Abstract
Anaphylaxis is a severe and potentially life-threatening allergic reaction that can occur in the dental practice. There are a number of dental-related causes including mouthwashes, local anaesthetics, latex and antibiotics. The dental team must be able to respond effectively and manage the life-threatening situation appropriately following Resuscitation Council UK guidelines. The timely administration of adrenaline is life-saving; any delays can lead to a poor outcome. With the current national supply issues with adrenaline auto-injector devices, there is an expectation that GDPs should be competent at drawing up adrenaline from an ampoule and administrating it intramuscularly. The aim of this article is to provide an update on the management of anaphylaxis in the dental practice with particular reference to the procedure for intramuscular injection of adrenaline.
Key points
Anaphylaxis is a life-threatening emergency and the incidence is on the increase.
Adrenaline needs to be administered promptly to optimise survival.
GDPs need to be competent at administering adrenaline intramuscularly using a syringe and needle.
Introduction
Anaphylaxis is a severe and potentially life-threatening allergic reaction1 which could occur in the dental practice.2 The GDC states that 'all registrants must be trained in dealing with medical emergencies, including resuscitation, and possess up-to-date evidence of capability'.3 The dental team must be able to manage anaphylaxis effectively following Resuscitation Council UK guidelines.4
The prompt administration of adrenaline is pivotal to a favourable outcome. However, due to the ongoing supply issues and product recalls relating to adrenaline auto-injector devices (AAIs),5,6,7,8 those who previously relied on using these simple devices now need to be competent at intramuscular (IM) injection of adrenaline using a syringe and needle, a nationally expected requirement.9,10
The aim of this article is to provide an update on the management of anaphylaxis in the dental practice.
Definition
Anaphylaxis is a severe, potentially life-threatening generalised allergic reaction characterised by rapidly developing symptoms and signs including skin changes (such as redness and itching), mucosal changes (swelling below the skin surface), swallowing and breathing difficulties (due to swollen mouth, throat or tongue), wheezing, tachypnoea, tachycardia and hypotension.11
Incidence
The incidence of anaphylaxis in the UK is increasing, with a reported increase in hospital admissions for anaphylaxis from one to seven cases per 100,000 population per year between 1992 and 2012.12 This 700% increase is fortunately not associated with an increase in mortality rates, which are still very rare - approximately 20 in the UK every year.4
Causes of anaphylaxis in dental practice
Antibiotics
Antibiotics are among the drugs most likely to cause anaphylaxis.13 Amoxicillin, phenoxymethylpenicillin and metronidazole are three antibiotics that are prescribed in dentistry.14 Amoxicillin is probably the one most commonly associated with anaphylaxis. Deaths from anaphylactic reactions to amoxicillin have been reported.15
Antibiotics are the main cause of perioperative anaphylaxis in the UK, being responsible for almost 50% of cases with an incidence of 4.0 per 100,000 administrations.16 One recent study in France identified 17 cases of anaphylaxis to amoxicillin administered for dental procedures.17
Chlorhexidine
Chlorhexidine is an effective antiseptic which is widely used in dentistry,18 being present in a number of dental products including some mouthwashes, toothpastes and dental implants.19,20 However, anaphylaxis to chlorhexidine has been increasingly reported worldwide, including two incidents in the UK where chlorhexidine-containing mouthwash had been used to irrigate tooth sockets following a recent tooth extraction;18 unfortunately, the resulting anaphylaxis resulted in the death of both patients.21,22
A national report23 investigating 266 cases of anaphylaxis in the perioperative period in UK hospitals found that chlorhexidine was:
The cause in nearly 10% of the cases
The third most common cause of anaphylaxis
Estimated to have an incidence of 0.78 per 100,000 exposures.
Interestingly, the authors also found that three cases could potentially have been avoided by better history-taking or by heeding a relevant history.23
A Medicines and Healthcare products Regulatory Agency (MHRA) alert in 201420 raised awareness about the risk of anaphylaxis to products containing chlorhexidine. The continued use of chlorhexidine as an irrigation solution for treating an established dry socket has been questioned.18 The SDCEP24 advises to consider saline to irrigate a dry socket because of the associated risks of anaphylaxis when using chlorhexidine and the lack of evidence to support its use. The latest advice during the COVID-19 pandemic advises patients to rinse with warm saltwater should the GDP suspect dry socket.25
Local anaesthetic
Anaphylactic reactions to local anaesthetic (LA) administered in the dental setting is very rare,26 but they have been reported.27,28,29 The actual incidence of adverse effects from LA is about 0.1-1%,30 with 1% of these cases being confirmed allergic reactions.26,31 Actual allergic reactions to LAs are either immediate hypersensitivity reactions (type I: systemic signs) or delayed hypersensitivity reactions (type IV: localised reaction at the injection site, contact dermatitis).26
Amide LA agents (for example, lignocaine) are most commonly used in dentistry, but allergic reactions are very rare.26 The least allergenic amide LA agents are mepivacaine and plain prilocaine.32 Allergic reactions are more common with ester LA agents because they are metabolised to para-aminobenzoic acid, a known allergenic compound.33 Benzocaine is the only ester LA used in dentistry (topical preparations applied before administration of LA).26
Many LA-induced allergic reactions are due to other constituents in the injection solution rather than to the drug itself.26 Excipients such as preservatives (for example, benzoates used in multi-dose vials) and antioxidants (for example, metabisulphites used in LA solutions containing adrenaline) can cause allergic reactions.27,33,34
It is also important to be aware of the risk of allergy to natural rubber latex (NRL) contained in bungs, gloves and dams, as well as other dental materials23 (see below). Contrary to some reports, the risk of latex allergies from LA carriages is minimal.35
Following administration of an LA, a few patients may suffer one of a range of unwanted symptoms which sometimes can be mistaken for allergic reactions and patients may be unnecessarily told they are allergic to the anaesthetic.26 Most adverse reactions are psychogenic or vasovagal.36
General anaesthetic
Anaphylaxis to anaesthetic drugs is not uncommon.16 There have been a number of cases in oral surgery theatres that have been reported.37
Latex
Allergy to NRL (latex) became increasingly common towards the end of the last century (partly due to increased use of latex gloves in healthcare settings), though it is now on the decline.38 It is estimated that approximately 1-6% of the UK's population have a latex allergy.35,39 Worldwide, reported data suggest that the average prevalence of latex allergy remains 9.7%, 7.2% and 4.3% among healthcare workers, susceptible patients and the general population, respectively.40
At-risk groups include spina bifida patients (67% chance of latex allergy), healthcare professionals, and patients with an existing allergy to Elastoplast and certain foods (particularly banana, kiwi and avocado).38,41 Latex allergy-induced anaphylaxis is more likely to be severe in patients with uncontrolled asthma.38
Although an allergy to latex is relatively common and may cause severe reactions,42 latex allergy-induced anaphylaxis is extremely rare.39 A review of the literature found only one case report in the last 20 years of latex-induced anaphylaxis in the dental practice - a patient who had a reaction to a rubber dam.43 Interestingly, a recent study examining 266 cases of perioperative anaphylaxis reported no cases were due to latex.16
Even rarer is fatal anaphylaxis due to a latex allergy, with only isolated case reports being found in the literature42 and none related to dentistry. The dental team will be aware of the importance of using latex-free products whenever possible and be alert to patients with latex allergies. Anaphylaxis Campaign has produced helpful guidance raising awareness of the presence of latex in dental products and listing latex-free alternatives (Table 1).44
Table 1.
Products in the dental practice containing latex and latex-free alternatives44
| Examples of dental equipment that may contain NRL | NRL-free alternative | Manufacturer |
|---|---|---|
| Amalgam carriers | Teflon amalgam carriers | Austinell |
| Aspirators | Yankauer plastic wide-bore |
Tyco-Healthcare Durr |
| Bunsen burner tubing | Heating device | |
| Dental dam |
Roeko flexi-dam Hygenic non-latex |
FE Cardozo Ltd Coltene/Whaledent |
| Hygenic Wedjets (dental dam stabilising cord) | Not currently available | |
| Endodontic* stops | Silicone stops | QED |
| Elastics (orthodontic) | GAC ELF Latex-Free elastics |
Orthocare TOC (The Orthodontic Co.) |
| Mixing bowls** (eg alginate nowls) | NRL-free bowls | Dentsply |
| Polishing equipment |
Prophy cups, prophy heads Disposable prophy angle to be used on nosecone of doriot-style handpiece |
Young Dental W+H Dental (UK) Ltd |
| Enhance polishing cups | Dentsply | |
| Shofu Greenie & Brownie polishing points | Minerva | |
| Temporary crowns and matrices |
Directa crowns (polycarbonate) Odus cervical matriza Odus Pella Crown Form |
Tower Dental Hawes Neos Austinall |
|
Key: NRL = natural rubber latex * = endodontic stop is a rubber stop on the hand files / rotary files that are used during root canal treatment and assists in the measuring of canals ** = Mixing bowls (it should read 'e.g. alginate bowls')- these are bowls in which the alginate impression material is hand mixed | ||
The incidence of latex allergy among healthcare workers is higher than in the general population,28,40,45 emphasising the importance for all dental team members to be particularly vigilant.35 The incidence of latex allergy in the workplace among healthcare workers is one that is clearly addressed in several specific areas of health and safety legislation.46
Toothpastes
Allergic reactions to toothpaste, including anaphylaxis47 and even fatal anaphylaxis, have been reported.48 In the US, a young person with a known dairy product allergy developed anaphylaxis and died after using a toothpaste containing Recaldent (a milk-derived protein) which had been recommended by her dentist.49
A similar case was reported recently in Australia where a child with a milk allergy developed anaphylaxis after a 'tooth mousse' containing Recaldent was used during dental treatment.50 The child began to complain of discomfort in her mouth, which progressed to difficulty swallowing which prompted her alert mother to administer her AAI immediately.49
Iodoform
Iodoform is in a number of endodontic products51 including Alvogyl.52 Alvogyl's product information stipulates that it shouldn't be used in patients with known allergies to procaine (novocaine)-type anaesthetic, iodine or compounds related to iodine.52 The authors are aware of two anecdotal reports of anaphylaxis to Alvogyl; in one case, the GDP discovered after the event that the patient was allergic to iodine.
Clinical features and diagnosis
The lack of a consistent clinical picture can sometimes make an accurate diagnosis difficult.4 Anaphylaxis is characterised by quick onset and rapid deterioration. As soon as possible, a detailed history should be taken and the patient assessed (and treated) following the ABCDE approach (Table 2) to help in the recognition and initial diagnosis of anaphylaxis.
Table 2.
| ABCDE | Signs and symptoms |
|---|---|
| Airway |
Swollen tongue Difficulty swallowing/speaking Throat tightness Hoarse voice Stridor |
| Breathing |
Difficult or noisy breathing Chest tightness Persistent coughing Wheeze Tachypnoea |
| Circulation |
Hypotension Tachycardia Pallor Collapse |
| Disability |
Feeling dizzy or faint Confusion Agitation Syncope Loss of consciousness |
| Exposure |
Skin changes: urticarial, angioedema and erythema Rhinitis and conjunctivitis Abdominal pain/cramps Nausea and vomiting Diarrhoea Sense of impending doom |
In a study of 593 cases of anaphylaxis, the most common findings were urticaria (Fig. 1) and angioedema (87%), shortness of breath/wheeze (59%) and symptoms of hypotension (33%).53
Fig. 1.
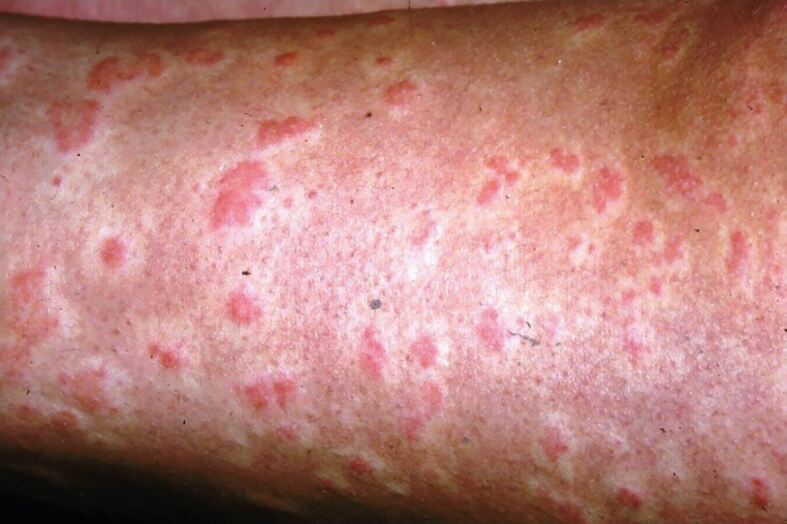
Urticaria (image courtesy of James Halpern, consultant dermatologist)
It is more likely to be anaphylaxis if all three of the following criteria are met:4
Sudden onset and rapid progression of symptoms
Life-threatening airway and/or breathing and/or circulation problems
Skin and/or mucosal changes (flushing, urticaria, angioedema).
Mistaken diagnosis
It is possible to mistake a panic attack or a vasovagal attack for anaphylaxis. Following the familiar ABCDE approach (Table 2) will help to distinguish between a panic attack and vasovagal syncope from anaphylaxis:
Panic attack: hyperventilation, tachycardia and anxiety-related erythematous (red) rash. The absence of urticaria, hypotension, pallor and dyspnoea helps to exclude anaphylaxis
Vasovagal syncope: pallor, sweating, hypotension, nausea, vomiting and bradycardia. The absence of a rash, tachycardia and dyspnoea helps to exclude anaphylaxis.
Adrenaline
Adrenaline remains the most important drug in anaphylaxis, but to be effective, it needs to be administered promptly. Delays in administering adrenaline increases the risk of death54,55 and failure to give adrenaline is the most common cause of death associated with anaphylaxis.56 A study of the UK registry found that, in almost 40% of all deaths due to anaphylaxis, adrenaline had not been administered.57
Actions
Adrenaline:
Reverses peripheral vasodilation
Reduces oedema
Dilates the airways
Increases myocardial contractility
Suppresses histamine and leukotriene release.
Dose
The recommended4 doses of adrenaline are as follows:
Adults: 500 micrograms IM (0.5 ml of 1:1,000)
Child >12 years: 500 micrograms IM (0.5 mL)
Child 6-12 years: 300 micrograms IM (0.3 ml of 1:1,000)
Child <6 years: 150 micrograms IM (0.15 ml of 1:1,000).
The dose can be repeated at five-minute intervals until there has been an adequate response.11
Route
Adrenaline should be administered intramuscularly, ideally into the anterolateral aspect of the middle third of the thigh.11
Injections through clothing
IM injections should ideally be administered into the bare leg. This may prove difficult in the dental practice if the patient is wearing clothing that is difficult to remove quickly or indeed is reluctant to be exposed.
AAIs can be administered through light clothing.58,59,60 This sensible and practical advice enables the prompt administration of adrenaline which otherwise could be delayed if the patient had to undress first to expose the leg. Sometimes, other injections (for example, insulin) are advocated to be administered through clothing.
The authors are aware of a number of anecdotal reports of adrenaline injections routinely administered through clothing in emergency departments without any difficulties and in fact this technique is recommended by some.61 It would therefore seem reasonable to consider this technique in a life-threatening, time-critical anaphylaxis situation in a dental practice if the patient is wearing light clothing, taking care to avoid the seam in the trousers and items in the patient's pocket.
Allergy to adrenaline
A question often asked is whether the patient can be allergic to the adrenaline injection.62 The answer is yes, but it is very rare and is usually due to hypersensitivity to sodium metabisulphite,63 a preservative which is found in adrenaline 1:1,000 solution (product literature) and in all three adrenaline self-injector devices prescribed in the UK (as well as in some drinks and food products).64
Adrenaline should still be administered to a patient in anaphylaxis who has hypersensitivity to sodium metabisulphite, as the need for the life-saving drug far outweighs any theoretical risk from sulphites.64,65
Adrenaline in dental medical emergency drugs kits
Adrenaline auto-injectors
Many dental practices stock AAIs in their medical emergency drugs kit. The AAIs currently available in the UK are Emerade, EpiPen and Jext, which are designed for self-use.60 The obvious perceived advantages of AAIs are that they are quick and relatively easy to use. If an AAI is used, it is important to follow the manufacturer's guidelines for its use, taking particular care to avoid accidental self-injection which has been reported.66
However, key concerns have been raised in the last two years concerning AAIs:
Ongoing supply issues
Recalls for Emerade following failed activations
EpiPen 300 mcg device: needle length alleged to be too short for IM use in adults67 (same will also apply to Jext)
EpiPen 300 mcg device: incorrect dose of adrenaline in adults67 (same will also apply to Jext).
The AAI supply issues led to the Chief Dental Officer for England (CDO) requesting dental practices that stock AAIs in their medical emergency drugs kit to renew them with adrenaline ampoules, in order to preserve national stocks of the devices.9 Also, both the CDO9 and the Department of Health10 stipulate that all healthcare professionals providing services where anaphylaxis treatment may be required (for example, dentistry) should have the competency to draw up and administer IM adrenaline from ampoules with a normal syringe and needle.
The Resuscitation Council UK has reiterated that, in anaphylaxis, healthcare professionals should be administering the recommended dose of adrenaline in anaphylaxis; that is, 500 mcg for a patient over 12 years of age (an AAI should only be used if it is the only available adrenaline preparation when treating anaphylaxis in a healthcare setting).68
Adrenaline 1:1,000
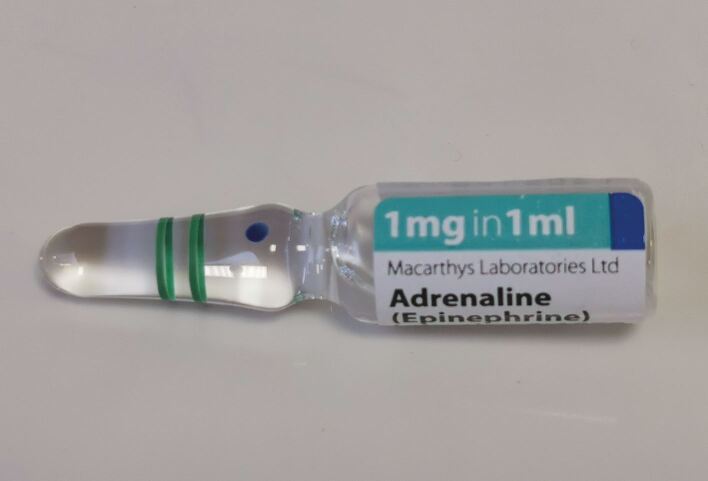
If not already, it is expected that dental practices will start stocking adrenaline 1:1,000 (1 mg/ml) ampoules (Fig. 2) in their emergency drugs kit (adrenaline 1:1,000 prefilled syringes are also available and are preferred by some to negate the need to draw up from an ampoule,68 though the authors are aware of recent supply issues with this product as well).
Fig. 2.
Adrenaline 1:1,000 ampoules (box of ten)
Dental practices are advised to follow Public Health England's69 guidelines, ensuring the following are available:
Adrenaline 1:1,000 (1 mg/ml) ampoules x2 (usually come in a box of ten)
Blue 23G 25 mm needles x4
Graduated 1 ml syringes x4.
The recommended needle length for IM injections is 25 mm (a blue 23G 25 mm needle) for all ages to ensure that the drug is injected into the muscle; a longer 21G 38 mm needle may be needed in some adults.4 Safety needles should ideally be used for IM injections to reduce the risk of needle-stick injury.70
Suggested procedure for IM injection of adrenaline
Which muscle?
IM injection into the vastus lateralis muscle (thigh) is advised because a high drug plasma level can be achieved within minutes.4,71 This is the ideal route.11
Although in the dental practice, the deltoid muscle (arm) is usually easier to access, the rate of absorption of adrenaline from this site is slower compared to the recommended vastus lateralis muscle.72 The deltoid muscle is therefore not routinely recommended,4 though it may need to be considered in a desperate situation.11
IM injections in the thigh: recent changes in advice
Wearing gloves routinely is not necessary,73 although dental staff will probably already be wearing them
Routinely using an 'alcohol swab' to clean the skin is no longer deemed necessary69
Leaving a small gap between the skin and the hub of the needle in case the needle breaks off during the injection is no longer advocated,71 as this may result in the drug being injected into subcutaneous fat, not muscle72
Aspirating or drawing back on a syringe to check if the needle is in a blood vessel is no longer advised for IM injections in the thigh69
Massaging the site following the injection is no longer advocated because it may dispel the drug out of the muscle tissue.74,75
Opening the glass adrenaline ampoule
Before opening the ampoule, it may be necessary to gently tap the top to dispel any remnants of the drug back to the bottom. Cuts and lacerations can occur when opening glass ampoules,76 particularly if the glass ampoule shatters due to too much pressure being applied. To minimise the risk of injury:77
Ideally, use a paper towel, piece of gauze or similar to protect the fingers and thumbs. Alternatively, use an ampoule-opening device
Only apply pressure to the 'neck' of the ampoule
Always apply pressure away from the coloured dot (Fig. 3) and never in any other direction
Apply sufficient light pressure to 'snap' the top off the ampoule
Don't apply any pressure to the base of the ampoule as this will probably cause it to shatter
Avoid pulling or twisting actions while applying pressure on the glass ampoule.
Fig. 3.
Adrenaline 1:1,000 ampoule
If the glass does shatter, then the broken glass and contents will need to be carefully discarded and another ampoule used.
Procedure for IM injection
The suggested procedure for IM injection below is a practical modification of the ideal procedure described comprehensively elsewhere, reflecting the urgency of the life-threating situation and the need to administer adrenaline without delay in order to save life:
Advise the patient of the urgency of the situation and that the adrenaline injection is required
Ask colleagues to prepare the patient; for example, expose the thigh if able. If not possible, plan to inject through light clothing
Draw up the required dose of adrenaline. Assemble the syringe and needle, safely open the adrenaline 1:1,000 ampoule (see above) and withdraw the required amount of drug (see above) from the ampoule; if present, quickly expel any large air bubbles
Change needle (ideally now use a safety needle),70 dispose of used needle in sharps container, and take syringe and needle in a tray to the patient
Use non-dominant hand to taut the skin (trickier to do this through clothing) to displace subcutaneous tissue at injection site
Advise patient to tense opposite leg (a distraction technique which also helps to relax the leg being used)
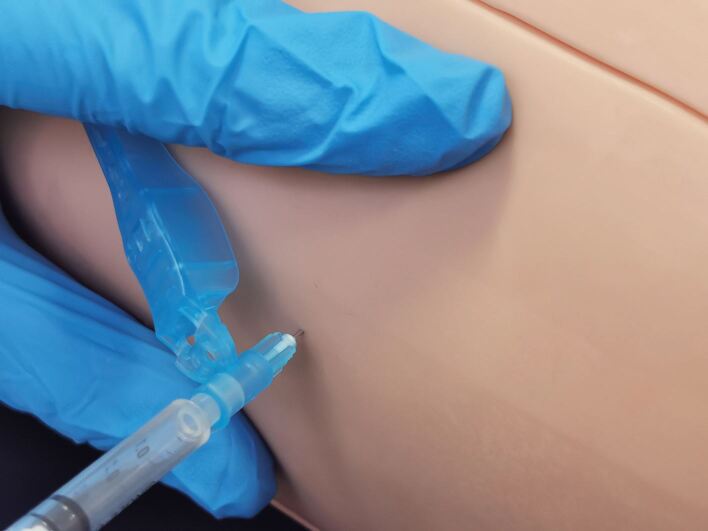
Insert the needle at 90-degree angle using a dart-like action71 and inject the adrenaline (Fig. 4)
Wait for two seconds (allows the drug to diffuse into the tissue)75
Withdraw the needle rapidly and dispose of sharps safely
Draw up another dose of adrenaline in case it is required.
Fig. 4.
IM injection - insert needle at 90 degrees
Management
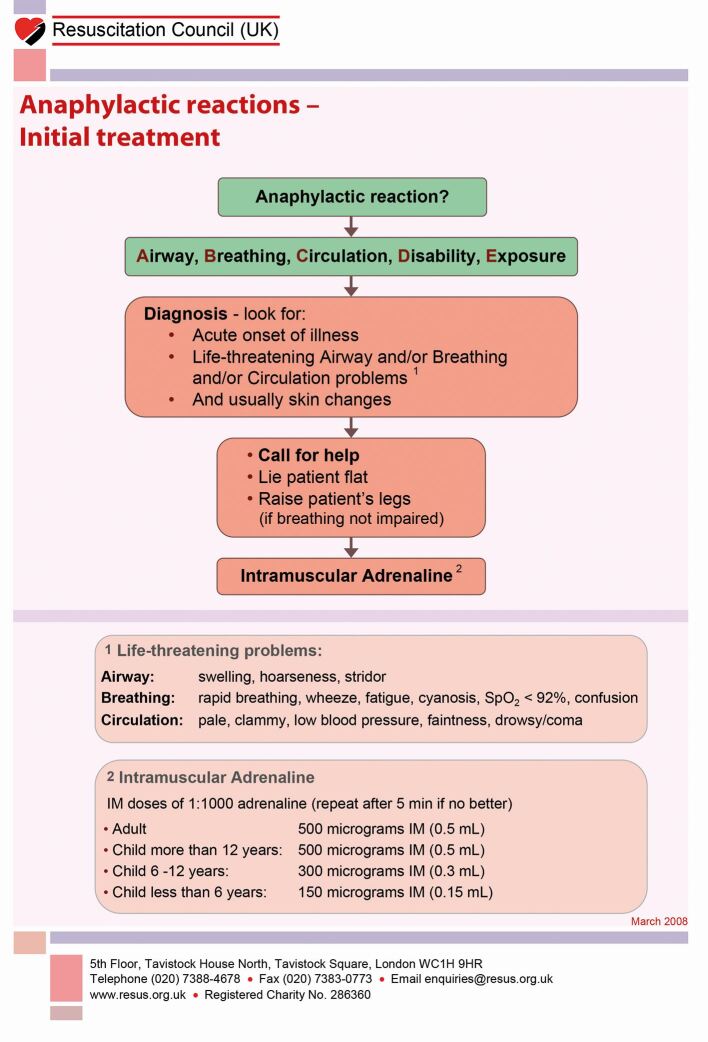
The Resuscitation Council UK algorithm for initial treatment of anaphylaxis is depicted in Figure 5. The management of suspected anaphylaxis is:
Call for help from colleagues and request the emergency drugs, oxygen and resuscitation kit
Assess the patient following the ABCDE approach
If anaphylaxis is suspected, call 999 for an ambulance and state anaphylaxis;11 communicate effectively following SBAR78 (Box 1)
Immediately stop the dental procedure, clear the airway of any materials and remove any contact of likely triggering agent from the patient79
Ensure the patient is in a comfortable position; patients with airway and breathing difficulties may prefer to sit up, while those with hypotension usually befit from lying down with legs raised4
Administer oxygen 15 litres/min through a non-rebreather oxygen mask (if available, establish oxygen saturation monitoring using a pulse oximeter)
For severe reactions where there are life-threatening airway and/or breathing and/or circulation problems (that is, hoarseness, stridor, severe wheeze, cyanosis, pale, clammy, drowsy, confusion or coma), administer IM adrenaline 500 mcg4
Closely monitor the patient following the ABCDE approach
Repeat IM adrenaline at five-minute intervals until there has been an adequate response11
Do not sit the patient up or stand them up if they are feeling faint or dizzy - they may be in profound shock and may then have a cardiac arrest.80
Fig. 5.
Initial management of anaphylaxis, reproduced with kind permission from the Resuscitation Council (UK)4
Box 1 SBAR communication tool for use in healthcare settings.
Situation
Background
Assessment
Recommendation
Information derived from:78
Debrief
A debrief should be planned and undertaken following any medical emergency to learn from the incident in order to try and improve for next time. A significant event analysis should also be done, but is probably best undertaken at a later date.
Documentation
Accurate record keeping will be expected.3,81 When able to do so, record the circumstances immediately before the onset of symptoms to help to identify the possible trigger of the anaphylactic reaction, the acute clinical features of the suspected anaphylactic reaction and the time of onset of the reaction.11
Reporting obligations
Anaphylaxis suspected to be related to a medication should be reported to the MHRA82 using the yellow card system. Fatal anaphylaxis in a dental practice may need to be reported to the CQC.83
Conclusion
Anaphylaxis can be life threatening. This article has described the management of anaphylaxis in the dental practice following Resuscitation Council UK guidelines, with particular reference to how to administer an IM injection.
References
- 1.Allergy UK. Anaphylaxis and severe allergic reaction. 2020. Available at https://www.allergyuk.org/information-and-advice/conditions-and-symptoms/33-anaphylaxis-and-severe-allergic-reaction (accessed May 2020).
- 2.Jevon P. Basic Guide to Medical Emergencies in the Dental Practice. 2nd ed. Oxford: Wiley, 2013.
- 3.GDC. Standards for the dental team. 2013. Available at https://standards.gdc-uk.org/Assets/pdf/Standards%20for%20the%20Dental%20Team.pdf (accessed May 2020).
- 4.Resuscitation Council UK. Emergency treatment of anaphylactic reactions: Guidelines for healthcare providers. 2017. Available online at https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment (accessed May 2020). [DOI] [PubMed]
- 5.Department of Health & Social Care. Supply Disruption Alert: SDA/2018/001 - EpiPen and EpiPen Junior (adrenaline auto-injector devices). 2018. Available online at https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=103102 (accessed May 2020).
- 6.MHRA. Adrenaline auto-injectors: recent action taken to support safety. 2019. Available online at https://www.gov.uk/drug-safety-update/adrenaline-auto-injectors-recent-action-taken-to-support-safety (accessed November 2020).
- 7.MHRA. Class 2 Medicines Recall: Emerade 300 micrograms solution for injection in pre-filled syringe, PL 33616/0014 (EL(20)A/20). 2020 Available at https://www.gov.uk/drug-device-alerts/class-2-medicines-recall-emerade-300-micrograms-solution-for-injection-in-pre-filled-syringe-pl-33616-0014-el-20-a-20 (accessed May 2020).
- 8.MHRA. Patients informed to exchange Emerade 500 micrograms adrenaline pens for a different brand. 2020. Available at https://www.gov.uk/government/news/patients-informed-to-exchange-emerade-500-micrograms-adrenaline-pens-for-a-different-brand (accessed May 2020).
- 9.Office of Chief Dental Officer England. Adrenaline for anaphylaxis kits - a reminder to Health Care Professionals. 2018. Available at http://www.bsdht.org.uk/News/20181009%20-%20EpiPen%20Advice%20-%20CDO%20England%20Final.pdf (accessed May 2020).
- 10.Department of Health & Social Care. Supply Disruption Alert: SDA/2019/004 - Emerade 500 microgram and 300 microgram adrenaline auto-injector devices. 2019. Available at https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=102885 (accessed May 2020).
- 11.NICE. Angio-ooedema and anaphylaxis. 2018.Available online at https://cks.nice.org.uk/topics/angio-oedema-anaphylaxis/ (accessed April 2020).
- 12.Turner P J, Gowland M H, Sharma V et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol 2015; DOI: 10.1016/j.jaci.2014.10.021. [DOI] [PMC free article] [PubMed]
- 13.Anaphylaxis Campaign. Drug Allergy: The Facts. 2017. Available at https://www.anaphylaxis.org.uk/wp-content/uploads/2019/07/Drug-Allergy-2017.pdf (accessed May 2020).
- 14.SDCEP. Drugs for the Management of Dental Problems During COVID-19 Pandemic. 2020. Available at https://www.sdcep.org.uk/wp-content/uploads/2020/05/SDCEP-MADP-COVID-19-drug-supplement-update-110520.pdf (accessed May 2020).
- 15.Pumphrey R S H. Fatal anaphylaxis in the UK, 1992-2001. Novartis Found Symp 2004; 257: 116-128; discussion 128-132, 157-160, 276-285. [PubMed]
- 16.Harper N, Cook T, Garcez T et al. Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6). Br J Anaesth 2018; 121: 159-171. [DOI] [PubMed]
- 17.Cloitre A, Duval X, Tubiana S et al. Antibiotic prophylaxis for the prevention of infective endocarditis for dental procedures is not associated with fatal adverse drug reactions in France. Med Oral Patol Oral Cir Bucal 2019; DOI: 10.4317/medoral.22818. [DOI] [PMC free article] [PubMed]
- 18.Pemberton M N. Allergy to chlorhexidine. Dent Update 2016; 43: 272-274. [DOI] [PubMed]
- 19.Anaphylaxis Campaign. Chlorhexidine. 2019 Available at https://www.anaphylaxis.org.uk/knowledgebase/chlorhexidine/ (accessed May 2020).
- 20.MHRA. All medical devices and medicinal products containing chlorhexidine - Risk of anaphylactic reaction due to chlorhexidine allergy. 2014.Available at https://www.gov.uk/drug-device-alerts/medical-device-alert-all-medical-devices-and-medicinal-products-containing-chlorhexidine-risk-of-anaphylactic-reaction-due-to-chlorhexidine-allergy (accessed May 2020).
- 21.BBC News. Mouthwash reaction killed Brighton dental patient. 2011. Available at https://www.bbc.co.uk/news/uk-england-sussex-14951073 (accessed May 2020).
- 22.Whitehaven News. Mouthwash linked to death of patient, 63. 2011. Available at https://www.whitehavennews.co.uk/news/17135703.mouthwash-linked-to-death-of-patient-63/ (accessed May 2020).
- 23.Harper N J N, Dixon T, Dugue P et al. Guidelines: Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia 2009; 64: 199-211. [DOI] [PMC free article] [PubMed]
- 24.SDCEP. Management of Acute Dental Problems: Guidance for healthcare professionals. 2013. Available at https://www.sdcep.org.uk/wp-content/uploads/2013/03/SDCEP+MADP+Guidance+March+2013.pdf (accessed May 2020).
- 25.SDCEP. Management of Acute Dental Problems during COVID-19. 2020. Available at https://www.sdcep.org.uk/wp-content/uploads/2020/03/SDCEP-MADP-COVID-19-guide-300320.pdf (accessed May 2020).
- 26.NHS, Specialist Pharmacy Service and UK Medicines Information. Allergy to local anaesthetic agents used in dentistry - what are the signs, symptoms, alternative diagnoses and management options? 2019. Available at https://www.sps.nhs.uk/wp-content/uploads/2019/08/UKMi_QA_Dental-local-anaesthetic-allergy-update_May-2019.pdf (accessed May 2020). [DOI] [PubMed]
- 27.Lee J, Lee J-Y, Kim H J, Seo K-S. Dental anaesthesia for patients with allergic reactions to lidocaine: two case reports. J Dent Anesth Pain Med 2016; 16: 209-212. [DOI] [PMC free article] [PubMed]
- 28.Gu J Q, Liu S, Zhi Y X. Provocation Test-confirmed chlorhexidine-induced anaphylaxis in dental procedure. Chin Med J (Engl) 2018; 131: 2893-2894. [DOI] [PMC free article] [PubMed]
- 29.Kim H, Lee J M, Seo K S, Kwon S M, Row H S. Anaphylactic reaction after local lidocaine infiltration for retraction of retained teeth. J Dent Anesth Pain Med 2019; 19: 175-180. [DOI] [PMC free article] [PubMed]
- 30.Fisher M M, Bowey C J. Alleged allergy to local anaesthetics. Anaesth Intensive Care 1997; 25: 611-614. [DOI] [PubMed]
- 31.Batinac T, Sotošek Tokmadžić V, Peharda V, Brajac I. Adverse reactions and alleged allergy to local anaesthetics: analysis of 331 patients. J Dermatol 2013; 40: 522-527. [DOI] [PubMed]
- 32.Renton T. Inferior Dental Blocks Versus Infiltration Dentistry: Is it time for change? Dent Update 2019; 46: 204-218.
- 33.Becker D E. Drug allergies and implications for dental practice. Anesth Prog 2013; 60: 188-197. [DOI] [PMC free article] [PubMed]
- 34.Wildsmith J A W, Mason A, McKinnon R P et al. Alleged allergy to local anaesthetic drugs. Br Dent J 1998; 184: 507-510. [DOI] [PubMed]
- 35.Sinclair E. Are you allergic to latex? BDJ Team 2017; DOI: 10.1038/bdjteam.2017.41.
- 36.Baluga J C, Casamayou R, Carozzi E et al. Allergy to local anaesthetics in dentistry. Myth or reality? Allergol Immunopathol (Madr) 2002; 30: 14-19. [DOI] [PubMed]
- 37.The Australian Dental Association. Available online at https://www.ada.org.au/ (accessed October 2020).
- 38.Anaphylaxis Campaign. Latex allery: the facts. 2019. Available at https://www.anaphylaxis.org.uk/knowledgebase/latex-allergy-the-facts/ (accessed May 2020).
- 39.Health and Safety Executive. Latex allergy in health and social care. 2019. Available at https://www.hse.gov.uk/healthservices/latex/ (accessed May 2020).
- 40.Wu M, McIntosh J, Liu J. Current prevalence rate of latex allergy: Why it remains a problem? J Occup Health 2016; 58: 138-144. [DOI] [PMC free article] [PubMed]
- 41.British Association of Dermatologists. Latex allergy. 2017. Available at https://www.bad.org.uk/shared/get-file.ashx?id=4349&itemtype=document (accessed May 2020).
- 42.Pumphrey R S, Duddridge M, Norton J. Fatal latex allergy. J Allergy Clin Immunol 2001; 107: 558. [DOI] [PubMed]
- 43.Chin S M, Ferguson J W, Bajurnow T. Latex allergy in dentistry. Review and report of case presenting as a serious reaction to latex dental dam. Aust Dent J 2004; 49: 146-148. [DOI] [PubMed]
- 44.Hamann C, Turjanmaa K, Rietschel R et al. Natural rubber latex hypersensitivity: incidence and prevalence of type I allergy in the dental professional. J Am Dent Assoc 1998; 129: 43-54. [DOI] [PubMed]
- 45.Anaphylaxis Campaign. Dental Practice: Management of a patient with Type 1 NRL allergy. 2019. Available at https://www.anaphylaxis.org.uk/hcp/natural-rubber-latex-nrl/dental-practice/ (accessed May 2020).
- 46.Anaphylaxis Campaign. Latex Allergy and the Law. 2019. Available at https://www.anaphylaxis.org.uk/living-with-anaphylaxis/living-with-natural-rubber-nrl-allergy/the-law/ (accessed May 2020).
- 47.Paiva M, Piedade S, Gaspar A. Toothpaste-induced anaphylaxis caused by mint (Mentha) allergy. Allergy 2010; 65: 1196-1204. [DOI] [PubMed]
- 48.Smith G. After Daughter's Fatal Reaction to Toothpaste, Mother Calls for Caution. Allergic Living 2019. Available at https://www.allergicliving.com/2019/04/15/after-daughters-fatal-reaction-to-toothpaste-mother-calls-for-caution/ (accessed November 2020).
- 49.Kekatos M. Girl, 11, dies after a severe allergic reaction to toothpaste that contained a milk protein. Daily Mail (London) 2019 April 18.
- 50.Australian Dental Association. Allergy: A dental cautionary tale. 2019 Available at https://www.ada.org.au/News-Media/News-and-Release/Latest-News/Allergy-A-dental-cautionary-tale (accessed May 2020).
- 51.Darvell B. Materials Science for Dentistry. 10th ed. London: Elsevier, 2018.
- 52.Septodont. Alvogyl patient information leaflet. 2006. Available at https://www.septodont.co.uk/sites/default/files/Alvogyl%20Patient%20information%20leaflet%20S%2005%2006%20047%2011%2000.pdf (accessed May 2020).
- 53.Webb L, Greene E, Lieberman P L. Anaphylaxis: a review of 593 cases. J Allergy Clin Immunol 2004; 113: S240.
- 54.Song T T, Lieberman P. Adrenaline in anaphylaxis: doubt no more. Curr Opin Allergy Clin Immunol 2015; 15: 323-328. [DOI] [PubMed]
- 55.Chooniedass R, Temple B, Becker A. Adrenaline use for anaphylaxis: too seldom, too late: current practices and guidelines in health care. Ann Allergy Asthma Immunol 2017; 119: 108-110. [DOI] [PubMed]
- 56.Lieberman P. Biphasic anaphylactic reactions. Ann Allergy Asthma Immunol 2005; 95: 217-226. [DOI] [PubMed]
- 57.Storey P, Fitzharris P. Adrenaline in anaphylaxis: overtreatment in theory, undertreatment in reality. Postgrad Med J 2015; 91: 1-2. [DOI] [PubMed]
- 58.Electronic Medicines Compendium.Adrenaline (Adrenaline) 1mg/ml (1:1000) solution for injection. Available online at https://www.medicines.org.uk/emc/product/3673/smpc (accessed May 2020).
- 59.NICE. British National Formulary. Available online at https://bnf.nice.org.uk/ (accessed May 2020).
- 60.Anaphylaxis Campaign. Anaphylaxis: the facts. 2019. Available at https://www.anaphylaxis.org.uk/wp-content/uploads/2019/07/Anaphylaxis-The-Facts-Feb-2019.pdf (accessed May 2020).
- 61.Cameron R, Little M, Mitra B, Deasy C. Textbook of Adult Emergency Medicine. 5th ed. London: Elsevier, 2019.
- 62.Roth J V, Shields A. A dilemma: how does one treat anaphylaxis in the sulphite allergic patient since Adrenaline contains sodium metabisulphite? Anesth Analg 2004; DOI: 10.1213/01.ane.0000120092.39021.f2. [DOI] [PubMed]
- 63.Australian Society of Clinical Immunology and Allergy. Sulfite Sensitivity. 2019. Available at https://www.allergy.org.au/patients/other-allergy/sulfite-allergy (accessed May 2020).
- 64.Anaphylaxis Campaign. Sulphites. 2019. Available at https://www.anaphylaxis.org.uk/wp-content/uploads/2019/11/Sulphites-2019.pdf (accessed May 2020).
- 65.Food Allergy Canada. Sulphites. 2020. Available at https://foodallergycanada.ca/food-allergy-basics/food-allergies-101/what-are-food-allergies/sulphites/ (accessed November 2020).
- 66.Ewan P, Brathwaite N, Leech S et al. BSACI guideline: prescribing an adrenaline auto-injector. Clin Exp Allergy 2016; 46: 1258-1280. [DOI] [PubMed]
- 67.Courts and Tribunal Judiciary. Regulation 28: Report to prevent further deaths. 2018. Available at https://www.judiciary.uk/wp-content/uploads/2018/10/Natasha-LAPEROUSE-2018-0279.pdf (accessed May 2020).
- 68.Travers A, Taylor K. Adrenaline use: The use of pre-filled adrenaline syringes in anaphylaxis kits. Br Dent J 2019; 226: 85. [DOI] [PubMed]
- 69.Public Health England. Immunisation against infectious diseases. 2014. Available online at https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book (accessed April 2020).
- 70.Health and Safety Executive. Health and Safety (Sharp Instruments in Healthcare) Regulations 2013: Guidance for employers and employees. 2013. Available online at https://www.hse.gov.uk/pubns/hsis7.htm (accessed May 2020).
- 71.Shepherd E. Injection technique 1: administering drugs via the intramuscular route. Nurs Times 2018; 114: 23-25.
- 72.Song T T, Nelson M R, Chang J H, Engler R J, Chowdhury B A. Adequacy of the Adrenaline autoinjector needle length in delivering adrenaline to the intramuscular tissues. Ann Allergy Asthma Immunol 2005; 94: 539-542. [DOI] [PubMed]
- 73.World Health Organisation. WHO guidelines on Hand Hygiene in Healthcare: a summary. 2009. Available at https://www.who.int/gpsc/5may/tools/who_guidelines-handhygiene_summary.pdf (accessed May 2020).
- 74.Greenway K. Rituals in Nursing: intramuscular injection. J Clin Nurs 2014; DOI: 10.1111/jocn.12627. [DOI] [PubMed]
- 75.Dougherty L, Lister S. The Royal Marsden Hospital Manual of Clinical Nursing Procedures. Oxford: Wiley-Blackwell, 2015.
- 76.Weenig C S. A better, safer, and inexpensive way to open glass ampules. Anaesthesiology 1998; 88: 838. [DOI] [PubMed]
- 77.University of Bristol. How to open a glass vial. 2018. Available at http://www.bristol.ac.uk/media-library/sites/vetscience/documents/clinical-skills/How%20to%20Open%20a%20Glass%20Vial.pdf (accessed November 2020).
- 78.ACT Academy. SBAR communication tool - situation, background, assessment, recommendation. 2018. Available at https://improvement.nhs.uk/documents/2162/sbar-communication-tool.pdf (accessed April 2020).
- 79.Maher N G, de Looze J, Hoffman G R. Anaphylaxis: an update for dental practitioners. Aust Dent J 2014; 59: 142-158. [DOI] [PubMed]
- 80.Pumphrey R S. Fatal posture in anaphylactic shock. J Allergy Clin Immunol 2003; 112: 451-452. [DOI] [PubMed]
- 81.CQC. Dental mythbuster 8: Dental care records. 2018. Available at https://www.cqc.org.uk/guidance-providers/dentists/dental-mythbuster-8-dental-care-records (accessed April 2020).
- 82.MHRA. Yellow card. Available online at https://yellowcard.mhra.gov.uk/ (accessed April 2020).
- 83.CQC. Dental mythbuster 11: Statutory notifications to CQC. 2020. Available at https://www.cqc.org.uk/guidance-providers/dentists/dental-mythbuster-11-statutory-notifications-cqc (accessed April 2020).