Abstract
Basal cell carcinoma (BCC) originate from Hedgehog/Patched signaling-activated epidermal stem cells. However, the chemically induced tumorigenesis of mice with a CD4Cre-mediated biallelic loss of the Hedgehog signaling repressor Patched also induces BCC formation. Here, we identified the cellular origin of CD4Cre-targeted BCC progenitors as rare Keratin 5+ epidermal cells and show that wildtype Patched offspring of these cells spread over the hair follicle/skin complex with increasing mouse age. Intriguingly, Patched mutant counterparts are undetectable in age-matched untreated skin but are getting traceable upon applying the chemical tumorigenesis protocol. Together, our data show that biallelic Patched depletion in rare Keratin 5+ epidermal cells is not sufficient to drive BCC development, because the spread of these cells is physiologically suppressed. However, bypassing the repression of Patched mutant cells, e.g., by exogenous stimuli, leads to an accumulation of BCC precursor cells and, finally, to tumor development.
Keywords: Patched receptor, basal cell carcinoma, keratin 5, epidermal cells, epidermis, hair follicle
1. Introduction
Epidermal stem cells of the hair follicle (HF) outer root sheath (ORS) [1], bulge [2], secondary hair germ [3,4,5] and/or the interfollicular epidermis (IFE) [1,6] can give rise to basal cell carcinoma (BCC). BCC are the most frequent skin neoplasia in humans [7], with mutations in the Hedgehog (Hh)-signaling inhibitor Patched (Ptch) appearing as one of the major driving forces in the development of this tumor entity [8]. We recently showed that a CD4Cre-mediated homozygous Patched deletion (Ptchf/f CD4Cre) does not affect T-cell functions in vitro and in vivo [9,10] but can result in BCC formation upon treatment with 7,12-Dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA) [11]. This was an unexpected observation, because CD4Cre-deleter mice express a Cre-recombinase gene under the control of the Cluster of differentiation 4 promoter/enhancer/silencer. However, Ptchf/f CD4Cre mice do not spontaneously develop BCC [11], demonstrating that homozygous Ptch depletion using the CD4Cre driver is not sufficient for BCC development. This is in contrast to BCC mouse models using “classical” BCC drivers [4,12,13], in which the Ptch mutation is simultaneously induced in a large proportion of HF stem cells [4] or basal IFE cells [1,4,13] and in which BCC develop spontaneously. These data suggest that the CD4Cre driver targets BCC progenitors with a lower tumorigenic potential and/or at lower frequency compared to “classical” BCC models. However, this also opens the question whether a certain quantity of Hh-activated BCC precursors is necessary for BCC development, which could have far-reaching consequences for the understanding and treatment of sporadic human BCC.
We here determined the CD4Cre-targeted BCC progenitor cell type and its frequency by following the nonhematopoietic progeny of wildtype Ptch and Ptch mutant CD4Cre-targeted cells in the adult skin by lineage tracing experiments. We show that the CD4Cre transgene is expressed in K5+ epidermal cells and that wildtype Ptch progenies of CD4Cre-targeted keratinocytes populate the adult HF/skin complex with increasing mouse age. In contrast, Ptch mutant progenies of CD4Cre-targeted keratinocytes are undetectable and do not accumulate like their wildtype Ptch counterparts under normal conditions. However, the exogenous stimulation of their survival can result in BCC development. Taking together, our data demonstrate that isolated BCC precursors with a homozygous Ptch depletion do not spread or accumulate and are not sufficient for BCC development under normal conditions.
2. Results
2.1. Wildtype Ptch Progeny of CD4Cre-Targeted Cells Spread over the HF/Skin Complex with Increasing Mouse Age
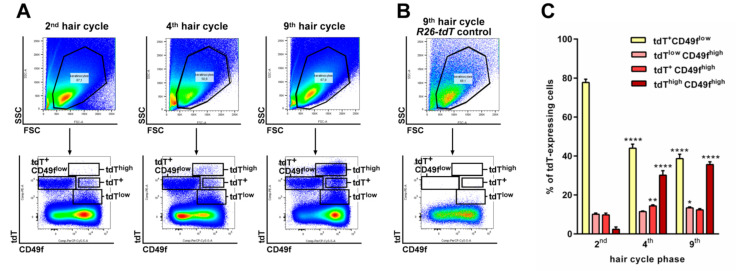
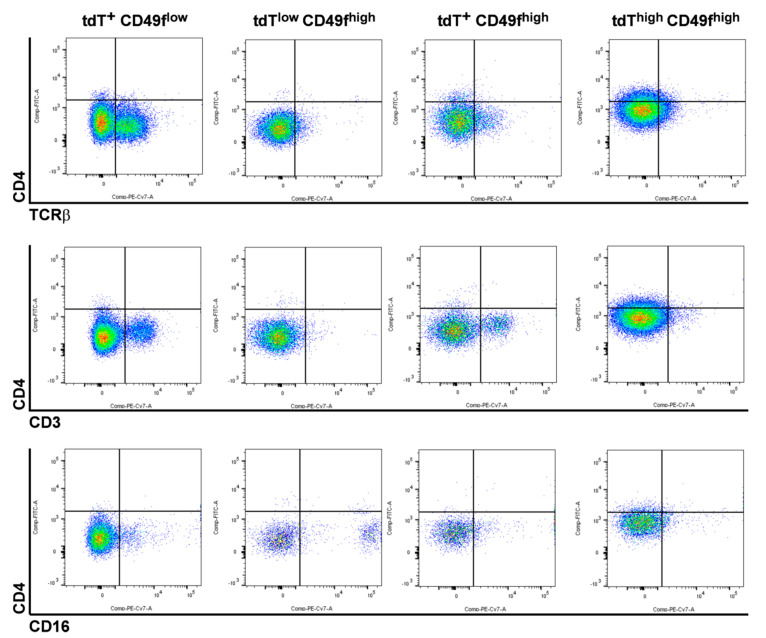
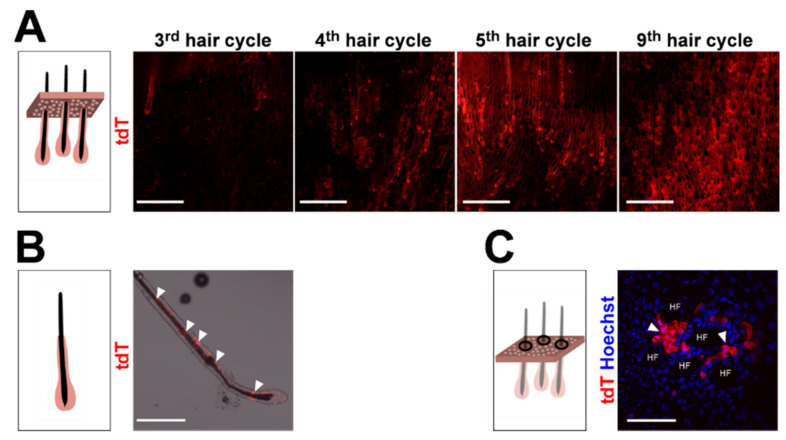
To characterize the cellular origin of Ptchf/f CD4Cre BCC, CD4Cre-targeted wildtype Ptch cells were traced in CD4Cre R26-tdT mice by their tdTomato (tdT) expression (see Appindix A, Figure A1 for tdT expression in CD4Cre R26-tdT thymus). Flow cytometric analyses revealed the existence of various tdT-expressing cell populations in back skin epidermal isolates of CD4Cre R26-tdT mice in comparison to the controls (Figure 1A,B). Based on the expression levels of tdT and the general keratinocyte marker CD49f, we observed three relatively stable tdT-expressing populations (tdT+ CD49flow, tdT+ CD49fhigh and tdTlow CD49fhigh) and a tdThigh CD49fhigh population, which strongly augments with increasing mouse age (Figure 1A,C). Further analyses revealed that the tdT+CD49flow population mainly consists of TCRβ-, CD3- or CD16-expressing immune cells (Figure 2), whereas the tdT+ CD49fhigh and the tdTlow CD49fhigh populations contain small numbers of CD16-expressing (e.g., macrophages; Figure 2) or TCRβ and CD3-expressing immune cells (T cells; Figure 2), respectively (for the verification of antibody specificity, see Appendix A Figure A2). Remarkably, tdThigh CD49fhigh cells do not express immune cell markers (Figure 2), indicating that this population has a pure keratinocyte identity. To verify our conclusion that the number of keratinocytes descending from CD4Cre-targeted cells increases with mouse age, we analyzed whole mount preparations from the back skin of differentially aged CD4Cre R26-tdT mice. Indeed, this approach showed that the skin of aged CD4Cre R26-tdT mice contains enormous numbers of wildtype Ptch tdT+ HF compared to younger mice (Figure 3A), whereas, in the third anagen of CD4Cre R26-tdT back skin (11 weeks old), only isolated tdT+ HF were detected; the numbers of tdT+ HF increased enormously from the fourth (16 weeks old), fifth (25 weeks old) and to the ninth anagen (55 weeks old) (Figure 3A). Thereby, tdT+ cells grow over the entire length of anagen HF (Figure 3B) and, also, in the IFE of CD4Cre R26-tdT back skin (Figure 3C), indicating that the CD4Cre transgene targets cells of the HF and of the IFE compartment.
Figure 1.
tdTomato (tdT)high CD49fhigh-expressing cells accumulate in CD4Cre R26-tdT skin with increasing mouse age. (A,B) Representative flow cytometric analyses of (A) 2,500,000 CD4Cre R26-tdT back skin isolates of the 2nd, 4th and 9th telogens and (B) 1,000,000 R26-tdT control back skin isolates of the 9th telogen stained with anti-CD49f-peridinin-chlorophyll-protein (PerCP)-Cy5.5 antibodies. Top: Forward scatter (FSC)/side scatter (SSC) plots for gating on living cells. Bottom: tdT (phycoerythrin [PE] channel)/CD49f plots for visualization of the tdT expression on the gated living cells. In CD4Cre R26-tdT back skin isolates, 4 different tdT-expressing populations (one CD49flow-expressing population: tdT+ CD49flow and 3 CD49fhigh-expressing populations: tdTlow, tdT+ and tdThigh) were distinguishable in differential aged mice, whereas no tdT+ cells were detected in R26-tdT back skin isolates using the PE channel (B). (C) Percentage share of tdT+ CD49flow and tdTlow, tdT+ and tdThigh cells (N2nd = 5, N4th = 3 and N9th = 3) at the indicated hair cycle phases (based on the flow cytometric analyses shown in (A)). Bars represent mean +/− SEM. Significant differences were calculated using a nonparametric Mann-Whitney test. * p > 0.05, ** p > 0.01 and **** p > 0.0001.
Figure 2.
tdThigh CD49fhigh-expressing cells of aged CD4Cre R26-tdT mice do not express immune cell markers. Representative plots of flow cytometric analyses of 2,500,000 CD4Cre R26-tdT back skin isolates of the 4th telogen stained with anti-CD4-fluorescein isothiocyanate (FITC); anti-CD49f-PerCP-Cy5.5 and anti-TCRβ-PE-Cy7 (top), CD3-PE-Cy7 (middle) or anti-CD16-PE-Cy7 (bottom) antibodies gated as shown in Figure 1A. Individual CD4/TCRβ, CD4/CD3 or CD34/CD16 analyses of the tdT+ CD49flow and the tdTlow CD49fhigh, tdT+ CD49fhigh and tdThigh CD49fhigh subpopulations revealed TCRβ+ and CD3+ immune cells in the tdT+ CD49flow and the tdT+ CD49fhigh populations, whereas CD16+ immune cells were detected in the tdT+ CD49flow and the tdTlow CD49fhigh populations. tdThigh CD49fhigh cells express none of the immune cell markers, except for a slightly increased CD4 protein level.
Figure 3.
Wildtype Ptch tdT+ cells accumulate in hair follicle (HF) and interfollicular epidermis (IFE) with increasing age of the CD4Cre R26-tdT mice. (A–C) Representative fluorescent analyses of (A) epidermal sheets of the back skin of CD4Cre R26-tdT mice at the indicated hair cycles (dermal view), (B) an individually isolated HF of the back skin of a CD4Cre R26-tdT mouse and (C) an epidermal preparation at late-anagen of the back skin of a CD4Cre R26-tdT mouse (top view). Scale bars: 1 mm (A) and 100 µm (B,C).
2.2. The CD4Cre-Deleter Targets Keratin 5+ Epidermal Cells That Are the Origin of DMBA/TPA-Induced BCC in Ptchf/f CD4Cre Mice
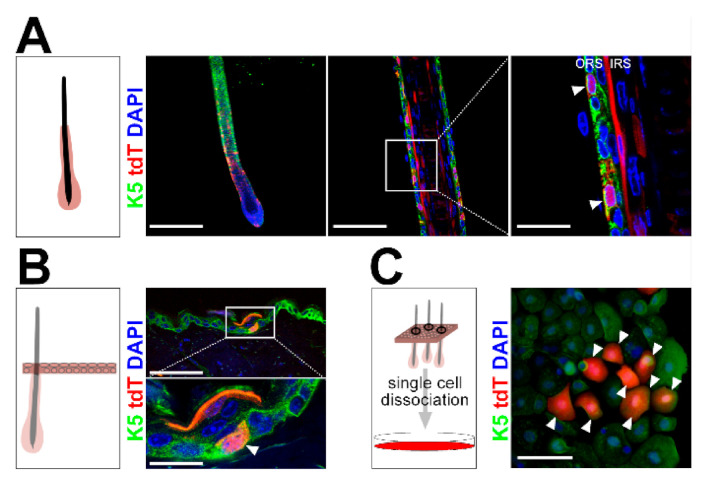
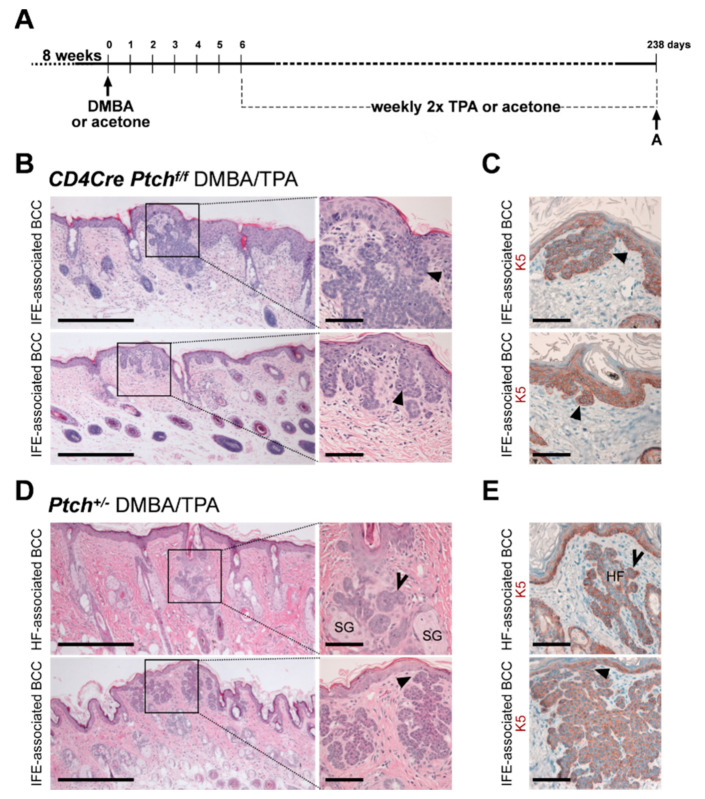
Both the ORS of HF and the basal layer of the IFE are characterized by Keratin 5 (K5) expression [1]. Thus, lineage tracing of the K5 promoter-driven CreERT-recombinase expression results in the labeling of cells in both compartments [14]. Furthermore, since K5+ cells can be the origin of BCC [1] (for review, see [15]), we hypothesized that the CD4Cre transgene targets K5+ epidermal cells. Indeed, immunofluorescence stainings of individually isolated tdT+ HF and cryo-sectioned back skin of CD4Cre R26-tdT mice revealed that K5+ cells of the ORS (Figure 4A), as well as of the basal layer, express tdT (Figure 4B). Moreover, in vitro cultured tdTneg keratinocytes from CD4Cre R26-tdT back skin started to express tdT 22 days post-seeding. The subsequent immunofluorescent staining verified that all newly recombined tdT+ cells express K5 (Figure 4C). Forty-two or 55 days after seeding, 0.47% or 0.68% of all K5+ cells were tdT+, respectively. Based on these data, we concluded that the CD4Cre driver targets rare K5+ keratinocytes, which most probably are also the origin of BCC in Ptchf/f CD4Cre mice. If this is the case, the histological appearance of the DMBA/TPA-induced BCC in Ptchf/f CD4Cre mice should mimic that of BCC from Ptchf/f K5CreERT mice. The latter arise from the lower HF and from the IFE [13,15] and express K5 (Appendix A Figure A3). However, BCC of DMBA/TPA-treated Ptchf/f CD4Cre skin (for experimental set-up, see Figure 5A) exclusively occur at the IFE in HF-near regions (Figure 5B,C) and express K5 (Figure 5C) but never grow as tumors of the bulge or secondary hair germ like in Ptchf/f K5CreERT skin [13] (Appendix A Figure A3). To evaluate the possibility that the chemical treatment may preferably induce BCC development from IFE cells, we furthermore analyzed BCC from DMBA/TPA-treated heterozygous Ptch+/− mice [16]. Contrarily to BCC in Ptchf/f CD4Cre skin, and similar to BCC from Ptchf/f K5CreERT, BCC from DMBA/TPA-treated Ptch+/− mice arise from HF and IFE (Figure 5D,E; for a comparison of BCC/mm skin in DMBA/TPA-treated Ptchf/f CD4Cre and Ptch+/− mice, see Appendix A Figure A4) and stain positive for K5 (Figure 5E). This shows that the origin of DMBA/TPA-induced BCC is not determined by the chemical treatment but, rather, by the compartment of the genetically targeted cell type, and thus, the CD4Cre-deleter most likely targets K5+ basal cells of HF-near IFE.
Figure 4.
Wildtype Ptch tdT+ cells of CD4Cre R26-tdT back skin descents from CD4Cre-targeted rare K5+ epidermal cells. (A,B) Representative fluorescent analyses of (A) an individually isolated HF of back skin and (B) cryo-sectioned back skin of CD4Cre R26-tdT mice stained against K5. (C) In vitro cultured tdTneg epidermal cells of CD4Cre R26-tdT back skin 55 days post-seeding stained against the outer root sheath (ORS) and basal cell marker K5. tdT+ K5+ double-positive cells are marked with arrow heads. Box: zoomed area. IRS: inner root sheath. Scale bars: 100 µm (A, left and C), 33 µm (A, middle and B, top) and 10 µm (A, right).
Figure 5.
Basal cell carcinoma (BCC)-like tumors of 7,12-Dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-treated Ptchf/f CD4Cre skin appear exclusively IFE-associated. (A) Experimental set-up of the DMBA/TPA carcinogenesis protocol. (B–E) Representative hematoxylin and eosin (H&E) stainings (B,D) and anti-K5 antibody stainings (C,E) of BCC from 42-week-old DMBA/TPA-treated Ptchf/f CD4Cre (B,C) and Ptch+/− back skin (D,E) (NPtchf/f CD4Cre = 13 and NPtch+/− = 8). Solid arrows: BCC associated to the IFE; open arrows: BCC associated to HF. HF: hair follicle, SG: sebaceous gland. Boxes: zoomed areas. Scale bars: 20 µm (B, left and D, left) and 100 µm (B, right, C and D, right and E).
2.3. Isolated Ptch Mutant Epidermal Cells do Not Spread Like Their Wildtype Ptch Counterparts
The CD4Cre-deleter targets rare K5-expressing epidermal IFE cells, in which a biallelic Ptch mutation did not, per se, result in BCC formation. This indicates that the quantity of K5+ Ptch mutant BCC precursors in untreated Ptchf/f CD4Cre skin is not sufficient for spontaneous BCC development, as seen in Ptchf/f K5CreERT mice. However, our lineage-tracing analyses showed that robust numbers of wildtype Ptch epidermal keratinocytes descending from CD4Cre-trageted cells grow in the skin of aged mice. Thus, one could speculate that the numbers of Ptch mutant keratinocytes growing in the skin of aged Ptchf/f CD4Cre mice are comparable, which would oppose the assumption that a low quantity of K5+ Ptch mutant BCC precursor is not sufficient for BCC development. To shed light on this discrepancy, we traced Ptch mutant progenies of CD4Cre-targeted cells in R26-LacZ Ptchf/f CD4Cre skin. Remarkably, and in contrast to wildtype Ptch skin (Figure 6A), this approach revealed that HF and IFE of 42-week-old R26-LacZ Ptchf/f CD4Cre mice (Figure 6B), as well as of R26-LacZ Ptchf/f CD4Cre mice transplanted with wildtype Ptch bone marrow (Figure 6C), are completely free of progenies of CD4Cre-targeted cells (control LacZ stainings, Appendix A Figure A5). In contrast, DMBA/TPA-treated R26-LacZ Ptchf/f CD4Cre skin shows Ptch mutant LacZ+ HF (Figure 6B) but at much lower numbers compared to the skin of age-matched untreated CD4Cre R26-tdT mice, which show numerous wildtype Ptch tdT+ HF (Figure 6A). Anti-K5 antibody staining furthermore revealed that the few LacZ+ cells in HF and HF-near IFE of DMBA/TPA-treated R26-LacZ Ptchf/f CD4Cre skin are positive for K5 or at least descend from K5+ cells (e.g., suprabasal layers of the IFE) (Figure 6D).
Figure 6.
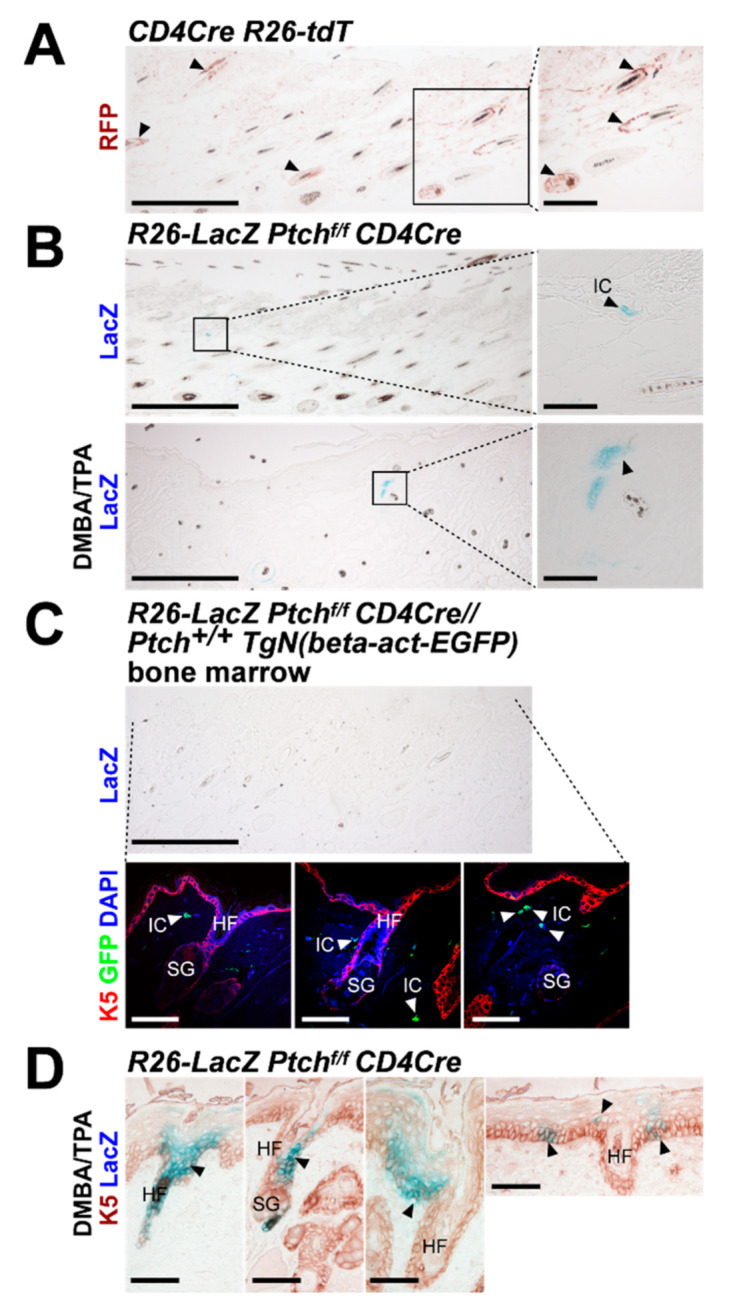
Ptch mutant descendants of CD4Cre-targeted epidermal cells do not populate the skin with increasing mouse age like their wildtype Ptch counterparts. (A,B) Representative anti-red fluorescent protein (RFP)-stained paraffin sections of (A) CD4Cre R26-tdT back skin and (B) LacZ-stained untreated (top) and DMBA/TPA-treated (bottom) R26-LacZ Ptchf/f CD4Cre back skin at an age of 42 weeks (NCD4Cre R26-tdT = 7; NR26-LacZ Ptchf/f CD4Cre = 6). The tdT protein was visualized using an anti-RFP antibody. (C) Representative anti-K5/anti-green fluorescent protein (GFP) antibody/LacZ-stained paraffin sections of untreated back skin of a R26-LacZ Ptchf/f CD4Cre mouse adoptively transplanted with wildtype Ptch TgN(beta-act-EnhancedGFP) bone marrow at an age of 42 weeks (30 weeks after transplantation). (D) Representative anti-K5 antibody/LacZ-stained paraffin sections of DMBA/TPA-treated R26-LacZ Ptchf/f CD4Cre back skin at an age of 42 weeks. Black arrow heads: tdT+ LacZ+ or double-positive cells/areas and white arrow heads: GFP+ immune cells. Boxes: zoomed areas. IC: immune cells; HF: hair follicle; SG, sebaceous gland. Scale bars: 20 µm (A, left, B, left and D, top); 100 µm (A, right, B, right and C) and 33 µm (D, bottom).
These findings indicate that isolated Ptch mutant descendants from K5+ epidermal cells do not contribute to normal skin homoeostasis, since the spreading of these rare epidermal cells (e.g., of CD4Cre-targeted cells) is suppressed under physiological conditions. Thus, as a result, the probability of spontaneous BCC development is strongly reduced in Ptchf/f CD4Cre skin. In addition, whereas the Ptch status of T cells does not influence this phenomenon, the DMBA/TPA treatment seems to enhance the survival probability of Ptch mutant K5+ epidermal cells and their offspring, which, finally, can result in BCC formation.
3. Discussion
The development of BCC is closely related to Ptch mutations and the subsequent activation of Hh/Ptch signaling in stem cells of the HF and the IFE [8]. However, genome-sequencing data from human BCC samples hint towards a more complex pathway interaction in BCC development [17]. In line with this assumption, our novel data show that homozygous Ptch depletion in rare K5+ epidermal cells is insufficient to drive BCC formation in mice, most likely due to the physiological erasure of the mutant cells.
In the skin, as an organ prone for Hh/Ptch-associated BCC development, a biallelic Ptch mutation, which occurs simultaneously in all stem cells of the HF or of the IFE, leads to BCC formation [4,13]. Thus, the lack of a spontaneous BCC development in Ptchf/f CD4Cre mice, together with our novel results that wildtype Ptch CD4Cre-targeted epidermal cells widely populate the HF/skin complex, were puzzling. However, our data also revealed that neither untreated nor DMBA/TPA-treated Ptchf/f CD4Cre skin contains comparable amounts of CD4Cre-targeted offspring, as seen in aged wildtype Ptch skin. Thus, under normal physiological conditions, BCC development in Ptchf/f CD4Cre skin seems to be repressed by the physiological inhibition of the spreading of rare Ptch mutant epidermal cells. Since CD4Cre-mediated Ptch depletion does not affect T cell (e.g., cytotoxic T cells [CTL]) function in vivo [9,10], and wildtype Ptch bone marrow transplantation does not induce a spread of Ptch mutant keratinocytes in Ptchf/f CD4Cre mice [11] (see Figure 6C), an immune cell-mediated suppression of Ptch mutant keratinocytes can be excluded. However, hypothetically, an epithelial defense against cancer (EDAC), which is based on cell competition between cells of different fitness [18], might mediate the inhibition of Ptch mutant keratinocyte spreading. One major prerequisite for the elimination of less fit cells (or mutant cells) by EDAC is that the genetically altered ones are surrounded by wildtype cells [18]. This is most likely the case for Ptch mutant epidermal cells in Ptchf/f CD4Cre skin. However, various parameters (e.g., ratio of normal/transformed cells and environmental factors) can affect the EDAC and potentially convert the antitumorigenic process to a super-competition, in which cells with an accumulation of a series of oncogenic mutations outcompete normal or single-mutant cells [18]. Indeed, we observed that the DMBA/TPA treatment of R26-LacZ Ptchf/f CD4Cre skin increases the probability to detect LacZ+ Ptch mutant epidermal cell clusters in isolated HF and IFE areas that have not yet developed to BCC. These observations indicate that the chemicals confer a survival benefit of the rare Ptch mutant K5+ epidermal cells in Ptchf/f CD4Cre skin, which might be due to an accumulation of oncogenic mutations and the suppression of normal cells. Probably, aberrant Ras signaling does not play a role in DMBA/TPA-induced BCC development in Ptchf/f CD4Cre mice [11], while the disruption of physiological apoptotic responses via the DMBA/TPA-mediated downregulation of p53 [19] is more likely [12,20,21]. Moreover, the induction of apoptosis in BCC cells, e.g., by Fas upregulation, reduces the development and the size of UV light-induced BCC [20]. Hence, in the case of a homozygous Ptch mutation in isolated BCC precursors, the DMBA/TPA treatment, similarly to UV light exposure [15,22,23], potentially may abrogate apoptotic processes, which normally can erase isolated Ptch mutant cells from the skin. Subsequently, Ptch mutant epidermal cells are multiplied, accumulate additional mutations and, thus, are predisposed to BCC development. Indeed, Ptch heterozygous SKH1 Hairless mice progressively develop spontaneous BCC, which are further boosted by UV light exposure. This suggests that, irrespective of aberrant Hh/Ptch signaling, age-related effects (e.g., altered DNA damage responses) play a crucial role in BCC formation [22,23].
The fact that a homozygous Ptch mutation in isolated K5+ epidermal cells is not sufficient for BCC development also questions the current BCC therapy based on Hh signaling inhibitors (e.g., vismodegib). Whereas these inhibitors only target Hh/Ptch signaling and suppress the growth of the initial BCC precursor cell, they will not target secondly accumulated tumor-promoting cascades. Indeed, cessation of the vismodegib treatment of Ptch/p53-mutant BCC not only leads to a loss of Hh-signaling marker gene expression but, also, to changes of HF stem cell-like to IFE- and isthmus-like expression profiles of the remaining BCC [21]. In humans, these inhibitors can lead to the development of BCC-adjacent cSCC (cutaneous squamous cell carcinoma) [24,25,26], which show decreased Hh/Ptch but increased Ras/MAPK signaling [27]. Moreover, the genetic differences of pretreatment BCC and post-treatment cSCC are minor (3%) [27], and mutations associated with cSCC development (e.g., in effectors of the Hippo-YAP pathway and in MYCN) [28,29,30,31] have also been identified in BCC [17]. This strongly argues for a scenario in which a complex pathway interaction drives the BCC development. Such a scenario is also supported by our data demonstrating that the spread of isolated biallelic Ptch mutant BCC precursors is suppressed under normal conditions (potentially by an EDAC) and that they only develop into BCC upon a second nonphysiological stimulus. Moreover, in light of the recently made postulation that, in human skin, spatial relationships and competitive behaviors amongst clones act as suppressors of malignant progression [32], the Ptchf/f CD4Cre BCC mouse model is more closely related to the human situation than “classical” BCC mouse models. This is due to the fact that the CD4Cre-deleter targets only single K5+ epidermal cells, whereas “classical” skin-specific drivers target all or large proportions of HF stem cells and/or basal cells simultaneously [4,13]. Thus, new BCC models such as Ptchf/f CD4Cre mice will not only allow to investigate BCC-initiating events beyond Hh/Ptch-signaling activation but, also, to develop new BCC treatment options.
4. Materials and Methods
4.1. Mice
All animal experiments were performed in compliance with German legal and ethical requirements and were approved by the Lower Saxony State Office for Consumer Protection and Food Safety (file numbers 33.9-42502-04-15/1926 from November 2015, 33.9-42502-04-11/0374 from April 2011 and 33.14-42502-04-100/07 from January 2008). The following mouse strains were used: Tg(Cd4-cre)1Cwi/Bflu (CD4Cre, JAX stock #017336) [33,34,35], K5CreERT [36], Gt(ROSA)26Sortm9(CAG-tdTomato)Hze (R26-tdT, JAX stock #007905) [37], Gt(ROSA)26Sor (R26-LacZ, JAX stock #003309) [38], Gt(ROSA)26Sortm1(cre/ERT2)Tyj (R26-CreERT2, JAX stock #008463) [39], TgN(beta-act-EGFP) [40], Ptch1tm1Zim (Ptch+/−) [41] and Ptch1tm1Hahn (Ptchf/f, JAX stock #012457) [42]. All used mouse strains were maintained on a C57BL/6 background. Genotyping was conducted by PCR on genomic DNA isolated from tail biopsies using primer pairs recommended by the providing scientists or by The Jackson Laboratory. Both genders of transgenic mice were used. No sex-specific differences were observed.
R26-LacZ and R26-CreERT2 mice were intraperitoneally (i.p.) injected with 1-mg tamoxifen in sunflower oil for 5 consecutive days at an age of 8 weeks [42]. DMBA/TPA treatment (Sigma-Aldrich Inc., St. Louis, MO, USA) and adoptive bone marrow transfer to irradiated mice were described previously [11].
4.2. Isolation of Keratinocytes, Epidermal Sheets and Individual Hair Follicles
Murine epidermal cells for flow cytometric analyses and in vitro culture were isolated using thermolysin as previously described [11]. Epidermal sheets of murine back skin and individual HF were isolated according to [43,44], respectively.
4.3. Tissue Embedding and Sectioning
Tissue samples were fixed in 4% paraformaldehyde/1x phosphate buffered saline (PBS) at 4 °C for 2 or 3 days and, depending on the subsequent analyses, either dehydrated and embedded in paraffin or equilibrated overnight in 20% sucrose/1x PBS at 4 °C and embedded in cryo-medium (Medite, Burgdorf, Germany). Paraffin-embedded or cryo-embedded samples were sectioned using a microtome or a cryotome, respectively, and used for antibody or H&E staining.
4.4. Primary Keratinocyte Culture
For primary keratinocyte culture, epidermal cells were isolated from telogen back skin of 19-week-old mice and were cultured under feeder-free conditions on collagen-coated dishes (Bovine Collagen Solution Type I, Sigma Aldrich Inc., St. Louis, MO, USA) in defined keratinocyte-serum-free medium (DK-SFM) basal medium (supplemented with DK-SFM growth supplements, Thermo Fisher Scientific, Waltham, MA, USA) for up to 55 days without passaging.
4.5. LacZ Staining, Antibody Staining, Flowcytometric Analyses and Microscopy
LacZ(β-galactosidase-) staining of tissue samples, immunohistological and immunofluorescent antibody stainings of paraffin and cryotome sections and fixed in vitro cultured cells, as well as flow cytometric analyses of keratinocytes, were described previously [11,45,46]. For antibody staining of individual HF, the samples were blocked for 30 min in 0.2% I-Block (Applied Biosystems, Foster City, CA, USA) and stained overnight at 4 °C with primary antibodies in 1 × tris buffered saline (TBS)/0.1% triton X-100, followed by a 4-h washing step in 1 × TBS/0.1% triton X-100. Samples were incubated overnight at 4 °C with secondary antibodies in 1 × TBS/0.1% triton X-100 and washed 4× for 1 h in 1 × TBS/0.1% triton X-100.
Fluorescent or immunohistological stainings or whole-organ analyses were documented on a confocal laser scanning microscope equipped with the software FluoView FV100 (Olympus Corporation, Shinjuku, Japan), Olympus BX60 microscope equipped with cellSense software or a fluorescent dissecting microscope (Leica M205FA) equipped with a digital camera (Leica DFC 450C) and the software Leica Application Suite, respectively. Flow cytometric analyses were conducted on a LSR II flow cytometer (BD Biosciences Pharmingen, San Jose, CA, USA). Data acquisition and analyses were performed using BD FacsDiva (BD Biosciences Pharmingen, USA) and FlowJo (Treestar, Ashland, OR, USA) software. If not otherwise stated, for each analysis, 2.5 × 106 keratinocytes were counted. For quantification of tdTlow CD49fhigh, tdT+ CD49fhigh and tdThigh CD49fhigh cell numbers, anti-CD49f-PerCP-Cy5.5 antibody-stained back skin isolates of differentially aged CD4Cre R26-tdT mice (N2nd hair cycle = 5, N4th hair cycle = 3 and N9th hair cycle = 3) were analyzed by flow cytometry. Used antibodies, antibody concentrations and retrieval methods are summarized in Appendix A Table A1 and Table A2.
5. Conclusions
The spread of rare Ptch mutant K5+ epidermal stem cells is physiologically suppressed. These cells only progress to BCC upon events that counteracted this suppression. These findings strengthen the more recent assumption that BCC rather develop due to a complex pathway interaction than to sole Hh/Ptch-signaling overactivation. Additionally, it indicates that mouse models for targeting isolated stem cells of the skin are needed to more precisely reflect sporadic human BCC development, which will help to better understand BCC-initiating events and to develop new, targeted BCC treatment options.
Acknowledgments
We are grateful for the excellent animal care by the animal caretakers and veterinarians of the Zentrale Tierexperimentelle Einrichtung of the University Medical Center, Göttingen, Germany. We furthermore thank the members of the Department of Cellular and Molecular Immunology for the opportunity to conduct flow cytometry.
Abbreviations
| AEC | 3-amino-9-ethylcarbazole |
| BCC | basal cell carcinoma |
| cSCC | cutaneous squamous cell carcinoma |
| CTL | cytotoxic T cells |
| DK-SFM | defined keratinocyte-serum-free medium |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| DN | double-negative |
| DP | double-positive |
| EDAC | epithelial defense against cancer |
| EGFP | enhanced GFP |
| FITC | fluorescein isothiocyanate |
| FSC | forward scatter |
| GFP | green fluorescent protein |
| H&E | hematoxylin and eosin |
| HF | hair follicle |
| Hh | hedgehog |
| IFE | interfollicular epidermis |
| IRS | inner root sheath |
| K5 | Keratin 5 |
| ORS | outer root sheath |
| PBS | phosphate buffered saline |
| PE | phycoerythrin |
| PerCP | peridinin-chlorophyll-protein |
| Ptch | Patched |
| RFP | red fluorescent protein |
| SG | sebaceous gland |
| SP | single-positive |
| SSC | side scatter |
| TBS | tris buffered saline |
| TCR | T cell receptor |
| tdT | tdTomato |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
Appendix A
Table A1.
Primary and secondary antibodies used for immunohistochemical or immunofluorescent stainings of individual isolated hair follicles, in vitro cultured cells and paraffine or cryotome sections.
| Antigen | Antibody | Reactivity | Manufacturer (Clone) | Antigen Retrieval | Dilution | Fluorochrome-Labeled Secondary Antibody (Dilution, Manufacturer, Catalogue Code) |
|---|---|---|---|---|---|---|
| GFP | g anti-GFP | Novus Biologicals, Littleton, CO, USA (NB100-1770) | citric acid, pH6 | 1:200 | bov anti-gt-Alexa488 (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA; #805-545-150) | |
| K5 | rb anti-K5 | m/h | Bio Legend, San Diego, CA, USA (Poly19055) | citric acid, pH6 | 1:1000 | d anti-rb-Alexa488 (1:200; Jackson ImmunoResearch; #711-545-152) d anti-rb-Cy3 (1:400; Jackson ImmunoResearch; #711-165-152) |
| RFP * | g anti-RFP | My BioSource, San Diego, CA, USA (MBS448122) | boric acid or citric acid, pH6 | 1:200 | d anti-g-FITC (1:200; Jackson ImmunoResearch; #705-095-147) bovine anti-g-Alexa488 (1:1000, Jackson ImmunoResearch; #805-545-150) |
|
| RFP * | rb anti-RFP | Rockland Immunochemicals, Inc., Gilbertsville, PA, USA (600-401-379S) |
boric acid or citric acid, pH6 | 1:500 | d anti-rb-Cy3 (1:400; Jackson ImmunoResearch; #711-165-152) g anti-rb-Cy3 (1:400; Jackson ImmunoResearch; #111-165-003) |
* detects tdTomato; m: mouse, r: rat; rb: rabbit; g: goat; d: donkey and bov: bovine. Immunohistological stainings were visualized using the EnVision system (Agilent Technologies, Santa Clara, CA, USA) and 3-amino-9-ethylcarbazole (AEC) chromogen.
Table A2.
Antibodies used for flow cytometry.
| Antigen | Antibody | Reactivity | Clone/Catalogue Code |
|---|---|---|---|
| CD16/CD32 | r anti-CD16/CD32-PE_Cy7 | m | 2.4G2 |
| CD3ε | ha anti-CD3e-PE-Cy7 | m | 145-2C11 |
| CD4 | r anti-CD4-FITC | m | RM4-5 |
| CD49f | r anti-CD49f-PerCP-Cy5.5 | m/h | GoH3 |
| TCRβ | ha anti-TCRβ-PE-Cy7 | m | H57-597 |
All antibodies were purchased from BD Bioscience. m: mouse, r: rat, ha: hamster and h: human.
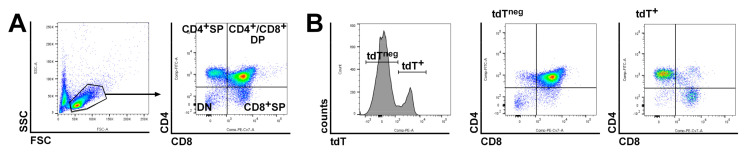
Figure A1.
Flow cytometric analysis of CD4Cre R26-tdT thymocytes. (A,B) Representative flow cytometric analyses of CD4Cre R26-tdT thymocytes stained with anti-CD4-FITC and anti-CD8-PerCP-Cy7 antibodies. (A) FSC/SSC plot for gating on thymocytes (left) and CD4/CD8 plot of the gated thymocytes for visualization of CD4/CD8 double-negative (DN), CD4 single-positive (CD4+ SP), CD8 single-positive (CD8+ SP) and CD4/CD8 double-positive (CD4+/CD8+ DP) T cells (right). (B) tdT expression analysis (left) of the gated thymocytes (see a left) and CD4/CD8 plots for individual CD4/CD8 expression analyses of tdTneg (middle) and tdT+ thymocytes (right). tdTneg thymocytes represent mainly DN and CD4+/CD8+ DP T cells (middle), whereas nearly all CD4+ SP and CD8+ SP thymocytes are tdT+ (right).
Figure A2.
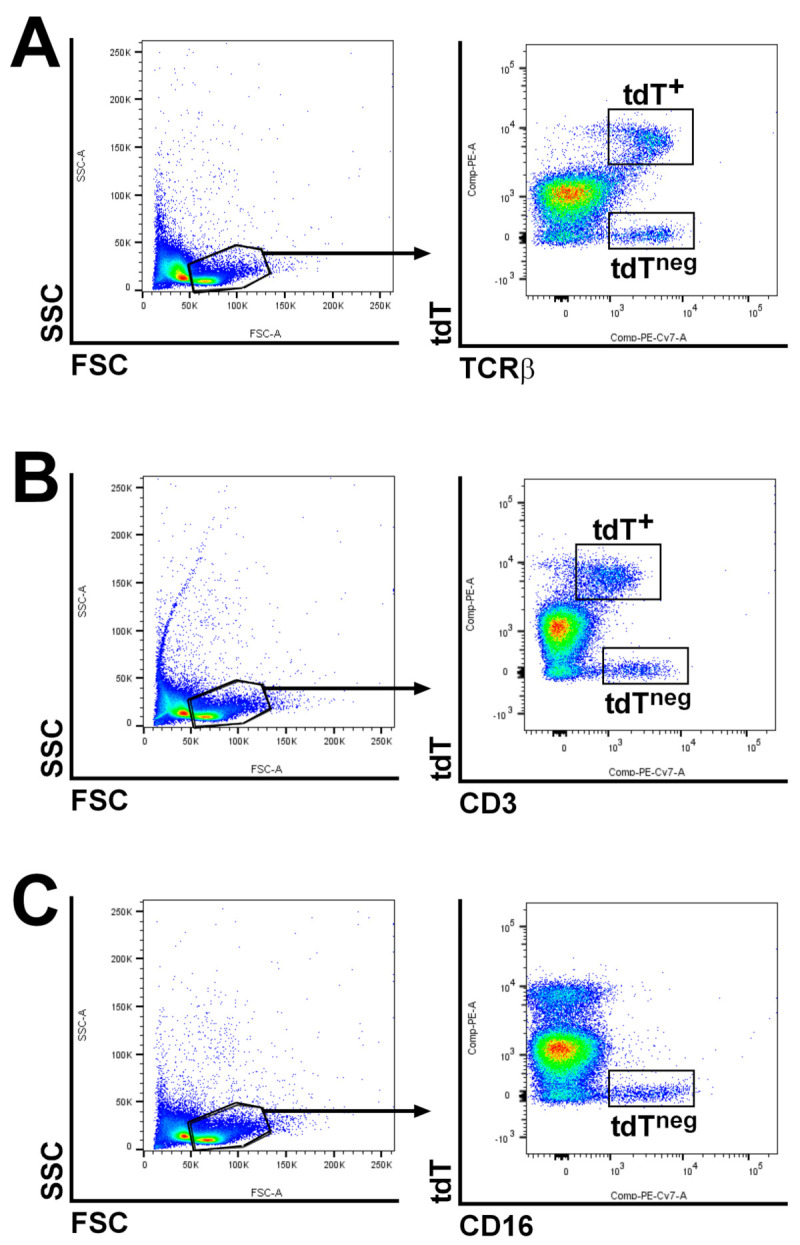
Flow cytometric analyses for the verification of antibody specificity. (A–C) Representative flow cytometric analyses of CD4Cre R26-tdT thymocytes stained with anti-CD4-FITC and (A) anti-TCRβ-PE-Cy7, (B) CD3-PE-Cy7 or (C) anti-CD16-PE-Cy7 antibodies. tdT was visualized using the PE channel. Left panels: FSC/SSC plots for gating on thymocytes. Right panels: individual tdT/TCRβ (A), tdT/CD3 (B) and tdT/CD16 (C) plots. The thymus of CD4Cre R26-tdT mice contains tdT+ TCRβ+ and tdT+ CD3+ T cells but only a small fraction of tdTneg CD16+ cells (dendritic cells, natural killer cells, macrophages, monocytes and granulocytes).
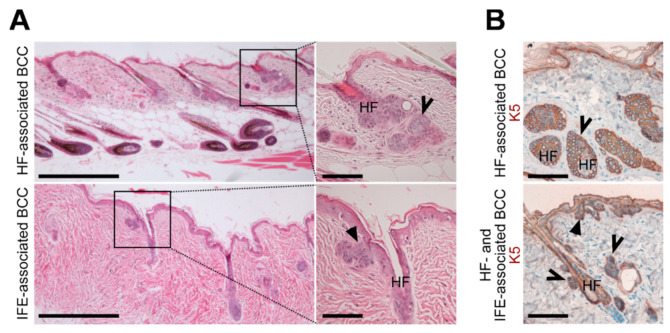
Figure A3.
BCC-like tumors of Ptchf/f K5CreERT skin arise from HF and IFE cells. (A,B) Representative (A) H&E stainings and (B) anti-K5 antibody stainings of BCC from 28-37-week-old Ptchf/f K5CreERT back skin (N = 5). Please note that the K5CreERT-deleter is leaky, and thus, BCC-like lesions also develop without tamoxifen induction [15]. Solid arrows: BCC associated to the IFE and open arrows: BCC associated to HF. HF, hair follicle. Boxes: zoomed areas. Scale bars: 20 µm (A, left) and 100 µm (A, right and B).
Figure A4.
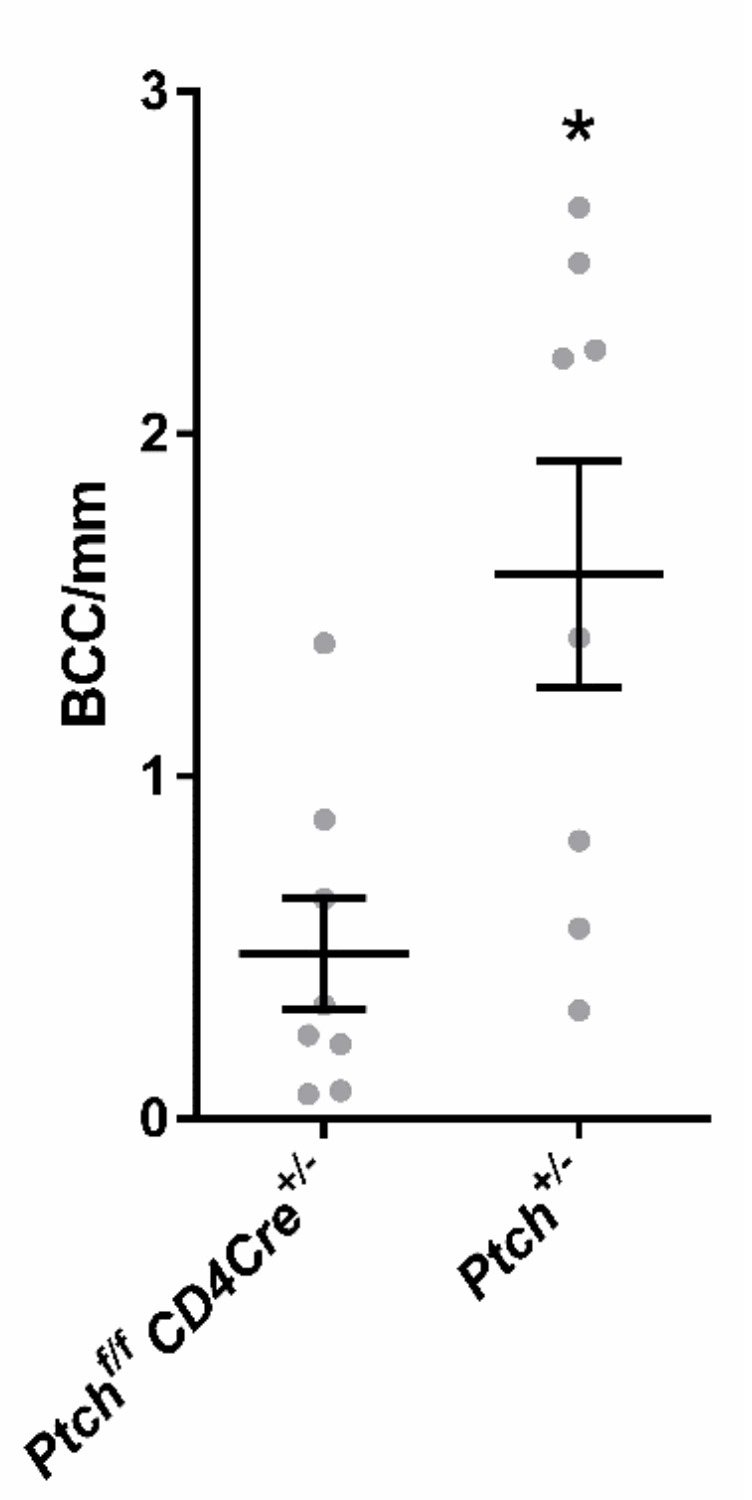
Comparison of BCC/mm skin in DMBA/TPA-treated Ptchf/f CD4Cre and Ptch+/− mice. BCC numbers per mm skin were counted from H&E-stained paraffine sections of 8 DMBA/TPA-treated Ptchf/f CD4Cre and 8 DMBA/TPA-treated Ptch+/− mice (each grey circle indicates the mean BCC number/mm of one animal). The significant difference was calculated using a nonparametric Mann-Whitney test. * p > 0.05.
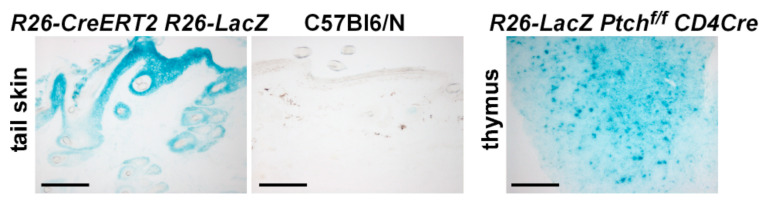
Figure A5.
LacZ stainings of control tissues. Representative images of paraffin-sectioned LacZ-stained tail skin of a tamoxifen-treated R26-CreERT2 R26-LacZ (left, positive control) and a C57Bl6/N (middle, negative control) mouse, as well as of a R26-LacZ Ptchf/f C D4Cre thymus (right, positive control). Scale bars: 100 µm.
Author Contributions
N.B.: Conception and design, collection and/or assembly of data, data analysis and interpretation, visualization, manuscript writing and final approval of the manuscript; S.H.M.: collection and/or assembly of data; D.S.B.: collection and/or assembly of data; W.M.: collection and/or assembly of data; A.M.: collection and/or assembly of data; H.S.: collection and/or assembly of data; S.Z.: collection and/or assembly of data; A.F.: collection and/or assembly of data; I.H.: collection and/or assembly of data; H.H.: provision of study material and manuscript writing and A.U.: conception and design, financial support, administrative support, provision of study material, supervision, collection and/or assembly of data, data analysis and interpretation, visualization, manuscript writing and final approval of the manuscript. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant UH227-7/1 of the Deutsche Forschungsgemeinschaft to A.U.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grachtchouk M., Pero J., Yang S.H., Ermilov A.N., Michael L.E., Wang A., Wilbert D., Patel R.M., Ferris J., Diener J., et al. Basal Cell Carcinomas in Mice Arise from Hair Follicle Stem Cells and Multiple Epithelial Progenitor Populations. J. Clin. Investig. 2011;121:1768–1781. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapouge G., Youssef K.K., Vokaer B., Achouri Y., Michaux C., Sotiropoulou P.A., Blanpain C. Identifying the Cellular Origin of Squamous Skin Tumors. Proc. Natl. Acad. Sci. USA. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang G.Y., Wang J., Mancianti M.-L., Epstein E. Basal Cell Carcinomas Arise from Hair Follicle Stem Cells in Ptch1+/− Mice. Cancer Cell. 2011;19:114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasper M., Jaks V., Are A., Bergström Å., Schwäger A., Svärd J., Teglund S., Barker N., Toftgård R. Wounding Enhances Epidermal Tumorigenesis by Recruiting Hair Follicle Keratinocytes. Proc. Natl. Acad. Sci. USA. 2011;108:4099–4104. doi: 10.1073/pnas.1014489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson S.C., Eberl M., Vagnozzi A.N., Belkadi A., Veniaminova N.A., Verhaegen M.E., Bichakjian C.K., Ward N.L., Dlugosz A.A., Wong S.Y. Basal Cell Carcinoma Preferentially Arises from Stem Cells Within Hair Follicle and Mechanosensory Niches. Cell Stem Cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong S.Y., Reiter J.F. From the Cover: Wounding mobilizes hair follicle stem cells to form tumors. Proc. Natl Acad Sci USA. 2011;108:4093–4098. doi: 10.1073/pnas.1013098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puig S., Berrocal A. Management of High-Risk and Advanced Basal Cell Carcinoma. Clin. Transl. Oncol. 2015;17:497–503. doi: 10.1007/s12094-014-1272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein E. Basal Cell Carcinomas: Attack of the Hedgehog. Nat. Rev. Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel K.D., Uhmann A., Dressel R., Brandt J.V.D., Hahn H., Reichardt H.M. The Hedgehog Receptor Patched1 in T Cells Is Dispensable for Adaptive Immunity in Mice. PLoS ONE. 2013;8:e61034. doi: 10.1371/journal.pone.0061034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhmann A., Brandt J.V.D., Dittmann K., Heß I., Dressel R., Binder C., Lühder F., Christiansen H., Fassnacht M., Bhandoola A., et al. T Cell Development Critically Depends on Prethymic Stromal Patched Expression. J. Immunol. 2011;186:3383–3391. doi: 10.4049/jimmunol.1001939. [DOI] [PubMed] [Google Scholar]
- 11.Uhmann A., Heß I., Frommhold A., König S., Zabel S., Nitzki F., Dittmann K., Lühder F., Christiansen H., Reifenberger J., et al. DMBA/TPA Treatment Is Necessary for BCC Formation from Patched Deficient Epidermal Cells in Ptch flox/Flox CD4Cre +/− Mice. J. Investig. Dermatol. 2014;134:2620–2629. doi: 10.1038/jid.2014.157. [DOI] [PubMed] [Google Scholar]
- 12.Adolphe C., Hetherington R., Ellis T., Wainwright B., Thebault S., Flourakis M., Vanoverberghe K., Vandermoere F., Roudbaraki M., Lehen’Kyi V., et al. Patched1 Functions As a Gatekeeper by Promoting Cell Cycle Progression. Cancer Res. 2006;66:2081–2088. doi: 10.1158/0008-5472.CAN-05-2146. [DOI] [PubMed] [Google Scholar]
- 13.Adolphe C., Nieuwenhuis E., Villani R., Li Z.J., Kaur P., Hui C.-C., Wainwright B. Patched 1 and Patched 2 Redundancy Has a Key Role in Regulating Epidermal Differentiation. J. Investig. Dermatol. 2014;134:1981–1990. doi: 10.1038/jid.2014.63. [DOI] [PubMed] [Google Scholar]
- 14.Reeves M.Q., Kandyba E., Harris S., Del Rosario R., Balmain A. Multicolour Lineage Tracing Reveals Clonal Dynamics of Squamous Carcinoma Evolution from Initiation to Metastasis. Nat. Cell Biol. 2018;20:699–709. doi: 10.1038/s41556-018-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitzki F., Becker M., Frommhold A., Schulz-Schaeffer W., Hahn H. Patched Knockout Mouse Models of Basal Cell Carcinoma. J. Ski. Cancer. 2012;2012:1–11. doi: 10.1155/2012/907543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pyczek J., Khizanishvili N., Kuzyakova M., Zabel S., Bauer J., Nitzki F., Emmert S., Schön M.P., Boukamp P., Schildhaus H.-U., et al. Regulation and Role of GLI1 in Cutaneous Squamous Cell Carcinoma Pathogenesis. Front. Genet. 2019;10:1185. doi: 10.3389/fgene.2019.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellegrini C., Maturo M.G., Di Nardo L., Ciciarelli V., García-Rodrigo C.G., Fargnoli M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017;18:2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanimura N., Fujita Y. Epithelial Defense against Cancer (EDAC) Semin. Cancer Biol. 2020;63:44–48. doi: 10.1016/j.semcancer.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Kong Y.-H., Xu S.-P. Salidroside Prevents Skin Carcinogenesis Induced by DMBA/TPA in a Mouse Model through Suppression of Inflammation and Promotion of Apoptosis. Oncol. Rep. 2018;39:2513–2526. doi: 10.3892/or.2018.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Athar M. Inhibition of Smoothened Signaling Prevents Ultraviolet B-Induced Basal Cell Carcinomas through Regulation of Fas Expression and Apoptosis. Cancer Res. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 21.Biehs B., Dijkgraaf G.J.P., Piskol R., Alicke B., Boumahdi S., Peale F., Gould S.E., De Sauvage F.J. A Cell Identity Switch Allows Residual BCC to Survive Hedgehog Pathway Inhibition. Nat. Cell Biol. 2018;562:429–433. doi: 10.1038/s41586-018-0596-y. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhary S.C., Tang X., Arumugam A., Li C., Srivastava R.K., Weng Z., Xu J., Zhang X., Kim A.L., McKay K., et al. Shh and p50/Bcl3 Signaling Crosstalk Drives Pathogenesis of BCCs in Gorlin Syndrome. Oncotarget. 2015;6:36789–36814. doi: 10.18632/oncotarget.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim A.L., Back J.H., Zhu Y., Tang X., Yardley N.P., Kim K.J., Athar M., Bickers D.R. AKT1 Activation Is Obligatory for Spontaneous BCC Tumor Growth in a Murine Model That Mimics Some Features of Basal Cell Nevus Syndrome. Cancer Prev. Res. 2016;9:794–802. doi: 10.1158/1940-6207.CAPR-16-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu G.A., Sundram U., Chang A.L.S. Two Different Scenarios of Squamous Cell Carcinoma within Advanced Basal Cell Carcinomas. JAMA Dermatol. 2014;150:970. doi: 10.1001/jamadermatol.2014.583. [DOI] [PubMed] [Google Scholar]
- 25.Orouji A., Goerdt S., Utikal J., Leverkus M. Multiple Highly and Moderately Differentiated Squamous Cell Carcinomas of the Skin during Vismodegib Treatment of Inoperable Basal Cell Carcinoma. Br. J. Dermatol. 2014;171:431–433. doi: 10.1111/bjd.12840. [DOI] [PubMed] [Google Scholar]
- 26.Aasi S., Silkiss R., Tang J.Y., Wysong A., Liu A., Epstein E., Oro A.E., Chang A.L.S. New Onset of Keratoacanthomas after Vismodegib Treatment for Locally Advanced Basal Cell Carcinomas: A Report of 2 Cases. JAMA Dermatol. 2013;149:242–243. doi: 10.1001/jamadermatol.2013.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X., Ponomaryov T., Ornell K.J., Zhou P., Dabral S.K., Pak E., Li W., Atwood S.X., Whitson R.J., Chang A.L.S., et al. RAS/MAPK Activation Drives Resistance to Smo Inhibition, Metastasis, and Tumor Evolution in Shh Pathway-Dependent Tumors. Cancer Res. 2015;75:3623–3635. doi: 10.1158/0008-5472.CAN-14-2999-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang N.J., Sanborn Z., Arnett K.L., Bayston L.J., Liao W., Proby C.M., Leigh I.M., Collisson E.A., Gordon P.B., Jakkula L., et al. Loss-of-Function Mutations in Notch Receptors in Cutaneous and Lung Squamous Cell Carcinoma. Proc. Natl. Acad. Sci. USA. 2011;108:17761–17766. doi: 10.1073/pnas.1114669108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering C.R., Zhou J.H., Lee J.J., Drummond J.A., Peng S.A., Saade R.E., Tsai K.Y., Curry J.L., Tetzlaff M.T., Lai S.Y., et al. Mutational Landscape of Aggressive Cutaneous Squamous Cell Carcinoma. Clin. Cancer Res. 2014;20:6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong X., Meng L., Liu P., Ji R., Su X., Xin Y., Jiang X. YAP/TAZ: A Promising Target for Squamous Cell Carcinoma Treatment. Cancer Manag. Res. 2019;11:6245–6252. doi: 10.2147/CMAR.S197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato H., Kurosawa K., Inoue Y., Tanuma N., Momoi Y., Hayashi K., Ogoh H., Nomura M., Sakayori M., Kakugawa Y., et al. Loss of Protein Phosphatase 6 in Mouse Keratinocytes Increases Susceptibility to Ultraviolet-B-Induced Carcinogenesis. Cancer Lett. 2015;365:223–228. doi: 10.1016/j.canlet.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Lynch M.D., Lynch C.N.S., Craythorne E., Liakath-Ali K., Mallipeddi R., Barker J.N., Watt F.M. Spatial Constraints Govern Competition of Mutant Clones in Human Epidermis. Nat. Commun. 2017;8:1119. doi: 10.1038/s41467-017-00993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfer A., Bakker T., Wilson A., Nicolas M., Ioannidis V., Littman D.R., Wilson C.B., Held W., Macdonald H.R., Radtke F. Inactivation of Notch1 in Immature Thymocytes Does Not Perturb CD4 or CD8 T Cell Development. Nat. Immunol. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 34.Lee P.P., Fitzpatrick D.R., Beard C., Jessup H.K., Lehar S., Makar K.W., Pérez-Melgosa M., Sweetser M.T., Schlissel M.S., Nguyen S., et al. A Critical Role for Dnmt1 and DNA Methylation in T Cell Development, Function, and Survival. Immun. 2001;15:763–774. doi: 10.1016/S1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 35.Sawada S., Scarborough J.D., Killeen N., Littman D.R. A Lineage-Specific Transcriptional Silencer Regulates CD4 Gene Expression During T Lymphocyte Development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 36.Indra A.K., Warot X., Brocard J., Bornert J.-M., Xiao J.-H., Chambon P., Metzger D. Temporally-Controlled Site-Specific Mutagenesis in the Basal Layer of the Epidermis: Comparison of the Recombinase Activity of the Tamoxifen-Inducible Cre-ERT and Cre-ERT2 Recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A Robust and High-Throughput Cre Reporting and Characterization System for the Whole Mouse Brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soriano P. Generalized LacZ Expression With the ROSA26 Cre Reporter Strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 39.Ventura A., Kirsch D.G., McLaughlin M.E., Tuveson D.A., Grimm J., Lintault L., Newman J.C.J., Reczek E.E., Weissleder R., Jacks T. Restoration of p53 Function Leads to Tumour Regression in Vivo. Nat. Cell Biol. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 40.Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. ‘Green mice’ As a Source of Ubiquitous Green Cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/S0014-5793(97)00313-X. [DOI] [PubMed] [Google Scholar]
- 41.Hahn H., Wojnowski L., Zimmer A.M., Hall J., Miller G., Zimmer A. Rhabdomyosarcomas and Radiation Hypersensitivity in a Mouse Model of Gorlin Syndrome. Nat. Med. 1998;4:619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 42.Uhmann A., Dittmann K., Nitzki F., Dressel R., Koleva M., Frommhold A., Zibat A., Binder C., Adham I., Nitsche M., et al. The Hedgehog Receptor Patched Controls Lymphoid Lineage Commitment. Blood. 2007;110:1814–1823. doi: 10.1182/blood-2007-02-075648. [DOI] [PubMed] [Google Scholar]
- 43.Braun K.M., Niemann C., Jensen U.B., Sundberg J.P., Silva-Vargas V., Watt F.M. Manipulation of Stem Cell Proliferation and Lineage Commitment: Visualisation of Label-Retaining Cells in Wholemounts of Mouse Epidermis. Dev. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 44.Sequeira I., Legue E., Capgras S., Nicolas J.-F. Microdissection and Visualization of Individual Hair Follicles for Lineage Tracing Studies. Advanced Structural Safety Studies. 2013;1195:247–258. doi: 10.1007/7651_2013_48. [DOI] [PubMed] [Google Scholar]
- 45.Pyczek J., Buslei R., Schult D., Hölsken A., Buchfelder M., Heß I., Hahn H., Uhmann A. Hedgehog Signaling Activation Induces Stem Cell Proliferation and Hormone Release in the Adult Pituitary Gland. Sci. Rep. 2016;6:24928. doi: 10.1038/srep24928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dräger J., Simon-Keller K., Pukrop T., Klemm F., Wilting J., Sticht C., Dittmann K., Schulz M., Leuschner I., Marx A., et al. LEF1 Reduces Tumor Progression and Induces Myodifferentiation in a Subset of Rhabdomyosarcoma. Oncotarget. 2017;8:3259–3273. doi: 10.18632/oncotarget.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]