Cellular H2S content is linked to macrophage functional status. H2S signalling regulates activation of macrophage. Targeting on H2S signalling modules represents a promising approach in treating macrophage‐related disorders.
Keywords: epigenetics, H2S, macrophage function, redox regulation, S‐sulphydration
Summary
Hydrogen sulphide (H2S) is the latest identified small gaseous mediator enabled by its lipophilic nature to freely permeate the biological membranes. Initially, H2S was recognized by its roles in neuronal activity and vascular relaxation, which makes it an important molecule involved in paracrine signalling pathways. Recently, the immune regulatory function of gasotransmitters, H2S in particular, is increasingly being appreciated. Endogenous H2S level has been linked to macrophage activation, polarization and inflammasome formation. Mechanistically, H2S‐induced protein S‐sulphydration suppresses several inflammatory pathways including NF‐κB and JNK signalling. Moreover, H2S serves as a potent cellular redox regulator to modulate epigenetic alterations and to promote mitochondrial biogenesis in macrophages. Here in this review, we intend to summarize the recent advancements of H2S studies in macrophages, and to discuss with focus on the therapeutic potential of H2S donors by targeting macrophages. The feasibility of H2S signalling component as a macrophage biomarker under disease conditions would be also discussed.
Abbreviations
- 3‐MST
3‐mercaptopyruvate sulphur transferase
- ATM
adipose tissue macrophage
- CBS
cystathionine β‐synthase
- CSE
cystathionine γ‐lyase
- H2S
hydrogen sulphide
- Hcy
homocysteine
- oxLDL
oxidized low‐density lipoprotein
Introduction
Gasotransmitters are a group of ubiquitous small gaseous signalling molecules, which mainly consist of nitric oxide (NO), carbon monoxide (CO) and hydrogen sulphide (H2S). 1 Their lipophilic nature allows them to freely permeate through the biological membranes and to play an essential role in the regulation of cellular processes. 1 , 2 Indeed, dysregulation of gasotransmitter system is associated with numerous diseases ranging from neurological disorders to musculoskeletal abnormalities. 3 , 4 , 5 Recently, encouraging results have further indicated a regulatory role for gasotransmitters in immune cells. 2 In particular, macrophage, as the patrolling sentinel in the immune system, is extensively regulated by these gaseous mediators. 6
Upon activation, the classically activated (M1) macrophages upregulate the expression of inducible nitric oxide synthase (iNOS), and catalyse the transformation of L‐arginine to NO. Elevated NO along with the production of reactive nitrogen species is indispensable for the optimal antimicrobial activity and the secretion of inflammatory cytokines such as IL‐6, TNF‐α and interferons. 7 , 8 On the other hand, the alternatively activated (M2) macrophages highly express the hallmark enzyme Arginase1 (Arg1), which outcompetes the activity of iNOS on l‐arginine availability and reduces the NO production. 9 Therefore, the fluctuation of NO metabolism serves as a key molecular switch for control of macrophage function to dynamically regulate the initiation or resolution of an inflammatory response. In contrast to NO, CO, a haem metabolism product produced by the haem oxygenase 1‐3 (HO 1‐3), attenuates macrophage activation, and therefore, HO‐1 overexpression in myeloid lineages favours M2 programme in macrophages and implies better outcome in liver transplant patients. 10 Consistently, HO‐1 deficiency leads to increased M1 macrophages along with enhanced inflammatory infiltration following ischaemia–reperfusion injury. 10 Similarly, CO suppresses lipopolysaccharide (LPS)‐induced macrophage activation and induces the secretion of IL‐10, which involves its effect on the activation of mitogen‐activated protein kinase kinase 3 (MKK3). 11
H2S, the latest identified gasotransmitter, was first recognized as a smelly and environmental toxic gas. 12 Past two decades of studies revealed that H2S can be generated endogenously and work as an autocrine signalling molecule. 3 , 13 , 14 In mammals, three enzymes including cystathionine γ‐lyase (CSE), cystathionine β‐synthase (CBS) and the 3‐mercaptopyruvate sulphur transferase (3‐MST) are responsible for H2S generation. 15 , 16 Specifically, CSE and CBS catalyse de‐sulphydration of cysteine to generate H2S, while MST induces H2S production by regulating the enzymatic activity of cysteine aminotransferase. 17 , 18 The essential role of H2S signalling in T‐cell biology has been well addressed, in which ablation of CBS and CSE leads to impaired T‐cell activation and proliferation. 19 Mice deficient in CBS also manifest reduced regulatory T cells along with massive inflammatory infiltration, which could be reversed by H2S donor supplementation. 20
Interestingly, unlike its effect on T cells, in macrophages, H2S signalling is clearly anti‐inflammatory in a variety of interesting ways. It seems that H2S actively impact macrophage on its activation, polarization and inflammasome formation through distinct mechanistic pathways. Particularly, macrophages likely also set the threshold for the activation of H2S signalling under various stimuli. Herein, we aim to summarize the regulatory mechanisms underlying H2S signalling and discuss with focus for the impact of H2S signalling on the regulation of macrophage functionality. We also discuss the potential that the cellular H2S content and the key H2S metabolic enzymes serve as ideal biomarkers to indicate distinct macrophage activation status.
The regulatory mechanisms underlying H2S signalling
H2S signalling plays a critical regulatory role in diverse immune responses, which involves H2S‐induced protein S‐sulphydration, cellular redox homeostasis and epigenetic chromatin remodelling (Fig. 1). In this section, we briefly summarize the above regulatory mechanisms underlying H2S signalling.
Figure 1.
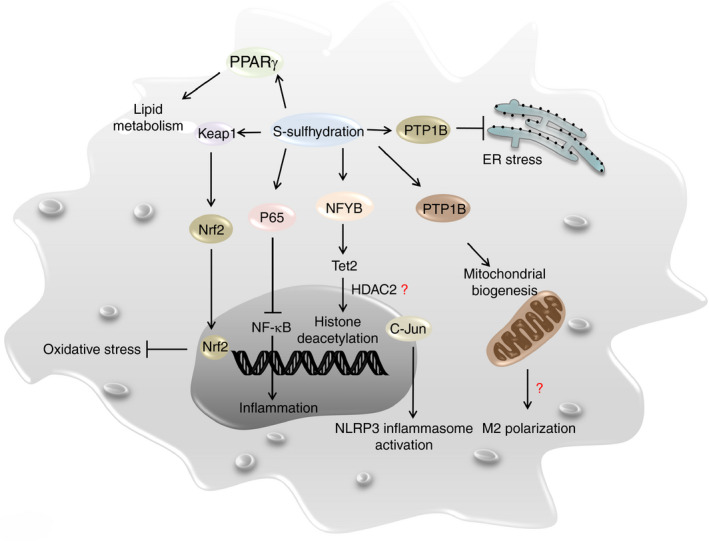
Potential regulatory mechanisms underlying H2S signalling. The mechanisms underlying H2S signalling in the regulation of macrophage function presumably involve direct mediation of protein S‐sulphydration, cellular redox homeostasis and epigenetic chromatin remodelling.
Protein S‐sulphydration
H2S‐induced protein S‐sulphydration is a novel post‐translational modification occurring on specific cysteine (Cys) residues of target proteins, by which it regulates the biological activity of targeted proteins. It is noteworthy that S‐sulphydration of key enzymes, receptors and transcriptional factors contributes a major part to H2S signalling and its regulatory function. Kir6.1, a subunit of ATP‐sensitive potassium channels (KATP), is S‐sulphydrated at Cys43, which promotes KATP channel activity and improves vasodilation. 21 Other ion channels such as voltage‐activated calcium channels, and transient receptor potential channel proteins TRPV6 and TRPV4, were also suggested to be S‐sulphydrated, thereby regulating calcium flux. 22 , 23 Together, these events perfectly explain the effect of endogenous H2S and exogenous H2S donors on vascular relaxation.
Metabolic reprogramming and stress responses including oxidative stress and endoplasmic reticulum (ER) stress are critical regulators in immune cells and their fate decision. Other than the well‐known role in cardiovascular system, H2S‐mediated protein S‐sulphydration also engages in the metabolic processes and cellular stress responses. S‐sulphydration of peroxisome proliferator‐activated receptor‐γ (PPARγ) at Cys139 enhances its DNA binding activity and the subsequent expression of adipogenic genes, thus increasing glucose uptake and lipid metabolism. 24 Additionally, H2S promotes the activities of PPARγ coactivator‐related protein (PPRC), alpha subunit of ATP synthase (ATP5A1) and interferon regulatory factor 1 via S‐sulphydration, by which it stimulates mitochondrial biogenesis and protects against mitochondrial dysfunction. 25 , 26 , 27 P66Shc is an upstream activator of mitochondrial redox signalling, and studies suggested that H2S protects neuronal cells against stress‐induced senescence by inducing its S‐sulphydration at Cys59 residue. 28 H2S also induces Keap1 S‐sulphydration (Cys151, Cys226 and Cys613) to promote the dissociation of Keap1‐Nrf2 complex, thereby releasing Nrf2 to transcribe the expression of antioxidant genes 29 , 30 . Similarly, PTP‐1B is a protein tyrosine phosphatase related to the deactivation of protein kinase RNA‐like ER kinase (PERK), while H2S mediates PTP‐1B S‐sulphydration at Cys215 to inhibit its enzymatic activity, thereby activating PERK pathway to alleviate ER stress. 31
It is worthy of note that some immune regulatory molecules are the direct targets for H2S‐induced S‐sulphydration. For example, S‐sulphydration of nuclear transcription factor Y subunit beta (NFYB) at Cys105 increases the transcription of the ten‐eleven translocation (Tet) genes. 32 Tet1 and Tet2 in turn bind to the regulatory regions within the Foxp3 gene to maintain the hypomethylation status of its promoter and the conserved non‐coding sequence 2 (CNS2) region, thereby ensuring Foxp3 expression and the stability of Treg cell lineage. 20 Similarly, S‐sulphydration of the free thiol group Cys38 in p65 inhibits NF‐κB activity in macrophages. 33 Moreover, S‐sulphydration of c‐Jun at Cys269 attenuates hydrogen peroxide (H2O2)‐induced NLRP3 inflammasome activation and reduces IL‐1β production in macrophages. 34
Cellular redox homeostasis
Theoretically, most H2S can dissolve in surface water and dissociate into HS− under normal circumstances (37°, pH = 7.4), 35 and HS− in turn could serve as a powerful one‐electron chemical reductant to scavenge ROS. In reality, however, the physiological concentration of H2S is at the sub‐micromolar level, 36 which is too low for H2S to act as a direct antioxidant. Nonetheless, low concentration of endogenous H2S can exert potent antioxidant effects in alternative manners. Specifically, other than the aforementioned Keap1 S‐sulphydration‐mediated pathway, hypoxia‐inducible factor 1α (HIF‐1α) also serves as another important molecule downstream of H2S signalling. 37 Studies in THP‐1 cells, a human macrophage cell line, revealed that H2S induces HIF‐1a nuclear translocation to enhance the expression of glucose transporter GLUT1 along with the abrogation of its pro‐inflammatory effect. 37 Consistently, it was also found that H2S could activate the antioxidant Nrf2/HO‐1 pathway by stimulating the p38 mitogen‐activated protein kinase (MAPK) activity. 37 Therefore, H2S has been found to attenuate LPS‐induced acute lung injury by reducing oxidative and nitrative species, 38 and H2S administration improves glutathione (GSH) level along with alleviated lipid peroxidation and allergic lung inflammation. 39 Collectively, as a negative regulator in cellular redox homeostasis, H2S exhibits anti‐inflammatory potency amid stress‐related inflammatory disorders.
Epigenetic chromatin remodelling
Another critical mechanism underlying H2S signalling is that H2S also manifests a remarkable capacity to regulate epigenetic chromatin remodelling. Apart from the above‐introduced NFYB‐Tet pathway, which mediates DNA demethylation of the Foxp3 regulatory regions in Treg cells, H2S exhibits high potency to remodel chromatin structure through regulation of histone modifications in macrophages.
The Jumonji domain‐containing protein 3 (JMJD3) is a histone 3 Lys27 (H3K27) demethylase and plays a critical role in chromatin remodelling. 40 There is evidence that LPS upregulates CSE expression in macrophages in a mouse model with septic shock, and enhanced CSE in turn inhibits JMJD3 expression to increase H3K27me3 levels, thereby attenuating LPS‐mediated inflammatory response. 41 Studies in macrophages further noted that H2S is capable of suppressing histone acetylation at the IL‐6 and TNF‐α promoter, by which it inhibits chromatin openness to repress the transcription of inflammatory cytokines following LPS stimulation. 42 Although no direct evidence shows the existence of H2S‐NFYB‐Tet pathway in macrophage, Tet2 resolves macrophage inflammatory response by recruiting HDAC2 and deacetylating permissive histone markers in the IL‐6 promoter, the mechanism of which is DNA methylation‐independent and quite different from what happens in Treg cells. 43 These results suggest that the CSE/H2S signalling could be vital to prevent uncontrolled macrophage inflammatory responses via epigenetic machineries.
Heretofore, the major mechanism underlying H2S signalling is likely attributed to the S‐sulphydration of substrate proteins (Table 1). Moreover, the impact of H2S signalling on the regulation of redox homeostasis and chromatin remodelling seems independent of S‐sulphydration, but additional studies would be necessary to fully address this issue. It should be also important to keep in mind that characterization of additional unidentified S‐sulphydration proteins would help to completely clarify the regulatory mechanisms.
Table 1.
Potential S‐sulphydration targets relevant to macrophage regulation
| Potential target | Modification site | Major cell types | Biological consequence | Reference |
|---|---|---|---|---|
| P65 | Cys38 | Macrophage | Inhibiting NF‐κB activity | 33 |
| c‐Jun | Cys269 | Macrophage | Attenuating inflammasome activation and IL‐1β production | 34 |
| Keap1 | Cys151, Cys226, Cys613 | Fibroblast | Dissociation of Keap1‐Nrf2 complex; antioxidative response | 29, 30 |
| PTP1B | Cys215 | 293T cell | Alleviating ER stress | 31 |
| PPARγ | Cys139 | Adipocyte | Enhancing DNA binding activity of PPARγ, increasing lipid metabolism | 24 |
| NFYB | Cys105 | Regulatory T cell | Promoting the transcription of Tet1/2 | 20, 32 |
Keap1, Kelch‐like ECH‐associated protein 1; NFYB, nuclear transcription factor Y subunit beta; PTP1B, protein tyrosine phosphatase 1B; Tet, tet methylcytosine dioxygenase 2.
H2S signalling in maintaining the M1/M2 homeostasis in macrophages
As described earlier, macrophages display different functional phenotypes depending on their residing environmental milieu. For simplicity, they are classified into two distinct subtypes: one is classically activated (M1) macrophages, and the other is alternatively activated (M2) macrophages. LPS and IFN‐γ induce the generation of M1 macrophages, which then augment the production of pro‐inflammatory cytokines. In contrast, M2 macrophages are elicited by glucocorticoids or type II cytokines such as IL‐4, IL‐13 and IL‐10. M2 macrophages are responsible for wound healing, tissue repair and the resolution of inflammation, thus generally regarded as an anti‐inflammatory cell type.
Recent studies provided compelling evidence that H2S signalling is implicated in dictating macrophage polarizations. Initially, the endogenous H2S was found to attenuate LPS‐induced oxidative stress and inflammatory damage by inhibiting NOX4‐ROS signalling pathway in macrophages. 44 GYY4137, a novel H2S‐releasing molecule, was confirmed to inhibit rat endotoxic shock and mucosal wound through abrogating M1 programme in macrophages. 45 , 46 Similarly, FW1256, another slow‐releasing H2S donor, was further noted to exhibit anti‐inflammatory properties by reducing the production of inflammatory mediators such as TNF‐α, IL‐6, PGE2, IL‐1β, COX‐2 and NO in macrophages. 47 Subsequent mechanistic studies demonstrated that NaHS promotes macrophage M2 polarization by enhancing mitochondrial biogenesis and fatty acid oxidation (FAO). 48 Similar results were also observed in the central nervous system, in which H2S exerts neuroprotection against hypoxia‐induced neurotoxicity through induction of M2 programme in microglia cells by inhibiting iNOS, NF‐κB, ERK and p38 MAPK signalling pathways. 49 Therefore, H2S signalling serves as a critical regulatory mechanism to maintain the homeostatic M1/M2 balance in the setting of inflammatory resolution.
H2S signalling in macrophage activation and inflammasome formation
It was noted that LPS‐stimulated macrophages and adipose tissue macrophages (ATMs) derived from diet‐induced obese mice manifest lower intracellular concentration of H2S, 50 suggesting that depletion of macrophage H2S content occurs during both acute (LPS‐induced) and chronic (obesity) inflammatory conditions. Indeed, oxidized low‐density lipoprotein (oxLDL) induces the CSE promoter to undergo DNA hypermethylation in macrophages, leading to attenuated CSE transcription and H2S production in favour of inflammatory responses, 51 which involves the activation of JNK/NF‐κB signalling. 52 Similarly, homocysteine (HCy) induces DNA hypermethylation in the CSE promoter in macrophages, through which it exaggerates inflammation by inhibiting CSE‐H2S signalling 53 (Fig. 2).
Figure 2.
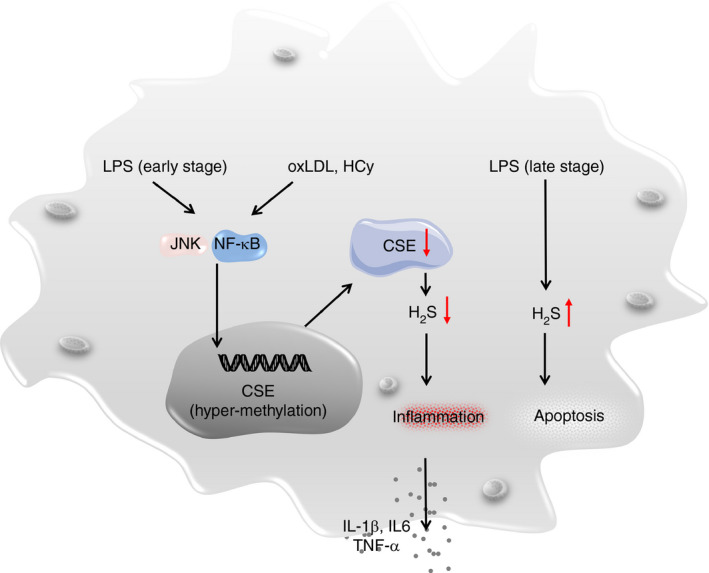
H2S signalling regulates macrophage functionality for the initiation and resolution of an inflammatory response. Upon stimulation (e.g. LPS and oxLDL), H2S production is shut down at the early stage to facilitate pro‐inflammatory cytokine secretion, while at the late stage, the H2S content becomes increased for induction of those mission‐completed macrophages to undergo apoptosis. Alerted H2S signalling would lead to the development of immune or metabolic disorders. LPS, lipopolysaccharide; oxLDL, oxidized low‐density lipoprotein.
In line with above observations, a time‐dependent change of H2S content in macrophages was found following activation. A decrease of H2S level in murine macrophages following 24 hr of LPS or IFN‐γ stimulation was observed (early phase), but the H2S content was restored to normal level after 48 hr of stimulation (late phase), which was associated with the feedback regulation between CBS and CSE. 54 It is worthy of note that H2S production was correlated with LPS‐induced macrophage late‐stage apoptosis, which could be blocked by the addition of H2S inhibitor. 55 Therefore, it is possible that sustained LPS stimulation renders macrophages that undergo apoptosis through the production of H2S (Fig. 2).
Macrophages not only sense exogenous pathogen‐associated molecular patterns (e.g. LPS) derived from micro‐organisms, but also respond to endogenous stimuli. The most commonly seen endogenous insults originate from harmful metabolites, such as excessive free fatty acids (FFAs) and oxLDL. Interestingly, these metabolites alone could lead to abnormal macrophage activation, while they could also serve as the second signals essential for inflammasome formation. Inflammasome is a complex of proteins found in macrophages that regulates the activation of caspase enzymes and induces the secretion of pro‐inflammatory cytokines (e.g. IL‐1β and IL‐18). Importantly, recent studies demonstrated that both exogenous and endogenous H2S inhibit NLRP3 inflammasome activation and reduce inflammatory cytokine production in macrophages. 56 In particular, upregulation of H2S content by treating the cells with NaHS reduces the expression level of inflammasome‐associated proteins such as TXNIP, NLRP3, ASC and caspase‐1 by inhibiting thioredoxin‐interacting protein–NLRP3 (TXNIP‐NLRP3) signalling pathway. 57 Taken together, H2S signalling not only directly represses macrophage activation, but also inhibits inflammasome formation, thereby attenuating inflammatory responses.
The therapeutic potential for targeting H2S signalling in macrophages
Macrophages are critical participants in the immune system, which are involved in innate immunity and also help to recruit other immune cells for adaptive immune responses. Macrophages can be found essentially in all tissues, and their dysfunction is linked to a variety of diseases. Dysregulation of macrophages is related to various diseases ranging from infection to metabolic disorders, wherein H2S donors exhibit significant therapeutic potential.
It has been well recognized that enhanced H2S signalling in macrophages abrogates the progression of septic shock, 45 a severe inflammatory disorder caused by bacterial infection and now faces up with limited therapeutics in clinic. Microglia, a specialized macrophage in the nervous system, is involved in the pathogenesis of Alzheimer’s and Parkinson’s disease. Given the role of H2S signalling in the resolution of neuronal inflammation, 58 , 59 , 60 H2S donors are proven to be effective in numerous neuronal disorders. 49 , 60 Similarly, as H2S reduces FFAs and oxLDL‐induced metabolic stress and inflammasome formation, H2S donors could inhibit foam cell formation and attenuate the release of pro‐inflammatory cytokines, thus leading to the amelioration of arterial atherosclerosis and other inflammasome‐associated diseases such as DSS‐induced colitis. 57 , 61 , 62 MicroRNA‐186 (miR‐186) plays an important role in atherosclerotic diseases. Mechanistic study revealed that miR‐186 directly binds to the 3’‐UTR of CSE and destabilizes the mRNA transcripts. As a result of decreased CSE‐H2S axis, the human macrophages take up more lipids and become pro‐inflammatory. 63 Exogenous administration of H2S donor NaHS or GYY4137 decreases the inflammatory cytokine secretion, prohibits lipid accumulation in macrophages and down‐tunes the expression of chemokine receptors (CX3CR1 in particular), thus demonstrating the effectiveness in atherosclerosis treatment. 64 , 65 As aforementioned, intracellular concentration of H2S was lower in ATMs of obese mice, and not surprisingly, exogenous supplementation of H2S donors could curb the development of obesity and the subsequent metabolic syndromes. 50
Alternatively activated M2 macrophages substantially participate in inflammation resolution and tissue repair. Given the role of H2S signalling played in M2 macrophages, it is not surprising that H2S would play a pivotal role in myocardial infarction (MI) and wound healing. Studies showed that H2S promotes macrophage migration towards the infracted area at the early stage, then induces M2 polarization by enhancing mitochondrial biogenesis and FAO, the two steps of which cooperatively accelerates the post‐MI recovery. 48 , 66 During the wound healing process, the local H2S content was found to significantly reduce amid injured tissue granulation. Replenishment of H2S inhibits macrophage activation and improves wound healing in both oral mucosal wound model and diabetic wound model. 46 , 67 In situ induction of M2 macrophages by employing the novel H2S‐releasing hydrogel greatly improves wound healing process, which displays a promising translational potential. 68 Together, these results support that targeting H2S signalling in macrophages could be a viable approach to fight against immune and metabolic disorders in clinical settings and to restore tissue homeostasis upon trauma.
Concluding remarks and perspectives
It would be important to note that the relationship between H2S signalling and macrophage functionality is reciprocal and dynamic. H2S actively modulates macrophage activation, polarization and inflammasome formation (Fig. 1), and macrophages in turn influence the intrinsic H2S synthetic machinery following external stimuli. Specifically, upon LPS stimulation, H2S production is shut down at the early stage to facilitate pro‐inflammatory cytokine secretion, while at the late stage, the H2S content becomes increased for induction of those mission‐completed macrophages to undergo apoptosis. This complex feedback loop underpins the multifaceted function of macrophages, which reflects a fine control of macrophage‐mediated immune response (Fig. 2).
Generally, H2S induces S‐sulphydration of key signalling molecules, such as p65 and c‐Jun, to impact on NF‐κB pathway and canonical NLRP3 inflammasome formation, while H2S also regulates cellular redox homeostasis and chromatin remodelling to affect macrophage function. However, we cannot exclude the possibility that additional unrecognized S‐sulphydrated proteins could be also engaged in H2S signalling. As for the regulation of cellular redox homeostasis, it is intriguing that H2S‐induced S‐sulphydration shares great similarity as GSH‐mediated S‐glutathionylation, 69 , 70 and both of which even possess the same substrate, PTP1B. 31 , 71 There is evidence that S‐glutathionylation regulates redox homeostasis, 72 and a typical example is MKP1, which has been verified to be a substrate for S‐glutathionylation. 73 It is therefore plausible that H2S could either directly mediates MKP1 S‐sulphydration to regulate macrophage redox homeostasis, or indirectly influences MKP1 S‐glutathionylation by elevating GSH levels, which could perfectly explain the inhibitory effect of H2S on MAPK signalling.
Macrophages demand distinct intracellular metabolic pathways depending on their functional state. The activation of M1 macrophages by LPS or IFN‐γ is associated with higher glycolysis along with attenuated tri‐carboxylic acid (TCA) cycle and mitochondrial oxidative phosphorylation (OXPHOS). 74 In contrast, M2 macrophages require higher mitochondrial biogenesis, fatty acid uptake and FAO. 75 , 76 Collectively, those discoveries support that H2S‐mediated metabolic reprogramming finely controls the initiation and resolution of an inflammatory response. 48 Therefore, a better understanding of the role for H2S signalling in macrophages would demonstrate great potential to develop therapies against either acute or chronic inflammatory responses in clinical settings of patients with immune or metabolic disorders. Indeed, some commonly prescribed drugs have already been indicated to affect endogenous H2S signalling pathway. For example, statins are able to modulate H2S metabolism 77 , 78 in the cardiovascular system, while the well‐known antidiabetic drug, metformin, could promote H2S production by elevating CSE. 79 These discoveries prompt us to rescrutinize the ‘new function of old drugs’ while pursuing for novel H2S regulating compounds in the future investigations.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
FS and JHL wrote the paper; TTY, FXW and CLY revised the manuscript; SZ gave us pivotal suggestions; XQW and CYW conceptualized and revised the manuscript. Our work was supported by the Ministry of Science and Technology (2016YFC1305002 and 2017YFC1309603), the National Natural Science Foundation of China (81530024, 91749207, 81920108009 and 81770823), the NHC Drug Discovery Program (2017ZX09304022‐07), the Integrated Innovative Team for Major Human Disease Programs of Tongji Medical College, Huazhong University of Science and Technology, and the Innovative Funding for Translational Research from Tongji Hospital.
Senior author: Fei Sun
Contributor Information
Xin‐Qiang Wang, Email: wangcy@tjh.tjmu.edu.cn.
Cong‐Yi Wang, Email: wangcy@tjh.tjmu.edu.cn.
Data Availability Statement
Data availability is not applicable.
References
- 1. Wang R. Gasotransmitters: growing pains and joys. Trends Biochem Sci 2014; 39:227–32. [DOI] [PubMed] [Google Scholar]
- 2. Fagone P, Mazzon E, Bramanti P, Bendtzen K, Nicoletti F. Gasotransmitters and the immune system: mode of action and novel therapeutic targets. Eur J Pharmacol 2018; 834:92–102. [DOI] [PubMed] [Google Scholar]
- 3. Paul BD, Snyder SH. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem Pharmacol 2018; 149:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang L, Wang Y, Li Y, Li L, Xu S, Feng X, et al Hydrogen sulfide (H2S)‐releasing compounds: therapeutic potential in cardiovascular diseases. Front Pharmacol 2018; 9:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallace JL, Ferraz JGP, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal 2012; 17:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol 2015; 15:731–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gautier EL, Yvan‐Charvet L. Understanding macrophage diversity at the ontogenic and transcriptomic levels. Immunol Rev 2014; 262:85–95. [DOI] [PubMed] [Google Scholar]
- 9. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593–604. [DOI] [PubMed] [Google Scholar]
- 10. Zhang M, Nakamura K, Kageyama S, Lawal AO, Gong KW, Bhetraratana M, et al Myeloid HO‐1 modulates macrophage polarization and protects against ischemia‐reperfusion injury. JCI Insight 2018; 3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Otterbein LE, Bach FH, Alam J, Soares M, Tao LuH, Wysk M, et al Carbon monoxide has anti‐inflammatory effects involving the mitogen‐activated protein kinase pathway. Nat Med 2000; 6:422–8. [DOI] [PubMed] [Google Scholar]
- 12. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 2012; 92:791–896. [DOI] [PubMed] [Google Scholar]
- 13. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996; 16:1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 2001; 20:6008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 2010; 285:21903–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powell CR, Dillon KM, Matson JB. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem Pharmacol 2018; 149:110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 2007; 6:917. [DOI] [PubMed] [Google Scholar]
- 18. Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Ann Rev Pharmacol Toxicol 2011; 51:169–87. [DOI] [PubMed] [Google Scholar]
- 19. Miller TW, Wang EA, Gould S, Stein EV, Kaur S, Lim L, et al Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem 2012; 287:4211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang R, Qu C, Zhou Y, Konkel JE, Shi S, Liu Y, et al Hydrogen sulfide promotes Tet1‐ and Tet2‐mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 2015; 43:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mustafa Asif K, Sikka G, Gazi Sadia K, Steppan J, Jung Sung M, Bhunia Anil K, et al Hydrogen sulfide as endothelium‐derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 2011; 109:1259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, et al Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 2014; 15:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naik JS, Osmond JM, Walker BR, Kanagy NL. Hydrogen sulfide‐induced vasodilation mediated by endothelial TRPV4 channels. Am J Physiol Heart Circ Physiol 2016; 311:H1437–H1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai J, Shi X, Wang H, Fan J, Feng Y, Lin X,et al Cystathionine γ lyase–hydrogen sulfide increases peroxisome proliferator‐activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes Biochim Biophys Acta 2016; 1861:419–29. [DOI] [PubMed] [Google Scholar]
- 25. Untereiner AA, Fu M, Módis K, Wang R, Ju Y, Wu L. Stimulatory effect of CSE‐generated H2S on hepatic mitochondrial biogenesis and the underlying mechanisms. Nitric Oxide 2016; 58:67–76. [DOI] [PubMed] [Google Scholar]
- 26. Módis K, Ju Y, Ahmad A, Untereiner AA, Altaany Z, Wu L, et al S‐Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol Res 2016; 113:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li S, Yang G. Hydrogen sulfide maintains mitochondrial DNA replication via demethylation of TFAM. Antioxid Redox Signal. 2015; 23:630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xie Z‐Z, Shi M‐M, Xie L, Wu Z‐Y, Li G, Hua F, et al Sulfhydration of p66Shc at Cysteine59 mediates the antioxidant effect of hydrogen sulfide. Antioxid Redox Signal 2014; 21:2531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, et al Hydrogen sulfide protects against cellular senescence via S‐sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 2012; 18:1906–19. [DOI] [PubMed] [Google Scholar]
- 30. Hourihan JM, Kenna JG, Hayes JD. The gasotransmitter hydrogen sulfide induces Nrf2‐target genes by inactivating the Keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between Cys‐226 and Cys‐613. Antioxid Redox Signal 2012; 19:465–81. [DOI] [PubMed] [Google Scholar]
- 31. Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S‐Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 2011; 4:ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oh Soyoung A, Li MO. TETs link hydrogen sulfide to immune tolerance. Immunity 2015; 43:211–3. [DOI] [PubMed] [Google Scholar]
- 33. Du J, Huang Y, Yan H, Zhang Q, Zhao M, Zhu M, et al Hydrogen sulfide suppresses oxidized low‐density lipoprotein (Ox‐LDL)‐stimulated monocyte chemoattractant protein 1 generation from macrophages via the nuclear factor κB (NF‐κB) pathway. J Biol Chem 2014; 289:9741–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin Z, Altaf N, Li C, Chen M, Pan L, Wang D, et al Hydrogen sulfide attenuates oxidative stress‐induced NLRP3 inflammasome activation via S‐sulfhydrating c‐Jun at Cys269 in macrophages. Biochim Biophys Acta Mol Basis Dis 2018;1864(9, Part B):2890–900. [DOI] [PubMed] [Google Scholar]
- 35. Mathai JC, Missner A, Kügler P, Saparov SM, Zeidel ML, Lee JK, et al No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci USA 2009; 106:16633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 2008; 295:R1479–R1485. [DOI] [PubMed] [Google Scholar]
- 37. Lohninger L, Tomasova L, Praschberger M, Hintersteininger M, Erker T, Gmeiner BMK, et al Hydrogen sulphide induces HIF‐1α and Nrf2 in THP‐1 macrophages. Biochimie 2015; 112:187–95. [DOI] [PubMed] [Google Scholar]
- 38. Zhang HX, Liu SJ, Tang XL, Duan GL, Ni X, Zhu XY, et al H2S attenuates LPS‐induced acute lung injury by reducing oxidative/nitrative stress and inflammation. Cell Physiol Biochem 2016; 40:1603–12. [DOI] [PubMed] [Google Scholar]
- 39. Campos D, Ravagnani FG, Gurgueira SA, Vercesi AE, Teixeira SA, Costa SKP, et al Increased glutathione levels contribute to the beneficial effects of hydrogen sulfide and inducible nitric oxide inhibition in allergic lung inflammation. Int Immunopharmacol 2016; 39:57–62. [DOI] [PubMed] [Google Scholar]
- 40. De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine‐27 demethylase Jmjd3 links inflammation to inhibition of polycomb‐mediated gene silencing. Cell 2007; 130:1083–94. [DOI] [PubMed] [Google Scholar]
- 41. Liu S, Wang X, Pan L, Wu W, Yang D, Qin M, et al Endogenous hydrogen sulfide regulates histone demethylase JMJD3‐mediated inflammatory response in LPS‐stimulated macrophages and in a mouse model of LPS‐induced septic shock. Biochem Pharmacol 2018; 149:153–62. [DOI] [PubMed] [Google Scholar]
- 42. Rios ECS, Szczesny B, Soriano FG, Olah G, Szabo C. Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int J Mol Med 2015; 35:1741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, et al Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL‐6. Nature 2015; 525:389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang XL, Pan LL, Long F, Wu WJ, Yan D, Xu P, et al Endogenous hydrogen sulfide ameliorates NOX4 induced oxidative stress in LPS‐stimulated macrophages and mice. Cell Physiol Biochem 2018; 47:458–74. [DOI] [PubMed] [Google Scholar]
- 45. Li L, Salto‐Tellez M, Tan C‐H, Whiteman M, Moore PK. GYY4137, a novel hydrogen sulfide‐releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med 2009; 47:103–13. [DOI] [PubMed] [Google Scholar]
- 46. Zhuang R, Guo L, Du J, Wang S, Li J, Liu Y. Exogenous hydrogen sulfide inhibits oral mucosal wound‐induced macrophage activation via the NF‐κB pathway. Oral Dis 2018; 24:793–801. [DOI] [PubMed] [Google Scholar]
- 47. Huang CW, Feng W, Peh MT, Peh K, Dymock BW, Moore PK. A novel slow‐releasing hydrogen sulfide donor, FW1256, exerts anti‐inflammatory effects in mouse macrophages and in vivo. Pharmacol Res 2016; 113:533–46. [DOI] [PubMed] [Google Scholar]
- 48. Miao L, Shen X, Whiteman M, Xin H, Shen Y, Xin X, et al Hydrogen sulfide mitigates myocardial infarction via promotion of mitochondrial biogenesis‐dependent M2 polarization of macrophages. Antioxid Redox Signal 2016; 25:268–81. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Q, Yuan L, Liu D, Wang J, Wang S, Zhang Q, et al Hydrogen sulfide attenuates hypoxia‐induced neurotoxicity through inhibiting microglial activation. Pharmacol Res 2014; 84:32–44. [DOI] [PubMed] [Google Scholar]
- 50. Velmurugan GV, Huang H, Sun H, Candela J, Jaiswal MK, Beaman KD, et al Depletion of H2S during obesity enhances store‐operated Ca2+ entry in adipose tissue macrophages to increase cytokine production. Sci Signal 2015;8:ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Du H‐P, Li J, You S‐J, Wang Y‐L, Wang F, Cao Y‐J, et al DNA methylation in cystathionine‐γ‐lyase (CSE) gene promoter induced by ox‐LDL in macrophages and in apoE knockout mice. Biochem Biophys Res Commun 2016; 469:776–82. [DOI] [PubMed] [Google Scholar]
- 52. Wang X‐H, Wang F, You S‐J, Cao Y‐J, Cao L‐D, Han Q, et al Dysregulation of cystathionine γ‐lyase (CSE)/hydrogen sulfide pathway contributes to ox‐LDL‐induced inflammation in macrophage. Cell Signal 2013; 25:2255–62. [DOI] [PubMed] [Google Scholar]
- 53. Li J‐J, Li Q, Du H‐P, Wang Y‐L, You S‐J, Wang F, et al Homocysteine triggers inflammatory responses in macrophages through inhibiting CSE‐H2S signaling via DNA hypermethylation of CSE promoter. Int J Mol Sci 2015; 16:12560–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bronowicka‐Adamska P, Jurkowska H, Gawda A, Skalska P, Nazimek K, Marcinkiewicz J, et al Expression and activity of hydrogen sulfide generating enzymes in murine macrophages stimulated with lipopolysaccharide and interferon‐γ. Mol Biol Rep 2019; 46:2791–2798. [DOI] [PubMed] [Google Scholar]
- 55. George L, Ramasamy T, Sirajudeen KNS, Manickam V. LPS‐induced apoptosis is partially mediated by hydrogen sulphide in RAW 264.7 murine macrophages. Immunol Invest 2019; 48:1–15. [DOI] [PubMed] [Google Scholar]
- 56. Castelblanco M, Lugrin J, Ehirchiou D, Nasi S, Ishii I, So A, et al Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J Biol Chem 2018; 293:2546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yue L‐M, Gao Y‐M, Han B‐H. Evaluation on the effect of hydrogen sulfide on the NLRP3 signaling pathway and its involvement in the pathogenesis of atherosclerosis. J Cell Biochem 2019; 120:481–92. [DOI] [PubMed] [Google Scholar]
- 58. Tang X‐Q, Fan L‐L, Li Y‐J, Shen X‐T, Zhuan Y‐Y, He J‐Q, et al Inhibition of hydrogen sulfide generation contributes to 1‐methy‐4‐phenylpyridinium ion‐induced neurotoxicity. Neurotox Res 2011; 19:403–11. [DOI] [PubMed] [Google Scholar]
- 59. Ghanbari F, Khaksari M, Vaezi G, Hojati V, Shiravi A. Hydrogen sulfide protects hippocampal neurons against methamphetamine neurotoxicity via inhibition of apoptosis and neuroinflammation. J Mol Neurosci 2019; 67:133–41. [DOI] [PubMed] [Google Scholar]
- 60. Ji J, Xiang P, Li T, Lan L, Xu X, Lu G, et al NOSH‐NBP, a novel nitric oxide and hydrogen sulfide‐ releasing hybrid, attenuates ischemic stroke‐induced neuroinflammatory injury by modulating microglia polarization. Front Cell Neurosci 2017; 11:154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61. Zhao Z‐Z, Wang Z, Li G‐H, Wang R, Tan J‐M, Cao X, et al Hydrogen sulfide inhibits macrophage‐derived foam cell formation. Exp Biol Med. 2011; 236:169–76. [DOI] [PubMed] [Google Scholar]
- 62. Qin M, Long F, Wu W, Yang D, Huang M, Xiao C, et al Hydrogen sulfide protects against DSS‐induced colitis by inhibiting NLRP3 inflammasome. Free Radic Biol Med 2019; 137:99–109. [DOI] [PubMed] [Google Scholar]
- 63. Yao Y, Zhang X, Chen H‐p, Li L, Xie W, Lan G, et al MicroRNA‐186 promotes macrophage lipid accumulation and secretion of pro‐inflammatory cytokines by targeting cystathionine γ‐lyase in THP‐1 macrophages. Atherosclerosis 2016; 250:122–32. [DOI] [PubMed] [Google Scholar]
- 64. Zhang H, Guo C, Wu D, Zhang A, Gu T, Wang L, et al Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS One 2012; 7:e41147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Z, Han Y, Li L, Lu H, Meng G, Li X, et al The hydrogen sulfide donor, GYY4137, exhibits anti‐atherosclerotic activity in high fat fed apolipoprotein E(‐/‐) mice. Br J Pharmacol 2013; 169:1795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miao L, Xin X, Xin H, Shen X, Zhu Y‐Z. Hydrogen sulfide recruits macrophage migration by integrin β1‐Src‐FAK/Pyk2‐Rac pathway in myocardial infarction. Sci Rep 2016; 6:22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao H, Lu S, Chai J, Zhang Y, Ma X, Chen J, et al Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J Diabetes Complications 2017; 31:1363–9. [DOI] [PubMed] [Google Scholar]
- 68. Wu J, Chen A, Zhou Y, Zheng S, Yang Y, An Y, et al Novel H(2)S‐releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials 2019; 222:119398. [DOI] [PubMed] [Google Scholar]
- 69. Zhang D, Du J, Tang C, Huang Y, Jin H. H2S‐induced sulfhydration: biological function and detection methodology. Front Pharmacol 2017; 8:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Short JD, Downs K, Tavakoli S, Asmis R. Protein Thiol Redox signaling in monocytes and macrophages. Antioxid Redox Signal 2016; 25:816–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barrett WC, DeGnore JP, König S, Fales HM, Keng Y‐F, Zhang Z‐Y, et al Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 1999; 38:6699–705. [DOI] [PubMed] [Google Scholar]
- 72. Kim HS, Asmis R. Mitogen‐activated protein kinase phosphatase 1 (MKP‐1) in macrophage biology and cardiovascular disease. A redox‐regulated master controller of monocyte function and macrophage phenotype. Free Radic Biol Med 2017; 109:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim HS, Ullevig SL, Zamora D, Lee CF, Asmis R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc Natl Acad Sci USA 2012; 109:E2803–E2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. O’Neill Luke AJ. A metabolic roadblock in inflammatory macrophages. Cell Rep 2016; 17:625–6. [DOI] [PubMed] [Google Scholar]
- 75. Rodríguez‐Prados J‐C, Través PG, Cuenca J, Rico D, Aragonés J, Martín‐Sanz P, et al Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 2010; 185:605. [DOI] [PubMed] [Google Scholar]
- 76. Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al Oxidative metabolism and PGC‐1β attenuate macrophage‐mediated inflammation. Cell Metab 2006; 4:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wiliński B, Wiliński J, Somogyi E, Piotrowska J, Góralska M. Atorvastatin affects the tissue concentration of hydrogen sulfide in mouse kidneys and other organs. Pharmacol Rep 2011; 63:184–8. [DOI] [PubMed] [Google Scholar]
- 78. Bełtowski J, Jamroz‐Wiśniewska A. Modulation of h(2)s metabolism by statins: a new aspect of cardiovascular pharmacology. Antioxid Redox Signal 2012; 17:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ma X, Jiang Z. Administration of metformin alleviates atherosclerosis by promoting H2S production via regulating CSE expression. J Cell Physiol 2020; 235:2102–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable.


