Abstract
Bisphenol A (BPA) is an endocrine disruptor that negatively affects spermatogenesis, a process where Sertoli cells play a central role. Thus, in the present study we sought to ascertain whether BPA could modulate the endocannabinoid (eCB) system in exposed mouse primary Sertoli cells. Under our experimental conditions, BPA turned out to be cytotoxic to Sertoli cells with an half-maximal inhibitory concentration (IC50) of ~6.0 µM. Exposure to a non-cytotoxic dose of BPA (i.e., 0.5 μM for 48 h) increased the expression levels of specific components of the eCB system, namely: type-1 cannabinoid (CB1) receptor and diacylglycerol lipase-α (DAGL-α), at mRNA level, type-2 cannabinoid (CB2) receptor, transient receptor potential vanilloid 1 (TRPV1) receptors, and DAGL-β, at protein level. Interestingly, BPA also increased the production of inhibin B, but not that of transferrin, and blockade of either CB2 receptor or TRPV1 receptor further enhanced the BPA effect. Altogether, our study provides unprecedented evidence that BPA deranges the eCB system of Sertoli cells towards CB2- and TRPV1-dependent signal transduction, both receptors being engaged in modulating BPA effects on inhibin B production. These findings add CB2 and TRPV1 receptors, and hence the eCB signaling, to the other molecular targets of BPA already known in mammalian cells.
Keywords: endocrine disruptor, inhibin B, male reproduction, receptor signalling, spermatogenesis
1. Introduction
Bisphenol A (BPA) is widely used to manufacture polycarbonate plastic and epoxy resin for the production of a multitude of consumer products, such as plastic bottles, food and drink containers, dental sealants, medical devices, and even toys and baby bottles [1]. Unsurprisingly, BPA has been detected in human colostrum [2], breast milk [3], urine [4], and saliva [5]. Unfortunately, BPA belongs to the family of endocrine disruptors (EDs), a kind of environmental chemical able to interfere with hormonal function, and hence disrupt hormone-dependent signaling with a severe impact on human health, reproductive events included [6,7,8,9]. Indeed, BPA and other EDs have been shown to negatively affect fertility, not only in females [10,11,12] but also in males [10,11,13,14,15,16].
In testis, Sertoli cells quantitatively and qualitatively support the spermatogenesis by providing a complex nutritional and regulative network indispensable for germ cell maintenance and development [17,18]. BPA specifically hits Sertoli cells by: (i) inducing their proliferation via the expression of metabolic enzymes and oxidative stress-related proteins [19]; (ii) promoting their apoptosis, and consequently that of germ cells, via different signaling pathways [20]; (iii) perturbing actin cytoskeleton [20]; and (iv) damaging tight junctions [14]. Other EDs too, such as dioxins, hamper Sertoli cells expression of secretory products and of specific proteins involved in cell–cell interaction [21]. Altogether, these studies support that the damage of Sertoli cells done by environmental pollutants contribute to the impairment of the spermatogenesis.
In recent years, lipid signals collectively termed “endocannabinoids (eCBs)” have emerged as key-regulators of diverse pathophysiologic processes, and have attracted considerable attention for their potential as diagnostic and/or therapeutic tools in human infertility. On the female side, eCBs regulate folliculogenesis, embryo oviductal transport, blastocyst development, implantation, decidualization, placentation and labor [22]. On the male side, they play a key role in controlling spermatogenesis, the acquisition of sperm fertilizing ability (i.e., sperm viability and motility), capacitation, and sperm-oocyte fusion [23]. The best-characterized eCBs are N-arachidonoylethanolamine, also known as anandamide (AEA), and 2-arachidonoylglycerol (2-AG). These bioactive lipids act by binding to type-1 and type-2 cannabinoid receptors (CB1 and CB2), to the G protein coupled orphan receptor 55 (GPR55), and to transient receptor potential vanilloid type 1 (TRPV1); in addition, the biological activity of eCBs is tightly controlled by metabolic enzymes that synthesize and cleave AEA (N-acylphosphatidylethanolamines-specific phospholipase D (NAPE-PLD), and fatty acid amide hydrolase (FAAH), respectively), or 2-AG (diacylglycerol lipases (DAGL) α and β, and monoacylglycerol lipase (MAGL), respectively) [24].
In previous studies, we have shown that Sertoli cells express the biochemical tools to metabolize AEA, in particular its cleaving enzyme FAAH and its binding CB2 receptor [25,26]. We also showed that AEA signaling plays a major role on Sertoli cell survival and death [17,26], with apparent consequences on the whole spermatogenesis [27,28,29].
Interestingly, BPA has been shown to derange eCB signaling in adult zebrafish and in human hepatocytes [30], much alike other EDs such as phthalates [31]. In addition, BPA has been shown to down-regulate CB1 expression in the hypothalamus of male mice, with subsequent anorexigenic effects [32]. Furthermore, environmentally relevant concentrations of BPA significantly impair spermatogenesis, not only in experimental animal models [33,34] but also in humans [35]. Of note, neonatal exposure to BPA inhibits the correct formation of blood testis barrier [20,36,37]. In any case, it is surprising that only a few studies aimed to identify the specific effects of BPA on Sertoli cells proliferation and functions. Thus, against this background we have been led to investigate whether in exposed mouse primary Sertoli cells BPA could alter the expression levels of specific eCB system components. Moreover, we tested two functional markers of Sertoli cells (transferrin and inhibin B) [38], with the aim of interrogating a possible impact of BPA on cell function through modulation of eCB signaling.
2. Results
2.1. Effect of BPA on Sertoli Cell Viability
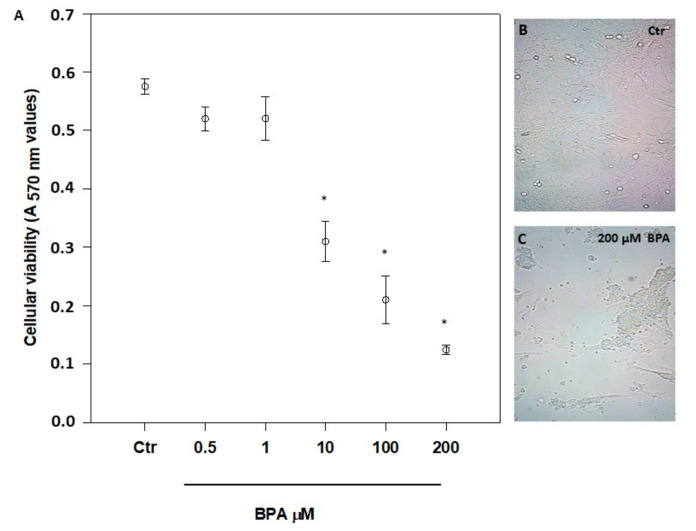
The possible cytotoxicity of BPA was checked by exposing 7-day-old mouse Sertoli cells for 48 h to a concentration range from 1 pM to 200 μM (Figure 1A, and data not shown), as reported elsewhere [14,19,20,30,34]. A significant cytotoxicity was evident only at concentrations >1 μM (p < 0.01 vs. Ctr), with an overt change of morphology at 200 µM BPA (Figure 1B,C). From the viability data shown in Figure 1A, a half maximal inhibitory concentration (IC50) value of 6.2 ± 2.0 μM was calculated. Based on these data, all further experiments were performed at the non-cytotoxic dose of 0.5 μM, in order to avoid cell poisoning and at the same time to reveal specific effects of BPA in living cells. Incidentally, the latter BPA concentration is very close to the value previously measured in human urine (0.65 µM) [39] and, most notably, in human follicular fluid (0.66 µM) [40].
Figure 1.
Effect of increasing concentrations of BPA on mouse Sertoli cell viability (A), and morphology (B,C), after 48 h of treatment. Magnification, ×200. Data are expressed as means ± SEM of 5 independent experiments. * p < 0.01 vs. vehicle-treated controls (Ctr).
2.2. Effect of BPA on Gene Expression of eCB System Elements
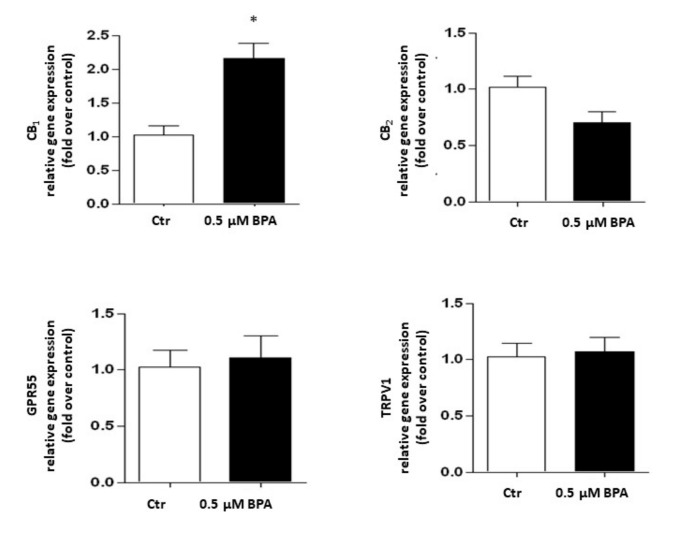
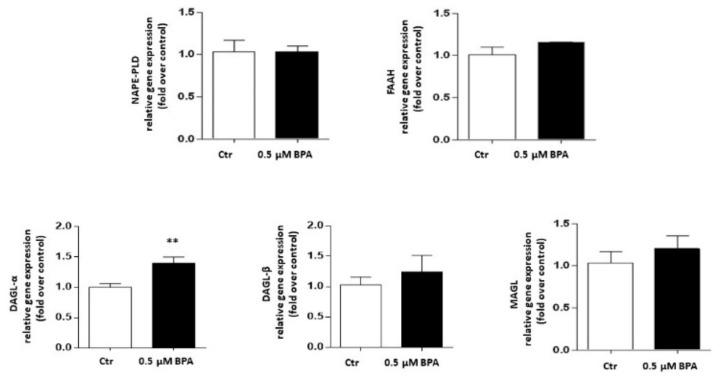
Gene expression of the major components of the eCB system was analyzed in Sertoli cells treated with 0.5 μM BPA or with a vehicle (Control, Ctr) for 48 h. As shown in Figure 2, a significant increase of mRNA level was observed only for the CB1 gene (p < 0.01 vs. Ctr), whereas levels of genes encoding for other eCB-binding receptors, i.e., CB2, GPR55 and TRPV1 channel, were almost comparable to controls (p > 0.05 vs. Ctr). Concerning the eCB metabolic enzymes, BPA significantly increased the mRNA level of DAGL-α, which synthesizes 2-AG (p < 0.05 vs. Ctr), leaving nearly unchanged the levels of either NAPE-PLD, which synthesizes AEA, or DAGL-β and MAGL, which synthesizes and cleaves 2-AG, respectively (p > 0.05 vs. Ctr; Figure 3). In this context it should be recalled that the receptors and metabolic enzymes tested here represent the major elements of the eCB system known so far [24].
Figure 2.
Effect of BPA (0.5 μM for 48 h) on mRNA levels of eCB-binding receptors (CB1, CB2, GPR55 and TRPV1) in exposed Sertoli cells, compared to vehicle-treated controls (Ctr). Gene expression is reported as 2−ΔΔCt values calculated by ΔΔCt method vs. Ctr, posed equal to 1.0. Expression levels of each gene were normalized to β-actin, and data were expressed as means ± SEM of 5 independent experiments. * p < 0.01 vs. Ctr.
Figure 3.
Effect of BPA (0.5 μM for 48 h) on mRNA levels of eCB metabolic enzymes (NAPE-PLD, FAAH, DAGL-α, DAGL-β and MAGL) in exposed Sertoli cells, compared to vehicle-treated controls (Ctr). Gene expression was reported as 2−ΔΔCt values calculated by ΔΔCt method vs. Ctr, posed equal to 1.0. Expression levels of each gene were normalized to β-actin, and data were reported as means ± SEM of 5 independent experiments. ** p < 0.05 vs. Ctr.
2.3. Effect of BPA on Protein Expression of eCB System Components
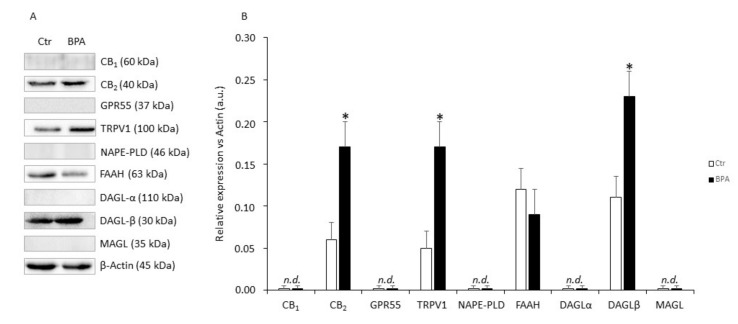
In these experiments, the effects of BPA (0.5 μM for 48 h) on protein expression of eCB system components were evaluated. As shown in Figure 4, in comparison with untreated controls Sertoli cells exposed to BPA expressed significantly higher levels of CB2 (+140%), TRPV1 (+250%), and DAGL-β (+130%) proteins (in all these cases: p < 0.05 vs. Ctr), and a trend towards reduced FAAH (−15%; p > 0.05 vs. Ctr). Conversely, CB1, GPR55, NAPE-PLD, DAGL-α and MAGL proteins in all cases were undetectable under our experimental conditions (p > 0.05).
Figure 4.
Protein expression levels of eCB system in mouse Sertoli cells. (A) Representative Western blot and (B) densitometric analysis of eCB-binding receptors and metabolic enzymes in Sertoli cells exposed to BPA (0.5 μM for 48 h) or to vehicle (Ctr). Data were expressed as mean values (arbitrary units a.u.) ± SEM (n = 3 independent experiments) after normalization with β-actin, used as loading control. * p < 0.05 vs. Ctr.
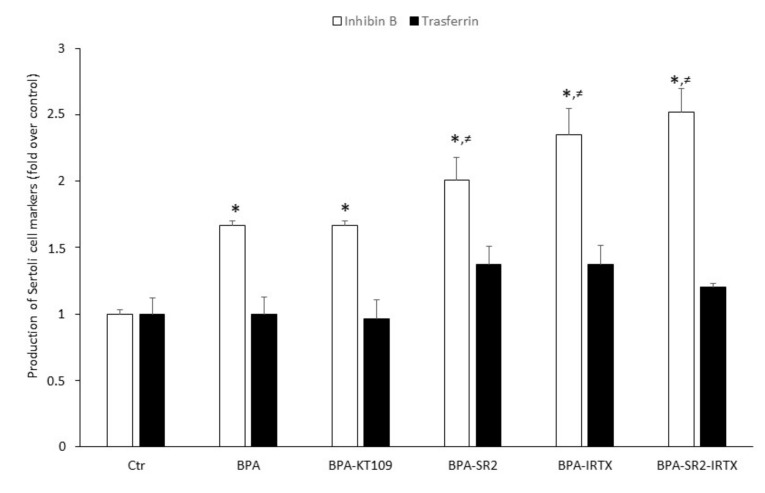
2.4. Effect of BPA on Production of Inhibin B and Transferrin by Sertoli Cells
To ascertain whether increased BPA-dependent expression of CB2, TRPV1 and DAGL-β proteins (and hence their functional activities) could modulate Sertoli cell functions, inhibin B and transferrin production were determined by means of quantitative ELISA tests with specific anti-inhibin B and anti-transferrin antibodies. To this end, Sertoli cells were exposed to 0.5 μM BPA for 48 h in the absence or in the presence of: (i) the selective DAGL-β inhibitor KT109 [41]; (ii) the selective CB2 antagonist SR144528 [42]; the selective TRPV1 antagonist iodoresinferatoxin [42]; or (iii) a combination of the last two compounds. BPA as such was found to significantly increase the production of inhibin B compared to vehicle-treated Sertoli cells (0.335 ± 0.040 vs.0.200 ± 0.025; p < 0.05). A similarly increased value was found when cells were treated with a combination of BPA and KT-109 (Figure 5). Although small, a still significantly higher increase of inhibin B production was detected when cells grown in the presence of BPA were exposed to the two CB2 and TRPV1 antagonists separately (SR144528: 0.412 ± 0.020 and iodoresinferatoxin: 0.469 ± 0.050, vs. 0.335 ± 0.040; p < 0.05) or both antagonists in combination (SR144528 + iodoresinferatoxin: 0.503 ± 0.08 vs. 0.335 ± 0.040; p < 0.05). Finally, transferrin production by Sertoli cells, grown in the absence or in the presence of BPA or a combination of BPA and inhibitor/antagonists, did not significantly increase compared to controls.
Figure 5.
Production of inhibin B and transferrin by mouse Sertoli cells. Cells were cultured for 48 h in the presence of vehicle (Ctr), or of 0.5 μM BPA alone or with 1 μM of KT109, SR144528 (SR2), IRTX, SR2 + IRTX. Results of 3 independent experiments were expressed as fold increase over controls, posed equal to 1.0. * p < 0.05 vs. Ctr; ≠ p < 0.05 vs. BPA-treated cells.
3. Discussion
Sertoli cells are targets of a variety of toxic EDs that induce reproductive dysfunctions [19,20,37]. Environmentally-relevant concentrations of BPA significantly impair spermatogenesis not only in experimental animal models [33,34] but also in humans [35]. In this scenario, mouse prepubertal Sertoli cells are commonly used to determine the impact of xenobiotics [43], because early postnatal life represents the most critical phase for the determination of the final number of Sertoli cells and spermatozoa, which establish the spermatogenic capacity of adult subjects [44,45].
Under our experimental conditions, BPA was cytotoxic at concentrations higher than 1 μM and with an IC50 value (6.2 ± 2.0 μM) comparable to one of the two IC50 values previously reported in mouse Sertoli TM4 cells [19]. However, in the latter immortalized cells BPA showed a biphasic effect [19], which was not apparent in our experiments. This feature could be due to the different experimental conditions used, and/or to a different sensitivity of the two cell types to BPA. Remarkably, in zebrafish male gonads BPA was effective at 0.04 µM [34], a dose that is 2 orders of magnitude lower than the IC50 value we found in mouse Sertoli cells. This finding highlights the limits of translating data from the zebrafish vertebrate model to mammals or even to humans [46]. At any rate, at the non-toxic concentrations utilized here, BPA was able to modulate gene and protein expression of specific eCB system components. Indeed, our results demonstrate that CB1 and DAGL-α mRNAs accumulate in exposed Sertoli cells (Figure 2 and Figure 3, respectively). However, in BPA-treated cells CB2, TRPV1 and DAGL-β protein contents were significantly higher than controls, and FAAH protein content was lower. Interestingly, the present findings are in keeping with our previous reports that documented CB2 and FAAH expression in Sertoli cells [25,26], and extend those data also to TRPV1 and DAGL-β. They also support the general notion that DAGL-α protein is predominantly expressed in the brain, whereas DAGL-β is more abundant in non-neuronal cells [47,48]. Intriguingly, similar disparities between changes in mRNA abundance and protein content have been already described in other cell systems [49], and have been previously observed also within the eCB system by others [50], and by us [51,52,53].
Overall, these data suggest that BPA enhanced eCB signaling triggered by CB2 and TRPV1, an effect that is possibly further supported by increased levels of 2-AG synthesized by DAGL-β. In fact, 2-AG is an agonist at both CB2 [54] and TRPV1 [55]. In keeping with these findings, 2-AG rather than AEA has been shown to contribute to normal progression of spermatogenesis via CB2 signaling [56,57], and also in zebrafish BPA has been shown to increase 2-AG levels [30].
The likely possibility that upregulation of CB2, TRPV1 and DAGL-β could be engaged in the effect of BPA on specific Sertoli cell functions has been confirmed by the finding that BPA significantly increases inhibin B, but not transferrin, production. Such an ability of BPA to increase inhibin B has been already described in immature rat Sertoli cells [58,59], and is shared with other environmental pollutants [60]. Interestingly, blockade of CB2 or TRPV1 receptors further enhanced the effect of BPA on inhibin B production, suggesting that both receptors are able to counterbalance it. Although signal transduction pathways triggered by CB2 and TRPV1 are largely different [42], blocking both receptors at the same time did not further potentiate BPA effect, suggesting that activation of one of them was enough to prevent BPA effect. Moreover, inhibition of DAGL-β in the presence of BPA had no effect on inhibin B production, probably due to a compensation of 2-AG biosynthesis by one of the alternative biosynthetic pathways known [24]. At any rate, the observation that eCB signaling is enhanced by BPA appears in line with the widely recognized pro-homeostatic role of eCBs [23], also in reproductive events [61,62,63,64]. From our results, CB2 and TRPV1 receptors can be added to the list of other known targets of BPA, such as estrogen receptors α [65] and β [66], androgen receptor [67], peroxisome proliferator-activated receptor γ and thyroid hormone receptor [68], G protein-coupled receptor 30 [69], membrane-bound estrogen receptor [70], and in Sertoli cells Fas/FasL, JNKs/p38 MAPK and NF-kB [71]. The molecular chain of events by which BPA stimulates inhibin B remains a relevant issue to be further investigated in independent studies. In this context, it seems noteworthy that previous reports have pointed to caspase 3 as an executioner of BPA in controlling mouse Sertoli cell morphology and viability [72]. Moreover, Sertoli cell adhesion molecules could be impaired upon BPA exposure [73], thus possibly contributing to its overall effect. At present, it remains to be ascertained whether engagement of caspase 3 and/or adhesion molecules might occur through eCB signaling. Certainly, a complex relationship exists among follicle-stimulating hormone (FSH), inhibin B and spermatogenesis. Inhibin B suppresses FSH secretion from the pituitary, thus contributing to the control of FSH-dependent stimulation of Sertoli cell proliferation. Indeed, circulating inhibin B can be considered as a “measure” of Sertoli cell number and sperm concentration [74]. In adult men, high levels of inhibin B are indicative of normal fertility, while low levels are associated with germ cell depletion [75]. However, when an abnormal rise of inhibin B level is accomplished by the decrease of FSH release, both markers indicate spermatogenic injury [76]. In mouse testis, a low level of inhibin B has been detected from post-natal day (PND) 1 to 6, and its expression raises from PND 18 onward [77]. Thus, since in our experiments we utilized Sertoli cells collected from 7-day-old mice, the rise of inhibin B content following BPA stimulation could be due to a deregulation of hypothalamic-pituitary-gonadal axis or of inhibin B expression. Since we found that CB2 and TRPV1 antagonism further increases inhibin B production, this might indicate a possible deregulation of FSH signaling by BPA, especially because of FSH-dependent modulation of eCB signaling in immature Sertoli cells [17,78]. Nevertheless, since inhibin B release is regulated not only by FSH but also by testosterone and even germ cells, the existence of multiple pathways acting synergistically to influence Sertoli cell differentiation makes the identification of a dysregulative mechanism more complex. Finally, the finding that transferrin level is not altered neither by BPA nor by BPA plus eCB system-targeting compounds is not surprising. Indeed, a constitutive secretion of transferrin occurs in primary Sertoli cell cultures from immature mice [79], and the lack of FSH in our culture conditions rules out well-known gonadotropin-dependent stimulative effects [80].
4. Materials and Methods
4.1. Chemicals
Chemicals were of the purest analytical grade. MEM, glutamine, PES, DMSO, collagenase, DNase1, trypsin, BPA (Bisphenol A), ABTS (2,2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt), and hyaluronidase were purchased from Sigma (St. Louis, MO, USA). Rabbit anti-CB1, anti-CB2, anti-GPR55, anti-FAAH, anti-DAGL-α, anti-DAGL-β, anti-MAGL, anti-NAPE-PLD polyclonal antibodies, and KT109 were purchased from Cayman Chemicals (Ann Arbor, MI, USA). Mouse anti-actin antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and rabbit anti-TRPV1 polyclonal antibody was from OriGene Technologies (Rockville, MD, USA). Rabbit anti-inhibin B polyclonal antibody was obtained from Elabscience Biotechnology (Houston, TX, USA), and rabbit anti-transferrin polyclonal antibody was from Proteintech Group (Chicago, IL, USA). Iodoresinferatoxin was obtained from Tocris Cookson (Bristol, UK), whereas SR144528 was a kind gift from Sanofi-Synthelabo (Montpellier, France).
4.2. Animals
Mouse (Mus musculus) Swiss CD1 males (7-day-old; Charles River Laboratories, Lecco, Italy) were housed in an animal facility under controlled temperature (21 ± 1 °C) and light (12 h light/day) conditions. A total number of 60 mice were used to perform the study.
4.3. Ethical Approval
All experimental procedures involving animals and their care were performed in conformity with national and international laws and policies (European Economic Community Council Directive 86/609,OJ 358, 1, 12 December, 1987; European Parliament Council Directive 2010/63/EU, OJ L 276, 20 October 2010; Italian Legislative Decree 116/92, Gazzetta Ufficiale della Repubblica Italiana n. 40, 18, February 1992; National Institutes of Health Guide for the Care and Use of Laboratory Animals, NIH publication no. 85–23, 1985). The method of euthanasia consisted of an inhalant overdose of carbon dioxide (CO2, 10–30%), followed by cervical dislocation. All efforts were made to minimize animal suffering.
4.4. Sertoli Cell Culture
Purified Sertoli cells were isolated from decapsulated testes of 7-day old CD1 mice, as previously described [25]. Sertoli cells (95% pure) were cultured at 34 °C and 5% CO2 for 48 h in serum and phenol-free Dulbecco’s Modified Eagle’s Medium-high glucose, as previously reported [25], supplemented with 2 mM glutamine and penicillin-streptomycin solution, in the presence of DMSO (0.1%) vehicle for controls (Ctr), or with the indicated concentrations of BPA, alone or in the presence of receptor antagonists (CB2 antagonist: 1 μM SR144528 [81]; TRPV1 antagonist: 1 μM iodoresinferatoxin [82,83]) or DAGL-β inhibitor: 1 μM KT109 [84].
4.5. Cytotoxicity Assay
The number of viable cells was evaluated by a colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay that assesses metabolic integrity [85]. Briefly, Sertoli cells were seeded in 96-well plates, and were treated with DMSO (0.1%) vehicle (Ctr), or with increasing concentrations of BPA (from 1 pM to 200 μM). After 48 h of treatment, 10 μL of MTT solution was added to each well at a final concentration of 0.5 mg/mL, and plates were incubated at 37 °C for 2 h. The amount of MTT-formazan product, dissolved in acidified isopropanol, was estimated by measuring the absorbance at 570 nm in a microplate reader (Model 550, BioRad, Hercules, CA, USA).
4.6. Analysis of Gene Expression
Messenger RNAs were extracted from control or BPA-exposed Sertoli cells using TRIzol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA, USA), as per manufacturer’s instructions, and were quantitated spectrophotometrically (NanoDrop, Thermo Scientific ND-2000c). One μg of total mRNA was reverse-transcribed to cDNA, using SuperScript Vilo™ Master Mix (Invitrogen, Carlsbad, CA, USA). Quantitative PCR analysis was performed using SYBR Green I Master and the LightCycler 480 System (Roche, Basel, Switzerland) on a DNA Engine Opticon 2 Continuous Fluorescence Detection System (BioRad, Hercules, CA, USA). The reaction was performed using the following qRT-PCR program: 95 °C for 10 min, followed by 40 amplification cycles of 95 °C for 10 s, optimal annealing temperature (see Table 1) for 30 s, and 72 °C for 30 s. The primers used for the amplification are listed in Table 1, with pertinent references. All data were normalized to the endogenous reference gene β-actin. Relative quantitation of mRNAs was performed by the comparative ΔΔCt method [22,86].
Table 1.
List of the primers used for qRT-PCR analysis.
| Gene | Corresponding Protein | PCR Primers | Annealing T (°C) |
Reference |
|---|---|---|---|---|
| Cnr1 | CB1 | Fw: 5′-CCAAGAAAAGATGACGGCAG-3′ Rev: 5′-AGGATGACACATAGCACCAG-3′ |
57 | [87] |
| Cnr2 | CB2 | Fw: 5′-TCGCTTACATCCTTCAGACAG-3′ Rev: 5′-TCTTCCCTCCCAACTCCTTC-3′ |
57 | [87] |
| Gpr55 | GPR55 | Fw: 5′-ATTCGATTCCGTGGATAAGC-3′ Rev: 5′- ATGCTGATGAAGTAGAGGC -3′ |
57 | [88] |
| Trpv1 | TRPV1 | Fw: 5′-TGAACTGGACTACCTGGAAC-3′ Rev: 5′-TCCTTGAAGACCTCAGCATC-3′ |
57 | [88] |
| Magl | MAGL | Fw: 5′-TTGTAGATACTGGAAGCCC-3′ Rev: 5′-ATGGTGTCCACGTGTTGCAGC-3′ |
62 | [52] |
| Faah | FAAH | Fw: 5′- AGATTGAGATGTATCGCCAG-3′ Rev: 5′- CTTCAGAATGTTGTCCCAC-3′ |
56 | [78] |
| Dagl-α | DAGL-α | Fw: 5′- AATGGCTATCATCTGGCTGAGC-3′ Rev: 5′-TTCCGAGGGTGACATTCTTAGC-3′ |
60 | [52] |
| Dagl-β | DAGL-β | Fw: 5′- TGTCAGCATGAGAGGAACCAT-3′ Rev: 5- CGCCAGGCGGATATAGAGC-3′ |
60 | Designed by Primer blast software |
| Nape-pld | NAPE-PLD | Fw: 5′-AAGTGTGTCTTCTAGGTTCTCC-3′ Rev: 5′-TTGTCAAGTTCCTCTTTGGAACC-3′ |
62 | [52] |
| Act-β | β-actin | Fw: 5′-TGTTACCAACTGGGACGA-3′ Rev: 5′-GTCTCAAACATGATCTGGGTC-3′ |
56 | [78] |
4.7. Western Blotting
At the end of 48 h culture, control or BPA-exposed Sertoli cells were resuspended in RIPA buffer containing a protease inhibitors cocktail for 30 min. The cell lysates were centrifugated for 15 min at 12,000× g at 4 °C, and the resulting supernatants were collected. Lysates (40 μg/sample) were separated on a 12.5% SDS-page and transferred to polyvinylidene difluoride (PVDF) (Hybond C Extra, Amersham, Little Chalfont, UK). Membranes were blocked with 5% (w/w) nonfat dry milk and incubated at 4 °C with specific primary antibodies: anti-actin (1:200); anti-CB1 (1:200); anti-CB2 (1:200); anti-GPR55 (1:200); anti-TRPV1 (1:1000); anti-MAGL (1:200); anti-FAAH (1:200); anti-DAGL-α (1:1000); anti-DAGL-β (1:1000); anti-NAPE-PLD (1:200); anti-inhibin B (1:400), anti-transferrin (1:200), and then for 1 h with a peroxidase-conjugated anti-mouse (1:20,000) or anti-rabbit (1:10,000) secondary antibody. After detection with a chemiluminescence reagent (ECL, Pierce, Rockfort, IL, USA), densitometric quantification was performed with the public domain software NIH Image v.1.62, and was standardized to β-actin (1:200) as a loading control.
4.8. ELISA Assay
Sertoli cells were cultured for 48 h, in the presence of vehicle (Ctr) or of BPA, alone or supplemented with 1 μM of: (a) KT-109; (b) SR144528 (SR2); (c) IRTX; or (d) the combination IRTX + SR2. Protein expression of inhibin B and transferrin was determined by enzyme linked immunosorbent assay (ELISA), as reported [89]. Briefly, wells were coated with Sertoli cell lysates (20 µg/well) in coating buffer (0.05 M Na2CO3, pH 9.6) for 2 h at room temperature. Then, they were incubated with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 1 h, and then for 2 h with anti-transferrin or anti-inhibin B polyclonal antibodies diluted to 1:500 and 1:1000, respectively, with 1% BSA in PBS. In preliminary experiments, these antibodies were found to recognize a single immunoreactive band in Sertoli cell extracts, of the molecular weight expected for inhibin B (45 kDa) and transferrin (77 kDa), respectively. After rinsing three times with 1% BSA in PBS-Tween 20, 100 µl horseradish peroxidase (HRP)-conjugated secondary antibody (diluted 1:5000) were added, and the ELISA plate was further incubated for 1 h at room temperature. Afterwards, enzymatic activity of HRP was determined by adding 100 µl/well of ABTS, then stopped with 100 µl of stop solution (1% SDS), and finally absorbance at 405 nm was measured in a Multiskan ELISA Microplate Reader (ThermoLabsystems, Bevery, MA, USA). The linearity ranges of ELISA against inhibin B and transferrin were ascertained by dose-dependence curves with different amounts (0–40 µg/well) of Sertoli cell extracts. All data were within these linearity ranges, and were expressed as fold increase over controls, posed equal to 1.0.
4.9. Statistical Analysis
Unless otherwise indicated, data are shown as mean ± SEM of at least three different experiments. The Prism 6 programme of GraphPad software (San Diego, CA, USA) was used to test the statistical significance of differences between group means. Comparisons between multiple groups were performed by ANOVA test, followed by Dunnett’s and Duncan’s test. Half-maximal inhibitory concentration (IC50) values were calculated using the OriginPro software version 8.5 (OriginLab Corporation, Northampton, Massachusetts). Changes in gene and protein expression were analyzed using unpaired t-test. Level of p < 0.05 was regarded as statistically significant.
5. Conclusions
Our present results provide unprecedented evidence that the endocrine disruptor BPA deranges the eCB system of primary mouse Sertoli cells towards CB2- and TRPV1-dependent signaling. They also show that both eCB-binding receptors contribute to the effect of BPA on inhibin B production, suggesting their engagement in the modulation of Sertoli cell function. Even though it seems premature to draw any conclusion on the molecular clues at the basis of the overall effect of BPA on Sertoli cells [90], our data add CB2 and TRPV1 to the already known molecular targets of BPA in mammalian cells.
Acknowledgments
The authors wish to thank Gianfranco Ciccone and Annarita Nardecchia for housing mice used in this study.
Abbreviations
| ABTS | 2:2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt |
| 2-AG | 2-Arachidonoylglycerol |
| AEA | N-Arachidonoylethanolamine |
| AU | Arbitrary units |
| BPA | Bisphenol A |
| BSA | Bovine serum albumin |
| CB1 | Type-1 cannabinoid receptor |
| CB2 | Type-2 cannabinoid receptor |
| Ctr | Control |
| DAGL-α | Diacylglycerol lipase-α |
| DAGL-β | Diacylglycerol lipase-β |
| DMSO | Dimethyl sulfoxide |
| eCBs | Endocannabinoids |
| ED | Endocrine disruptors |
| ELISA | Enzyme-linked immunosorbent assay |
| FAAH | Fatty acid amide hydrolase |
| GPR55 | G protein-coupled receptor 55 |
| HRP | Horseradish peroxidase |
| IRTX | Iodoresinferatoxin |
| MAGL | Monoacylglycerol lipase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
| NAPE-PLD | N-acyl phosphatidylethanolamine-specific phospholipase D |
| PBS | Phosphate-buffered saline |
| SR144528 (SR2) | 5-(4-Chloro-3-methylphenyl)-1-(4-methylbenzyl)-N-((1S,2S,4R)-1,3,3-trimethylbicyclo [2.2.1]heptan-2-yl)-1H-pyrazole-3-carboxamide |
| TMB | Tetramethylbenzidine |
| TRPV1 | Transient receptor potential vanilloid type 1 channel |
Author Contributions
Conceptualization, M.M.; Data curation, G.R., B.D., A.R.L., C.L. and A.P.; Funding acquisition, S.C. and M.M.; Methodology, F.F. and R.I.; Software, R.I.; Supervision, G.D. and E.D.; Writing—original draft, G.R. and M.M.; Writing—review & editing, G.R., G.D., S.C. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the University of L’Aquila (RIA 2016-17 project to SC), and by the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 2015 competitive project to MM).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Staples C.A., Dome P.B., Klecka G.M., Oblock S.T., Harris L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36:2149–2173. doi: 10.1016/S0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- 2.Kuruto-Niwa R., Tateoka Y., Usuki Y., Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66:1160–1164. doi: 10.1016/j.chemosphere.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 3.Vandenberg L.N., Chahoud I., Heindel J.J., Padmanabhan V., Paumgartten F.J.R., Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat A.M., Weuve J., Ye X., Jia L.T., Hu H., Ringer S., Hutter K., Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giesbrecht G.F., Ejaredar M., Liu J., Thomas J., Letourneau N., Campbell T., Martin J.W., Dewey D., APrON Study Team Prenatal bisphenol A exposure and dysregulation of infant hypothalamic-pituitary-adrenal axis function: Findings from the APrON cohort study. Environ. Health. 2017;16:47. doi: 10.1186/s12940-017-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colborn T., Vom Saal F., Soto A. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochester J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Rivollier F., Krebs M.O., Kebir O. Perinatal exposure to environmental endocrine disruptors in the emergence of neurodevelopmental psychiatric diseases: Asystematic review. Int. J. Environ. Res. Public Health. 2019;16:1318. doi: 10.3390/ijerph16081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chianese R., Troisi J., Richards S., Scafuro M., Fasano S., Guida M., Pierantoni R., Meccariello R. Bisphenol A in reproduction: Epigenetic effects. Curr. Med. Chem. 2018;25:748–770. doi: 10.2174/0929867324666171009121001. [DOI] [PubMed] [Google Scholar]
- 11.Brehm E., Flaws J.A. Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology. 2019;160:1421–1435. doi: 10.1210/en.2019-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rattan S., Flaws J.A. The epigenetic impacts of endocrine disruptors on female reproduction across generations. Biol. Reprod. 2019;101:635–644. doi: 10.1093/biolre/ioz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong J., Chen F., Wang X., Bai Y., Zhou R., Li Y., Chen L. Exposure of preimplantation embryos to low-dose bisphenol A impairs testes development and suppresses histone acetylation of StAR promoter to reduce production of testosterone in mice. Mol. Cell. Endocrinol. 2016;427:101–111. doi: 10.1016/j.mce.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Tian J., Ding Y., She R., Ma L., Du F., Xia K., Chen L. Histologic study of testis injury after bisphenol A exposure in mice. Toxicol. Ind. Health. 2017;33:36–45. doi: 10.1177/0748233716658579. [DOI] [PubMed] [Google Scholar]
- 15.Lymperi S., Giwercman A. Endocrine disruptors and testicular function. Metabolism. 2018;86:79–90. doi: 10.1016/j.metabol.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Castellini C., Totaro M., Parisi A., Settimio D’Andrea S., Lucente L., Cordeschi G., Francavilla S., Francavilla F., Barbonetti A. Bisphenol A and male fertility: Myths and realities. Front. Endocrinol. 2020;11:353. doi: 10.3389/fendo.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaldi P., Rossi G., Catanzaro G., Maccarrone M. Modulation of the endocannabinoid-degrading enzyme fatty acid amide hydrolase by follicle-stimulating hormone. Vitam. Horm. 2009;81:231–261. doi: 10.1016/S0083-6729(09)81010-8. [DOI] [PubMed] [Google Scholar]
- 18.Kopera I.A., Bilinska B., Cheng C.Y., Mruk D.D. Sertoli–germ cell junctions in the testis: A review of recent data. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:1593–1605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge L.C., Chen Z.J., Liu H., Zhang K.S., Su Q., Ma X.Y., Huang H.B., Zhao Z.D., Wang Y.Y., Giesy J.P., et al. Signaling related with biphasic effects of bisphenol A (BPA) on Sertoli cell proliferation: Acomparative proteomic analysis. Biochim. Biophys. Acta. 2014;1840:2663–2673. doi: 10.1016/j.bbagen.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y., Mruk D.D., Cheng C.Y. Sertoli cells are the target of environmental toxicants in the testis—A mechanistic and therapeutic insight. Expert Opin. Ther. Targets. 2015;19:1073–1090. doi: 10.1517/14728222.2015.1039513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai K.P., Wong M.H., Wong K.C. Effects of TCDD in modulating the expression of Sertoli cell secretory products and markers for cell–cell interaction. Toxicology. 2005;206:111–123. doi: 10.1016/j.tox.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Cecconi S., Rapino C., Di Nisio V., Rossi G., Maccarrone M. The (endo) cannabinoid signaling in female reproduction: What are the latest advances? Prog. Lipid Res. 2020;77:101019. doi: 10.1016/j.plipres.2019.101019. [DOI] [PubMed] [Google Scholar]
- 23.Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maccarrone M. Missing pieces to the endocannabinoid puzzle. Trends Mol. Med. 2020;26:263–272. doi: 10.1016/j.molmed.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Maccarrone M., Cecconi S., Rossi G., Battista N., Pauselli R., Finazzi-Agrò A. Anandamide activity and degradation are regulated by early postnatal aging and follicle-stimulating hormone in mouse Sertoli cells. Endocrinology. 2003;144:20–28. doi: 10.1210/en.2002-220544. [DOI] [PubMed] [Google Scholar]
- 26.Rossi G., Gasperi V., Paro R., Barsacchi D., Cecconi S., Maccarrone M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary Sertoli cells. Endocrinology. 2007;148:1431–1439. doi: 10.1210/en.2006-0969. [DOI] [PubMed] [Google Scholar]
- 27.Grimaldi P., Di Giacomo D., Geremia R. The endocannabinoid system and spermatogenesis. Front. Endocrinol. (Lausanne) 2013;4:192. doi: 10.3389/fendo.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carvalho R.K., Santos M.L., Souza M.R., Rocha T.L., Guimarães F.S., Anselmo-Franci J.A., Mazaro-Costa R. Chronic exposure to cannabidiol induces reproductive toxicity in male Swiss mice. J. Appl. Toxicol. 2018;38:1215–1223. doi: 10.1002/jat.3631. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen J.E., Rolland A.D., Rajpert-De Meyts E., Janfelt C., Jørgensen A., Winge S.B., Kristensen D.M., Juul A., Chalmel F., Jégou B., et al. Characterisation and localisation of the endocannabinoid system components in the adult human testis. Sci. Rep. 2019;9:12866. doi: 10.1038/s41598-019-49177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martella A., Silvestri C., Maradonna F., Gioacchini G., Allarà M., Radaelli G., Overby D.R., Di Marzo V., Carnevali O. Bisphenol A induces fatty liver by an endocannabinoid-mediated positive feedback loop. Endocrinology. 2016;157:1751–1763. doi: 10.1210/en.2015-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forner-Piquer I., Maradonna F., Gioacchini G., Santangeli S., Allarà M., Piscitelli F., Habibi H.R., Di Marzo V., Carnevali O. Dose-specific effects of di-isononyl phthalate on the endocannabinoid system and on liver of female Zebrafish. Endocrinology. 2017;158:3462–3476. doi: 10.1210/en.2017-00458. [DOI] [PubMed] [Google Scholar]
- 32.Suglia A., Chianese R., Migliaccio M., Ambrosino C., Fasano S., Pierantoni R., Cobellis G., Chioccarelli T. Bisphenol A induces hypothalamic down-regulation of the cannabinoid receptor 1 and anorexigenic effects in male mice. Pharmacol. Res. 2016;113:376–383. doi: 10.1016/j.phrs.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Cabaton N.J., Wadia P.R., Rubin B.S., Zalko D., Schaeberle C.M., Askenase M.H., Gadbois J.L., Tharp A.P., Whitt G.S., Sonnenschein C., et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ. Health Perspect. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forner-Piquer I., Beato S., Piscitelli F., Santangeli S., Di Marzo V., Habibi H.R., Maradonna F., Carnevali O. Effects of BPA on zebrafish gonads: Focus on the endocannabinoid system. Environ. Pollut. 2020;264:114710. doi: 10.1016/j.envpol.2020.114710. [DOI] [PubMed] [Google Scholar]
- 35.Meeker J.D., Ehrlich S., Toth T.L., Wright D.L., Calafat A.M., Trisini A.T., Ye X.Y., Hauser R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010;30:532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng C.Y., Wong E.W.P., Lie P.P.Y., Li M.W.M., Su L., Siu E.R., Yan H.H.N., Mannu J., Mathur P.P., Bonanomi M., et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson K.J. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2015;4:e979106. doi: 10.4161/21565562.2014.979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharya I., Basu S., Pradhan B.S., Sarkar H., Nagarajan P., Majumdar S.S. Testosterone augments FSH signaling by upregulating the expression and activity of FSH-receptor in pubertal primate Sertoli cells. Mol. Cell. Endocrinol. 2019;482:70–80. doi: 10.1016/j.mce.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poormoosavi S.M., Behmanesh M.A., Janati S., Najafzadehvarzi H. Level of Bisphenol A in follicular fluid and serum and oocyte morphology in patients undergoing IVF treatment. J. Fam. Reprod. Health. 2019;13:154–159. doi: 10.18502/jfrh.v13i3.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkerson J.L., Ghosh S., Bagdas D., Mason B.L., Crowe M.S., Hsu K.L., Wise L.E., Kinsey S.G., Damaj M.I., Cravatt B.F., et al. Diacylglycerol lipase β inhibition reverses nociceptive behaviour in mouse models of inflammatory and neuropathic pain. Br. J. Pharmacol. 2016;173:1678–1692. doi: 10.1111/bph.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G., Mackie K., et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB₁ and CB₂. Pharmacol. Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moody S., Goh H., Bielanowicz A., Rippon A.P., Loveland K.L., Itman C. Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-nbutyl phthalate. Endocrinology. 2013;154:3460–3475. doi: 10.1210/en.2012-2227. [DOI] [PubMed] [Google Scholar]
- 44.Griswold M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 45.Meroni S.B., Galardo M.N., Rindone G., Gorga A., Riera M.F., Cigorraga S.B. Molecular mechanisms and signaling pathways involved in Sertoli cell proliferation. Front. Endocrinol. (Lausanne) 2019;10:224. doi: 10.3389/fendo.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho S.J., Park E., Baker A., Reid A.Y. Age bias in Zebrafish models of epilepsy: What can we learn from old fish? Front. Cell Dev. Biol. 2020;8:573303. doi: 10.3389/fcell.2020.573303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bisogno T., Howell F., Williams G., Minassi A., Cascio M.G., Ligresti A. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu K.-L., Tsuboi K., Adibekian A., Pugh H., Masuda K., Cravatt B.F. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 2012;8:999–1007. doi: 10.1038/nchembio.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janitz M. Assigning functions to genes-the main challenge of the post genomics era. Rev. Physiol Biochem. Pharmacol. 2007;159:115–129. doi: 10.1007/112_2007_0703. [DOI] [PubMed] [Google Scholar]
- 50.Colombo G., Rusconi F., Rubino T., Cattaneo A., Martegani E., Parolaro D., Bachi A., Zippel R. Transcriptomic and proteomic analyses of mouse cerebellum reveals alterations in Ras GRF1 expression following in vivo chronic treatment with delta 9-tetra-hydrocannabinol. J. Mol. Neurosci. 2009;37:111–122. doi: 10.1007/s12031-008-9114-2. [DOI] [PubMed] [Google Scholar]
- 51.Pasquariello N., Catanzaro G., Marzano V., Amadio D., Barcaroli D., Oddi S., Federici G., Urbani A., Finazzi Agrò A., Maccarrone M. Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J. Biol. Chem. 2009;284:29413–29426. doi: 10.1074/jbc.M109.044412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bari M., Tedesco M., Battista N., Pasquariello N., Pucci M., Gasperi V., Scaldaferri M.L., Farini D., De Felici M., Maccarrone M. Characterization of the endocannabinoid system in mouse embryonic stem cells. Stem. Cells Dev. 2011;20:139–147. doi: 10.1089/scd.2009.0515. [DOI] [PubMed] [Google Scholar]
- 53.Cecconi S., Rossi G., Oddi S., Di Nisio V., Maccarrone M. Role of major endocannabinoid-binding receptors during mouse oocyte maturation. Int. J. Mol. Sci. 2019;20:2866. doi: 10.3390/ijms20122866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugiura T., Kishimoto S., Oka S., Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Zygmunt P.M., Ermund A., Movahed P., Andersson D.A., Simonsen C., Jönsson B.A.G., Blomgren A., Birnir B., Bevan S., Eschalier A., et al. Monoacylglycerols activate TRPV1—A link between phospholipase C and TRPV1. PLoS ONE. 2013;8:e81618. doi: 10.1371/journal.pone.0081618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grimaldi P., Orlando P., Di Siena S., Lolicato F., Petrosino S., Bisogno T., Geremia R., De Petrocellis L., Di Marzo V. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:11131–111316. doi: 10.1073/pnas.0812789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Giacomo D., De Domenico E., Sette C., Geremia R., Grimaldi P. Type 2 cannabinoid receptor contributes to the physiological regulation of spermatogenesis. FASEB J. 2016;30:1453–1463. doi: 10.1096/fj.15-279034. [DOI] [PubMed] [Google Scholar]
- 58.Depuydt C.E., Mahmoud A.M., Dhooge W.S., Schoonjans F.A., Comhaire F.H. Hormonal regulation of inhibin B secretion by immature rat Sertoli cells in vitro: Possible use as a bioassay for estrogen detection. J. Androl. 1999;20:54–62. [PubMed] [Google Scholar]
- 59.Monsees T.K., Franz M., Gebhardt S., Winterstein U., Schill W.B., Hayatpour J. Sertoli cells as a target for reproductive hazards. Andrologia. 2000;32:239–246. doi: 10.1046/j.1439-0272.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 60.Reis M.M.S., Moreira A.C., Sousa M., Mathur P.P., Oliveira P.F., Alves M.G. Sertoli cell as a model in male reproductive toxicology: Advantages and disadvantages. J. Appl. Toxicol. 2015;35:870–883. doi: 10.1002/jat.3122. [DOI] [PubMed] [Google Scholar]
- 61.Cobellis G., Meccariello R., Chianese R., Chioccarelli T., Fasano S., Pierantoni R. Effects of neuroendocrine CB1 activity on adult Leydig cells. Front. Endocrinol. (Lausanne) 2016;7:47. doi: 10.3389/fendo.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barchi M., Innocenzi E., Giannattasio T., Dolci S., Rossi P., Grimaldi P. Cannabinoid receptors signaling in the development, epigenetics, and tumours of male germ cells. Int. J. Mol. Sci. 2019;21:25. doi: 10.3390/ijms21010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carvalho R.K., Andersen M.L., Mazaro-Costa R. The effects of cannabidiol on male reproductive system: A literature review. J. Appl. Toxicol. 2020;40:132–150. doi: 10.1002/jat.3831. [DOI] [PubMed] [Google Scholar]
- 64.Cecconi S., Rossi G., Castellucci A., D’Andrea G., Maccarrone M. Endocannabinoid signaling in mammalian ovary. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;178:6–11. doi: 10.1016/j.ejogrb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Gould J.C., Leonard L.S., Maness S.C., Wagner B.L., Conner K., Zacharewski T., Safe S., McDonnell D.P., Gaido K.W. Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998;142:203–214. doi: 10.1016/S0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 66.Kuiper G.G., Lemmen J.G., Carlsson B., Corton J.C., Safe S.H., van der Saag P.T., van der Burg B., Gustafsson J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 67.Lee H.J., Chattopadhyay S., Gong E.Y., Ahn R.S., Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol. Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- 68.Richter A.C., Birnbaum L.S., Farabollini F., Newbold R.R., Rubin B.S., Talsness C.E., Vandenbergh J.G., Walser-Kuntz D.R., vom Saal F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong S., Terasaka S., Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ. Pollut. 2011;159:212–218. doi: 10.1016/j.envpol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Viñas R., Jeng W.J., Watson C.S. Non-genomic effects of xenoestrogen mixtures. Int. J. Environ. Res. Public Health. 2012;9:2694–2714. doi: 10.3390/ijerph9082694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qi S., Fu W., Wang C., Liu C., Quan C., Kourouma A., Yan M., Yu T., Duan P., Yang K. BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod. Toxicol. 2014;50:108–116. doi: 10.1016/j.reprotox.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 72.Iida H., Maehara K., Doiguchi M., Mōri T., Yamada F. Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod. Toxicol. 2003;17:457–464. doi: 10.1016/S0890-6238(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 73.Menegazzo M., Zuccarello D., Luca G., Ferlin A., Calvitti M., Mancuso F., Calafiore R., Foresta C. Improvements in human sperm quality by long-term in vitro co-culture with isolated porcine Sertoli cells. Hum. Reprod. 2011;26:2598–2605. doi: 10.1093/humrep/der248. [DOI] [PubMed] [Google Scholar]
- 74.Luisi S., Florio P., Reis F.M., Petraglia F. Inhibins in female and male reproductive physiology: Role in gametogenesis, conception, implantation and early pregnancy. Hum. Reprod. Update. 2005;11:123–135. doi: 10.1093/humupd/dmh057. [DOI] [PubMed] [Google Scholar]
- 75.Stewart J., Turner K.J. Inhibin B as a potential biomarker of testicular toxicity. Cancer Biomark. 2005;1:75–91. doi: 10.3233/CBM-2005-1109. [DOI] [PubMed] [Google Scholar]
- 76.Meachem S.J., Nieschlag E., Simoni M. Inhibin B in male reproduction: Pathophysiology and clinical relevance. Eur. J. Endocrinol. 2001;145:561–571. doi: 10.1530/eje.0.1450561. [DOI] [PubMed] [Google Scholar]
- 77.Kim Y., Kim J.S., Song M.S., Seo H.S., Kim J.C., Bae C.S., Kim S., Shin T., Kim S.H., Moon C. The expression and localization of inhibin isotypes in mouse testis during postnatal development. J. Vet. Sci. 2008;9:345–349. doi: 10.4142/jvs.2008.9.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grimaldi P., Pucci M., Di Siena S., Di Giacomo D., Pirazzi V., Geremia R., Maccarrone M. The faah gene is the first direct target of estrogen in the testis: Role of histone demethylase LSD1. Cell Mol. Life Sci. 2012;69:4177–4190. doi: 10.1007/s00018-012-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huleihel M., Zeyse D., Lunenfeld E., Zeyse M., Mazor M. Induction of transferrin secretion in murine Sertoli cells by FSH and IL-1: The possibility of different mechanism(s) of regulation. Am. J. Rep. Immun. 2002;47:112–117. doi: 10.1034/j.1600-0897.2002.0o054.x. [DOI] [PubMed] [Google Scholar]
- 80.Sylvester F.R., Griswold M.D. Molecular biology of iron transport in the testis. In: David K., editor. Molecular Biology of the Male Reproductive System. 1st ed. Academic Press; London, UK: 2012. p. 315. [Google Scholar]
- 81.Dhopeshwarkar A., Mackie K. Functional selectivity of CB2 cannabinoid receptor ligands at a canonical and noncanonical pathway. J. Pharmacol. Exp. Ther. 2016;358:342–351. doi: 10.1124/jpet.116.232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bashashati M., Fichna J., Piscitelli F., Capasso R., Izzo A.A., Sibaev A., Timmermans J.P., Cenac N., Vergnolle N., Di Marzo V., et al. Targeting fatty acid amide hydrolase and transient receptor potential vanilloid-1 simultaneously to modulate colonic motility and visceral sensation in the mouse: A pharmacological intervention with N-arachidonoyl-serotonin (AA-5-HT) Neurogastroenterol. Motil. 2017;29:e13148. doi: 10.1111/nmo.13148. [DOI] [PubMed] [Google Scholar]
- 83.Zhang M., Liu Y., Hu Z., Zhou Y., Pi Y., Guo L., Wang X., Chen X., Li G., Zhang L. TRPV1 attenuates intracranial arteriole remodeling through inhibiting VSMC phenotypic modulation in hypertension. Histochem. Cell Biol. 2017;147:511–521. doi: 10.1007/s00418-016-1512-x. [DOI] [PubMed] [Google Scholar]
- 84.Luk J., Lu Y., Ackermann A., Peng X., Bogdan D., Puopolo M., Komatsu D.E., Tong S., Ojima I., Rebecchi M.J., et al. Contribution of diacylglycerol lipase β to pain after surgery. J. Pain Res. 2018;11:473–482. doi: 10.2147/JPR.S157208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 86.Pucci M., Pasquariello N., Battista N., Di Tommaso M., Rapino C., Fezza F., Maccarrone M. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012;287:15466–15478. doi: 10.1074/jbc.M111.314880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Compagnucci C., Di Siena S., Bustamante M.B., Di Giacomo D., Di Tommaso M., Maccarrone M., Sette C. Type-1 (CB1) cannabinoid receptor promotes neuronal di_erentiation and maturation of neural stem cells. PLoS ONE. 2013;8:e54271. doi: 10.1371/journal.pone.0054271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pucci M., D’Addario C. Assessing gene expression of the endocannabinoid system. Methods Mol. Biol. 2016;1412:237–246. doi: 10.1007/978-1-4939-3539-0_24. [DOI] [PubMed] [Google Scholar]
- 89.Gasperi V., Fezza F., Pasquariello N., Bari M., Oddi S., Agrò A.F., Maccarrone M. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell. Mol. Life Sci. 2007;64:219–229. doi: 10.1007/s00018-006-6445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maccarrone M., Rapino C., Francavilla F., Barbonetti A. Cannabinoid signalling and effects of cannabis on the male reproductive system. Nat. Rev. Urol. 2020 doi: 10.1038/s41585-020-00391-8. [DOI] [PubMed] [Google Scholar]