Abstract
Organisms have evolved various physiological mechanisms to cope with unfavourable environmental conditions. The ability to tolerate non-optimal thermal conditions can be substantially improved by acclimation. In this study, we examined how an early-life acclimation to different temperatures (19 °C, 25 °C and 29 °C) influences thermal reaction norms for energy stores in Drosophila adults. Our results show that acclimation temperature has a significant effect on the amount of stored fat and glycogen (and their relative changes) and the optimal temperature for their accumulation. Individuals acclimated to 19 °C had, on average, more energy reserves than flies that were initially maintained at 25 °C or 29 °C. In addition, acclimation caused a shift in optimal temperature for energy stores towards acclimation temperature. We also detected significant population differences in this response. The effect of acclimation on the optimal temperature for energy stores was more pronounced in flies from the temperate climate zone (Slovakia) than in individuals from the tropical zone (India). Overall, we found that the acclimation effect was stronger after acclimation to low (19 °C) than to high (29 °C) temperature. The observed sensitivity of thermal reaction norms for energy reserves to acclimation temperature can have important consequences for surviving periods of food scarcity, especially at suboptimal temperatures.
Subject terms: Ecophysiology, Fat metabolism
Introduction
Since all biological processes are influenced by temperature, any change in the thermal environment has a strong effect on the animal’s physiology1–3. Acclimation is a physiological response to environmental changes that reduces stress and/or improves organismal performance4,5. Thermal acclimation may encompass multiple, diverse responses, such as changes in membrane lipid composition (e.g. Ref.6,7), enzyme activities (e.g.8,9), expression of heat-shock proteins (e.g.10,11), or behavioural changes (e.g.12). Acclimation is usually the result of long-term exposure (days or weeks), as opposed to hardening, which is the physiological reaction to a brief exposure to a stressful environment13,14. According to Collier et al.15, acclimation responses occur in two phases. During the first, acute phase, various metabolic and physiological responses (including heat shock response) take place at the cellular and systemic level; the second, long-term phase involves substantial changes in transcriptome, proteome and metabolome (e.g.16–18). However, not all changes elicited by temperature, or any other environmental stimuli, are necessarily adaptive; some alterations are just a result of stressful conditions19. For example, prolonged exposure to low or high temperatures can cause cellular damage and apoptosis (e.g.20).
The relationship between temperature and a physiological process is typically described by a thermal performance curve, which is usually unimodal and often asymmetrical21–23. Thermal performance initially rises with temperature, reaches a maximum at the optimal temperature, and gradually decreases with a further temperature increase21–23. Both position and shape of the thermal performance curve can be altered by acclimation23,24. Theoretically, the beneficial effect of thermal acclimation can lead to an adaptive shift in the thermal performance (i.e. the optimal temperature) towards the acclimation temperature25. Although the adaptive character of acclimation is well-documented in the case of thermal tolerance (e.g.7,17,26–31), the evidence for beneficial effects of thermal acclimation on other fitness-related traits is often contradictory (e.g.4,32–37). According to Ayrinhac et al.27, the fact that acclimation might have opposite effects on different traits or physiological processes only demonstrates “the complexity and diversity of plastic responses”.
The animal’s ability to survive periods of food shortage depends strongly on the amount of stored fat and glycogen38. Under optimal conditions, the positive energy balance allows accumulation of energy stores, whereas suboptimal or stressful conditions lead to their reduction39–41. Previous studies in the fruit fly Drosophila melanogaster have shown that the fat and glycogen content is significantly influenced by temperature40,41. The relationship between temperature and the amount of stored lipids and glycogen can be described by a quadratic function with a maximum value reached at approximately 21°C41. At intermediate temperatures (15–27 °C), flies can build up energy stores, whereas exposure to lower or higher temperatures decreases the amount of stored fat and glycogen41. In the present work, we have tested whether early-life acclimation affects thermal reaction norms for energy reserves in D. melanogaster. We have used outbred flies from two populations originating from distinct climate zones: tropical (India) and temperate (Slovakia). Experimental individuals were acclimated during early life to three different temperatures (19, 25 and 29 °C), subsequently exposed as adults to eleven temperatures in the range of 11 °C to 31 °C, and examined for the amount of stored fat and glycogen. We have hypothesised that acclimation might significantly affect thermal reaction norms for energy stores. Based on the assumptions of the beneficial acclimation hypothesis4,25,42, acclimation might shift these reaction norms in accordance with the temperature of acclimation, i.e. flies acclimated to a lower temperature might have a lower optimal temperature for energy stores than individuals acclimated to a higher temperature (and vice versa). Finally, we have also examined whether temperature-induced changes in fat reserves are associated with underlying changes in the lipid droplets—fat storage organelles43,44.
Results
Acclimation temperature affects thermal reaction norms for fat reserves
To examine the effect of acclimation temperature on thermal reaction norms for energy reserves and to account for potential intraspecific differences, we used individuals from outbred, wild-caught populations of D. melanogaster from the temperate (Slovakia) and the tropical (India) climate zone. Since our previous study41 has shown that the relationship between temperature and the amount of stored fat and glycogen is best described by a quadratic function, we used multiple nonlinear (quadratic) regression to analyse the effects of population origin, the acclimation and test temperatures, and their interactions on the amount of stored fat. All the factors, including their interactions (except two three-way interactions), had a significant effect on both the absolute fat content and the relative changes in the fat content (ratio between the fat content measured after and before exposure to a given temperature) (Supplementary Tables S1, S2). Similar results were also obtained for the fat content normalised to protein content (Supplementary Tables S3, S4).
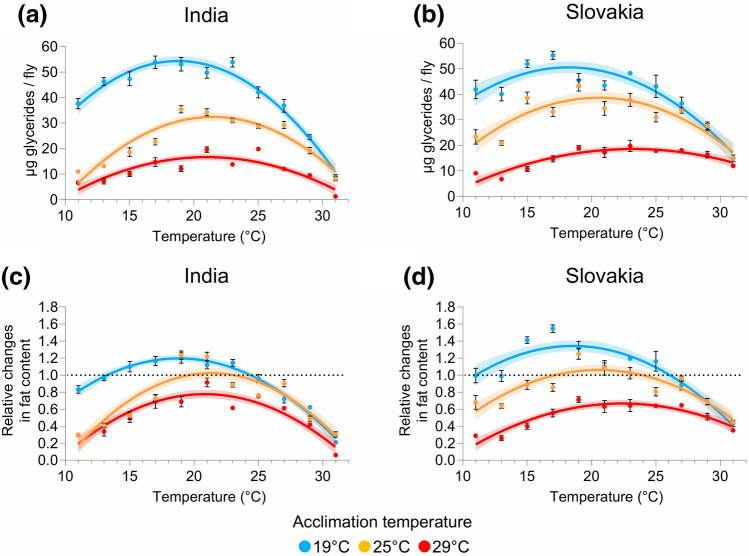
In order to find shared patterns and potential differences between the populations, we analysed the effect of acclimation and test temperatures on the fat content for each population separately (Supplementary Table S5). Overall, individuals from both populations that were reared at 19 °C before the exposure to different test temperatures had, on average, a higher amount of stored fat than flies that were initially maintained at higher temperatures (Fig. 1a,b; Supplementary Fig. S1). Consistently, the cold-acclimated flies had significantly higher estimated maximum value of fat content (Supplementary Fig. S2). Importantly, individuals from both populations that were acclimated to 19 °C increased their fat content when exposed to temperatures between 15 °C and 23/25 °C, whereas flies that were acclimated to 25 °C were able to accumulate fat only when exposed to a temperature around 19–21 °C (Figs. 1c,d; S3). In contrast, the amount of stored fat decreased at all experimental temperatures in individuals acclimated to 29 °C (Fig. 1c,d; Supplementary Fig. S3).
Figure 1.
Acclimation temperature affects thermal reaction norms for fat reserves. (a, b) Absolute fat content (µg glycerides per fly) in flies from India (a) and Slovakia (b). (c, d) Relative changes in the absolute fat content (calculated as a ratio between the final values and the mean of initial values) in flies from India (c) and Slovakia (d). Data points are mean values ± s.e.m.. Lines represent a quadratic fit to data with the 95% confidence band. For statistical analyses, see Supplementary Tables S1, S2, S5.
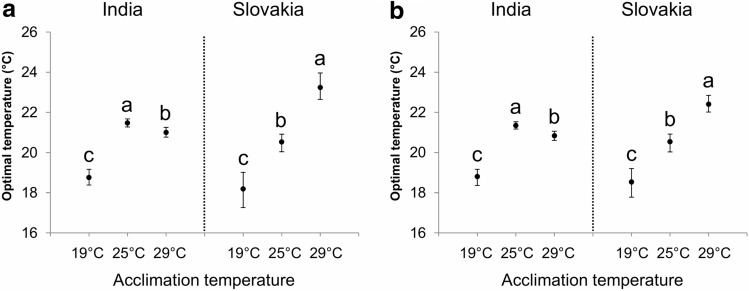
Based on the fitted curves for the absolute fat content and the relative changes in the fat content, we also calculated the optimal temperature (i.e. the temperature at which the given trait reaches its maximum value), 75% performance breadth (i.e. the thermal range over which the amount of stored fat was at least 75% of the maximum value), and individual parameters of each reaction norm (quadratic coefficient, slope, and intercept). In general, there were only small differences among the estimated optimal temperatures for the absolute fat content (Fig. 2a), the relative changes in the fat content (Fig. 2b), the absolute fat content normalised to protein (Supplementary Fig. S4a), or the relative changes in the fat content normalised to protein (Supplementary Fig. S4b). In the Indian population, flies that were initially kept at 25 °C had a higher optimal temperature for the amount of stored fat (the calculated optimal temperature for the absolute fat content/the relative changes in the fat content is 21.5 °C/21.3 °C) than flies that were acclimated to either 19 °C (18.8 °C/18.8 °C) or 29 °C (21.0 °C/20.8 °C) (Fig. 2). In contrast, flies from the Slovak population that were initially maintained at 29 °C had a higher optimal temperature (23.2 °C/22.4 °C) than flies acclimated to 25 °C (20.5 °C/20.5 °C); individuals acclimated to 19 °C had the lowest optimal temperature for the amount of stored fat (18.2 °C/18.5 °C) (Fig. 2). These results are consistent also with those calculated based on the fat content normalised to protein content (Supplementary Fig. S4). In the Indian population, we also found significant differences in the performance breadth; flies acclimated to low temperature had a broader performance breadth than those acclimated to higher temperatures (Supplementary Fig. S5). Such trend was relatively weak or absent in flies from the Slovak population. Consistent with the above-mentioned findings, we detected significant differences also in the parameters of individual thermal reaction norms (Supplementary Fig. S6, S7). Overall, there seemed to be a weak trend towards a less concave (i.e. less steep) thermal reaction norm at higher acclimation temperature (as indicated by the significantly lower absolute value of the quadratic coefficient—a) (Supplementary Figs. S6a,d, S7a,d).
Figure 2.
The optimal temperatures for fat reserves estimated based on (a) the absolute fat content (µg glycerides per fly), and (b) the relative changes in the absolute fat content. Error bars represent 95% confidence intervals. Values with different letters are significantly different from each other (α = 0.05).
Acclimation temperature has a significant impact on thermal reaction norms for glycogen stores
Using the same approach as for the fat reserves, we next examined the glycogen stores (Supplementary Tables S6–S10). The population, the acclimation and test temperatures, and three of their interactions (population × acclimation temperature; acclimation temperature × test temperature; acclimation temperature × (test temperature)2) significantly affected the absolute glycogen content and also the relative changes in the glycogen content (Supplementary Tables S6, S7). In the case of glycogen content normalised to the amount of proteins, we obtained similar outcome (Supplementary Tables S8, S9); however, two of the interactions (acclimation temperature × test temperature; acclimation temperature × (test temperature)2) were not statistically significant when we analysed the relative changes in the glycogen stores normalised to protein content (Supplementary Table S9).
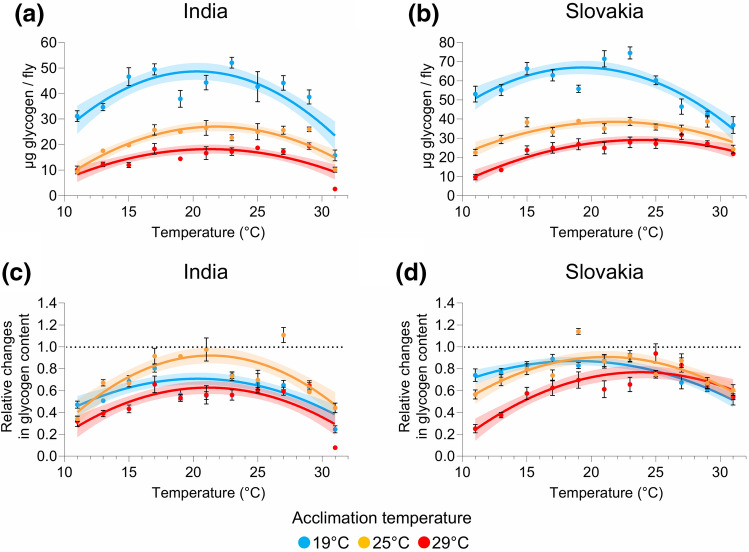
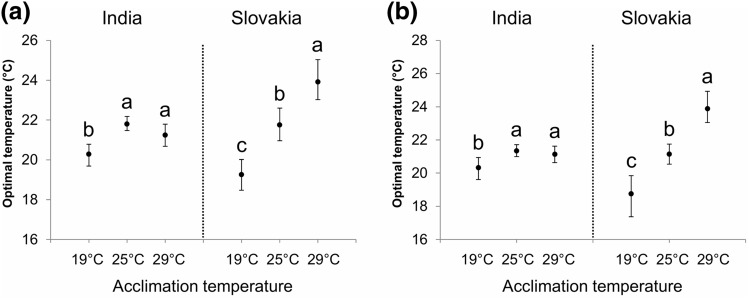
Similar to fat stores, the amount of glycogen (as well as the glycogen content normalised to protein content) (Fig. 3a, b; Supplementary Fig. S8), and its estimated maximum values (Supplementary Fig. S9) were inversely related to the acclimation temperature. In contrast, the glycogen content tended to decrease at all temperatures (Fig. 3c,d; Supplementary Fig. S10). Moreover, in comparison to fat stores, the acclimation temperature seemed to have a weaker (but still significant) effect on the relative changes in glycogen content (Fig. 3c,d; Supplementary Fig. S10). Nevertheless, at temperatures below 25 °C, the individuals that were initially kept at 29 °C seemed to deplete their glycogen reserves slightly more than the flies acclimated to 19 °C and 25 °C (Fig. 3c,d; Supplementary Fig. S10). The estimated optimal temperatures for the glycogen content and the relative changes in the glycogen content were very similar to those obtained based on the changes in fat reserves. Indian flies acclimated to 25 °C had comparable optimal temperatures for glycogen reserves (the calculated optimal temperature for the absolute glycogen content/the relative changes in the glycogen content: 21.8 °C/21.3 °C) as flies acclimated to 29 °C (21.2 °C/21.1 °C), whereas the estimated optimal temperatures for individuals acclimated to 19 °C was slightly lower (20.3 °C/20.3 °C) (Fig. 4). The effect of the acclimation temperature on the optimal temperature for glycogen reserves was again more pronounced in the Slovak population; there was a clear positive relation between the acclimation temperature and the optimal temperature (acclimation at 19 °C: optimal temperature = 19.3 °C/18.7 °C; acclimation at 25 °C: optimal temperature = 21.8 °C/21.1 °C; acclimation at 29 °C: optimal temperature = 23.9 °C/23.9 °C) (Fig. 4). Analogous results were obtained for the glycogen content normalised to protein content (Supplementary Fig. S11). Unlike the fat reserves, the acclimation temperature did not have substantial effect on the performance breadth (Supplementary Fig. S12). Finally, we did not find any clear trend in the estimated parameters of thermal reaction norms for glycogen stores in response to acclimation temperature (Supplementary Figs. S13, S14). Moreover, when the relative changes in glycogen stores were compared, the differences among the parameters were small or absent (Supplementary Figs. S13d–f, S14d–f).
Figure 3.
Acclimation temperature has a significant effect on thermal reaction norms for glycogen stores. (a, b) Glycogen content (µg glycogen per fly) in flies from India (a) and Slovakia (b). (c, d) Relative changes in the glycogen content (calculated as a ratio between the final values and the mean of initial values) in flies from India (c) and Slovakia (d). Data points are mean values ± s.e.m.. Lines represent a quadratic fit to data with the 95% confidence band. For statistical analyses, see Supplementary Tables S6, S7, S10.
Figure 4.
The optimal temperatures for glycogen stores estimated based on (a) the absolute glycogen content (µg glycogen per fly), and (b) the relative changes in the absolute glycogen content. Error bars represent 95% confidence intervals. Values with different letters are significantly different from each other (α = 0.05).
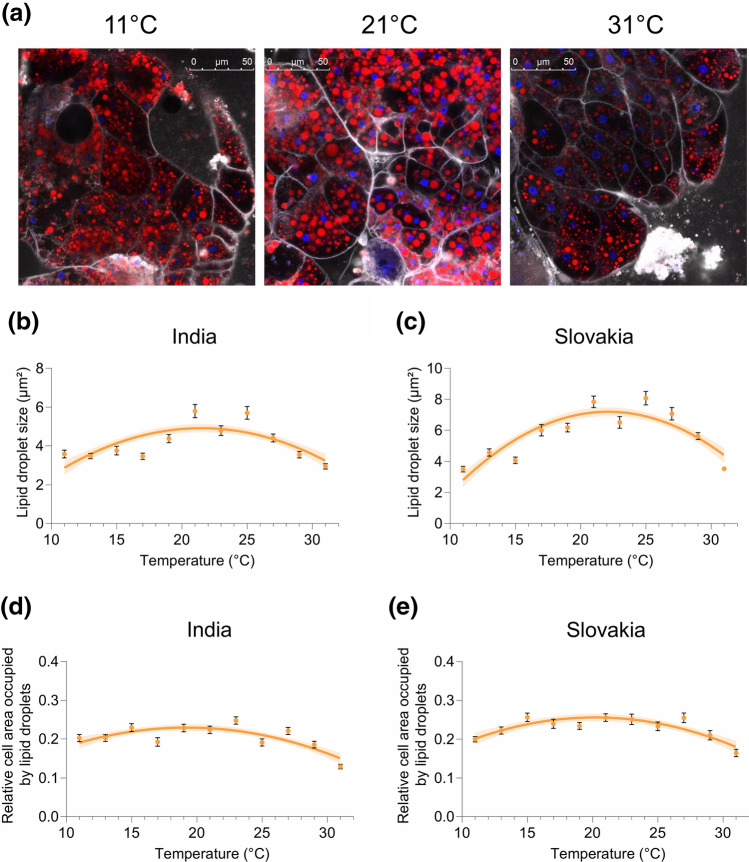
Temperature affects lipid droplet size and the relative cell area occupied by lipid droplets
In insects, the central storage deposit of lipids is the fat body45. Fat is stored in the fat body adipocytes in specialised cellular organelles—lipid droplets (e.g.43). Lipid droplets are sensitive to changes in metabolism and nutritional status44. We, therefore, investigated to what extent the ambient temperature affects their size and relative volume (as a proxy we used the relative cell area occupied by lipid droplets measured in a cross-section) in the fat body adipocytes. We found that the relationship between temperature and the lipid droplet size, and the relative cell area occupied by lipid droplets can be described by a unimodal (quadratic) function (Supplementary Table S11). Temperatures below 15 °C or above 29 °C decreased both the size (Fig. 5a–c) and the relative cell area occupied by lipid droplets, i.e. adipocytes contained smaller lipid droplets at these presumably non-optimal temperatures, and lipid droplets tended to occupy (especially at 31 °C) a smaller relative volume in a cell (Fig. 5a,d,e). Interestingly, we also detected significant intraspecific differences in the thermal reaction norms for lipid droplet size (indicated by significant interactions between ‘population’ and ‘test temperature’, and ‘population’ and ‘(test temperature)2’); in the Slovak population, the relationship between temperature and the lipid droplet size was described by a quadratic function that was more concave (i.e. more pronounced differences between the lipid droplet size at intermediate vs low/high temperatures) than in the Indian population (Fig. 5b,c; Supplementary Table S12). In contrast, we did not detect any significant intraspecific differences in the thermal reaction norms for the relative cell area occupied by lipid droplets (Fig. 5d,e; Supplementary Table S12). Finally, we observed that the changes in lipid droplet size and the relative cell area occupied by lipid droplets are consistent, to some extent, with the temperature-induced changes in the amount of stored fat (as indicated by significant positive correlations between these variables) (Supplementary Table S13).
Figure 5.
Temperature affects lipid droplet size and the relative cell area occupied by lipid droplets. (a) Confocal images of the fat body of Drosophila exposed to different temperatures; lipid droplets in red (Bodipy 493/503), cell membranes in white/grey (CellMask Deep Red), and DNA in blue (Hoechst 33342). (b, c) The relationship between temperature and lipid droplet size in flies from India (b) and Slovakia (c). (d, e) The effect of temperature on the relative cell area occupied by lipid droplets (a ratio between the area covered by lipid droplets and the total area of a given cell measured in a single optical section) in flies from India (d) and Slovakia (e). Data points are mean values ± s.e.m.. Lines represent a quadratic fit to data with the 95% confidence band. For statistical analyses, see Supplementary Tables S11, S12.
Discussion
In this study, we examined how acclimation temperature affects thermal reaction norms for energy reserves in Drosophila adults. In general, the accumulation of energy reserves can occur only when energy intake outweighs energy expenditure, and in comparison to other energy-requiring processes, such as growth or reproduction, the accumulation of energy stores is of relatively lower priority39. Thus, increased deposition of fat and glycogen might indicate optimal environmental conditions39. Conversely, their significant reduction may be a marker of stressful conditions39,40,46,47. The amount of stored fat and glycogen might, therefore, represent an important indicator of physiological state39. Our results show that the acclimation temperature affects the absolute fat and glycogen content (and their relative changes) and the optimal temperature for their accumulation. Overall, the shape of the thermal reaction norms for fat stores tended to be more sensitive to acclimation temperature than thermal reaction norms for glycogen reserves. Individuals from both examined populations that were exposed to low temperatures early in life had, on average, higher fat and glycogen content than flies acclimated to 25 °C or 29 °C. In addition, the estimated optimal temperature for both fat and glycogen was lower in flies that were acclimated to 19 °C than in flies that were acclimated to higher temperatures (25 °C or 29 °C). Such a shift in the optimal temperature towards acclimation temperature might be adaptive and in accordance with the beneficial acclimation hypothesis4,25. In contrast, the performance breadth was affected by acclimation only in the case of fat reserves in the Indian flies.
Although theory suggests that acclimation may alter different parameters of the thermal performance curve, optimal temperature and maximum performance are usually less sensitive to acclimation than performance breadth28,48. For example, Deere and Chown25 found only minor variation in optimal temperature for locomotor performance, and no consistent changes in maximum performance and performance breadth in response to acclimation in five mite species. Other studies in different arthropods did not find any association between acclimation and optimal temperature28,49–51. On the other hand, shifts in optimal temperature or changes in maximum performance and/or performance breadths in response to acclimation have been repeatedly documented in ectothermic vertebrates (e.g.52–54). The substantial interspecific variation in acclimation responses may result from differences in selection pressures, thermoregulatory abilities, intrinsic constraints, and environmental predictability (reviewed in55). In addition, acclimation can have a different effect on different physiological functions56. Since complex traits depend on numerous physiological processes that might be affected by acclimation in different ways, the overall effect of acclimation on these traits might be neutral or even negative56. Altogether, in comparison to other studies on various traits in arthropods25,28,49–51, our results show that thermal reaction norms for energy reserves in Drosophila are relatively sensitive to acclimation.
Interestingly, we also found significant intraspecific differences in the acclimation response. The effect of acclimation on the optimal temperature for energy stores was more prominent in the Slovak population. In the Indian population, individuals acclimated to 29 °C had lower or comparable optimal temperatures for fat and glycogen in comparison to flies acclimated to 25 °C; whereas the optimal temperature for energy reserves for flies from Slovakia that were acclimated to 29 °C was higher than for individuals initially maintained at 25 °C. Another difference was that the estimated thermal performance breadth for fat reserves in flies from the Indian population was significantly narrower at higher acclimation temperatures (25 °C and 29 °C) than at 19 °C; in flies from the Slovak population, such trend was relatively weak or absent. These findings might indicate that flies from Slovakia have greater acclimation ability than the Indian flies. According to Gabriel and Lynch57, organisms that experience higher levels of environmental variance between generations may have a greater capacity to acclimate in comparison to organisms from more stable environments. Consequently, organisms from the temperate climate zone, which experience seasonal temperature fluctuations, should exhibit higher thermal plasticity than organisms from the tropical zone (e.g.58–60). Moreover, this effect should be more pronounced in species that complete several generations per year (such as Drosophila) where offspring may encounter different thermal conditions than their parents did57,61. Experimental studies, however, provide mixed support for this idea (e.g.58,60–63). For example, temperate mayflies (Ephemeroptera) tend to have greater acclimation ability than tropical mayflies60. In contrast, Hoffmann and Watson62 did not detect any significant differences in the acclimation response to heat and cold in tropical and temperate populations of D. melanogaster and D. simulans. Similarly, Cooper et al.58 found no evidence for divergence in thermal acclimation capacity of fecundity in D. melanogaster from a highly versus less seasonal environment. Overall, it seems that the majority of studies in Drosophila do not provide strong evidence for intraspecific variation in acclimation capacities58,61–63. Because we examined flies from only two populations, further study with a larger number of populations is needed before making broader generalizations about differences in the acclimation response of energy stores in individuals from different climate zones.
In accordance with the previous studies40,41, we found that exposure to non-optimal temperatures can cause a substantial depletion of energy reserves, which might have a significant negative impact on survival in times of food scarcity40. Despite the observed shift in the optimal temperature, thermal acclimation did not seem to have an effect on the rate of depletion of energy stores at higher temperatures. In contrast, acclimation to 19 °C appeared to substantially diminish the reduction of fat reserves at low temperatures (< 15 °C). This finding might correspond with previous studies indicating that low-temperature tolerance is more responsive to acclimation than tolerance to high temperature (e.g.28,64,65). Moreover, cold-acclimated flies were able to accumulate additional fat at intermediate temperatures, whereas warm-acclimated (29 °C) individuals depleted their fat reserves even at presumably optimal thermal conditions. This observation supports the notion that whereas acclimation to non-stressful environments can have beneficial effects on fitness, exposure to stressful conditions (high temperature in this case) during early life might have a long-term negative impact on adult performance66. Although the underlying cause of this phenomenon is unclear, it may involve the increased rate of cell death of the fat body adipocytes40 or their reduced capacity to store lipids.
Fat reserves are stored in specialised cellular organelles, lipid droplets43 that occur predominantly in cells (adipocytes) of the fat body, which is the major organ of fat storage in insects45. Number and size of lipid droplets increase or decrease in response to nutrient availability44, and therefore, these characteristics are sometimes used as indicators of imbalance between energy intake and energy expenditure. Since the effect of temperature on the abundance and size of the lipid droplets has not been analysed yet, we tested whether and how these organelles respond to ambient temperature. In agreement with the results on the fat content, we found a unimodal relationship between temperature and both the lipid droplet size and the relative cell area occupied by lipid droplets. We found that exposure to either lower (˂ 15 °C) or higher (˃ 29 °C) temperatures decreases lipid droplet size, as well as the relative area occupied by lipid droplets in the fat body cells. Although our study did not test how thermal acclimation affects lipid droplets, it is evident that the size and abundance of these organelles respond to ambient temperature. Since the cellular mechanisms that regulate lipid droplet size and numbers are still poorly understood43,44, it is not clear whether the fat body cells store less lipids at non-optimal temperatures due to damage or inhibition of formation of lipid droplets, or simply due to a shifted balance between energy intake and expenditure. Moreover, it seems that these changes might not be solely responsible for the observed changes in the total fat content; there are very likely also other processes involved, such as changes in the number of fat body adipocytes40. However, the fact that temperature affects characteristics of lipid droplets might suggest that the temperature-driven changes in fat reserves are, at least partially, caused by the altered balance between energy input and output. As has been discussed previously41, there are several mechanisms that could potentially explain the temperature-driven changes in energy stores. For example, it has been observed that stressful temperatures induce apoptosis in Drosophila adipocytes, which can cause a decline in body fat40. Non-optimal conditions may also impose on organism additional energetic costs, for instance, due to increased proteolysis and protein synthesis, metabolic perturbations, damage repair etc.17,31,39,67. Other potential causes that can lead to increased utilisation of energy reserves include lower efficiency of energy acquisition68,69 and reduced assimilation efficiency (the relative amount of energy obtained per unit of consumed food)70. Previous studies indicate that the temperature-induced changes in energy stores in Drosophila might result from a combination of altered assimilation efficiency and stress-induced apoptosis40,41.
In summary, we found that early-life acclimation significantly affects the absolute amount of stored fat and glycogen. Moreover, we revealed that the optimal temperature for energy reserves positively correlates with the acclimation temperature. Our findings suggest that whereas cold-acclimation has a beneficial effect on overall energy balance at low temperatures, acclimation to high temperature (29 °C) has rather deleterious effects on physiological processes related to energy storage. Overall, our results demonstrate that thermal reaction norms for Drosophila energy reserves are sensitive to acclimation temperature, which can have a direct impact on survival and fitness, especially under nutritionally poor conditions.
Materials and methods
Fly populations and maintenance
We used two wild-caught populations of D. melanogaster from the tropical (collected in Mysore, Karnataka, India; July 2017), and the temperate (collected in Bratislava, Slovakia; October 2017) climate zone. Both laboratory populations were initiated from at least 300 freshly collected flies and were kept in the laboratory as outbred populations (at population sizes of approx. 1500–2000 adults) at 25 °C (12 h:12 h light–dark cycle, 60% relative humidity) for 21 (population from India)/ 18 (population from Slovakia) months prior to the experiments (for further details see32). All flies (including experimental individuals) were maintained on a standard Drosophila medium (6 g agar, 50 g yeast, 50 g sucrose, 70 g maize flour, 5.12 ml propionic acid and 1.3 g methylparaben per 1 L of medium).
Thermal treatments
To obtain experimental individuals for testing the effect of acclimation temperature on the amount of stored fat and glycogen, parental flies (approx. one week old; approx. 100 individuals) were allowed to lay eggs into vials (68 ml) containing standard medium during a three-hour period. Vials with intermediate egg density (approx. 150 eggs per 68 ml vial) were placed either in an incubator at 19 °C, 25 °C, or 29 °C. Males collected within 12 h after emergence were placed on the standard medium (approx. 30 males per 68 ml vial; three vials per acclimation temperature, test temperature, and population) and kept at the same temperature as under development for four days. This additional period was necessary to ensure complete removal of the larval fat body, which is still present in newly eclosed flies but depleted and replaced within few days after eclosion71,72. The four-day old experimental individuals were then exposed to one of eleven temperatures (11 °C, 13 °C, 15 °C, 17 °C, 19 °C, 21 °C, 23 °C, 25 °C, 27 °C 29 °C, 31 °C) at a 12 h:12 h light–dark cycle, and 60% relative humidity. Flies were maintained at their given experimental temperatures for eight days with daily food exchange. Samples for the lipid and glycogen quantification (five males per sample; six samples (two samples from each vial) per acclimation temperature, test temperature, and population) were collected at the beginning and at the end of this eight-day period41.
Fat and glycogen quantification
To determine the amount of fat (glycerides), glycogen and proteins, we used 4–6 samples (5 males per sample) per acclimation temperature, test temperature, and population. Samples were homogenised in 600 μL of 0.05% Tween-20 using TissueLyser II (Qiagen) at 30 s−1 for 1 min., heat-inactivated at 70 °C for 5 min and centrifuged at 3000g for 3 min73. Fat content was determined by a coupled colorimetric assay using the Triglycerides (liquid) assay (Randox, TR1697) as described in Gáliková et al.74. Fat content is expressed either as absolute fat content (μg glycerides per fly) or as fat content normalised to protein content (μg glycerides per mg protein)75. The quantification of proteins was performed using the Pierce Coomassie (Bradford) Protein Assay Kit (Thermo Scientific, 23200) according to the manufacturer's protocol. The amount of glycogen was measured by the GO kit (Sigma, GAGO)75 as described in Gáliková et al.74. Relative changes in fat or glycogen content were calculated as a ratio between the final value (measured at the end of the eight-day period of exposure to a given temperature) and the average of initial values (measured right before the exposure to a given temperature). Values > 1 signify accumulation of fat or glycogen, whereas values < 1 indicate depletion of energy reserves.
Lipid droplet analysis
Lipid droplets were analysed in males that were initially kept at 25 °C and then exposed to eleven different temperatures for eight days as described above. To stain lipid droplets in the fat body, 10–12 individuals were dissected in 1xPBS (phosphate-buffered saline), and their carcass was subsequently embedded in 1xPBS containing Bodipy 493/503 (1:100; Invitrogen, D3922) for visualising lipid droplets, Hoechst 33342 (1:100; Invitrogen, H3570) for nuclei staining, and CellMask Deep Red (1:100; Invitrogen, C10046) for plasma membrane staining. Samples were viewed on a confocal microscope Leica TCS SPE (Leica Microsystems, Germany). Lipid droplets were examined as described in Gáliková et al.76. In brief, confocal images were analysed using ImageJ v1.52p. In order to smooth the edges of lipid droplets, a Gaussian blur function (2 pixel radius) was applied to a single optical section. Next, a binary image was created by automatic thresholding; clustered lipid droplets were separated by applying watershed segmentation. Lipid droplet size was measured by a particle analyser (size (µm2): 0.0 → ∞; circularity: 0.01–1.0). For each test temperature and population, lipid droplets from 40–60 cells (originated from several different individuals, approx. 4–12) were analysed. The relative cell area occupied by lipid droplets was calculated as a ratio between the cell area covered by lipid droplets and the area of a given cell in a single optical section.
Statistical analyses
The previous study41 has shown that the relationship between temperature and energy reserves is best described by a quadratic function. Thus, to examine the effect of thermal acclimation and population origin on thermal reaction norms for the amount of fat and glycogen, or for the relative changes in their amounts, we used multiple nonlinear (polynomial—quadratic) regression analyses with two categorical factors (‘acclimation temperature’, ‘population’), two continuous factors (‘test temperature’, ‘(test temperature)2’), and seven interactions (see Tables S1–S4, S6–S9). In addition, we also performed similar analyses for each population separately. The optimal temperature (Topt) for each thermal reaction norm was calculated from the formula Topt = − b/2a, where b is the slope, and a the quadratic coefficient of the fitted quadratic function77. The mean values and confidence intervals for optimal temperatures, maximum values, performance breadths and coefficients of fitted quadratic functions were obtained by nonparametric bootstrapping: (1) we used random resampling with replacement, (2) fitted a quadratic function, (3) calculated the parameters, and (4) repeated this procedure 1000 times. To test whether the pairwise differences between the estimated parameters of the three acclimation groups are statistically significant (α = 0.05), we assessed bootstrap confidence intervals for each pairwise difference (if a given 95% confidence interval for differences between two groups includes zero, then the difference is not statistically significant at the 0.05 significance level)78. To examine the relationship between temperature and lipid droplet size and the relative cell area occupied by lipid droplets, we first fitted linear, quadratic, and cubic functions to our data (using mean values), and based on the Akaike information criterion (AICc), we chose the function with the lowest AICc value. In the case of lipid droplet size, the AICc value for a cubic function fitted to data obtained from the Slovak population was slightly lower than for a quadratic function, which has scored the lowest AICc value in the Indian population; however, for the sake of consistency and to facilitate comparisons, we used a quadratic function for both populations. The relationship between temperature and the relative cell area covered by lipid droplets was best described by a quadratic function in both populations. Next, we analysed lipid droplet size and the relative cell area occupied by lipid droplets by multiple nonlinear (quadratic) regression analyses with one categorical (‘population’), two continuous factors (‘test temperature’, ‘(test temperature)2’), and two interactions (‘population × test temperature’, ‘population × (test temperature)2’). Finally, we calculated Pearson's correlation coefficients (Pearson's r) between the fat content and the lipid droplet size, and the fat content and the relative cell area occupied by lipid droplets. All statistical analyses were performed using JMP v.15 (SAS, Raleigh, NC, USA) (multiple nonlinear regression analyses, curve fitting) and Excel (Microsoft) (nonparametric bootstrapping). Graphs were generated in GraphPad Prism 8 (GraphPad Software Inc.) and Excel (Microsoft).
Supplementary information
Acknowledgements
We would like to thank Dr. Ľubomír Vidlička for the flies collected in Slovakia and Prof. Ravi Parkash and Dr. B. E. Pradeep for their help in obtaining the flies from India. This work was supported by the Slovak Research and Development Agency under the Contract No. APVV-19-0196 and by the Slovak Academy of Sciences [MVTS-1219].
Author contributions
P.K. conceived the study; P.K., T.N.G. and M.G. designed the experiments; P.K. and M.G. performed the experiments; P.K. analysed the data; P.K., T.N.G. and M.G. wrote the manuscript.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78726-z.
References
- 1.Cossins AR, Bowler K. Temperature Biology of Animals. London: Chapman and Hall; 1987. [Google Scholar]
- 2.Hochachka PW, Somero GN. Biochemical Adaptation. Oxford: Oxford University Press; 2002. [Google Scholar]
- 3.Wilmer P, Stone G, Johnston I. Environmental Physiology of Animals. Oxford: Blackwell Publishing; 2005. [Google Scholar]
- 4.Huey RB, Berrigan D, Gilchrist GW, Herron JC. Testing the adaptive significance of acclimation: a strong inference approach. Am. Zool. 1999;39:323–336. doi: 10.1093/icb/39.2.323. [DOI] [Google Scholar]
- 5.IUPS Thermal Commission Glossary of terms for thermal physiology. Third edition. J. Therm. Biol. 2003;28:75–106. doi: 10.1016/S0306-4565(02)00055-4. [DOI] [Google Scholar]
- 6.Hazel JR. Influence of thermal acclimation on membrane lipid composition of rainbow trout liver. Am. J. Physiol. 1979;236:R91–101. doi: 10.1152/ajpregu.1979.236.1.R91. [DOI] [PubMed] [Google Scholar]
- 7.Overgaard J, et al. Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J. Insect Physiol. 2008;54:619. doi: 10.1016/j.jinsphys.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Moon TW, Hochachka PW. Temperature and enzyme activity in poikilotherms. Biochem. J. 1971;123:695–705. doi: 10.1042/bj1230695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storey KB, Storey JM. Biochemical strategies of overwintering in the gall gly larva, Eurosta solidaginis: effect of low temperature acclimation on the activities of enzymes of intermediary metabolism. J. Comp. Physiol. 1981;144:191–199. doi: 10.1007/BF00802757. [DOI] [Google Scholar]
- 10.Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J. Exp. Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- 11.Colinet H, Overgaard J, Com E, Sørensen JG. Proteomic profiling of thermal acclimation in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2013;43:352–365. doi: 10.1016/j.ibmb.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Lagerspetz KYH, Vainio LA. Thermal behaviour of crustaceans. Biol. Rev. 2006;81:237–258. doi: 10.1017/S1464793105006998. [DOI] [PubMed] [Google Scholar]
- 13.Bowler K. Acclimation, heat shock and hardening. J. Therm. Biol. 2005;30:125–130. doi: 10.1016/j.jtherbio.2004.09.001. [DOI] [Google Scholar]
- 14.Loeschcke V, Sørensen JG. Acclimation, heat shock and hardening—a response from evolutionary biology. J. Therm. Biol. 2005;30:255–257. doi: 10.1016/j.jtherbio.2004.12.005. [DOI] [Google Scholar]
- 15.Collier RJ, Baumgard LH, Zimbelman RB, Xiao Y. Heat stress: physiology of acclimation and adaptation. Anim. Front. 2019;9:12–19. doi: 10.1093/af/vfy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier RJ, et al. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle. J. Anim. Sci. 2006;84:E1–13. doi: 10.2527/2006.8413_supplE1x. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen TN, Kjeldal H, Schou MF, Nielsen JL. Proteomic data reveal a physiological basis for costs and benefits associated with thermal acclimation. J. Exp. Biol. 2016;219:969–976. doi: 10.1242/jeb.132696. [DOI] [PubMed] [Google Scholar]
- 18.MacMillan HA, et al. Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Sci. Rep. 2016;6:28999. doi: 10.1038/srep28999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. doi: 10.1111/j.1365-2435.2007.01283.x. [DOI] [Google Scholar]
- 20.Yao CL, Somero GN. The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and Mytilus californianus): DNA damage, membrane integrity, apoptosis and signaling pathways. J. Exp. Biol. 2012;215:4267–4277. doi: 10.1242/jeb.073577. [DOI] [PubMed] [Google Scholar]
- 21.Huey RB, Stevenson RD. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am. Zool. 1979;19:357–366. doi: 10.1093/icb/19.1.357. [DOI] [Google Scholar]
- 22.Huey RB, Kingsolver JG. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 1989;4:131–135. doi: 10.1016/0169-5347(89)90211-5. [DOI] [PubMed] [Google Scholar]
- 23.Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. J. Therm. Biol. 2002;27:249–268. doi: 10.1016/S0306-4565(01)00094-8. [DOI] [Google Scholar]
- 24.Schulte PM, Healy TM, Fangue NA. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 2011;51:691–702. doi: 10.1093/icb/icr097. [DOI] [PubMed] [Google Scholar]
- 25.Deere JA, Chown SL. Testing the beneficial acclimation hypothesis and its alternatives for locomotor performance. Am. Nat. 2006;168:630–644. doi: 10.1086/508026. [DOI] [PubMed] [Google Scholar]
- 26.Gibert P, Huey RB. Chill-coma temperature in Drosophila: effects of developmental temperature, latitude, and phylogeny. Physiol. Biochem. Zool. 2001;74:429–434. doi: 10.1086/320429. [DOI] [PubMed] [Google Scholar]
- 27.Ayrinhac A, et al. Cold adaptation in geographical populations of Drosophila melanogaster: phenotypic plasticity is more important than genetic variability. Funct. Ecol. 2004;18:700–706. doi: 10.1111/j.0269-8463.2004.00904.x. [DOI] [Google Scholar]
- 28.Lachenicht MW, Clusella-Trullas S, Boardman L, Le Roux C, Terblanche JS. Effects of acclimation temperature on thermal tolerance, locomotion performance and respiratory metabolism in Acheta domesticus L. (Orthoptera: Gryllidae) J. Insect Physiol. 2010;56:822–830. doi: 10.1016/j.jinsphys.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Colinet H, Hoffmann AA. Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster. Funct. Ecol. 2012;26:84–93. doi: 10.1111/j.1365-2435.2011.01898.x. [DOI] [Google Scholar]
- 30.Kellermann V, van Heerwaarden B, Sgrò CM. How important is thermal history? Evidence for lasting effects of developmental temperature on upper thermal limits in Drosophila melanogaster. Proc. Biol. Sci. 2017;31:20170447. doi: 10.1098/rspb.2017.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schou MF, Kristensen TN, Pedersen A, Karlsson BG, Loeschcke V, Malmendal A. Metabolic and functional characterization of effects of developmental temperature in Drosophila melanogaster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;312:R211–R222. doi: 10.1152/ajpregu.00268.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klepsatel P, Girish TN, Dircksen H, Gáliková M. Reproductive fitness of Drosophila is maximised by optimal developmental temperature. J. Exp. Biol. 2019;222:jeb202184. doi: 10.1242/jeb.202184. [DOI] [PubMed] [Google Scholar]
- 33.Frazier MR, Harrison JF, Kirkton SD, Roberts SP. Cold rearing improves cold-flight performance in Drosophila via changes in wing morphology. J. Exp. Biol. 2008;211:2116–2122. doi: 10.1242/jeb.019422. [DOI] [PubMed] [Google Scholar]
- 34.Kristensen TN, Hoffmann AA, Overgaard J, Sørensen JG, Hallas R, Loeschcke V. Costs and benefits of cold acclimation in field-released Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:216–221. doi: 10.1073/pnas.0708074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamudio KR, Huey RB, Crill WD. Bigger isn't always better: body size, developmental and parental temperature and male territorial success in Drosophila melanogaster. Anim. Behav. 1995;49:671–677. doi: 10.1016/0003-3472(95)80200-2. [DOI] [Google Scholar]
- 36.Zwaan BJ, Bijlsma R, Hoekstra RF. On the developmental theory of ageing. II. The effect of developmental temperature on longevity in relation to adult body size in D. melanogaster. Heredity. 1992;68:123–130. doi: 10.1038/hdy.1992.19. [DOI] [PubMed] [Google Scholar]
- 37.Gibert P, Huey RB, Gilchrist GW. Locomotor performance of Drosophila melanogaster: interactions among developmental and adult temperatures, age, and geography. Evolution. 2001;55:205–209. doi: 10.1111/j.0014-3820.2001.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 38.Rion S, Kawecki TJ. Evolutionary biology of starvation resistance: what we have learned from Drosophila. J. Evol. Biol. 2007;20:1655–1664. doi: 10.1111/j.1420-9101.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- 39.Sokolova IM. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 2013;53:597–608. doi: 10.1093/icb/ict028. [DOI] [PubMed] [Google Scholar]
- 40.Klepsatel P, Gáliková M, Xu Y, Kühnlein RP. Thermal stress depletes energy reserves in Drosophila. Sci. Rep. 2016;6:33667. doi: 10.1038/srep33667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klepsatel P, Wildridge D, Gáliková M. Temperature induces changes in Drosophila energy stores. Sci. Rep. 2019;9:5239. doi: 10.1038/s41598-019-41754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leroi AM, Bennett AF, Lenski RE. Temperature acclimation and competitive fitness: an experimental test of the beneficial acclimation assumption. Proc. Natl. Acad. Sci. USA. 1994;91:1917–1921. doi: 10.1073/pnas.91.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beller M, Thiel K, Thul PJ, Jäckle H. Lipid droplets: a dynamic organelle moves into focus. FEBS Lett. 2010;584:2176–2182. doi: 10.1016/j.febslet.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 2010;55:207–255. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giesy JP, Graney RL. Recent developments in and intercomparisons of acute and chronic bioassays and bioindicators. Hydrobiologia. 1989;188(189):21–60. doi: 10.1007/BF00027770. [DOI] [Google Scholar]
- 47.Smolders R, Bervoets L, De Coen W, Blust R. Cellular energy allocation in zebra mussels exposed along a pollution gradient: linking cellular effects to higher levels of biological organization. Environ. Pollut. 2004;129:99–112. doi: 10.1016/j.envpol.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 48.Angilletta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press; 2009. [Google Scholar]
- 49.Schuler MS, Cooper BS, Storm JJ, Sears MW, Angilletta MJ. Isopods failed to acclimate their thermal sensitivity of locomotor performance during predictable or stochastic cooling. PLoS ONE. 2011;6:e20905. doi: 10.1371/journal.pone.0020905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson LV, Heinrichs DE, Sinclair BJ. Paradoxical acclimation responses in the thermal performance of insect immunity. Oecologia. 2016;181:77–85. doi: 10.1007/s00442-015-3529-6. [DOI] [PubMed] [Google Scholar]
- 51.MacLean HJ, et al. Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:20180548. doi: 10.1098/rstb.2018.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson T, Bennett A. The thermal acclimation of burst escape performance in fish: an integrated study of molecular and cellular physiology and organismal performance. J. Exp. Biol. 1995;198:2165–2175. doi: 10.1242/jeb.198.10.2165. [DOI] [PubMed] [Google Scholar]
- 53.Seebacher F, Ducret V, Little AG, Adriaenssens B. Generalist-specialist trade-off during thermal acclimation. R. Soc. Open. Sci. 2015;2:140251. doi: 10.1098/rsos.140251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.da Silva CRB, Riginos C, Wilson RS. An intertidal fish shows thermal acclimation despite living in a rapidly fluctuating environment. J. Comp. Physiol. B. 2019;189:385–398. doi: 10.1007/s00360-019-01212-0. [DOI] [PubMed] [Google Scholar]
- 55.Kingsolver JG, Huey RB. Selection and evolution of morphological and physiological plasticity in thermally varying environments. Am. Zool. 1998;38:545–560. doi: 10.1093/icb/38.3.545. [DOI] [Google Scholar]
- 56.Woods HA, Harrison JF. The beneficial acclimation hypothesis versus acclimation of specific traits: physiological change in water-stressed Manduca sexta caterpillars. Physiol. Biochem. Zool. 2001;74:32–44. doi: 10.1086/319302. [DOI] [PubMed] [Google Scholar]
- 57.Gabriel W, Lynch M. The selective advantage of reaction norms for environmental tolerance. J. Evol. Biol. 1992;5:41–59. doi: 10.1046/j.1420-9101.1992.5010041.x. [DOI] [Google Scholar]
- 58.Cooper BS, Czarnoleski M, Angilletta MJ. Acclimation of thermal physiology in natural populations of Drosophila melanogaster: a test of an optimality model. J. Evol. Biol. 2010;23:2346–2355. doi: 10.1111/j.1420-9101.2010.02095.x. [DOI] [PubMed] [Google Scholar]
- 59.Nilsson-Örtman V, Johansson F. The rate of seasonal changes in temperature alters acclimation of performance under climate change. Am. Nat. 2017;190:743–761. doi: 10.1086/694412. [DOI] [PubMed] [Google Scholar]
- 60.Shah AA, Funk WC, Ghalambor CK. Thermal acclimation ability varies in temperate and tropical aquatic insects from different elevations. Integr. Comp. Biol. 2017;57:977–987. doi: 10.1093/icb/icx101. [DOI] [PubMed] [Google Scholar]
- 61.Angilletta MJ, Condon C, Youngblood JP. Thermal acclimation of flies from three populations of Drosophila melanogaster fails to support the seasonality hypothesis. J. Therm. Biol. 2019;81:25–32. doi: 10.1016/j.jtherbio.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann AA, Watson M. Geographical variation in the acclimation responses of Drosophila to temperature extremes. Am. Nat. 1993;142:S93–S113. doi: 10.1086/285525. [DOI] [PubMed] [Google Scholar]
- 63.Bubliy OA, Riihimaa A, Norry FM, Loeschcke V. Variation in resistance and acclimation to low-temperature stress among three geographical strains of Drosophila melanogaster. J. Therm. Biol. 2002;27:337–344. doi: 10.1016/S0306-4565(01)00098-5. [DOI] [Google Scholar]
- 64.Chown SL. Physiological variation in insects: hierarchical levels and implications. J. Insect Physiol. 2001;47:649–660. doi: 10.1016/S0022-1910(00)00163-3. [DOI] [PubMed] [Google Scholar]
- 65.Terblanche JS, Sinclair BJ, Klok CJ, McFarlane ML, Chown SL. The effects of acclimation on thermal tolerance, desiccation resistance and metabolic rate in Chirodica chalcoptera (Coleoptera: Chrysomelidae) J. Insect Physiol. 2005;51:1013–1023. doi: 10.1016/j.jinsphys.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 66.Woods HA, Harrison JF. Interpreting rejections of the beneficial acclimation hypothesis: when is physiological plasticity adaptive? Evolution. 2002;56:1863–1866. doi: 10.1111/j.0014-3820.2002.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 67.Somero GN. Comparative physiology: a ‘crystal ball’ for predicting consequences of global change. Am. J. Physiol. Regul. 2011;I(301):R1–R14. doi: 10.1152/ajpregu.00719.2010. [DOI] [PubMed] [Google Scholar]
- 68.Abele D, Heise K, Pörtner HO, Puntarulo S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 2002;205:1831–1841. doi: 10.1242/jeb.205.13.1831. [DOI] [PubMed] [Google Scholar]
- 69.Martinez E, Menze MA, Agosta SJ. Reduced mitochondrial efficiency explains mismatched growth and metabolic rate at supraoptimal temperatures. Physiol. Biochem. Zool. 2017;90:294–298. doi: 10.1086/689871. [DOI] [PubMed] [Google Scholar]
- 70.Kukal O, Dawson TE. Temperature and food quality influences feeding behavior, assimilation efficiency and growth rate of arctic woolly-bear caterpillars. Oecologia. 1989;79:526–532. doi: 10.1007/BF00378671. [DOI] [PubMed] [Google Scholar]
- 71.Butterworth FM. Adipose tissue of Drosophila melanogaster. V. Genetic and experimental studies of an extrinsic influence on the rate of cell death in the larval fat body. Dev. Biol. 1972;28:311–325. doi: 10.1016/0012-1606(72)90016-4. [DOI] [PubMed] [Google Scholar]
- 72.Aguila JR, Suszko J, Gibbs AG, Hoshizaki DK. The role of larval fat cells in adult Drosophila melanogaster. J. Exp. Biol. 2007;210:956–963. doi: 10.1242/jeb.001586. [DOI] [PubMed] [Google Scholar]
- 73.Gáliková M, Klepsatel P, Xu Y, Kuhnlein RP. The obesity-related Adipokinetic hormone controls feeding and expression of neuropeptide regulators of Drosophila metabolism. Eur. J. Lipid Sci. Technol. 2017;119:1600138. doi: 10.1002/ejlt.201600138. [DOI] [Google Scholar]
- 74.Gáliková M, Klepsatel P, Münch J, Kühnlein RP. Spastic paraplegia-linked phospholipase PAPLA1 is necessary for development, reproduction, and energy metabolism in Drosophila. Sci Rep. 2017;7:46516. doi: 10.1038/srep46516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tennessen JM, Barry WE, Cox J, Thummel CS. Methods for studying metabolism in Drosophila. Methods. 2014;68:105–115. doi: 10.1016/j.ymeth.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gáliková M, et al. Energy homeostasis control in Drosophila Adipokinetic hormone mutants. Genetics. 2015;201:665–683. doi: 10.1534/genetics.115.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waner S, Constenoble S. Finite Mathematics and Applied Calculus. 7. Boston, MA: Cengage Learning; 2017. [Google Scholar]
- 78.Bruce PS. Introductory Statistics and Analytics: A Resampling Perspective. Hoboken: Wiley; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.