunctional components have been reported, linking an acid with an amine and an acid with a carbonyl group. The bifunctional components were either resin-bound or in solution (Figure 4).
Figure 4.
Bifunctional BBs used in U-4C-3CR.
8.1. Resin-Bound Bifunctional Reagents
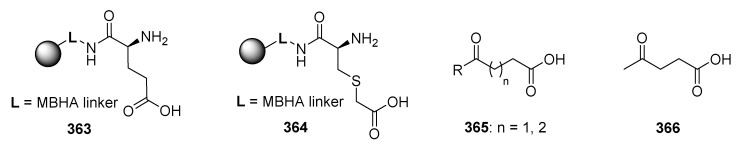
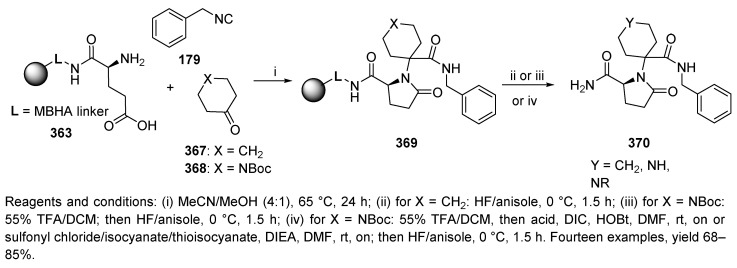
Liu and Nefzi described the synthesis of N-substituted pyrrolidinone-tethered N-substituted piperidines via U-4C-3CR [47]. The synthesis sequence started with MBHA-resin-supported glutamic acid 363, which reacted with two ketones and four isocyanides (Scheme 61). However, the Ugi reaction was not successful with resin-bound aspartic acid as the bifunctional reagent.
Scheme 61.
Synthesis of N-substituted pyrrolidinone by U-4C-3CR.
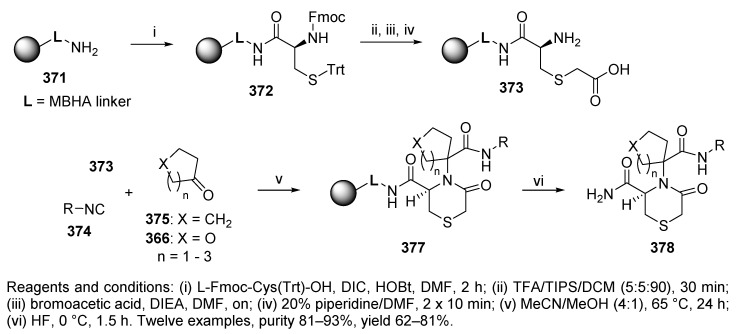
Liu and Nefzi also carried out the synthesis of thiomorpholines via Ugi 4C-3CR [48]. MBHA resin-bound bifunctional BB 373 was prepared via four steps and reacted with four symmetrical ketones and three different isocyanides at 65 °C overnight (Scheme 62). The target enantiopure thiomorpholines 377 were cleaved with HF.
Scheme 62.
Synthesis of N-substituted thiomorpholinones by U-4C-3CR.
8.2. Bifunctional Reagents in Solutions
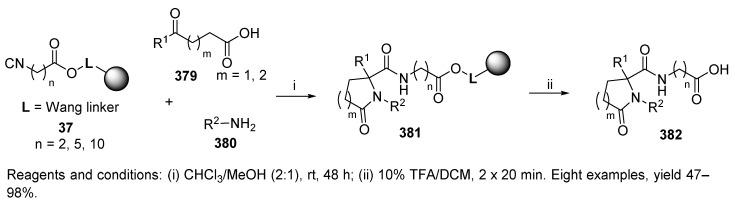
Keto acids with different chain lengths 379 served as bifunctional reagents in the synthesis of lactams 382 (Scheme 63) [54]. Their reaction with resin-bound isocyanide 37 [50] and amines yielded ɣ- and δ-lactams 381, which were released with 10% TFA in DCM. The reaction was also performed in the solution phase.
Scheme 63.
U-4C-3CR leading to lactams.
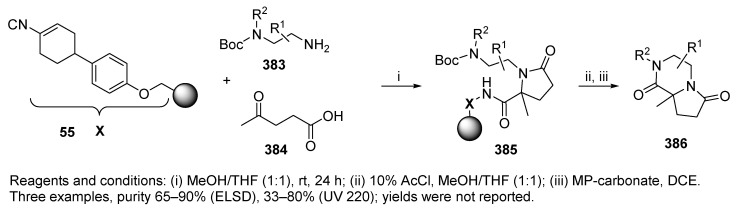
Hulme et al. [1] reported on U-4C-3CR using cyclohexenyl isocyanide resin 55 (Scheme 64), N-Boc diamines 383 [97], and a bifunctional reagent, levulinic acid 384. The Ugi reaction was followed by post-condensation modification affording fused lactam-ketopiperazines 386.
Scheme 64.
Synthesis of bicyclic γ-lactams.
9. Non-Traditional Ugi Reaction
In this section, we include IMCRs with one of the inputs, amine or acid, replaced by a different reactant. The amine was replaced with sulfonamide, and the acid was replaced with thiocarboxylic acid, alcohol, or inorganic acid, including HOCN, HSCN, or HN3. In addition, we incorporate modified Ugi reactions that did not fit into any of the above-used categories.
9.1. Alternative Amine Input
Sulfonamide
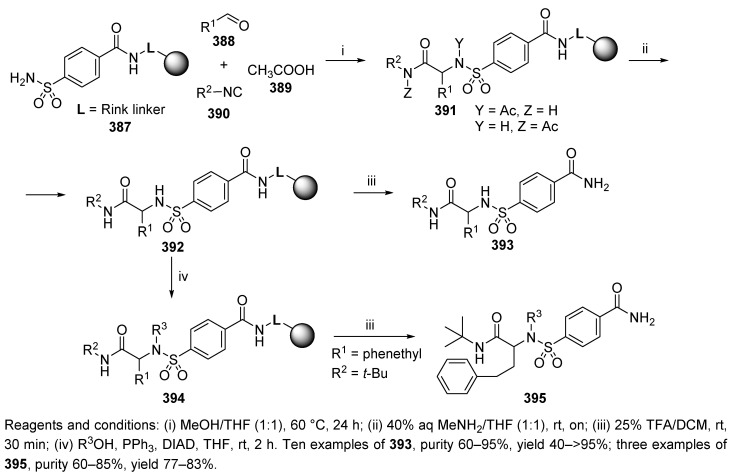
The two following examples were reported by the Lou laboratory [43]. α-Amino acid sulfonamides were synthesized by Ugi-type 4-CC utilizing polymer-supported sulfonamide as the amine input (387, Scheme 65). The synthesis was carried out in the solution phase and was not successful. Even after 3 days, the majority of the sulfonamide was unreacted, and the only a trace amount of the product was observed. The SPS improved the reaction rate, and the best results were obtained at elevated temperature (60 °C) in 24 h. In addition to the expected product 391, a small amount of deacetylated product was also observed. Cleavage of the acetyl group was then accomplished in 40% aqueous (aq) MeNH2 and THF at a ratio of 1:1 (resin 392). The target products 393 were obtained with 25% TFA in DCM. The diversity of the products was expanded by N-alkylation under Mitsunobu conditions (product 395).
Scheme 65.
Ugi-type 4-CC involving polymer-supported arylsulfonamide.
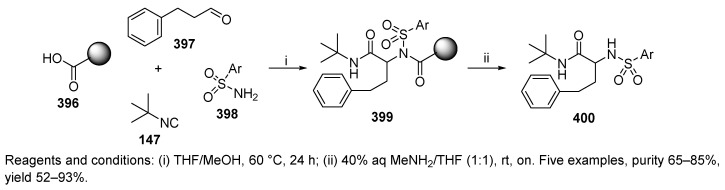
Analogous sulfonamides, structurally complementary to the products obtained with the first approach (Scheme 65), were achieved by using carboxy PS 396 (Scheme 66) [43]. Ugi 4-CC was followed by traceless cleavage to result in the target products 400.
Scheme 66.
Ugi-type 4-CC involving arylsulfonamide in solution.
9.2. Alternative Acid Input
9.2.1. Thiocarboxylic Acid
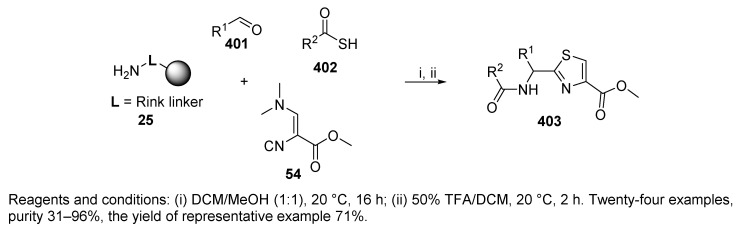
In addition to the previously reported solution-phase U-4CR leading to thiazoles [98,99], Dömling et al. [51] conducted another study on the solid-phase Ugi reaction of 3-N,N-(dimethylamino)-2-isocyanoacrylate 54 (Scheme 67) and thiocarboxylic acid 402, which yielded 4-carboxy-2-acylaminomethylthiazoles 403. Reagent 54, described for the first time in 1979 [100], represents rich functional isocyanide, bearing an isocyano functionality, a Michael acceptor, and dimethylamino leaving group.
Scheme 67.
Synthesis of thiazoles on Rink amide resin.
The synthesis was compatible with both aliphatic and aromatic thiocarboxylic acids as well as with 16 tested aliphatic and (hetero)aromatic aldehydes. A potential drawback of the usage of thiocarboxylic acids is their limited commercial availability [98]. On the other hand, there are many methods for their preparation (e.g., Reference [101]).
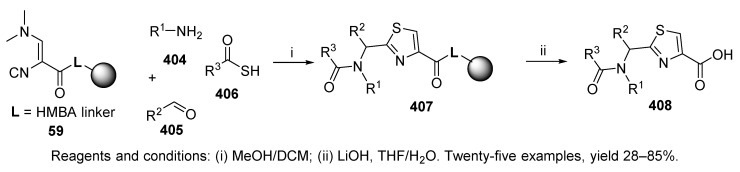
In a follow-up synthesis, Dömling et al. applied 3-N,N-(dimethylamino)-2-isocyanoacrylate attached to resin 59 (for its preparation, cf. Scheme 13) to synthesize thiazoles 408 (Scheme 68) [52]. Thiobenzoic acid afforded slightly higher yields than thioacetic acid.
Scheme 68.
Synthesis of thiazoles via resin-bound 3-N,N-(dimethylamino)-2-isocyanoacrylate.
9.2.2. (Thio)cyanic Acids and Hydrogen Azide
Replacing carboxylic acids with inorganic acids, such as HOCN, HSCN, or HN3, leads to alternative nitrogenous heterocycles [53,54].
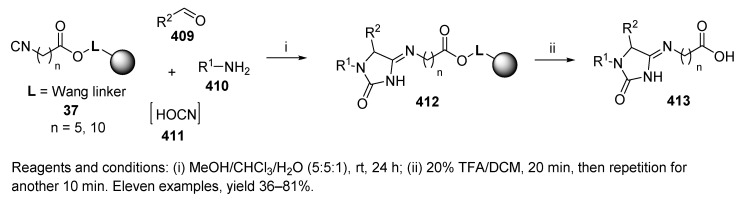
U-4CC of Wang resin-bound isocyanide 37 [50], aldehyde, amine, and in situ generated HOCN 411 (from pyridine hydrochloride and KOCN), led to hydantoin 4-imides 412 (Scheme 69) [53]. Several amines and aldehydes were tested. While the reaction tolerated different types of amines, it was highly dependent on the nature of the aldehyde. Aliphatic aldehydes (branched and unbranched) worked for this reaction; in contrast, aromatic aldehydes did not afford the products. One year later, the chemistry was extended and also described in the solution phase [54].
Scheme 69.
Preparation of hydantoin 4-imides by U-4CC.
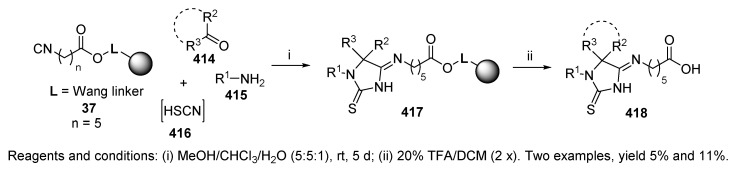
This synthetic strategy was adopted for the preparation of 2-thiohydantoin 4-imides 418 (Scheme 70). Cyanic acid was replaced by thiocyanic acid 416 (prepared in situ), and aldehyde was replaced by ketone (aldehyde was reportedly unreactive). Disappointingly, the Ugi reaction with these components afforded very low yields of 418 (5% and 11%).
Scheme 70.
Synthesis of 2-thiohydantoin 4-imides by U-4CR.
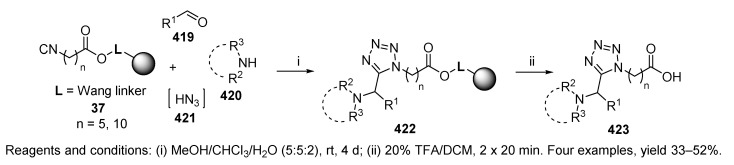
Mjalli et al. [54] investigated the reaction of polymer-supported isocyanide resin 37 (Scheme 71) [50], aldehyde, primary or secondary amine, and hydrogen azide 421 generated in situ. The Ugi reaction resulted in the formation of resin-bound tetrazoles 422, which were cleaved with 20% TFA in DCM. Both primary and secondary amines were tolerated as well as p-bromoaniline. In contrast to the Ugi reaction producing hydantoin 4-imides 413 and 418 (Scheme 69 and Scheme 70), aromatic aldehydes were compatible, although the yields were mediocre.
Scheme 71.
Synthesis of 5-(1′-aminoalkyl)tetrazoles.
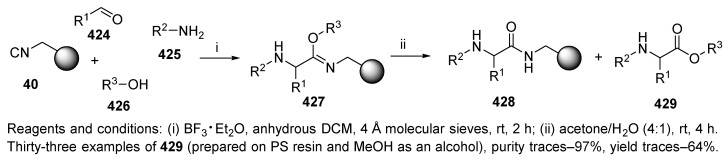
9.2.3. Alcohol
Henkel and coworkers employed 4-CR to synthesize N-substituted α-amino acid esters [39]. The outcome of the synthesis was based on the aqueous cleavage of imino-ethers, which depended on the reaction conditions. In addition to α-amino acid amides 428 (Scheme 72), which were generated in most cases, α-amino acid esters 429 were also formed.
Scheme 72.
4CR in the synthesis of N-substituted α-amino acid esters.
The Ugi reaction of isocyanide resin 40 [74] with aldehydes, amines, and alcohols, led to polymer-supported imino-ethers 427, which are key intermediates. When isocyanide tethered to aminomethyl PS resin was used, product 429 was not formed unless the reaction was catalyzed. Therefore, the synthesis was performed in the presence of a Lewis acid catalyst, boron trifluoride diethyl etherate. Cleavage in an aqueous acetone solution liberated α-amino acid esters 429, while the products of hydrolysis, α-amino acid amides 428, remained on the resin.
Both yields and purities were higher when the aldehyde and amine were pre-condensed to the imine. Additionally, aromatic amines led to better results with respect to purity and yield than aliphatic amines. In contrast, the type of alcohol had no influence on the reaction outcome (methanol, isopropanol, and phenylpentanol resulted in comparable outcomes). The reaction was also performed by using TentaGel resin, which improved the results compared to hydrophobic PS resin. Seventeen of the thirty-three successfully prepared α-amino acid methyl esters were obtained in ≥90% crude purity. However, the yields were mediocre.
9.2.4. Azide
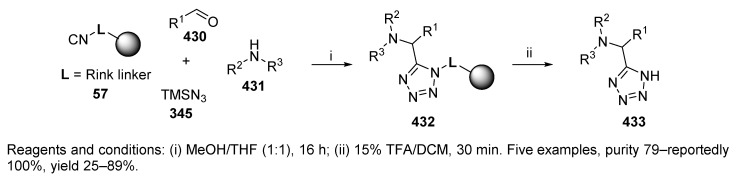
Four-component reactions involving aldehydes, amines, isocyanides, and trimethylsilyl azide, leading to the formation of tetrazoles, are referred to as Ugi-azide-4CRs. Tetrazoles are considered bioisosteres of carboxylic acids and were shown to preserve or improve biological activities. The first example is the previously mentioned repetitive Ugi reaction (cf. Scheme 58), and the second example is the solid-phase Ugi-azide-4CR generating 5-substituted tetrazoles by using convertible Rink-isocyanide resin [6]. Resin-bound tetrazoles 432 underwent acidic cleavage to produce the target 5-substituted tetrazoles 433 (Scheme 73). Notably, secondary amine was used instead of traditional primary amine. This is an example of traceless synthesis [90].
Scheme 73.
5-Substituted 1H-tetrazoles prepared on universal Rink-isocyanide resin.
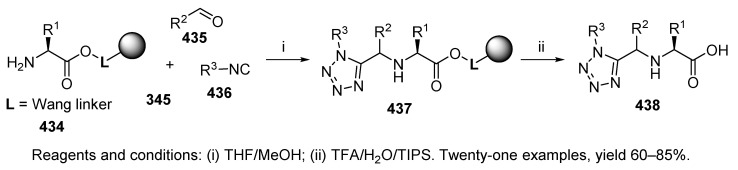
Rivera et al. used Ugi-azide-4-CR to synthesize a library of tetrazole-peptidomimetics, which are potent inhibitors of the Escherichia coli M1-aminopeptidase [5]. α-Amino acid attached to Wang resin 434 (Scheme 74) was reacted with aldehyde, isocyanide, and trimethylsilyl azide 345. Four different amino acids, five aldehydes, and three isocyanides were used to generate a set of 1,5-disubstituted tetrazoles 438. The products were obtained as a mixture of two diastereomers (dr was from 1:1 to 4.7:1).
Scheme 74.
Synthesis of tetrazole-peptidomimetics by Ugi-azide 4CR.
9.3. U-4CR Using Preformed Schiff Base
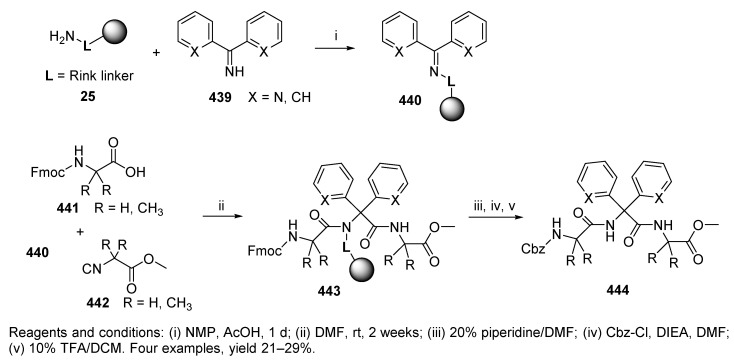
In addition to the classical U-4CR, a modified approach was investigated for the preparation of tripeptides with α,α-disubstituted glycine with two pyridine rings 444 (Scheme 75) [44]. The difficulty of reacting sterically hindered amine and diaryl ketone was overcome by preforming Schiff base iminium ions to participate in the Ugi-type reaction (with isocyanide and carboxylic acid).
Scheme 75.
Synthesis of tripeptides by modified Ugi reaction.
Polymer-supported Schiff base 440 was reacted with Fmoc-amino acids (Fmoc-Gly-OH or Fmoc-4-aminoisobutyric acid) and isocyanide 442. The Fmoc group of the resulting resin-bound Ugi product 443 was replaced with a Cbz protecting group, and the product was cleaved from the resin to yield tripeptide 444. The yields of bis-pyridyl tripeptides obtained from the solid-phase approach were higher than those obtained from solution-phase synthesis. The bis-phenyl derivatives showed the opposite trend. The effect of the solvent used in the Ugi reaction was investigated and the best results were obtained with DCM and NMP or 2,2,2-trifluoroethanol as a cosolvent. The drawback of this synthesis is its very long reaction time. It is worth noting that α,α-disubstituted glycine with two pyridine rings served as a very effective peptide backbone constraint [102].
10. Other IMCRs
10.1. Passerini Three-Component Reaction (P-3CR)
The Passerini reaction is a three-component reaction between aldehydes, carboxylic acids, and isocyanides, which leads to α-acyloxy carboxamides. Passerini and Ugi reactions are concurrent reactions, and the Passerini product is often observed as a side-product of U-4CC. In contrast to the Ugi reaction, which is typically performed in polar protic solvents, the Passerini reaction is favored in nonpolar solvents. The two reported examples from the same laboratory [10,11] aimed at the preparation of β-acyalamino-α-hydroxyamides and β-acyalamino-α-oxoamides containing peptides, known as protease inhibitors.
The sequence of the reported transformations involved the Passerini reaction, amine deprotection, and acyl migration (PADAM). P-3CR was performed on aminomethyl Lantern® 445 (Scheme 76) acylated with 2-(4-(hydroxymethyl)phenoxy)acetic acid to form Wang-type Lanterns 446. Solid-supported isocyanides 448 were prepared via three steps. The resin-bound linker was acylated with 3-formylaminopropionic acid followed by dehydration to form Lantern-bound isocyanide 448. Treatment of Passerini product 451 with piperidine induced Fmoc cleavage and simultaneous acyl migration. Intermediate 453 was then cleaved, and β-acylamino-α-hydroxyamide 453 was achieved with dr 6:4. Oxidation of 452 followed by treatment with TFA led to the target ketopeptide 454 [11]. Twelve products were prepared from the isocyanides derived from three amino acids (β-Ala, Ala, Leu), two aldehydes derived from Fmoc-Phe and Fmoc-Ala, and two acids (phenylacetic and hippuric acids). The same synthetic strategy was applied to the synthesis of peptidomimetics on aminomethyl PS modified with a photocleavable linker [10].
Scheme 76.
Synthesis of oligopeptides through P-3CR.
10.2. Groebke-Blackburn-Bienaymé Three-Component Reaction (GBB-3CR)
GBB-3CR is a reaction between aldehyde, isocyanide, and amine-containing NH2-C=N moieties in their cyclic structure (2-aminoazine or amidine). This reaction was efficient in the preparation of imidazo[1,2-a]-annulated pyridines, pyrazines, and pyrimidines as core structures of many marketed drugs [103]. Chen et al., who first reported the preparation of universal convertible Rink-isocyanide resin 57 (cf. Scheme 11), used this support for the traceless synthesis of 3-acylamino imidazo[1,2-a]pyridines 458 (Scheme 77) [8]. Acylation with acid chlorides and spontaneous cleavage at 50 °C yielded the target acylated products 458. Attempts to carry out base-mediated acylation or sulfonation were not successful.
Scheme 77.
Synthesis of 3-substituted imidazo[1,2-a]pyridines by GBB-3CR.
10.3. Miscellaneous
The first example in this section is the formation of α-(dialkylamino)amides 461 (Scheme 78), which are known to be formed in solution-phase synthesis when carboxylic acid is not present in the MCRs [7]. In the solid phase, addition of a catalytic amount of acetic acid was required in the reaction; otherwise, no product was formed. If other acidic catalysts or equivalents of acetic acid were used instead, a mixture of products (including Passerini-type adducts) was obtained.
Scheme 78.
Synthesis of α-(dialkylamino)amides.
Other IMCRs involved N-acylazinium salts as a source of iminium ions [9]. The reaction was initially studied in the solution phase. Treatment of azines (such as quinolines, isoquinolines, and phenanthridine) with activating agents (chloroformates, acid halides, or sulfonyl halides), isocyanide, and water, yielded 1,2-dihydroazine-1-carboxamides. In the solid phase, N-acyl isoquinoline ion 463 (Scheme 79) was reacted with tert-butyl isocyanide and water, and the target isoquinoline-1-carboxamide 466 was liberated by oxidative cleavage in 70% yield. Only one example was given.
Scheme 79.
IMCR involving N-acylazinium ion.
11. Conclusions and Future Perspectives
In conclusion, U-4CC has become an established and robust synthetic method, as documented by numerous reports, including syntheses of sizable libraries for drug discovery. There are several avenues to fully exploit their potential, and they particularly include the following four areas of research, which are mostly focused on drug discovery:
(i) The advanced intermediates prepared by U-4CC with three or four diversity positions can be further modified by derivatization, which can lead to structurally complex chiral frameworks with three-dimensional architecture. In addition to the previously reported Diels-Alder reaction [3,96], the cyclic iminium–nucleophilic addition tandem reaction shows great potential [104].
(ii) DNA-encoded libraries represent a cutting-edge technology in drug discovery and are used by numerous pharmaceutical companies. The current restriction of the technology is the need to use mild reaction conditions compatible with DNA. Due to their mild reaction conditions, U-4CC open a new potential in this avenue [18].
(iii) Ugi reactions are already carried out under environmentally friendly reaction conditions. 2-MeTHF as a solvent is applied for SP [15]. In addition, the MCRs do not require the use of any additional reagents, and high atom economy and mild reaction conditions are additional attributes of sustainable organic synthesis [14].
(iv) The formation of a new asymmetric center, particularly in the synthesis of novel α-amino acids, is probably the most useful BB in the synthesis of novel structures. Chiral auxiliary has already been shown to enable direct enantio-enriched compounds [19,20].
IMCRs play an important role in organic synthesis, including the drug discovery process. High atom economy, high yields of products, mild reaction conditions, and no need for additional reagents or special laboratory equipment belong to the most indisputable highlights of these reactions. The solid-phase approach provides a huge advantage in the possibility of producing of chemical libraries in a short time with simple isolation of products that may markedly facilitate searching for new pharmacologically relevant molecules.
Acknowledgments
The work was supported by the Department of Organic Chemistry, Palacký University in Olomouc, Czech Republic.
Author Contributions
N.C.; writing—original concept and draft preparation, V.K.; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hulme C., Ma L., Cherrier M.P., Romano J.J., Morton G., Duquenne C., Salvino J., Labaudiniere R. Novel applications of convertible isonitriles for the synthesis of mono and bicyclic γ-lactams via a UDC strategy. Tetrahedron Lett. 2000;41:1883–1887. doi: 10.1016/S0040-4039(00)00052-6. [DOI] [Google Scholar]
- 2.Kim S.W., Bauer S.M., Armstrong R.W. Construction of combinatorial chemical libraries using a rapid and efficient solid phase synthesis based on a multicomponent condensation reaction. Tetrahedron Lett. 1998;39:6993–6996. doi: 10.1016/S0040-4039(98)01547-0. [DOI] [Google Scholar]
- 3.Lee D., Sello J.K., Schreiber S.L. Pairwise Use of Complexity-Generating Reactions in Diversity-Oriented Organic Synthesis. Org. Lett. 2000;2:709–712. doi: 10.1021/ol005574n. [DOI] [PubMed] [Google Scholar]
- 4.Hulme C., Ma L., Kumar N.V., Krolikowski P.H., Allen A.C., Labaudiniere R. Novel applications of resin bound α-amino acids for the synthesis of benzodiazepines (via Wang resin) and ketopiperazines (via hydroxymethyl resin) Tetrahedron Lett. 2000;41:1509–1514. doi: 10.1016/S0040-4039(99)02326-6. [DOI] [Google Scholar]
- 5.Méndez Y., De Armas G., Pérez I., Rojas T., Valdés-Tresanco M.E., Izquierdo M., Alonso del Rivero M., Álvarez-Ginarte Y.M., Valiente P.A., Soto C., et al. Discovery of potent and selective inhibitors of the Escherichia coli M1-aminopeptidase via multicomponent solid-phase synthesis of tetrazole-peptidomimetics. Eur. J. Med. Chem. 2019;163:481–499. doi: 10.1016/j.ejmech.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 6.Chen J.J., Golebiowski A., Klopfenstein S.R., West L. The universal Rink-isonitrile resin: Applications in Ugi reactions. Tetrahedron Lett. 2002;43:4083–4085. doi: 10.1016/S0040-4039(02)00700-1. [DOI] [Google Scholar]
- 7.Ugi I. Neuere Methoden der präparativen organischen Chemie IV: Mit Sekundär-Reaktionen gekoppelte α-Additionen von Immonium-Ionen und Anionen an Isonitrile. Angew. Chem. 1962;74:9–22. doi: 10.1002/ange.19620740103. [DOI] [Google Scholar]
- 8.Chen J.J., Golebiowski A., McClenaghan J., Klopfenstein S.R., West L. Universal Rink-isonitrile resin: Application for the traceless synthesis of 3-acylamino imidazo[1,2-a]pyridines. Tetrahedron Lett. 2001;42:2269–2271. doi: 10.1016/S0040-4039(01)00159-9. [DOI] [Google Scholar]
- 9.Díaz J.L., Miguel M., Lavilla R. N-Acylazinium Salts: A New Source of Iminium Ions for Ugi-Type Processes. J. Org. Chem. 2004;69:3550–3553. doi: 10.1021/jo049823n. [DOI] [PubMed] [Google Scholar]
- 10.Banfi L., Basso A., Guanti G., Riva R. Passerini reaction—Amine Deprotection—Acyl Migration (PADAM): A convenient strategy for the solid-phase preparation of peptidomimetic compounds. Mol. Divers. 2003;6:227–235. doi: 10.1023/B:MODI.0000006778.42751.7f. [DOI] [PubMed] [Google Scholar]
- 11.Basso A., Banfi L., Riva R., Piaggio P., Guanti G. Solid-phase synthesis of modified oligopeptides via Passerini multicomponent reaction. Tetrahedron Lett. 2003;44:2367–2370. doi: 10.1016/S0040-4039(03)00238-7. [DOI] [Google Scholar]
- 12.Touré B.B., Hall D.G. Natural Product Synthesis Using Multicomponent Reaction Strategies. Chem. Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]
- 13.Dömling A., Wang W., Wang K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cioc R.C., Ruijter E., Orru R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014;16:2958–2975. doi: 10.1039/C4GC00013G. [DOI] [Google Scholar]
- 15.Lawrenson S., North M., Peigneguy F., Routledge A. Greener solvents for solid-phase synthesis. Green Chem. 2017;19:952–962. doi: 10.1039/C6GC03147A. [DOI] [Google Scholar]
- 16.Reguera L., Méndez Y., Humpierre A.R., Valdés O., Rivera D.G. Multicomponent Reactions in Ligation and Bioconjugation Chemistry. Acc. Chem. Res. 2018;51:1475–1486. doi: 10.1021/acs.accounts.8b00126. [DOI] [PubMed] [Google Scholar]
- 17.Reguera L., Rivera D.G. Multicomponent Reaction Toolbox for Peptide Macrocyclization and Stapling. Chem. Rev. 2019;119:9836–9860. doi: 10.1021/acs.chemrev.8b00744. [DOI] [PubMed] [Google Scholar]
- 18.Kunig V.B.K., Ehrt C., Dömling A., Brunschweiger A. Isocyanide Multicomponent Reactions on Solid-Phase-Coupled DNA Oligonucleotides for Encoded Library Synthesis. Org. Lett. 2019;21:7238–7243. doi: 10.1021/acs.orglett.9b02448. [DOI] [PubMed] [Google Scholar]
- 19.Oertel K., Zech G., Kunz H. Stereoselective Combinatorial Ugi-Multicomponent Synthesis on Solid Phase. Angew. Chem. Int. Ed. 2000;39:1431–1433. doi: 10.1002/(SICI)1521-3773(20000417)39:8<1431::AID-ANIE1431>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Zech G., Kunz H. Synthesis of a Polymer-Bound Galactosylamine and Its Application as an Immobilized Chiral Auxiliary in Stereoselective Syntheses of Piperidine and Amino Acid Derivatives. Chem. Eur. J. 2004;10:4136–4149. doi: 10.1002/chem.200400253. [DOI] [PubMed] [Google Scholar]
- 21.Arcadia C.E., Kennedy E., Geiser J., Dombroski A., Oakley K., Chen S.L., Sprague L., Ozmen M., Sello J., Weber P.M., et al. Multicomponent molecular memory. Nat. Commun. 2020;11:691. doi: 10.1038/s41467-020-14455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugi I. Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem. 2001;73:187–191. doi: 10.1351/pac200173010187. [DOI] [Google Scholar]
- 23.Dömling A., Ugi I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Lieke W. Ueber das Cyanallyl. Justus Liebigs Ann. Chem. 1859;112:316–321. doi: 10.1002/jlac.18591120307. [DOI] [Google Scholar]
- 25.Gautier A. Ueber die Einwirkung des Chlorwasserstoffs u. a. auf das Aethyl- und Methylcyanur. Justus Liebigs Ann. Chem. 1867;142:289–294. doi: 10.1002/jlac.18671420304. [DOI] [Google Scholar]
- 26.Ugi I., Dömling A., Gruber B., Almstetter M. Multicomponent reactions and their libraries—A New approach to preparative organic chemistry. Croat. Chem. Acta. 1997;70:631–647. [Google Scholar]
- 27.Ugi I. The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions. Angew. Chem. Int. Ed. Engl. 1962;1:8–21. doi: 10.1002/anie.196200081. [DOI] [Google Scholar]
- 28.Ugi I., Meyr R., Fetzer U., Steinbrückner C. Versuche mit Isonitrilen. Angew. Chem. 1959;71:386. [Google Scholar]
- 29.Arshady R., Ugi I. Solid Phase Peptide Synthesis by Four Component Condensation: Peptide Formation on an Isocyano Polymer Support. Z. Für Nat. B. 1981;36:1202–1203. doi: 10.1515/znb-1981-0933. [DOI] [Google Scholar]
- 30.Bienaymé H., Bouzid K. A New Heterocyclic Multicomponent Reaction for the Combinatorial Synthesis of Fused 3-Aminoimidazoles. Angew. Chem. Int. Ed. 1998;37:2234–2237. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Blackburn C., Guan B., Fleming P., Shiosaki K., Tsai S. Parallel synthesis of 3-aminoimidazo[1,2–a]pyridines and pyrazines by a new three-component condensation. Tetrahedron Lett. 1998;39:3635–3638. doi: 10.1016/S0040-4039(98)00653-4. [DOI] [Google Scholar]
- 32.Groebke K., Weber L., Mehlin F. Synthesis of Imidazo[1,2-a] Annulated Pyridines, Pyrazines, and Pyrimidines by a Novel Three-Component Condensation. Synlett. 1998;6:661–663. doi: 10.1055/s-1998-1721. [DOI] [Google Scholar]
- 33.La Venia A., Lemrová B., Krchňák V. Regioselective Incorporation of Backbone Constraints Compatible with Traditional Solid-Phase Peptide Synthesis. ACS Comb. Sci. 2013;15:59–72. doi: 10.1021/co300125m. [DOI] [PubMed] [Google Scholar]
- 34.Cheng J.F., Chen M., Arrhenius T., Nadzan A. A convenient solution and solid-phase synthesis of Δ5-2-oxopiperazines via N-acyliminium ions cyclization. Tetrahedron Lett. 2002;43:6293–6295. doi: 10.1016/S0040-4039(02)01403-X. [DOI] [Google Scholar]
- 35.Tempest P.A., Brown S.D., Armstrong R.W. Solid-Phase, Parallel Syntheses by Ugi Multicomponent Condensation. Angew. Chem. Int. Ed. Engl. 1996;35:640–642. doi: 10.1002/anie.199606401. [DOI] [Google Scholar]
- 36.Golebiowski A., Jozwik J., Klopfenstein S.R., Colson A.O., Grieb A.L., Russell A.F., Rastogi V.L., Diven C.F., Portlock D.E., Chen J.J. Solid-Supported Synthesis of Putative Peptide β-Turn Mimetics via Ugi Reaction for Diketopiperazine Formation. J. Comb. Chem. 2002;4:584–590. doi: 10.1021/cc020029u. [DOI] [PubMed] [Google Scholar]
- 37.Hoel A.M.L., Nielsen J. Microwave-assisted solid-phase Ugi four-component condensations. Tetrahedron Lett. 1999;40:3941–3944. doi: 10.1016/S0040-4039(99)00616-4. [DOI] [Google Scholar]
- 38.Lin Q., O’Neil J.C., Blackwell H.E. Small Molecule Macroarray Construction via Ugi Four-Component Reactions. Org. Lett. 2005;7:4455–4458. doi: 10.1021/ol051684o. [DOI] [PubMed] [Google Scholar]
- 39.Henkel B., Weber L. A Novel Four-Component Synthesis of N-Substituted Amino Acid Esters. Synlett. 2002:1877–1879. doi: 10.1055/s-2002-34864. [DOI] [Google Scholar]
- 40.Obrecht D., Abrecht C., Grieder A., Villalgordo J.M. A Novel and Efficient Approach for the Combinatorial Synthesis of Structurally Diverse Pyrimidines on solid support. Helv. Chim. Acta. 1997;80:65–72. doi: 10.1002/hlca.19970800106. [DOI] [Google Scholar]
- 41.Ma X., Zhou Y., Song Q. Synthesis of β-Aminoenones via Cross-Coupling of In-Situ-Generated Isocyanides with 1,3-Dicarbonyl Compounds. Org. Lett. 2018;20:4777–4781. doi: 10.1021/acs.orglett.8b01888. [DOI] [PubMed] [Google Scholar]
- 42.El Kaim L., Grimaud L., Schiltz A. “Isocyanide-free” Ugi reactions. Org. Biomol. Chem. 2009;7:3024–3026. doi: 10.1039/b908541f. [DOI] [Google Scholar]
- 43.Campian E., Lou B., Saneii H. Solid-phase synthesis of α-sulfonylamino amide derivatives based on Ugi-type condensation reaction using sulfonamides as amine input. Tetrahedron Lett. 2002;43:8467–8470. doi: 10.1016/S0040-4039(02)02085-3. [DOI] [Google Scholar]
- 44.Hanyu M., Murashima T., Miyazawa T., Yamada T. Synthesis of tripeptides containing a very crowded α-,α-disubstituted glycine with pyridine rings by solid-phase Ugi reaction. Tetrahedron Lett. 2004;45:8871–8874. doi: 10.1016/j.tetlet.2004.09.171. [DOI] [Google Scholar]
- 45.Habashita H., Kokubo M., Hamano S.I., Hamanaka N., Toda M., Shibayama S., Tada H., Sagawa K., Fukushima D., Maeda K., et al. Design, Synthesis, and Biological Evaluation of the Combinatorial Library with a New Spirodiketopiperazine Scaffold. Discovery of Novel Potent and Selective Low-Molecular-Weight CCR5 Antagonists. J. Med. Chem. 2006;49:4140–4152. doi: 10.1021/jm060051s. [DOI] [PubMed] [Google Scholar]
- 46.Szardenings A.K., Burkoth T.S., Lu H.H., Tien D.W., Campbell D.A. A simple procedure for the solid phase synthesis of diketopiperazine and diketomorpholine derivatives. Tetrahedron. 1997;53:6573–6593. doi: 10.1016/S0040-4020(97)00218-4. [DOI] [Google Scholar]
- 47.Liu Z., Nefzi A. Solid-Phase Synthesis of N-Substituted Pyrrolidinone-Tethered N-Substituted Piperidines via Ugi Reaction. J. Comb. Chem. 2010;12:566–570. doi: 10.1021/cc100054u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z., Nefzi A. Ugi four-center three-component reaction for the parallel solid-phase synthesis of N-substituted thiomopholinones. Tetrahedron Lett. 2012;53:1013–1014. doi: 10.1016/j.tetlet.2011.12.074. [DOI] [Google Scholar]
- 49.Aguiam N.R., Castro V.I., Ribeiro A.I.F., Fernandes R.D.V., Carvalho C.M., Costa S.P.G., Pereira-Lima S.M.M.A. α-,α-Dialkylglycines obtained by solid phase Ugi reaction performed over isocyanide functionalized resins. Tetrahedron. 2013;69:9161–9165. doi: 10.1016/j.tet.2013.08.002. [DOI] [Google Scholar]
- 50.Zhang C., Moran E.J., Woiwode T.F., Short K.M., Mjalli A.M.M. Synthesis of tetrasubstituted imidazoles via α-(N-acyl-N-alkylamino)-β-ketoamides on Wang resin. Tetrahedron Lett. 1996;37:751–754. doi: 10.1016/0040-4039(95)02310-0. [DOI] [Google Scholar]
- 51.Henkel B., Sax M., Dömling A. A new and efficient multicomponent solid-phase synthesis of 2-acylaminomethylthiazoles. Tetrahedron Lett. 2003;44:3679–3682. doi: 10.1016/S0040-4039(03)00662-2. [DOI] [Google Scholar]
- 52.Henkel B., Westner B., Dömling A. Polymer-Bound 3-N,N-(Dimethylamino)-2-isocyanoacrylate for the Synthesis of Thiazoles via a Multicomponent Reaction. Synlett. 2003:2410–2412. doi: 10.1055/s-2003-43331. [DOI] [Google Scholar]
- 53.Short K.M., Ching B.W., Mjalli A.M.M. The synthesis of hydantoin 4-imides on solid support. Tetrahedron Lett. 1996;37:7489–7492. doi: 10.1016/0040-4039(96)01699-1. [DOI] [Google Scholar]
- 54.Short K.M., Ching B.W., Mjalli A.M.M. Exploitation of the Ugi 4CC reaction: Preparation of small molecule combinatorial libraries via solid phase. Tetrahedron. 1997;53:6653–6679. doi: 10.1016/S0040-4020(97)00223-8. [DOI] [Google Scholar]
- 55.Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 56.Piscopio A.D., Miller J.F., Koch K. Ring closing metathesis in organic synthesis: Evolution of a high speed, solid phase method for the preparation of β-turn mimetics. Tetrahedron. 1999;55:8189–8198. doi: 10.1016/S0040-4020(99)00300-2. [DOI] [Google Scholar]
- 57.Sutherlin D.P., Stark T.M., Hughes R., Armstrong R.W. Generation of C-Glycoside Peptide Ligands for Cell Surface Carbohydrate Receptors Using a Four-Component Condensation on Solid Support. J. Org. Chem. 1996;61:8350–8354. doi: 10.1021/jo960119j. [DOI] [PubMed] [Google Scholar]
- 58.Portlock D.E., Naskar D., West L., Ostaszewski R., Chen J.J. Solid-phase synthesis of five-dimensional libraries via a tandem Petasis-Ugi multi-component condensation reaction. Tetrahedron Lett. 2003;44:5121–5124. doi: 10.1016/S0040-4039(03)01119-5. [DOI] [Google Scholar]
- 59.Golebiowski A., Klopfenstein S.R., Shao X., Chen J.J., Colson A.O., Grieb A.L., Russell A.F. Solid-Supported Synthesis of a Peptide β-Turn Mimetic. Org. Lett. 2000;2:2615–2617. doi: 10.1021/ol006145s. [DOI] [PubMed] [Google Scholar]
- 60.Constabel F., Ugi I. Repetitive Ugi reactions. Tetrahedron. 2001;57:5785–5789. doi: 10.1016/S0040-4020(01)00516-6. [DOI] [Google Scholar]
- 61.Lin Q., Blackwell H.E. Rapid synthesis of diketopiperazine macroarrays via Ugi four-component reactions on planar solid supports. Chem. Commun. 2006:2884–2886. doi: 10.1039/b604329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell J., Blackwell H.E. Efficient Construction of Diketopiperazine Macroarrays through a Cyclative-Cleavage Strategy and Their Evaluation as Luminescence Inhibitors in the Bacterial Symbiont Vibrio fischeri. J. Comb. Chem. 2009;11:1094–1099. doi: 10.1021/cc900115x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcaccini S., Torroba T. The use of the Ugi four-component condensation. Nat. Protoc. 2007;2:632–639. doi: 10.1038/nprot.2007.71. [DOI] [PubMed] [Google Scholar]
- 64.Banfi L., Guanti G., Riva R., Basso A. Multicomponent Reactions in Solid-Phase Synthesis. Curr. Opin. Drug Discov. Dev. 2007;10:704–714. [PubMed] [Google Scholar]
- 65.Ramón D.J., Yus M. Asymmetric Multicomponent Reactions (AMCRs): The New Frontier. Angew. Chem. Int. Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]
- 66.Hlaváč J., Soural M., Krchňák V. Solid-Phase Organic Synthesis. Wiley; Hoboken, NJ, USA: 2011. Practical Aspects of Combinatorial Solid-Phase Synthesis; pp. 95–130. [DOI] [Google Scholar]
- 67.Wang S.-S. p-Alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments. J. Am. Chem. Soc. 1973;95:1328–1333. doi: 10.1021/ja00785a052. [DOI] [PubMed] [Google Scholar]
- 68.Rink H. Solid-phase synthesis of protected peptide fragments using a trialkoxy-diphenyl-methylester resin. Tetrahedron Lett. 1987;28:3787–3790. doi: 10.1016/S0040-4039(00)96384-6. [DOI] [Google Scholar]
- 69.Xiaodong C., Moran E.J., Siev D., Lio A., Ohashi C., Mjalli A.M.M. Synthesis of NH-acyl-α-aminoamides on Rink resin: Inhibitors of the hematopoietic protein tyrosine phosphatase (HePTP) Bioorg. Med. Chem. Lett. 1995;5:2953–2958. doi: 10.1016/0960-894X(95)00533-6. [DOI] [Google Scholar]
- 70.Pulici M., Quartieri F., Felder E.R. Trifluoroacetic Anhydride-Mediated Solid-Phase Version of the Robinson-Gabriel Synthesis of Oxazoles. J. Comb. Chem. 2005;7:463–473. doi: 10.1021/cc049831h. [DOI] [PubMed] [Google Scholar]
- 71.Li Z., Yeo S.L., Pallen C.J., Ganesan A. Solid-phase synthesis of potential protein tyrosine phosphatase inhibitors via the Ugi four-component condensation. Bioorg. Med. Chem. Lett. 1998;8:2443–2446. doi: 10.1016/S0960-894X(98)00408-9. [DOI] [PubMed] [Google Scholar]
- 72.Sun D., Lee R.E. Solid-Phase Synthesis Development of a Thymidinyl and 2′-Deoxyuridinyl Ugi Library for Anti-bacterial Agent Screening. Tetrahedron Lett. 2005;46:8497–8501. doi: 10.1016/j.tetlet.2005.10.012. [DOI] [Google Scholar]
- 73.Henkel B., Sax M., Dömling A. New method for the preparation of solid-phase bound isocyanocarboxylic acids and Ugi reactions therewith. Tetrahedron Lett. 2003;44:7015–7018. doi: 10.1016/S0040-4039(03)01793-3. [DOI] [Google Scholar]
- 74.Arshady R., Ugi I. Synthesis and characterization of polymer supports carrying isocyano groups. Polymer. 1990;31:1164–1169. doi: 10.1016/0032-3861(90)90268-4. [DOI] [Google Scholar]
- 75.Ugi I., Rosendahl F.K., Isonitrile X.V. Δ1-Cyclohexenyl-isocyanid. Justus Liebigs Ann. Chem. 1963;666:65–67. doi: 10.1002/jlac.19636660109. [DOI] [Google Scholar]
- 76.Keating T.A., Armstrong R.W. Molecular Diversity via a Convertible Isocyanide in the Ugi Four-Component Condensation. J. Am. Chem. Soc. 1995;117:7842–7843. doi: 10.1021/ja00134a044. [DOI] [Google Scholar]
- 77.Hulme C., Morrissette M.M., Volz F.A., Burns C.J. The solution phase synthesis of diketopiperazine libraries via the Ugi reaction: Novel application of Armstrong’s convertible isonitrile. Tetrahedron Lett. 1998;39:1113–1116. doi: 10.1016/S0040-4039(97)10795-X. [DOI] [Google Scholar]
- 78.Keating T.A., Armstrong R.W. Postcondensation Modifications of Ugi Four-Component Condensation Products: 1-Isocyanocyclohexene as a Convertible Isocyanide. Mechanism of Conversion, Synthesis of Diverse Structures, and Demonstration of Resin Capture. J. Am. Chem. Soc. 1996;118:2574–2583. doi: 10.1021/ja953868b. [DOI] [Google Scholar]
- 79.Miller J.F., Koch K., Piscopio A.D. Preparation and synthetic application of an immobilized, convertible isonitrile. Abstr. Pap. Am. Chem. Soc. 1997;214:232. [Google Scholar]
- 80.Hulme C., Peng J., Morton G., Salvino J.M., Herpin T., Labaudiniere R. Novel safety-catch linker and its application with a Ugi/De-BOC/Cyclization (UDC) strategy to access carboxylic acids, 1,4-benzodiazepines, diketopiperazines, ketopiperazines and dihydroquinoxalinones. Tetrahedron Lett. 1998;39:7227–7230. doi: 10.1016/S0040-4039(98)01593-7. [DOI] [Google Scholar]
- 81.Kennedy A.L., Fryer A.M., Josey J.A. A New Resin-Bound Universal Isonitrile for the Ugi 4CC Reaction: Preparation and Applications to the Synthesis of 2,5-Diketopiperazines and 1,4-Benzodiazepine-2,5-diones. Org. Lett. 2002;4:1167–1170. doi: 10.1021/ol0256015. [DOI] [PubMed] [Google Scholar]
- 82.Oikawa M., Takeda Y., Sasaki M. 2-Oxo-1,2-ethylenedioxy group as a linker for solution-, liquid-, and solid-phase syntheses to discover drug-like small molecules. Tetrahedron Lett. 2005;46:4667–4670. doi: 10.1016/j.tetlet.2005.04.138. [DOI] [Google Scholar]
- 83.Strocker A.M., Keating T.A., Tempest P.A., Armstrong R.W. Use of a convertible isocyanide for generation of Ugi reaction derivatives on solid support: Synthesis of α-acylaminoesters and pyrroles. Tetrahedron Lett. 1996;37:1149–1152. doi: 10.1016/0040-4039(96)00012-3. [DOI] [Google Scholar]
- 84.Chéron N., Ramozzi R., Kaim L.E., Grimaud L., Fleurat-Lessard P. Challenging 50 Years of Established Views on Ugi Reaction: A Theoretical Approach. J. Org. Chem. 2012;77:1361–1366. doi: 10.1021/jo2021554. [DOI] [PubMed] [Google Scholar]
- 85.Holden C.M., Greaney M.F. Modern Aspects of the Smiles Rearrangement. Chem. Eur. J. 2017;23:8992–9008. doi: 10.1002/chem.201700353. [DOI] [PubMed] [Google Scholar]
- 86.Dömling A., Ugi I. The Seven-Component Reaction. Angew. Chem. Int. Ed. Engl. 1993;32:563–564. doi: 10.1002/anie.199305631. [DOI] [Google Scholar]
- 87.Pirrung M.C., Sarma K.D. Multicomponent Reactions Are Accelerated in Water. J. Am. Chem. Soc. 2004;126:444–445. doi: 10.1021/ja038583a. [DOI] [PubMed] [Google Scholar]
- 88.Gil C., Brase S. Traceless and multifunctional linkers for the generation of small molecules on solid supports. Curr. Opin. Chem. Biol. 2004;8:230–237. doi: 10.1016/j.cbpa.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Wiehn M.S., Jung N., Brase S. Safety-catch and traceless linkers in solid phase organic synthesis. In: Tulla-Puche J., Albericio F., editors. Power Functional Resins in Organic Synthesis. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2008. pp. 437–465. [Google Scholar]
- 90.Cankařová N., Schütznerová E., Krchňák V. Traceless Solid-Phase Organic Synthesis. Chem. Rev. 2019;119:12089–12207. doi: 10.1021/acs.chemrev.9b00465. [DOI] [PubMed] [Google Scholar]
- 91.Nixey T., Tempest P., Hulme C. Two-step solution-phase synthesis of novel quinoxalinones utilizing a UDC (Ugi/de-Boc/cyclize) strategy. Tetrahedron Lett. 2002;43:1637–1639. doi: 10.1016/S0040-4039(02)00101-6. [DOI] [Google Scholar]
- 92.Scott B.O., Siegmund A.C., Marlowe C.K., Pei Y., Spear K.L. Solid phase organic synthesis (SPOS): A novel route to diketopiperazines and diketomorpholines. Mol. Divers. 1996;1:125–134. doi: 10.1007/BF01721328. [DOI] [PubMed] [Google Scholar]
- 93.Mjalli A.M.M., Sarshar S., Baiga T.J. Solid phase synthesis of pyrroles derived from a four component condensation. Tetrahedron Lett. 1996;37:2943–2946. doi: 10.1016/0040-4039(96)00365-6. [DOI] [Google Scholar]
- 94.Nahm S., Weinreb S.M. N-methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 1981;22:3815–3818. doi: 10.1016/S0040-4039(01)91316-4. [DOI] [Google Scholar]
- 95.Dinh T.Q., Armstrong R.W. Synthesis of ketones and aldehydes via reactions of Weinreb-type amides on solid support. Tetrahedron Lett. 1996;37:1161–1164. doi: 10.1016/0040-4039(95)02400-X. [DOI] [Google Scholar]
- 96.Paulvannan K. Preparation of tricyclic nitrogen heterocycles via tandem four-component condensation/intramolecular Diels-Alder reaction. Tetrahedron Lett. 1999;40:1851–1854. doi: 10.1016/S0040-4039(99)00072-6. [DOI] [Google Scholar]
- 97.Hulme C., Peng J., Louridas B., Menard P., Krolikowski P., Kumar N.V. Applications of N-BOC-diamines for the solution phase synthesis of ketopiperazine libraries utilizing a Ugi/De-BOC/Cyclization (UDC) strategy. Tetrahedron Lett. 1998;39:8047–8050. doi: 10.1016/S0040-4039(98)01770-5. [DOI] [Google Scholar]
- 98.Kolb J., Beck B., Almstetter M., Heck S., Herdtweck E., Dömling A. New MCRs: The first 4-component reaction leading to 2,4-disubstituted thiazoles. Mol. Divers. 2003;6:297–313. doi: 10.1023/B:MODI.0000006827.35029.e4. [DOI] [PubMed] [Google Scholar]
- 99.Heck S., Domling A. A Versatile Multi-Component One-Pot Thiazole Synthesis. Synlett. 2000:424–426. doi: 10.1055/s-2000-6517. [DOI] [Google Scholar]
- 100.Schöllkopf U., Porsch P.H., Lau H.H. Synthesen mit α-metallierten Isocyaniden, XLIV. Notiz über β-Dimethylamino-α-isocyanacrylsäureester und ihre Verwendung in der Heterocyclenchemie. Liebigs Ann. Chem. 1979:1444–1446. doi: 10.1002/jlac.197919790918. [DOI] [Google Scholar]
- 101.Mckervey M.A., O’Sullivan M.B., Myers P.L., Green R.H. Reductive acylation of α-keto azides derived from L-amino acids using N-protected L-aminothiocarboxylic S-acids. J. Chem. Soc. Chem. Commun. 1993:94–96. doi: 10.1039/C39930000094. [DOI] [Google Scholar]
- 102.Venkatraman J., Shankaramma S.C., Balaram P. Design of Folded Peptides. Chem. Rev. 2001;101:3131–3152. doi: 10.1021/cr000053z. [DOI] [PubMed] [Google Scholar]
- 103.Boltjes A., Dömling A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019:7007–7049. doi: 10.1002/ejoc.201901124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gunawan S., Hulme C. Construction of functionalized tricyclic dihydropyrazino-quinazolinedione chemotypes via an Ugi/N-acyliminium ion cyclization cascade. Tetrahedron Lett. 2013;54:4467–4470. doi: 10.1016/j.tetlet.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]