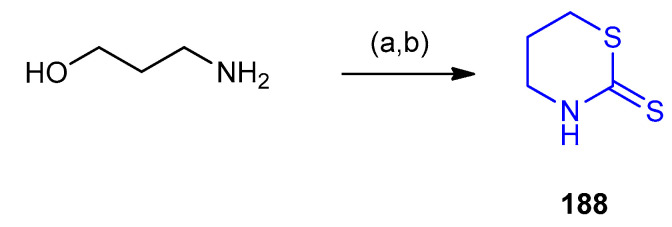Abstract
Thiazinanes and its isomeric forms represent one of the most important heterocyclic compounds, and their derivatives represented a highly potent drug in disease treatment such as, 1,1-dioxido-1,2-thiazinan-1,6-naphthyridine, which has been shown to have anti-HIV activity by a mechanism that should work as anti-AIDS treatment, while (Z)-methyl 3-(naphthalen-1-ylimino)- 2-thia-4-azaspiro[5 5]undecane-4-carbodithioate showed analgesic activity, cephradine was used as antibiotic and chlormezanone was utilized as anticoagulants. All publications were interested in the chemistry of thiazine (partially or fully unsaturated heterocyclic six-membered ring containing nitrogen and sulfur), but no one was dealing with thiazinane itself which encouraged us to shed new light on these interesting heterocycles. This review was focused on the synthetic approaches of thiazinane derivatives and their chemical reactivity.
Keywords: biologic activity, fused-heterocycles, spiro compounds, structures, thiazinanes
1. Introduction
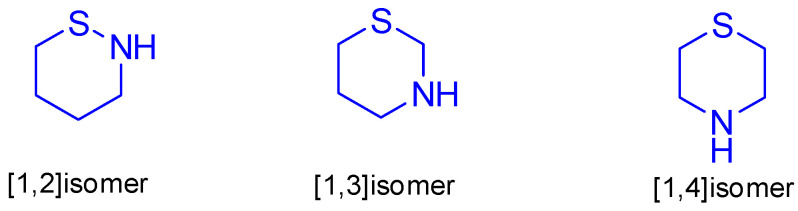
Nitrogen–sulfur containing heterocycles represent a widespread group of heterocyclic compounds. These types of heterocycles constructed a large number of drugs used in the treatment of a variety of diseases. Thiazinane resembles a compound containing nitrogen and sulfur on its structure. It is a fully saturated thiazine six-membered ring containing two hetero-atoms nitrogen and sulfur in a three isomeric structures [1,2]thiazinane, [1,3]thiazinane and [1,4]thiazinane as mentioned below (Figure 1).
Figure 1.
Isomeric forms of thiazinane.
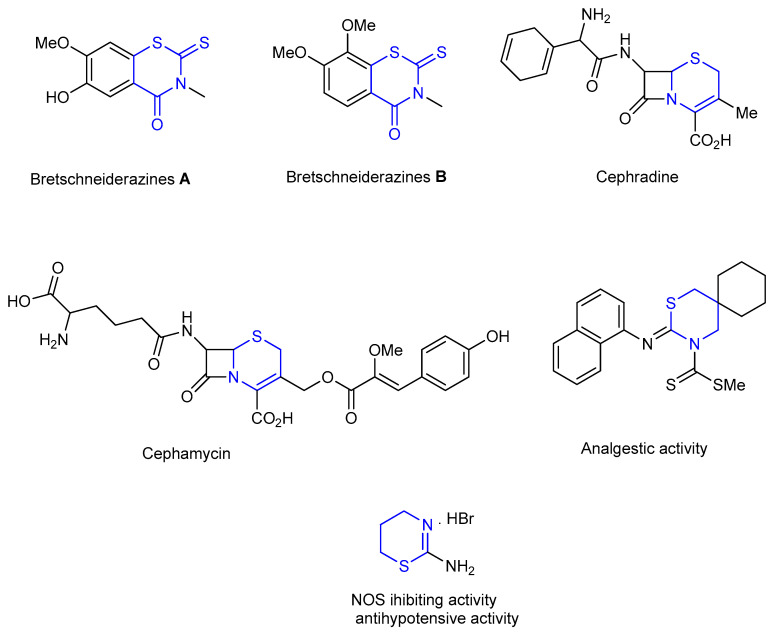
1,3-Thiazine framework represented an important structural motif presented in natural products (bretschneiderazines A & B) [1] (Figure 2) and bioactive compounds [2,3,4]. The well-known antibiotics, cephamycin and cephradine (cephalosporin class of β-lactam antibiotics) containing a 1,3-thiazine skeleton [2] (Figure 2).
Figure 2.
Natural products and bioactive molecules with the 1,3-thiazine framework.
In addition, several synthetic 1,3-thiazine derivatives shown various biologic activities such as analgesic [3], antihypotensive [4] and NOS (nitric oxide synthases) inhibiting activities (Figure 2) [4].
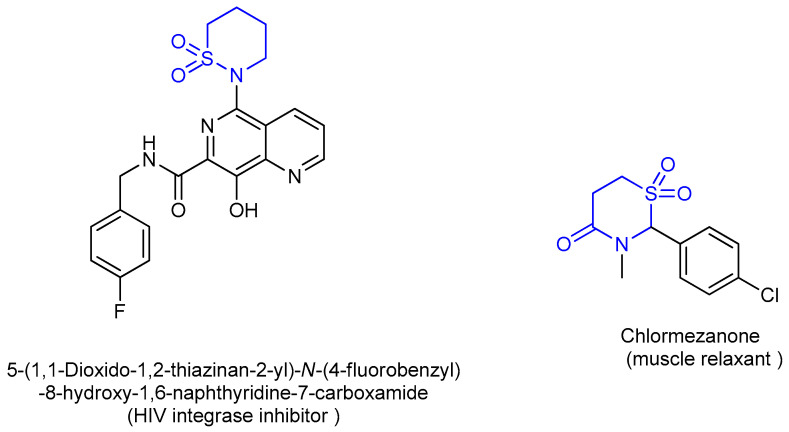
1,1-Dioxido-1,2-thiazinan-1,6-naphthyridine is an HIV integrase inhibitor currently undergoing evaluation for the treatment of AIDS (acquired immune deficiency syndrome) (Figure 3) [5].
Figure 3.
Structure of 1,1-dioxido-1,2-thiazinan-1,6-naphthyridine chlormezanone.
Chlormezanone (Figure 3) is a centrally acting muscle relaxant [6]. It was introduced into human therapy as a racemic monosubstance, later also in combination with codeine phosphate and paracetamol. Chloromezanone (Figure 3) was widely used as anticoagulant [7]. Other derivatives have shown wide range activities as antimicrobial [8,9] and peptic ulcer treatment [10] and anti-inflammatory [11].
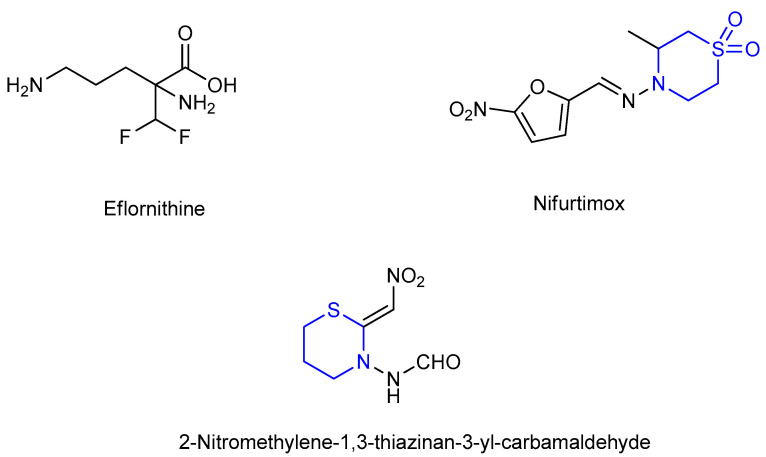
Eflornithine (α-difluoromethylornithine), an ornithine decarboxylase inhibitor, is active against second-stage Trypanosoma brucei gambiensis [12] and has been used in conjunction with nifurtimox against Trypanosoma brucei [13,14] (Figure 4). In addition, 2-nitromethylene-1,3-thiazinan-3-yl- carbam-aldehyde was used as an insecticide [15] (Figure 4).
Figure 4.
Molecular structure of some bioactive thiazinane derivatives.
On the other hand, thiazinanones, are very interesting compounds due to their important role in medicinal chemistry [16,17,18]. It has been reported that, substituted thiazinanones exhibited antitumor [19], antifungal activity [20] and antimalarial activity [21], as well as antioxidant activity [22]. Reactions of amine, carbonyl compounds and a mercapto acid in one-pot three-component condensation or a two-step process afforded thiazinanone derivatives [20].
2. Chemistry of Thiazinanes
2.1. Synthesis of Thiazinanes
2.1.1. Synthesis of 1,2-thiazinanes
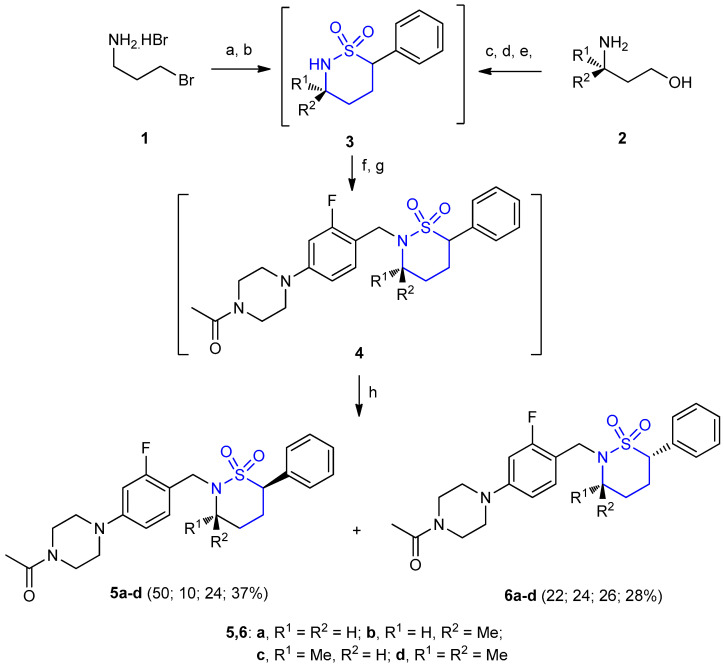
1,2-Thiazinane-1,1-dioxide derivatives 5a–d (yields 10–50%) and 6a–d (yields 22–28%) as diastereoisomers were synthesized from the corresponding amino-halides 1 or amino-alcohols 2. The sultam rings were constructed according to the method of Lee et al. [23] Compounds 1 were reacted with phenylmethanesulfonyl chloride in presence of triethylamine (Et3N) gave the secondary sulfonamides, treatment with base facilitate cyclization to the sultam ring intermediates 3. Similar to 1, derivatives of compound 2 were reacted with phenylmethanesulfonyl chloride and triethylamine, followed by treatment with NaCl yielded the alkyl bromide intermediates. The latter were treated with a base gave the sultam ring intermediates 3. Treatment of 3 with sodium hydride and 4-bromo-1-(bromomethyl)-2-fluorobenzene gave N-benzyl sultam intermediates 4. Intermediates 4 were subjected to Buchwald–Hartwig amination by reacting 2-dicyclohexyl phosphino-2′,6′-diisopropoxybiphenyl (RuPhos) (as a reagent in palladium-catalyzed cross-coupling) [24] with N-acetylpiperazine to give the sultam products as mixtures of enantiomers and diastereomers 5 and 6, which has been separated using chiral supercritical fluid chromatography (SFC) (Scheme 1) [11].
Scheme 1.
Diasereoselective synthesis of 1,2-thiazinane-1,1-dioxide derivatives 5,6a–d. (a) BnSO2Cl, Et3N, THF, 0–23 °C; (b) n-BuLi, (i–Pr)2NH, phenanthroline, THF, −78 °C, 42.59% over 2 steps; (c) BnSO2Cl, Et3N, THF, 0–23 °C; (d) NaCl, DMF, 80 °C; (e) n-BuLi, (i–Pr)2NH, phenanthroline, THF, −78 °C, 21.42% over 3 steps; (f) 4-bromo-1-(bromomethyl)-2-fluorobenzene, NaH, DMF, 0 °C; (g) Pd(OAc)2, RuPhos, Cs2CO3, N-acetyl-piperazine, 1,4-dioxane, 80 °C, 16–73% over 2 steps; (h) chiral column SFC purification.
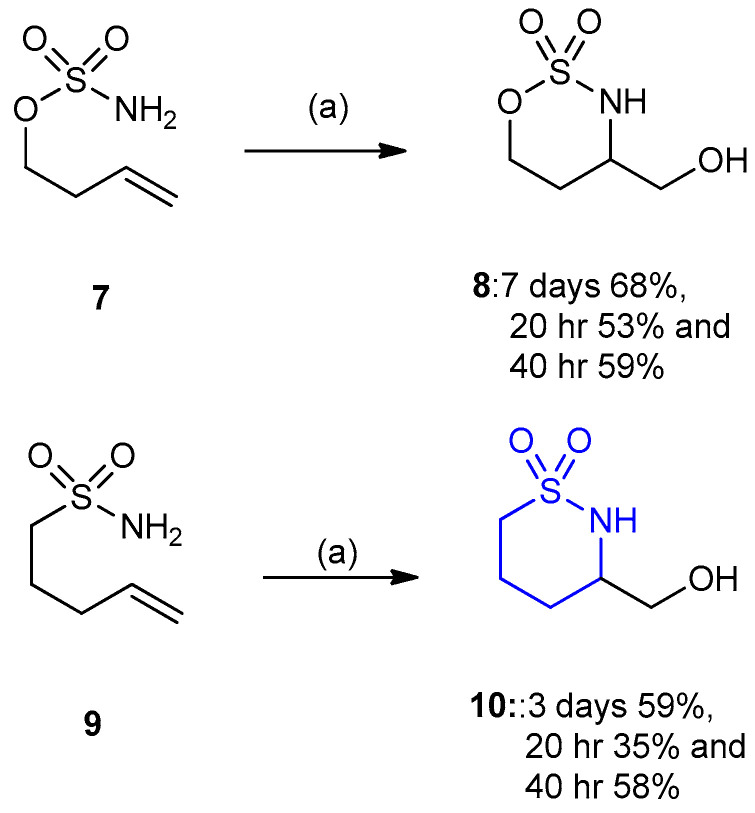
Homo-allylic sulfamate ester 7 and sulfonamide 9 were useful substrates for the Tethered Aminohydroxylation (TA) reaction. The sulfamate ester 7 was underwent the TA reaction giving 1,2,3-oxathiazinane product 8 (yields 53–68%). In contrast, the sulfonamide (pent-4-ene-1- sulfonamide) 9 gave 1,2-thiazinane product (1,1-dioxo-[1,2]thiazinan-3-yl) methanol) 10 (yields 35–59%) under the same conditions (Scheme 2) [25].
Scheme 2.
Synthesis of (1,1-dioxo-[1,2]thiazinan-3-yl)methanol 10. (a) n-PrOH–H2O, NaOH (0.92 equiv.), t-BuOCl (1.0 equiv.), EtN(i-Pr)2 (5 mol%), K2OsO4·2H2O (4 mol%).
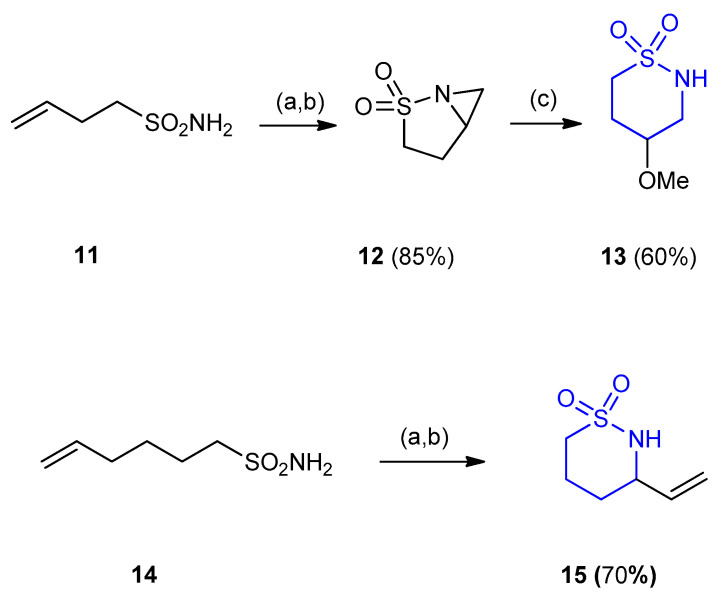
But-3-ene-1-sulfonamide 11 underwent intramolecular aziridination to give the bicyclic aziridines 12. Reaction of 5-hexenyl-substituted sulfonamide 14 only furnished the product derived from allylic insertion 3-vinyl [1,2]thiazinane-1,1-dioxide 15 (yield 70%). Treatment of azabicyclic sulfonamide 12 (2-thia-1-azabicyclo-[3,1,0]hexane-2,2-dioxide) with p-toluenesulfonic acid (p-TsOH) resulted in ring-opening of the aziridine 12 at the more substituted position affording the six-membered ring product 4-methoxy-1,2-thiazinane-1,1-dioxide (13) (yield 60%). The aziridination ring-opening was facilitated in the presence of Lewis acid (Scheme 3) [26].
Scheme 3.
Synthesis of 4-methoxy-1,2-thiazinane-1,1-dioxide (13) and 3-vinyl [1,2]thiazinane- 1,1-dioxide (15). (a) PhI(OAc)2; (b) Rh2(OAc)4, MgO, CH2Cl2, 45 °C, under argon atmosphere, 48 h; (c) p-TsOH, MeOH, H+.
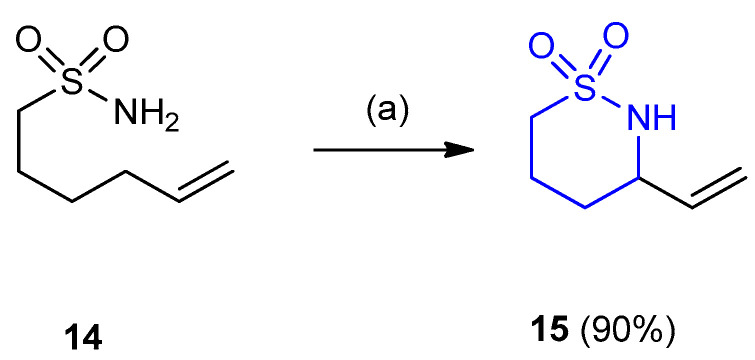
Unsaturated sulfonamide (hex-5-ene-1-sulfonamide) (14) underwent intramolecular aziridination catalyzed by Rh2(OAc)4 with PhI(OAc)2 and Al2O3 to give the corresponding 3-vinyl-1,2-thiazinane-1,1-dioxide (15) (yield 90%) (Scheme 4) [27].
Scheme 4.
Synthesis of 3-vinyl [1,2]thiazinane-1,1-dioxide (15). (a) PhI(OAc)2 (0.02 equiv.), Rh2(OAc)4 (1.5 equiv.), Al2O3 (2.5 equiv.), CH2Cl2, 40 °C, 3 h.
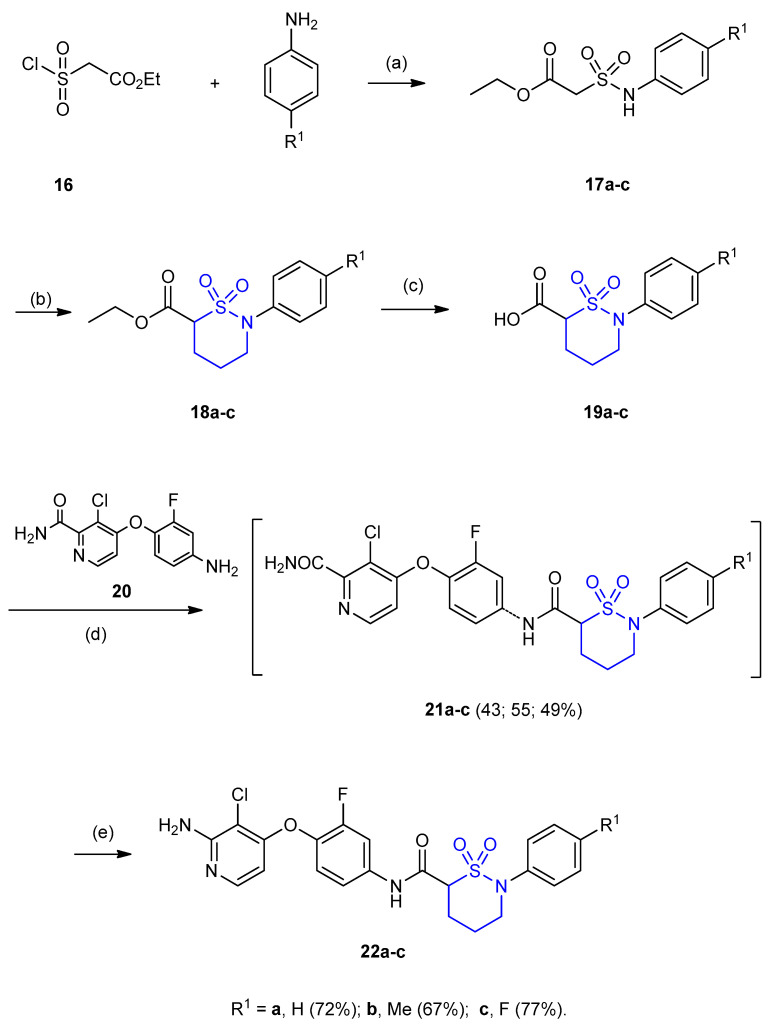
Reactions of ethyl 2-(chlorosulfonyl) acetate (16) with amines furnished sulfonamides 17a–c. Upon treatment of 17a–c with 1-bromo-3-chloropropane in DMF and in presence of K2CO3 gave the six-membered cyclic sulfamoyl acetamide esters (ethyl 2-aryl-1,2-thiazinane-6-carboxylate-1,1-di-oxide) 18a–c. Hydrolysis of 18a–c using methanolic KOH gave 19a–c. Coupling of 19a–c with 4-(4-amino-2-fluorophenoxy)-3-chloropicolinamide (20), under 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/HCl and N,N-dimethylpyridin-4-amine (DMAP) conditions in THF yielding intermediates 2-substituted-1,2-thiazinane-6-carboxamide-1,1-dioxide 21a–c (yields 43–55%). Compound 21a–c underwent Hoffman rearrangement using iodobenzenediacetate furnished 2-amino-3-chloropyridin 2-substituted-1,2-thiazinane-6-carboxamide-1,1-dioxides 22a–c in yields 58–68% (Scheme 5) [28].
Scheme 5.
Synthesis of 2-amino-3-chloropyridin 2-substituted-1,2-thiazinane-6-carboxamide-1,1-dioxides 22a–c. (a) THF, Et3N, 0 °C, then at rt 1 h; (b) 1-bromo-3-chloropropane, K2CO3, DMF; (c) NaOH, MeOH/H2O, 3 h; (d) EDC, HCl, DMAP; (e) ethyl acetate/CH3CN/H2O (2:2:1), PhI(OAc)2, rt, 2 h.
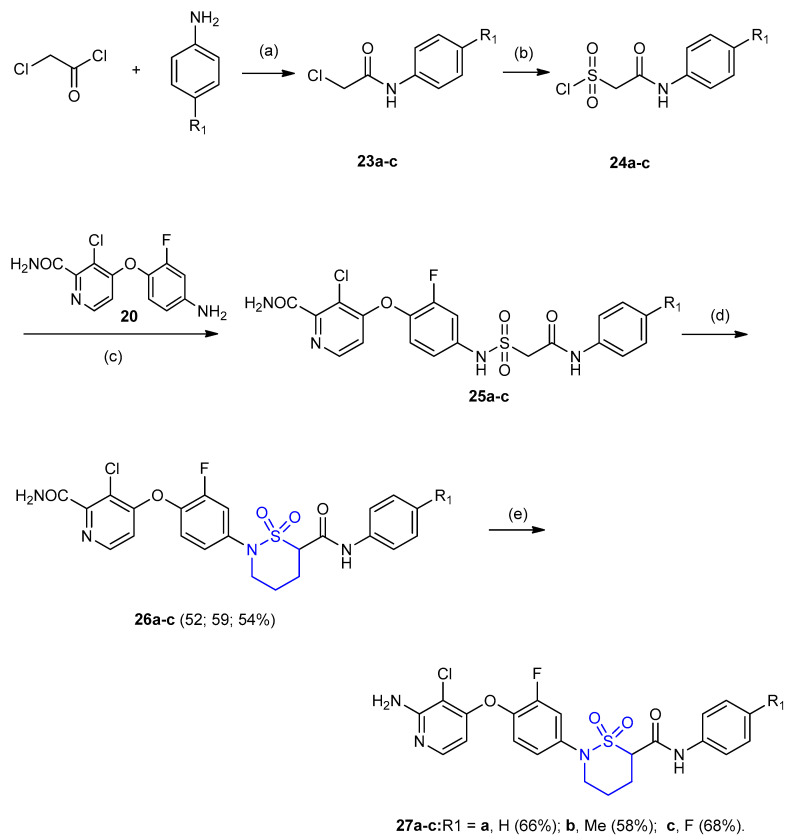
In addition, the isomeric six-membered sulfamoyl acetamides 27a–c were obtained from coupling between chloroacetyl chloride and substituted anilines to give compounds 23a–c, which were converted to sulfamoyl chlorides 24a–c in the presence of sodium sulfite followed by phosphorous pentachloride (PCl5). Coupling of substituted anilines (4-(4-amino-2-fluorophenoxy) -3-chloropicolinamide) 20a–c with sulfamoyl chlorides 24a–c gave sulfamoyl acetamides 25a–c in presence of N,N-diisopropylethylamine (DIPEA) in dry THF. Treatment of 25a–c with 1,3-bromochloropropane in the presence of potassium carbonate gave cyclic sulfamoyl acetamides 26a–c (yields 52–59%). Hoffman rearrangement in compounds 26a–c using PhI(OAc)2 as a mediator yielded sulfamoyl acetamides 27a–c in moderate yields 58–68% (Scheme 6) [28].
Scheme 6.
Synthesis of sulfamoyl acetamides 27a–c. (a) Et3N, toluene; (b) (i) Na2SO3, EtOH, (ii) PCl5; (c) DIPEA THF, 1 h; (d) 1-bromo-3-chloropropane, K2CO3, DMF, 60 °C; (e) ethyl acetate/CH3CN/H2O, PhI(OAc)2, rt. 2 h.
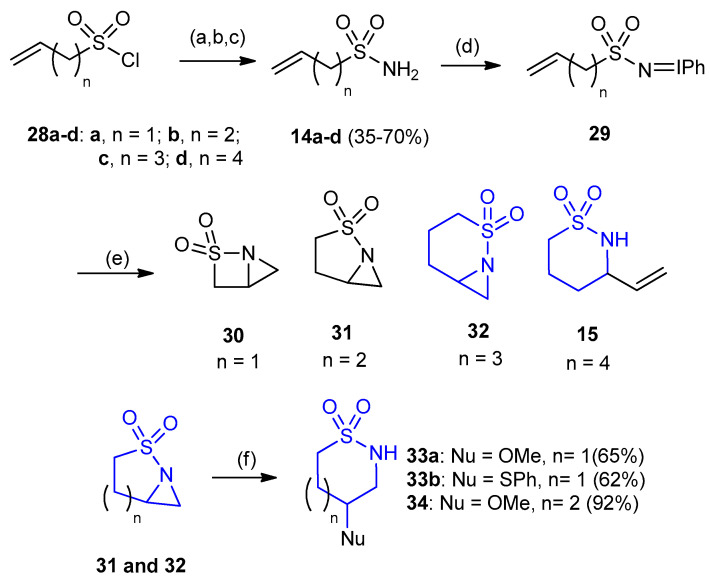
ω-Alkene-1-sulfonamides 14a–d was prepared by aminolysis of ω-alkene-1-sulfonyl chlorides 28a–d. Allylsulfonamide (29a) did not lead to the highly strained bicyclic [2.1.0] structure 30. In contrast, the higher homologues 14b,c gave bicyclic aziridines 31 and 32, respectively. However, sulfonamide 28d under the same conditions gave rise to the allylic insertion product (3-vinyl-1,2-thiazinane-1,1-dioxide) 15. Using different types of nucleophiles (alcohol, thiophenol, allyl magnesium bromide, benzylamine) afforded aziridine ring-opened products in good yields with C–O, C–S, C–C or C–N bond formation. Ring-opening of the aziridine at the more substituted site take place in case of compounds 31 and 32, leading to six- and seven-membered ring products 4-methoxy-1,2-thiazinane-1,1-dioxide (33a) (Yield 65%), 4-(phenylthio)-1,2-thiazinane-1,1-dioxide (33b) (Yield 62%) and 4-methoxy-1,2-thiazepan-1,1-dioxide (34) (yield 92%), respectively using copper (I) or (II) trifluoromethanesulfonate (Cu (I or II) OTf) and sodium hydride as reagents (Scheme 7) [29].
Scheme 7.
Synthesis of 4-(phenylthio)-1,2-thiazinane-1,1-dioxide (33b) and 4-methoxy-1,2-thiazepan- 1,1-dioxide (34). (a) Na2SO3, H2O, 60–125 °C; (b) POCl3, 130 °C; (c) aq. NH3, CH3CN, 0 °C; (d) PhI(OAc)2, KOH, MeOH; (e) 10% Cu (I or II) OTf, CH3CN; (f) NaH, BF3·OEt2.
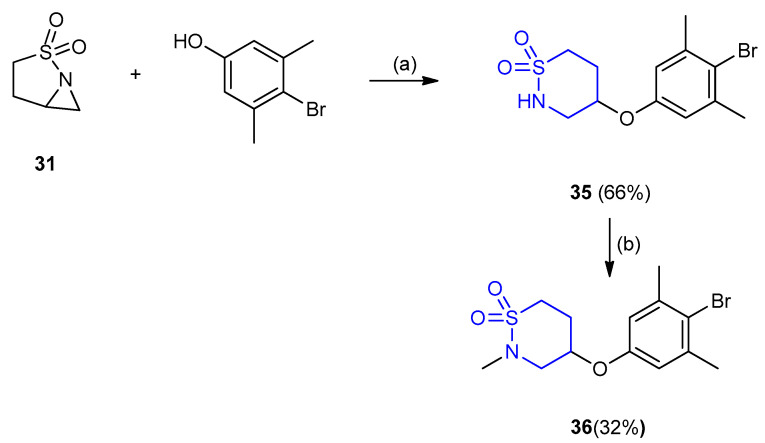
4-(4-Bromo-3,5-dimethylphenoxy)-1,2-thiazinane-1,1-dioxide (35) was prepared in 66% yield, from the reaction between 2-thia-1-aza-bicyclo[3.1.0]hexane-2,2-dioxide (31) and 4-bromo-3,5-dimethylphenol in N,N-dimethylacetamide (DMAc) via ring opening–ring closure interaction. The thiazinane 35 when treated with NaH in N,N-dimethylacetamide and iodomethane gave 4-(4-bromo-3,5-dimethylphenoxy)-2-methyl[1,2]thiazinane-1,1-dioxide (36) (yield 32%) (Scheme 8) [30].
Scheme 8.
Synthesis of 4-(4-bromo-3,5-dimethylphenoxy)-2-methyl[1,2]thiazinane-1,1-dioxide (36). (a) DMAc, 130 °C, 5 h; (b) DMAc, NaH, CH3I, rt., 3 h.
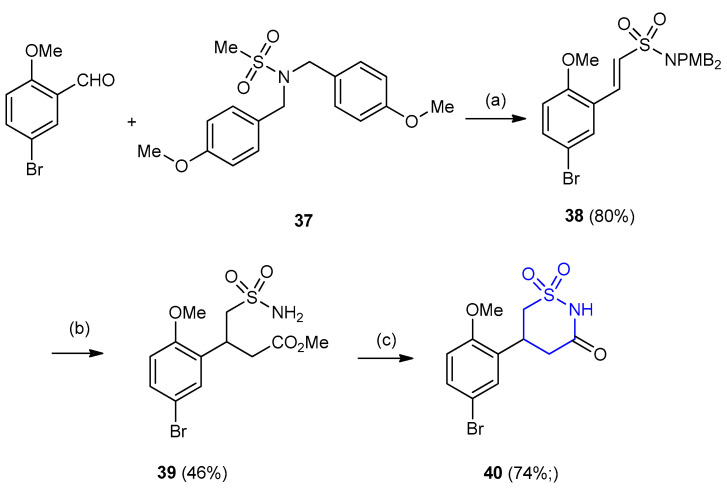
The sulfonamide (N,N-bis(4-methoxybenzyl)methanesulfonamide) (37) was treated with lithium hexamethyldisilazide (LiHMDS), followed by addition of diethyl chlorophosphate and quenched with 5-bromo-2-methoxybenzaldehyde to form alkenyl sulfonamide 38 in 80% yield. Compound 38 was subjected to Michael-addition using dimethyl malonate to form the diester. Decarboxylation and sulfonamide deprotection of 38 formed the sulfonamide 39 (yield 46%). Cyclisation of 39 using standard NaOMe furnished 5-aryl-1,2-thiazinan-3-one-1,1-dioxide 40 in good yield 74%, after Suzuki coupling with phenylboronic acid (Scheme 9) [31].
Scheme 9.
Synthesis of 5-aryl-1,2-thiazinan-3-one-1,1-dioxide 40. (a) LiHMDS (2 equiv.), −20 °C, 30 min then ClPO(OEt)2, 1 h then RCHO,−20 °C to r.t., 1 h, 80%; (b) (i) dimethyl malonate, NaOMe-MeOH, MeCN, 18 h, reflux, 85%; (ii) DMF, NaCl, H2O, reflux, 5 h; (iii) TFA–CH2Cl2 (1:1), 18 h, r.t., 46% (2 steps); (c) (i) NaOMe–MeOH, r.t., 1 h, 93%; (ii) PhB(OH)2, DME–H2O (2:1), Pd(PPh3)4 (5 mol%), Cs2CO3 (4 equiv.), 74%.
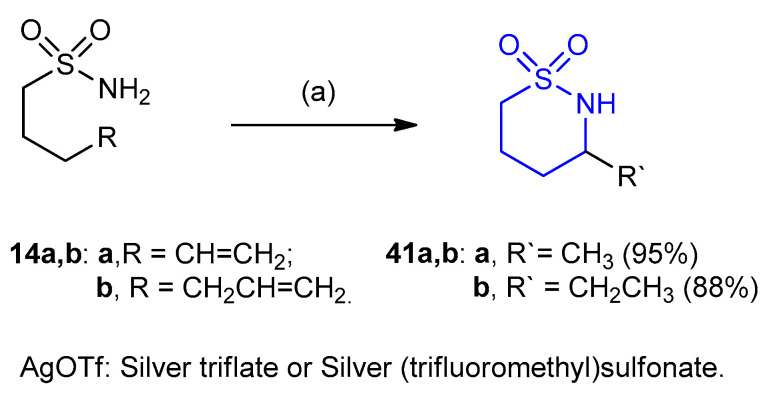
Terminal alkenes and hydroamination of inactivated alkenes have been isomerized using phosphine gold (I) complexes as a catalyst under both thermal and microwave conditions. Sulfonamides 14a,b readily underwent intramolecular hydroamination to give thiazinane-1,1-dioxides 41a,b (yields 95% and 88%), respectively (Scheme 10) [32].
Scheme 10.
Synthesis of thiazinane-1,1-dioxides 41a,b. (a) toluene, 5-mol% (PPh3)AuCl/AgOTf.
2.1.2. Syntheses of 1,3-thiazinanes
Syntheses of N-tosyl-1,3-thiazinanes
N-Tosyldiazoketamine 42 was converted to the corresponding E (5%)/Z (95%)-α-phenyl-β-enamino ester 43 via decomposition of 42 through losing of N2 to form carbine followed by 1,2-phenyl migration under two different catalytic conditions, Rh2(OAc)4 and p-TsOH. For the reaction catalyzed by Rh2(OAc)4, E-isomer 43b (91%) was found to be the major product along with the formation of very small quantities of the Z-isomer of 1,2-phenyl migration product 43a (5%) and 1,2-hydride migration product 44 (4%). The ratio of 43a/43b/44 was found to be 5:91:4. In contrast, the 1,2-hydride migration product 44 could not be detected in reactions catalyzed by p-TsOH. Moreover, in the latter case, the Z-α-phenyl-β-enamino ester 43a was formed as the major product (43a/43b = 95:5). Mitsunobu adduct 46 was obtained via premixing DEAD (Diethyl azodicarboxylate) and PPh3, followed by addition of Z-α-phenyl-β-enamino ester 43a and alcohol 45. The cyclized products 47 (yield 79%) were obtained from alkenylthiols 46 in one pot using trifluoroacetic acid (TFA) in diastereoselectivities (86:14) (Scheme 11) [33].
Scheme 11.
Synthesis of N-tosyl-1,3-thiazinane 47 via 1,2-phenyl migration. (a) p-TsOH, CH2Cl2, rt, 30 min, 89%; (b) Rh2(OAc)4, CH2Cl2; (c) Ph3P, DEAD; (d) TFA,CH2Cl2.
Synthesis of Epipyridazinoanthracen-1,3-thiazinane Propanenitrile
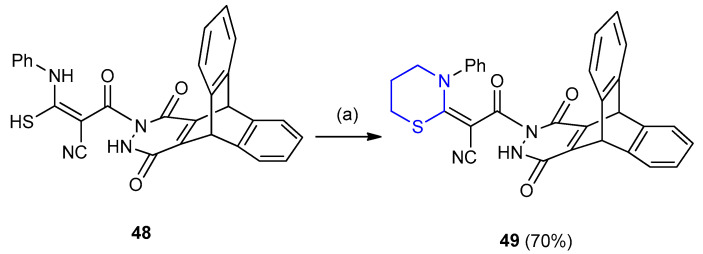
Reaction between thiocarbamoyl derivative 48 and 1,3-dibromopropane in presence of Et3N furnished the stereoselective product cyclic ketene S,N-acetal ((E)-3-((9s,10s)-12,15-dioxo- 9,11,12,14,15,16-hexahydro-9,10-[4,5]epipyridazinoantracen-13(10H)-yl)-3-oxo-2-(3-phenyl-1,3-thiazinan-2-ylidene)propanenitrile) (49) in 70% yield (Scheme 12) [34].
Scheme 12.
Synthesis of [4,5]epipyridazinoanthracen-1,3-thiazinan propanenitrile 49. (a) 1,3-dibromopropane, DMF/TEA, reflux 10 h.
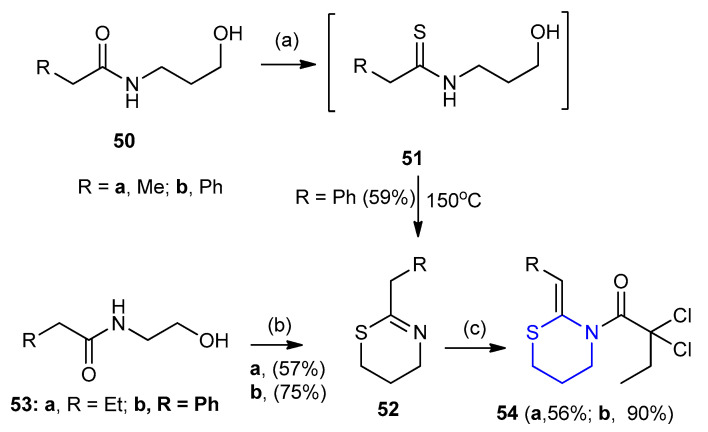
Cornia et al. [35] utilized Berzelius reagent P4S10 (phosphorus decasulfide or phosphorus pentasulfide) for thionation. 3-Hydroxypropane amide 50 combined with hexamethyldisiloxane (HMDO) gave thioamide 51. Cyclization of intermediate 51 to 1,3-thiazine 52 (59%), which acylated using 2,2-dichloropropanoyl chloride to give (Z)-2,2-dichloro-1-(2-propylidene-1,3-thiazinan-3-yl) butan-1-one 54a and (Z)-1-(2-benzylidene-1,3-thiazinan-3-yl)-2,2-dichloropropan-1-one 54b in 56% and 90% yield, respectively. In addition, 2-ethyl-5,6-dihydro-4H-1,3-thiazines 52a,b (57% and 75%) were prepared via the treatment of the N-(2-hydroxyethyl)propionamide 53 with the Lawesson’s reagent followed by exposure to a solution of K2CO3 (Scheme 13) [35,36].
Scheme 13.
Synthesis of (Z)-2,2-dichloro-1-(2-propylidene/benzylidene-1,3-thiazinan-3-yl) butan-1-one 54a,b. (a) P4S10/HMDO, CH2Cl2, reflux; (b) (i) LR (Lawesson’s reagent), toluene, reflux 1 h, under N2, (ii) 2-M K2CO3; (c) CH3CH2CCl2COCl, TEA, CH2Cl2, rt.
Synthesis of 2-imino-1,3-thiazinane Derivatives
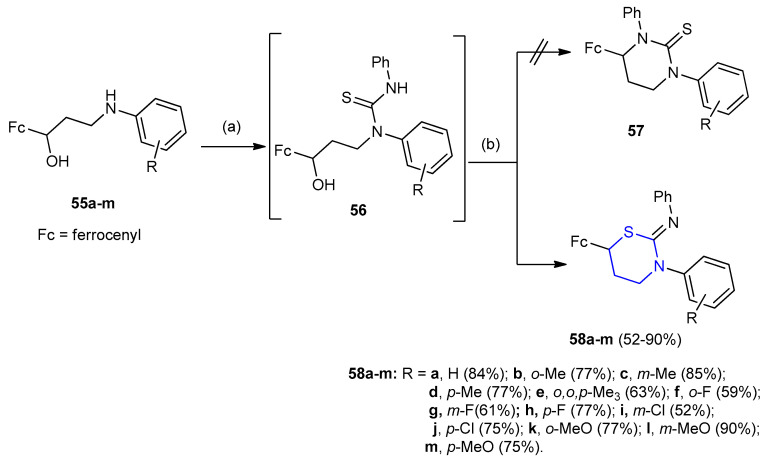
Chemoselective synthesis of ferrocene-containing 1,3-thiazinan-2-imines 58a–m via the reaction between 3-aryl-amino-1-ferrocenylpropan-1-ols 55a–m and phenyl isothiocyanate in acidic medium. The intermediate β-hydroxy thioureas 56 were generated in situ using ultrasound irradiation and the cyclizations were achieved by the addition of acetic acid to give the corresponding 3-aryl-6-ferrocenyl-N-phenyl-1,3-thiazinan-2-imines 58a–m (yields 52–90%) instead of 3-arylamino-1-ferrocenylpropan-1-ols 57 (Scheme 14) [37].
Scheme 14.
Synthesis of 3-aryl-6-ferrocenyl-N-phenyl-1,3-thiazinan-2-imines 58a–m. (a) PhNCS, ultrasound irradiation (hν); (b) AcOH, ultrasound irradiation (hν).
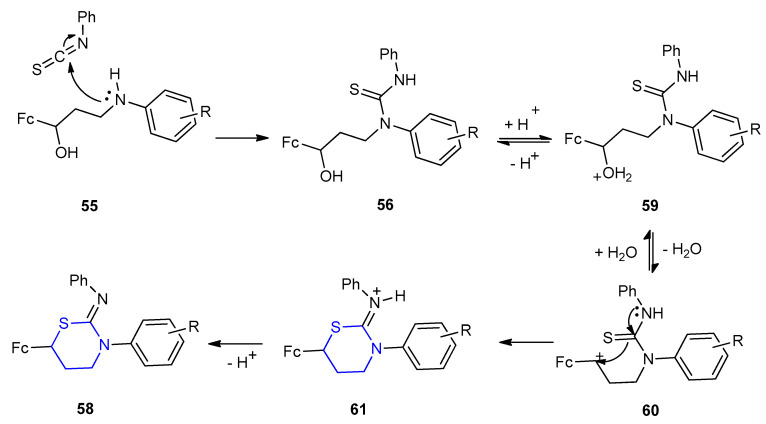
The mechanism for the formation of ferrocenyl 1,3-thiazinane-2-imine 58a–m was illustrated in Scheme 15. Thiourea derivatives 56 were via nucleophilic attack of the amine 55 on the isothiocyanate. Under acidic conditions, the thiourea cyclized via the thione-group with the elimination of H2O molecule to give intermediate 61 through intermediates 59 and 60, respectively. Intermediate 61 was deprotonated to give 58 (Scheme 15).
Scheme 15.
Mechanism for the formation of 3-aryl-6-ferrocenyl-N-phenyl-1,3-thiazinan-2-imines 58a–m.
Synthesis of 1,3-thiazinane-4-one Derivatives
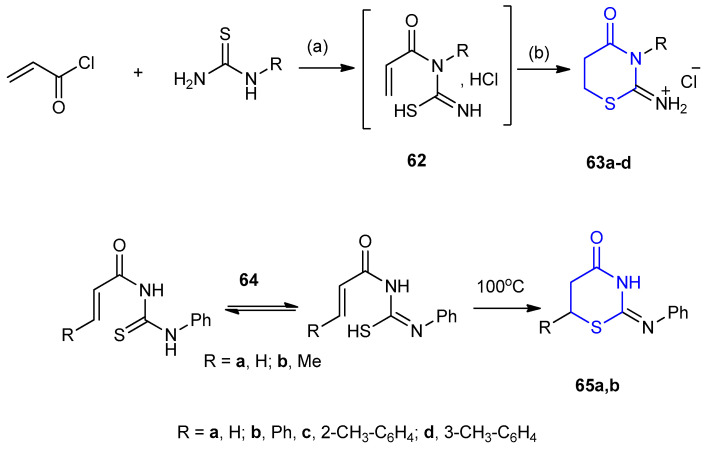
A variety of methods were made to synthesize 2-imino-1,3-thiazinane, based on the cyclization of acyl thioureas containing an α,β-unsaturated acid fragment. Reactions of acryloyl chloride with thiourea or with N-substituted thioureas, no N-acryloylthioureas 62 were isolated and hydrochlorides of 3-substituted-1,3-thiazinane-4-one 63a–d were obtained. 2-Imino-1,3-thiazinane -4-one 65a,b with a substituent on the exocyclic N-atom, were synthesized via thermal cyclization of methacryloyl thioureas 64a,b (Scheme 16) [38].
Scheme 16.
Synthesis of 3-substituted-1,3-thiazinane-4-one hydrochlorides 63a–d and 2-Imino-1,3-thiazinane-4-one 65a,b. (a) CH3CN, 12 h, r.t.; (b) heating 100 °C, 4–6 h.
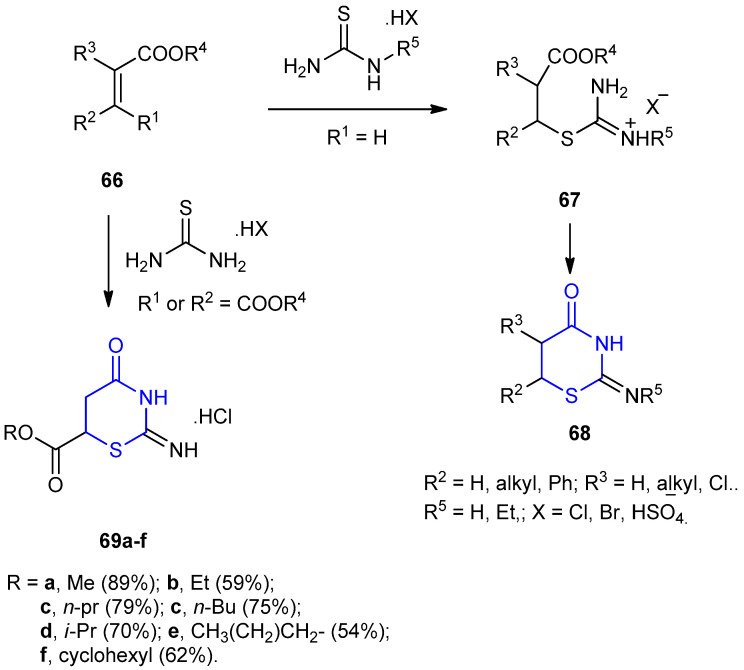
The syntheses of 3-unsubstituted 2-imino-1,3-thiazinan-4-ones 68 and 69, were based on the reaction of α,β-unsaturated carboxylic esters 66 with thioureas, including isolation and subsequent cyclization of hydrochlorides or sulfates 67 in the presence of aqueous ammonia or sodium acetate [39]. In the case of maleic or fumaric acids, hydrochlorides of 2-imino-thiazinans 69 were obtained in one-pot synthesis (Scheme 17) [40].
Scheme 17.
Synthesis of 3-unsubstituted 2-imino-1,3-thiazinan-4-ones 68 and 69.
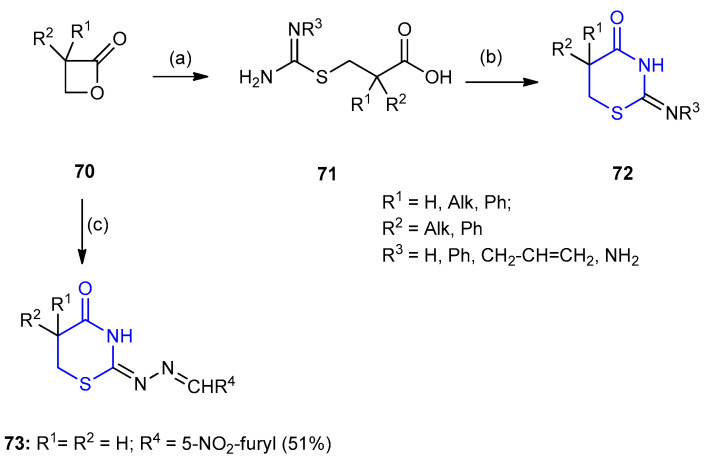
6-Unsubstituted 2-imines-1,3-thiazinane-4-one 72, were synthesized via reaction of β-propiolactone 70 [41,42] and its derivatives with thioureas. At the first step, acids 71 were isolated; the cyclization of 71 in acetic anhydride or its mixture with pyridine gave thiazinan-4-ones 72. Thiosemicarbazones reacted similarly to give 1,3-thiazinan-4-ones ((E)-2-((E)-((5-nitrofuran-2-yl) methylene)hydrazono)-1,3-thiazinan-4-one) (73) (51%) in one pot procedure [43] (Scheme 18).
Scheme 18.
Synthesis of 6-Unsubstituted 2-imines-1,3-thiazinane-4-one 72 2-hydrazono- 1,3-thiazinan-4-one 73. (a) Thiourea, H2O, 30 °C, standing 2 h at 10 °C (90%); (b) Ac2O/pyridine; (c) Thiosemicarbazones, EtOH, AcOH, 75 °C, then reflux 30 min.
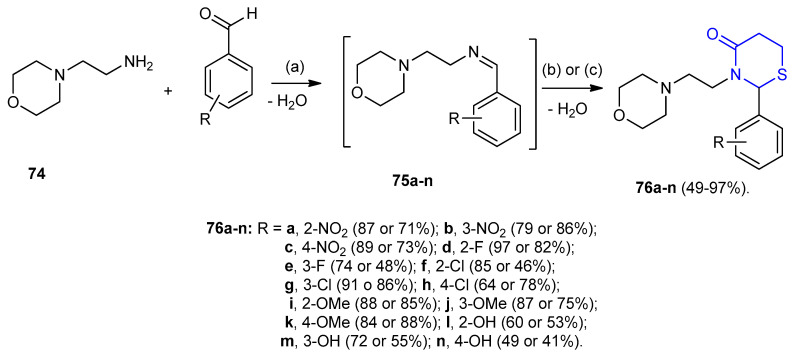
Thiazinanones 76a–n were synthesized via three-component reactions between aldehydes, 2-morpholinoethanamine (74) and 3-mercaptopropionic acid under both thermal and ultrasonication conditions. The products were formed via the intermediates 75a–n [44] (Scheme 19).
Scheme 19.
Synthesis of N-morpholinoethane-1,3-thiazinane-4-one 76a–n. (a) Toluene, 110 °C, 3 h; (b) HSCH2CH2COOH, 110 °C, 16 h. OR (c) Toluene, HSCH2CH2COOH, ultrasound, r.t., 25 min.
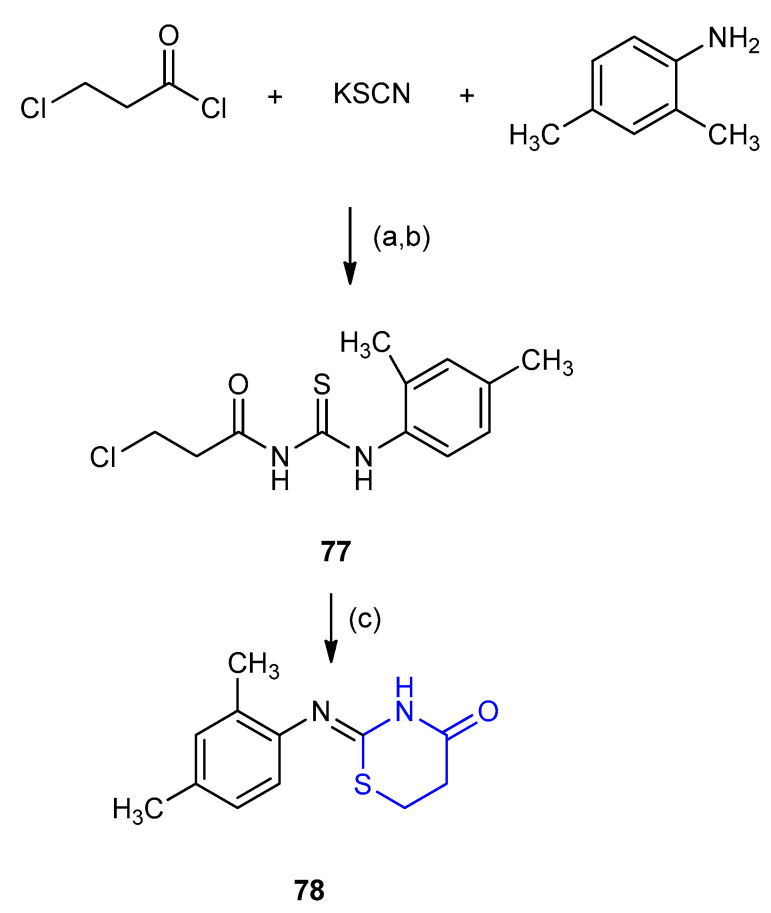
(Z)-2-[(2,4-Dimethylphenyl)imino]-1,3-thiazinan-4-one 78 was prepared according to the procedure reported by Mansuroğlu et al.[45]. 3-Chloropropionyl chloride was reacted with potassium thiocyanate and 2,4-dimethylaniline, after acidification N-(3-chloropropionyl)-N’- (2,4-di-methylphenyl)thiourea (77) was formed. The substituted thiourea 77 was refluxed in toluene/acetone media to afford (Z)-2-[(2,4-dimethyl-phenyl)imino]-1,3-thiazinan-4-one (78) (Scheme 20) [46].
Scheme 20.
Synthesis of (Z)-2-[(2,4-dimethylphenyl)imino]-1,3-thiazinan-4-one 78. (a) acetone, reflux, 30 min; (b) Stirring 2 h, HCl 0.2 N; (c) toluene/acetone, reflux 4 h.
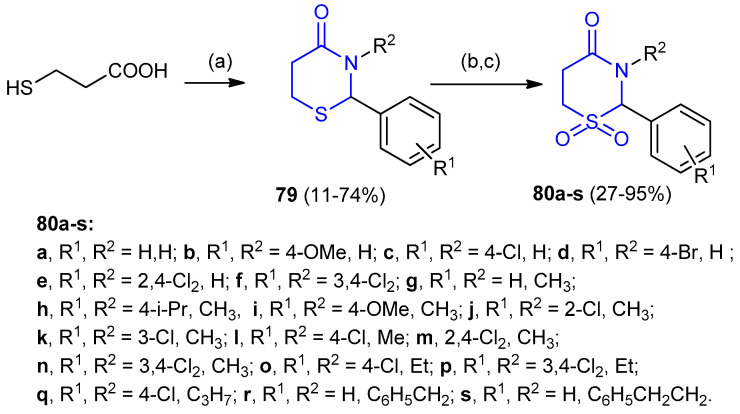
3-Mercaptopropionic acid reacted with ammonia or primary amines and aryl aldehydes to give 2- and 2,3-substituted-1,3-thiazinan-4-ones 79a–s. The corresponding 1,3-thiazinan-4-one- 1,1-dioxide derivatives 80 (27–95%) were obtained from the synthesized substituted 1,3-thiazinan-4-ones 79 (11–74%) via oxidation using KMnO4 (Scheme 21) [6].
Scheme 21.
Synthesis of 1,3-thiazinan-4-one-1,1-dioxide derivatives 80. (a) aldehyde (R1C6H4CHO), primary amine (R2NH2), benzene, reflux 48 h; (b) AcOH, KMnO4, >30 °C; (c) NaHCO3.
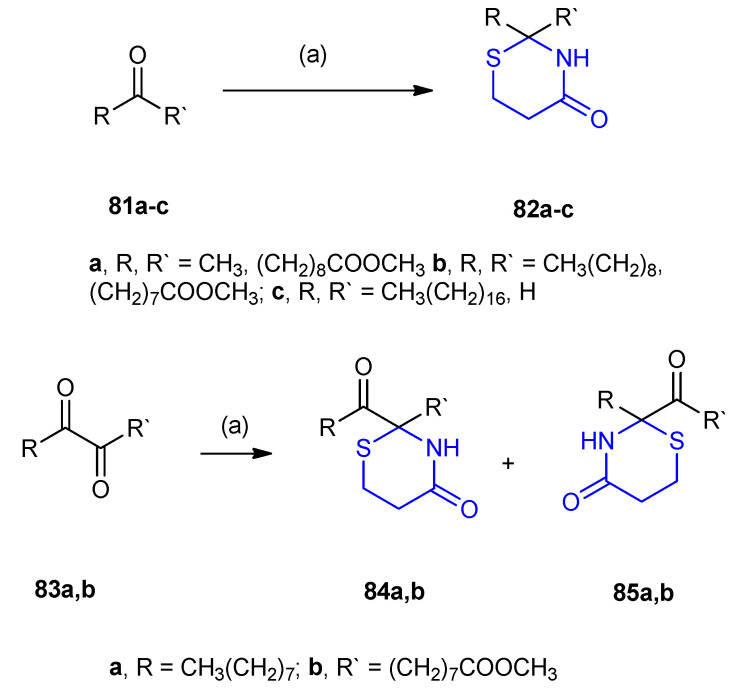
The reaction of keto fatty acids and long-chain aldehydes with 3-mercapto-propionic acid in the presence of ammonium carbonate resulted in the formation of thiazanone derivatives. The treatment of methyl 10-oxoundecanoate 81a, methyl 9-oxostearate 81b and octadecanal 81c with 3-mercaptopropionic acid in the presence of ammonium carbonate ((NH4)2CO3) the thiazanone derivatives were obtained, 9-(2-methyl-4-oxo-1,3-thiazinan-2-yl)nonanoic acid 82a, 8-(2-nonyl-4-oxo-1,3-thiazinan-2-yl)octanoic acid 82b and 2-heptadecyl-1,3-thiazinan-4-one 82c, respectively. Under the same conditions thiazanones 84a,b and 85a,b were obtained from the vicinal-dioxo ester 83a,b (Scheme 22) [47].
Scheme 22.
Synthesis of thiazinanones with long-chain substituents at 2-position. (a) 3-mercaptopropionic acid, (NH4)2CO3.
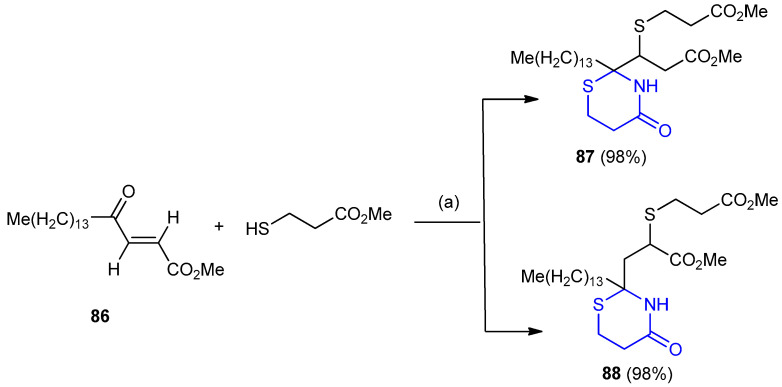
Azeotropic reflux of (E)-methyl 4-oxo-octadec-2-enoate (86) [48] with methyl 3-mercaptopropionate and ammonium carbonate afforded the thiazinane, as a mixture of isomers 87 and 88 (Scheme 23) [49].
Scheme 23.
Synthesis of 1,3-thiazinane-4-one derivatives 87 and 88. (a) (NH4)2CO3, benzene, azeotropic reflux.
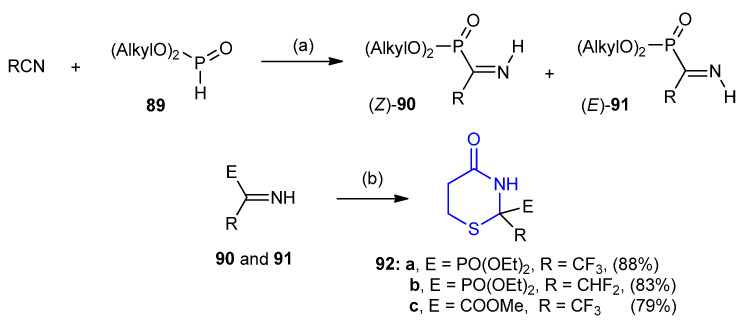
Dialkyl phosphites 89 reacted with difluoro- or trifluoroacetonitriles in the presence of a catalytic amount of nitrogen base to form iminophosphonates 90 and 91 as diastereoisomers. Cyclo-condensation of iminophosphonates 90 and 91 with 3-mercaptopropionic acid furnished 1,3-thiazinan-4-ones 92a–c in good yields 79–88% (Scheme 24) [50].
Scheme 24.
Synthesis of diethyl phosphonate of 1,3-thiazinan-4-ones. (a) Et3N, rt., 7 days; (b) 3-mercaptopropionic acid, benzene, reflux 2–4 h.
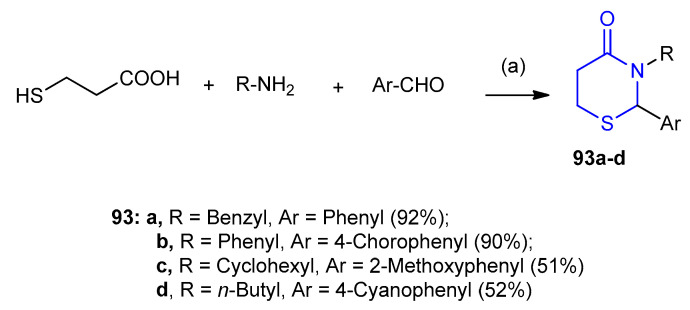
Three-component reactions between amines or amino acids, aldehydes and 3-mercaptopropionic acid were catalyzed dicyclohexylcarbodimide (DCC) afforded metathiazanones 93a–d in yields 51–92% (Scheme 25) [51].
Scheme 25.
Synthesis of thiazinanones 93a–d. (a) N,N-dicyclohexylcarbodimide (DCC)/THF, 0 °C.
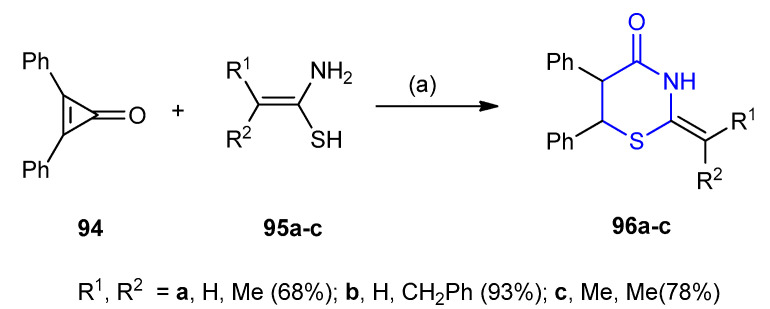
The ring enlargement of 2,3-diphenylcyclopropenone 94 using 1-amino-2-substituted alkene- 1-thiols 95a–c afforded different 5,6-diphenyl-2-(substituted-2-ylidene)-1,3-thiazinan-4-one 96a–c (68–93%) (Scheme 26) [52].
Scheme 26.
Synthesis of 5,6-diphenyl-2-(substituted-2-ylidene)-1,3-thiazinan-4-one 96a–c. (a) CH3CN, r.t. overnight.
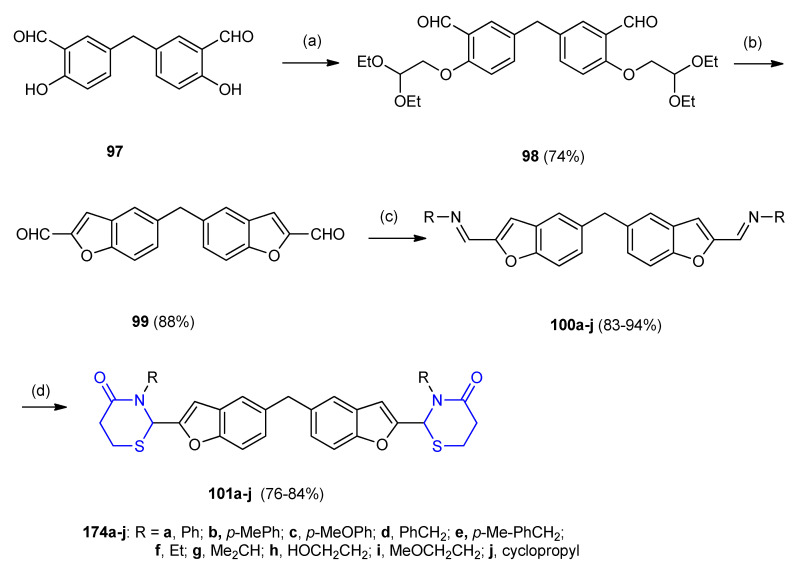
Coupling of bis-5,5′-methylenebis(2-hydroxybenzaldehyde) (97) with bromo-acetaldehyde diethyl ether furnished the desired diacetal (5,5′-methylenebis(2-(2,2-diethoxyethoxy)benzaldehyde)) (98) in 74% yield. Deacetylation the diacetal 98 followed by intramolecular aldol condensation and acid-catalyzed dehydration afforded benzofuran-2-al dimer (5,5′-methylenebis(benzofuran-2- carbaldehyde)) 99 (88%). Condensation of 99 (in excess) with alkyl-, cycloalkyl-, aryl- and aralkyl amines gave bis-imines 100a–j (83–94%). Subsequent cyclization of bis-imines 100a–j through condensation with 3-mercaptopropionic acid furnished bis-(benzofurane-1,3-thiazinan-4-one) derivatives 101a–j (Scheme 27) [53].
Scheme 27.
Synthesis of bis-(benzofurane-1,3-thiazinan-4-one) derivatives 101a–j. (a) K2CO3, DMF, 120 °C; (b) AcOH, 110 °C; (c) arylamine, MeOH, 75 °C; (d) 3-mercaptopropionic acid, DCC/THF.
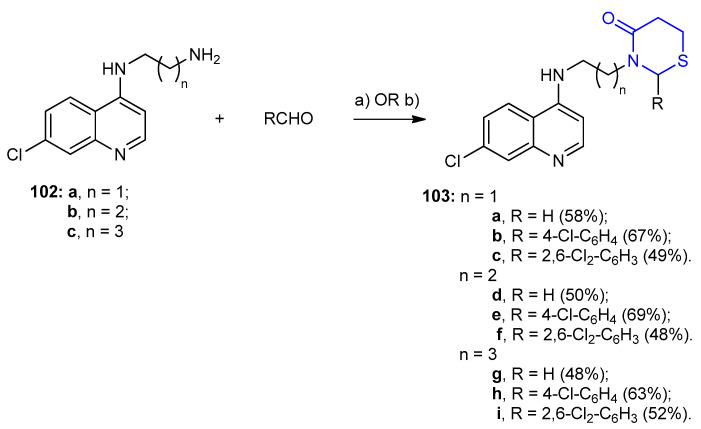
N1-(7-Chloroquinolin-4-yl)alkane diamines 102a–c reacted with aldehydes in THF under ice-cold conditions, followed by addition of 3-mercaptopropanoic acid in presence of dicyclohexylcarbodimide (DCC) or in toluene under reflux afforded 2-(alkyl/aryl)-3-(2-((7-chloro quinolin-4-yl)amino)ethyl)-1,3-thiazinan-4-one derivatives 103a–i in 48–67% yields (Scheme 28) [54].
Scheme 28.
Synthesis of 2-(alkyl/aryl)-3-(2-((7-chloroquinolin-4-yl)amino)ethyl)-1,3-thiazinan-4-one derivatives 103a–c. (a) 3-mercaptopropionic acid, DCC, THF, rt.; OR (b) toluene, reflux.
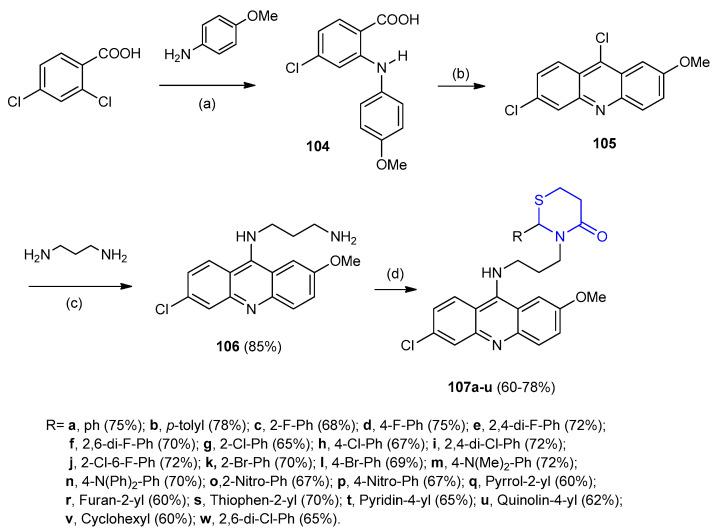
The reaction of 2,4-dichlorobenzoic acid with p-methoxyaniline gave diphenylamine 104 on treatment with POCl3 cyclized to 6,9-dichloro-2-methoxyacridine (105) (85%). The acridine 105 reacted with 1,3-propandiamine afforded N1-(6-chloro-2-methoxyacridin-9-yl)propane-1,3-diamine (106). Compound 106 reacted with aldehydes and 3-mercaptopropionic acid in the presence of dicyclohexylcarbodimide (DCC) as a dehydrating agent furnished quinacrine[1,3]-thiazinan-4-one derivatives 107 in yields 60–78% (Scheme 29) [55].
Scheme 29.
Synthesis of quinacrine[1,3]-thiazinan-4-one derivatives 107. (a) LiNH2, THF, 8 h; (b) POCl3, 120–130 °C, 3 h; (c) Et3N, 120–130 °C, 6 h; (d) Aldehyde, 3-mercaptopropionic acid, DCC, THF, rt 1 h.
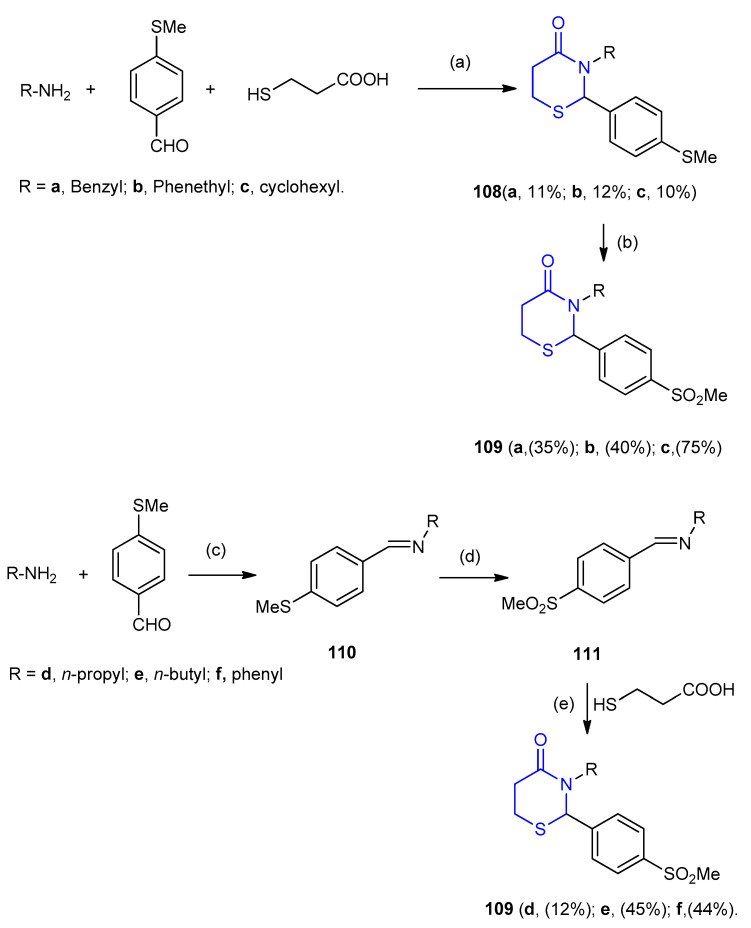
3-Alkyl-2-aryl-1,3-thiazinan-4-one derivatives 109a–c were synthesized via the routes outlined in Scheme 30. Treatment of amines with 4-methylthiobenz-aldehyde and thioglycolic acid in dry toluene in the presence of p-TsOH under reflux afforded 3-alkyl-2-(4-methylthiophenyl)- 1,3-thiazinan-4-one (108). Oxidation of 108 using 30% H2O2 in methanol in the presence of trace amount of tungsten oxide (WO3) gave 3-alkyl-2-(4-methylsulfonylphenyl)-1,3-thiazinan-4-one 109a–c (35–75%). For low boiling point amines, the intermediate imine products 110-were obtained by the reaction with 4-methylthiobenzaldehyde in anhydrous DMF. Subsequent oxidation 110 with hydrogen peroxide and WO3 in methanol solution afforded the (E)-N-(4-(methylsulfonyl benzylidene)alkyl-1-amine 111. Reaction of 111 with mercaptopropionic acid under reflux gave 109d–f (12–45%) (Scheme 30) [56].
Scheme 30.
Synthesis of 3-alkyl-2-(4-methylsulfonylphenyl)-1,3-thiazinan-4-one 109a–c. (a) Toluene, reflux, 72 h; (b) H2O2 30%, WO3, 25 °C, 6 h; (c) DMF, 25 °C, 24 h; (d) H2O2 30%, WO3, 25 °C, 4 h; (e) toluene, reflux, 24 h.
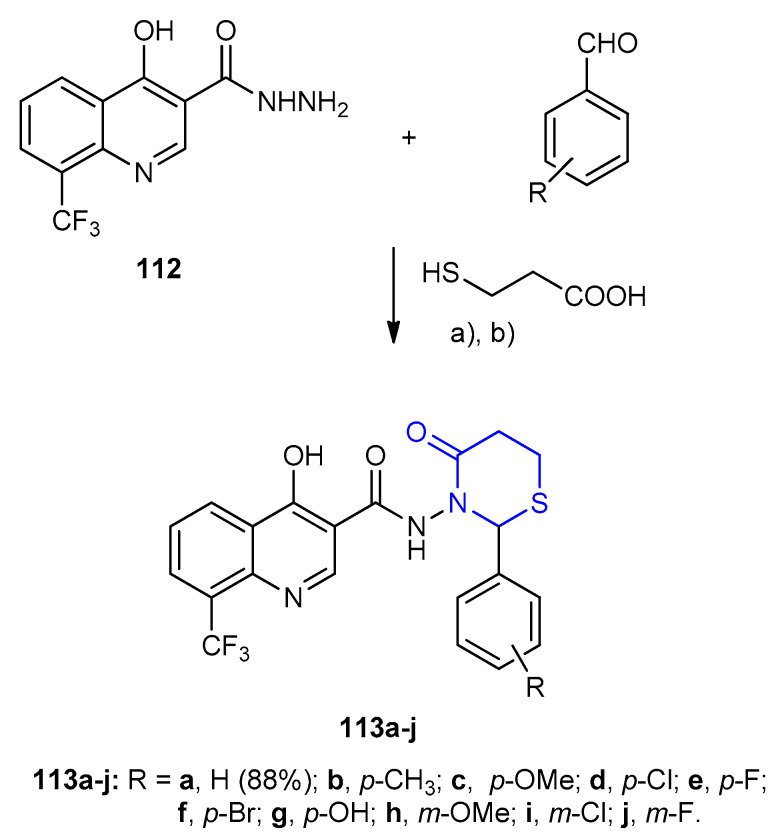
3-Hydroxy-N-(4-oxo-2-phenyl-1,3-thiazinan-3-yl)-8-(trifluoromethyl)quino-line-2-carboxamide derivatives 113a–j were synthesized by one-pot three component cyclocondensation reaction between quinoline hydrazide 112, substituted benzaldehyde and 3-mercaptopropionic acid in the presence of 1-ethyl-3-(3-dimethylamino-propyl)carbodiimide EDC (Scheme 31) [57].
Scheme 31.
Synthesis of 3-hydroxy-N-(4-oxo-2-phenyl-1,3-thiazinan-3-yl)-8-(trifluoromethyl) quinoline-2-carboxamide derivatives 113a–j. (a) THF, - 5 °C; (b) EDC, 7–9 h.
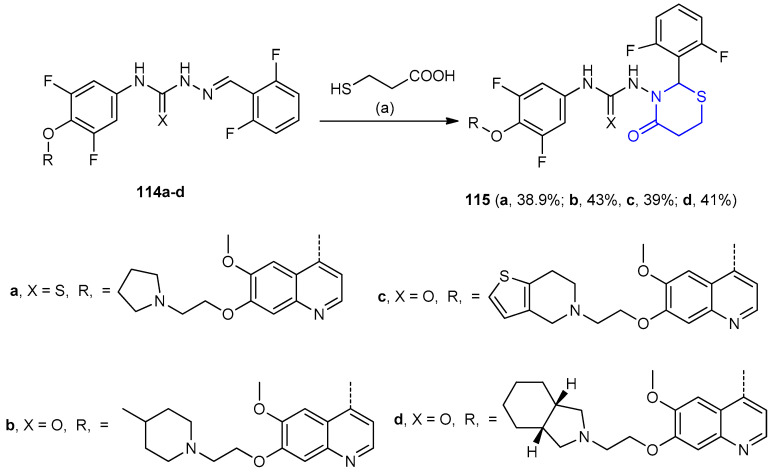
Hydrazinecarboxamides 114a–d reacted with 3-mercaptopropionic acid in presence of SiCl4 gave 1,3-thiazinan-4-one as urea derivatives 115a–d (39–43%), (Scheme 32) [58].
Scheme 32.
Synthesis of 1,3-thiazinan-4-one urease derivatives 115a–d. (a) SiCl4, CH2CH2, 40 °C, 5 h.
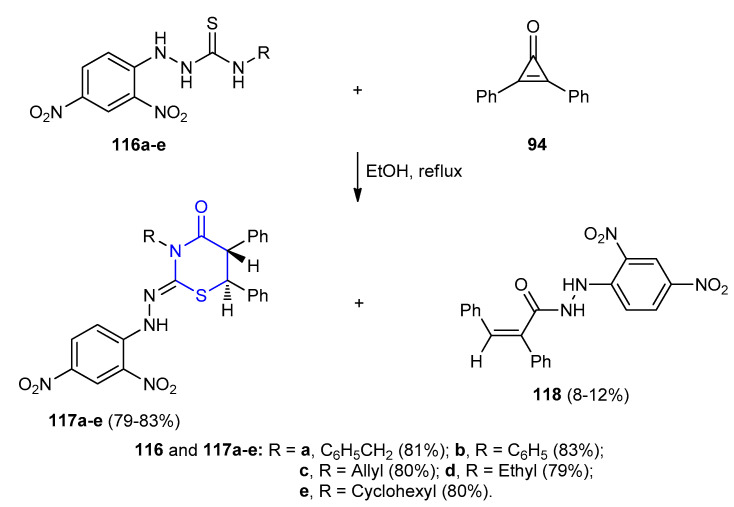
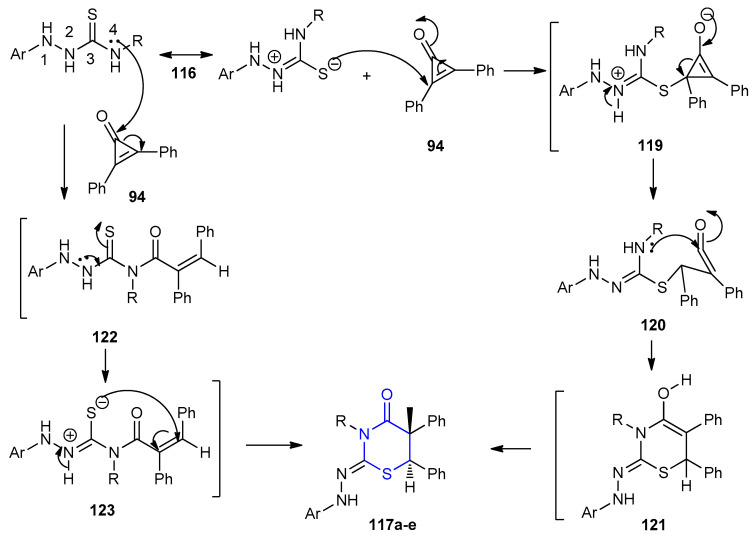
Hassan et al. reported that diastereoselective reaction between 4-substituted 1-(2,4-dinitrophenyl)thiosemicarbazides 116a–e and 2,3-diphenylcycloprop-2-enone 94 under refluxing ethanol furnished racemic 2-(2,4-dinitrophenyl)hydrazono)-5,6-diphenyl- 1,3-thiazinan-4-ones 117a–e (79–83%) as a major product and (Z)-N‘-(2,4-dinitrophenyl)- 2,3-diphenylacrylo hydrazide 118 (8–12%) as minor product (Scheme 33) [59].
Scheme 33.
Synthesis of racemic 2-(2,4-dinitrophenyl)hydrazono)-5,6-diphenyl-1,3-thiazinan-4-ones 117a–e. (a) EtOH, reflux, 4–6 h.
The mechanism for the formation of products 117a–e is presented in Scheme 34. The sulfur atom attacks the conjugate double bond of 94 forming the intermediate 119. The intermediate 119 underwent ring opening to compound 120. Intramolecular nucleophilic attack of N-4 on C=O afforded the intermediate 121 which rearranged to give 117a–e. On the other hand, N-4 attacks the carbonyl group of 94 with the formation of 117a–e via intermediates 122 and 123 (Scheme 34).
Scheme 34.
Mechanism for the formation of racemic 2-(2,4-dinitrophenyl) hydrazono)-5,6- diphenyl-1,3-thiazinan-4-ones 117a–e.
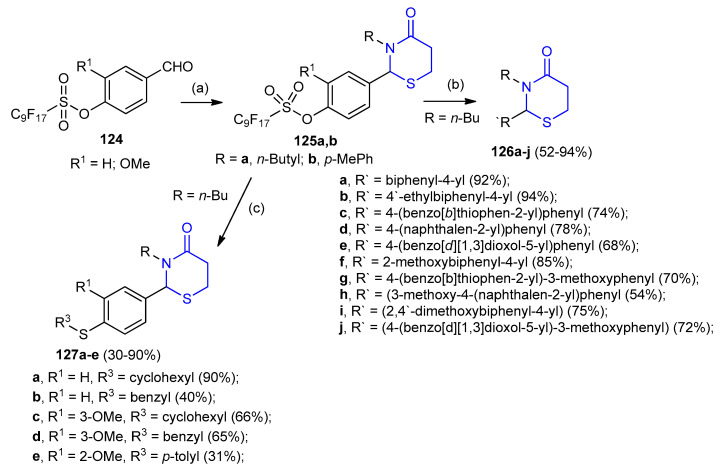
One-pot, three-component reactions of fluoro substituted benzaldehydes 124a,b with amines and mecaptopropanoic acid afforded 1,3-thiazinan-4-one 125a,b. Under microwave-assisted palladium-catalyzed coupling reactions in presence of boronic acid, thiazinanone 125a,b gave the biaryl thiazinanones 126 and thioarylthiazinanones 127. The microwave-assisted reactions were carried out using Pd(dppf)Cl2 [(1,1′-bis(diphenylphosphino)ferrocene) dichloro palladium(II)] as a catalyst, K2CO3 as a base and 4:4:1 acetone/toluene/water as a co-solvent (Scheme 35) [60].
Scheme 35.
Synthesis of biaryl thiazinanones 126 and thioarylthiazinanones 127. (a) Amine, 3-mercaptopropionic, DCC, THF, rt.; (b) R4B(OH)2, Pd(dppf)Cl2, K2CO3, MW 150 °C, 20 min; (c) R3SH, Pd(dppf)Cl2, K2CO3, MW 150 °C, 20 min.
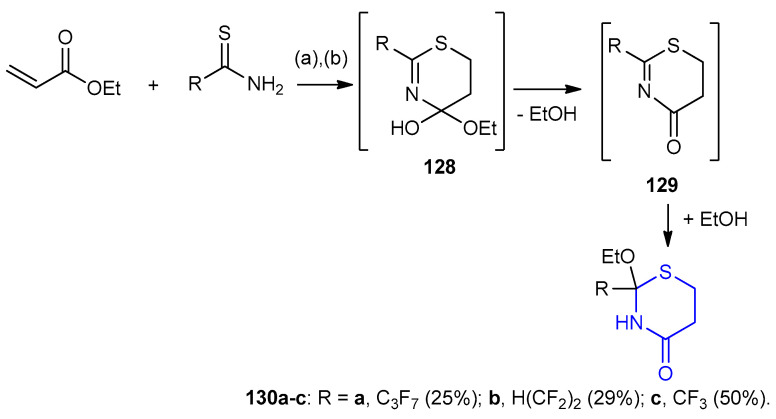
Polyfluoroalkanethioamides using BF3 in diethyl ether and ethyl acrylate were reacted and afforded 1,3-thiazinan-4-one 130a–c (25–50%) through the formation of intermediates 128 and 129 (Scheme 36) [61].
Scheme 36.
Synthesis of polyfluoroalkane 1,3-thiazinan-4-one 130a–c. (a) Toluene, BF3·Et2O, rt, 15days; (b) NaHCO3/H2O.
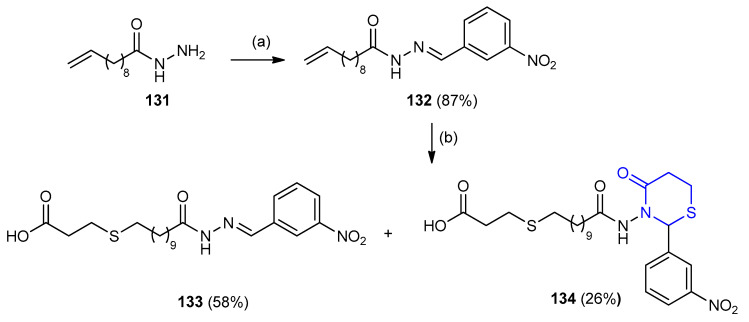
4-Oxo-1,3-thiazinan-11-oxoundecensulfanyl propanoic acid 134 was prepared in two steps: The hydrazine (N’-(3-nitrobenzylidene)undec-10-enehydrazide) (132) was first prepared by refluxing 10-undecenoic acid hydrazide 131 with m-nitrobenzaldehyde in anhydrous benzene. The compound 132 was then reacted with 3-mercaptopropionic acid, uncyclized adduct 133 (58%) was formed as aside product along with 4-oxo-1,3-thiazinan-11-oxoundecyl thiopropanoic acid 134 (26%) (Scheme 37) [62].
Scheme 37.
Synthesis of 4-oxo-1,3-thiazinan-11-oxoundecensulfanyl propanoic acid 134. (a) m-Nitrobenzaldehyde, anhydrous benzene, reflux, 5 h. (b) HSCH2CH2COOH, anhydrous benzene, reflux, 26 h.
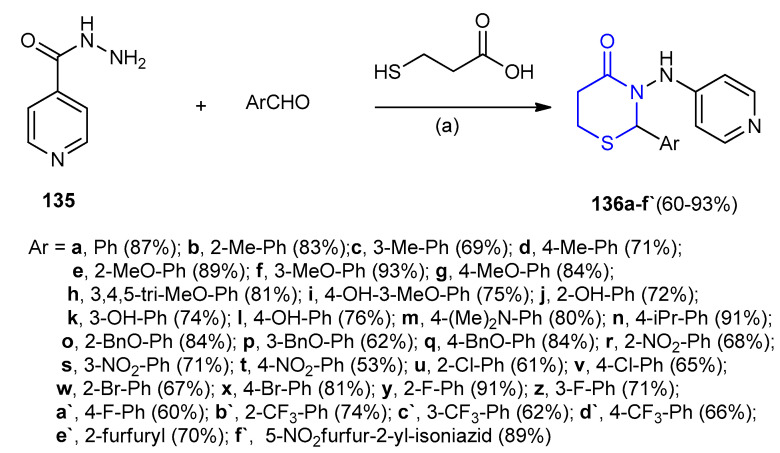
Isonicotinohydrazide (135) was reacted with aldehydes and 3-mercaptopropionic acid in presence of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) gave 1,3-(thiazinan-3-yl)- isonicotinamides 136a–f’ in moderate to high yields 60–93% (Scheme 38) [63].
Scheme 38.
(a) 1-ethyl-3-(3-dimethylaminoprop-yl)carbodiimide (EDC), THF, 0-rt, 5–6 h.
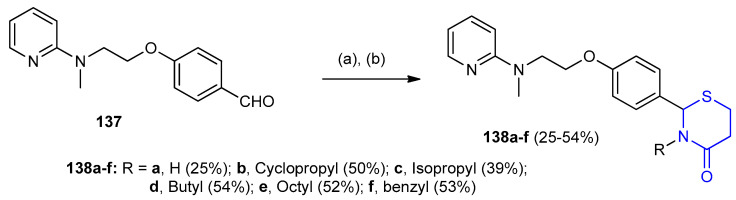
Similarly 4-(2-(methyl(pyridin-2-yl)amino)ethoxy)benzaldehyde (137) was reacted with appropriate primary amines (RNH2) and 3-mercaptopropinoic acid in presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) at room temperature to give thiazinan-4-ones 138a–f (25–54%) (Scheme 39) [64].
Scheme 39.
Synthesis of 4-(2-(methyl(pyridin-2-yl)amino)ethoxy)benzaldehyde (137). (a) RNH2, 10 min, 0 °C; (b) 3-mercaptopropionic acid, 10 min, EDC, 0 °C to rt., 5–6 h.
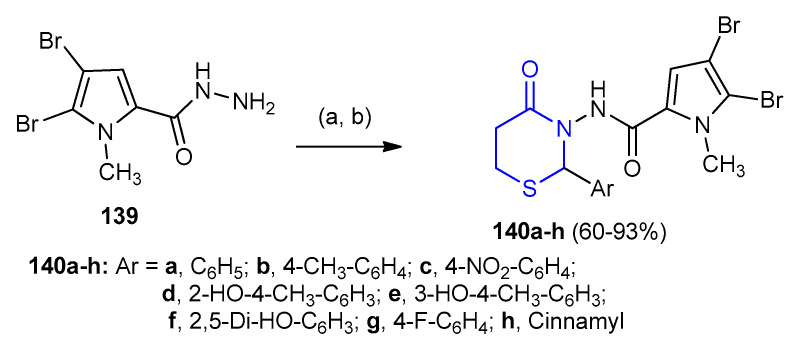
On the other hand, 4,5-dibromo-1-methyl-N-(4-oxo-2-aryl-1,3-thiazinan-3-yl)-1H-pyrrole-2-carboxamide 140a–h were synthesized in a quantitative yields via one-pot three component condensation between 4,5-dibromo-1-methyl-1H-pyrrole-2-carbohydrazide (139), aromatic aldehydes and 3-mercaptopropionic acid in the ratio 1:2:3 in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Scheme 40) [65].
Scheme 40.
Synthesis of 4,5-dibromo-1-methyl-N-(4-oxo-2-aryl-1,3-thiazinan-3-yl) -1H-pyrrole-2-carboxamide 140a–h. (a) THF, Ar-CHO, 0 °C; (b) 3-mercaptopropionic acid, EDC, THF, 0 °C-rt, 5–6 h.
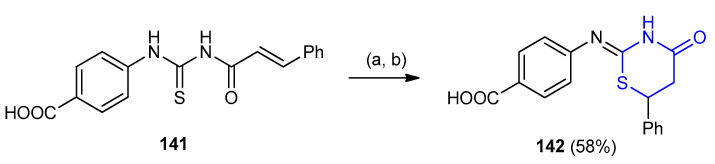
4-(4-Oxo-6-phenyl-1,3-thiazinan-2-ylideneamino)benzoic acid (142) were obtained during the stirring of (E)-4-(3-cinnamoylthioureido) benzoic acid (141) with sodium ethoxide at room temperature, then the reaction mixture was neutralized by HCl (Scheme 41) [66].
Scheme 41.
Synthesis of 4-(4-oxo-6-phenyl-1,3-thiazinan-2-ylideneamino)benzoic acid (142). (a) NaOEt/EtOH, rt overnight; (b) neutralization with HCl.
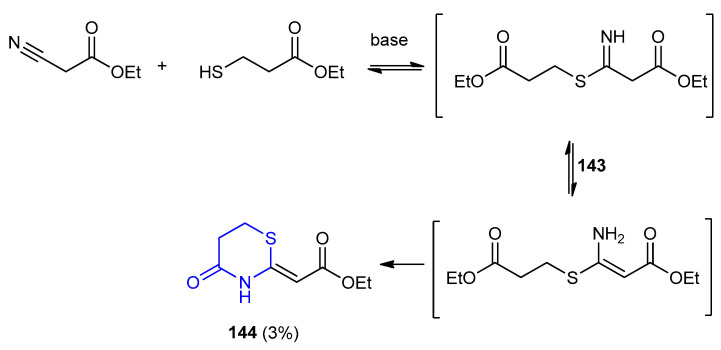
The base-catalyzed reactions of β-oxonitriles (ethyl 2-cyanoacetate) with ethyl 3-mercaptopropanoate were illustrated in Scheme 42. The more reactive β-oxonitrile reacted with β-mercaptoester afforded ethyl (E)-4-oxo-[1,3]thiazinan-2-ylidene)ethanoate (144), after the cyclization of the intermediate ethyl (Z)-3-amino-3-(2-ethoxycarbonylethylsulfanyl)propenoate (143) (Scheme 42) [67].
Scheme 42.
Synthesis of ethyl (E)-4-oxo-[1,3]thiazinan-2-ylidene)ethanoate (144). K2CO3, EtOH, reflux, 7 h.
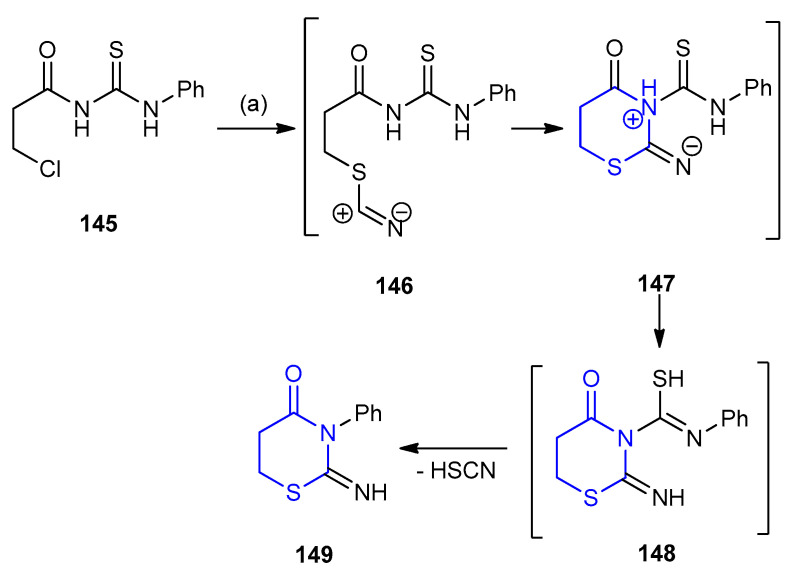
The reaction of acyl thiourea 145 with potassium thiocyanate via an unusual thiocyanic acid elimination through the formation of intermediates 146–148 afforded 2-imino-3-phenyl -1,3-thiazinan-4-one (149) (Scheme 43) [68].
Scheme 43.
Synthesis of 2-imino-3-phenyl-1,3-thiazinan-4-one 149. (a) KSCN, EtOH, reflux.
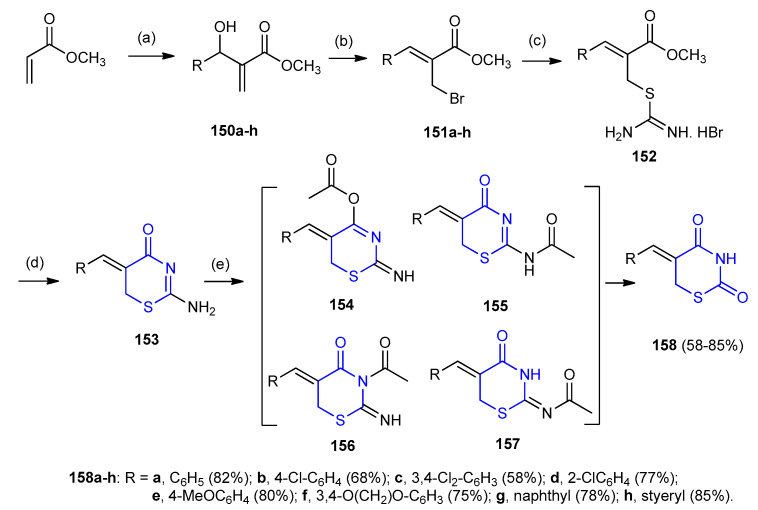
Allylic bromides 151a–h were prepared from 150a–h and reacted with thiourea in a 3:1 mixture of acetone:water at room temperature then reacted with an aqueous base of isothiouronium salts 152 gave 2-amino-1,3-thiazin-4-ones 153 as insoluble solids [69,70]. Transformation of 2-aminothiazin-4-one 153a into thiazinane-2,4-dione 158a was achieved by hydrolysis in an acidic medium [71]. The development of this method was achieved via a two-step (one-pot) method, initially: acetylation of 2-amino-thiazinan-4-one 153a followed by mild hydrolysis of the acetylated intermediates. 2-Iminothiazinan-4-one 153a was acetylated using acetic anhydride to give an approximately 1:1 mixture of two (out of four) possible acetylated isomers 154–157. Acetylation/hydrolysis protocol was then extended to other thiazine-4-ones 153 with the formation of the expected 1,3-thiazinane-2,4-diones 158a–h (58–85%) (Scheme 44) [72].
Scheme 44.
Synthesis of 1,3-thiazinane-2,4-diones 158a–h. (a) RCHO, DABCO; (b) LiBr, H+, CH3CN; (c) H2NCSNH2, acetone/H2O; (d) NaHCO3, H2O; (e) Ac2O, EtOH, 25 °C, then HCl (1 M), rt 1–3 h.
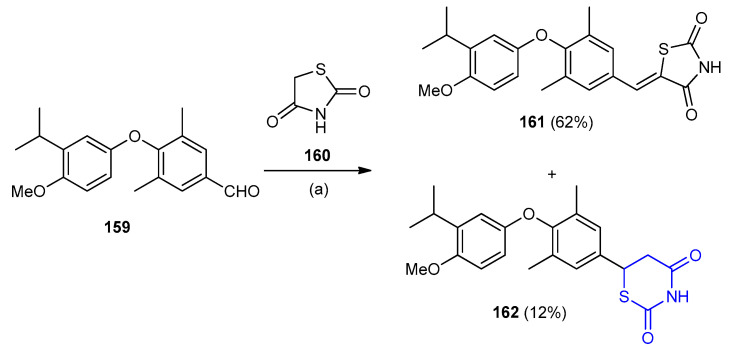
The condensation of 4-(3-isopropyl-4-methoxyphenoxy)-3,5-dimethylbenz-aldehyde (159) with thiazolidine-2,4-dione (160) under basic conditions gave the rearranged thiazinane-2,4-dione 162 (12%) in addition to thiazolidine-2,4-dione (161) (62%) (Scheme 45) [73].
Scheme 45.
Synthesis of thiazinane-2,4-dione 162. (a) piperidine, benzoic acid, toluene and reflux.
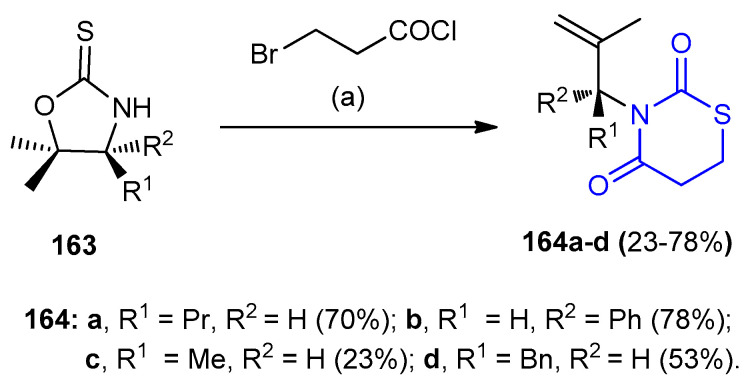
Under ice-condition reactions of oxazolidinethiones 163 with 3-bromo-propionyl chloride in methylene chloride gave 1,3-thiazinane-2,4-diones 164a–d (23–78%) (Scheme 46) [74].
Scheme 46.
1,3-thiazinane-2,4-diones 164a–d. (a) NaH, CH2Cl2, 0 °C, 4 h.
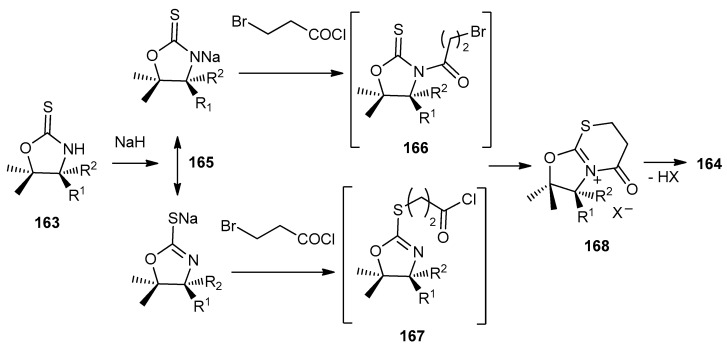
The formation of 1,3-thiazinane-2,4-diones 164a–d took place through the formation of both, the bromoamide 166 and S-alkylated intermediate 167 via N-acylation or intramolecular substitution reaction, respectively. Both intermediates 166 and 167 gave the immonium salts 168, which lost HX molecule with ring-opening to give 164a–d (Scheme 47).
Scheme 47.
The mechanism for the formation of 1,3-thiazinane-2,4-diones 164a–d.
Synthesis of 1,3-thiazinane-2-thione-4-one Derivatives
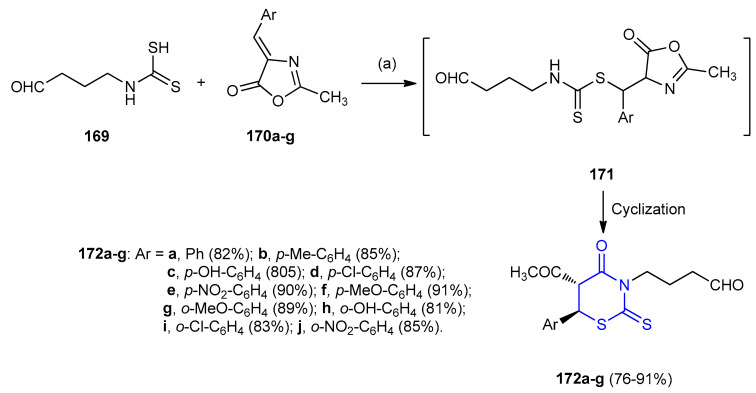
Arylideneoxazalones 170a–g were added to (4-oxobutyl)carbamodithioic acid (169) and the mixture was subjected to microwave irradiation in presence of montmorillonite K10 (SiO2/Al2O3), basic and neutral alumina and silica gel-forming Michael adducts 171 which were cyclized to 1,3-thiazinane derivatives 172a–g in yields 76–91% (Scheme 48) [75].
Scheme 48.
Synthesis of 1,3-thiazinane derivatives 172a–g. (a) K10/MW, 2 min.
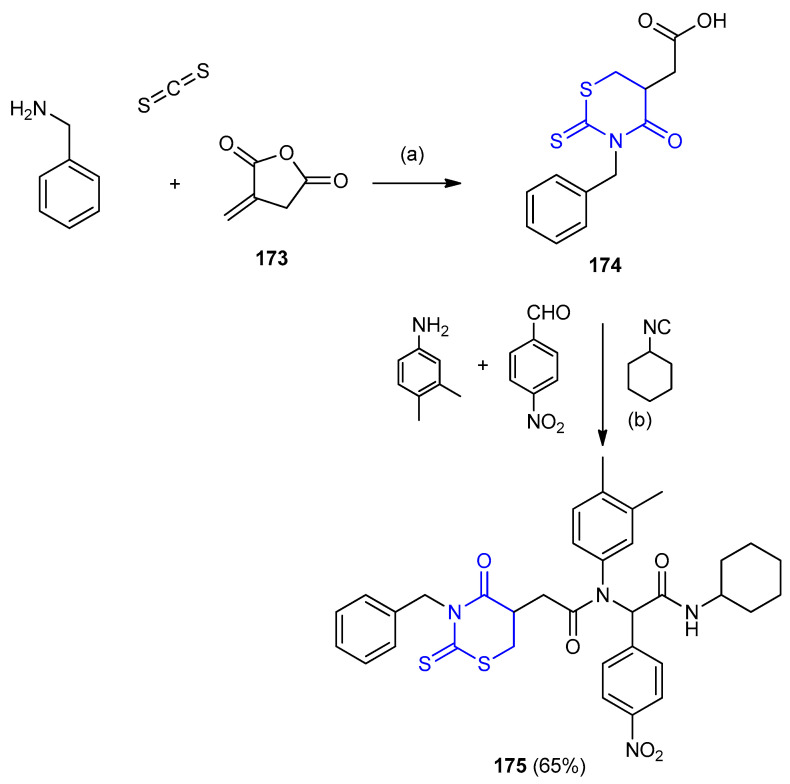
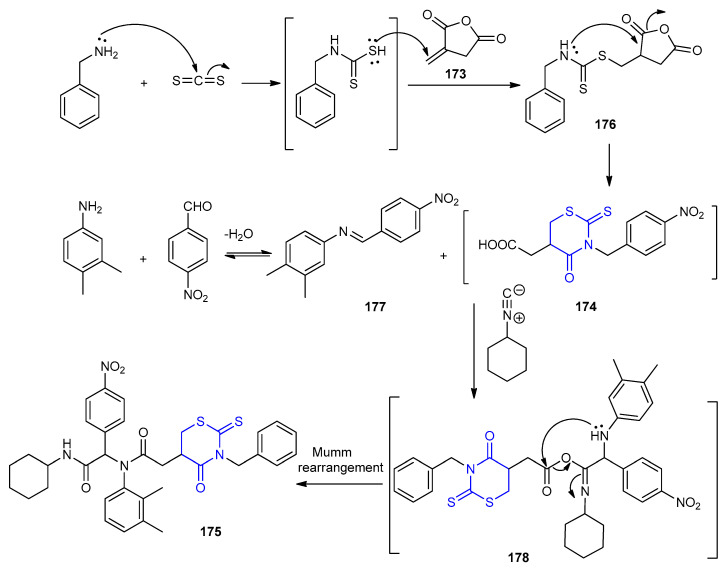
Pseudo-peptide containing 4-oxo-2-thioxo-1,3-thiazinane 175 in 65% yield, was obtained via Isocyanide-based six-component reactions with itaconic anhydride 173 (Scheme 49) [76].
Scheme 49.
Synthesis of 4-oxo-2-thioxo-1,3-thiazinane 175. Water/US (45 kHz), rt. (a) for 15 min. (b) for 75 min.
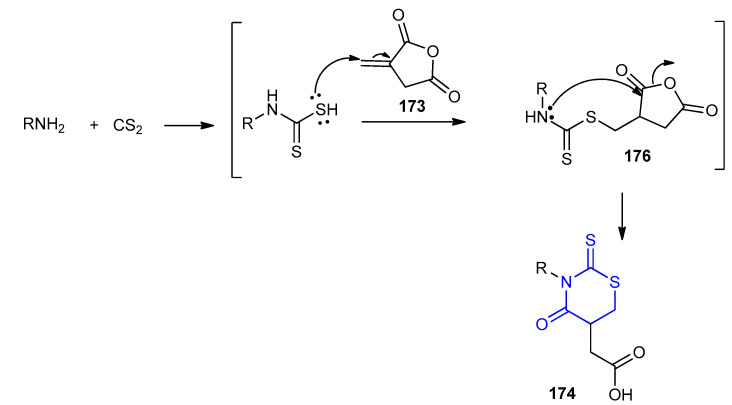
Initially, carbamodithioic acid was formed from a primary amine and carbon disulfide. Then, Michael addition of carbamodithioic acid (in situ prepared) to itaconic anhydride 173 afforded the intermediate 176, which underwent an intramolecular cyclization to give 174. The addition of the carbenoid-C atom of the isocyanides onto the iminium group followed by the addition of the carboxylate ion onto the C -atom of the nitrilium ion leads to the formation of the adduct 178, which underwent intramolecular acylation (Mumm rearrangement) [77] to give 175 (Scheme 50).
Scheme 50.
The mechanism for the formation of 4-oxo-2-thioxo-1,3-thiazinane 175.
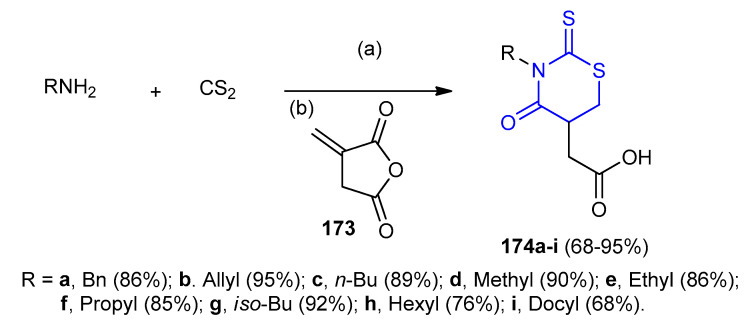
Similarly, one-pot three-component reaction of primary amines (RNH2), carbon disulfide (CS2) and itaconic anhydride (173) in water resulted in the formation of 2-(3-alkyl-4-oxo-2-thioxo- 1,3-thiazinan-5-yl) acetic acid derivatives 174a–i in 68–95% yields (Scheme 51) [78].
Scheme 51.
Synthesis of 2-(3-alkyl-4-oxo-2-thioxo-1,3-thiazinan-5-yl) acetic acid derivatives 174a–i. (a) H2O, 15 min; (b) rt 1 h.
Carbamodithioic acid was formed from a primary amine and carbon disulfide. Then it underwent Michael addition to itaconic anhydride 173 to give intermediate 176, which underwent an intramolecular cyclization to afforded 174 (Scheme 52).
Scheme 52.
The mechanism for the formation of 2-(3-alkyl-4-oxo-2-thioxo-1,3-thiazinan-5-yl) acetic acid derivatives 174a–i.
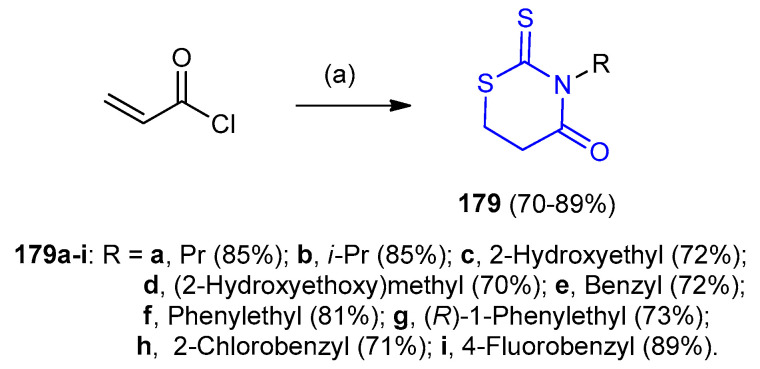
On the other hand, the one-pot reaction between primary amines and carbon disulfide in the presence of acryloyl chloride afforded 2-thioxo-1,3-thiazinane-4-one derivatives 179 in 70–89% yields (Scheme 53) [79].
Scheme 53.
Synthesis of 2-thioxo-1,3-thiazinane-4-one derivatives 179. (a) RNH2, CS2, solvent-free, r.t., 15 min.
Synthesis of 1,3-thiazinane-2-thione Derivatives
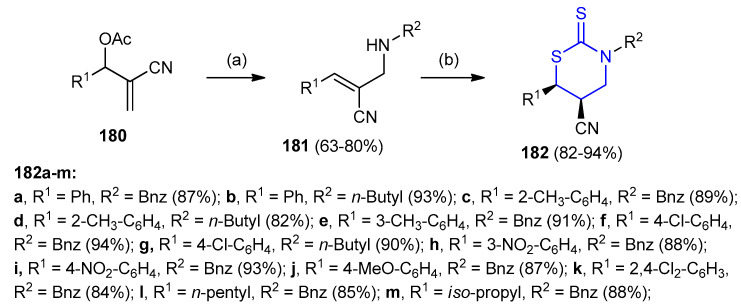
Allylamines 181, which easily, obtained via the reaction of acetates 180 of Baylis–Hillman alcohols with appropriate primary amines. The allylamine 181 was transformed into cis-5,6-disubstituted-1,3-thiazinane-2-thione derivatives 182 (82–94%) via the reaction with carbon disulfide in the presence of dimethylaminopyridine (DMAP) (Scheme 54) [80].
Scheme 54.
Synthesis of cis-5,6-disubstituted-1,3-thiazinane-2-thione derivatives 182. (a) R2NH2, EtOH, rt 3 h. (b) CS2, MeOH, DMAP, rt 15 min.
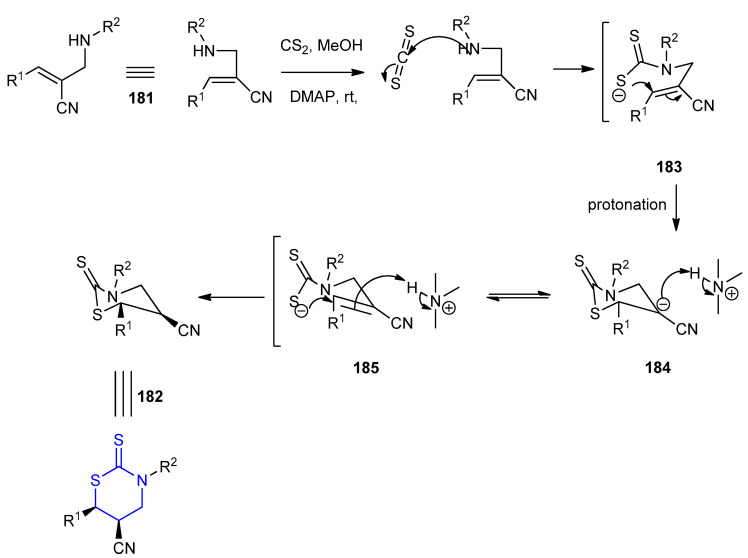
A plausible mechanism for the formation of thiazinanes 182a–m, with 5,6-cis-stereochemistry, is presented in Scheme 55. Nucleophilic attack of amine 181 onto CS2 gave the intermediate thiocarbamate ion (S=C–S−) 183, which underwent Michael addition to the α,β-unsaturated nitrile moiety to give the carbanion 184. Then protonation of the carbanion species 184 from the less hindered side, gave the thiazinanes 182 with 5,6-cis-stereoselectivity.
Scheme 55.
The mechanism for the formation of thiazinanes 182a–m.
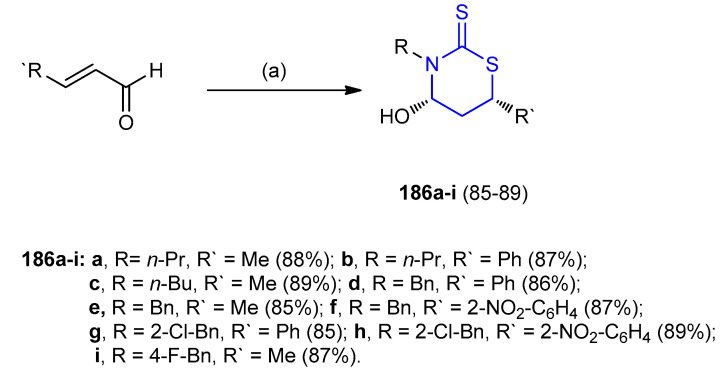
Solvent-free one-pot stereoselective synthesis of 1,3-thiazinane-2-thione derivatives 186 (85–89%) was achieved through the interaction between primary amines, carbon disulfide and α,β-unsaturated aldehydes (Scheme 56) [81].
Scheme 56.
Synthesis of 1,3-thiazinane-2-thione derivatives 186. (a) RNH2, CS2, solvent-free, r.t, 5 min.
The 1,3-thiazinane-2-thione 186 was formed upon the nucleophilic addition of the amine to carbon disulfide (S=C=S) and formation of dithiocarbamate, followed by addition to the α,β–unsaturated aldehyde to form intermediate 188, which underwent intramolecular nucleophilic cyclization on the carbonyl group to afforded thiazinane-2-thione 186 (Scheme 57).
Scheme 57.
The mechanism for the formation of 1,3-thiazinane-2-thione derivatives 186.
N-Alkyl-1,3-thiazine-2-thiones 188 was prepared from the reaction of 3-bromopropylamines [82] or substituted thiourea via iminothiazines 189 [83] with carbon disulfide. In addition, it was obtained from dithiocarbamic acids with 1,3-dibromopropane [84] (Scheme 58) [85].
Scheme 58.
Synthesis of N-Alkyl-1,3-thiazine-2-thiones 188. (a) CS2, base; (b) CS2, heat; (c) 1,3-dibromopropane, base.
1,3-Thiazinane-2-thione 188 (50% yield) was prepared via the treatment of 3-aminopropan-1-ol with sulfochloridic acid followed by carbon disulfide (CS2) (Scheme 59) [86].
Scheme 59.
Synthesis of 1,3-Thiazinane-2-thione 188. (a) ClSO3H, CCl4, MeOH, 0 °C; (b) CS2, NaOH, EtOH (50%), 0 °C, then reflux 30–40 min.
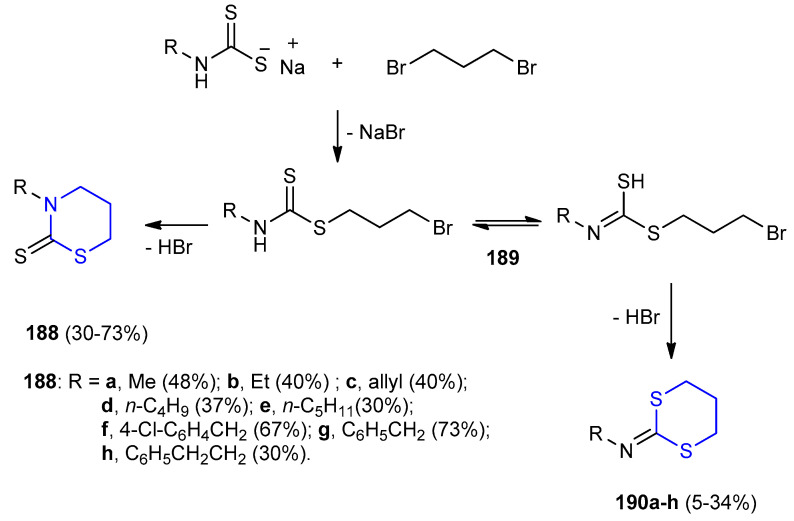
Dithiocarbamates were reacted with 1,3-dibromopropane in basic medium gave 3-bromopropyl alk/arylcarbamodithioate 189, which cyclized to both 1,3-thiazinan-2-thione derivatives 188a–h (30–73%) and 2-imino-1,3-dithian derivatives 190a–h (5–34%) (Scheme 60) [85].
Scheme 60.
Synthesis of 1,3-thiazinan-2-thione derivatives 188a–h and 2-imino-1,3-dithian derivatives 190a–h. EtOH, base, reflux.
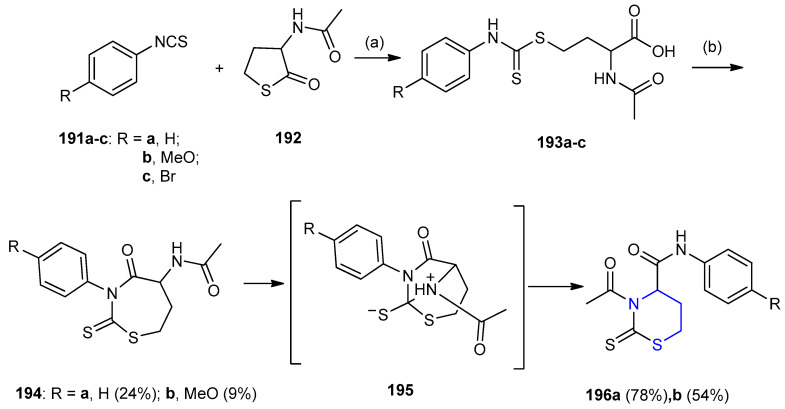
2-Oxo-thiophen acetamide 192 was reacted with aryl isothiocyanates 191a–c yielding butyric acid derivatives 193a–c. Cyclization of 193a,b in the presence of dicyclohexylcarbodimide (DCC) and 4-pyrrolidinopyridine yielded 1,3-thiazipane derivatives 194, which underwent ring transformation to afford 1,3-thiazinan-2-thione derivatives 196a,b (78% and 54%) (Scheme 61) [87].
Scheme 61.
Synthesis of 1,3-thiazinan-2-thione derivatives 196a,b. (a) dioxane, NaOH, 1 h, 75 °C then HCl; (b) DDC, 4-pyrrolidinopyridine, CH2Cl2, 15–20 h.
Synthesis of 1,3-thiazinane-4-carboxylic Acid Derivatives
The well-known cyclization reactions of β/γ-aminoalkylthiols (containing both SH and NH2 groups) with organic aldehydes, which form the thiazolidine and thiazinane derivatives, have been widely used to design fluorescent probes for the detection of the concentration of Cys and HCys in living tissues.
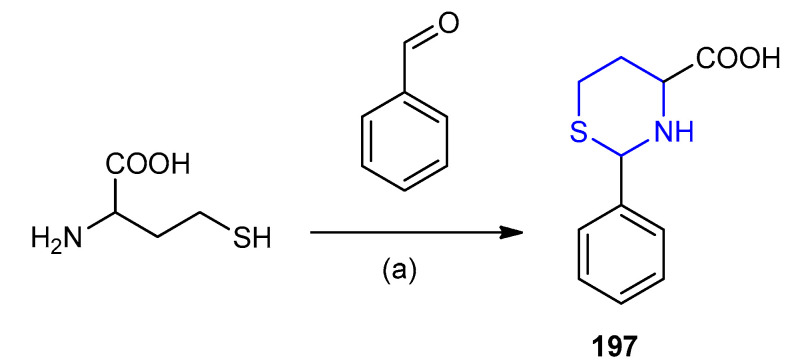
DL-Homocysteine was reacted with benzaldehyde in absolute ethanol for three days, afforded the stereoisomers (2S,4R)-, (2S,4S)-, (2R,4R)-, (2R,4S)-2-phenyl-1,3-thiazinane-4-carboxylic acid (197) (Scheme 62) [88].
Scheme 62.
Synthesis of (2S,4R)-, (2S,4S)-, (2R,4R)-, (2R,4S)-2-phenyl-1,3-thiazinane-4-carboxylic acid (197). (a) EtOH/H2O, 3 d, r.t.
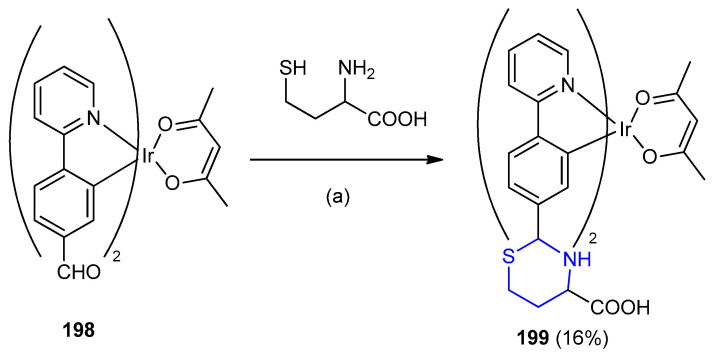
The reaction of Ir(pba)2(acac) 198 (Hpba = 4-(2-pyridyl)benzaldehyde; acac = acetylacetone) with homocysteine under stirring for 12 days in mixture of CH2Cl2/MeOH as solvent (2:1 v/v) afforded Iridium complex of thiazinane 199 (16%) (Scheme 63) [89].
Scheme 63.
Synthesis of Iridium complex of 1,3-thiazinane-4-carboxylic acid 199. (a) CH2Cl2 and MeOH (2:1v/v), 12 h.
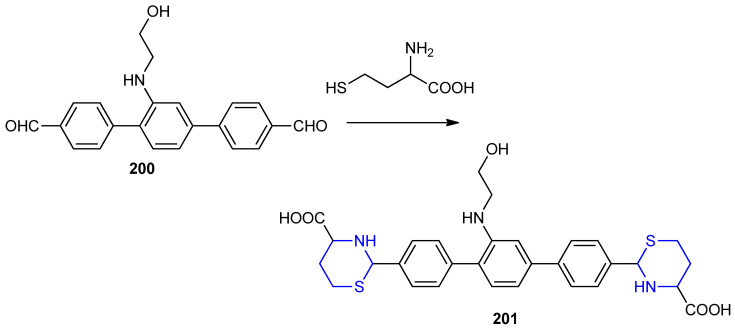
Homocysteine was reacted with 2′-((2-hydroxyethyl)amino)-[1,1′:4′,1″-terphenyl]-4,4″-dicarb-aldehyde 200 bearing electron-donating group (-NH(CH2)2OH) and electron withdrawing group (-CHO) gave 2,2′-(2′-((2-hydroxyethyl)amino)-[1,1′:4′,1″-terphenyl]-4,4″-diyl)bis(1,3-thiazinane-4-carboxylic acid) 201 (Scheme 64) [90].
Scheme 64.
Synthesis of 2,2′-(2′-((2-hydroxyethyl)amino)-[1,1′:4′,1″-terphenyl]-4,4″-diyl) bis.(1,3-thiazinane-4-carboxylic acid) 201. DMSO, r.t.
4-(6,11-Dioxo-6,11-dihydro-1H-anthra[1,2-d] imidazol-2-yl)benzaldehyde (203) was synthesized through condensation between 1,2-diaminoanthraquinone (202) and terephthalaldehyde. In addition, imidazophenanthrolin benzaldehyde 206 was obtained by refluxing a mixture of 1,10-phen anthroline-5,6-dione 205 and terephthalaldehyde. The two ligands 202 and 206 were cyclized with homocysteine furnished anthra[1,2-d]imidazolyl-1,3-thiazinane-4-carboxylic acid 204 and imidazophenanthrolin-1,3-thiazinane-4-carboxylic acid 207, respectively (Scheme 65) [91].
Scheme 65.
Synthesis of anthra[1,2-d]imidazolyl-1,3-thiazinane-4-carboxylic acid 204 and imidazophenanthrolin-1,3-thiazinane-4-carboxylic acid 207. (a) Terephthalaldehyde, EtOH, CF3CO2H, reflux, 4 h. (b) H2O/DMSO, 75 °C, 6 h. (c) NH4OOCCH3, AcOH, heat, 100 °C, 30 min. (d) H2O/DMSO, 75 °C, 6 h.
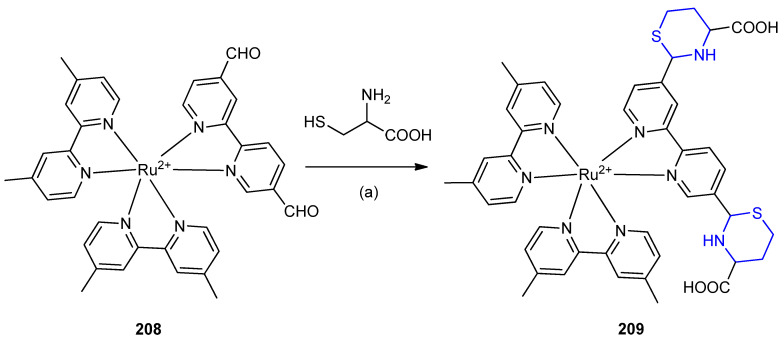
Ruthenium (II( complexes containing aldehyde groups 208 were characterized to recognize homocysteine via the formation of thiazinane 209. A strong luminescence response was found upon the reaction of the ruthenium (II) chromophore 208 with homocysteine (Scheme 66) [92].
Scheme 66.
Synthesis of Ruthenium (II( complexes of 1,3-thiazinane-4-carboxylic acid 209. (a) CH3CN, buffer, 10 min, r.t.
Tetraphenylethylenedialdehyde (210) was used for the detection of homo-cysteine via the formation of ((E)-2,2′-((1,2-diphenylethene-1,2-diyl)-bis(4,1-phenylene))bis(1,3-thiazinane-4- carboxylic acid)) 211 in DMSO under buffering conditions (pH = 7.4) as shown in Scheme 67 [93].
Scheme 67.
Synthesis of ((E)-2,2′-((1,2-diphenylethene-1,2-diyl)-bis(4,1-phenylene)) bis(1,3-thiazinane-4-carboxylic acid)) 211. (a) DMSO, buffer (pH 7.4), r.t.
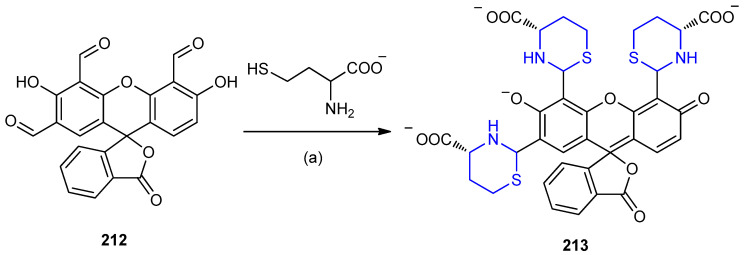
The trialdehyde 212 showed high selectivity for homocysteine at pH = 6.0 via the formation of thiazinane 213 (Scheme 68) [94].
Scheme 68.
Synthesis of thiazinane 213. (a) Phosphate buffer, DMSO, 20 °C.
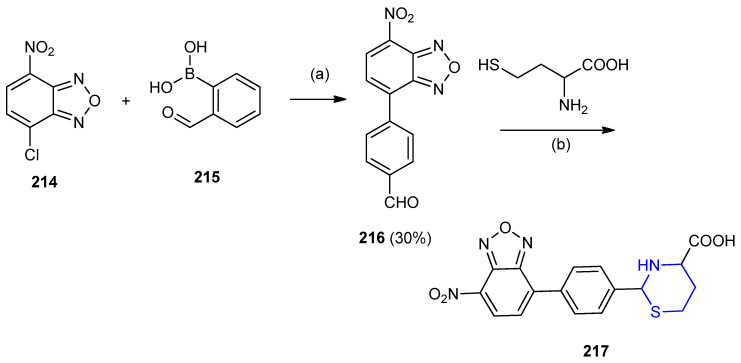
Suzuki–Miyaura–cross-coupling [95] of 4-chloro-7-nitrobenzo[1,2,5]-oxadiazole 214 with 4-formylphenylboronic acid 215 yielded (4-(7-nitrobenzo-[c][1,2,5]oxadiazol-4-yl)benzaldehyde) (216), which was reacted with homocysteine afforded a highly fluorescent compound oxadiazolyl-1,3-thiazinane-4-carboxylic acid 217 through the cyclization with the aldehydic group (Scheme 69) [96].
Scheme 69.
Synthesis of oxadiazolyl-1,3-thiazinane-4-carboxylic acid 217. (a) KF, 2-(dicyclohexylphosphino)-2′-methylbiphenyl, pd(OAc)2, toluene, 70 °C, 12 h; (b) DMSO/H2O, Phosphate buffer, pH 7.4.
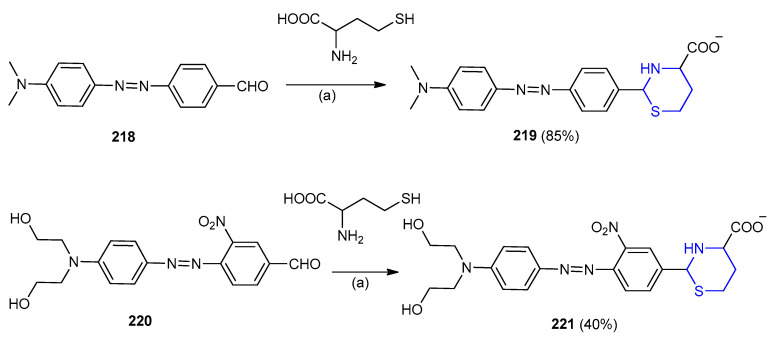
The azo dyes 4-[[40-(N,N-dimethylamino)phenyl-10-]azo]benzaldehyde 218 and 4-[[4′-(bis(2-hydroxyethyl)amino)phenyl-10-]azo]-3-nitrobenzaldehyde 220 were reacted with cysteine and homocysteine. The reaction of 218 and 220 with homocysteine afforded very stable derivatives thiazinane 219 and 221 under neutral pH conditions (Scheme 70) [97].
Scheme 70.
Synthesis of thiazinane 219 and 221. (a) DMF-H2O v/v (9:1), buffer pH 7.0, r.t.
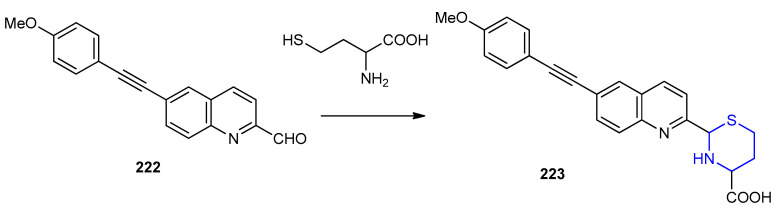
Quinoline derivative 222 was also used to detect homocysteine depending on, the formation of thiazinane 223 ring through cyclization reaction (Scheme 71) [98].
Scheme 71.
Synthesis of thiazinane 223. DMSO/PBS (9:1) buffer pH 7.4, r.t.
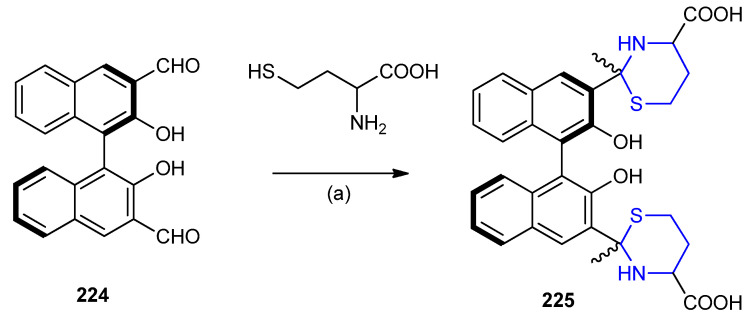
1,10-Bi-2-naphthol 224 based dialdehyde was found to exhibit selective fluorescent response towards cellular thiols, cysteine and homocysteine. 2,2′-Dihydroxy-[1,1′-binaphthalene]-3,3′-dicarb aldehyde (224) reacted with homocysteine, resulted in the formation of thiazinane 225 (Scheme 72) [99].
Scheme 72.
Synthesis of thiazinane 225. (a) MeOH, Zn(OAc)2, r.t.
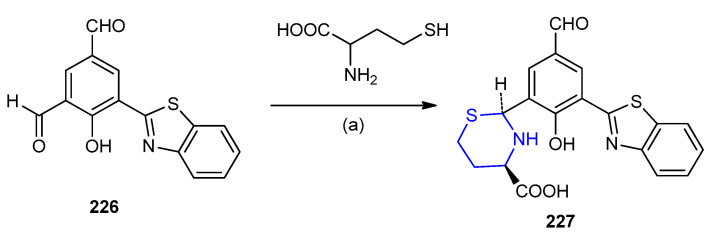
The addition of homocysteine into 5-(benzothiazol-2-yl)-4-hydroxyiso-phthalaldehyde (226) o-aldehyde group was transformed into (2S,4R)-benzo[d]thiazol-1,3-thiazinane-4-carboxylic acid derivatives 227 (Scheme 73) [100].
Scheme 73.
Synthesis of (2S,4R)-benzo[d]thiazol-1,3-thiazinane-4-carboxylic acid derivatives 227. (a) H2O, HEPES buffer pH 7.4.
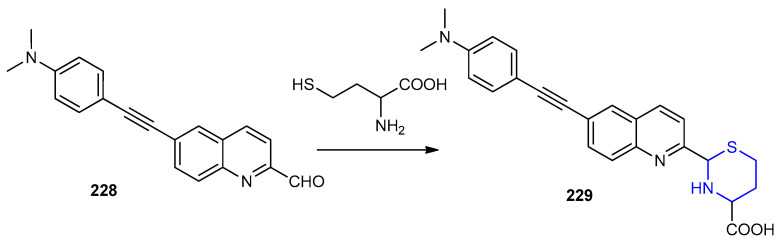
6-((4-(Dimethylamino)phenyl)ethynyl)quinoline-2-carbaldehyde (228) showed high selectivity in the detection of cysteine and homocysteine, because of the formation of thiazolidine and thiazinane derivatives. The quinoline-2-carbaldehyde 228 was reacted with homocysteine afforded 2-(6-((4-(dimethylamino)-phenyl)ethynyl)quinolin-2-yl)-1,3-thiazinane-4-carboxylic acid 229 (Scheme 74) [101].
Scheme 74.
Synthesis of 2-(6-((4-(dimethylamino)-phenyl)ethynyl)quinolin-2-yl)-1,3-thiazinane -4-carboxylic acid 229. DMSO-H2O, PBS buffer pH 7.4.
2.1.3. Synthesis of 1,4-thiazinane Derivatives
From Diazabutadiene and Butylaminoethanethiol
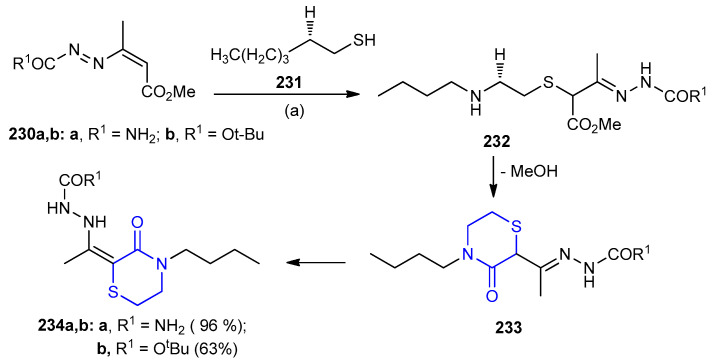
Addition of 2-(butylamino)ethanethiol 231 to 1,2-diaza-1,3-butadiene 230 resulted in the formation of hydrazone 1,4-adduct intermediate 232. The reaction between 231 and 1,2-diaza-1,3-butadienes 230 containing an ester group in position 4 of the heterodiene system gave 2-[1-(4-butyl-3-oxo-1,4-thiazinan-2-yliden)ethyl]-1-hydrazinecarboxylates 234a,b (96% and 63%) via intermediates 232 and 233 (Scheme 75) [102].
Scheme 75.
Synthesis of 2-[1-(4-butyl-3-oxo-1,4-thiazinan-2-yliden)ethyl]-1-hydrazinecarboxylates 234a,b. (a) MeOH, rt 10 min.
From Cyclic Sulfamidates
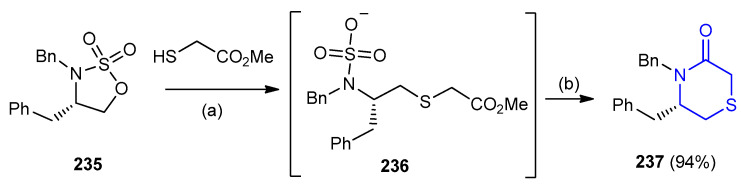
Cyclic sulfates and cyclic sulfamidates represented a versatile class of functionalized and enantiomerically pure electrophiles. A six-ring N-heterocycle ((S)-4,5-dibenzyl-1,4-thiazinane-3-one) 237 (94%) was formed through a regioselective nucleophile displacement on 235 via reaction with methyl 2-mercaptoacetate and subsequent lactamization of (S)-benzyl(1-((2-methoxy-2-oxoethyl) thio)-3-phenylpropan-2-yl)sulfamate (236) (Scheme 76) [103].
Scheme 76.
Synthesis of ((S)-4,5-dibenzyl-1,4-thiazinane-3-one) 237. (a) Na2CO3, THF; (b) toluene, 5-M HCl.
From Diethyl 2,2-sulfonyldiacetate
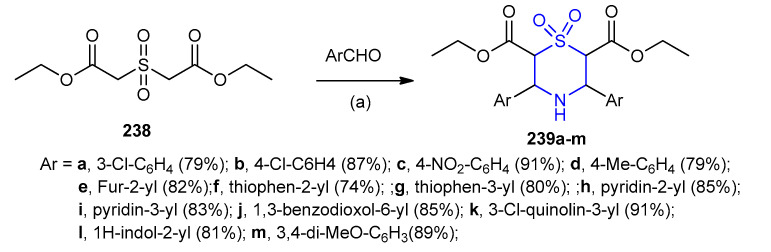
Diethyl 3,5-diphenyl-1,4-thiazinane-2,6-dicarboxylate 1,1-dioxide 239a–m (79–91%) were formed by reacting diethyl 2,2-sulfonyldiacetate (238) and aryl/heteroyl aldehydes in water, in the presence of ammonium acetate (Scheme 77) [104].
Scheme 77.
Synthesis of diethyl 3,5-diphenyl-1,4-thiazinane-2,6-dicarboxylate 1,1-dioxide 239a–m. (a) NH4OAc, H2O/80 °C, 3 h.
From Ethyl 2-[(2-oxo-2-arylethyl)sulfonyl]acetate
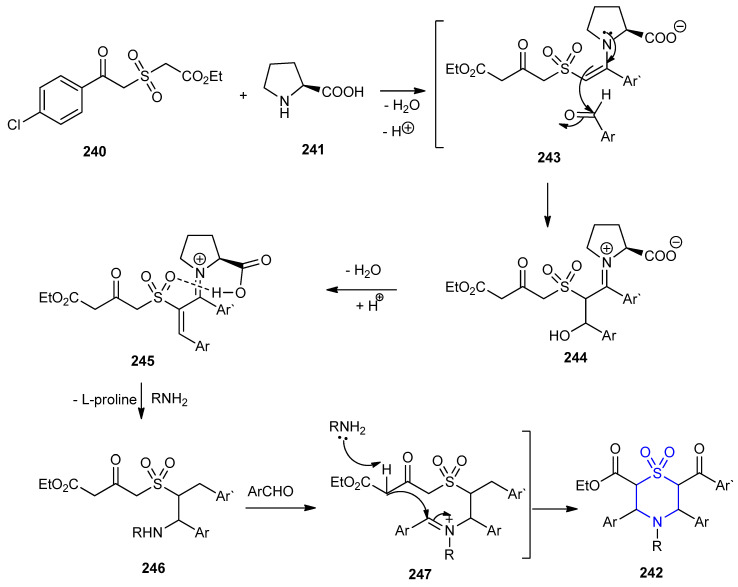
The reaction of sulfonylacetate 240, aromatic aldehydes and amines in presence of L-proline (241) as green catalyst furnished 1,1-dioxo-1,4-thiazinane-2-carboxylates 242a–a’ (72–90%) (Scheme 78) [105].
Scheme 78.
Synthesis of 1,1-dioxo-1,4-thiazinane-2-carboxylates 242a–a’. (a) 2ArCHO, RNH2, EtOH, rt., 2–4 h.
L-Proline catalyzed the reaction between sulfonyl acetate and aromatic aldehyde via the formation of enamine-imine intermediates 243 and 244, respectively, followed by dehydration of 244 to give intermediate 245. Losing of, proline moiety of 245 via the attack of the amine result in the formation of intermediate 246, which was condensed with another molecule of the aldehyde followed by intramolecular cyclization with deprotonation to furnish 242 (Scheme 79).
Scheme 79.
The Mechanism for the formation of 1,1-dioxo-1,4-thiazinane-2-carboxylates 242a–a’.
2.1.4. Synthesis of Fused Thiazinane Derivatives
Synthesis of Tetrahydrocyclopenta[e][1,3]thiazinan-2,4-dione
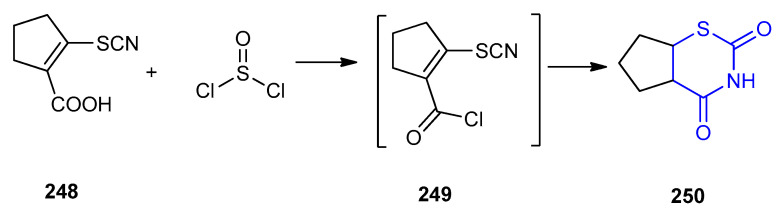
Tetrahydrocyclopenta[e][1,3]thiazinan-2,4-dione 250 was formed by reacting 2-thio cyanatocycIopent-1-ene-1-carboxylic acid (248) and thionyl chloride at room temperature via ring closure of the intermediate carboxylic acid chloride 249 (Scheme 80) [106].
Scheme 80.
Synthesis of tetrahydrocyclopenta[e][1,3]thiazinan-2,4-dione 250. (a) H2O, rt allow to stand.
Synthesis of 1,3-benzothiazinan-4-one Derivatives
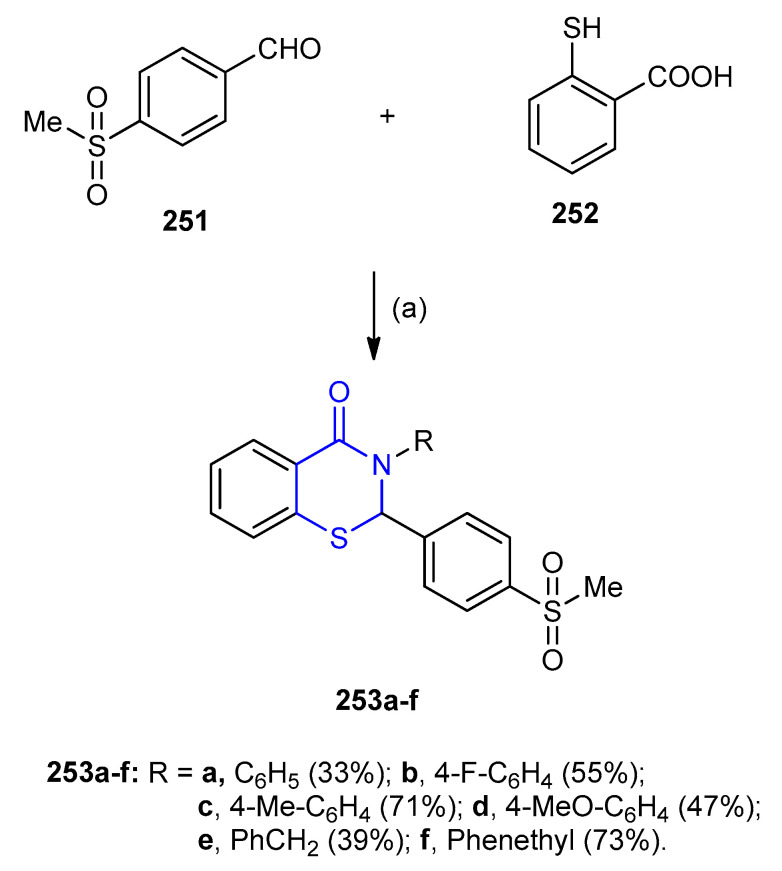
4-Methylsulfonylbenzaldehyde (251) was reacted with aromatic amines and thiosalicylic acid (252) in the presence of p-TsOH gave 2-(4-methylsulfonylphenyl)-3-substituted-1,3-benzothiazinan- 4-one 253a–f (33–73/5) (Scheme 81) [107].
Scheme 81.
Synthesis of 2-(4-methylsulfonylphenyl)-3-substituted-1,3-benzothiazinan-4-one 253a–f. (a) RNH2, toluene, p-TsOH, reflux 48 h.
Synthesis of Tetrahydropyrido[2,1-b]-[1,3]thiazine-7-carboxylate
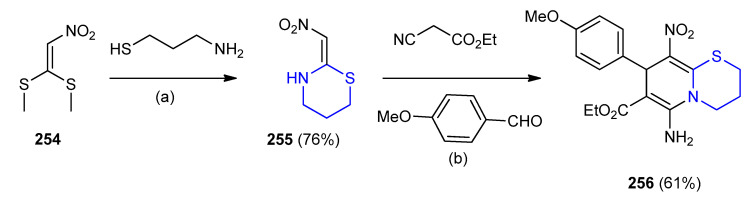
In multicomponent reactions, ethyl 6-amino-8-(4-methoxy phenyl)-9-nitro-2,3,4,8-tetrahydro- pyrido[2,1-b][1,3]thiazine-7-carboxylate 256 were synthesized. Initially, 3-aminopropanethiol was reacted with (2-nitroethene-1,1-diyl)bis-(methylsulfane) (254) in dry ethanol afforded 2-(nitro-methylene)-1,3-thiazinane (255). In the second step, compound 255 reacted with ethyl cyanoacetate and p-methoxybenzaldehyde furnished ethyl 6-amino-8-(4-methoxyphenyl)-9-nitro- 2,3,4,8-tetrahydropyrido[2,1-b][1,3]thiazine-7-carboxylate (256) (Scheme 82) [108].
Scheme 82.
Synthesis of ethyl 6-amino-8-(4-methoxyphenyl)-9-nitro-2,3,4,8-tetrahydropyrido [2,1-b][1,3]thiazine-7-carboxylate (256). (a) EtOH, rt., 18 h; (b) (i) EtOH, rt. 3 min then piperidine, 3 h. (ii) Reflux 20 h.
Synthesis of [1,3]thiazino[3,2-a]indole
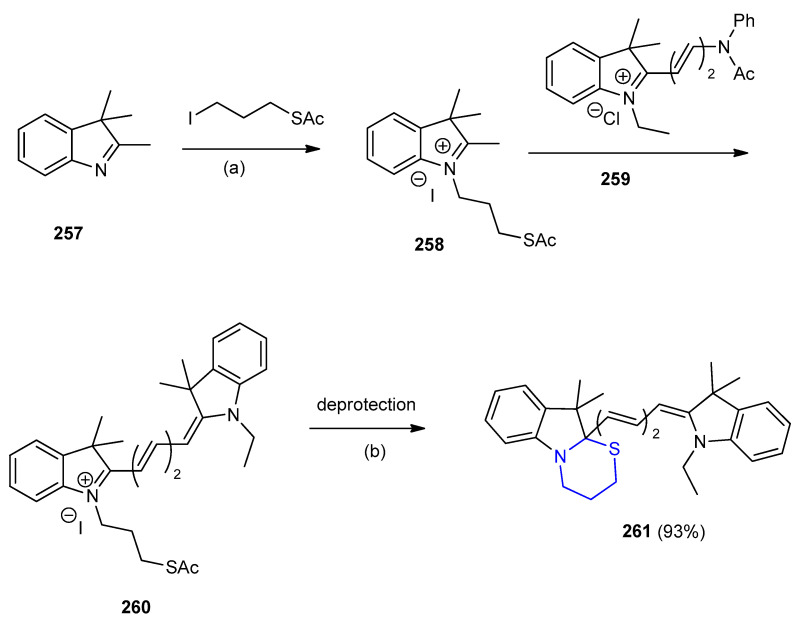
Thiazinane[3,2-a] indole 261 was synthesized from 1-(3-(acetylthio)propyl)-2,3,3-trimethyl-3H- indol-1-ium iodide (258). N-Substituted-3H-indoles (258) were obtained via nucleophilic substitution of 2,3,3-trimethyl-3H-indole (257) with alkyl halides (S-(3-iodopropyl)ethanethioate). Condensation of 258 with the reactive cyanine derivative ((E)-1-ethyl-3,3-dimethyl-2-(2-(N-phenylacetamido) vinyl)-3H-indol-1-ium chloride) (259) afforded protected 1-(3-(acetylthio)propyl)-2-((1E,3E)-3-(1-ethyl-3,3-dimethylindolin-2-ylidene)prop-1-en-1-yl)-3,3-dimethyl-3H-indol-1-ium iodide) (260). After deprotection under basic conditions [1,3]thiazino[3,2-a] indole 261 was obtained in high yield 93% (Scheme 83) [109].
Scheme 83.
Synthesis of [1,3]thiazino[3,2-a] indole 261. (a) MeCN; (b) base.
Synthesis of [1,3]dioxolo[4′,5′:3,4]pyrido[2,1-b][1,3]thiazinanone
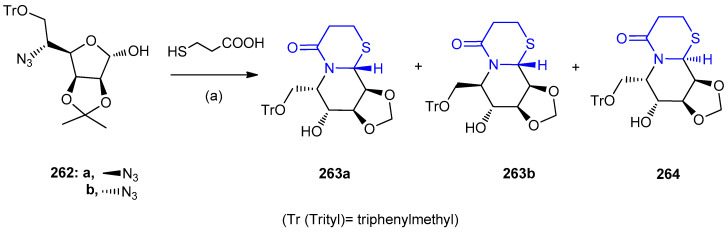
Microwave-assisted one-pot Staudinger/aza-Wittig/cyclization reaction using 262a and 262b as the starting materials afforded two diastereoisomers of the bi/tricyclic azasugars 263a,b and 264 in satisfying yields with low stereoselectivity (in total yields 62%) (Scheme 84) except the case of the reaction of 262b with 3-mercaptopropionic acid stereospecifically afforded a single diastereoisomer ((3aS,4R,5R,-10aR,10bS)-4-hydroxy-5-((trityloxy)methyl)hexahydro-[1,3]dioxolo-[4′,5′:3,4]pyrido-[2,1-b][1,3]-thiazin-7(3aH)-one) (263b, 71%), possibly due to the synergistic hindrance effects of the cis neighboring cyclic 2,3-isopropylidene and 5β-group in 262b, which made a dominant exo-attack of the sulfur atom to the intermediate imine (Scheme 84) [110].
Scheme 84.
Synthesis of diastereoisomers of the bi/tricyclic azasugars 263a,b and 264. (a) DCC, Ph3P, MW 10 min, 100 °C.
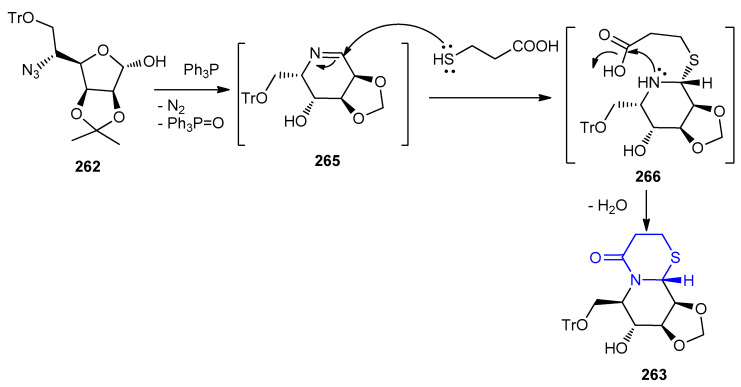
The aza-sugar 262 was cyclized to intermediate 265 in presence of PPh3 with losing of N2 and Ph3P=O. Nucleophilic attack of the thiol–group of the mercaptopropionic acid on the imine carbon of 265 gave intermediate 266. Intramolecular cyclization of intermediate 266 afforded 263 (Scheme 85).
Scheme 85.
The mechanism for the formation of diastereoisomers of the bi/tricyclic azasugars 263a,b and 264.
Synthesis of Octahydrobenzo[f][1,3]thiazino[2,3-b]quinazoline
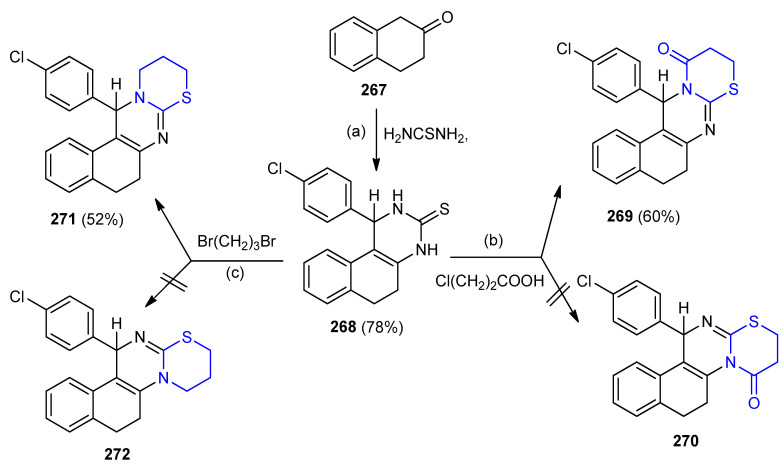
Unsymmetrical quinazoline-3-thione (1-(4-chlorophenyl)-1,2,5,6-tetrahydro-benzo[f]quinaz oline-3(4H)-thione) 268 (78%) was obtained from one-pot condensation of 2-tetralone 267, p-chlorobenzaldehyde and thiourea in acidic medium. Condensation of quinazoline-3-thione 268 with 3-chloropropionic acid and 1,3-dibromopropane furnished thiazinoquinazoline derivatives 269 and 271 in 60% and 52% yield, respectively, instead of their regioisomers 270 and 272 (Scheme 86) [111].
Scheme 86.
Synthesis of thiazinoquinazoline derivatives 269 and 271. (a) 4-Cl-C6H4CHO, thiourea, AcOH, 100 °C, 4 h; (b) NaOAc, AcOH, (AcO)2O, reflux 10–12 h; (c) EtOH, reflux 5 h.
2.1.5. Synthesis of Spirothiazinane Derivatives
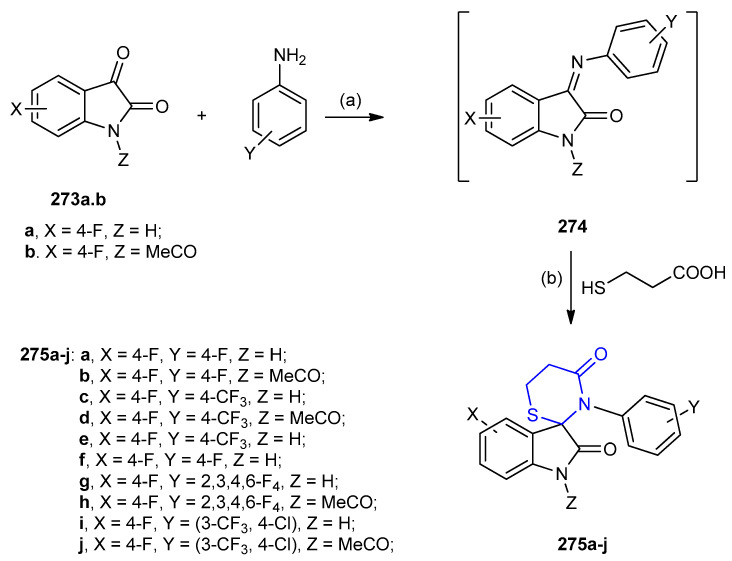
Condensation of fluorinated indole-2,3-diones 273a and 1-acetylindole-2,3-diones 273b with fluorinated aniline afforded 3-arylimino-2H-indol-2-ones 274, which, in situ, were cyclized with 3-mercaptopropanoic acid to afford the spiro compounds 275. In a few cases, intermediates isatin-3-anil 274 were isolated (Scheme 87) [112,113,114]. In addition, it was reported that fluorinated spiro[indoline-3,2′-[1,3]thiazinane]-2,4′-diones 275, were synthesized via one-step synthesis through the formation of Schiff’s bases followed by cyclization with 3-mercaptopropanoic acid, both thermally and under microwave irradiation. The reactions were studied under different reaction conditions. It was observed that the yield was improved when the reaction was carried out under microwave irradiation [115]. Furthermore, Dandia et al. reported, a one-pot solvent-free synthesis of spiro[indole-3,2-[1,3]thiazinane]-2,4-diones 275a (4 min, 140 °C (85%); 6 min, 135 °C (93%)) [108] from the reaction of intermediate 274 with 3-mercaptopropionic acid (Scheme 87) [112,113,114,115,116,117].
Scheme 87.
Synthesis of spiro[indole-3,2-[1,3]thiazinane]-2,4-diones 275a–j. (a) solvent-free, MW 30 s, 640 W; (b) MW 4 min, 640 W (85%) or montmorillonite KSF, MeOH, MW 6 min, 640 W (93%).
2.2. Reactions of Thiazinanes
2.2.1. Reactions of 1,2-thiazinanes
N-Arylation of 1,2-thiazinane
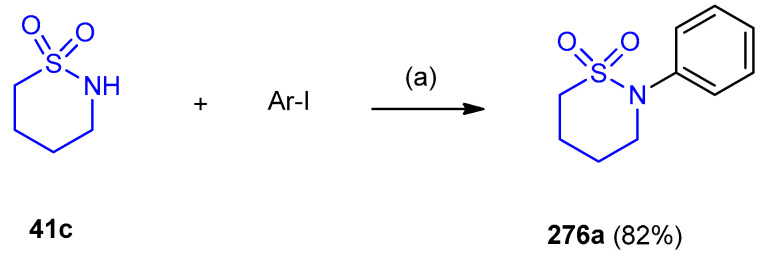
N-Arylation reaction of 1,2-thiazinane-1,1-dioxide 41c using Cu2O and Cs2CO3 in water gave N-arylmethanesulfonamide (2-phenyl-1,2-thiazinane-1,1-dioxide) 276 (82%) (Scheme 88) [118].
Scheme 88.
Synthesis of N-arylmethanesulfonamide (2-phenyl-1,2-thiazinane-1,1-dioxide) 276. (a) Cu2O (2 mol.%), CsCO3 (2 equiv.), H2O, 130 °C.
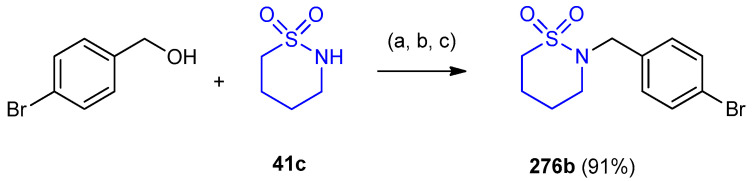
2-(4-Bromobenzyl)-1,2-thiazinane-1,1-dioxide 276b was prepared via direct sulfonamidation of (4-bromophenyl)methanol. The reaction between (4-bromophenyl)methanol and 1,2-thiazinane- 1,1-dioxide 41c was carried out using 2,3,4,5-tetrafluorophenylboronic acid, oxalic acid dihydrate and HFIP (hexafluoroisopropanol)/nitromethane mixture to afford 276b (Scheme 89) [119].
Scheme 89.
Synthesis of 2-(4-bromobenzyl)-1,2-thiazinane-1,1-dioxide 276b. (a) 2,3,4,5-Tetrafluorophenylboronic acid; (b) oxalic acid dehydrate; (c) HFIP/MeNO2 (4:1), 80 °C, 6 h.
2.2.2. Reactions of 1,3-thiazinanes
Ring-opening of N-substituted 1,3-thiazinanes and Synthesis of Thioesters
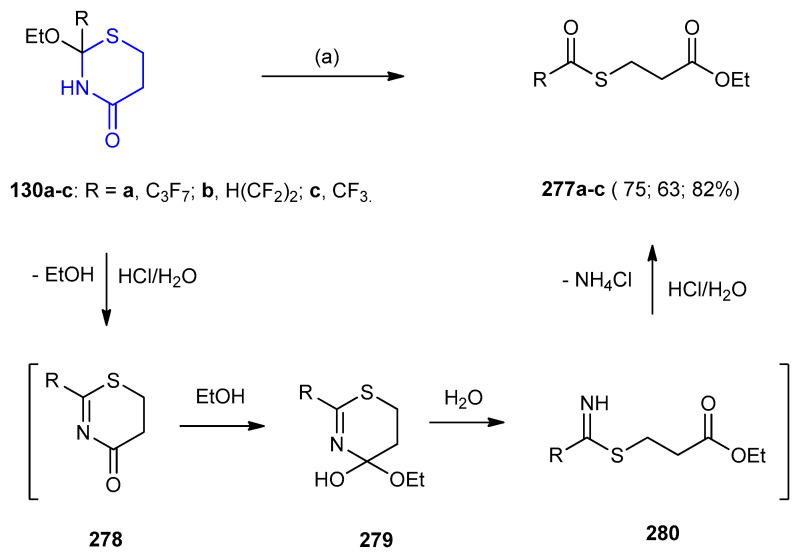
1,3-Thiazinane-4-ones 130a–c relatively stable in aqueous alkaline medium and are easily hydrolyzed under acidic conditions. Treatment of 130a–c with conc. HCl resulted in, the formation of thioester derivatives 277a–c. The possible reaction mechanism includes the elimination of ethanol from 130a–c catalyzed by HCl with its subsequent addition to 278 giving intermediate 279. Hydrolysis of the latter led to acyclic imine 280, which was converted into 277a–c (63–82%) under acidic conditions (Scheme 90) [61].
Scheme 90.
Synthesis of thioester derivatives 277a–c. (a) conc. HCl (36%), 4 d, rt.
N-Alkylation of 1,3-thiazinane-2-thione
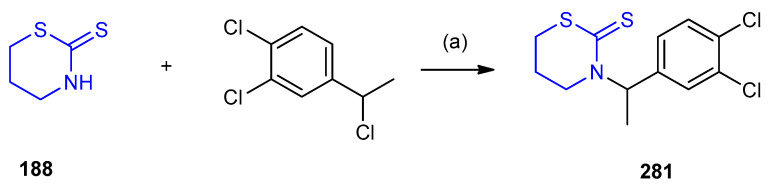
1,3-Thiazinane-2-thione (188) reacted with 1,2-dichloro-4-(1-chloroethyl)-benzene in presence of sodium hydride afforded 3-substituted 1,3-thiazinane-2-thione 281 (Scheme 91) [120].
Scheme 91.
Synthesis of 3-substituted 1,3-thiazinane-2-thione 281. (a) CH3CN, 80 °C, 3 h.
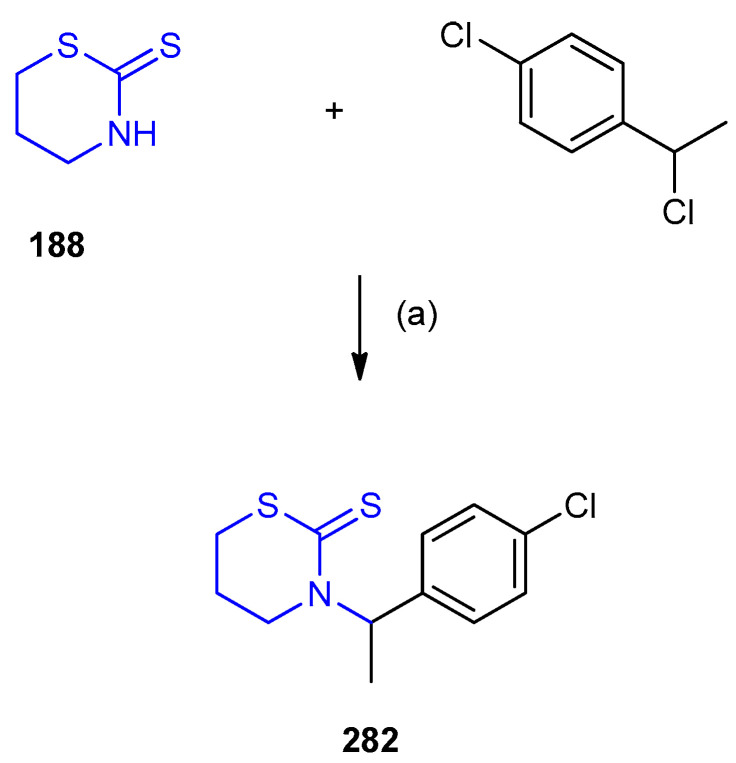
Similarly, 1,3-thiazinane-2-thione (188) was reacted with 1-chloro-4-(1-chloroethyl)benzene in presence of K2CO3 afforded 3-[1-(4-chlorophenyl)ethyl]-1,3-thiazinane-2-thione 282 (Scheme 92) [121].
Scheme 92.
Synthesis of 3-[1-(4-chlorophenyl)ethyl]-1,3-thiazinane-2-thione 282. (a) K2CO3 (5.0 mmol), CH3CN, 160 °C, 8 h.
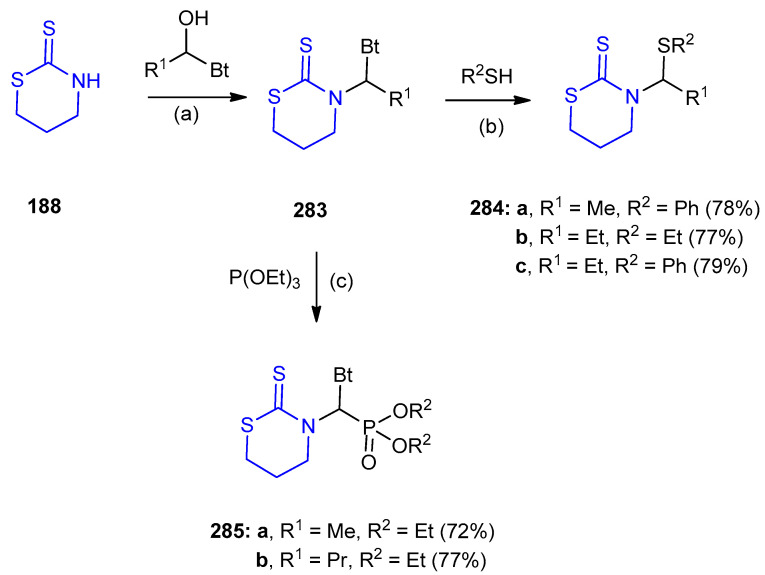
In addition, 1,3-thiazinane-2-thione 188 [82] was condensed with 1-(1-hydroxyalkyl) benzotriazoles in the presence of boron trifluoride gave 3-(1-benzotriazolylalkyl)thiazine-2-thiones 283. Nucleophilic substitution of benzotriazolyl group in 3-(1-benzotriazolyl alkyl)-1,3- thiazinane-2-thiones 283 using thiol compounds and in the presence of ZnBr2, 3-[1-(substituted sulfanyl)alkyl]-1,3-thiazinane-2-thiones 284a–c were formed (78; 77; and 79%). On the other hand, 1,3-thiazinane-2-thiones 283 reacted with triethyl phosphite catalyzed by ZnBr2 in CH2Cl2 under reflux furnished 1-(2-thioxo-1,3-thiazinan-3-yl)alkylphosphonates 285a,b (72; 77%) (Scheme 93) [85].
Scheme 93.
Synthesis of 1-(2-thioxo-1,3-thiazinan-3-yl)alkylphosphonates 285a,b. (a) BF3, THF, 12 h, 25 °C; (b) Et2O, ZnBr2, reflux 12 h; (c) CH2Cl2, 20–25 °C, ZnBr2, reflux 14 h.
Synthesis of Bis-pyrrol and Bis-pyrrolothiazole
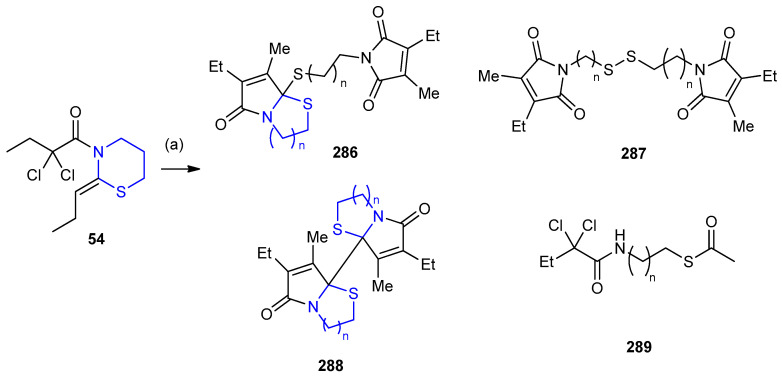
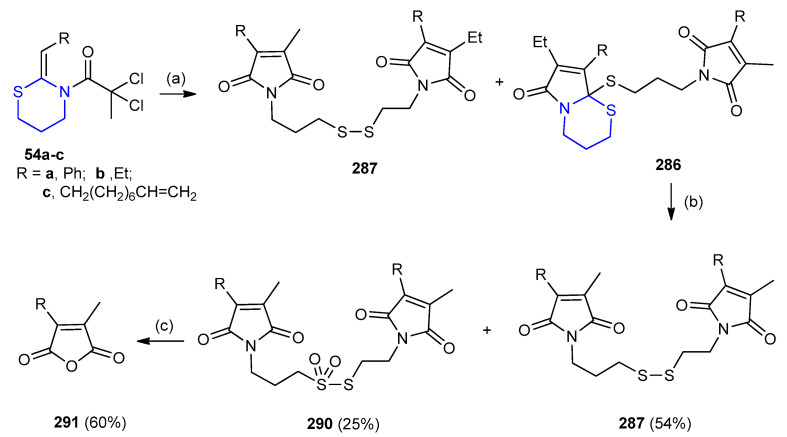
(Z)-2,2-Dichloro-1-(2-ethylidene-1,3-thiazinan-3-yl)butan-1-one 54a underwent stereoselective copper-catalyzed radical cyclization (RC) under reaction conditions: CuCl with TMEDA or PMDETA in MeCN to give a 9:1 mixture of dimers 286 and 287, respectively. Only traces of thioester 289 were indicated with the absence of dimer 288 (Scheme 94) [122].
Scheme 94.
Stereoselective copper catalyzed radical cyclization of (Z)-2,2-dichloro-1-(2-ethylidene-1,3-thiazinan-3-yl)butan-1-one 54a. (a) CuCl, TMEDA or PMDETA, 10 mol% in CH3CN.
Synthesis of Maleic Anhydride
Radical cyclization of (Z)-3-(2,2-dichloropropanoyl)-2-pentadecylidene-1,3-thiazinane 54 giving, thioacetal 286 and disulfide 288 (Scheme 94) [37]. Acetal 286 was oxidized to disulfide 287 using KI in water via the liberation of iodine (I2) as illustrated in scheme b. N-(3-Hydroxypropyl)-undec-10-enamide was applied in the reaction yield enhancement, which subjected to radical cyclization, followed by hydrolysis to furnish maleic anhydride 291 (60%) (Scheme 95) [123].
Scheme 95.
Synthesis of maleic anhydride 291 from radical cyclization and hydrolysis of 54a–c. (a) CuCl (10-mol %), TMEDA (20-mol %), MeCN, Na2CO3; (b) silica/sulfuric acid, NaNO3, SiO2/H2O (3:2), CH2Cl2; (c) H2SO4/AcOH (1:1), 140 °C.
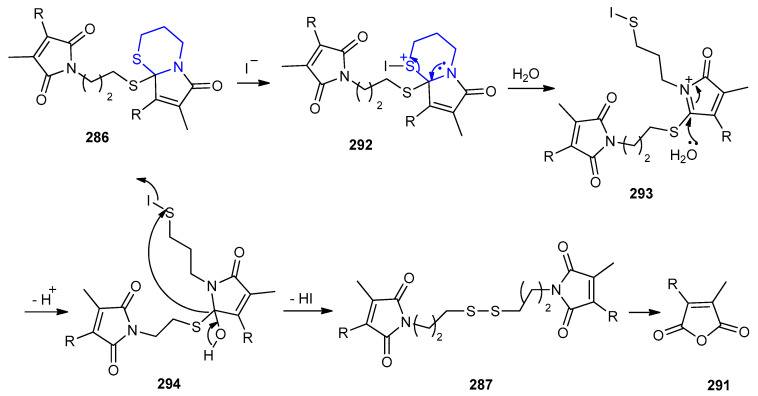
The liberation of iodine catalyzed radical oxidation of S,S-acetal 286 by accelerating ring-opening of intermediate 292 to 293. In presence of H2O as a nucleophile attacked the imine-carbon in 293 to give the hydroxylated intermediate 294. The liberation of HI from 294 gave disulfide 287, which hydrolyzed to maleic anhydride 291 (Scheme 96).
Scheme 96.
The mechanism of the radical cyclization and iodine hydrolysis of 54a–c to form maleic anhydride.
Synthesis of Bis-thiazinethioether
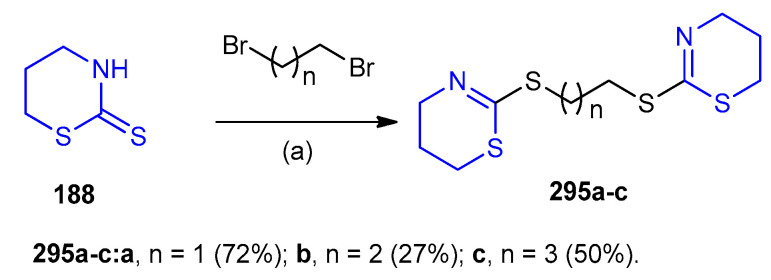
Wang et al. made a series of multithioether derivatives 295a–c (27–72%) using the reaction of thiazinan-2-thiones (188) with alkyl dibromides. The synthesized compounds were tested for antitumor activity (Scheme 97) [124].
Scheme 97.
Synthesis of multithioether derivatives 295a–c. (a) EtOH, KOH, 72 h at 25 °C.
Synthesis of Benzo[4,5]thieno[3,2-b][1,5]thiazocin-6(3H)-one
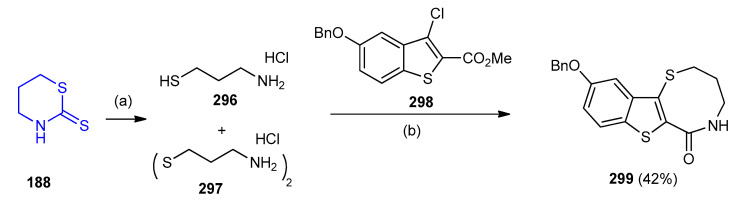
8-Membered thiazepinone ring analog (10-(benzyloxy)-4,5,6a,11b-tetrahydro-2H-benzo[4,5]- thieno[3,2-b][1,5]thiazocin-6(3H)-one) (299) was prepared from with the synthesis of 296 and 297 via the ring opening of 1,3-thiazinane-2-thione (188). Aminothiol 296 and 297 was isolated as a thiol/disulfide mixture and used directly in the aforementioned cyclo-condensation-deprotection sequence with methyl 5-(benzyloxy)-3-chlorobenzo[b]thiophene-2-carboxylate (298) to provide the desired 8-membered thiazepinone analogs (10-(benzyloxy)-4,5-dihydro-2H-benzo[4,5]thieno- [3,2-b][1,5]thiazocin-6(3H)-one) 299 (42%) (Scheme 98) [125].
Scheme 98.
Synthesis of (10-(benzyloxy)-4,5-dihydro-2H-benzo[4,5]thieno[3,2-b][1,5]thiazocin-6(3H)-one) 299. (a) conc. HCl, reflux, N2; (b) DBU, DMF, rt to 70 °C.
3. Conclusions
This review was focused on the chemistry of 1,2-, 1,3- and 1,4-thiazinane derivatives, synthesis and reactions such as arylation, alkylation, ring-opening and dimerizations were presented. Some mechanisms were illustrated to evaluate the reaction pathways for the formation of thiazinane derivatives as well as their interactions. In addition, abbrev was demonstrated about the structure, biologic activities and the commercial drugs containing thiazinane rings on their structure cores.
Acknowledgments
Authors acknowledge support by the KIT-Publication Fund of the Karlsruhe Institute of Technology.
Author Contributions
Writing, editing and submitting, A.A.H. supervision, A.A.A.; writing and editing, S.B. draft writing, H.N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu C.M., Li B., Shen Y.H., Zhung W.D. Heterocyclic Compounds and Aromatic Diglycosides from Bretschneidera sinensis. J. Nat. Prod. 2010;73:1582–1585. doi: 10.1021/np1002934. [DOI] [PubMed] [Google Scholar]
- 2.Glasby J.S. Encyclopaedia of Antibiotics. John Wiley & Sons; London, UK: 1976. pp. 124–125. [Google Scholar]
- 3.Kai H., Morioka Y., Tomida M., Takahashi T., Hattori M., Hanasaki K., Koike K., Chiba H., Shinohara S., Kanemasa T., et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 2: Orally bioavailable compounds. Bioorg. Med. Chem. Lett. 2007;17:3925–3929. doi: 10.1016/j.bmcl.2007.04.099. [DOI] [PubMed] [Google Scholar]
- 4.Trofimova T.P., Zefirova O.N., Mandrugin A.A., Fedoseev V.M., Peregud D.I., Onufriev M.V., Gulyaeva N.V., Proskuryakov S.Y. Synthesis and Study of NOS-Inhibiting Activity of 2-N-Acylamino-5,6-dihydro-4H-1,3-thiazine. Mosc. Univ. Chem. Bull. 2008;63:274–277. doi: 10.3103/S0027131408050088. [DOI] [Google Scholar]
- 5.Hazuda D.J., Anthony N.J., Gomez R.P., Jolly S.M., Wai J.S., Zhuang L., Fisher T.E., Embrey M., Guare J.P., Egbertson M.S., et al. A naphthyridine carboxamide provides evidence for discordant resistance between mechanistically identical inhibitors of HIV-1 integrase. Proc. Natl. Acad. Sci. USA. 2004;101:11233–11238. doi: 10.1073/pnas.0402357101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surrey A.R., Webb W.G., Gesler R.M. Central Nervous System Depressants. The Preparation of Some 2-Aryl-4-metathiazanones. J. Am. Chem. Soc. 1958;80:3469–3471. doi: 10.1021/ja01546a065. [DOI] [Google Scholar]
- 7.Chang P., Hung M.C. Synthetic studies on chlormezanone. ZhonghuaYaoxchang Zazhi. 1991;43:437. [Google Scholar]
- 8.El-Sayed O.A., Aboul-Enein H.Y. Synthesis and Antimicrobial Activity of Novel Pyrazolo[3,4-b]quinolone Derivatives. Arch. Pharma. 2001;334:117–120. doi: 10.1002/1521-4184(200104)334:4<117::AID-ARDP117>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Salama M.A., El-Essa S.A. Synthesis and reactions of some new substituted phenylhydrazono indeno-thiazolo[3,2-b]pyrimidine-3-ones of possible antimicrobial activity. Indian J. Chem. 2001;40B:678–681. [Google Scholar]
- 10.Kawasaki T., Immaru D., Osaka Y., Tsuchiya T., Ono S. Eur. patent 50003 (Cl. C07D279/06), 1982. Eur. patent 50003 (Cl. C07D279/06) Chem. Abstr. 1982;97:92300b.
- 11.Fauber B.P., René O., Deng Y., DeVoss J., Eidenschenk C., Everett C., Ganguli A., Gobbi A., Hawkins J., Johnson A.R., et al. Discovery of 1-{4-[3-Fluoro-4-((3S,6R)-3-methyl-1,1-dioxo-6-phenyl[1,2] -thiazinan-2-yl-methyl) phenyl] piperazin-1-yl}ethanone (GNE-3500): A Potent, Selective, and Orally Bioavailable Retinoic Acid Receptor-Related Orphan Receptor C (RORc or RORγ) Inverse Agonist. J. Med. Chem. 2015;58:5308–5322. doi: 10.1021/acs.jmedchem.5b00597. [DOI] [PubMed] [Google Scholar]
- 12.Steverding D. The development of drugs for treatment of sleeping sickness: A historical review. Parasites Vectors. 2010;3:15–24. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alirol E., Schrumpf D., Heradi J.A., Riedel A., de Patoul C., Quere M., Chappuis F. Nifurtimox-eflornithine combination therapy for second-stage gambiense human African trypanosomiasis: Médecins Sans Frontières experience in the Democratic Republic of the Congo. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;56:195–203. doi: 10.1093/cid/cis886. [DOI] [PubMed] [Google Scholar]
- 14.Botero A., Keatley S., Peacock C., Thompson R.C.A. In vitro drug susceptibility of two strains of the wildlife trypanosome, Trypanosoma copemani: A comparison with Trypanosoma cruzi. Int. J. Parasitol. 2017;7:34–41. doi: 10.1016/j.ijpddr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris M., Price R.N., Robinson J., May T.E., Wadayama N. WL108477—A novel neurotoxic insecticide. Proc. Br. Crop Prot. Conf. 1986:115–122. [Google Scholar]
- 16.Rawal R.K., Tripathi R., Katti S.B., Pannecouque C., De Clercq E. Synthesis and evaluation of 2-(2,6-dihalophenyl)-3-pyrimidinyl-1,3-thiazolidin-4-one analogues as anti-HIV-1 agents. Bioorg. Med. Chem. 2007;15:3134–3142. doi: 10.1016/j.bmc.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 17.Rawal R.K., Tripathi R., Katti S.B., Pannecouque C., De Clercq E. Design, synthesis, and evaluation of 2-aryl-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Bioorg. Med. Chem. 2007;15:1725–1731. doi: 10.1016/j.bmc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Verma A., Saraf S.K. 4-Thiazolidinone-A biologically active scaffold. Eur. J. Med. Chem. 2008;43:897–905. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Kamel M.M., Ali H.I., Anwar M.M., Mohamed N.A., Soliman A.M. Synthesis, antitumor activity and molecular docking study of novel Sulfonamide-Schiff’s bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur. J. Med. Chem. 2010;45:572–580. doi: 10.1016/j.ejmech.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Verma A., Verma S.S., Saraf S.K. A DIC mediated expeditious small library synthesis and biological activity of thiazolidin-4-one and 1,3-thiazinan-4-one derivatives. J. Heterocycl. Chem. 2010;47:1084–1089. doi: 10.1002/jhet.429. [DOI] [Google Scholar]
- 21.Kumawat M.K., Singh U.P., Singh B., Prakash A., Chetia D. Synthesis and antimalarial activity evaluation of 3-(3-(7-chloroquinolin-4-ylamino)propyl)-1,3-thiazinan-4-one derivatives. Arabian J. Chem. 2016;9:S643–S647. doi: 10.1016/j.arabjc.2011.07.007. [DOI] [Google Scholar]
- 22.Bosenbecker J., Bareño V.D.O., Difabio R., Vasconcellos F.A., Dutra F.S.P., Oliveira P.S., Barschak A.G., Stefanello F.M., Cunico W.J. Synthesis and Antioxidant Activity of 3-(Pyridin-2-ylmethyl)-1,3- thiazinan(thiazolidin)-4-ones. Biochem. Mol. Toxicol. 2014;28:425–432. doi: 10.1002/jbt.21581. [DOI] [PubMed] [Google Scholar]
- 23.Lee J., Zhong Y.L., Reamer R.A., Askin D. Practical Synthesis of Sultams via Sulfonamide Dianion Alkylation: Application to the Synthesis of Chiral Sultams. Org. Lett. 2003;5:4175–4177. doi: 10.1021/ol0356183. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L., Buchwald S. Palladium-Catalyzed Aromatic Carbon-Nitrogen Bond Formation. In: De Meijere A., Diederich F., editors. Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. Volume 2. Wiley-VCH; Weinheim, Germany: 2003. pp. 699–760. [Google Scholar]
- 25.Kenworthy M.N., Taylor R.J.K. Tethered aminohydroxylation using acyclic homo-allylic sulfamate esters and sulfonamides as substrates. Org. Biomol. Chem. 2005;3:603–611. doi: 10.1039/b416477f. [DOI] [PubMed] [Google Scholar]
- 26.Padwa A., Flick A.C., Leverett C.A., Stengel T. Rhodium (II)-Catalyzed Aziridination of Allyl-Substituted Sulfonamides and Carbamates. J. Org. Chem. 2004;69:6377–6386. doi: 10.1021/jo048990k. [DOI] [PubMed] [Google Scholar]
- 27.Liang J.L., Yuan S.X., Chan P.W.H., Che C.M. Rhodium (II,II) Dimer as an Efficient Catalyst for Aziridination of Sulfonamides and Amidation of Steroids. Org. lett. 2004;4:4507–4510. doi: 10.1021/ol0270475. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W., Ai J., Shi D., Peng X., Ji Y., Liu J., Geng M., Li Y. Discovery of novel type II c-Met inhibitors based on BMS-777607. Eur. J. Med. Chem. 2014;80:254–266. doi: 10.1016/j.ejmech.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 29.Dauban P., Dodd R.H. Synthesis of Cyclic Sulfonamides via Intramolecular Copper-Catalyzed Reaction of Unsaturated Iminoiodinanes. Org. Lett. 2000;2:2327–2329. doi: 10.1021/ol000130c. [DOI] [PubMed] [Google Scholar]
- 30.Eckhardt M., Frattini S. New Indanyloxy Dhydrobenzofuranylacetic Acid. 0,252,937 A1. U.S. Patent. 2013 Sep 26
- 31.Campbell A.D., Birch A.M. Expedient Syntheses of Sulfonylhydantoins and Two Six-Membered Analogues. Synlett. 2005;5:0834–0838. doi: 10.1055/s-2005-863735. [DOI] [Google Scholar]
- 32.Liu X.Y., Li C.H., Che C.M. Phosphine Gold (I)-Catalyzed Hydroamination of Alkenes under Thermal and Microwave-Assisted Conditions Org. J. Am. Chem. Soc. 2006;8:2707–2710. doi: 10.1021/ol060719x. [DOI] [PubMed] [Google Scholar]
- 33.Khan H.P.A., Chakraborty T.K. Diversity-Oriented Approach to N-Heterocyclic Compounds from α-Phenyl-β-enamino Ester via a Mitsunobu-Michael Reaction Sequence. J. Org. Chem. 2018;83:2027–2039. doi: 10.1021/acs.joc.7b02962. [DOI] [PubMed] [Google Scholar]
- 34.Khalil A.M., Berghot M.A., Gouda M.A. Synthesis and antibacterial activity of some new thiazole and thiophene derivatives. Eur. J. Med. Chem. 2009;44:4434–4440. doi: 10.1016/j.ejmech.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Cornia A., Felluga F., Frenna V., Ghelfi F., Parsons A.F., Pattarozzi M., Roncaglia F., Spinelli D. CuCl-catalyzed radical cyclisation of N-a-perchloroacyl-ketene-N,S-acetals: A new way to prepare disubstituted maleic anhydrides. Tetrahedron. 2012;68:5863–5881. doi: 10.1016/j.tet.2012.04.117. [DOI] [Google Scholar]
- 36.Fuganti C., Gatti F.G., Serra S. A general method for the synthesis of the most powerful naturally occurring Maillard flavors. Tetrahedron. 2007;63:4762–4767. doi: 10.1016/j.tet.2007.03.089. [DOI] [Google Scholar]
- 37.Minić A., Bugarinović J.P., Pejović A., Komatina D.I., Bogdanović G.A., Damljanović I., Stevanović D. Synthesis of novel ferrocene-containing 1,3-thiazinan-2-imines: One-pot reaction promoted by ultrasound irradiation. Tetrahedron Lett. 2018;59:3499–3502. doi: 10.1016/j.tetlet.2018.08.029. [DOI] [Google Scholar]
- 38.Sineokov A.P., Sergeeva M.E. [C. A. 66(1967)55150s] Zh. Org. Khim. 1967;3:1468. [Google Scholar]
- 39.Taborsky R. Thiol Addition of Thiourea in Heterocyclic Ring Formation: Preparation of 5-Ethyl-6-phenyl- meta-thiazane-2,β-dion. J. Org. Chem. 1958;23:1779–1780. doi: 10.1021/jo01105a604. [DOI] [Google Scholar]
- 40.Zimmermann R. A Simple Synthesis of 2-Imino-4-oxo-3,5H-1,3-thiazine-6-carboxylic Esters. Angew. Chem. Int. Ed. 1962;1:663. doi: 10.1002/ange.19620742220. [DOI] [Google Scholar]
- 41.Gresham T.L., Jansen J.E., Shaver F.W., Gregory J.T. β-Propiolactone. III. Reactions with Dithiocarbamic Acids, their Salts and Thiourea. J. Am. Chem. Soc. 1948;70:1001–1002. doi: 10.1021/ja01183a032. [DOI] [Google Scholar]
- 42.Misra A.I. Certain Thiazolo-Benzimidazoles and Thiazino-Benzimidazoles. J. Org. Chem. 1958;23:897–899. doi: 10.1021/jo01100a602. [DOI] [Google Scholar]
- 43.Ebetino F.F., Gever G. Chemotherapeutic Nitrofurans. VII. The Formation of 5-Nitrofurfurylidene Derivatives of Some Aminoguanidines, Aminotriazoles, and Related Compounds. J. Org. Chem. 1962;27:188–191. doi: 10.1021/jo01048a047. [DOI] [Google Scholar]
- 44.Gouvêa D.P., Berwaldt G.A., Neuenfeldt P.D., Nunes R.J., Almeida W.P., Cunico W. Synthesis of Novel 2-Aryl-3-(2-morpholinoethyl)-1,3-thiazinan-4-ones Via Ultrasound Irradiation. J. Braz. Chem. Soc. 2016;27:1109–1115. doi: 10.5935/0103-5053.20160009. [DOI] [Google Scholar]
- 45.Mansuroğlu D.S., Arslan H., Van Derveer D., Binzet G. Phosphorus Sulfur Silicon and Related Compounds. Phosphorus Sulfur Silicon. 2009;184:3221–3230. doi: 10.1080/10426500902979743. [DOI] [Google Scholar]
- 46.Zhao H.R., Meng X.W. (Z)-2-[(2,4-Dimethylphenyl)imino]-1,3-thiazinan-4-one. Acta Cryst. 2011;E67:o110. doi: 10.1107/S1600536810051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan A., Husain S.R., Ahmad F., Siddiqui M.S., Pimlott W. Derivatization of keto fatty acids, X: Synthesis of thiazanones. Lipids. 1987;22:578–582. doi: 10.1007/BF02537284. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama M., Shinke S., Matsushita Y., Ohira S., Hayashi S. Allylic Oxidation of Methyl 2-Alkenoates. Bull. Chem. Soc. Jpn. 1979;52:184–185. doi: 10.1246/bcsj.52.184. [DOI] [Google Scholar]
- 49.Lie Ken Jie M.S.F. The synthesis of rare and unusual fatty acids. Prog. Lipid Res. 1993;32:151–194. doi: 10.1016/0163-7827(93)90014-N. [DOI] [PubMed] [Google Scholar]
- 50.Rassukana Y.V., Yelenich I.P., Synytsya A.D., Onys’ko P.P. Fluorinated NH-iminophosphonates and iminocarboxylates: Novel synthons for the preparation of biorelevant α-aminophosphonates and carboxylates. Tetrahedron. 2014;70:2928–2937. doi: 10.1016/j.tet.2014.03.030. [DOI] [Google Scholar]
- 51.Srivastava T., Haq W., Katti S.B. Carbodiimide mediated synthesis of 4-thiazolidinones by one-pot three-component condensation. Tetrahedron. 2002;58:7619–7624. doi: 10.1016/S0040-4020(02)00866-9. [DOI] [Google Scholar]
- 52.Walter W., Krohn J. The Reaction of Thioamides with Diphenylcyclopropenone. Justus Liebigs Ann. Chem. 1971;752:136–141. doi: 10.1002/jlac.19717520116. [DOI] [Google Scholar]
- 53.Hwu J.R., Gupta N.K., Tsay S.C., Huang W.C., Albulescu I.C., Kovacikova K., Van Hemert M.J. Bis(benzofuranethiazolidinone)s and bis(benzo-furanethiazinanone)s as inhibiting agents for chikungunya virus. Antivir. Res. 2017;146:96–101. doi: 10.1016/j.antiviral.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Solomon V.R., Haq W., Srivastava K., Puri S.K., Katti S.B. Synthesis and Antimalarial Activity of Side Chain Modified 4-Aminoquinoline Derivatives. J. Med. Chem. 2007;50:394–398. doi: 10.1021/jm061002i. [DOI] [PubMed] [Google Scholar]
- 55.Solomon V.R., Pundir S., Le H.T., Lee H. Design and synthesis of novel quinacrine-[1,3]-thiazinan-4-one hybrids for their antibreast cancer activity. Eur. J. Med. Chem. 2018;143:1028–1038. doi: 10.1016/j.ejmech.2017.11.097. [DOI] [PubMed] [Google Scholar]
- 56.Zebardast T., Zarghi A., Daraie B., Hedayati M., Dadrass O.G. Design and synthesis of 3-alkyl-2-aryl-1,3-thiazinan-4-one derivatives as selective cyclooxygenase (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2009;19:3162–3165. doi: 10.1016/j.bmcl.2009.04.125. [DOI] [PubMed] [Google Scholar]
- 57.Umamatheswaria S., Sankar C. Synthesis, Identification and in vitro biological evaluation of some novel quinolone incorporated 1,3-thiazinan-4-one derivatives. Bioorg. Med. Chem. Lett. 2017;27:695–699. doi: 10.1016/j.bmcl.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 58.Qi B., Yang Y., Gong G., He H., Yue X., Xu X., Hu Y., Li J., Chen T., Wan X., et al. Discovery of N1-(4-((7-(3-(4-ethylpiperazin-1-yl)propoxy)-6-methoxyquinolin-4-yl)oxy)-3,5-difluoro- phenyl)-N3-(2-(2,6-difluorophenyl)-4-oxothiazolidin-3-yl)urea as a multi-tyrosine kinase inhibitor for drug-sensitive and drug-resistant cancers treatment. Eur. Med. Chem. 2019;163:10–27. doi: 10.1016/j.ejmech.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 59.Hassan A.A., Mohamed N.K., Aly1 A.A., Tawfeek H.N., Hopf H., Bräse S., Nieger M. Convenient diastereoselective synthesis of annulated 3 substituted (5S*,6S*,Z) 2 (2 (2,4 dinitrophenyl)hydrazono) 5,6 diphenyl 1,3 thiazinan 4 ones. Mol. Divers. 2019;23:821–828. doi: 10.1007/s11030-018-09912-5. [DOI] [PubMed] [Google Scholar]
- 60.Zhou H., Liu A., Li X., Ma X., Feng W., Zhang W., Yan B. Microwave-Assisted Fluorous Synthesis of 2-Aryl-Substituted 4-Thiazolidinone and 4-Thiazinanone Libraries. J. Comb. Chem. 2008;10:303–312. doi: 10.1021/cc700164u. [DOI] [PubMed] [Google Scholar]
- 61.Mykhaylychenko S.S., Pikun N.V., Rusanov E.B., Shermolovich Y.G. Synthesis of fluorine-containing 1,3-thiazine derivatives from primary Polyfluoroalkanethioamides. J. Fluorine Chem. 2014;168:105–110. doi: 10.1016/j.jfluchem.2014.09.006. [DOI] [Google Scholar]
- 62.Rahman V.P.M., Mukhtar S., Ansari W.H., Lemiere G. Synthesis, stereochemistry and biological activity of some novel long alkyl chain substituted thiazolidin-4-ones and thiazan-4-one from 10-undecenoic acid hydrazide. Eur. J. Med. Chem. 2005;40:173–184. doi: 10.1016/j.ejmech.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Ramani A.V., Monika A., Indira V.L., Karyavardhi G., Venkatesh J., Jeankumar V.U., Manjashetty T.H., Yogeeswari P., Sriram D. Synthesis of highly potent novel anti-tubercular isoniazid analogues with preliminary pharmacokinetic evaluation. Bioorg. Med. Chem. Lett. 2012;22:2764–2767. doi: 10.1016/j.bmcl.2012.02.091. [DOI] [PubMed] [Google Scholar]
- 64.Raza S., Srivastava S.P., Srivastava D.S., Srivastava A.K., Haq W., Katti S.B. Thiazolidin-4-one and Thiazinan-4-one Derivatives Analogous to Rosiglitazone as Potential Antihyperglycaemic and Antidyslipidemic Agents. Eur. J. Med. Chem. 2013;63:611–620. doi: 10.1016/j.ejmech.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 65.Rane R.A., Sahu N.U., Shah C.P. Synthesis and antibiofilm activity of marine natural product-based 4-thiazolidinones derivatives. Bioorg. Med. Chem. Lett. 2012;22:7131–7134. doi: 10.1016/j.bmcl.2012.09.073. [DOI] [PubMed] [Google Scholar]
- 66.Haggam R.A., Assy M.G., Sherif M.H., Galahom M.M. Facile synthesis of some condensed 1,3-thiazines and thiazoles under conventional conditions: Antitumor activity. Res. Chem. Intermed. 2017;43:6299–6315. doi: 10.1007/s11164-017-2990-8. [DOI] [Google Scholar]
- 67.Marković R., Baranac M., Džambaski Z., Stojanović M., Steel P.J. High regioselectivity in the heterocyclization of β-oxonitriles to 4-oxothiazolidines: X-ray structure proof. Tetrahedron. 2003;59:7803–7810. doi: 10.1016/S0040-4020(03)01146-3. [DOI] [Google Scholar]
- 68.Pohloudek-Fabini R., Schkopl E. [C. A. 71(1969)124387s] Pharmazie. 1969;24:96. [PubMed] [Google Scholar]
- 69.Sá M.M., Fernandes L., Ferreira M., Bortoluzzi A.J. Synthesis of allylic thiocyanates and novel 1,3-thiazin-4-ones from 2-(bromomethyl)alkenoates and S-nucleophiles in aqueous medium. Tetrahedron Lett. 2008;49:1228–1232. doi: 10.1016/j.tetlet.2007.12.029. [DOI] [Google Scholar]
- 70.Sá M.M., Ferreira M., Bortoluzzi A.J., Fernandes L., Cunha S. Exploring the reaction of multifunctional allylic bromides with N,S-dinucleophiles: Isothiouronium salts and analogs as useful motifs to assemble the 1,3-thiazine core. Arkivoc. 2010;xi:303–321. doi: 10.3998/ark.5550190.0011.b24. [DOI] [Google Scholar]
- 71.Turkevich N.M., Vladzimirskaya E.V., Vengrinovich L.M. UV absorption spectra and reactivity of 4-oxo and 4-thioxo derivatives of 1,3-thiazane. Chem. Heterocycl Compd. 1969;5:376–378. doi: 10.1007/BF00471149. [DOI] [Google Scholar]
- 72.Ferreira M, Assunção LS, Filippin-Monteiro FB, Creczynski-Pasa TB, Sá MM (2013) Synthesis of 1,3-thiazine-2,4-diones with potential anticancer activity. Eur. J. Med. Chem. 1972;70:411–418. doi: 10.1016/j.ejmech.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 73.Haning H., Mueller U., Schmidt G., Schmeck G., Voehringer V., Kretschmer A., Bischoff H. Novel heterocyclic thyromimetics. Part 2. Bioorg. Med. Chem. Lett. 2007;17:3992–3996. doi: 10.1016/j.bmcl.2007.04.085. [DOI] [PubMed] [Google Scholar]
- 74.Sabala R., Hernández J., Carranza V., Meza-León R.L., Bernès S., Sansinenea E., Ortiz A. Rearrangement of oxazolidinethiones to thiazolidinediones or thiazinanediones and their application for the synthesis of chiral allylic ureas and α-methyl-β-amino acids. Tetrahedron. 2010;66:111–120. doi: 10.1016/j.tet.2009.11.035. [DOI] [Google Scholar]
- 75.Siddiqui I.R., Singh P.K., Srivastava V., Singh J. Organic synthesis using clay and clay-supported catalysts. J. Chem. 2010;49B:512–520. doi: 10.1016/j.clay.2010.09.016. [DOI] [Google Scholar]
- 76.Shaabani A., Hooshmand S.E. Diversity-oriented catalyst-free synthesis of pseudopeptides containing rhodanine scaffolds via a one-pot sequential isocyanide-based sixcomponent reactions in water using ultrasound irradiation. Ultrason. Sonochem. 2018;40:84–90. doi: 10.1016/j.ultsonch.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 77.Mumm O. Umsetzung von Säureimidchloriden mit Salzen organischer Säuren und mit Cyankalium Ber. Dtsch. Chem. Ges. 1910;43:886–893. doi: 10.1002/cber.191004301151. [DOI] [Google Scholar]
- 78.Halimehjani A.Z., Nosood Y.L. Investigation of the Reaction of In Situ Prepared Dithiocarbamic Acids with Itaconic Anhydride in Water. J. Heterocycl. Chem. 2017;54:3372–3376. doi: 10.1002/jhet.2957. [DOI] [Google Scholar]
- 79.Nasiri F., Zolali A., Ahmadiazar M. Solvent Free One-Pot Synthesis of 2-thioxo-1,3-thiazinane-4-one Derivatives. Phosphorus Sulfur Silicon Relat. Compd. 2014;189:180–184. doi: 10.1080/10426507.2013.788002. [DOI] [Google Scholar]
- 80.Basavaiah D., Pal S., Veeraraghavaiah G., Bharadwaj K.C. The BayliseHillman acetates as a source of ambiphilic molecules: A simple synthesis of 1,3-thiazinane-2-thione frameworks. Tetrahedron. 2015;71:4659–4664. doi: 10.1016/j.tet.2015.04.087. [DOI] [Google Scholar]
- 81.Nasiri F., Zolali A., Kadkhoda J. Stereoselective Solvent-Free Synthesis of 4-Hydroxy-1,3-thiazinane-2-thiones. J. Heterocycl. Chem. 2016;53:937–940. doi: 10.1002/jhet.1846. [DOI] [Google Scholar]
- 82.Felder E., Fumaģalli L., Pitré D. Eine Synthese des (+)-Homopantethins. Helv. Chim. Acta. 1963;46:752–757. doi: 10.1002/hlca.19630460307. [DOI] [Google Scholar]
- 83.Hanefeld W., Bercin E. Untersuchungen an 1,3-Thiazinen, 15. Mitt. Neue Synthese für N-substituierte Tetrahydro-1,3-thiazin-2-thione. Arch. Pharm. 1981;314:413–419. doi: 10.1002/ardp.19813140507. [DOI] [Google Scholar]
- 84.Hanefeld W. Untersuchungen an 1,3-Thiazinen, VIII. Konkurrierende Bildung von Tetrahydro-1,3-thiazin-2-thionen und 2-Imino-1,3-dithianen. Arch. Pharm. 1981;310:409–417. doi: 10.1002/ardp.19773100511. [DOI] [PubMed] [Google Scholar]
- 85.Katritzky A.R., Singh S., Mohapatra P.P., Clemens N., Kirichenko K. Synthesis of functionalized dithiocarbamates via N-(1-benzotriazolylalkyl)dithiocarbamates. Arkivoc. 2005;9:63–79. doi: 10.3998/ark.5550190.0006.908. [DOI] [Google Scholar]
- 86.Amir N., Motonishi M., Fujita M., Miyashita Y., Fujisawa K., Okamoto K.I. Synthesis of Novel S-Bridged Heterotrinuclear Complexes Containing Six-Membered Chelate Rings: Structural, Spectroscopic, and Electrochemical Properties of [Co{Rh(apt)3}2]3+ (apt = 3-Aminopropanethiolate) Eur. J. Inorg. Chem. 2006;2006:1041–1049. doi: 10.1002/ejic.200500701. [DOI] [Google Scholar]
- 87.Hanefeld W., Schütz H. 2-Thioxo-1,3-thiazepan-4-ones, a novel class of cyclic dithiourethanes with a 7-membered ring system. J. Heterocycl Chem. 1999;36:1167–1174. doi: 10.1002/jhet.5570360509. [DOI] [Google Scholar]
- 88.Kim W.S., Kim W.K., Choi N., Suh W., Lee J., Kim D.D., Kim I., Sung J.H. Development of S-Methylmethionine Sulfonium Derivatives and Their Skin-Protective Effect against Ultraviolet Exposure. Biomol. Ther. 2018;26:306–312. doi: 10.4062/biomolther.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H., Zhao Q., Wu Y., Li F., Yang H., Yi T., Huang C. Selective Phosphorescence Chemosensor for Homocysteine Based on an Iridium(III) Complex. Inorg. Chem. 2007;46:11075–11081. doi: 10.1021/ic7010887. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y., Xiao J., Wang S., Yang B., Ba X. Tunable fluorescent sensing of cysteine and homocysteine by intramolecular charge transfer. Supramol. Chem. 2010;22:380–386. doi: 10.1080/10610271003759139. [DOI] [Google Scholar]
- 91.Das P., Mandal A.K., Chandar N.B., Baidya M., Bhatt H.B., Ganguly B., Ghosh S.K., Das A. New Chemodosimetric Reagents as Ratiometric Probes for Cysteine and Homocysteine and Possible Detection in Living Cells and in Blood Plasma. Chem. Eur. J. 2012;18:15382–15393. doi: 10.1002/chem.201201621. [DOI] [PubMed] [Google Scholar]
- 92.Li M.J., Zhan C.Q., Nie M.J., Chen G.N., Chen X. Selective recognition of homocysteine and cysteine based on new ruthenium(II) complexes. J. Inorg. Biochem. 2011;105:420–425. doi: 10.1016/j.jinorgbio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Mei J., Wang Y., Tong J., Wang J., Qin A., Sun J.Z., Tang B.Z. Discriminatory Detection of Cysteine and Homocysteine Based on Dialdehyde-Functionalized Aggregation-Induced Emission Fluorophores. Chem. Eur. J. 2013;19:613–620. doi: 10.1002/chem.201202969. [DOI] [PubMed] [Google Scholar]
- 94.Barve A., Lowry M., Escobedo J.O., Thainashmuthu J., Strongin R.M. Fluorescein Tri-Aldehyde Promotes the Selective Detection of Homocysteine. J. Fluoresc. 2016;26:731–737. doi: 10.1007/s10895-015-1762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Len C., Bruniaux S., Delbecq F., Parmar V.S. Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow. Catalysts. 2017;7:146. doi: 10.3390/catal7050146. [DOI] [Google Scholar]
- 96.Mahapatra A.K., Manna S., Karmakar P., Maiti K., Maji R., Mandal D., Uddin R., Mandal S. Installation of efficient quenching groups of a fluorescent probe for the specific detection of cysteine and homocysteine over glutathione in solution and imaging of living cells. Supramolecular Chemistry. Supramol. Chem. 2016;29:59–68. doi: 10.1080/10610278.2016.1170127. [DOI] [Google Scholar]
- 97.Zhang D., Zhang M., Liu Z., Yu M., Li F., Yi T., Huang C. Highly selective colorimetric sensor for cysteine and homocysteine based on azo derivatives. Tetrahedron Lett. 2006;47:7093–7096. doi: 10.1016/j.tetlet.2006.07.080. [DOI] [Google Scholar]
- 98.Meng X., Ye W., Wang S., Feng Y., Chen M., Zhu M., Guo Q. A ratiometric two-photon fluorescent probe for cysteine andhomocysteine in living cells. Sens. Actuators. 2014;B201:520–525. doi: 10.1016/j.snb.2014.05.042. [DOI] [Google Scholar]
- 99.Iqbal S., Yu S., Zhao F., Wang Y., Tian J., Jiang L., Du Y., Yu X., Pu L. Discriminating three biothiols by using one fluorescent probe. Tetrahedron Lett. 2018;59:3397–3400. doi: 10.1016/j.tetlet.2018.07.060. [DOI] [Google Scholar]
- 100.Goswami S., Manna A., Paul S., Das A.K., Nandi P.K., Maity A.K., Saha P. A turn on ESIPT probe for rapid and ratiometric fluorogenic detection of homocysteine and cysteine in water with live cell-imaging. Tetrahedron Lett. 2014;55:490–494. doi: 10.1016/j.tetlet.2013.11.055. [DOI] [Google Scholar]
- 101.Wei X., Yang X., Feng Y., Ning P., Yu H., Zhu M., Xi X., Guo Q., Meng X. A TICT based two-photon fluorescent probe for cysteine andhomocysteine in living cells. Sens. Actuators. 2016;B231:285–292. doi: 10.1016/j.snb.2016.03.027. [DOI] [Google Scholar]
- 102.Attanasi O.A., Filippone P., Lillini S., Mantellini F., Nicolini S., De los Santos J.M., Ignacio R., Aparicio D., Palacios F. Reactions of 1,2-diaza-1,3-dienes with thiol derivatives: A versatile construction of nitrogen/sulfur containing heterocycles. Tetrahedron. 2008;64:9264–9274. doi: 10.1016/j.tet.2008.07.030. [DOI] [Google Scholar]
- 103.Williams A.J., Chakthong S., Gray D., Lawrence R.M., Gallagher T. 1,2-Cyclic Sulfamidates as Versatile Precursors to Thiomorpholines and Piperazines. Org. Lett. 2003;5:811–814. doi: 10.1021/ol027418h. [DOI] [PubMed] [Google Scholar]
- 104.Edayadulla N., Ramesh P. Synthesis of 2,6-dicarbethoxy-3,5-diaryltetrahydro-1,4-thiazine-1,1-dioxide derivatives as potent anticonvulsant agents. Eur. J. Med. Chem. 2015;106:44–49. doi: 10.1016/j.ejmech.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Indumathi S., Perumal S., Banerjee D., Yogeeswari P., Sriram D. L-Proline-catalysed facile green protocol for the synthesis and antimycobacterial evaluation of [1,4]-thiazines. Eur. J. Med. Chem. 2009;44:4978–4984. doi: 10.1016/j.ejmech.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 106.Zahn G., Kirrbach S., Schulze B. Crystal structure of 5,6-dihydro-(4H)-cyclopenta-l,3-thiazine-2,4-dione, C7H7NO2S. Z. Krist. 1996;211:847–848. doi: 10.1524/zkri.1996.211.11.847. [DOI] [Google Scholar]
- 107.Zarghi A., Zebardast T., Daraie B., Hedayati M. Design and synthesis of new 1,3-benzthiazinan-4-one derivatives as selective cyclooxygenase (COX-2) inhibitors. Bioorg. Med. Chem. 2009;17:5369–5373. doi: 10.1016/j.bmc.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 108.Li D., Tian Z., Wang G. Synthesis, biological activity and crystal structure of ethyl 6-amino-8-(4-methoxy phenyl)-9-nitro-2,3,4,8-tetrahydropyrido[2,1-b][1,3]thiazine-7-carboxylate. Res. Chem. Intermed. 2013;39:2435–2443. doi: 10.1007/s11164-012-0769-5. [DOI] [Google Scholar]
- 109.Oe M., Miki K., Mu H., Harada H., Morinibu A., Ohe K. pH-Responsive Cy5 dyes having nucleophilic substituents for molecular imaging. Tetrahedron Lett. 2018;59:3317–3321. doi: 10.1016/j.tetlet.2018.07.044. [DOI] [Google Scholar]
- 110.Chen H., Hao L., Zhu M., Yang T., Wei S., Qin Z., Zhang P., Li X. Synthesis of bi-/tricyclic azasugars fused thiazinan-4-one and their HIV-RT inhibitory Activity. Bioorg. Med. Chem. Lett. 2014;24:3426–3429. doi: 10.1016/j.bmcl.2014.05.079. [DOI] [PubMed] [Google Scholar]
- 111.Gautam D., Gautam P., Chaudhary R.P. Spectral, DFT and X-ray diffraction studies on regioselective synthesis of thiazolo-quinazoline system. J. Mol. Struct. 2017;1145:268–277. doi: 10.1016/j.molstruc.2017.05.109. [DOI] [Google Scholar]
- 112.Dandia A., Sharma C.S., Saha M. FACILE Synthesis of some new fluorine containing spiro [3H-indole-3,2′tetrahydro-1,3-thiazine]. 2,4′(1H)-diones. Phosphorus Sulfur Silicon Relat. Elem. 1998;139:57–66. doi: 10.1080/10426509808035677. [DOI] [Google Scholar]
- 113.Dandia A., Sehgal V., Singh P. Elegant one-pot synthesis of some novel biodynamic spiro-indoline derivatives. Pharmazie. 1994;49:364–365. [Google Scholar]
- 114.Joshi K.C., Dandia A., Ahmed N. Studies in Spiroheterocycles: Part IX: A New Elegant Synthesis and Reactions of Some Novel Fluorine-containing Spiro[3H-indole-3,2′-tetrahydro-1,3-thiazine]- 2,4′(1H)-diones. Heterocycles. 1986;24:2479–2485. doi: 10.3987/R-1986-09-2479. [DOI] [Google Scholar]
- 115.Chandrasekhar B. α/β-Mercaptoalkanoic acids: Versatile synthons in the syntheses of fused ring 4-thiazolidinones/thiazolinones/thiazinanones ring system (s) J. Sulfur Chem. 1986;33:439–503. doi: 10.1080/17415993.2012.693490. [DOI] [Google Scholar]
- 116.Dandia A., Saha M., Rani B. Microwave-induced Synthesis of Spiro[indoline-3,2′-[1,3] thiazinane]-2,4′-diones. J. Chem. Res. 1998:360–361. doi: 10.1039/a706678c. [DOI] [Google Scholar]
- 117.Dandia A., Singh R., Merienne C., Morgant G., Loupy A. Solvent-free one-pot synthesis and crystal structure of a spiro[indole-thiazine] Sulfur Lett. 2003;26:201–207. doi: 10.1080/02786110310001637617. [DOI] [Google Scholar]
- 118.Tan B.Y.H., Teo Y.C., Seow A.H. Low Catalyst Loadings for Ligand-Free Copper(I)-Oxide-Catalyzed N-Arylation of Methanesulfonamide in Water. Eur. J. Org. Chem. 2014;2014:1541–1546. doi: 10.1002/ejoc.201301561. [DOI] [Google Scholar]
- 119.Verdelet T., Ward R.M., Hall D.G. Direct Sulfonamidation of Primary and Secondary Benzylic Alcohols Catalyzed by a Boronic Acid/Oxalic Acid System. Eur. J. Org. Chem. 2017;2017:5729–5738. doi: 10.1002/ejoc.201700621. [DOI] [Google Scholar]
- 120.Yana F.F., Liang C.J. 3-[1-(3,4-Dichlorophenyl)ethyl]-1,3-thiazinane-2-thione. Acta. Cryst. 2009;E65:o3067. doi: 10.1107/S1600536809046352. [DOI] [Google Scholar]
- 121.Gong Y.-Y., Zhang P., Wang M.-H. 3-[1-(4-Chlorophenyl)ethyl]-1,3-thiazinane-2-thione. Acta Cryst. 2011;E67:o514. doi: 10.1107/S1600536811002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pattarozzi M., Ghelfi F., Roncaglia F., Pagnoni U.M., Parsons A.F. Synthesis of the Disubstituted Maleic Anhydride Frame Using a Novel Tandem Radical–Polar Reaction. Synlett. 2009;13:2172–2176. doi: 10.1055/s-0029-1217569. [DOI] [Google Scholar]
- 123.Bellesia F., Choi S.R., Felluga F., Fiscaletti G., Ghelfi F., Menziani M.C., Parsons A.F., Poulter C.D., Roncaglia F., Sabbatini M., et al. Novel route to chaetomellic acid A and analogues: Serendipitous discovery of a more competent FTase inhibitor. Bioorg. Med. Chem. 2013;21:348–358. doi: 10.1016/j.bmc.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang W., Zhao B., Xu C., Wu W. Synthesis and Antitumor Activity of the Thiazoline and Thiazine Multithioether. Int. J. Org. Chem. 2012;2:117–120. doi: 10.4236/ijoc.2012.22018. [DOI] [Google Scholar]
- 125.George K.M., Frantz M.C., Bravo-Altamirano K., LaValle C.R., Tandon M., Leimgruber S., Sharlow E.R., Lazo J.S., Wang Q.J., Wipf P. Design, Synthesis, and Biological Evaluation of PKD Inhibitors. Pharmaceutics. 2011;3:186–228. doi: 10.3390/pharmaceutics3020186. [DOI] [PMC free article] [PubMed] [Google Scholar]