Abstract
Kidney disease is expected to become the fifth leading cause of premature death globally by 2040. Uric acid level is a risk factor for kidney disease. The current study aims to investigate the association between uric acid levels and kidney function in the Korean population. The data of 11,042 participants of the 2016–2017 Korea National Health and Nutrition Examination Survey were analysed. The estimated glomerular filtration rate was calculated using the modification of diet in renal disease formula for Koreans. For each sex, uric acid levels were divided into five subsequent categories of increasing levels (Q1, Q2, Q3, Q4, and hyperuricemia). The association between uric acid level and kidney function was investigated using multiple logistic regression. The results showed that the higher the uric acid levels, the greater the odds of reduced kidney function in both sexes. In men, the adjusted odds ratios (95% confidence intervals) for reduced eGFR comparing the hyperuricemia group to the lowest serum uric acid quartile was 5.55 (3.27–9.44), and in women, the odds ratios (95% confidence intervals) was 7.52 (4.39–12.87). Normal weight or underweight in men and overweight in women, as well as diabetes mellitus, hypertension, and physical inactivity were highly associated with reduced kidney function. Our study revealed a dose–response relationship between uric acid levels and kidney function. Therefore, high uric acid level should be considered as a factor that is potentially related to kidney dysfunction in the Korean population.
Subject terms: Diseases, Endocrinology
Introduction
Kidney disease is a common non-communicable condition that currently affects around 850 million people worldwide. Kidney disease is expected to become the fifth leading cause of premature deaths globally by 2040. One in 10 adults has chronic kidney disease (CKD), and every year, millions die prematurely from complications related to CKD1,2. CKD is a cause of substantial healthcare expenditure, and the global burden of CKD is increasing3. CKD requires hemodialysis or kidney transplantation, reduces quality of life, and involves a high cost for the patient4. Additionally, the risk of cardiovascular complications such as heart attack, stroke, and myocardial infarction is increased in CKD patients5,6.
The kidneys play a major role in filtering body waste and toxins from the blood and discharging them into the urine as well as in maintaining the body’s moisture, electrolyte, and acidity levels and controlling blood pressure. If these kidney functions are impaired and waste is not removed from the blood, a variety of uremic symptoms occur, some which may be life-threatening. Importantly, the kidney has a limited capacity to recover from chronic damage. The kidney performs major functions in the body, and attention should be paid to the fact that by the time symptoms manifest, kidney function is often already reduced by approximately 50%. In the face of this global public health problem, it is important to prevent the side effects of CKD by identifying and managing modifiable risk factors, such as diabetes or high blood pressure7,8.
As the population ages, the number of CKD patients is expected to increase at an accelerated pace, but unfortunately the lack of early diagnosis leads to the loss of opportunities for prevention9. The main indicator of kidney function is the blood creatinine level; creatinine accumulates in the blood, leading to elevated creatinine levels when kidney function is reduced. Kidney function can be best measured using the glomerular filtration rate (GFR), which is a measure of blood filtration by the kidneys. GFR can be easily estimated (eGFR) by measuring the blood creatinine level and considering age, ethnicity, and sex.
Diabetes, hypertension, glomerulonephritis, obesity, and aging have been consistently reported as the risk factors for CKD10–12. The situation with respect to uric acid level, however, is remained widely unexplored. Previously, a few studies have been conducted to investigate the relationship between serum uric acid levels and CKD suggesting that a high uric acid level could play a potential role in onset or progression of kidney disease13–15. According to a study examining the association between serum uric acid levels and kidney disease in Korean men, elevated serum uric acid levels were independently associated with an increased likelihood of CKD16. In addition, a recent study examining the association between gout and the risk of advanced CKD found that patients with gout had a higher incidence of advanced CKD than those without17.
Although studies on the relationship between uric acid levels and kidney function have shown controversial results for a long time, an insufficient number of studies have been performed in the Korean population. Furthermore, previous study has focused mainly on hyperuricemia18,19. Thus, the present study aimed to validate the association between serum uric acid level and CKD and clarify what other factors might play a potential role in developing CKD in the general South Korean population.
Materials and methods
Data collection and study population
Data were derived from the Korea National Health and Nutrition Examination Survey (2016–2017)18. KNHANES is a cross-sectional study design of nationally representative to evaluate the health and nutritional status of the Korean population aged 1 year and over. This survey is conducted annually by the Korean Centers for Disease Control and Prevention (KCDC). KNHANES employs a stratified and multistage cluster sampling design based on geographic area, gender, and age, to select household units. KNHANES contains reliable data on the general population of Korea, and is a valuable resource for development and evaluation health policies and programs in Korea.
The initial study population comprised 16,277 individuals. Among them, 16,232 subjects were selected, and those who were diagnosed with kidney failure by doctor were excluded. In addition, the subjects with missing data on uric acid levels, sex, age, socioeconomic status, or health-related factors were excluded from the study, resulting in a final sample of 11,042 individuals for analysis. KNHANES data is publicly accessible and ethical approval is not required for the use of the data. In addition, as the respondent’s information is completely anonymous, there is no need for prior consent for research purposes.
Measurement of kidney function using the eGFR for the Korean population
The main objective of this study was assessment of kidney function. GFR is an essential aspect of kidney function evaluation. This measurement determines the level of creatinine in the blood and calculates a kidney function score that indicates how well the kidneys are functioning. Glomerular filtration rate indicates the amount of blood filtered per minute by the glomeruli. If kidney function decreases due to injury or disease, the filtration rate decreases, and waste products begin to accumulate in the blood. Clinically, the eGFR is mainly used to detect kidney status. eGFR is calculated on the basis of a serum creatinine test. Creatinine is a muscle waste product that is filtered out of the blood by the kidneys and is released into the urine at a relatively constant rate.
The eGFR was calculated using the modification of diet in renal disease (MDRD) formula. In 2010, a Korean MDRD formula was developed, which was reported to measure the glomerular filtration rate of Koreans more accurately than the conventional MDRD formula. The Korean MDRD formula for the eGFR is as follows: 107.904 × (Creatinine in mg/dL)−1.009 × (age)−0.02 × (0.667 if female)19. Therefore, in this study, kidney function was confirmed using the Korean MDRD formula. The normal range of glomerular filtration rate is about 90–120 mL per minute. A reduction of the GFR can be interpreted as impaired kidney function. According to the eGFR formula, participants with a GFR of less than 90 mL were considered to have reduced kidney function. In order to identify malfunction of kidney function, the dependent variable was designed in the form of a binary division with a cut-off of 90 mL (i.e., decreased function group: < 90 mL/min/1.73 m2, normal group: ≥ 90 mL/min/1.73 m2)2.
Uric acid levels
The main exposure of interest was serum uric acid levels (SUA). Venous blood samples were collected in the morning after an overnight fast. In the KNHANES, SUA was measured by means of a Hitachi automatic analyzer 7600-210 using a colorimetric enzymatic method. SUA levels were then divided into five categories in both sexes: one group for hyperuricemia (> 7.00 mg/dL for men; > 6.00 mg/dL for women20,21), and four groups (Q1, Q2, Q3, and Q4) within the reference range according to sex-specific quartiles. The SUA quartiles were as follows: < 4.80 mg/dL, 4.80–5.49 mg/dL, 5.50–6.09 mg/dL, 6.10–6.99 mg/dL for men; and < 3.70 mg/dL, 3.70–4.19 mg/dL, 4.20–4.79 mg/dL, 4.80–5.99 mg/dL for women. The groups under 4.80 mg/dL (men) and 3.70 mg/dL (women) were set as the reference groups.
Covariates
The following covariates were included in the fully adjusted models because they could be associated with kidney function and uric acid level: age (< 40, 40–49, 50–59, 60–69, ≥ 70), household income group (low, medium–low, medium–high, or high), educational level (high school or under, university or above), region (metropolitan or rural), and occupational categories (white collar, pink collar, blue collar, or unemployed). Household income groups classified as quartiles were calculated by dividing household income by the square root of the number of household members, a standard method recommended by the Organisation for Economic Cooperation and Development22. Occupations were categorised according to the Korean version of the Standard Classification of Occupations based on the International Standard Classification of Occupations by the International Labor Organization23. We restructured the classification into four categories: white (office work), pink (sales and service), blue (agriculture, forestry, fishery, and armed forces occupations), and unemployed.
Health-related covariates included body mass index (normal or underweight, overweight, or obese), waist circumference (normal circumference or abdominal obesity), diabetes mellitus, hypertension, dyslipidaemia, smoking status (non-smoker, ex-smoker, or current smoker), frequency of drinking (never, occasionally, or frequently), physical activity (active or inactive). Waist circumference was divided using the Korean abdominal obesity criteria, which is 90 cm for men and 85 cm for women24. The diagnoses of diabetes mellitus, hypertension, and dyslipidaemia were confirmed by an experienced physician. Physical activity was assessed by the ability of the subject to engage in a moderate-intensity activity for more than 150 min, a high intensity activity for more than 75 min, or a combination of both moderate and high intensity-activity (1 min of high intensity and 2 min of moderate intensity) per week25.
Statistical analysis
Owing to the considerable sex differences in physical functions such as the capacity of female hormones to effectively reduce uric acid levels, all analyses have been stratified by sex. Sampling weights were applied in all data analyses to perform multistage stratified probability sampling of KNHANES. Descriptive analysis was performed to examine the distribution of the general characteristics of the study population. We calculated the frequency and percentages for each variable through the chi-square test. The statistical significance level was set as p-value of lower than 0.05. To identify the association between uric acid levels and kidney function, a multiple logistic regression analysis was performed after adjusting sociodemographic and health-related covariates. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated in order to perform comparisons between subjects with uric acid levels below 4.80 mg/dL (males) or 3.70 mg/dL (females) and subjects with different levels of uric acid (males: 4.80–5.49 mg/dL, 5.50–6.09 mg/dL, 6.10–6.99 mg/dL, over 7.00 mg/dL; females: 3.70–4.19 mg/dL, 4.20–4.79 mg/dL, 4.80–5.99 mg/dL, over 6.00 mg/dL). Subgroup analysis was performed by body mass index (BMI), waist circumference, age, previously diagnosed diabetes, previously diagnosed hypertension, previously diagnosed dyslipidaemia. In addition, multicollinearity was tested using the variance inflation factors. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Table 1 presents the general characteristics of the gender-stratified study population. Overall, 922 (19.0%) of the 4,848 men and 403 (6.5%) of the 6194 women included in the study were considered hyperuricemic respectively. In general, among both men and women, higher uric acid levels were associated with lower kidney function (p < 0.001 for both men and women). Specifically, the percentages of decreased kidney function among men were 6.6%, 9.3%, 10.8%, 15.4%, and 24.8% in the < 4.80, 4.80–5.49, 5.50–6.09, 6.10–6.99, and ≥ 7.00 mg/dL groups, respectively, while among women, the proportions of decreased kidney function were 19.8, 25.9, 35.5, 43.1, and 61.8% in the < 3.70, 3.70–4.19, 4.20–4.79, 4.80–5.99, and ≥ 6.00 mg/dL groups, respectively.
Table 1.
General characteristics of the study population.
| Kidney function: eGFR (mL/min per 1.73 m2)ª | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 4848) | Women (n = 6194) | |||||||||||||
| TOTAL | Decreased functionc | Normal functiond | P value | TOTAL | Decreased function | Normal function | P value | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |||
| Uric acid level (mg/dL)b | < 0.0001 | < 0.0001 | ||||||||||||
| Q1 | 951 | 19.6 | 63 | 6.6 | 888 | 93.4 | 1367 | 22.1 | 270 | 19.8 | 1097 | 80.2 | ||
| Q2 | 949 | 19.6 | 88 | 9.3 | 861 | 90.7 | 1318 | 21.3 | 341 | 25.9 | 977 | 74.1 | ||
| Q3 | 945 | 19.5 | 102 | 10.8 | 843 | 89.2 | 1506 | 24.3 | 535 | 35.5 | 971 | 64.5 | ||
| Q4 | 1081 | 22.3 | 166 | 15.4 | 915 | 84.6 | 1600 | 25.8 | 689 | 43.1 | 911 | 56.9 | ||
| Hyperuricemia | 922 | 19.0 | 229 | 24.8 | 693 | 75.2 | 403 | 6.5 | 249 | 61.8 | 154 | 38.2 | ||
| BMIe | < 0.0001 | 0.001 | ||||||||||||
| Underweight or Normal (< 23.0) | 1610 | 33.2 | 166 | 10.3 | 1444 | 89.7 | 3031 | 48.9 | 950 | 31.3 | 2081 | 68.7 | ||
| Overweight (23.0–24.9) | 1267 | 26.1 | 173 | 13.7 | 1094 | 86.3 | 1267 | 20.5 | 456 | 36.0 | 811 | 64.0 | ||
| Obese (≥ 25.0) | 1971 | 40.7 | 309 | 15.7 | 1662 | 84.3 | 1896 | 30.6 | 678 | 35.8 | 1218 | 64.2 | ||
| Waist circumference | < 0.0001 | |||||||||||||
| Normal | 2133 | 44.0 | 226 | 10.6 | 1907 | 89.4 | 4457 | 72.0 | 1430 | 32.1 | 3027 | 67.9 | ||
| Abdominal obesity | 2715 | 56.0 | 422 | 15.5 | 2293 | 84.5 | 1737 | 28.0 | 654 | 37.7 | 1083 | 62.3 | ||
| Age | < 0.0001 | < 0.0001 | ||||||||||||
| < 40 | 1403 | 28.9 | 116 | 8.3 | 1287 | 91.7 | 1739 | 28.1 | 426 | 24.5 | 1313 | 75.5 | ||
| 40–49 | 911 | 18.8 | 95 | 10.4 | 816 | 89.6 | 1175 | 19.0 | 346 | 29.4 | 829 | 70.6 | ||
| 50–59 | 906 | 18.7 | 85 | 9.4 | 821 | 90.6 | 1206 | 19.5 | 378 | 31.3 | 828 | 68.7 | ||
| 60–69 | 842 | 17.4 | 133 | 15.8 | 709 | 84.2 | 1072 | 17.3 | 422 | 39.4 | 650 | 60.6 | ||
| ≥ 70 | 786 | 16.2 | 219 | 27.9 | 567 | 72.1 | 1002 | 16.2 | 512 | 51.1 | 490 | 48.9 | ||
| Household income | < 0.0001 | < 0.0001 | ||||||||||||
| Low | 828 | 17.1 | 166 | 20.0 | 662 | 80.0 | 1233 | 19.9 | 515 | 41.8 | 718 | 58.2 | ||
| Mid-low | 1157 | 23.9 | 154 | 13.3 | 1003 | 86.7 | 1523 | 24.6 | 491 | 32.2 | 1032 | 67.8 | ||
| Mid-high | 1373 | 28.3 | 165 | 12.0 | 1208 | 88.0 | 1699 | 27.4 | 501 | 29.5 | 1198 | 70.5 | ||
| High | 1490 | 30.7 | 163 | 10.9 | 1327 | 89.1 | 1739 | 28.1 | 577 | 33.2 | 1162 | 66.8 | ||
| Educational level | 0.0002 | < 0.0001 | ||||||||||||
| ≤ High school | 2394 | 49.4 | 364 | 15.2 | 2030 | 84.8 | 3704 | 59.8 | 1353 | 36.5 | 2351 | 63.5 | ||
| ≥ College | 2454 | 50.6 | 284 | 11.6 | 2170 | 88.4 | 2490 | 40.2 | 731 | 29.4 | 1759 | 70.6 | ||
| Region | 0.278 | 0.012 | ||||||||||||
| Metropolitan | 2138 | 44.1 | 273 | 12.8 | 1865 | 87.2 | 2725 | 44.0 | 963 | 35.3 | 1762 | 64.7 | ||
| Rural | 2710 | 55.9 | 375 | 13.8 | 2335 | 86.2 | 3469 | 56.0 | 1121 | 32.3 | 2348 | 67.7 | ||
| Occupational categoriesf | < 0.0001 | < 0.0001 | ||||||||||||
| White | 1417 | 29.2 | 159 | 11.2 | 1258 | 88.8 | 1346 | 21.7 | 410 | 30.5 | 936 | 69.5 | ||
| Pink | 483 | 10.0 | 30 | 6.2 | 453 | 93.8 | 890 | 14.4 | 251 | 28.2 | 639 | 71.8 | ||
| Blue | 1620 | 33.4 | 199 | 12.3 | 1421 | 87.7 | 960 | 15.5 | 305 | 31.8 | 655 | 68.2 | ||
| Unemployed | 1328 | 27.4 | 260 | 19.6 | 1068 | 80.4 | 2998 | 48.4 | 1118 | 37.3 | 1880 | 62.7 | ||
| Diabetes mellitus | < 0.0001 | < 0.0001 | ||||||||||||
| Yes | 513 | 10.6 | 121 | 23.6 | 392 | 76.4 | 539 | 8.7 | 262 | 48.6 | 277 | 51.4 | ||
| No | 4335 | 89.4 | 527 | 12.2 | 3808 | 87.8 | 5655 | 91.3 | 1822 | 32.2 | 3833 | 67.8 | ||
| Hypertension | < 0.0001 | < 0.0001 | ||||||||||||
| Yes | 1278 | 26.4 | 307 | 24.0 | 971 | 76.0 | 1374 | 22.2 | 648 | 47.2 | 726 | 52.8 | ||
| No | 3570 | 73.6 | 341 | 9.6 | 3229 | 90.4 | 4820 | 77.8 | 1436 | 29.8 | 3384 | 70.2 | ||
| Dyslipidemia | < 0.0001 | < 0.0001 | ||||||||||||
| Yes | 757 | 15.6 | 143 | 18.9 | 614 | 81.1 | 1215 | 19.6 | 509 | 41.9 | 706 | 58.1 | ||
| No | 4091 | 84.4 | 505 | 12.3 | 3586 | 87.7 | 4979 | 80.4 | 1575 | 31.6 | 3404 | 68.4 | ||
| Smoking status | < 0.0001 | 0.316 | ||||||||||||
| Current smoker | 1712 | 35.3 | 178 | 10.4 | 1534 | 89.6 | 303 | 4.9 | 90 | 29.7 | 213 | 70.3 | ||
| Ex-smoker | 2004 | 41.3 | 326 | 16.3 | 1678 | 83.7 | 353 | 5.7 | 122 | 34.6 | 231 | 65.4 | ||
| Non-smoker | 1132 | 23.3 | 144 | 12.7 | 988 | 87.3 | 5538 | 89.4 | 1872 | 33.8 | 3666 | 66.2 | ||
| Drinking status | < 0.0001 | < 0.0001 | ||||||||||||
| Frequently | 1772 | 36.6 | 204 | 11.5 | 1568 | 88.5 | 708 | 11.4 | 184 | 26.0 | 524 | 74.0 | ||
| Occasionally | 2256 | 46.5 | 286 | 12.7 | 1970 | 87.3 | 3350 | 54.1 | 1077 | 32.1 | 2273 | 67.9 | ||
| Never | 820 | 16.9 | 158 | 19.3 | 662 | 80.7 | 2136 | 34.5 | 823 | 38.5 | 1313 | 61.5 | ||
| Physical activity | 0.044 | 0.071 | ||||||||||||
| Active | 2288 | 47.2 | 282 | 12.3 | 2006 | 87.7 | 2589 | 41.8 | 838 | 32.4 | 1751 | 67.6 | ||
| Inactive | 2560 | 52.8 | 366 | 14.3 | 2194 | 85.7 | 3605 | 58.2 | 1246 | 34.6 | 2359 | 65.4 | ||
| Year | 0.335 | 0.014 | ||||||||||||
| 2016 | 2391 | 49.3 | 331 | 13.8 | 2060 | 86.2 | 3136 | 50.6 | 1074 | 34.2 | 2062 | 65.8 | ||
| 2017 | 2457 | 50.7 | 317 | 12.9 | 2140 | 87.1 | 3058 | 49.4 | 1010 | 33.0 | 2048 | 67.0 | ||
| Total | 4848 | 100.0 | 648 | 13.4 | 4200 | 86.6 | 6194 | 100.0 | 2084 | 33.6 | 4110 | 66.4 | ||
ªGlomerular filtration rate for the Korean population, estimated using the Modification of Diet in Renal Disease equation (mL/min per 1.73 m2) = 107.904 × (Creatinine in mg/dL)−1.009 × (age)−0.02 × 0.667 [if female].
bQ1: < 4.80 mg/dL, < 3.70 mg/dL; Q2: 4.80–5.49 mg/dL, 3.70–4.19 mg/dL; Q3: 5.50–6.09 mg/dL, 4.20–4.79 mg/dL; Q4: 6.10–6.99 mg/dL, 4.80–5.99 mg/dL; Hyperuricemia: ≥ 7.00 mg/dL, ≥ 6.00 mg/dL, respectively.
cDecreased function = eGFR < 90 mL/min per 1.73 m2; dNormal function = eGFR 90 ≥ mL/min per 1.73 m2.
eBMI body mass index; Obesity status defined by BMI based on the 2018 Clinical Practice Guidelines for Overweight and Obesity in Korea.
fThree groups (white, pink, and blue) based on the International Standard Classification of Occupations Codes. Unemployed group includes housewives.
Table 2 shows the logistic regression analysis results for both men and women, adjusted for all covariates. We observed a dose–response relationship between uric acid levels and kidney function in both men and women. In particular, compared with the reference group, the odds ratios (95% CIs) for kidney dysfunction in men were as follows: OR = 1.49 [95% CI 0.92–2.39] for 4.80–5.49 mg/dL; OR = 1.70 [95% CI 1.10–2.64] for 5.50–6.09 mg/dL; OR = 3.02 [95% CI 1.96–4.67] for 6.10–6.99 mg/dL; OR = 5.49 [95% CI 3.64–8.29] for ≥ 7.00 mg/dL. Among women, the odds ratios (95% Cis) across the uric acid level groups in women were as follows: OR = 1.41 [95% CI 1.12–1.77] for 3.70–4.19 mg/dL; OR = 2.24 [95% CI 1.82–2.76] for 4.20–4.79 mg/dL; OR = 3.12 [95% CI 2.52–3.86] for 4.80–5.99 mg/dL; OR = 5.79 [95% CI 4.37–7.66] for ≥ 6.00 mg/dL. In other words, the higher the uric acid levels, the higher the odds ratios for reduced eGFR. The odds ratio was significant in all groups for both sexes except for men in the 4.80–5.49 mg/dL group. The hyperuricemia groups in both genders showed the highest risk of kidney function reduction.
Table 2.
Odds ratio for decreased kidney function.
| Variables | Kidney function: eGFR (mL/min per 1.73 m2)ª | |||
|---|---|---|---|---|
| Men | Women | |||
| Adjusted ORb | 95% CI | Adjusted ORb | 95% CI | |
| Uric acid level (mg/dL)c | ||||
| Q1 | 1.00 | 1.00 | ||
| Q2 | 1.49 | (0.92–2.39) | 1.41 | (1.12–1.77) |
| Q3 | 1.70 | (1.10–2.64) | 2.24 | (1.82–2.76) |
| Q4 | 3.02 | (1.96–4.67) | 3.12 | (2.52–3.86) |
| Hyperuricemia | 5.49 | (3.64–8.29) | 5.79 | (4.37–7.66) |
| BMId | ||||
| Normal or underweight (< 23.0) | 1.00 | 1.00 | ||
| Overweight (23.0–24.9) | 1.45 | (1.05–2.00) | 0.87 | (0.73–1.04) |
| Obese (≥ 25.0) | 1.61 | (1.17–2.22) | 0.74 | (0.60–0.90) |
| Waist circumference | ||||
| Normal | 1.00 | 1.00 | ||
| Abdominal obesity | 0.80 | (0.60–1.05) | 0.90 | (0.73–1.11) |
| Age | ||||
| < 40 | 1.00 | 1.00 | ||
| 40–49 | 1.58 | (1.15–2.18) | 1.42 | (1.17–1.72) |
| 50–59 | 1.36 | (0.93–1.98) | 1.61 | (1.31–1.98) |
| 60–69 | 2.30 | (1.55–3.43) | 2.24 | (1.75–2.88) |
| ≥ 70 | 4.63 | (3.11–6.90) | 3.14 | (2.37–4.17) |
| Household income | ||||
| Low | 1.00 | 1.00 | ||
| Mid-low | 0.94 | (0.66–1.34) | 0.91 | (0.74–1.12) |
| Mid-high | 0.92 | (0.66–1.29) | 0.93 | (0.75–1.15) |
| High | 0.77 | (0.55–1.08) | 1.09 | (0.87–1.36) |
| Educational level | ||||
| ≤ Highschool | 1.00 | 1.00 | ||
| ≥ College | 1.37 | (1.04–1.79) | 1.05 | (0.88–1.25) |
| Region | ||||
| Metropolitan | 1.00 | 1.00 | ||
| Rural | 1.04 | (0.84–1.29) | 0.80 | (0.70–0.92) |
| Occupational categoriese | ||||
| White | 1.05 | (0.77–1.44) | 1.06 | (0.87–1.28) |
| Pink | 0.52 | (0.33–0.81) | 0.81 | (0.66–0.99) |
| Blue | 0.87 | (0.66–1.14) | 0.89 | (0.73–1.09) |
| Unemployed | 1.00 | 1.00 | ||
| Diabetes | ||||
| Yes | 1.30 | (0.98–1.74) | 1.38 | (1.08–1.77) |
| No | 1.00 | 1.00 | ||
| Hypertension | ||||
| Yes | 2.19 | (1.70–2.82) | 1.28 | (1.04–1.56) |
| No | 1.00 | 1.00 | ||
| Dyslipidemia | ||||
| Yes | 0.92 | (0.70–1.23) | 0.96 | (0.80–1.14) |
| No | 1.00 | 1.00 | ||
| Smoking status | ||||
| Current smoker | 1.03 | (0.78–1.37) | 1.37 | (1.03–1.83) |
| Ex-smoker | 0.93 | (0.69–1.25) | 0.92 | (0.69–1.24) |
| Non-smoker | 1.00 | 1.00 | ||
| Drinking status | ||||
| Frequently | 0.91 | (0.70–1.18) | 0.99 | (0.86–1.14) |
| Occasionally | 0.67 | (0.49–0.92) | 0.83 | (0.64–1.08) |
| Never | 1.00 | 1.00 | ||
| Physical activity | ||||
| Active | 1.00 | 1.00 | ||
| Inactive | 0.96 | (0.77–1.18) | 0.97 | (0.84–1.10) |
| Year | ||||
| 2016 | 1.04 | (0.85–1.28) | 1.08 | (0.94–1.25) |
| 2017 | 1.00 | 1.00 | ||
ªModification of Diet in Renal Disease estimated glomerular filtration rate for Korean population (mL/min per 1.73 m2) = 107.904 × (Creatinine in mg/dL)−1.009 × (age)−0.02 × 0.667 [if female].
bOR adjusted for all sociodemographic, economic, health-related factors considered in the study.
Q1: < 4.80 mg/dL, < 3.70 mg/dL; Q2: 4.80–5.49 mg/dL, 3.70–4.19 mg/dL; Q3: 5.50–6.09 mg/dL, 4.20–4.79 mg/dL; Q4: 6.10–6.99 mg/dL, 4.80–5.99 mg/dL; Hyperuricemia: ≥ 7.00 mg/dL, ≥ 6.00 mg/dL, respectively.
dBMI body mass index; Obesity status defined by BMI based on 2018 Clinical Practice Guidelines for Overweight and Obesity in Korea.
eThree groups (white, pink, blue) based on the International Standard Classification Occupations codes. Unemployed group includes housewives.
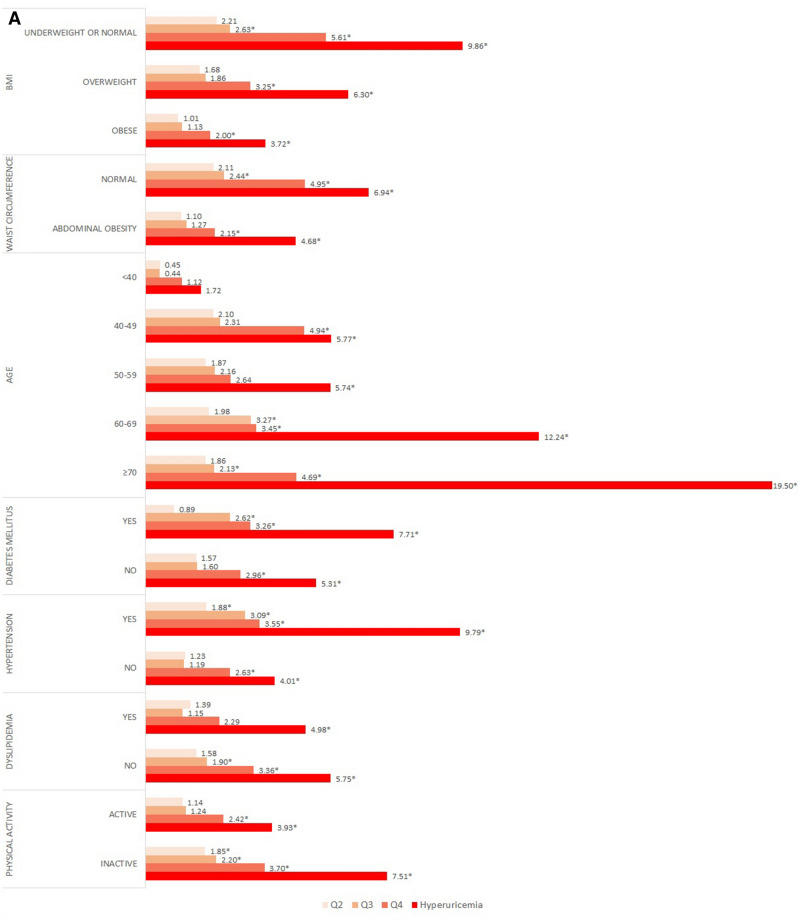
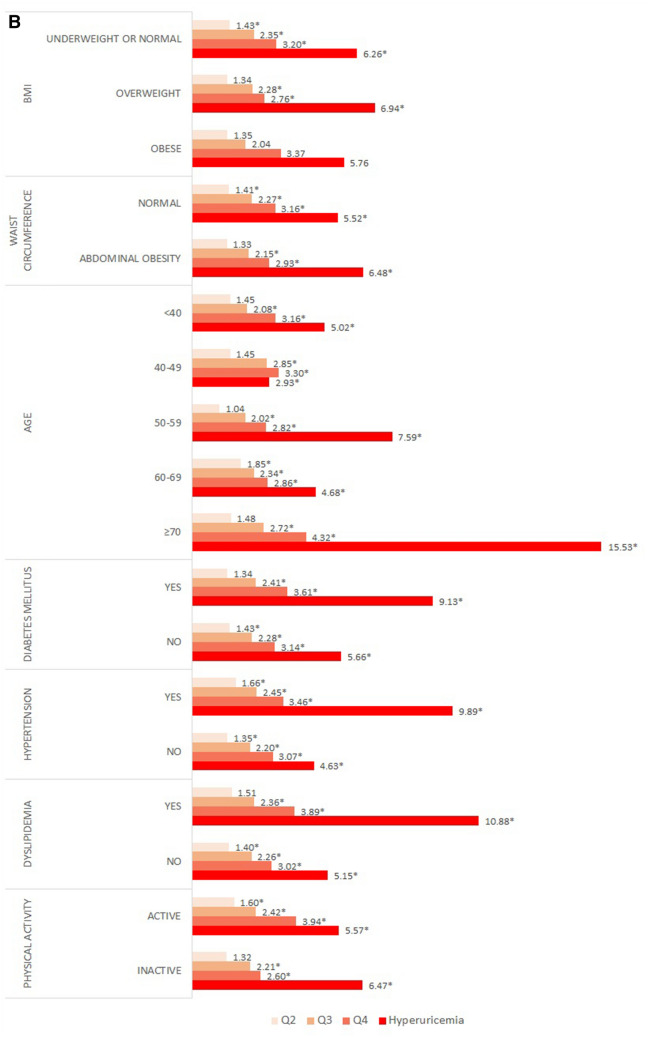
Fig. 1A and B present the results of the subgroup analysis stratified by gender. For BMI, both genders maintained the dose–response relationship in all BMI categories. Among men, the ORs of the normal or underweight group were significantly higher than those of the other groups. In particular, the OR of the hyperuricemia group was 9.86 times higher than that of normal subjects. In contrast, among women, the OR of the hyperuricemia subjects in the overweight group was 6.94 times higher than that of normal subjects.
Figure 1.
Subgroup analysis presenting odds ratio for decreased kidney function stratified by covariates in (A) men, (B) women. †Analysis was adjusted for all sociodemographic, economic, health-related factors considered in the study. ‡* indicate statistically significant results (P < .05).
In the subgroup analysis according to waist circumference and BMI, a dose–response relationship was observed in both the normal and the abdominal obesity groups. Among women, those who had abdominal obesity and hyperuricemia were 6.48 times more likely than normal controls to show decreased kidney function. The likelihood of decreased kidney function in both men and women increased with advancing age. Men who were previously diagnosed with diabetes or hypertension showed significantly higher ORs than did normal subjects; in particular, men with hyperuricemia were 7.71, and 9.79 times higher than the reference group. Women who were previously diagnosed with diabetes, hypertension, and dyslipidaemia had a dose–response relationship between uric acid levels and decreased kidney function. Particularly, in the hyperuricemia group, ORs were the highest at 9.13, 9.89, and 10.88 times. Moreover, subjects with limited physical activity were more likely to have decreased kidney function.
Discussion
The purpose of this study was to examine the association between uric acid levels and kidney function in the Korean population using the Korean MDRD formula using representative KNHANES data. We also conducted a subgroup analysis according to BMI, waist circumference, diabetes, hypertension, dyslipidemia, and physical activity, which are factors related to kidney disease.
In this study, we measured the GFR using the MDRD formula for the Korean population to evaluate kidney function. While the direct measurement of GFR is considered to be the most accurate way to detect changes in kidney function, direct measurement requires special skills and involves complex measurements. Therefore, the eGFR is often used in clinical practice. The MDRD formula was derived from studies on the relationship between protein intake and kidney failure progression in patients with CKD in the United States. However, since the MDRD formula was derived mainly from research conducted in populations of European descent, the accuracy of the formula was not verified for other races26,27. In 2010, a Korean MDRD formula was developed, allowing for more accurate GFR measurement in Koreans than that with the conventional MDRD formula19.
We observed a dose–response relationship between uric acid levels and kidney function in both sexes. Our results indicate that an increasing uric acid level is significantly associated with an increased odds ratio of decreased kidney function. These results are similar to those of earlier studies confirming that high uric acid levels are a risk factor for kidney dysfunction15,27–29. Animal studies have also shown that elevated blood uric acid levels can cause kidney damage26,30.
Although there is controversy about the association between uric acid levels and CKD, there are several possible mechanisms by which higher uric acid levels could play a role in the development of CKD. According to previous studies, increased levels of uric acid, especially hyperuricemia, can contribute to endothelial dysfunction by inducing antiproliferative effects on the endothelium and inhibiting the production of nitric oxide31–33. Over time, damage to the lining of the blood vessels can lead to CKD34. A cohort study indicated that early treatment of hyperuricemia could potentially control CKD35.
Women had a slightly higher risk of kidney dysfunction than men, which is consistent with the findings of previous studies20,36,37. However, other studies have reported that gout and hyperuricemia affect men more commonly38. This is possibly related to the female hormone estrogen, which increases uric acid clearance; indeed, premenopausal women have lower uric acid levels than men, and uric acid levels in women start to rise in the fifth decade of life38–40. In particular, kidney function was significantly reduced in both men and women in the hyperuricemia group. Patients with CKD often have hyperuricemia29,41,42. A recent study revealed that even asymptomatic hyperuricemia that does not develop into gout can affect kidney function unless it is treated with uric acid-lowering drugs43.
BMI and waist circumference are known to affect uric acid levels44. Our subgroup analysis revealed that women with overweight and abdominal obesity have a more pronounced association with kidney disease. Previous studies have also shown that people with obesity have a higher risk of kidney disease45. In addition, uric acid levels and the likelihood of kidney disease development increased with age, as in previous research studies46. Diabetes, hypertension, and dyslipidemia are also powerful risk factors for kidney disease6,34,47. In addition, physical activity was found to be positively associated with kidney function48,49.
This study has several limitations that should be considered when interpreting the results. First, cross-sectional data cannot be used to infer any causal relationships. However, unlike a previous cross-sectional study that utilized hospitalized patients50, our study explored the relationship between uric acid level and kidney function using nationally representative samples of South Korea. Further research is still needed to characterize the longitudinal relationship between uric acid levels and kidney diseases in the South Korean population. Second, factors related to health and behavior, including previously diagnosed diseases, were self-reported. Thus, the possibility of recall bias cannot be discarded, despite the efforts of the surveying agency to reduce such bias. Third, although we adjusted for covariates related to uric acid levels and kidney function, the confounding effect cannot be completely excluded because other confounding variables might exist. Particularly, we did not consider the use of uric acid-lowering drugs such as allopurinol and febuxostat. Fourth, additional formulas, such as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, are available for measuring the eGFR. However, this study did not compare the differences in results using different formulas.
Despite these limitations, our research has several strengths. The use of nationally representative data allows our results to be generalized to the general adult population of South Korea. To the best of our knowledge, few studies has been conducted to investigate the association between uric acid levels and kidney function in the Korean population. Thus, this study is meaningful in that it investigated the correlation between uric acid levels and kidney function according to sex. Furthermore, blood samples were collected using standardized laboratory procedures, thus ensuring an accurate estimate of serum creatinine and uric acid levels.
Conclusion
Our study demonstrated a significant inverse association between high uric acid level and kidney function in the South Korean population. In particular, in the analysis stratified by obesity status, the association was stronger in BMI ≥ 23 group and abdominally obese participants compared with the normal group. Our research shows that uric acid level has a potential role in developing kidney disease. This could help health policymakers and professionals to implement strategies of prevention and intervention for CKD, such as blood screening for patients at a high risk of CKD.
Supplementary information
Author contributions
H.J.J., G.R.K., D.-W.C., J.H.J., and E.-C.P. contributed to the study concept and design. H.J.J., G.,R.K. analyzed and interpreted the data. H.J.J. wrote the manuscript. H.J.J., G.R.K., D.-W.C., J.H.J., and E.-C.P. critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript submitted.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77702-x.
References
- 1.Nasri H. World Kidney Day 2014; Chronic kidney disease and aging: a global health alert. Iran. J. Public Health. 2014;43:126–127. [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 3.Feehally J. Health burden of kidney disease recognized by UN. Nat. Rev. Nephrol. 2012;8:12–13. doi: 10.1038/nrneph.2011.191. [DOI] [PubMed] [Google Scholar]
- 4.Oh TR, et al. Association between health related quality of life and progression of chronic kidney disease. Sci. Rep. 2019;9:19595. doi: 10.1038/s41598-019-56102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 6.Gansevoort RT, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 7.Remuzzi G, Ruggenenti P, Perico N. Chronic renal diseases: renoprotective benefits of renin–angiotensin system inhibition. Ann. Intern. medicine. 2002;136:604–615. doi: 10.7326/0003-4819-136-8-200204160-00010. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, et al. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann. Intern. Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Haroun MK, et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington county, Maryland. J. Am. Soc. Nephrol. 2003;14:2934–2941. doi: 10.1097/01.Asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 11.Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int. Suppl. 2013;3:368–371. doi: 10.1038/kisup.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, et al. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J. Korean Med. Sci. 2009;24(Suppl):S11–S21. doi: 10.3346/jkms.2009.24.S1.S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellomo G, et al. Association of uric acid with change in kidney function in healthy normotensive individuals. Am. J. Kidney Dis. 2010;56:264–272. doi: 10.1053/j.ajkd.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Oh TR, et al. Hyperuricemia has increased the risk of progression of chronic kidney disease: propensity score matching analysis from the KNOW-CKD study. Sci. Rep. 2019;9:6681. doi: 10.1038/s41598-019-43241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obermayr RP, et al. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 2008;19:2407–2413. doi: 10.1681/asn.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryoo J-H, Choi J-M, Oh C-M, Kim M-G. The association between uric acid and chronic kidney disease in Korean men: a 4-year follow-up study. J. Korean Med. Sci. 2013;28:855–860. doi: 10.3346/jkms.2013.28.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stack AG, et al. Gout and the risk of advanced chronic kidney disease in the UK health system: a national cohort study. BMJ Open. 2019;9:e031550. doi: 10.1136/bmjopen-2019-031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kweon S, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES) Int. J. Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CS, et al. Ethnic coefficients for glomerular filtration rate estimation by the modification of diet in renal disease study equations in the Korean population. J. Korean Med. Sci. 2010;25:1616–1625. doi: 10.3346/jkms.2010.25.11.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang J, Alderman MH. Serum uric acid and cardiovascular mortalitythe NHANES I epidemiologic follow-up study, 1971–1992. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 21.Bae JS, et al. The impact of serum uric acid level on arterial stiffness and carotid atherosclerosis: the Korean multi-rural communities cohort study. Atherosclerosis. 2013;231:145–151. doi: 10.1016/j.atherosclerosis.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Oh SS, Jang J-E, Lee D-W, Park E-C, Jang S-I. Cigarette type or smoking history: Which has a greater impact on the metabolic syndrome and its components? Sci. Rep. 2020;10:10467. doi: 10.1038/s41598-020-67524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korea, S. Korean standard classification of occupations. (2000).
- 24.Lee S, et al. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J. Obes. 2006;15:1–9. [Google Scholar]
- 25.An K-Y. Physical activity level in Korean adults: the Korea national health and nutrition examination survey 2017. Epidemiol. Health. 2019;41:e2019047–e2019047. doi: 10.4178/epih.e2019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teo BW, et al. GFR estimating equations in a multiethnic Asian population. Am. J. Kidney Dis. 2011;58:56–63. doi: 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- 27.Satirapoj B, et al. Relationship between serum uric acid levels with chronic kidney disease in a Southeast Asian population. Nephrology (Carlton) 2010;15:253–258. doi: 10.1111/j.1440-1797.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 28.Mohandas R, Johnson RJ. Uric acid levels increase risk for new-onset kidney disease. J. Am. Soc. Nephrol. 2008;19:2251–2253. doi: 10.1681/ASN.2008091012. [DOI] [PubMed] [Google Scholar]
- 29.Chonchol M, et al. Relationship of uric acid with progression of kidney disease. Am. J. Kidney Dis. 2007;50:239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Mazzali M, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 31.Khosla UM, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanellis J, Kang D-H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin. Nephrol. 2005;25:39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Kang D-H, et al. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13:2888–2897. doi: 10.1097/01.ASN.0000034910.58454.FD. [DOI] [PubMed] [Google Scholar]
- 34.Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin. Chim. Acta. 2010;411:1412–1420. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Mok Y, et al. Serum uric acid and chronic kidney disease: the Severance cohort study. Nephrol. Dial. Transpl. 2011;27:1831–1835. doi: 10.1093/ndt/gfr530. [DOI] [PubMed] [Google Scholar]
- 36.Levine W, Dyer AR, Shekelle RB, Schoenberger JA, Stamler J. Serum uric acid and 11.5-year mortality of middle-aged women: findings of the Chicago heart association detection project in industry. J. Clin. Epidemiol. 1989;42:257–267. doi: 10.1016/0895-4356(89)90061-9. [DOI] [PubMed] [Google Scholar]
- 37.Carrero J-J, Hecking M, Ulasi I, Sola L, Thomas B. Chronic kidney disease, gender, and access to care: a global perspective. Semin. Nephrol. 2017;37:296–308. doi: 10.1016/j.semnephrol.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Akizuki S. Serum uric acid levels among thirty-four thousand people in Japan. Ann. Rheumat. Dis. 1982;41:272–274. doi: 10.1136/ard.41.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nan H, et al. The prevalence of hyperuricemia in a population of the coastal city of Qingdao, China. J. Rheumatol. 2006;33:1346–1350. [PubMed] [Google Scholar]
- 40.Mikkelsen WM, Dodge HJ, Valkenburg H, Himes S. The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia: Tecumseh, Michigan 1959–1960. Am. J. Med. 1965;39:242–251. doi: 10.1016/0002-9343(65)90048-3. [DOI] [PubMed] [Google Scholar]
- 41.Ohno I. Relationship between hyperuricemia and chronic kidney disease. Nucleosides Nucleotides Nucleic Acids. 2011;30:1039–1044. doi: 10.1080/15257770.2011.611484. [DOI] [PubMed] [Google Scholar]
- 42.Li L, et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato Y, et al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat. Rev. Nephrol. 2019;15:767–775. doi: 10.1038/s41581-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 44.Ritz E. Metabolic syndrome and kidney disease. Blood Purif. 2008;26:59–62. doi: 10.1159/000110566. [DOI] [PubMed] [Google Scholar]
- 45.Postorino M, Marino C, Tripepi G, Zoccali C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J. Am. Coll. Cardiol. 2009;53:1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 46.Lai SW, Tan CK, Ng KC. Epidemiology of hyperuricemia in the elderly. Yale J. Biol. Med. 2001;74:151–157. [PMC free article] [PubMed] [Google Scholar]
- 47.Bakris GL, Ritz E. The message for World Kidney Day 2009: hypertension and kidney disease—a marriage that should be prevented. J. Hum. Hypertens. 2009;23:222–225. doi: 10.1038/jhh.2008.169. [DOI] [PubMed] [Google Scholar]
- 48.Hawkins MS, et al. Association between physical activity and kidney function: national health and nutrition examination survey. Med. Sci. Sports Exerc. 2011;43:1457–1464. doi: 10.1249/MSS.0b013e31820c0130. [DOI] [PubMed] [Google Scholar]
- 49.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (NHANES III) Clin. J. Am. Soc. Nephrol. 2009;4:1901. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung D-H, Lee Y-J, Lee H-R, Lee J-H, Shim J-Y. Association of renal manifestations with serum uric acid in Korean adults with normal uric acid levels. J. Korean Med. Sci. 2010;25:1766–1770. doi: 10.3346/jkms.2010.25.12.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.