Abstract
Despite the availability of various clinical trials that used different diagnostic methods to identify diabetic sensorimotor polyneuropathy (DSPN), no reliable studies that prove the associations among diagnostic parameters from two different methods are available. Statistically significant diagnostic parameters from various methods can help determine if two different methods can be incorporated together for diagnosing DSPN. In this study, a systematic review, meta-analysis, and trial sequential analysis (TSA) were performed to determine the associations among the different parameters from the most commonly used electrophysiological screening methods in clinical research for DSPN, namely, nerve conduction study (NCS), corneal confocal microscopy (CCM), and electromyography (EMG), for different experimental groups. Electronic databases (e.g., Web of Science, PubMed, and Google Scholar) were searched systematically for articles reporting different screening tools for diabetic peripheral neuropathy. A total of 22 studies involving 2394 participants (801 patients with DSPN, 702 controls, and 891 non-DSPN patients) were reviewed systematically. Meta-analysis was performed to determine statistical significance of difference among four NCS parameters, i.e., peroneal motor nerve conduction velocity, peroneal motor nerve amplitude, sural sensory nerve conduction velocity, and sural sensory nerve amplitude (all p < 0.001); among three CCM parameters, including nerve fiber density, nerve branch density, and nerve fiber length (all p < 0.001); and among four EMG parameters, namely, time to peak occurrence (from 0 to 100% of the stance phase) of four lower limb muscles, including the vastus lateralis (p < 0.001), tibialis anterior (p = 0.63), lateral gastrocnemius (p = 0.01), and gastrocnemius medialis (p = 0.004), and the vibration perception threshold (p < 0.001). Moreover, TSA was conducted to estimate the robustness of the meta-analysis. Most of the parameters showed statistical significance between each other, whereas some were statistically nonsignificant. This meta-analysis and TSA concluded that studies including NCS and CCM parameters were conclusive and robust. However, the included studies on EMG were inconclusive, and additional clinical trials are required.
Subject terms: Medical research, Neurology
Introduction
Diabetic sensorimotor polyneuropathy (DSPN) is a common and costly complication that is experienced by patients with diabetes; this complication, which involves disruption in the anatomy of the nerve and blood vessels that subsequently leads to the dysfunction of the motor, sensory, and autonomic nerves, has an estimated prevalence of 50%1–3. The variance in attributes and symptoms of nerve injury in patients with DSPN makes diagnostic strategies challenging. DSPN causes dispersed regular and length-dependent damage to peripheral nerves, sensation loss, and foot muscle dysfunction, thus leading to increased healthcare cost and decreased quality of life; it is also an early indicator of nonhealing diabetic wounds, infections, diabetic foot ulcers, amputations, and death3–5. Early detection and improved classification tools can allow the correct diagnosis and treatment of DSPN, as well as timely intervention to prevent foot ulceration, amputation, and other diabetic complications, hence reducing the possibilities of mortalities due to DSPN6–8.
A large number of specialized screening and diagnostic tests for the assessment of DSPN are available, and in most cases, neurological history, physical examination, and electrophysiological tests are combined for the accurate conventional assessment of DSPN9,10. Some of the most common clinical and electrophysiological diagnostic methods for DSPN are vibration sensation with a 128 Hz tuning fork, monofilament test, quantitative sensory testing (QST)11,12, skin biopsy13, nerve conduction study (NCS)14, corneal confocal microscopy (CCM)15, and electromyography (EMG)16.
Given the lack of reliable estimates for the frequency of DSPN in different populations and the absence of clear diagnostic guidelines17,18, different clinical studies have been conducted by using various screening methods to identify DSPN19–40. QST, neuropathy disability score, Michigan neuropathy screening method, vibration sensing with a 128 Hz tuning fork, and monofilament test are used for assessing pain, touch, vibration, and temperature sensation loss due to DSPN9–12. However, the change in nerve and muscle function due to DSPN and the progression of muscle and nerve dysfunction with the advancement of DSPN cannot be clearly observed by using these methods. Electrophysiological tests, such as NCS, CCM, and EMG, provide information regarding nerve and muscle dysfunction due to DSPN. NCS has been considered as the gold standard for clinical research or trials on patients with DSPN because of its advantages of objectivity, sensitivity, reliability, noninvasiveness, and association with small coefficients of variation9,14,41. CCM is a new rapid, regenerable, and noninvasive method for accurately detecting small-fiber neuropathy. This method allows studying the structure of the human cornea in vivo and has immense potential for studying different corneal diseases9. Given that small nerve fibers are the first to be damaged due to DSPN, CCM has shown reasonable diagnostic utility for the assessment of small-fiber DSPN19–31,33,42. According to the European Federation of Neurological Society guideline in 2005, skin biopsy is one of the most accurate diagnostic methods for small-fiber DSPN; however, this method cannot be advocated for routine use because it is an invasive and highly specialized procedure that requires electron microscopy and professional expertise43,44. Diabetic neuropathy leads to the progressive loss of somatosensory sensitivity, especially in the lower limbs; this effect may cause functional gait variations and is predominantly related to reductions in joint movement range and active muscle power and changes in gait mechanics45. EMG has been widely used by researchers to observe muscle activities and diagnose DSPN in patients36–40. Thus, in this study, the three noninvasive electrophysiological diagnostic methods NCS, CCM, and EMG were considered for meta-analysis and systematic review.
In numerous studies, CCM and NCS have been used together to identify DSPN19,24,26–28,30. NCS and EMG46–50 are also widely used to diagnose neuromuscular diseases, and their severity such as DSPN. However, the significance of relationships among these different methods and their parameters and threshold values have not been determined uniformly in existing studies. Another major drawback of existing studies is their differences in sample sizes, patient’s characteristics, environments, and diagnostic tools. These factors affect their results and introduce bias. A review of the existing literature revealed the absence of studies with a large sample size from which standardized values of different parameters can be established for these diagnostic tests. An existing clinical study on DSPN with a large sample size can be considered as a reference for understanding the characteristics of different patient groups and the baseline values of different diagnostic parameters for those groups. A meta-analysis can be a very powerful tool for summarizing results from different studies and obtaining conclusive results from different reported studies. To our knowledge, no meta-analysis has been conducted on DSPN screening methods to observe the baseline values for diagnostic parameters and to identify the statistical significance between screening parameters. Therefore, this study aimed to conduct a meta-analysis to assess the significance among the different parameters of the most commonly used electrophysiological screening methods for DSPN (NCS, CCM, and EMG) in clinical research. It also aimed to summarize the results of different studies to produce a single estimate of the major effect with enhanced accuracy for the different diagnostic parameters of patients with DSPN when compared with those of healthy controls and diabetic patients without DSPN (non-DSPN). We conducted trial sequential analysis (TSA) to validate the meta-analysis and to identify the effect of the included studies for different DSPN diagnostic methods.
Methods
Literature search strategy
Electronic databases (Web of Science, PubMed, Scopus, and Google Scholar) were searched systematically for articles published between January 2007 and June 2019. The search strategy was based on a combination of terms: (1) diabetic neuropathy. (2) Electromyography* AND diabetic neuropathy. (3) Nerve conduction studies AND diabetic neuropathy. (4) Corneal confocal microscopy AND diabetic neuropathy. The following inclusion criteria were adopted: (i) published between January 2007 and June 2019; (ii) included at least 10 adult patients with DSPN; (iii) reported any of the three diagnostic methods for DSPN, namely, NCS, CCM or EMG; (iv) reported the values of at least two diagnostic parameters out of the four NCS parameters, three CCM parameters, and four EMG parameters selected for this study. Exclusion criteria included abstracts from conferences, articles that did not report diagnostic parameters, diagnostic parameter data presented graphically that could not be retrieved, case reports, comments, and reviews.
Selection of studies and data extraction
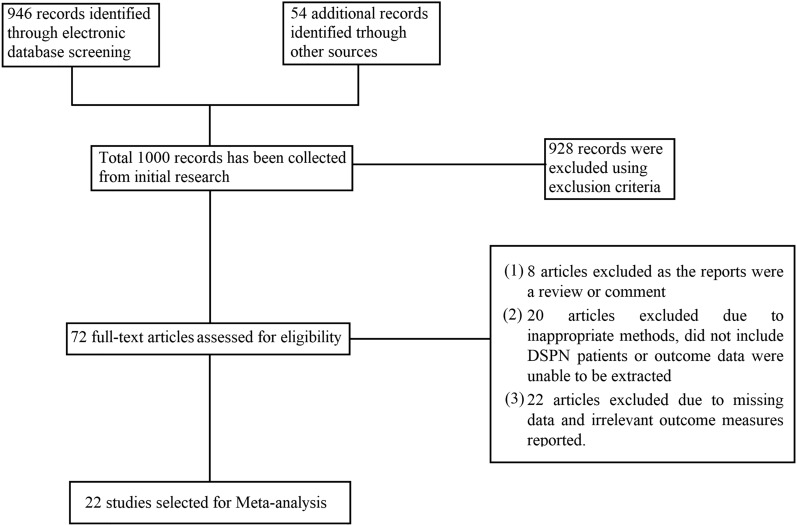
The titles and abstracts retrieved from the initial database search were screened on the basis of the literature search strategy. The full text was reviewed for articles that remained relevant after the initial screening on the basis of inclusion criteria. All studies meeting the exclusion criteria were removed from the review. The following data were extracted from the eligible articles: study details (title, author list, year of publication, journal of publication, citation, method used, major finding, and a short summary); patient characteristics (number of patients, experimental group, age, sex, DM duration, HbA1c [%], and body mass index); and diagnostic parameters for NCS, namely, peroneal motor nerve conduction velocity (PMNCV), peroneal motor nerve amplitude (PMNamp), sural sensory nerve conduction velocity (SSNCV), and sural sensory nerve amplitude (SSNamp); parameters for CCM, including nerve fiber density (NFD), nerve branch density (NBD), nerve fiber length (NFL); and parameters for EMG, including time to peak occurrence (from 0 to 100% of the stance phase) for the vastus lateralis (VL), tibialis anterior (TA), lateral gastrocnemius (LG), gastrocnemius medialis (GM) muscles, and vibration perception threshold (VPT). Extracted data were recorded in a tabular manner to prepare a summary form for each study. Figure 1 shows the flow chart of the selection of the studies for meta-analysis. The first author was responsible for the study selection, study design, data extraction, and meta-analysis, and all authors were involved in result analysis, data representation, and manuscript preparation.
Figure 1.
Study selection process for meta-analysis.
Meta-analysis
Data were transformed into standardized units of measure in the form of mean ± standard deviation (SD) for comparison and statistical analysis when possible. Meta-analysis was carried out on individual outcome measures when more than two studies reported the particular individual outcome measure. Statistical significance (p) between different diagnostic parameters was calculated with Student’s t-test. Here, p < 0.05 was considered to be statistically significant. The standard mean difference and the corresponding 95% confidence intervals (95% CIs) for all diagnostic parameters were calculated. Heterogeneity was measured by using the I2 statistic, and I2 > 50% was considered significantly heterogeneous. All the statistical analyses were performed with Minitab version 18.0 software (Minitab LLC, State College, Pennsylvania, USA). Meta-analysis was performed by using the Review Manager (RevMan) 5.3 computer program (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Trial sequential analysis (TSA)
A meta-analysis with a small sample size may lead to a false negative or positive conclusion even with a statistical significance. TSA (TSA, version 0.9 beta, http://www.ctu.dk/tsa/) (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark)51 was performed to avoid that type of error in this meta-analysis and to validate the conclusion from the meta-analysis. TSA uses a combination of statistical analyses to identify the required information size (RIS), which helps evaluate if sufficient information has been included and whether the outcomes of a meta-analysis are reliable or not51. A decision is considered conclusive from the meta-analysis if the Z-curve crosses the TSA boundary or enters the futility area, indicating that further studies are not required in that meta-analysis or else the meta-analysis is inconclusive and additional studies should be required. The RIS was calculated on the basis of an alpha risk of 5% error, 90% statistical power, and a two-sided boundary type for continuous data.
Results
Study selection
Overall, 1000 unique records were originally identified. However, 928 articles were excluded for a variety of reasons, such as unsuitable study design, inappropriate comparison groups, undesired diagnostic methods, missing data, irrelevant data, or inability to extract data. Thus, 72 articles were considered as potentially relevant, and their full texts were retrieved. Upon reviewing the full texts, 50 studies were excluded because (1) the report was a review or commentary (n = 8); (2) the study did not include patients with DSPN or outcome data could not be extracted (n = 20); and (3) the study did not include the parameters desired for the meta-analysis (n = 22). Finally, 22 remaining studies were eligible for inclusion. Several studies used CCM and NCS methods19,24,26–28,30. A total of 13 studies on CCM19–31, 10 on NCS19,24,26–28,30,32–35, and five on EMG36–40 were selected for this analysis.
Participants’ characteristics
Table 1 displays a summary of the characteristics and sociodemographic variables of the participants in the included studies. The 22 studies included for meta-analysis had 2394 participants (801 patients with DSPN, 702 controls, and 891 non-DSPN patients) in total from three different experimental groups19–40. The mean group size was 44.21 and ranged from 10 to 164 participants. The age range of participants in the control, non-DSPN, and DSPN groups from the included studies were 47.76 ± 15.28, 48.34 ± 15.56, and 55.40 ± 12.24 years, respectively. The mean diabetes duration in the DSPN group was higher than that in the non-DSPN group. Table 1 shows that patients with DSPN had higher HbA1c (%) than other participants, and no significance difference in BMI (kg/m2) between the control and non-DSPN groups were observed.
Table 1.
| Study | Diagnostic Method | Group | N | Age (years) | Sex (M/F) | Duration of diabetes (years) | HbA1c (%) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|
| Ahmed et al.19 |
NCS CCM |
Control | 64 | 38.9 ± 17.6 | 30/34 | – | 5.5 ± 0.4 | 24.7 ± 4.6 |
| No-DSPN | 56 | 34.9 ± 14.8 | 27/29 | 17.6 ± 14.0 | 7.4 ± 1.3 | 25.3 ± 4.4 | ||
| DSPN | 33 | 50.0 ± 14.3 | 16/17 | 31.4 ± 13.5 | 8.7 ± 2.1 | 28.9 ± 5.0 | ||
| Akashi et al.38 | EMG | Control | 16 | 51.1 ± 8.3 | 8/8 | – | – | 23.9 ± 2.9 |
| DSPN | 19 | 57.6 ± 8.5 | 11/8 | 12.6 ± 5.3 | – | 26.6 ± 4.2 | ||
| DSPN-U | 10 | 53.8 ± 7.9 | 5/5 | 16.4 ± 8.5 | – | 27.8 ± 4.6 | ||
| Alam et al.33 |
NCS CCM |
Control | 27 | 41 ± 14.9 | 16/11 | - | 5.5 ± 0.31 | 26.9 ± 4.0 |
| No- DSPN | 30 | 38.8 ± 12.5 | 13/14 | 17.2 ± 12.0 | 7.7 ± 1.92 | 26.3 ± 4.4 | ||
| DSPN | 31 | 53.3 ± 11.9 | 19/12 | 37.2 ± 13.1 | 8.6 ± 1.55 | 27.2 ± 4.2 | ||
| Chen et al.30 |
NCS CCM |
Control | 26 | 44 ± 15 | – | 5.5 ± 0.3 | 26.8 ± 4.0 | |
| No-DSPN | 46 | 44 ± 13 | – | 23 ± 15 | 8.2 ± 1.4 | 26.4 ± 4.5 | ||
| DSPN | 17 | 59 ± 11 | – | 39 ± 14 | 8.5 ± 1.3 | 27.5 ± 3.5 | ||
| Edwards et al.20 | CCM | Control | 61 | 52 ± 14 | 27/34 | – | 5.4 ± 0.3 | 26.1 ± 5.1 |
| No-DSPN | 143 | 48 ± 16 | 66/77 | 14 ± 12 | 7.8 ± 1.2 | 27.6 ± 5.3 | ||
| DSPN | 88 | 58 ± 9 | 57/31 | 23 ± 14 | 8.2 ± 1.7 | 30.7 ± 7.2 | ||
| Gomes et al.39 | EMG | Control | 23 | 55 ± 8 | 9/14 | – | – | – |
| DSPN | 23 | 56 ± 8 | 9/14 | 14.4 ± 6.5 | – | – | ||
| Hertz et al.21 | CCM | Control | 20 | 41.4 ± 17.3 | 5/15 | — | 5.5 ± 0.4 | – |
| DM-1 | 26 | 42.8 ± 16.9 | 18/8 | 22.7 ± 16.4 | 8.0 ± 1.9 | – | ||
| Hussain et al.34 | NCS | No-DSPN | 22 | 51.90 ± 6.46 | 10/12 | 2 ± 1.7 | 6.67 ± 0.98 | 25.38 ± 2.93 |
| DSPN | 64 | 54.68 ± 7.59 | 28/36 | 7.59 ± 4.77 | 7.9 ± 2.2 | 25.63 ± 3.19 | ||
| Li et al.31 |
NCS CCM |
Control | 24 | 68.63 ± 5.19 | 9/15 | – | 5.88 ± 0.82 | 25.41 ± 3.57 |
| No-DSPN | 49 | 67.12 ± 6.01 | 18/30 | 9.79 ± 7.09 | 7.07 ± 0.96 | 24.56 ± 2.93 | ||
| DSPN | 79 | 70.15 ± 7.34 | 46/33 | 12.58 ± 7.28 | 7.94 ± 1.86 | 25.38 ± 3.40 | ||
| Malik et al.22 | CCM | Control | 18 | 57.8 ± 11.5 | – | – | < 6.5 | – |
| DSPN | 18 | 57.82 ± 11.90 | – | 22.93 ± 11.79 | 8.07 ± 1.34 | – | ||
| Mehra et al.23 | CCM | Control | 15 | 46 ± 3 | – | – | – | – |
| DSPN | 20 | 41 ± 1 | 20/0 | 27 ± 2 | 8.9 ± 1.4 | – | ||
| Petropoulos et al.24 |
NCS CCM |
Control | 55 | 51.7 ± 11.4 | 28/27 | – | 5.5 ± 0.3 | 25.6 ± 4.6 |
| No-DSPN | 86 | 50.4 ± 14.1 | 108/78 | 24.2 ± 21.2 | 7.7 ± 1.6 | 27.2 ± 5.2 | ||
| DSPN | 100 | 34.4 ± 17.3 | 7.9 ± 1.6 | 27.6 ± 5.8 | ||||
| Pritchard et al.25 | CCM | Control | 154 | 46 ± 15 | 70/84 | – | 5.5 ± 0.3 | 28 ± 6 |
| No-DSPN | 168 | 43 ± 16 | 85/83 | 20 ± 15 | 8.0 ± 1.2 | 26 ± 4 | ||
| DSPN | 74 | 57 ± 11 | 41/33 | 34 ± 16 | 8.6 ± 1.8 | 26 ± 5 | ||
| Quattrini et al.26 | CCM | Control | 15 | 55.0 ± 18.5 | 6/9 | – | – | – |
| No-DSPN | 10 | 53.5 ± 10.2 | 6/4 | 16.7 ± 4.44 | 7.16 ± 0.40 | – | ||
| DSPN | 44 | 59.05 ± 3.18 | 36/8 | 21.22 ± 4.24 | 8.01 ± 0.42 | – | ||
| Sivaskandarajah et al.27 |
CCM NCS |
Control | 64 | 38.3 ± 16.4 | 34/30 | – | 5.6 ± 0.4 | 24.7 ± 5.0 |
| No-DSPN | 63 | 32.7 ± 13.6 | 29/34 | 17.3 ± 12.2 | 7.5 ± 1.2 | 24.9 ± 3.6 | ||
| DSPN | 33 | 48.5 ± 13.7 | 14/19 | 32.3 ± 13.1 | 8.4 ± 1.6 | 27.7 ± 6.1 | ||
| De Souza et al.32 | NCS | Control | 51 | 38.5 ± 14.2 | 24/27 | – | ≤ 6% | – |
| No-DSPN | 50 | 46.4 ± 16.5 | 25/25 | 9.3 ± 15.1 | 8.1 | – | ||
| DSPN | 52 | 57.3 ± 11.7 | 27/25 | 10.9 ± 8 | 9.2 | – | ||
| Sacco et al.36 | EMG | Control | 21 | 50.9 ± 7.3 | – | – | – | 24.3 ± 2.6 |
| DSPN | 24 | 55.2 ± 7.9 | – | – | – | 27.0 ± 4.4 | ||
| Sawacha et al.37 | EMG | Control | 10 | 61.2 ± 5.07 | – | – | – | 24.4 ± 2.8 |
| No-DSPN | 20 | 56.53 ± 13.29 | – | 23.3 ± 13.7 | – | 26.4 ± 2.5 | ||
| DSPN | 20 | 61.2 ± 7.7 | – | 13 ± 6.5 | – | 26.8 ± 3.4 | ||
| Tavakoli et al.28 |
NCS CCM |
Control | 17 | 55 ± 4.8 | 8/9 | – | < 6.5 | – |
| No-DSPN | 34 | 55 ± 1.9 | 19/15 | 10.7 ± 1.82 | 8.1 ± 0.27 | – | ||
| DSPN | 67 | 58.87 ± 2.46 | 54/13 | 17.03 ± 3.02 | 8.10 ± 0.38 | – | ||
| Tavakoli et al.29 | CCM | Control | 18 | 57.0 ± 3.0 | 10/8 | – | 5.7 ± 0.1 | – |
| DSPN | 25 | 52.0 ± 2.0 | 20/5 | 26.5 ± 2.5 | 8.1 ± 0.3 | – | ||
| Weisman et al.35 | NCS | No-DSPN | 84 | 56 ± 9 | 48/36 | 10 ± 11 | 7.8 ± 1.7 | 29.9 ± 5.15 |
| DSPN | 25 | 55 ± 10 | 16/9 | 10 ± 7 | 9.0 ± 1.7 | 28.9 ± 5.82 | ||
| Watari et al.40 | EMG | Control | 30 | 54.1 ± 7.5 | 14/16 | – | – | 25.7 ± 3.9 |
| No-DSPN | 43 | 56.7 ± 6.8 | 25/18 | 8.1 ± 7.2 | – | 28.4 ± 3.9 | ||
| DSPN | 74 | 56.7 ± 6.0 | 45/29 | 13.4 ± 7.1 | 28.8 ± 4.2 | |||
| Total | Control | 702 | 47.76 ± 15.28 | – | – | 5.53 ± 0.38 | 25.21 ± 6.85 | |
| No-DSPN | 891 | 48.34 ± 15.56 | – | 15.69 ± 14.41 | 7.77 ± 1.29 | 26.80 ± 4.68 | ||
| DSPN | 801 | 55.40 ± 12.24 | – | 23.85 ± 15.43 | 8.29 ± 1.64 | 27.43 ± 5.56 | ||
Nerve conduction study (NCS)
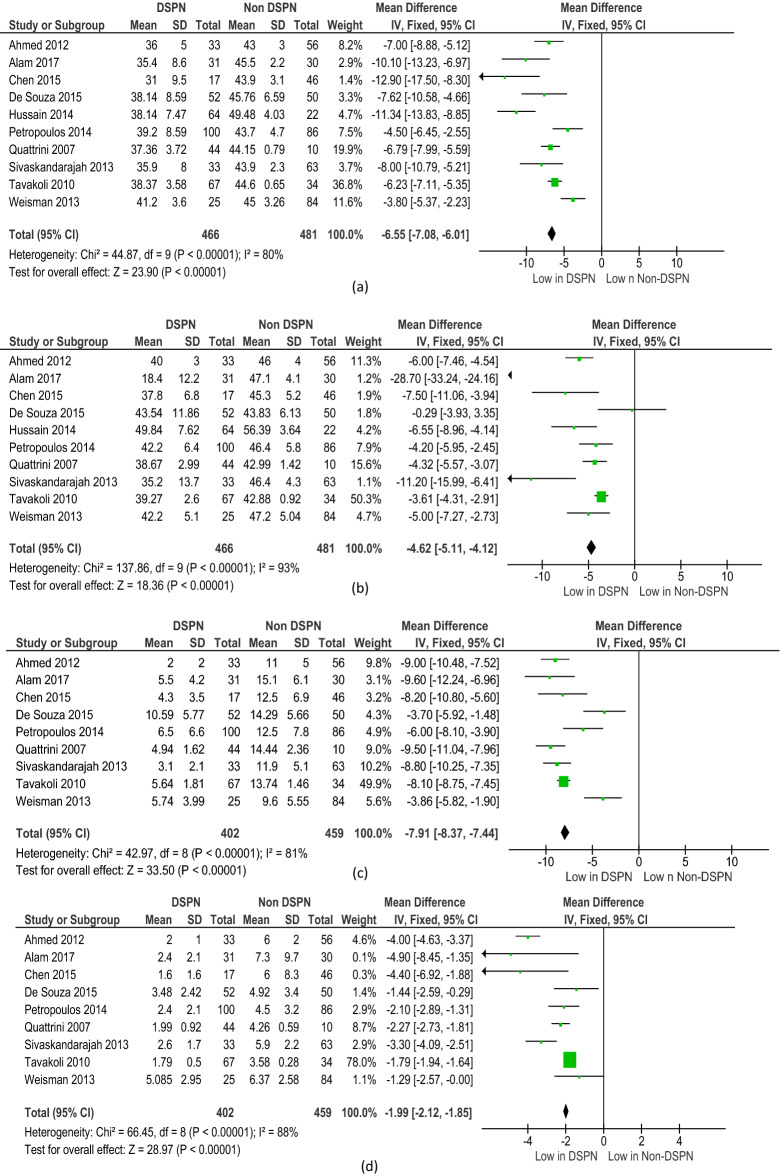
NCS has been used as a standardized clinical test for diagnosing DSPN and for validating other diagnostic methods. In 10 studies19,24,26–28,30,32–35, a total of 1231 participants (471 patients with DSPN, 292 controls, and 468 non-DSPN patients) underwent NCS. In all the included studies, the main observed parameters were the nerve conduction velocity (NCV) and nerve amplitude (Namp) for peroneal motor (PM) and sural sensory (SS) nerves. All the NCS parameters are listed in Table 2. However, median nerve32, ulnar nerve32,34, and tibial nerve35 parameters were reported in a few studies which were insufficient for a conclusive meta-analysis. Thus, only PM and SS parameters were considered for meta-analysis. Quattrini et al.26 reported the NCS results for five different classes (control, non-DSPN, mild DSPN, moderate DSPN, and severe DSPN). Given that only three experimental groups are considered in this study, the three reported26 DSPN severity groups (mild, moderate, and severe) were considered as the DSPN group on the basis of the Cochrane Guidelines for Systematic Review52. The PMNCV (m/s) values of patients with DSPN (37.80 ± 6.48) were significantly lower than those of the controls (48.57 ± 3.82) and non-DSPN patients (44.40 ± 4.10). Similarly, the SSNCV (m/s) [49.96 ± 5.10 for Control, 44.57 ± 10.20 for Non-DSPN and 38.61 ± 13.72 for DSPN], SSNamp (μV) [18.67 ± 7.91 for Control, 11.00 ± 6.66 for Non-DSPN, 5.88 ± 4.99 for DSPN] , PMNamp (μV) [5.90 ± 2.25 for control, 5.10 ± 3.77 for Non-DSPN, 2.52 ± 1.95 for DSPN] values of the DSPN group were significantly reduced compared with those of other two experimental groups. A few studies have considered VPT24,28,30,33 alongside NCS to observe the change in the vibration sensation of patients with DSPN. These studies showed that VPT (V) values drastically increased for the DSPN group (23.53 ± 11.75) but not for the control (6.51 ± 4.66) or non-DSPN (8.83 ± 5.55) groups (Table 3). All of the NCS parameters of the DSPN groups were significantly reduced in comparison with those of the other two experimental groups, and VPT (V) values drastically increased for DSPN but not for the control or non-DSPN groups.
Table 2.
| Study | Group | N | Peroneal motor nerve conduction velocity (PMNCV) (m/s) | Sural sensory nerve conduction velocity (SSNCV) (m/s) | Sural sensory nerve amplitude (SSNamp) (μV) | Peroneal motor nerve amplitude (PMNamp) (μV) |
|---|---|---|---|---|---|---|
| Ahmed et al.19 | Control | 64 | 48 ± 3 | 51 ± 5 | 18 ± 8 | 6 ± 2 |
| No-DSPN | 56 | 43 ± 3 | 46 ± 4 | 11 ± 5 | 6 ± 2 | |
| DSPN | 33 | 36 ± 5 | 40 ± 3 | 2 ± 2 | 2 ± 1 | |
| Petropoulos et al.24 | Control | 55 | 48.8 ± 3.3 | 51.0 ± 4.8 | 20.0 ± 9.7 | 5.2 ± 1.8 |
| No-DSPN | 86 | 43.7 ± 4.7 | 46.4 ± 5.8 | 12.5 ± 7.8 | 4.5 ± 3.2 | |
| DSPN | 100 | 39.2 ± 6.1 | 42.2 ± 6.4 | 6.5 ± 6.6 | 2.4 ± 2.1 | |
| Quattrini et al.26 | Control | 15 | 45.71 ± 0.99 | 46.52 ± 1.87 | 20.25 ± 3.76 | 4.27 ± 0.64 |
| No-DSPN | 10 | 44.15 ± 0.79 | 42.99 ± 1.42 | 14.44 ± 2.36 | 4.26 ± 0.59 | |
| DSPN | 44 | 37.36 ± 3.72 | 38.67 ± 2.99 | 4.94 ± 1.62 | 1.99 ± 0.92 | |
| Sivaskandarajah et al.27 | Control | 64 | 48.0 ± 3.5 | 50.8 ± 4.5 | 17.5 ± 8.5 | 6.4 ± 2.3 |
| No-DSPN | 63 | 43.9 ± 2.3 | 46.4 ± 4.3 | 11.9 ± 5.1 | 5.9 ± 2.2 | |
| DSPN | 33 | 35.9 ± 8.0 | 35.2 ± 13.7 | 3.1 ± 2.1 | 2.6 ± 1.7 | |
| Tavakoli et al.28 | Control | 17 | 49.26 ± 1.63 | 47.85 ± 2.62 | 18.62 ± 2.55 | 5.58 ± 1.02 |
| No-DSPN | 34 | 44.60 ± 0.65 | 42.88 ± 0.92 | 13.74 ± 1.46 | 3.58 ± 0.28 | |
| DSPN | 67 | 38.37 ± 3.58 | 39.27 ± 2.60 | 5.64 ± 1.81 | 1.79 ± 0.50 | |
| Chen et al.30 | Control | 26 | 49.1 ± 3.4 | 50.9 ± 3.9 | 19.7 ± 8.3 | 6 ± 2.4 |
| No-DSPN | 46 | 43.9 ± 3.1 | 45.3 ± 5.2 | 12.5 ± 6.9 | 6.0 ± 8.3 | |
| DSPN | 17 | 31.0 ± 9.5 | 37.8 ± 6.8 | 4.3 ± 3.5 | 1.6 ± 1.6 | |
| Souza et al.32 | Control | 51 | 50.08 ± 5.78 | 47.71 ± 6.66 | 18.59 ± 6.70 | 6.46 ± 2.97 |
| No-DSPN | 50 | 45.76 ± 6.59 | 43.83 ± 6.13 | 14.29 ± 5.66 | 4.92 ± 3.40 | |
| DSPN | 52 | 38.9 ± 8.59 | 43.54 ± 11.86 | 10.59 ± 5.77 | 3.48 ± 2.42 | |
| Alam et al.33 | Control | 27 | 49.2 ± 3.7 | 50.6 ± 2.0 | 20.2 ± 8.8 | 6.1 ± 2.4 |
| No-DSPN | 30 | 45.5 ± 2.2 | 47.1 ± 4.1 | 15.1 ± 6.1 | 7.3 ± 9.7 | |
| DSPN | 31 | 35.4 ± 8.6 | 18.4 ± 12.2 | 5.5 ± 4.2 | 2.4 ± 2.1 | |
| Hussain et al.34 | No-DSPN | 22 | 49.48 ± 4.03 | 56.39 ± 3.64 | – | – |
| DSPN | 64 | 38.14 ± 7.47 | 49.84 ± 7.62 | – | – | |
| Weisman et al.35 | No-DSPN | 84 | 45.0 ± 3.26 | 47.2 ± 5.04 | 9.6 ± 5.55 | 6.37 ± 2.58 |
| DSPN | 25 | 41.2 ± 3.6 | 42.2 ± 5.10 | 5.74 ± 3.99 | 5.08 ± 2.95 | |
| Total | Control | 292 | 48.57 ± 3.82 | 49.96 ± 5.10 | 18.67 ± 7.91 | 5.90 ± 2.25 |
| No-DSPN | 468 | 44.40 ± 4.10 | 44.57 ± 10.20 | 11.00 ± 6.66 | 5.10 ± 3.77 | |
| DSPN | 471 | 37.80 ± 6.48 | 38.61 ± 13.72 | 5.88 ± 4.99 | 2.52 ± 1.95 |
Table 3.
| Study | Group | N | Vibration perception threshold (VPT) (V) |
|---|---|---|---|
| Petropoulos et al.24 | Control | 55 | 5.8 ± 4.6 |
| No-DSPN | 86 | 9.2 ± 6.5 | |
| DSPN | 100 | 22.3 ± 12.6 | |
| Tavakoli et al.28 | Control | 17 | 9.58 ± 0.93 |
| No-DSPN | 34 | 9.56 ± 0.84 | |
| DSPN | 67 | 24.93 ± 9.82 | |
| Chen et al.30 | Control | 26 | 6 ± 5.5 |
| No-DSPN | 46 | 7.6 ± 5.5 | |
| DSPN | 17 | 25.2 ± 13.4 | |
| Alam et al.33 | Control | 27 | 5.3 ± 4.1 |
| No-DSPN | 30 | 5.6 ± 2.5 | |
| DSPN | 31 | 18.4 ± 12.2 | |
| Total | Control | 125 | 6.51 ± 4.66 |
| No-DSPN | 196 | 8.83 ± 5.55 | |
| DSPN | 215 | 23.53 ± 11.75 |
Corneal confocal microscopy (CCM)
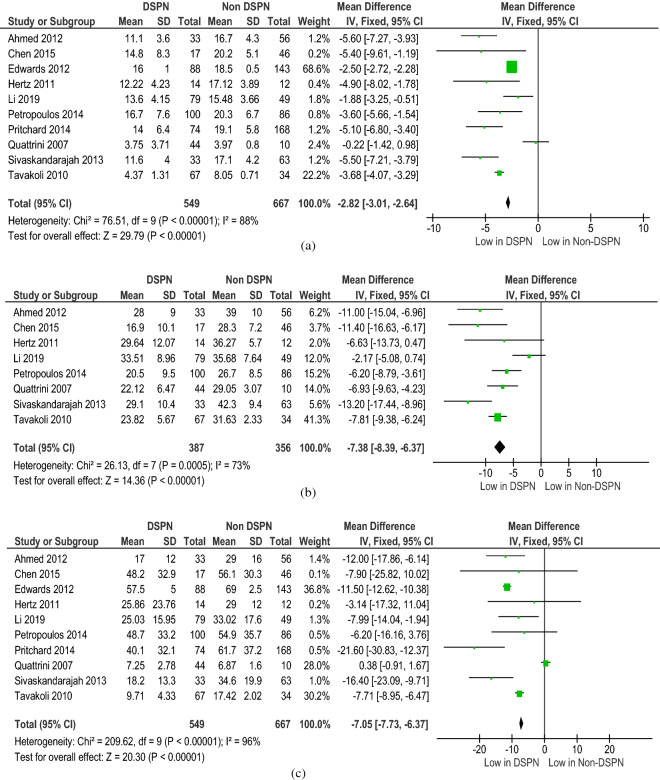
CCM is another commonly used noninvasive method in clinical studies on DSPN. The main corneal nerve parameters studied with CCM include NFD, NBD, and NFL. Recent studies have assessed the screening and monitoring of DPN in clinical studies by using CCM19–31. According to Akashi et al.19, the NFL parameter can identify patients with DSPN more accurately then the other parameters. Most studies stated that CCM19–31 parameters progressively decrease with the increasing severity of neuropathy. Our meta-analysis found similar results. Few studies24,30,31 have compared manual CCM parameters with automated CCM measurements. Automated corneal nerve fiber measurements are slightly lower than corresponding manual measurements. Thus, in this meta-analysis, only manual CCM was considered for these studies24,30,31. A total of 13 studies19–31 involving 1830 participants (612 patients with DSPN, 551controls, and 667 non-DSPN patients) were included. The measured manual CCM parameters, namely, NFD, NFL, and NBD, from these studies are listed in Table 4. The average of each CCM parameter for the control, non-DSPN, and DSPN groups was determined. The NFL (no./mm2) in the DSPN group (11.76 ± 6.65) was significantly lower than that in the control (19.94 ± 6.64) and non-DSPN (17.72 ± 5.51) groups. The mean NFD (fiber/mm2) values for the control, non-DSPN, and DSPN groups were 41.14 ± 9.91, 33.70 ± 9.97, and 24.60 ± 10.18, respectively. The NBD (branches/mm2) in the DSPN group (30.91 ± 27.24) was significantly lower than that in the control (63.66 ± 39.12) and non-DSPN (50.92 ± 30.72) experimental groups. The CCM parameter values of patients with DSPN had reduced compared with those of the non-DSPN and control groups.
Table 4.
| Study | Group | N | Corneal nerve fiber length (NFL) (mm/mm2) | Corneal nerve fiber density (NFD) (fibers/mm2) | Corneal branch density (NBD) (branches/mm2) | |||
|---|---|---|---|---|---|---|---|---|
| Ahmed et al.19 | Control | 64 | 18.4 ± 4.4 | 43 ± 11 | 35 ± 14 | |||
| No-DSPN | 56 | 16.7 ± 4.3 | 39 ± 10 | 29 ± 16 | ||||
| DSPN | 33 | 11.1 ± 3.6 | 28 ± 9 | 17 ± 12 | ||||
| Edwards et al.20 | Control | 61 | 20 ± 1 | – | 80 ± 8 | |||
| No-DSPN | 143 | 18.5 ± 0.5 | – | 69 ± 2.5 | ||||
| DSPN | 88 | 16 ± 1 | – | 57.5 ± 5 | ||||
| Hertz et al.21 | Control | 20 | 16.15 ± 4.13 | 31.9 ± 9.4 | 37.2 ± 17.7 | |||
| No-DSPN | 12 | 17.12 ± 3.89 | 36.27 ± 5.7 | 29.0 ± 12. 7 | ||||
| DSPN | 14 | 12.22 ± 4.23 | 29.64 ± 12.07 | 25.86 ± 23.76 | ||||
| Malik et al.22 | Control | 18 | 13.5 ± 0.3 | 44.5 ± 14.1 | 78.9 ± 30.4 | |||
| DSPN | 18 | 6.99 ± 2.21 | 26 ± 14.99 | 26.31 ± 19.51 | ||||
| Mehra et al.23 | Control | 15 | 9.69 ± 0.7 | 42.04 ± 3.2 | 26.73 ± 2.5 | |||
| DSPN | 20 | 2.23 ± 0.28 | 13.88 ± 2.1 | 4.04 ± 1.5 | ||||
| Petropoulos et al.24 | Control | 55 | 26.4 ± 5.6M | 21.2 ± 3.5A | 37.2 ± 6.7M | 30.0 ± 6.9 A | 92.7 ± 38.6M | 50.4 ± 24.7 A |
| No-DSPN | 86 | 20.3 ± 6.7M | 17.1 ± 4.5 A | 26.7 ± 8.5M | 20.1 ± 8.7 A | 54.9 ± 35.7M | 31.4 ± 25.6 A | |
| DSPN | 100 | 16.7 ± 7.6M | 13.7 ± 5.2 A | 20.5 ± 9.5 M | 14.4 ± 8.9 A | 48.7 ± 33.2M | 20.1 ± 18.7 A | |
| Pritchard et al.25 | Control | 154 | 23.2 ± 6.3 | – | 83.5 ± 45.8 | |||
| No-DSPN | 168 | 19.1 ± 5.8 | – | 61.7 ± 37.2 | ||||
| DSPN | 74 | 14.0 ± 6.4 | – | 40.1 ± 32.1 | ||||
| Quattrini et al.26 | Control | 15 | 6.14 ± 1.22 | 42.10 ± 4.31I [μm] | 43.20 ± 5.05 | 11.21 ± 0.84I (no/mm) | 27.39 ± 3.31 | 139.66 ± 23.42I |
| No-DSPN | 10 | 3.97 ± 0.80 | 32.64 ± 2.78 I [μm] | 29.05 ± 3.07 | 7.22 ± 1.04I (no/mm) | 6.87 ± 1.60 | 44.99 ± 8.93I | |
| DSPN | 44 | 3.75 ± 3.71 | 28.61 ± 10.20I [μm] | 22.12 ± 6.47 | 4.90 ± 3.27I (no/mm) | 7.25 ± 2.78 | 31.79 ± 15.25I | |
| Sivaskandarajah et al.27 | Control | 64 | 18.8 ± 4.5 | 45.3 ± 12.0 | 39.7 ± 16.9 | |||
| No-DSPN | 63 | 17.1 ± 4.2 | 42.3 ± 9.4 | 34.6 ± 19.9 | ||||
| DSPN | 33 | 11.6 ± 4.0 | 29.1 ± 10.4 | 18.2 ± 13.3 | ||||
| Tavakoli et al.28 | Control | 17 | 11.21 ± 0.88 | 45.60 ± 4.47 | 25.38 ± 2.99 | |||
| No-DSPN | 34 | 8.05 ± 0.71 | 31.63 ± 2.33 | 17.42 ± 2.02 | ||||
| DSPN | 67 | 4.37 ± 1.31 | 23.82 ± 5.67 | 9.71 ± 4.33 | ||||
| Tavakoli et al.29 | Control | 18 | 13.5 ± 0.8 | 46.0 ± 3.8 | 35.6 ± 6.7 | |||
| DSPN | 25 | 8.3 ± 0.9 | 18.8 ± 2.1 | 6.9 ± 1.5 | ||||
| Chen et al.30 | Control | 26 | 26.7 ± 3.7M | 17.7 ± 2.8A | 36.8 ± 5.3M | 31.3 ± 6.5A | 92.8 ± 36.4M | 44.6 ± 17.2A |
| No-DSPN | 46 | 20.2 ± 5.1M | 13.4 ± 3.3A | 28.3 ± 7.2M | 22.6 ± 7.3A | 56.1 ± 30.3M | 26.2 ± 15.1A | |
| DSPN | 17 | 14.8 ± 8.3M | 8.8 ± 4.7A | 16.9 ± 10.1M | 13.5 ± 9.1A | 48.2 ± 32.9M | 15.4 ± 12.1A | |
| Li et al.31 | Control | 24 | 17.81 ± 3.19M | 14.66 ± 2.31A | 35.32 ± 5.55M | 23.18 ± 5.77A | 41.48 ± 16.50M | 36.20 ± 12.87A |
| No-DSPN | 49 | 15.48 ± 3.66M | 13.37 ± 3.65A | 35.68 ± 7.64M | 18.98 ± 7.21A | 33.02 ± 17.60M | 32.96 ± 19.30A | |
| DSPN | 79 | 13.60 ± 4.15M | 11.92 ± 3.51A | 33.51 ± 8.96M | 16.88 ± 7.39A | 25.03 ± 15.95M | 23.66 ± 15.60A | |
| Total | Control | 551 | 19.94 ± 6.64 | 41.14 ± 9.91 | 63.66 ± 39.12 | |||
| No-DSPN | 655 | 17.72 ± 5.51 | 33.70 ± 9.97 | 50.92 ± 30.72 | ||||
| DSPN | 624 | 11.76 ± 6.65 | 24.60 ± 10.18 | 30.91 ± 27.24 | ||||
IIntra-epidermal nerve fiber (IENF).
AAutomated IVCCM.
MManual IVCCM; IVCCM: in vivo CCM.
Electromyography (EMG)
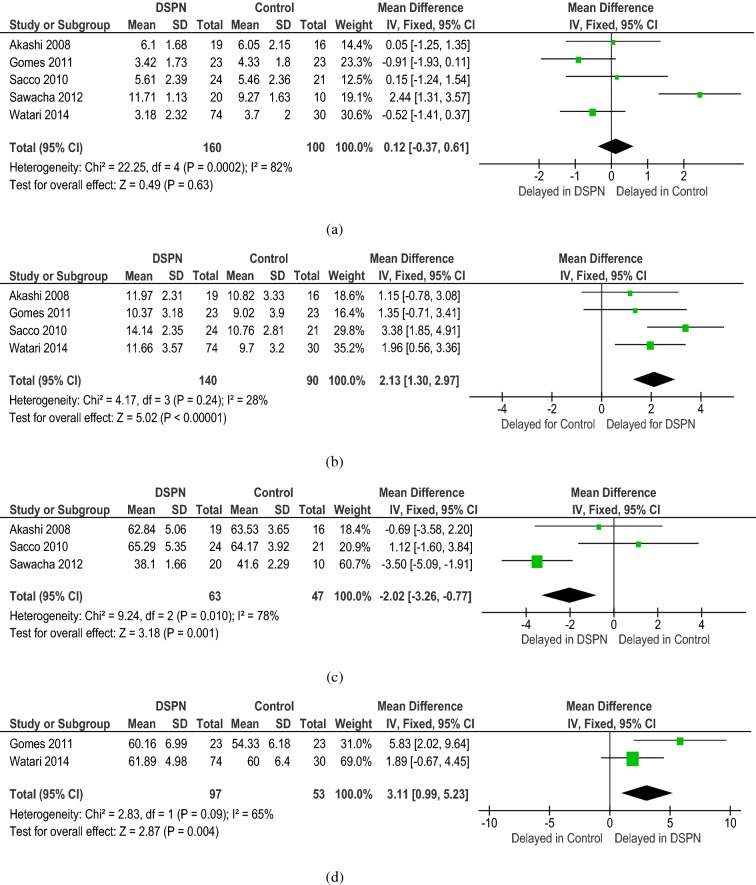
EMG36–40 is widely used in different clinical research and trials to diagnose DSPN and observe the biomechanics changes in different muscle activities due to DSPN. EMG activities from lower limb muscles were used in five studies36–40 with a total of 333 participants (170 patients with DSPN, 100 controls, and 63 non-DSPN patients). In all the included studies, time to peak occurrence (from 0 to 100% of stance phase) for VL, TA, LG, and GM were used as diagnostic parameters for all three experimental groups (Table 5). The time of muscle peak activity occurrence was longer for VL (12.11 ± 3.42), LG (57.57 ± 12.91), and GM (61.48 ± 5.53) but reduced for TA (4.97 ± 3.37) in the DSPN group compared with those in the control group (9.97 ± 3.36 for VL, 59.15 ± 9.86 for LG and 57.54 ± 6.86 for GM). In the DSPN and non-DSPN groups, meta-analysis suggested a non-significantly longer time for peak occurrence in TA (4.97 ± 3.37, 5.08 ± 2.44) and GM (61.48 ± 5.53, 60.5 ± 5.1) and no difference in VL. Given the absence of studies on the LG muscle in non-DSPN patients, meta-analysis was not possible.
Table 5.
| Study | Group | N | Time of peak occurrence (%) | |||
|---|---|---|---|---|---|---|
| Vastus lateralis (VL) | Tibialis anterio (TA) | Lateral gastrocnemius (LG) | Gastrocnemius medialis (GM) | |||
| Sacco et al.36 | Control | 21 | 10.76 ± 2.81 | 5.46 ± 2.36 | 64.17 ± 3.92 | – |
| DSPN | 24 | 14.14 ± 2.35 | 5.61 ± 2.39 | 65.29 ± 5.35 | – | |
| Sawacha et al.37 | Control | 10 | – | 9.27 ± 1.63 | 41.60 ± 2.29 | – |
| No-DSPN | 20 | – | 6.96 ± 1.10 | 35.9 ± 1.38 | – | |
| DSPN | 20 | – | 11.71 ± 1.13 | 38.1 ± 1.66 | – | |
| Akashi et al.38 | Control | 16 | 10.82 ± 3.33 | 6.05 ± 2.15 | 63.53 ± 3.65 | – |
| DSPN | 19 | 11.97 ± 2.31 | 6.10 ± 1.68 | 62.84 ± 5.06 | – | |
| DSPN-U | 10 | 14.83 ± 3.53 | 4.64 ± 1.59 | 68.00 ± 4.78 | – | |
| Gomes et al.39 | Control | 23 | 9.02 ± 3.90 | 4.33 ± 1.80 | – | 54.33 ± 6.18 |
| DSPN | 23 | 10.37 ± 3.18 | 3.42 ± 1.73 | – | 60.16 ± 6.99 | |
| Watari et al.40 | Control | 30 | 9.7 ± 3.2 | 3.7 ± 2.0 | – | 60.0 ± 6.4 |
| No-DSPN | 43 | 12.1 ± 2.3 | 4.2 ± 2.4 | – | 60.5 ± 5.1 | |
| DSPN | 74 | 11.66 ± 3.57 | 3.18 ± 2.32 | – | 61.89 ± 4.98 | |
| Total | Control | 100 | 9.97 ± 3.36 | 5.15 ± 2.57 | 59.15 ± 9.86 | 57.54 ± 6.86 |
| No-DSPN | 63 | 12.1 ± 2.3 | 5.08 ± 2.44 | – | 60.5 ± 5.1 | |
| DSPN | 170 | 12.11 ± 3.42 | 4.97 ± 3.37 | 57.57 ± 12.91 | 61.48 ± 5.53 | |
Meta-analysis of the diagnostic parameters of DSPN
PMNCV, PMNamp, SSNCV, and SSNamp for NCS; NFD, NBD and NFL for CCM; and time to peak occurrence for the VL, TA, LG, and GM and VTP for EMG were subjected to statistical analysis to find significant differences between different screening variables, which can be considered as a substitute for statistically significant paired parameters. Table S1 shows that that for control group, the SSNamp and NFL (p = 0.45); time to peak occurrence for LG and NFD (p = 0.37); PMNCV and SSNCV (p = 0.82); and PMNamp and VPT (p = 1.00) were not statistically significant. For the non-DSPN experimental groups, PMNCV and SSNCS (p = 0.12); time to peak occurrence for VL and SSNamp (p = 0.71); PMNamp and VPT (p = 0.28); PMNamp and time to peak occurrence for TA (p = 0.16); time to peak occurrence for LG and SSNamp (p = 0.47); and time to peak occurrence for VL and LG (p = 0.47) were not statistically significant (Table S2). For the DSPN experimental group, PMNCV and NFD (p = 0.29); time to peak occurrence for VL and NFL (p = 0.68); VPT and NFD (p = 0.44); VPT and PMNCV (p = 0.16); and SSNamp and time to peak occurrence for TA (p = 0.15) (Table S3) were statistically insignificant.
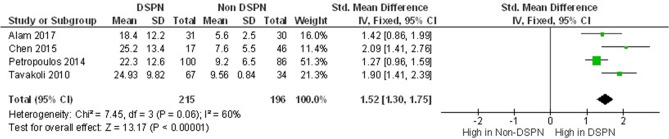
All the diagnostic parameters from the included studies were subjected to meta-analysis. The meta-analysis showed good heterogeneity for the following NCS parameters: PMNCV (p < 0.001), SSNCV (p < 0.001), PMNamp (p < 0.001), and SSNamp (p < 0.001) (Fig. 2). The meta-analysis of the CCM parameters of DSPN and non-DSPN groups exhibited good heterogeneity for CNFL (p < 0.001), CNFD (p < 0.001), and CNBD (p < 0.001) (Fig. 3). Given that not all the included studies reported the EMG parameters for non-DSPN patients, meta-analysis was conducted with EMG parameters for the control and DSPN groups. Four different lower limb muscles, namely, the TA (p = 0.63), VL (p < 0.001), LG (p = 0.001), and GM (p = 0.004), were considered for meta-analysis as illustrated in Fig. 4. The time to achieve muscle activation peak for the TA muscle in the DSPN and control groups was not statistically significant, and the meta-analysis indicated low heterogeneity for the VL muscle. GM and LG muscles have moderate and Ta muscles showed good heterogeneity. VPT (p < 0.001) also exhibited moderate heterogeneity (Fig. 5).
Figure 2.
Forest plots for NCS diagnosis parameters comparing DSPN and Non-DSPN groups (a) PMNCV, (b) SSNCV, (c) SSNamp, (d) PMNamp.
Figure 3.
Forest plots for CCM diagnosis parameters comparing DSPN and Non-DSPN groups (a) CNFL, (b) CNFD, (c) CNBD.
Figure 4.
Forest plots for EMG parameters of time for muscle activation peak for four different lower limb muscles (a) TA, (b) VL, (c) LG, (d) GM for DSPN and control groups.
Figure 5.
Forest plots for vibration perception threshold (VPT) comparing DSPN and Non-DSPN groups.
TSA for DSPN diagnostic parameters
TSA was conducted on the NCS and CCM parameters of DSPN and non-DSPN groups and on the EMG parameters of the DSPN and control groups. TSA was performed on all 12 diagnostic parameters that were selected for meta-analysis. Although the pooled effective size did not exceed the RIS, the TSA established sufficient and conclusive evidence. Figures S1 and S2 illustrate the TSA results for NCS and the CCM parameters of the DSPN and non-DSPN groups. The TSA on all NCS and CCM parameters indicated that the cumulative Z-curve crossed the conventional boundary for benefit and the trial sequential monitoring boundary for benefit, demonstrating that the results were robust and conclusive and further studies were not required. TSA exhibited conclusive results for the EMG parameter of the time to obtain the activation peak in the VL muscle (Fig. S3). However, studies on the TA, LG, and GM muscles of the DSPN and control groups were not conclusive because the Z-curves were located between the TSA monitoring boundaries, indicating the involvement of insufficient information in the meta-analysis (Fig. S3). Additional relevant studies are necessary to prove the significance of EMG in the diagnosis of DSPN. Figure S4 shows the TSA results for VPT for the DSPN and non-DSPN groups. The Z-value crossed the TSA monitoring boundaries, indicating that the studies included for meta-analysis were conclusive.
Discussion
Diabetic Neuropathy (DN), one of the major complications of patients with DM53, has attracted the attention of researchers for past few decades. DSPN is the most common distal and symmetrical form of DN. Over the years, a vast range of diagnostic tools for DSPN (symptom scores, QST, and electrophysiology) have been introduced by researchers. The evaluation of DSPN by using clinical assessment instruments is simple and inexpensive, but the obtained results vary during reproduction. Thus, their accuracy remains questionable. According to the position statement of the American Diabetic Association (ADA)54, combining clinical history and examination is highly suggested for the clinical diagnosis of DSPN. However, the evaluation of DSPN through clinical history and examination varies due to the lack of standardized baselines. The identification of the appropriate patient population is critical for the valid and careful diagnosis of DSPN in clinical research. The ADA recommended the use of validated clinical instruments combined with electrophysiology and measurements of small-fiber damage and repair obtained via NCS or CCM55. Therefore, researchers and health professionals have conducted different clinical studies on DSPN by using different screening methods and, in many cases, two or more methods, to diagnose DSPN accurately9,10. Therefore, this work aimed to analyze the existing literature on clinical studies on DSPN to help understand the characteristics of patients and the nature of different screening parameters for control, non-DSPN, and DSPN groups. This review also focused on finding statistically significant relationships among different diagnostic parameters that have been reported in the literature for identifying DSPN.
Three noninvasive electrophysiological methods for the diagnosis of DSPN, i.e., NCS, CCM, and EMG, were considered for this review and meta-analysis because this work aimed to understand the effect of diagnostic parameters in the control, non-DSPN, and DSPN groups. The following diagnostic parameters were considered for meta-analysis and systematic review: PMNCV, PMNamp, SSNCV, and SSNamp for NCS; NFD, NBD, and NFL for CCM; and time to peak occurrence (from 0 to 100% of the stance phase) for VL, TA, LG, and GM muscles for EMG and VPT.
The 19th annual Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes (NEURODIAB) identified NCS as the first objective quantitative indication of DSPN56. NCS, a noninvasive method, has been recommended for epidemiologic surveys or controlled clinical trials on DSPN as an early and reliable indicator of the occurrence of this neuropathy56. NCS has been used as a standardized method for identifying patients with DSPN and validating the performances of other methods19,24,26–28,30,46–50. This meta-analysis revealed that the DSPN group showed reduced NCV and NA in SS and PM nerves compared with the other two experimental groups. This result satisfied and validated the accuracy of the included studies for NCS.
CCM is a new noninvasive method that has been widely used in clinical studies on identifying small-fiber neuropathy19–31. CCM involves the use of in vivo images to study the corneal structure in corneal disease identification. Small-fiber DSPN affects sensitive nerve fibers in the human cornea, and CCM has shown good sensitivity in identifying small-fiber DSPN at a very early stage. Many review studies have been conducted to describe different approaches for CCM imaging and observed the clinical correlation of CCM in the assessment of DSPN15,57,58. The use of CCM is increasing rapidly given its advantages in the assessment of DSPN at an early stage. Therefore, we considered CCM in our meta-analysis as one of the methods for DSPN diagnosis. The meta-analysis revealed that the CCM parameters of the DSPN group were drastically reduced compared with those of the non-DSPN and control groups.
EMG55,56,59–62 is an electrophysiological method that measures the electrical activity of muscles. It36–40 has been used to evaluate the change in a muscle’s electrical activity to diagnose DSPN in clinical research. Compared with other groups, the DSPN group showed greater stance phase time63–65 and decreased and delayed lower limb muscle activity; in particular, the VL, TA, and GM are the most affected by the progression of neuropathy38,66. Thus, EMG36–40 is widely used in different clinical research and trials to diagnose DSPN and observe the biomechanics changes in different muscle activities due to DSPN. Akashi et al.38 reported that patients with DSPN and ulceration show delayed activation peak in the VL and LG muscles. Gomes et al.39 reported delayed activation peak in the VL, TA, GM, and fibularis longus muscles during gait.
In this meta-analysis, the time to peak occurrence (from 0 to 100% of the stance phase) for four lower limb muscles (VL, TA, LG, and GM) were considered as diagnostic parameters for all three experimental groups. All these muscles showed changes in activities due to DSPN. The time of peak muscle activity occurrence was longer for the VL, LG, and GM but reduced for the TA in the DSPN group compared with those in the control group. Our meta-analysis suggested that compared with that in the DSPN and control groups, the peak occurrence in the TA and GM was non-significantly longer and that for VL in the DSPN group did not differ. A meta-analysis was not possible due to lack of studies on the LG muscle of the non-DSPN groups.
All the parameters’ mean values, which were calculated from all the included studies, were subjected to Student’s t-test to observe statistically significant differences between the parameters of three diagnostic methods (NCS, CCM, and EMG) for different experimental groups (control, non-DSPN, and DSPN groups). For the DSPN experimental group, PMNCV and NFD (p = 0.29); time to peak occurrence for VL and NFL (p = 0.68); VPT and NFD (p = 0.44); VPT and PMNCV (p = 0.16); and SSNA and time to peak occurrence for TA (p = 0.15) were statistically non-significant. For each experimental group, most of the parameters showed statistically significant difference between each other. However, no specific pattern for statistically non-significant parameter pairs was found among the three different experimental groups. This analysis indicated that the accuracy of the diagnostic methods is doubtable if any two methods with statistically non-significant parameters are considered for the diagnosis of DSPN. Further analysis is required to understand the difference in statistical patterns for all the screening variables among the three experimental groups.
Given that the values of diagnostic parameters changed depending on the different conditions of the patients and environments, obtaining a baseline value for each experimental group can be challenging. All the diagnostic parameter values from the included studies were used to find the summarized value of each parameter. After finding the summarized value of each parameter, meta-analysis was used to identify the heterogeneity of this observation. We conducted our meta-analysis for each parameter of three different diagnostic methods (NCS, CCM, and EMG) that have been widely used for clinical researches on DSPN. The studies included in our meta-analysis on NCS and CCM parameters (all p < 0.001) for DSPN and non-DSPN groups showed good heterogeneity, indicating that the effect of the included studies were acceptable for obtaining a conclusion on the baseline values for each diagnostic parameters. However, for EMG, few studies have reported the time to delay in activation peak for the non-DSPN group. This situation prevented us from conducting a meta-analysis. Therefore, we conducted the meta-analysis on the DSPN and control groups for EMG, and all three lower limb muscles (TA, LG and GM), except for VL, showed good heterogeneity. Moreover, we recommend adding clinical trials for studying patients EMG from lower limbs to understand the change in muscle activity due to DSPN. The time to muscle activation peak in DSPN and control group for the TA muscle was not statistically significant, and the meta-analysis exhibited low heterogeneity for the VL and GM muscles. Meta-analysis on the included studies reporting VPT revealed moderate heterogeneity, indicating that studies must be added to obtain robust conclusion. Good heterogeneity indicates that the included studies have variations in the data and that the baseline values calculated from the included studies can be considered as reliable standardized values.
A number of reviews have been conducted on different diagnostic methods of DSPN. Jiang et al.8 conducted a meta-analysis on CCM for the assessment of DSPN. They found that all the CCM parameters, except for nerve fiber tortuosity coefficient, were significantly reduced in the DSPN group relative to that in the control and non-DSPN groups. In our meta-analysis, we also observed that all the CCM parameters decreased in the DSPN group. Fernando et al.45 reviewed the biomechanical characteristics of DSPN. Although they considered the EMG dynamics of the three studies, in their meta-analysis, they only observed the TA muscle of the DSPN and control group. We considered five studies and the time to activation peak occurrence of four lower limb muscles in three different experimental groups. Li et al.67 observed the correlation among three diabetic microvascular diseases, namely, DN, diabetic retinopathy, and diabetic kidney disease, but did not focus on diagnostic methods. One drawback of all these studies is that they did not conduct TSA to verify the conclusiveness of their meta-analysis. Shabeeb et al.68 systematically reviewed electrophysiological examinations for DSPN. They summarized the list of studies using NCS and EMG diagnostic methods reported over 2008 to 2018 and suggested the use of electrophysiological studies for the assessment of DSPN. However, their study have not conducted any meta-analysis. To the best of our knowledge, this is the first study that have conducted meta-analysis with trial sequential analysis and observed statistically significant differences among noninvasive electrophysiological methods for the assessment of DSPN.
TSA was conducted to validate the meta-analysis and hence prove the validity of the calculated baseline values of each diagnostic parameter for three experimental groups. TSA is used to decide if the results from any meta-analysis are conclusive or not. All the 12 diagnostic parameters that were selected for meta-analysis were subjected to TSA. For NCS, CCM and VPT parameters, TSA has been observed for DSPN and non-DSPN groups. Studies involving DSPN and control groups were considered for the TSA of EMG parameters. Although the pooled effective size did not exceed the RIS, TSA established sufficient and conclusive evidence and indicated that no further observational trials are required for NCS and CCM parameters, the meta-analysis depicted conclusive observational evidence, and the analytical findings are sufficiently robust as baseline values for future studies. However, the results for the EMG parameters of four different muscles were inconclusive, and additional trials are needed to understand the effect of DSPN on lower limb muscles. TSA results for VPT were conclusive. Moderate heterogeneity from the meta-analysis and a conclusive TSA for VPT indicated that even though the included studies exhibited visible difference in the data of patient groups, additional studies should be included in the meta-analysis to obtain a solid conclusive result.
One major limitation of this study is that only five study have been found in the literature those have considered lower limb EMG to investigate change in muscle activity due to DSPN as a diagnosis criteria, which leads to the poor heterogeneity, and inconclusive meta-analysis for EMG parameters. Additional studies must be conducted to observe the effect of time to peak occurrence in lower limb muscles due to the progression of DSPN. Another drawback of this study is all the sensory and motor nerve parameters should be studied to understand the propagation of DSPN in different nervous systems. In conclusion, this systematic review and meta-analysis considered a larger sample size for each diagnostic method than individual studies. The results for NCS and CCM showed that the included studies had potentially variable data, and the meta-analysis showed good heterogeneity. This study can be a have a promising effect for the upcoming research work to understand the effect of the three noninvasive electrophysiological methods on DSPN identification.
Supplementary Information
Acknowledgements
This study was made possible by Universiti Kebangsaan Malaysia (UKM) Research grant, Grant Number: number DIP-2020-004, and DIP-2018-017 and the Qatar National Research Fund grant, Grant Number: NPRP12s-0227-190164.
Abbreviations
- ADA
American Diabetic Association
- BMI
Body Mass Index
- CCM
Corneal confocal microscopy
- CI
Confidence interval
- DN
Diabetic neuropathy
- DSPN
Diabetic sensorimotor polyneuropathy
- EMG
Electromyography
- FL
Fibularis longus
- GM
Gastrocnemius medialis
- HbA1c
Glycated hemoglobin
- IENF
Intra-epidermal nerve fiber
- LG
Lateral gastrocnemius
- NCV
Nerve conduction velocity
- NCS
Nerve conduction studies
- NA
Nerve amplitude
- NFD
Nerve fiber density
- NBD
Nerve branch density
- NFL
Nerve fiber length
- PM
Peroneal motor
- PMNCV
Peroneal motor nerve conduction velocity
- PMNamp
Peroneal motor nerve amplitude
- QST
Quantitative sensory testing
- RIS
Required information size
- SD
Standard deviation
- SMD
Standard mean difference
- SS
Sural sensory
- SSNCV
Sural sensory nerve conduction velocity
- SSNamp
Sural sensory nerve amplitude
- TA
Tibialis anterior
- TSA
Trial sequential analysis
- VPT
Vibration perception threshold
- VL
Vastus lateralis
Author contributions
F.H. contributed to acquisition of data, interpreted the data carried out for the meta-analysis, statistical analysis of the study, and drafted the manuscript. M.B.I.R. participated in the design of the study and contributed to data analysis and manuscript preparation. S.H.M.A. and N.A. contributed to revise the article and the new information inclusion based on the suggestion of the reviewers and M.E.H.C. participated in design and coordination of this study and helped to draft the manuscript and finalize the manuscript for submission. All authors read and approved the final manuscript.
Funding
Study Funded by Universiti Kebangsaan Malaysia (UKM) under the Grant Number DIP-2020-004, and DIP-2018-017 and the Qatar National Research Fund, Grant Number: NPRP12s-0227-190164).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mamun Bin Ibne Reaz, Email: mamun@ukm.edu.my.
Muhammad Enamul Hoque Chowdhury, Email: mchowdhury@qu.edu.qa.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78787-0.
References
- 1.Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathyCl vs. NPhys trial. Muscle Nerve. 2010;42:157–164. doi: 10.1002/mus.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817–824. doi: 10.1212/WNL.43.4.817. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin RH, Malone DC, Panarites CJ, Armstrong EP, Pham SV. Impact of postherpetic neuralgia and painful diabetic peripheral neuropathy on health care costs. J. Pain. 2010;11:360–368. doi: 10.1016/j.jpain.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Contin. Lifelong Learn. Neurol. 2012;18:192–198. doi: 10.1212/01.CON.0000411562.03591.74. [DOI] [PubMed] [Google Scholar]
- 6.Hsu WC, Chiu SY, Yen AM, Chen LS, Fann CY, et al. Somatic neuropathy is an independent predictor of all- and diabetes-related mortality in type 2 diabetic patients: a population-based 5-year follow-up study (KCIS No. 29) Eur. J. Neurol. 2012;19(9):1192–1198. doi: 10.1111/j.1468-1331.2011.03659.x. [DOI] [PubMed] [Google Scholar]
- 7.Soedamah-Muthu S, Chaturvedi N, Witte D, Stevens L, Porta M, et al. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB prospective complications study (PCS) Diabetes Care. 2008;31:1360–1366. doi: 10.2337/dc08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang MS, Yuan Y, Gu ZX, Zhuang SL. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysi. Br. J. Ophthalmol. 2016;100(1):9–14. doi: 10.1136/bjophthalmol-2014-306038. [DOI] [PubMed] [Google Scholar]
- 9.Petropoulos IN, Ponirakis G, Khan A, Almuhannadi H, Gad H, Malik RA. Diagnosing diabetic neuropathy: something old, something new. Diabetes Metab. J. 2018;42(4):255–269. doi: 10.4093/dmj.2018.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle Nerve. 2002;25(2):212–217. doi: 10.1002/mus.10044. [DOI] [PubMed] [Google Scholar]
- 11.Hoitsma E, Reulen JP, de Baets M, Drent M, Spaans F, Faber CG. Small fiber neuropathy: a common and important clinical disorder. J. Neurol. Sci. 2004;227:119–130. doi: 10.1016/j.jns.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Nebuchennykh M, Loseth S, Lindal S, Mellgren SI. The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J. Neurol. 2009;256:1067–1075. doi: 10.1007/s00415-009-5065-y. [DOI] [PubMed] [Google Scholar]
- 13.Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology. 2009;54:273–285. doi: 10.1111/j.1365-2559.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- 14.Perkins B, Bril V. Electrophysiologic testing in diabetic neuropathy. Handb. Clin. Neurol. 2014;126:235–248. doi: 10.1016/B978-0-444-53480-4.00018-7. [DOI] [PubMed] [Google Scholar]
- 15.Tavakoli M, Petropoulos IN, Malik RA. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J. Diabet. Sci. Technol. 2013;7(5):1179–1189. doi: 10.1177/193229681300700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reaz MBI, Hussain MS, Mohd-Yasin F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proc. Online. 2006;8(1):11–35. doi: 10.1251/bpo115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy”. Endocrinol. Metab. 2016;31(2):230–238. doi: 10.3803/EnM.2016.31.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, et al. Prevalence and clinical characteristics of diabetic peripheral neuropathy in hospital patients with type 2 diabetes in Korea. Diabet. Med. 2012;29:290–296. doi: 10.1111/j.1464-5491.2012.03697.x. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Bril V, Orszag A, Paulson J, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35(4):821–828. doi: 10.2337/dc11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin. Exp. Optom. 2012;95(3):348–354. doi: 10.1111/j.1444-0938.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 21.Hertz P, Bril V, Orszag A, Ahmed A, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet. Med. 2011;28(10):1253–1260. doi: 10.1111/j.1464-5491.2011.03299.x. [DOI] [PubMed] [Google Scholar]
- 22.Malik RA, Kallinikos P, Abbott CA, Van Schie CHM, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 23.Mehra S, Tavakoli M, Kallinikos PA, Efron N, et al. Transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30(7):2608–2612. doi: 10.2337/dc07-0870. [DOI] [PubMed] [Google Scholar]
- 24.Petropoulos IN, Alam U, Fadavi H, Marshall A, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014;55(4):2062–2070. doi: 10.1167/iovs.13-12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchard N, Edwards K, Dehghani C, Fadavi H, et al. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res. Clin. Pract. 2014;104(2):248–256. doi: 10.1016/j.diabres.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–2154. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 27.Sivaskandarajah GA, Halpern EM, Lovblom LE, et al. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care. 2013;36(9):2748–2755. doi: 10.2337/dc12-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavakoli M, Quattrini C, Abbott C, Kallinikos P, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792–1797. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavakoli M, Kallinikos P, Iqbal A, Herbert A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet. Med. 2011;28(10):1261–1267. doi: 10.1111/j.1464-5491.2011.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Che X, Graham J, Dabbah MA, Petropoulos IN, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138–1144. doi: 10.2337/dc14-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Zhong Y, Zhang T, Zhang R, et al. Quantitative analysis of corneal nerve fibers in type 2 diabetics with and without diabetic peripheral neuropathy: comparison of manual and automated assessments. Diabetes Res. Clin. Pract. 2019;151:33–38. doi: 10.1016/j.diabres.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 32.De Souza RJ, De Souza A, Nagvekar MD. Nerve conduction studies in diabetics presymptomatic and symptomatic for diabetic polyneuropathy. J. Diabetes Complic. 2015;29(6):811–817. doi: 10.1016/j.jdiacomp.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS ONE. 2017;12:1–16. doi: 10.1371/journal.pone.0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain G, Rizvi SAA, Singhal S, Zubair M, Ahmad J. Cross sectional study to evaluate the effect of duration of type 2 diabetes mellitus on the nerve conduction velocity in diabetic peripheral neuropathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2014;8(1):48–52. doi: 10.1016/j.dsx.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Weisman A, Bril V, Ngo M, Lovblom LE, et al. Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PLoS ONE. 2013;8(3):1–9. doi: 10.1371/journal.pone.0058783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacco C, Akashi PM, Hennig EM. A comparison of lower limb EMG and ground reaction forces between barefoot and shod gait in participants with diabetic neuropathic and healthy controls. BMC Musculoskelet. Disord. 2010 doi: 10.1186/1471-2474-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawacha Z, Spolaor F, Guarneri G, Contessa P, et al. Abnormal muscle activation during gait in diabetes patients with and without neuropathy. Gait Posture. 2012;35(1):101–105. doi: 10.1016/j.gaitpost.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Akashi PMH, Sacco ICN, Watari R, Hennig E. The effect of diabetic neuropathy and previous foot ulceration in EMG and ground reaction forces during gait. Clin. Biomech. 2008;23(5):584–592. doi: 10.1016/j.clinbiomech.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Gomes AA, Onodera AN, Otuzi MEI, Pripas D, et al. Electromyography and kinematic changes of gait cycle at different cadences in diabetic neuropathic individuals. Muscle Nerve. 2011;44(2):258–268. doi: 10.1002/mus.22051. [DOI] [PubMed] [Google Scholar]
- 40.Watari R, Sartor CD, Picon AP, Butugan MK, et al. Effect of diabetic neuropathy severity classified by a fuzzy model in muscle dynamics during gait. J. Neuroeng. Rehabil. 2014;11(1):1–9. doi: 10.1186/1743-0003-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins BA, Bril V. Diabetic neuropathy: a review emphasizing diagnostic methods. Clin. Neurophysiol. 2003;114(7):1167–1175. doi: 10.1016/S1388-2457(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 42.Sveen KA, Karimé B, Jørum E, Mellgren SI, Fagerland MW, Monnier VM, et al. Small- and large-fiber neuropathy after 40 years of type 1 diabetes: associations with glycemic control and advanced protein glycation: the Oslo study. Diabetes Care. 2013;36:3712–3717. doi: 10.2337/dc13-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauria G, Devigili G. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat. Clin. Pract. Neurol. 2007;3:546–557. doi: 10.1038/ncpneuro0630. [DOI] [PubMed] [Google Scholar]
- 44.Lauria G, Hsieh ST, Johansson O, Kennedy WR, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Eur. J. Neurol. 2010;17:903–912. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 45.Fernando M, Crowther R, Lazzarini P, Sangla K, et al. Biomechanical characteristics of peripheral diabetic neuropathy: a systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clin. Biomech. 2013;28(8):831–845. doi: 10.1016/j.clinbiomech.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Andersen H, Stålberg E, Gjerstad MD, Jakobsen J. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle Nerve. 1998;21:1647–1654. doi: 10.1002/(SICI)1097-4598(199812)21:12<1647::AID-MUS4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 47.Kane NM, Oware A. Nerve conduction and electromyography studies. J. Neurol. 2012;259:1502–1508. doi: 10.1007/s00415-012-6497-3. [DOI] [PubMed] [Google Scholar]
- 48.Al-Shekhlee A, Shapiro BE, Preston DC. Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve. 2003;27:517–526. doi: 10.1002/mus.10315. [DOI] [PubMed] [Google Scholar]
- 49.Lesser EA, Starr J, Kong X, Megerian JT, Gozani SN. Point-of-service nerve conduction studies: an example of industry-driven disruptive innovation in health care. Perspect. Biol. Med. 2007;50:40–53. doi: 10.1353/pbm.2007.0007. [DOI] [PubMed] [Google Scholar]
- 50.Rubin DI. Technical issues and potential complications of nerve conduction studies and needle electromyography. Neurol. Clin. 2012;30:685–710. doi: 10.1016/j.ncl.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA) Copenhagen: Copenhagen Trial Unit, Centre for Clinical Intervention Research; 2011. pp. 1–115. [Google Scholar]
- 52.Higgins J. P. T., Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. Accessed 3 December 2020.
- 53.Sinnreich M, Taylor BV, Dyck PJB. Diabetic neuropathies: Classification, clinical features, and pathophysiological basis. Neurologist. 2005;11(2):63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
- 54.Pop-Busui R, Boulton AJM, Feldman EL, Bril V, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(1):136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussain MS, Reaz MBI, Mohd-Yasin F, Ibrahimy MI. Electromyography signal analysis using wavelet transform and higher order statistics to determine muscle contraction. Expert Syst. 2009;26(1):35–48. doi: 10.1111/j.1468-0394.2008.00483.x. [DOI] [Google Scholar]
- 56.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P, Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic. Curr. Diabet. Rep. 2013;13:488–499. doi: 10.1007/s11892-013-0390-z. [DOI] [PubMed] [Google Scholar]
- 58.Tavakoli M, Petropoulos IN, Malik RA. Assessing corneal nerve structure and function in diabetic neuropathy. Clin. Exp. Optom. 2012;95(3):338–347. doi: 10.1111/j.1444-0938.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 59.Mamun M, Al-Kadi M, Marufuzzaman M. Effectiveness of wavelet denoising on electroencephalogram signals. J. Appl. Res. Technol. 2013;11(1):156–160. doi: 10.1016/S1665-6423(13)71524-4. [DOI] [Google Scholar]
- 60.Hussain MS, Mamun M. Effectiveness of the wavelet transform on the surface EMG to understand the muscle fatigue during walk. Meas. Sci. Rev. 2012;12(1):28–33. doi: 10.2478/v10048-012-0005-x. [DOI] [Google Scholar]
- 61.Ng, C. L. & Reaz, M. B. I. Capacitive electromyography biosensor with wearable material as an insulator. In 2016 International Conference on Advances in Electrical, Electronic and Systems Engineering, ICAEES, 165–169 (2017).
- 62.Ng CL, Reaz MBI. Evolution of a capacitive electromyography contactless biosensor: design and modelling techniques. Meas. J. Int. Meas. Confed. 2019;145:460–471. doi: 10.1016/j.measurement.2019.05.031. [DOI] [Google Scholar]
- 63.Shaw JE, Van Shie CHM, Carrington AL, Abbott CA, Boulton AJM. An analysis of dynamic forces transmitted through the foot in diabetic neuropathy. Diabetes Care. 1998;21(11):1955–1959. doi: 10.2337/diacare.21.11.1955. [DOI] [PubMed] [Google Scholar]
- 64.Sacco IC, Amadio AC. A study of biomechanical parameters in gait analysis and sensitive cronaxie of diabetic neuropathic patients. Clin. Biomech. 2000;15:196–202. doi: 10.1016/S0268-0033(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 65.Kwon OY, Minor SD, Maluf KS, Mueller MJ. Comparison of muscle activity during walking in subjects with and without diabetic neuropathy. Gait Posture. 2003;18:105–113. doi: 10.1016/S0966-6362(02)00166-2. [DOI] [PubMed] [Google Scholar]
- 66.Abboud RJ, Rowley DI, Newton RW. Lower limb muscle dysfunction may contribute to foot ulceration in diabetic patients. Clin. Biomech. 2000;15:37–45. doi: 10.1016/S0268-0033(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 67.Li J, et al. Correlations among diabetic microvascular complications: a systematic review and meta-analysis. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shabeeb D, et al. Electrophysiological measurements of diabetic peripheral neuropathy: a systematic review. Diabetes Metab. Syndr. Clin. Res. and Rev. 2018;12:591–600. doi: 10.1016/j.dsx.2018.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.