Abstract
Background
Patients with symptomatic and asymptomatic heart failure undergoing noncardiac surgery may benefit from the haemodynamic profile of etomidate. However, the safety of etomidate in this population is unknown. We examined anaesthesiologist variation in etomidate use and assessed its safety using an instrumental variable approach to account for differences in treatment selection.
Methods
A retrospective cohort study of 19 714 patients with heart failure undergoing noncardiac surgery at two tertiary care institutions from January 2006 to December 2017 was performed. The proportion of etomidate use among 294 anaesthesiologists was examined and adjusted risk differences (aRD) for in-hospital and 30-day mortality were calculated using physician preference for etomidate as an instrumental variable.
Results
Etomidate was used in 14.3% (2821/19 714) of patients. Preference for etomidate varied substantially among individual anaesthesiologists with the lowest and highest quartile users using etomidate in 0–4.7% and 20.4–66.7% of their own heart failure patients, respectively. The adjusted instrumental variable analysis showed no significant differences in the risk of in-hospital (aRD –0.2%; 95% confidence interval, –2.4%–1.9%; P=0.83) or 30 day mortality (aRD 0.2%; 95% confidence interval, –2.5%–2.9%; P=0.90). Anaesthesiologists with higher preferences for etomidate were more experienced (greater heart failure and total case volume) than anaesthesiologists with lower preferences for etomidate.
Conclusions
We found substantial variability in anaesthesiologists' preference for etomidate for use in patients with heart failure undergoing noncardiac surgery. There was no association between etomidate use and in-hospital or 30-day mortality. Etomidate is not inferior to other currently used options for induction of general anaesthesia in patients with heart failure.
Keywords: etomidate, heart failure, instrumental variable analysis, mortality, noncardiac surgery
Editor's key points.
-
•
It is currently not known which induction agent is best for patients with heart failure requiring general anaesthesia.
-
•
Some anaesthesiologists feel strongly that etomidate must always be used in such situations because it does not cause myocardial depression or hypotension, whereas others feel strongly that it should never be used in vulnerable patients because of its suppressant effect on stress hormone release from the adrenal cortex.
-
•
In this large retrospective two-centre study, there was substantial variation in practice, and etomidate, compared predominantly with propofol, was neither associated with increased nor decreased postoperative mortality.
-
•
The choice of anaesthetic induction agent for patients with heart failure is unlikely to have clinically important consequences in relation to serious adverse events; clinicians should probably not base their choice of induction agent on safety considerations.
Etomidate is a sedative–hypnotic drug valued for its haemodynamic stability during induction of general anaesthesia in patients with diminished cardiovascular reserve. However, even single-dose etomidate induces adrenal insufficiency and inhibits steroidogenesis for approximately 48–72 h1,2 by reversibly inhibiting 11 beta-hydroxylase in the adrenal gland.3 The clinical significance of this effect is controversial.4, 5, 6
Patients with symptomatic or asymptomatic heart failure (HF) undergoing noncardiac surgery represent a growing population of high-risk patients7, 8, 9 who may benefit from the haemodynamic profile of etomidate. However, there is a lack of knowledge regarding the safety of etomidate in the HF population undergoing surgery. Several retrospective studies have investigated the impact of etomidate on perioperative outcomes in other populations with conflicting results.2,10, 11, 12, 13, 14 One retrospective study of ASA 3/4 patients undergoing noncardiac surgery concluded that etomidate was associated with a 2.5 increased odds of 30 day mortality10; however, HF status was not available as a covariate. Another retrospective study concluded that etomidate was not associated with in-hospital mortality in cardiac surgery patients,11 but patients with HF composed a minority of this study population.
As such, there is considerable disagreement among physicians regarding the safety of etomidate.15, 16, 17, 18, 19 The first goal of this study was to examine anaesthesiologist variation in etomidate use; contingent on the presence of substantial practice variation, the second goal of this study was to assess the safety of etomidate use in patients with symptomatic/asymptomatic HF undergoing noncardiac surgery using an instrumental variable approach to account for differences in treatment selection. We hypothesised that there would be substantial variability in anaesthesiologists' preference for etomidate, and that use of etomidate in this population would not be associated with in-hospital or 30-day mortality.
Material and methods
Data sources
The study cohort was derived from perioperative clinical data through multiple hospital registry databases from Beth Israel Deaconess Medical Center (BIDMC; January 1, 2006–September 30, 2017) and Massachusetts General Hospital (MGH; January 1, 2007–December 31, 2015) in Boston, Massachusetts (Supplemental Methods). The study was approved by the BIDMC Institutional Review Board (2019P000391) and the Partners Human Research Committee, and written informed consent was waived.
Study cohort
Adults (≥18 yr) undergoing surgery with general anaesthesia who were diagnosed with HF or cardiomyopathy (defined by International Classification of Diseases, 9th/10th Revision, Clinical Modification [ICD-9/10-CM] codes) (Supplementary Table S1) within 1 yr before the procedure were included in the analysis. Validation studies have demonstrated good sensitivity (0.86), specificity (0.83), and positive predictive value (0.97) with the group of codes used to identify the diagnosis of HF.20, 21, 22, 23, 24 The Study Population was defined by excluding patients who underwent cardiac surgery or had surgery within 4 weeks before the index case, cases without a documented anaesthesiologist, and anaesthesiologists with an HF case volume ≤25th percentile of all operators (≤7 patients over the study period) in order to study a group of practitioners who regularly engaged with this population. The Instrumental Variable Analysis Population was defined by excluding patients with missing values.
Study exposures and outcomes
The primary exposure was intraoperative etomidate use. The primary outcomes for this analysis were in-hospital and 30-day mortality.
Covariates
Available patient characteristics included age, sex, BMI, American Society of Anesthesiologists (ASA) physical status/Charlson comorbidity index (CCI), nine comorbidities (including coronary artery disease [CAD], hypertension, diabetes mellitus, atrial fibrillation, and chronic kidney disease [CKD]), smoking status, left ventricular ejection fraction (LVEF; within 1 yr before the procedure), and prescriptions for seven medications such as steroids and therapies impacting HF survival (e.g. beta blockers, angiotensin-converting enzyme [ACE] inhibitors) (Supplementary Table S2). Procedural characteristics included intraoperative use of sedative–hypnotics including propofol and ketamine, and duration and type of surgery, presence of neuraxial anaesthesia, admission type, emergency status, age-adjusted minimum alveolar concentration (MAC), intraoperative total fluid volume (defined as the total volume of crystalloids and colloids using an effective volume expansion ratio of 1:1.5),25 estimated blood loss, urine output, packed red blood cells, and vasopressors (defined in mg norepinephrine equivalents). Work relative value units (RVU), a measure of surgical complexity, were also included; RVUs are assigned by the American Medical Association's Specialty Society Relative Value Update Committee and reflect the estimated time, effort, and skill associated with each procedure with higher RVUs reflecting longer and more intense surgeries.26 Anaesthesiologist characteristics included their case volume of patients with HF undergoing noncardiac surgery with general anaesthesia.
Statistical analysis
Categorical variables were presented as counts and percentages and continuous variables were presented as means and standard deviations or medians and inter-quartile ranges (IQRs) as appropriate. Given the large sample size and therefore the potential of small, clinically non-meaningful differences to be statistically significant, standardised differences (STDs) were reported with a threshold of greater than 10% used to define a significant difference between groups. We compared patient, procedural, and anaesthesiologist characteristics among patients with symptomatic/asymptomatic HF undergoing noncardiac surgery receiving intraoperative etomidate vs no etomidate. Unadjusted primary outcomes were compared between etomidate vs non-etomidate groups by calculating risk differences (RD). We also examined the proportion of etomidate use among anaesthesiologists, which could range from 0% to 100% of their own HF patients.
Our prespecified primary analytic strategy was an instrumental variable approach27 owing to potential confounding of etomidate and non-etomidate comparisons from treatment selection bias. Confounders that could influence the selection of etomidate for induction of anaesthesia such as patient frailty are rarely quantified in databases. These unmeasured confounders subsequently cannot be accounted for within models traditionally used in observational methods (e.g. multivariable regression or propensity scores analysis) and may lead to residual confounding by indication. Instrumental variable methods have been used to overcome treatment selection bias by using an instrument that is related to treatment but not directly to outcome to achieve ‘quasi-randomisation’ of groups and a balance of both measured and unmeasured confounders for a valid comparison of outcomes.
Anaesthesiologist preference for etomidate (i.e. the proportion of etomidate use by each anaesthesiologist) was used as the instrumental variable.28,29 In this case, the heterogeneity of practice patterns in etomidate use creates a ‘marginal population’ of patients who would likely receive etomidate when randomly assigned to an anaesthesiologist who has a high preference for etomidate or otherwise would be unlikely to receive etomidate if randomly assigned to an anaesthesiologist who has a low preference for etomidate. In this way, the natural variation in etomidate use among anaesthesiologists can be harnessed to estimate a causal relationship between etomidate and perioperative mortality so long as this variation is not otherwise related to other factors that correlate with outcomes. We performed the instrumental variable analysis using the two-stage least squares methodology as previously described30,31 (Supplemental Methods).
The patient and procedural variables in Table 1 (excluding ejection fraction, intraoperative agents, estimated blood loss, urine output, vasopressors, and service type) were used for adjustment in both stages. In addition, it is possible that links between receipt of etomidate and the outcome outside of physician preference may exist owing to the institution (e.g. use or non-use of etomidate determined by institution or institutions as a whole having better or worse outcomes in noncardiac surgery) or owing to anaesthesiologist experience or subspecialty training affecting both treatment preference and outcomes. To address these possibilities, we also adjusted for hospital site and anaesthesiologist experience in both stages of the instrumental variable analysis. The Wald F-statistic was calculated to assess the strength of the instrument to predict actual etomidate use with an F-statistic >10 indicating a strong instrument. The effectiveness of the instrument for balancing clinical characteristics was assessed by comparing characteristics across quartiles of anaesthesiologist etomidate use.
Table 1.
Baseline patient and procedural characteristics of cases stratified by etomidate use. ∗Total fluid volume defined as the volumes of crystalloid plus one-and-a-half times colloid administered intraoperative exclusive of PRBCs. All comorbidities are within 1 yr of procedure date. All medications are prescriptions within 30 days of procedure except steroids (1 yr before). †Vasopressors in milligrams norepinephrine equivalents = total amount epinephrine + total amount norepinephrine + (total amount phenylephrine/10) + (total amount dopamine/weight [kg]/2). CAD, coronary artery disease; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CCI, Charlson comorbidity index; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; MAC, minimal alveolar concentration; PRBC, packed red blood cells; RVU, relative value unit of main procedure; sd, standard deviation; ENT, ear, nose, throat.
| Descriptor | Total (N=19 714) | No etomidate (N=16 893) | Etomidate (N=2821) | Standardised difference (%) |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (yr), mean (range) | 67.5 (18–107) | 66.7 (18–107) | 72.1 (18–104) | 40.0 |
| BMI (kg m−2), mean (sd) | 29.5 (7.8) | 29.7 (7.9) | 28.4 (7.0) | –17.4 |
| Female sex, no. (%) | 8745 (44.4) | 7591 (44.9) | 1154 (40.9) | –8.1 |
| ASA physical status, median (IQR) | 3 (3, 3) | 3 (3, 3) | 3 (3, 4) | 63.7 |
| Hypertension, no. (%) | 14 792 (75.0) | 12 734 (75.4) | 2058 (73.0) | –5.5 |
| Hyperlipidaemia, no. (%) | 11 863 (60.2) | 10 141 (60.0) | 1722 (61.0) | 2.1 |
| Diabetes mellitus, no. (%) | 8182 (41.5) | 6830 (40.4) | 1352 (47.9) | 15.2 |
| On insulin, no. (%) | 4472 (22.7) | 3938 (23.3) | 534 (18.9) | –10.8 |
| CAD, no. (%) | 10 282 (52.2) | 8383 (49.6) | 1899 (67.3) | 36.5 |
| Atrial fibrillation, no. (%) | 7789 (39.5) | 6480 (38.4) | 1309 (46.4) | 16.2 |
| PVD, no. (%) | 3751 (19.0) | 2950 (17.5) | 801 (28.4) | 26.1 |
| Ischaemic stroke, no. (%) | 1446 (7.3) | 1262 (7.5) | 184 (6.5) | –3.9 |
| COPD, no. (%) | 4636 (23.5) | 3881 (23.0) | 755 (26.8) | 8.8 |
| CKD, no. (%) | 6723 (34.1) | 5570 (33.0) | 1153 (40.9) | 16.4 |
| CCI, median (IQR) | 5 (3, 7) | 5 (3, 7) | 5 (3, 7) | 6.0 |
| Smoking, no. (%) | 4589 (23.3) | 4107 (24.3) | 482 (17.1) | –17.8 |
| Beta blocker, no. (%) | 8193 (41.6) | 7318 (43.3) | 875 (31.0) | –25.7 |
| ACE inhibitor/ARB, no. (%) | 7357 (37.3) | 6303 (37.3) | 1054 (37.4) | 0.2 |
| Hydralazine/nitrates, no. (%) | 2698 (13.7) | 2316 (13.7) | 382 (13.5) | –0.6 |
| Aldosterone antagonists, no. (%) | 1587 (8.1) | 1308 (7.7) | 279 (9.9) | 7.8 |
| Digoxin, no. (%) | 1498 (7.6) | 1212 (7.2) | 286 (10.1) | 10.3 |
| Steroid use, no. (%) | 3402 (17.3) | 3159 (18.7) | 243 (8.6) | –29.7 |
| Antiplatelet use, no. (%) | 8611 (43.7) | 7485 (44.3) | 1126 (39.9) | –8.9 |
| Anticoagulant use, no. (%) | 4802 (24.4) | 4149 (24.6) | 653 (23.2) | –3.3 |
| Ejection fraction (EF), mean (sd) no. (%) | 55.6 (15.0) | 57.2 (14.0) | 46.2 (17.5) | –69.4 |
| EF >40% | 7542 (38.3) | 6777 (40.1) | 765 (27.1) | –27.8 |
| EF 20–40% | 1602 (8.1) | 1112 (6.6) | 490 (17.4) | 33.7 |
| EF <20% | 117 (0.6) | 51 (0.3) | 66 (2.3) | 17.7 |
| Missing | 10 453 (53.0) | 8.953 (53.0) | 1500 (53.2) | 0.4 |
| Procedural characteristics | ||||
| Intraoperative etomidate use, no. (%) | 2821 (14.3) | – | – | – |
| Intraoperative agent use, no. (%) | ||||
| Etomidate only | 1244 (6.3) | 0 | 1244 (44.1) | 125.6 |
| Propofol only | 15 539 (78.8) | 15 539 (95.2) | 0 | –629.8 |
| Ketamine only | 30 (0.2) | 30 (5.3) | 0 | –33.5 |
| Etomidate and propofol | 1508 (7.6) | 0 | 1508 (53.5) | 151.7 |
| Etomidate and ketamine | 27 (0.1) | 0 | 27 (1.0) | 4.5 |
| Propofol and ketamine | 791 (4.0) | 791 (4.7) | 0 | –31.4 |
| All three | 42 (0.2) | 0 | 42 (1.5) | 17.5 |
| No agent | 533 (2.7) | 533 (3.2) | 0 | –25.7 |
| Propofol (total) | 17 880 (90.7) | 16 330 (96.7) | 1550 (55.0) | –111.6 |
| Ketamine (total) | 890 (4.5) | 821 (4.9) | 69 (2.5) | –12.7 |
| Emergency status, no. (%) | 1664 (8.4) | 1253 (7.4) | 411 (14.6) | 23.2 |
| Admission type, no. (%) | ||||
| Ambulatory | 2812 (14.3) | 2623 (15.5) | 189 (6.7) | –28.3 |
| Same-day admit | 8860 (44.9) | 7668 (45.4) | 1192 (42.3) | –6.3 |
| Inpatient | 8042 (40.8) | 6602 (39.1) | 1440 (51.1) | 24.3 |
| Neuraxial anaesthesia, no. (%) | 793 (4.0) | 737 (4.4) | 56 (2.0) | –13.7 |
| Age adjusted MAC, mean (sd) | 0.84 (0.35) | 0.83 (0.35) | 0.85 (0.34) | 5.8 |
| Total fluid volume (ml),∗ median (IQR) | 1703 (900, 3000) | 1500 (825, 2750) | 2250 (1250, 3750) | 29.1 |
| Estimated blood loss (ml), median (IQR) | 0 (0, 50) | 0 (0, 100) | 0 (0, 0) | –17.5 |
| Urine output (ml), median (IQR) | 0 (0, 200) | 0 (0, 200) | 0 (0, 0) | –23.7 |
| PRBC (units), mean (sd) | 0.18 (0.81) | 0.16 (0.77) | 0.31 (1.02) | 16.6 |
| Total vasopressors, mg norepinephrine equivalents,† median (IQR) | 0.10 (0.01, 0.40) | 0.10 (0.01, 0.40) | 0.15 (0.02, 0.47) | 0.7 |
| Duration of surgery (min), mean (sd) | 167.9 (110.4) | 167.1 (111.7) | 172.6 (101.9) | 5.1 |
| Work RVU, median (IQR) | 14.5 (7.4, 20.8) | 14.1 (7.2, 20.8) | 16.7 (10.5, 21.8) | 14.9 |
| Service, no. (%) | ||||
| Orthopaedic surgery | 3818 (19.4) | 3166 (18.7) | 652 (23.1) | 10.8 |
| Vascular surgery | 2449 (12.4) | 1816 (10.8) | 633 (22.4) | 31.6 |
| Thoracic surgery | 2186 (11.1) | 1990 (11.8) | 196 (7.0) | –16.5 |
| Urology | 1560 (7.9) | 1359 (8.0) | 201 (7.1) | –3.4 |
| General surgery | 1378 (7.0) | 1234 (7.3) | 144 (5.1) | –9.1 |
| Anaesthesiology | 1233 (6.3) | 1132 (6.7) | 101 (3.6) | –14.1 |
| Neurosurgery | 1172 (6.0) | 1070 (6.3) | 102 (3.6) | –12.5 |
| Transplant | 1063 (5.4) | 949 (5.6) | 114 (4.0) | –7.5 |
| Acute care surgery | 991 (5.0) | 879 (5.2) | 112 (4.0) | –5.7 |
| Gynaecology | 490 (2.5) | 448 (2.7) | 42 (1.5) | –8.4 |
| Surgical oncology | 437 (2.2) | 408 (2.4) | 29 (1.0) | –10.9 |
| Plastic surgery | 336 (1.7) | 308 (1.8) | 28 (1.0) | –6.8 |
| Radiology | 241 (1.2) | 226 (1.3) | 15 (0.5) | –6.8 |
| ENT | 207 (1.1) | 188 (1.1) | 19 (0.7) | –4.2 |
| Colorectal | 159 (0.8) | 134 (0.8) | 25 (0.9) | 1.1 |
| Burn | 134 (0.7) | 134 (0.8) | 0 (0) | –12.7 |
| Other | 579 (2.9) | 519 (3.1) | 60 (2.1) | –6.3 |
| Missing | 1281 (6.5) | 933 (5.5) | 348 (12.3) | 24.1 |
We performed multiple sensitivity analyses to assess the influence of missing data, alternative definitions of symptomatic/asymptomatic HF, and time trends on our results. We also assessed anaesthesiologists' total institutional case volume as a comparison to HF case volume. First, the instrumental variable analysis was repeated in the Study Population including the 2200 patients originally excluded from the primary analysis because of missing data (necessarily omitting from the models variables with missing data including BMI, emergent status, admission type, duration of surgery, work RVUs, and ASA physical status). Second, LVEF, which was excluded from the primary analysis because of a high rate of missingness, was added to the model as a categorical variable with a missing category. Third, analyses were repeated using alternative definitions of symptomatic/asymptomatic HF including ejection fraction ≤40% and systolic HF (using ICD-9/10-CM codes for only systolic HF coupled with evidence of HF therapy as defined by beta blocker, ACE inhibitor, or angiotensin receptor blocker use). Fourth, time effect was assessed by analysing the subgroup of cases occurring in the latter half of the acquisition period, 2012–17. Fifth, we evaluated whether there were changes in anaesthesiologist preference over time from 2007 to 2015 (during which data were available for both institutions). Finally, we assessed anaesthesiologists' total institutional case volume in order to compare overall anaesthesiologists' experience with HF case volume. Total institutional case volume counts all cases performed at the anaesthesiologists' institution before application of inclusion and exclusion criteria and includes all ages, diagnoses, and anaesthetics (e.g. sedation), and cardiac surgery and repeat cases. A two-sided P-value <0.05 was used to define statistical significance. Analyses were performed using STATA Version 15 (StataCorp LLC, College Station, TX, USA) and SAS Version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patterns of etomidate use
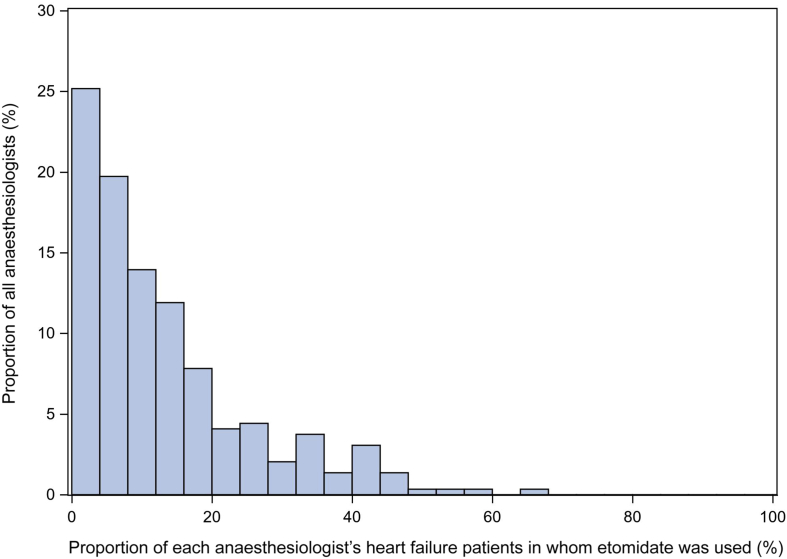
A total of 30 585 patients with 396 anaesthesiologists were identified. Of these, 19 714 patients and 294 anaesthesiologists met the study criteria (Supplementary Fig. S1). Etomidate was used in 14.3% (2821/19 714) of patients with a mean dose of 0.22 (0.2) mg kg−1. The preference for etomidate varied substantially among individual anaesthesiologists (Fig 1) with the lowest and highest quartile users using etomidate in 0–4.7% and 20.4–66.7% of their HF patients, respectively.
Fig 1.
Anaesthesiologist use of etomidate with heart failure patients in noncardiac cases. Proportion of noncardiac cases among individual anaesthesiologists in which etomidate was used. Overall preference for etomidate varied widely across individual anaesthesiologists (0–67%).
Patient, procedural, and anaesthesiologist characteristics by etomidate use
Patients who received etomidate were older and had higher rates of diabetes, CAD, atrial fibrillation, peripheral vascular disease, and CKD (Table 1). These patients also had lower rates of beta blocker, insulin, and digoxin use. Steroid use was significantly lower in the etomidate group compared with the patients who did not receive etomidate (8.6% vs 18.7%; STD –29.7%).
In the non-etomidate group, propofol was used intraoperatively in 96.7% of patients. Of patients who received etomidate, 44% received etomidate alone whereas 53.5% received both etomidate and propofol in the intraoperative period. Patients who received etomidate were more frequently emergency cases (14.6% vs 7.4%; STD 23.2%) and inpatient admissions (51.1% vs 39.1%; STD 24.3%). They received more total fluid volume (2250 ml [IQR, 1250 to 3750] vs 1500 ml [IQR, 825 to 2750]; STD 29.1%) and PRBC units (0.31 (1.02) units vs 0.16 (0.77) units; STD 16.6%). Surgical complexity (by RVUs) was higher in the etomidate group compared with the non-etomidate group (16.7 units [IQR, 10.5 to 21.8] vs 14.1 units [IQR, 7.2 to 20.8]; STD 14.9%). The etomidate group underwent more orthopaedic (23.1% vs 18.7%; STD 10.8) and vascular surgeries (22.4% vs 10.8%; STD 31.6%) and fewer thoracic surgeries (7.0% vs 11.8%; STD –16.5%). Overall, anaesthesiologists managed 51 (IQR, 23 to 112) HF patients undergoing noncardiac surgery; median anaesthesiologist experience was higher among anaesthesiologists who used etomidate in at least one patient (n=248; 61 HF patients [IQR, 29 to 119]) compared with anaesthesiologists who never used etomidate (n=46; 21 HF patients [IQR, 12 to 36], STD 94.2%) (Table 2).
Table 2.
Anaesthesiologist heart failure case volume stratified by etomidate use. IQR, inter-quartile range.
| Descriptor | Total (N=294) | Etomidate never used in HF cases (N=46) | Etomidate used in ≥1 HF case (N=248) | Standardised difference (%) |
|---|---|---|---|---|
| Heart failure cases, median (IQR) | 51 (23, 112) | 21 (12, 36) | 61 (29, 119) | 94.2 |
Unadjusted outcomes
The crude rate of in-hospital death was higher in the etomidate group compared with the group who did not receive etomidate (4.0% vs 2.3%; RD 1.7%, P<0.0001). Crude 30-day mortality was also higher in the etomidate group compared with the non-etomidate group (6.7% vs 3.5%, RD 3.2%; P<0.0001).
Assessment of the instrumental variable
The stage 1 Wald F-statistic was 1855.0 (P<0.0001) consistent with a strong instrumental variable that was highly predictive of observed etomidate use. After application of the instrumental variable, there was improved balance in the rates of CAD, atrial fibrillation, peripheral vascular disease, and CKD across quartiles of anaesthesiologist preference for etomidate (Table 3). However, imbalances remained in the rates of diabetes and in the use of insulin, beta blocker, digoxin, and steroids.
Table 3.
Patient and procedural characteristics of cases stratified by groups of increasing anaesthesiologist etomidate use. ∗Total fluid volume defined as the volumes of crystalloid plus one-and-a-half times colloid administered intraoperative exclusive of PRBCs. All comorbidities are within 1 yr of procedure date. All medications are prescriptions within 30 days of procedure except steroids (1 yr before). †Vasopressors in milligrams norepinephrine equivalents = total amount epinephrine + total amount norepinephrine + (total amount phenylephrine/10) + (total amount dopamine/weight [kg]/2). CAD, coronary artery disease; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CCI, Charlson Comorbidity Index; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; MAC, minimal alveolar concentration; PRBC, packed red blood cells; RVU, relative value unit of main procedure; sd, standard deviation.
| Descriptor | Etomidate use by anaesthesiologist (%) |
|||
|---|---|---|---|---|
| Quartile 1 0 to 4.7 (N=4721) |
Quartile 2>4.7 to 11.1 (N=5029) | Quartile 3>11.1 to 20.4 (N=5027) | Quartile 4>20.4 to 66.7 (N=4937) | |
| Patient characteristics | ||||
| Age (yr), mean (range) | 66.2 (18–100) | 67.3 (18–102) | 67.2 (18–107) | 69.1 (18–102) |
| BMI (kg m−2), mean (sd) | 29.4 (7.9) | 29.3 (7.6) | 29.9 (7.9) | 29.5 (7.8) |
| Female sex, no. (%) | 1993 (42.2) | 2249 (44.7) | 2220 (44.2) | 2283 (46.2) |
| ASA physical status, median (IQR) | 3 (3, 3) | 3 (3, 3) | 3 (3, 3) | 3 (3, 3) |
| Hypertension, no. (%) | 3652 (77.4) | 3830 (76.2) | 3693 (73.5) | 3617 (73.3) |
| Hyperlipidaemia, no. (%) | 2848 (60.3) | 3017 (60.0) | 3043 (60.5) | 2955 (59.9) |
| Diabetes mellitus, no. (%) | 1776 (37.6) | 1903 (37.8) | 2176 (43.3) | 2327 (47.1) |
| On insulin, no. (%) | 1365 (28.9) | 1294 (25.7) | 992 (19.7) | 821 (16.6) |
| CAD, no. (%) | 2426 (51.4) | 2634 (52.4) | 2521 (50.2) | 2701 (54.7) |
| Atrial fibrillation, no. (%) | 1783 (37.8) | 1925 (38.3) | 2136 (42.5) | 1945 (39.4) |
| PVD, no. (%) | 901 (19.1) | 937 (18.6) | 797 (15.9) | 1116 (22.6) |
| Ischaemic stroke, no. (%) | 438 (9.3) | 439 (8.7) | 307 (6.1) | 262 (5.3) |
| COPD, no. (%) | 1094 (23.2) | 1125 (22.4) | 1197 (23.8) | 1220 (24.7) |
| CKD, no. (%) | 1526 (32.3) | 1634 (32.5) | 1789 (35.6) | 1774 (35.9) |
| CCI, median (IQR) | 5 (3, 8) | 5 (3,7) | 5 (3,7) | 5 (3,7) |
| Smoking, no. (%) | 1303 (27.6) | 1270 (25.3) | 1150 (22.9) | 866 (17.5) |
| Beta blocker, no. (%) | 2759 (58.4) | 2711 (53.9) | 1701 (33.8) | 1022 (20.7) |
| ACE inhibitor, no. (%) | 1725 (36.5) | 1881 (37.4) | 1930 (38.4) | 1821 (36.9) |
| Hydralazine/nitrates, no. (%) | 859 (18.2) | 751 (14.9) | 597 (11.9) | 491 (10.0) |
| Aldosterone antagonists, no. (%) | 451 (9.6) | 416 (8.3) | 398 (7.9) | 322 (6.5) |
| Digoxin, no. (%) | 422 (8.9) | 457 (9.1) | 351 (7.0) | 268 (5.4) |
| Steroid use, no. (%) | 1259 (26.7) | 1059 (21.1) | 687 (13.7) | 397 (8.0) |
| Antiplatelet use, no. (%) | 2512 (53.2) | 2430 (48.3) | 1965 (39.1) | 1704 (34.5) |
| Anticoagulant use, no. (%) | 1234 (26.1) | 1357 (27.0) | 1266 (25.2) | 945 (19.1) |
| Ejection fraction, mean (sd) no. (%) | 58.5 (14.3) | 57.3 (14.8) | 54.3 (15.1) | 51.7 (15.1) |
| EF >40% | 2133 (45.2) | 2058 (40.9) | 1810 (36.0) | 1541 (31.2) |
| EF 20–40% | 316 (6.7) | 370 (7.4) | 435 (8.7) | 481 (9.7) |
| EF <20% | 15 (0.3) | 25 (0.5) | 36 (0.7) | 41 (0.8) |
| Missing | 2257 (47.8) | 2576 (51.2) | 2746 (54.6) | 2874 (58.2) |
| Procedural characteristics | ||||
| Emergency status, no. (%) | 258 (5.5) | 340 (6.8) | 468 (9.3) | 598 (12.1) |
| Admission type, no. (%) | ||||
| Ambulatory | 669 (14.2) | 612 (12.2) | 808 (16.1) | 723 (14.6) |
| Same-day admit | 2083 (44.1) | 2372 (47.2) | 2282 (45.4) | 2123 (43.0) |
| Inpatient | 1969 (41.7) | 2045 (40.7) | 1937 (38.5) | 2091 (42.4) |
| Neuraxial anaesthesia, no. (%) | 363 (7.7) | 253 (5.0) | 109 (2.2) | 68 (1.4) |
| Age adjusted MAC, mean (sd) | 0.80 (0.32) | 0.83 (0.34) | 0.85 (0.37) | 0.86 (0.36) |
| Total fluid volume,∗ median (IQR) | 1000 (550, 2000) | 1500 (750, 2500) | 2000 (1000, 3250) | 2500 (1250, 3750) |
| Estimated blood loss (ml), median (IQR) | 0 (0, 150) | 0 (0, 150) | 0 (0, 30) | 0 (0, 0) |
| Urine output (ml), median (IQR) | 0 (0, 290) | 0 (0, 290) | 0 (0, 150) | 0 (0, 0) |
| PRBC units, mean (sd) | 0.14 (0.67) | 0.15 (0.60) | 0.19 (1.02) | 0.25 (0.88) |
| Total vasopressors, mg norepinephrine equivalents,† median (IQR) | 0.15 (0.02, 0.45) | 0.11 (0.01, 0.40) | 0.09 (0.01, 0.42) | 0.07 (0, 0.35) |
| Duration of surgery, min mean (sd) | 167.4 (115.4) | 172.9 (109.4) | 170.7 (115.0) | 160.3 (100.7) |
| Work RVU, median (IQR) | 14.1 (6.8, 20.9) | 14.9 (8.0, 20.9) | 13.6 (7.2, 20.4) | 15.3 (7.5, 20.9) |
| Service, no. (%) | ||||
| Orthopaedic surgery | 636 (13.5) | 1119 (22.3) | 981 (19.5) | 1082 (21.9) |
| Vascular surgery | 544 (11.5) | 603 (12.0) | 391 (7.8) | 911 (18.5) |
| Thoracic surgery | 698 (14.8) | 434 (8.6) | 476 (9.5) | 578 (11.7) |
| Urology | 327 (6.9) | 493 (9.8) | 367 (7.3) | 373 (7.6) |
| General surgery | 528 (11.2) | 349 (6.9) | 308 (6.1) | 193 (3.9) |
| Anaesthesiology | 450 (9.5) | 290 (5.8) | 421 (8.4) | 72 (1.5) |
| Neurosurgery | 326 (6.9) | 319 (6.3) | 293 (5.8) | 234 (4.7) |
| Transplant | 183 (3.9) | 241 (4.8) | 390 (7.8) | 249 (5.0) |
| Acute care surgery | 236 (5.0) | 257 (5.1) | 301 (6.0) | 197 (4.0) |
| Gynaecology | 106 (2.3) | 177 (3.5) | 111 (2.2) | 96 (1.9) |
| Surgical oncology | 130 (2.8) | 118 (2.4) | 116 (2.3) | 73 (1.5) |
| Plastic surgery | 54 (1.1) | 108 (2.2) | 85 (1.7) | 89 (1.8) |
| Radiology | 79 (1.7) | 117 (2.3) | 41 (0.8) | 4 (0.1) |
| ENT | 17 (0.4) | 38 (0.8) | 80 (1.6) | 72 (1.5) |
| Colorectal | 16 (0.3) | 32 (0.6) | 51 (1.0) | 60 (1.2) |
| Burn | 35 (0.7) | 64 (1.3) | 28 (0.6) | 7 (0.1) |
| Other | 260 (5.5) | 127 (2.5) | 100 (2.0) | 92 (1.9) |
| Missing | 96 (2.0) | 143 (2.8) | 487 (9.7) | 555 (11.2) |
Among procedural characteristics, there was improved balance with inpatient admission rates and RVUs but persistent imbalances with emergency status and total fluid volume. Anaesthesiologist experience also remained imbalanced ranging from 36 patients (IQR, 19 to 69) in the lowest etomidate use group to 64 patients (IQR, 35 to 126) in the highest use group (Table 4).
Table 4.
Anaesthesiologist heart failure case volume stratified by groups of increasing anaesthesiologist etomidate use.
| Descriptor | Etomidate use by anaesthesiologist (%) |
|||
|---|---|---|---|---|
| Quartile 1 0 to 4.7 (N=86) |
Quartile 2>4.7 to 11.1 (N=80) | Quartile 3>11.1 to 20.4 (N=68) | Quartile 4>20.4 to 66.7 (N=60) | |
| Heart failure cases, median (IQR) | 36 (19, 69) | 51 (22,92) | 66 (25, 132) | 64 (35, 126) |
IQR, inter-quartile range.
Instrumental variable outcomes
Adjusted instrumental variable analysis demonstrated no significant difference in the rate of in-hospital mortality associated with etomidate compared with non-etomidate use (adjusted RD [aRD] –0.2% [–2.4%–1.9%]; P=0.83). In addition, there was no significant difference in the rate of 30 day mortality, as well (aRD 0.2% [–2.5%–2.9%]; P=0.90) (Table 5).
Table 5.
Primary outcomes – unadjusted and instrumental variable-based outcomes. Adjusted instrumental variable model includes hospital site and all covariates in Table 1 except ejection fraction, intraoperative agents, estimated blood loss, urine output, vasopressors, and service type. CI, confidence interval.
| Outcomes | Unadjusted outcomes |
Adjusted instrumental variable analysis |
||||
|---|---|---|---|---|---|---|
| No etomidate | Etomidate | Risk difference | P value | Risk difference (95% CI) | P value | |
| In-hospital mortality, % | 2.3 | 4.0 | 1.7 | <0.0001 | –0.2 (–2.4 to 1.9) | 0.83 |
| 30-day mortality, % | 3.5 | 6.7 | 3.2 | <0.0001 | 0.2 (–2.5 to 2.9) | 0.90 |
Sensitivity analyses
Ten percent (2200/21 914) of the Study Population had missing values. The characteristics of patients with missing data included lower rates of hypertension, hyperlipidaemia, and diabetes, and higher rates of atrial fibrillation and stroke and use of insulin, beta blocker, digoxin, and anticoagulants (Supplementary Table S3). Patients with missing data had lower intraoperative MAC and received less fluids. Notably, patients with and without missing data had similar rates of etomidate use (14.3 vs 14.6%, respectively; STD, 0.85%), LVEF category, and median anaesthesiologist experience (57 patients [IQR, 28 to 117] vs 51 patients [IQR, 23 to 112], respectively; STD, 6.6%) (Supplementary Table S4). A repeat adjusted instrumental variable analysis using the Study Population (N=21 914) excluding the six covariates associated with missing data showed no significant differences in mortality between the etomidate and non-etomidate group (in-hospital mortality aRD 0.8% [–1.4%–2.9%]; P=0.49 and 30 day mortality aRD 1.2% [–1.5%–3.8%]; P=0.39) (Supplementary Table S5).
Overall, 53% (10 453/19 714) of patients had missing values for LVEF. The characteristics of these patients included older age and lower rates of hypertension, CAD, atrial fibrillation, ischaemic stroke, and CKD (Supplementary Tables S6 and S7). Patients with missing values for LVEF also had lower CCI scores, less frequent use of insulin, beta blocker, ACE inhibitors/angiotensin receptor blockers, aldosterone antagonists, digoxin, steroids, antiplatelets, and anticoagulants, and were less frequently inpatients. Use of etomidate was similar in both groups (14.3% vs 14.4%, respectively; STD, 0.3%). The addition of LVEF as a categorical covariate to the adjusted instrumental variable analysis also did not change the results for in-hospital mortality (aRD –0.3% [–2.4%–1.9%]; P=0.79) and 30 day mortality (aRD 0.04% [–2.7%–2.7%]; P=0.98).
Adjusted instrumental variable analyses of subgroups using alternative definitions of symptomatic/asymptomatic HF including ejection fraction ≤40% and ICD-9/10 code-defined systolic HF with treatment did not show a statistically significant association between etomidate and in-hospital and 30 day mortality. An analysis of cases occurring between 2012 and 2017 also did not show a statistically significant association between etomidate and the primary outcomes. Anaesthesiologist preference for etomidate declined progressively over time with median use decreasing from 13.3% (IQR, 6.2 to 26.7; years 2007–9) to 8.0% (IQR, 3.8 to 15.5; years 2014–15) (Supplementary Table S8, Supplementary Fig. S2). The median total institutional case volume among the 294 anaesthesiologists over the study period was 908 cases (IQR, 433 to 2041) (Supplementary Table S9). Total institutional case volume, similar to HF case volume, was higher among anaesthesiologists who had used etomidate in at least one patient compared with anaesthesiologists who never used etomidate (1034 total cases [IQR, 556 to 2335] vs 395 total cases [IQR, 189 to 700], respectively). As with HF case volume, total institutional case volume also increased with higher preference for etomidate and ranged from a median of 685 total cases (IQR, 350 to 1156) in the lowest etomidate use group to 1480 total cases (IQR, 896 to 3122) in the highest use group (Supplementary Table S10).
Discussion
Our study provides the largest, multicentre analysis to date examining provider variability in the use of etomidate and its impact on symptomatic/asymptomatic HF patients undergoing noncardiac surgery. We found that anaesthesiologists' preferences for etomidate varied widely ranging from 0% to 67% of individual practitioner's HF caseloads. In addition, we demonstrated with an adjusted instrumental variable analysis that there was no evidence of increased in-hospital or 30 day mortality associated with the use of etomidate.
Provider variability in the use of etomidate has not been previously assessed. We observed a large degree of provider variability with some anaesthesiologists never using etomidate and others using etomidate in up to two-thirds of their noncardiac patients with symptomatic/asymptomatic HF patients. This heterogeneity in practice pattern is unsurprising given the lack of consensus among anaesthesiologists regarding the risk/benefit ratio of etomidate in the perioperative setting. Interestingly, anaesthesiologists with higher preferences for etomidate were more experienced (greater HF and total case volume) than anaesthesiologists with lower preferences for etomidate. Positive experiences with etomidate accrued over an anaesthesiologist's cumulative practice may have strengthened these preferences.
Much of the hesitation in the use of etomidate stems from concerns over its impact on mortality.2,4, 5, 6,10 In a retrospective study of ASA 3/4 patients undergoing noncardiac surgery, Komatsu and colleagues10 propensity-matched 2144 patients who received etomidate with 5233 patients who received propofol and found a 4.0% RD in 30 day mortality (6.5% [etomidate group] vs 2.5% [propofol group]). Although they matched on 17 patient and procedural characteristics, variables such as HF, LVEF, and CKD were not incorporated into the propensity score. In addition, in-hospital mortality was not assessed, an important outcome given that the adrenal suppression effect of etomidate appears to diminish by postoperative day 22 rendering effects on 30 day mortality less biologically plausible. We found a 3.2% crude RD for 30 day mortality between the etomidate and non-etomidate groups that attenuated to 0.2% after adjusted instrumental variable analysis, suggesting the presence of significant treatment selection bias contributing to the excess mortality observed in our unadjusted comparison.
Wagner and colleagues11 assessed the impact of etomidate on in-hospital mortality in 3127 cardiac surgical patients using both logistic regression and propensity score modelling. They reported a crude RD of –1.2% (3.0% [etomidate group] vs 4.2% [no etomidate group]); after logistic regression adjustment for 17 characteristics including ACE inhibitor use, type of surgery, LVEF, HF, and preoperative creatinine, they found that etomidate was not associated with a higher odds of in-hospital mortality (adjusted odds ratio=0.75; 95% CI, 0.45 to 1.24). Patients with HF composed a minority of this study population (17–23%). In our population of noncardiac surgery patients, we found an in-hospital mortality RD of 1.7% (4.0% [etomidate group] vs 2.3% [no etomidate group]) that, similar to our 30 day mortality results, also attenuated to –0.2%.
Our findings suggest that etomidate is safe for induction of anaesthesia in patients with symptomatic/asymptomatic HF with significant comorbidity burden in elective/urgent settings throughout a diversity of noncardiac surgical procedures. These results may easily extend to situations where induction of anaesthesia is required for brief procedures (e.g. cardioversions for atrial fibrillation) and for non-procedural intubations (e.g. urgent intubations for respiratory distress).
Although a large and multicentre study, there are several important limitations to our study. Physician preference was an imperfect instrumental variable as it did not achieve balance of all covariates across the quartiles of etomidate use. The persistence of imbalances after application of the instrument suggests that complete pseudo-randomisation may not have been achieved, which could result in the continued presence of unmeasured confounders and residual bias. Our approach adjusted for multiple important confounders in both models of the two-stage regression, but it is nevertheless possible that residual bias may exist. ICD-9/10-CM definitions of symptomatic/asymptomatic HF may be subject to coding errors and misclassification. The ICD-9/10-CM definitions of HF utilised included more than one mechanism (e.g. reduced and preserved ejection fraction); however, we did not find a statistically significant association between etomidate and mortality in sensitivity analyses of systolic HF-only subgroups.
Even though the etomidate and non-etomidate groups were clearly delineated, propofol and ketamine were used across both groups creating heterogeneity in the comparisons. However, propofol, the most commonly used agent, is not associated with clinically significant adrenocortical suppression32; as such, the overlap in use of intraoperative propofol across groups should not impact our analysis of the effect of exposure to etomidate. Notably, there are few data assessing the effects of ketamine on adrenocortical activity in humans, but the proportion of patients in the cohort exposed to ketamine was small (<5%). Causes of death could not be ascertained. Patients with chronic adrenal insufficiency or pre-existing hypothalamic–pituitary–adrenal axis dysfunction were not specifically identified, but patients with prescriptions for steroids with possible secondary adrenal insufficiency were able to be captured and incorporated into the analysis. Similarly, patients with sepsis were not specifically investigated. Finally, emergency cases only constituted 8% of the cohort limiting extension of our findings to this subgroup.
Prospective studies of etomidate in the perioperative period have been performed but are limited in their ability to detect differences in mortality because of the sample size.2,12, 13, 14 Given the small mortality effect sizes in our study and in the literature, a large RCT would be necessary to detect a difference between the treatment and control groups, underscoring the importance of harnessing the power available in retrospective datasets and developing methods such as instrumental variables to minimise the biases inherent in these analyses. Future studies investigating impact of etomidate use in the perioperative setting specifically in patients with sepsis and those presenting for emergency surgery are needed.
In this large, two-centre study, we found substantial variability in anaesthesiologists' preference for etomidate use in patients with symptomatic/asymptomatic HF undergoing noncardiac surgery. There was no association between etomidate and in-hospital or 30-day mortality. Etomidate should be considered an acceptable option for induction of general anaesthesia in this patient population.
Funding
National Institutes of Health (T32-GM007592) (MC). There was funding for projects outside of this submitted work from the National Heart, Lung, and Blood Institute (1K23HL144907) (JS).
Authors' contributions
Study conception and design: MC, CS, RY
Acquisition of data: PS, DR
Drafting of the article: MC
Final approval of the version to be published: MC, PS, DR, YZ, TZ, JS, TH, CS, ME, RY
All authors were involved in the analysis and interpretation of data and in critical revisions for intellectual content; approved final version to be published; and agreed to be accountable for all aspects of the work.
Declarations of interest
MC is funded by Medtronic outside the submitted work. JS is a consultant for Philips Healthcare outside the submitted work. TH is a consultant for GlaxoSmithKline and for Eli Lily. ME reports a patent for calabadion, a reversal agent for anaesthetics. The other authors do not report any relevant conflicts of interest.
Acknowledgements
The authors are grateful to Linda Valsdottir.
Handling editor: Michael Avidan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.06.059.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vinclair M., Broux C., Faure P. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–719. doi: 10.1007/s00134-007-0970-y. [DOI] [PubMed] [Google Scholar]
- 2.Morel J., Salard M., Castelain C. Haemodynamic consequences of etomidate administration in elective cardiac surgery: a randomized double-blinded study. Br J Anaesth. 2011;107:503–509. doi: 10.1093/bja/aer169. [DOI] [PubMed] [Google Scholar]
- 3.Wagner R.L., White P.F., Kan P.B., Rosenthal M.H., Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–1421. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- 4.Chan C.M., Mitchell A.L., Shorr A.F. Etomidate is associated with mortality and adrenal insufficiency in sepsis: a meta-analysis. Crit Care Med. 2012;40:2945–2953. doi: 10.1097/CCM.0b013e31825fec26. [DOI] [PubMed] [Google Scholar]
- 5.Gu W.J., Wang F., Tang L., Liu J.C. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. 2015;147:335–346. doi: 10.1378/chest.14-1012. [DOI] [PubMed] [Google Scholar]
- 6.Jabre P., Combes X., Lapostolle F. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300. doi: 10.1016/S0140-6736(09)60949-1. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group M., Mozaffarian D., Benjamin E.J. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich P.A., Albert N.M., Allen L.A. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammill B.G., Curtis L.H., Bennett-Guerrero E. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108:559–567. doi: 10.1097/ALN.0b013e31816725ef. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu R., You J., Mascha E.J., Sessler D.I., Kasuya Y., Turan A. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013;117:1329–1337. doi: 10.1213/ANE.0b013e318299a516. [DOI] [PubMed] [Google Scholar]
- 11.Wagner C.E., Bick J.S., Johnson D. Etomidate use and postoperative outcomes among cardiac surgery patients. Anesthesiology. 2014;120:579–589. doi: 10.1097/ALN.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basciani R.M., Rindlisbacher A., Begert E. Anaesthetic induction with etomidate in cardiac surgery: a randomised controlled trial. Eur J Anaesthesiol. 2016;33:417–424. doi: 10.1097/EJA.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 13.Hannam J.A., Mitchell S.J., Cumin D. Haemodynamic profiles of etomidate vs propofol for induction of anaesthesia: a randomised controlled trial in patients undergoing cardiac surgery. Br J Anaesth. 2019;122:198–205. doi: 10.1016/j.bja.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Iribarren J.L., Jimenez J.J., Hernandez D. Relative adrenal insufficiency and hemodynamic status in cardiopulmonary bypass surgery patients. A prospective cohort study. J Cardiothorac Surg. 2010;5:26. doi: 10.1186/1749-8090-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn G., Shehabi Y. Pro/con debate: is etomidate safe in hemodynamically unstable critically ill patients? Crit Care. 2012;16:227. doi: 10.1186/cc11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz J., Greenberg S. Etomidate is NOT a first-line induction agent in critically ill patients: primum non nocere–above all, do no harm. Crit Care Med. 2018;46:1495–1496. doi: 10.1097/CCM.0000000000003291. [DOI] [PubMed] [Google Scholar]
- 17.Lynde G.C., Jabaley C.S. Etomidate is a first-line induction agent in critically ill patients. Crit Care Med. 2018;46:1492–1494. doi: 10.1097/CCM.0000000000003290. [DOI] [PubMed] [Google Scholar]
- 18.Ray D.C., McKeown D.W. Etomidate for critically ill patients. Pro: yes we can use it. Eur J Anaesthesiol. 2012;29:506–510. doi: 10.1097/EJA.0b013e32835819b0. [DOI] [PubMed] [Google Scholar]
- 19.de la Grandville B., Arroyo D., Walder B. Etomidate for critically ill patients. Con: do you really want to weaken the frail? Eur J Anaesthesiol. 2012;29:511–514. doi: 10.1097/EJA.0b013e32835819ca. [DOI] [PubMed] [Google Scholar]
- 20.Birman-Deych E., Waterman A.D., Yan Y., Nilasena D.S., Radford M.J., Gage B.F. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 21.Saczynski J.S., Andrade S.E., Harrold L.R. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz S.E., Rothwell D.M., Chen Z., Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33:160–166. [PubMed] [Google Scholar]
- 23.So L., Evans D., Quan H. ICD-10 coding algorithms for defining comorbidities of acute myocardial infarction. BMC Health Serv Res. 2006;6:161. doi: 10.1186/1472-6963-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick N., Lacaille D., Bhole V., Avina-Zubieta J.A. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orbegozo Cortes D., Gamarano Barros T., Njimi H., Vincent J.L. Crystalloids versus colloids: exploring differences in fluid requirements by systematic review and meta-regression. Anesth Analg. 2015;120:389–402. doi: 10.1213/ANE.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 26.Merkow R.P., Bentrem D.J., Cohen M.E. Effect of cancer surgery complexity on short-term outcomes, risk predictions, and hospital comparisons. J Am Coll Surg. 2013;217:685–693. doi: 10.1016/j.jamcollsurg.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Rassen J.A., Brookhart M.A., Glynn R.J., Mittleman M.A., Schneeweiss S. Instrumental variables I: instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. J Clin Epidemiol. 2009;62:1226–1232. doi: 10.1016/j.jclinepi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rassen J.A., Brookhart M.A., Glynn R.J., Mittleman M.A., Schneeweiss S. Instrumental variables II: instrumental variable application-in 25 variations, the physician prescribing preference generally was strong and reduced covariate imbalance. J Clin Epidemiol. 2009;62:1233–1241. doi: 10.1016/j.jclinepi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brookhart M.A., Wang P.S., Solomon D.H., Schneeweiss S. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006;17:268–275. doi: 10.1097/01.ede.0000193606.58671.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh R.W., Vasaiwala S., Forman D.E. Instrumental variable analysis to compare effectiveness of stents in the extremely elderly. Circ Cardiovasc Qual Outcome. 2014;7:118–124. doi: 10.1161/CIRCOUTCOMES.113.000476. [DOI] [PubMed] [Google Scholar]
- 31.Secemsky E.A., Kirtane A., Bangalore S. Use and effectiveness of bivalirudin versus unfractionated heparin for percutaneous coronary intervention among patients with ST-segment elevation myocardial infarction in the United States. JACC Cardiovasc Interv. 2016;9:2376–2386. doi: 10.1016/j.jcin.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Fragen R.J., Weiss H.W., Molteni A. The effect of propofol on adrenocortical steroidogenesis: a comparative study with etomidate and thiopental. Anesthesiology. 1987;66:839–842. doi: 10.1097/00000542-198706000-00026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.