Abstract
Mammalian sperm acquire fertilization capacity in the female reproductive tract in a process known as capacitation. During capacitation, sperm change their motility pattern (i.e., hyperactivation) and become competent to undergo the acrosome reaction. We have recently shown that, in the mouse, sperm capacitation is associated with increased uptake of fluorescently labeled deoxyglucose and with extracellular acidification (ECAR) suggesting enhanced glycolysis. Consistently, in the present work we showed that glucose consumption is enhanced in media that support mouse sperm capacitation suggesting up-regulation of glucose metabolic pathways. The increase in glucose consumption was modulated by bicarbonate and blocked by protein kinase A and soluble adenylyl cyclase inhibitors. Moreover, permeable cAMP agonists increase glucose consumption in sperm incubated in conditions that do not support capacitation. Also, the increase in glucose consumption was reduced when sperm were incubated in low calcium conditions. Interestingly, this reduction was not overcome with cAMP agonists. Despite these findings, glucose consumption of sperm from Catsper1 knock-out mice was similar to the one from wild type suggesting that other sources of calcium are also relevant. Altogether, these results suggest that cAMP and calcium pathways are involved in the regulation of glycolytic energy pathways during murine sperm capacitation.
Keywords: sperm, cAMP, capacitation, glycolysis, glucose consumption, ATP
1. INTRODUCTION
Mammalian sperm acquire the ability to fertilize in the female tract in a process known as capacitation (Austin, 1951; Chang, 1951). Functionally, capacitation is associated with changes in the sperm motility pattern, a process known as hyperactivation, and with sperm preparation to undergo a physiologically-induced acrosome reaction (Gervasi & Visconti, 2016; Yanagimachi, 1994). Sperm capacitation can be mimicked in vitro in a well-defined media with absolute requirement for HCO3−, Ca2+, a protein source (usually serum albumin) and energy sources. Omission of any of these molecules in the sperm incubation media blocks their ability to acquire fertilizing capacity. Importantly, multiple evidence suggests that, in vitro, only a fraction of sperm undergo capacitation. Although these sperm are able to fertilize metaphase II arrested eggs in vitro, the similarities and differences between sperm in vitro and in vivo capacitation remains to be elucidated.
At the molecular level, several signaling pathways which have both independent and also convergent effects are activated to produce a capacitated state. Capacitation starts when sperm come into contact with high HCO3− and Ca2+ present in the seminal fluid or in capacitation-supporting media (Gervasi & Visconti, 2016). These ions activate the atypical adenylyl cyclase Adcy10 (also known as sAC) and consequently increase intracellular cAMP levels and stimulates protein kinase A (PKA) which initiates a series of signaling processes leading to capacitation (Gervasi & Visconti, 2016). Genetic and pharmacological loss-of-function experiments have demonstrated that activation of cAMP-dependent pathways is essential for capacitation. For example, mice lacking either Adcy10 (Esposito et al., 2004; Hess et al., 2005; Xie et al., 2006) or the sperm-specific PKA catalytic subunit splicing variant alpha 2 (Cα2) (Nolan et al., 2004) are sterile, and sperm from these mice cannot undergo capacitation. Also, pharmacological studies using Adcy10 (Ramos-Espiritu et al., 2016) or PKA inhibitors (Tateno et al., 2013) are consistent with these findings. Another essential second messenger involved in capacitation process is calcium (Ca2+) (Gervasi & Visconti, 2016). In addition to contributing to Adcy10 activation, Ca2+ regulates hyperactivation and the acrosome reaction (Navarrete et al., 2016; Tateno et al., 2013). Consistently, mice lacking the sperm-specific Ca2+ channel complex CatSper are sterile in vitro and in vivo (Ren et al., 2001). Finally, BSA, another essential component of capacitation media (Visconti et al., 1995), contributes to capacitation via modulation of cholesterol fluxes (Visconti, Galantino-Homer, et al., 1999; Visconti, Ning, et al., 1999), but the specific signaling cascade mediating capacitation downstream from BSA remains unclear.
In addition to these signaling pathways, sperm capacitation requires production of energy. Similar to somatic cells, both glycolysis and mitochondrial oxidative phosphorylation contribute to the ATP pools in sperm (Mukai & Travis, 2012). In mouse sperm, while either glycolysable (i.e., glucose and fructose) and non-glycolysable (i.e., lactate and pyruvate) substrates can maintain sperm motility and ATP levels, glycolysable substrates were uniquely able to support hyperactivation and the capacitation-induced increase in tyrosine phosphorylation (Goodson et al., 2012). These studies suggest that in mouse sperm, glycolysis and local production of ATP in the principal piece are essential for the sperm to gain fertilizing capacity. Moreover, mice lacking the sperm-specific glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase (GAPDS) (Miki et al., 2004) or phosphoglycerate kinase 2 (PGK2) (Danshina et al., 2010) are sterile. Sperm from these mice are unable to maintain intracellular ATP concentrations and consequently lose their motility within minutes. In summary, both cAMP- and Ca2+- dependent signaling pathways as well as glycolysis are required for capacitation, but the relationship between signaling and metabolism is still not well understood.
Recently, to investigate the role of capacitation in the regulation of energy pathways we used Seahorse XF. These experiments indicate that both extracellular acidification (ECAR) and oxygen consumption (OCR) rates are enhanced by addition of cAMP agonists to sperm incubated in conditions that do not support capacitation (Balbach et al., 2020). However, because Seahorse XF ECAR is based on measurements of extracellular pH close to the plasma membrane, these experiments preclude the use of HCO3− (Traba, Miozzo, Akkaya, Pierce, & Akkaya, 2016). In addition, we observed that uptake of fluorescently labeled deoxyglucose was accelerated in conditions that support capacitation (Balbach et al., 2020). These experiments allowed the use of full capacitation media containing HCO3−; however, saturation levels were obtained in a very short period of time preventing quantitative analyses. On the other hand, glucose can be measured quantitatively using glucose oxidase coupled to Amplex Red (de Raemy-Schenk et al., 2006). Using this method, we observed that in vitro capacitation conditions increased sperm glucose consumption and that this increase was mainly dependent on the presence of HCO3− and Ca2+ in the capacitation media. Not surprisingly, HCO3− effect was mediated by cAMP; however, the role of Ca2+ was independent of this pathway. Unexpectedly, sperm from CatSper1 knock out (KO) mice consume similar amounts of glucose than wild type sperm.
2. MATERIALS AND METHODS
2.1. Materials
Chemicals were purchased from the following sources (codes between parenthesis indicate the catalog number of the respective compound): Sodium bicarbonate (NaHCO3) (S-5761), Bovine serum albumin (BSA, fatty acid-free) (A0281), Ethylene glycol-bis (2-aminoethylether)-N,N,N,N’tetraacetic acid (EGTA) (E-3889), N6,2’-O-Dibutyryladenosine 3’,5’-cyclic monophosphate sodium salt (db-cAMP) (d-0627), 3-Isobutyl-1-methylxanthine (IBMX) (I-5879), 4-Br calcium Ionophore A23187 (C7272), Tween-20 (P7949) and fish skin gelatin (G-7765) were purchased from Sigma (St. Louis, MO). Dihydrochloride H-89 (130964-39-5) was purchased from Cayman chemical (Ann Arbor, Michigan). Protein kinase inhibitor-(14-22)-amide (PKI) was purchased from Tocris (Minneapolis, USA) (2546). Anti-phosphotyrosine (anti-pY) monoclonal antibody (clone 4G10) was obtained from Millipore (Billerica, MA). Rabbit monoclonal anti-phospho PKA substrates (anti-pPKAS) antibody (clone 100G7E) was purchased from Cell Signaling (Danvers, MA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgGs were purchased from Jackson Immuno Research Laboratories (West Grove, PA) and GE Life Sciences (Pittsburgh, PA), respectively. Acrylamide/Bis solution (30%) (161-0138) and β-Mercaptoethanol (BP176-100) were obtained from Biorad (Hercules, CA). HEPES (BP310-100) was purchased from Roche (Hatfield, PA, USA) and Amplex®Red glucose/Glucose Oxidase Assay Kit from Invitrogen (Grand Island, NY) (A22189). LRE1 was produced as previously described (Ramos-Espiritu et al., 2016).
2.2. Animals and spermatozoa collection
The animal study was reviewed and approved by the University of Massachusetts, Amherst Institutional Animal Care and Use Committee (IACUC), protocol 2016-0026. Cauda epididymal mouse sperm were collected from CD1 retired male breeders (Charles River Laboratories, Wilmington, MA) and KO mice genetic model CatSper1 KO (Ren et al., 2001) that were obtained from Dr. Clapham and Dr. Chung from Harvard University, Boston (Massachusetts, USA). Both epididymides were placed in 1 ml of a modified Toyoda–Yokoyama–Hosi (TYH) medium (Toyoda, Yokoyama, & Hosi, 1971). Briefly, this medium contained the following compounds (concentrations are given in parenthesis): NaCl (119,3 mM), KCl (4.7 mM), CaCl2x2H2O (1,71 mM), KH2PO4 (1.2 mM), MgSO4x7H2O (1.2 mM), Glucose (5.56 mM), Na Pyruvate (0.51 mM), Hepes Acid (20 mM) and Gentamicin (10 mg/ml). This medium does not support mouse sperm capacitation. When glucose was measured, sperm were retrieved in glucose- and pyruvate-free medium and then supplemented with different glucose concentrations according to the experimental design. Once the cauda epididymis was immersed in the retrieving media, a cut was made with a sharp razor blade, in this way sperm are released. This method is known as “swim-out” and average of ~ 1,5 x 107 sperm cells is routinely obtained. After 10 min incubation at 37°C (swim-out), epididymides were discarded and the suspension washed twice with the respective media by gentle centrifugation and adjusted with the same media to a final concentration of 1x107 cells/ml with the appropriate glucose concentration. Experiments were conducted with one fourth of this suspension diluted in the appropriate media depending on the experiment. Sperm were then incubated under air in closed 1.5 mL Eppendorf tube in a water bath at 37°C for up-to 3 h in conditions that support or not capacitation. To start capacitation, the non-cap TYH media was supplemented with 15 mM NaHCO3 and 5 mg/ml BSA (CAP). All media were adjusted to pH 7.2-7.4. Sperm collection for glucose consumption experiments is detailed below. When used, inhibitors were added to sperm 15 minutes before the start of capacitation. All the experiments were conducting using ~1 milllion sperm suspended in a final volume of 400 ul.
2.3. Glucose consumption
To evaluate glucose consumption, spermatozoa were allowed to swim-out in 2 mL of modified TYH in absence of energy sources (no glucose, no pyruvate) for 10 min. Then spermatozoa were washed twice in modified TYH in the absence of energy sources for 5 min at 850 and 640 g respectively. For those experiments where glucose consumption was tested in presence of 4Br-A23187 slight modifications of the protocol were made as it stated below. Spermatozoa were then incubated at 37°C in different conditions at a final concentration of 1 mM of glucose. A 30 μl aliquot of diluted sperm suspension was taken every hour for 3 hours of incubation. Sperm were then removed by centrifugation at 12,000 x g for 3 min, and the supernatant was recovered and frozen at −80°C until analysis. In each experiment the exact number of sperm was quantified and was considered for the final calculation of glucose consumption. Glucose concentration was measured using a fluorescent Amplex®Red glucose/Glucose Oxidase Assay Kit (Invitrogen #A22189). Fluorescence was measured in duplicate in 96-well plates (Corning®#3915, New York, USA). In each well, 3 μl of sample were added to 47 μl of 1x Reaction Buffer followed by 50 μl of reaction mix. The reaction mix contained (concentrations are given in parenthesis): Amplex® Red reagent (100 μM), Horseradish peroxidase (0.2 U/ml) and Glucose Oxidase (2 U/ml). After 30 min of incubation at 23°C, fluorescence was measured using fluorimeter capabilities of a POLARstar Omega equipment (BMG LABTECH, Germany) with wavelength excitation of 540 nm and wavelength emission of 590 nm. For each experimental condition, independent glucose concentration curves were used as standard. Except for BSA, we did not find that any of the other compounds used (e.g. H89, LRE1, PKI, Ca2+, EGTA, etc) changed the fluorescent values. On the other hand, BSA did quench Amplex® Red fluorescence; therefore, in all experiments in which BSA was used, two independent glucose concentration curves were done; one in the absence and one in the presence of BSA, as shown in supplementary Figure 1. Each series of assays included a “blank” sample containing TYH media without glucose and standard concentration curves done, as mentioned above, in presence or in the absence of BSA with the respective incubation buffers. After correction of all relative light units (RLU) values for background (blank sample), RLU values were averaged for each sample and the glucose concentration determined by using the linear equation of the glucose standard curve (y=mx + b), where “y” was RLU, “x” is glucose concentration, “m” is slope and “b” is y-intercept. To calculate glucose consumption, we subtracted the initial glucose concentration, equal to 1 mM (time 0) to the remnant glucose concentration in the media that we measured after 1, 2 and 3 hours of incubation. These glucose concentration data were represented over time. Each equation line was calculated, and the slope represented the average of glucose consumption by unit time (hour) over 3 hours incubation period corrected by the sperm concentration in each experiment. This value was used to calculate the average ± SEM of each independent measurement as indicated in figure legends. The number of independent mice used for each determination is given in the figure legend.
2.4. Calcium ionophore treatment
To evaluate the role of Ca2+ ions, 4Br-A23187 was used at a final concentration of 20 μM as previously described by Tateno et al. (2013). Briefly, sperm were recovered by swim-out and washed in non-cap TYH (glucose and pyruvate free) as explained above. Once in these media, 4Br-A23187 was added to a final concentration of 20 μM and sperm incubated for 10 min at 37°C. After this time, sperm were immediately rendered motionless due to a massive entrance of Ca2+ into the sperm (Sanchez-Cardenas et al., 2018). Subsequently, spermatozoa were washed twice by centrifugations (5 min at 600 x g) in non-cap TYH (glucose and pyruvate free) to remove 4Br-A23187 from the media. After these washes, sperm were resuspended in non-cap or cap TYH (containing glucose 1 mM) and kept in the same conditions for 3 hours at 37°C. Every hour an aliquot from the media was taken to measure glucose consumption as it was stated in the previous section.
2.5. Western Blotting
Sperm were collected by centrifugation, washed in 1 ml of PBS, resuspended in Laemmli sample buffer (Laemmli, 1970) without β-mercapthoethanol, boiled (5 min) and centrifuged at 12,100 g. Supernatants were then supplemented with 5% β-mercaptoethanol and boiled (3 min). Protein extracts were separated by SDS-PAGE and electro-transferred to PVDF membranes (Millipore). Immunoblotting was conducted with anti-pPKAs (clone 100G7E) and anti-pY (clone 4G10) sequentially as previously described (Krapf et al., 2010). Briefly, PVDF membranes were blocked with 5% fat-free milk in TBS (Tris-HCl 20 mM, NaCl 150 mM, pH 7.5) containing 0.1% Tween 20 (T-TBS) for anti-pPKAs. After development, the same PVDF membranes used for pPKAs were stripped at 55°C for 20 min in 2% SDS, 0.74% β-mercaptoethanol, 62.5 mM Tris, pH 6.5, and then washed six times for 5 min each in T-TBS. Then, membranes were blocked once more with 5% fish gelatin in T-TBS and used for Western blots with anti-PY antibodies. In both cases, antibodies were used at a final concentration of 1:10.000. Secondary antibodies were diluted in T-TBS (1:10.000) for anti-pPKAs and anti-pY respectively. Enhanced chemiluminescent (ECL) Plus Kit (GE Healthcare) was used for detection of pPKAs signal. ECL regular was used for detection of pY signal. Tyrosine phosphorylated hexokinase served as a loading control (Porambo, Salicioni, Visconti, & Platt, 2012; Visconti et al., 1995). Image analysis was conducted using the free software ImageJ (http://imagej.nih.gov/ij). Western blotting regions of interest (ROIs) used for quantification are indicated by a vertical bar on the left of the respective western blot. Images shown are representative of experiments repeated three times (n=3) using three different animals.
2.6. Statistics
All analyses were performed using SPSS v20.0 for Windows software (SPSS Inc. Chicago, IL). Data were first tested for normal distribution with a Kolmogorov-Smirnov test and for homoscedasticity with a Levene test. Logarithmic transformation was applied to those parameters that did’t meet normality and/or homoscedasticity. Glucose consumption and protein phosphorylation data were statistically analyzed by one-way analysis of variance including the experiment as random factor (ANOVA, randomized blocks). F and p values are given for all figure panels; the number subscript between parenthesis indicate the degree of freedom for treatments. When significance was found, post-hoc comparisons between treatments were made through Bonferroni’s test. Data of glucose consumption rate over time were statistically analyzed by a two tailed paired Students’ t-test for each time point; t and p values for each time point are given; numbers in subscript between parenthesis indicate degrees of freedom. In all cases, the level of significance was set at p < 0.05.
3. RESULTS
3.1. Glucose is consumed at higher rates in sperm incubated in capacitating conditions.
We evaluated glucose consumption by directly measuring the amount of glucose remaining in the sperm incubation media at various times during incubation in both non-capacitating (absence of HCO3− and BSA) and capacitating-supporting conditions (presence of HCO3− and BSA). As part of this method, to increase the sensitivity of the assay, instead of using 5 mM glucose in the capacitation media, we used 1 mM. This lower glucose concentration was compatible with phosphorylation of PKA substrates and for the capacitation-associated increase in tyrosine phosphorylation (Fig. 1 A). Using this concentration, glucose consumption remained linear for at least three hours in both conditions (Fig. 1 B), and glucose was consumed at approximately twice as fast in capacitating media as it was in non-capacitating media (Fig. 1 B; 1h: t(16)= −3.8, p = 0.002; 2h: t(16)= −6.4, p < 0.001; 3h: t(16)= −6.5, p < 0.001 ). To quantify changes in rate, the slope of each independent experiment was determined and the average glucose consumption ± SEM calculated in the presence or absence of pyruvate (Fig. 1 C). These data showed significant increases between non-capacitating and capacitating-supporting media in both conditions indicating that glucose consumption rate was not modified by the presence of pyruvate in the media (Fig. 1 C; F(3)= 58.4, p < 0.001).
Figure 1. Glucose is consumed at higher rates in sperm incubated in capacitating conditions.
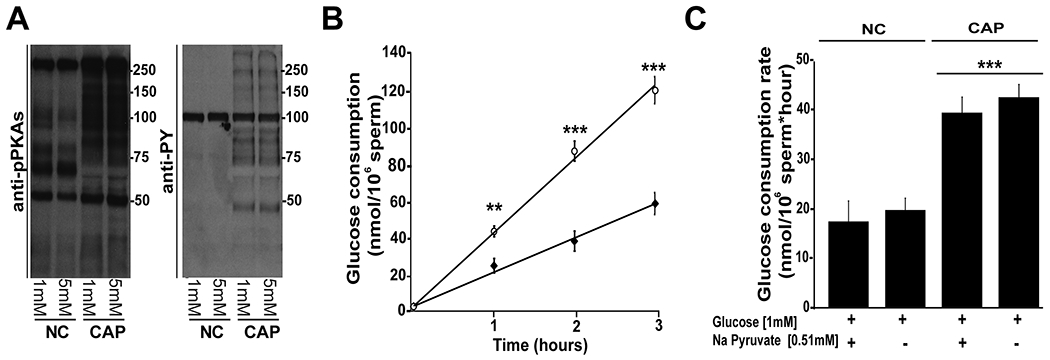
A) Comparison of two different glucose concentrations (5 and 1 mM) in the media. Phosphorylation pathways remain active when sperm are incubated with 1 mM glucose in the capacitation medium. Cauda epididymal sperm were incubated for 1 hour in conditions that support (CAP) or not capacitation (NC), protein extracted and phosphorylation of PKA substrates and tyrosine residues evaluated by Western blots as described in Methods. A representative membrane is shown (n=3). B) Cauda epididymal sperm were incubated in the presence of glucose (1mM). At different times (0, 1, 2 and 3 hours), the remaining glucose in the consumption was measured as described in Methods through 3 hours of incubation in the presence (white circle, Cap conditions) or absence (black diamond, Non-Cap conditions) of 15 mM HCO3− and 5 mg/mL BSA. To quantify the changes in glucose rate consumption, the slope of each independent experiment was determined and the average ± SEM calculated for 9 independent experiments. Differences between conditions at corresponding time points were analyzed using two-tailed, paired t-test **p < 0.01, ***p < 0.001 show differences versus non capacitating (NC) conditions (n = 9). C) Glucose consumption values were obtained based on linear-regression analyses. In this graph, data corresponding to glucose consumption in capacitating (CAP) or non-capacitating (NC) conditions were tested in the presence or absence of 0.51 mM pyruvate. Differences between treatments were analyzed by Bonferroni post-hoc test, ***p < 0.001 indicates differences versus non capacitating (NC) control conditions (1 mM glucose and 0.51 mM pyruvate).
The only difference between capacitating and non-capacitating conditions in our experiments was the presence of HCO3− and BSA, both of which have been shown to be essential for initiating the signaling cascades necessary for capacitation (Gervasi & Visconti, 2016). To evaluate their individual roles, glucose consumption rates were assessed in sperm incubated in the absence or presence of BSA and in the absence or presence of HCO3− and significant differences between treatments were found (Fig. 2 A; F(3) = 21.7, p < 0.001). While BSA alone slightly increased glucose consumption, HCO3− was the predominant mediator of the increased glucose consumption during capacitation (Fig. 2A; Bonferroni test, p<0.001 vs NC).
Figure 2. Glucose consumption is up-regulated downstream of cAMP pathways.
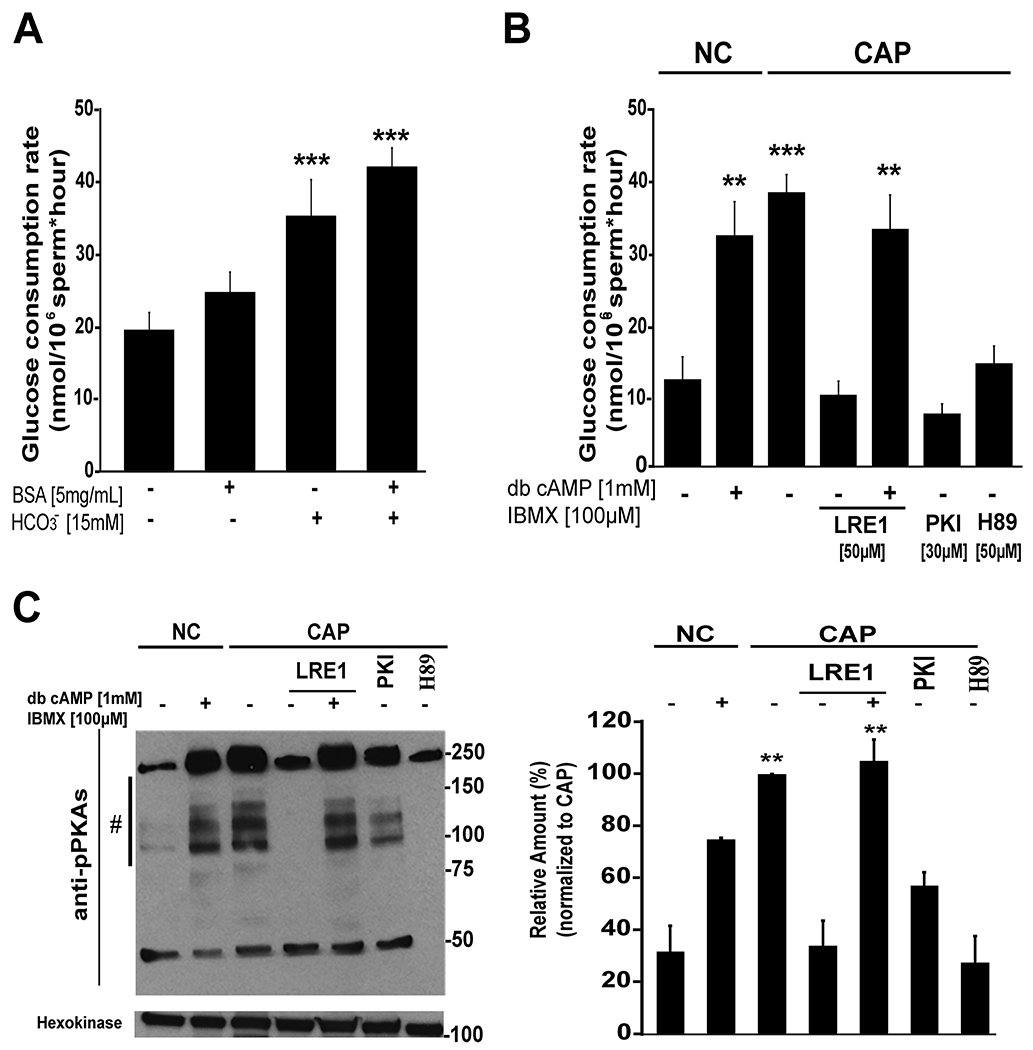
A) Role of HCO3− and BSA in the capacitation media on glucose consumption. Bars show average ± SEM of 9 independent experiments. Differences between treatments were analyzed by Bonferroni post-hoc test, ***p <0.001 indicates differences versus no capacitating conditions (NC). B) Role of cAMP in the regulation of glucose consumption rates. As indicated in Methods section, cauda epididymal sperm were incubated in conditions that support (CAP) or not capacitation (NC) in the presence or absence of cAMP agonists dibutyryl cAMP (1 mM) and IBMX (100 μM), and in the presence or absence of inhibitors of Adcy10 (LRE1, 50 μM) or PKA (PKI, 30 μM; or H89, 50 μM). Spermatozoa were incubated in these conditions for 3 hours and glucose consumption was determined by measuring glucose every hour as described in Methods. Bars represent the average ± SEM of at least 6 independent experiments. Differences between treatments were analyzed by Bonferroni post-hoc test, **p < 0.01 and ***p < 0.001 indicate differences versus no capacitating conditions (NC). C) Left panel: western blot using anti-PKA substrates antibodies of mouse sperm incubated for 1 hour in the same conditions than in section B, in all cases, tyrosine phosphorylated hexokinase was used as loading control. Right panel: western blots were analyzed using ImageJ. For comparison between blots, pixels for each lane contained in the region marked by # were quantified and normalized using the CAP lane as reference (100 %). Bars represent the average ± SEM of 3 independent experiments. Differences between treatments were analyzed by Bonferroni post-hoc test, **p < 0.01 indicates differences versus non capacitating (NC) conditions.
Once inside the cell, HCO3− ions stimulate Adcy10 activity and consequently increase cAMP levels (see model below in the discussion section). To evaluate whether the HCO3− effect on glucose consumption was mediated by a cAMP pathway, sperm were incubated with or without cAMP agonists and inhibitors and significant differences were found between these treatments (Fig. 2 B; F(6) = 22.2, p < 0.001). On one hand, addition of permeable cAMP agonists to sperm incubated in the absence of HCO3− induced glucose consumption although less than those observed for capacitated sperm (Fig. 2 B; Bonferroni test, p = 0.005 vs NC). On the other hand, two different PKA inhibitors, H89 and PKI, and a recently described Adcy10 inhibitor, LRE1 (Ramos-Espiritu et al., 2016), blocked the increase in glucose consumption (Fig. 2 B). Moreover, cAMP agonists restored glucose consumption in sperm treated with LRE1 (Fig. 2 B; Bonferroni test, p = 0.005 vs NC). As capacitation controls, the effect of these treatments on the phosphorylation of PKA substrates was evaluated in each of these experimental conditions by Western blot using anti phospho-PKA substrates antibodies (Krapf et al., 2010) (Fig. 2 C, left panel) and quantified using ImageJ (Fig. 2 C, right panel; F(6) = 21.7, p < 0.001). These data indicate that the Adcy10-cAMP-PKA signaling cascade is at least partially sufficient to mediate the capacitation-induced elevation of glucose consumption and raise the possibility that other capacitation-induced signaling cascades are involved in this event.
Ca2+ is another second messenger essential for capacitation. To test the role of Ca2+ in glucose consumption, sperm were incubated in conditions that support or not capacitation in the presence or absence of this ion. The capacitation-associated increase in glucose consumption was significantly decreased when sperm were incubated in a media prepared without Ca2+ addition and also when the medium without Ca2+ was supplemented with 1 mM EGTA (Fig. 3 A). In neither of these low Ca2+ conditions, cAMP agonists were able to restore the rate of glucose consumption observed in complete media (Fig. 3 A) suggesting an additional Ca2+ need for this process. In all these conditions, the level of PKA-dependent phosphorylation was evaluated by Western blot (Fig. 3 B, left panel) and quantified using ImageJ (Fig. 3 B, right panel; F(7) = 75.4, p <0.001). The increase in PKA-dependent phosphorylation observed in Fig. 3 B Western blots is consistent with the biphasic role of Ca2+ in the regulation of cAMP-dependent pathways in sperm (Battistone et al., 2014; Navarrete et al., 2015). Altogether, these results indicate that cAMP and Ca2+ are both needed to up-regulate glucose consumption during capacitation.
Figure 3. Ca2+ in the capacitation media modulates glucose consumption.
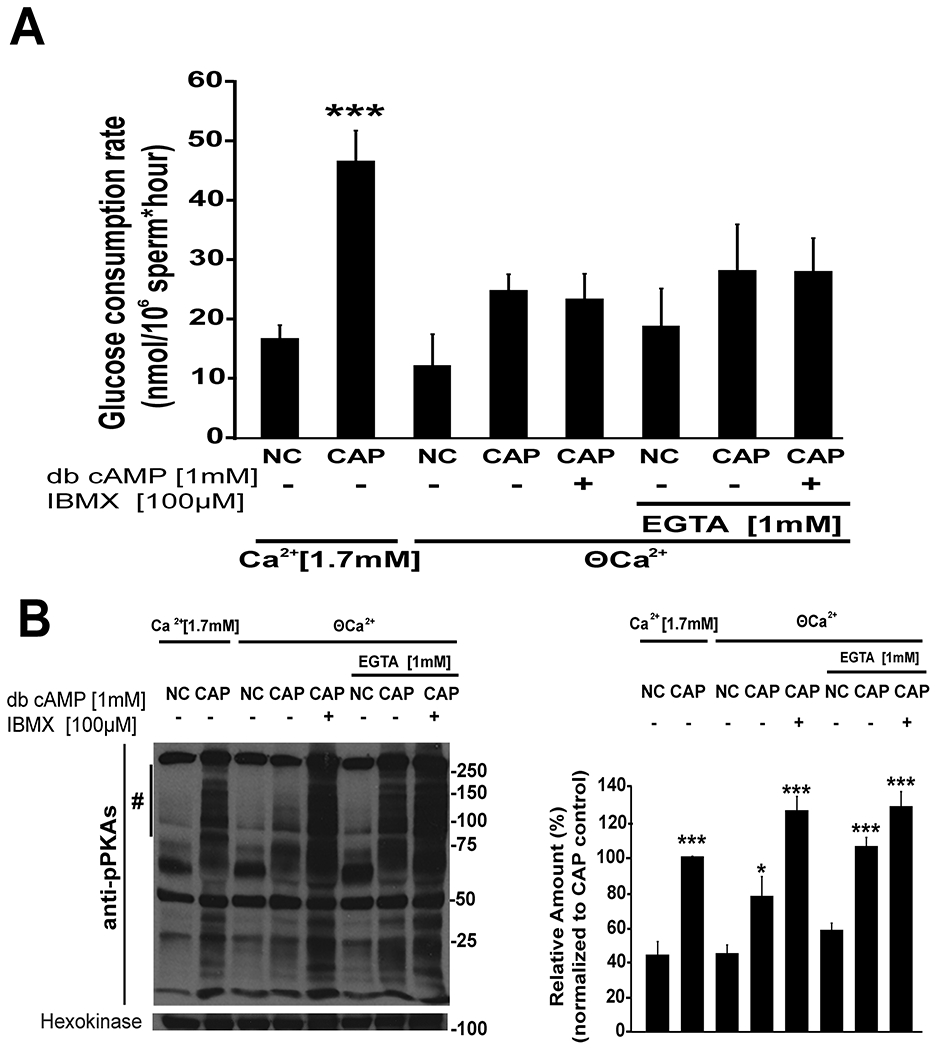
A) Cauda epididymal sperm were recovered by swim-out in NC media without Ca2+ (nominal zero Ca2+ (ϴ Ca2+)), sperm were then centrifuged and resuspended in media that support (CAP, 15mM HCO3− and 5mg/mL BSA) or not (NC) capacitation in the presence (Ca2+ [1.7 mM]) or in the absence (ϴ) of Ca2+ and in the presence of absence of EGTA (1mM) as indicated in the figure text. To analyze the role of cAMP, db-cAMP (1 mM) and IBMX (100 μM) were added to sperm incubated in low Ca2+ conditions. Glucose consumption was determined as it was described in Methods (n≥6). Differences between treatments were analyzed by Bonferroni post-hoc test, ***p < 0.001 indicates differences versus NC control (1.7 mM Ca2+). B) Left panel: a representative western blot using anti-phospho-PKA substrates antibodies of mouse sperm incubated for 1 hour in the same conditions described in section A with tyrosine phosphorylated hexokinase used as a loading control (n=3). Right panel: western blots were analyzed using ImageJ. For comparison between blots, pixels for each lane contained in the region marked by # were quantified and normalized using the CAP (1.7 mM Ca2+) lane as reference (100 %) (n=3). Differences between treatments were analyzed by Bonferroni post-hoc test, ***p < 0.001 and *p < 0.05 indicate differences versus NC control (1.7 mM Ca2+).
The sperm-specific Ca2+ channel complex CatSper mediates a subset of Ca2+-dependent signaling events during capacitation; specifically, CatSper is essential for sperm to undergo hyperactivated motility. However, the capacitation-induced increase in glucose consumption was unaffected in sperm from CatSper1 KO mice (Fig. 4 A, F(3) = 9.4, p = 0.001; Bonferroni test p < 0.05 vs NC control). We also evaluated whether a temporary elevation of intracellular Ca2+ was sufficient to increase glucose consumption. Incubating sperm in the presence of calcium ionophore for 10 min., which transiently increases intracellular Ca2+ (Tateno et al., 2013), did not increase glucose consumption rates (Fig. 4 B). As described (Sanchez-Cardenas et al., 2018; Tateno et al., 2013), while ionophore exposure completely stopped sperm motility in minutes, sperm motility was recovered immediately after its removal. Thus, although Ca2+ plays a role in stimulating glucose consumption, the temporal increase of its intracellular concentration is not sufficient to elicit the increase in glucose consumption observed during capacitation. Longer period of ionophore treatment are detrimental to sperm viability and were not assessed.
Figure 4. Glucose consumption in sperm from CatSper1 KO and in sperm treated with A23187.
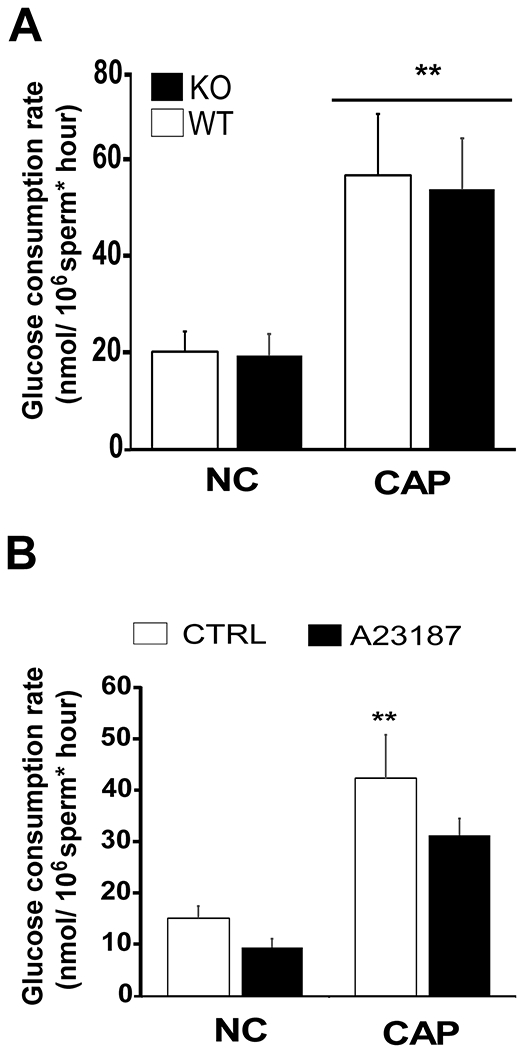
A) Cauda epididymal sperm from wild-type (WT, white column) or from CatSper1 KO mice (KO, black column) were separately obtained by swim-out and incubated in media that support (CAP) or not (NC) capacitation for 3 hours and glucose consumption ratio was determined as described in Methods. Data are shown as mean ± SEM of 7 independent experiments. Differences between treatments were analyzed by Bonferroni post-hoc test, **p < 0.01 indicates differences versus wild-type NC control. B) Spermatozoa obtained from CD-1 retired male breeders were allowed to swim-out in non-capacitating media, washed, suspended in the same media and treated with 20 μM 4Br-A23187 (black column) or with DMSO (white columns) for 10 min. At that point sperm become motionless in less than 1 minute. Ten minutes later, sperm were washed twice to remove the excess of 4Br-A23187 from the media and suspended in media supporting (CAP) (HCO3− 15 mM and 5 mg/mL BSA) or not (NC) capacitation. Sperm were then incubated for 3 hours and glucose consumption rates determined as described in Methods (n = 4). Differences between treatments were analyzed by Bonferroni post-hoc test, *p < 0.05 indicates differences versus NC control.
4. DISCUSSION
Capacitation is a lengthy process associated with sequential and concomitant molecular changes in different sperm compartments (for review, see Gervasi and Visconti 2016). Moreover, capacitation-induced related events require energy in the form of ATP which is obtained from nutrient molecules present in the surrounding environment. Nevertheless, how metabolic pathways are coupled to the regulation of signaling pathways in sperm is not well understood. On one hand, pharmacologic and genetic loss-of-function experiments in mouse models provided strong evidence that glycolysis is essential to maintain sperm motility in these species. Mice lacking the sperm-specific glycolytic enzymes Gapds (Miki et al., 2004) or Pgk2 (Danshina et al., 2010) are sterile. On the other hand, although oxidative phosphorylation is able to maintain sperm motility, in mouse sperm, glucose is required for hyperactivation (Goodson et al., 2012). Therefore, in this work, we prioritize the study of glucose consumption and evaluated the extent by which capacitation conditions affect the use of this metabolite.
Our results showed that sperm incubated in conditions that support capacitation consumed glucose at higher rates than those incubated in media that do not support capacitation. The difference between capacitating vs non-capacitating media is the presence of HCO3− and BSA. While HCO3− stimulates the Adcy10-cAMP-PKA signaling cascade essential for capacitation (Gervasi & Visconti, 2016), BSA is involved in regulating sperm plasma membrane cholesterol (Visconti, Galantino-Homer, et al., 1999; Visconti, Ning, et al., 1999) and Ca2+ (Blackmore & Eisoldt, 1999; Espinosa et al., 2000; Sakata et al., 2002). In our experiments, both HCO3− and BSA were needed to maximize sperm glucose consumption; however, the HCO3− contribution was the most significant. Consistent with the role of HCO3− in Adcy10 activation, cAMP permeable agonists were able to induce glucose consumption in sperm incubated in conditions that do not support capacitation. In addition, the increase in glucose consumption was blocked in the presence of PKI and H89, two different types of PKA inhibitors which have different mode of action. While PKI is a peptide substrate competitive inhibitor, H89 is a small molecule that competes with ATP binding. Glucose consumption was also blocked when a recently described Adcy10 inhibitor (Ramos-Espiritu et al., 2016), LRE1, was present in the capacitation media. Moreover, the LRE1-dependent inhibition was overcome with the addition of cAMP permeable agonists that directly activate PKA. Altogether, these data indicate that cAMP-dependent signaling pathways play a role in the regulation of glucose consumption.
Another compound needed for capacitation is Ca2+ and this divalent cation plays a biphasic role in the regulation of sperm signaling pathways (Navarrete et al., 2015). In media prepared without addition of external Ca2+, PKA-dependent phosphorylation is reduced. Media devoid of external Ca2+, however, still contains micromolar concentrations of this cation (Marin-Briggiler et al., 2005). To further reduce Ca2+ concentration in the incubation media, it is necessary to chelate this ion. We have previously shown that when sperm are incubated in very low extracellular Ca2+ (by addition of EGTA), PKA and tyrosine phosphorylation pathways are up-regulated (Battistone et al., 2014; Navarrete et al., 2015). This biphasic effect can be explained by a dual Ca2+ role in activating enzymes that play opposite roles in cAMP pathways (discussed in (Navarrete et al., 2015). Similarly, in the present work, we observed that addition of EGTA to sperm incubated in capacitation-supporting conditions increased phosphorylation of PKA substrates. However, despite the increase in PKA-dependent phosphorylation observed in capacitation-supporting conditions, EGTA did not induce an increase in glucose consumption. This experiment suggests that Ca2+ ions are required independently or downstream of cAMP-dependent pathways. Consistent with this possibility, permeable cAMP agonists in conditions of low extracellular Ca2+ (with or without EGTA) were not able to increase glucose consumption rates.
Despite these observations, a temporary exposure to Ca2+ ionophore did not increase glucose consumption in the absence of capacitation suggesting that Ca2+ alone is not sufficient for this process. On the other hand, sperm from CatSper1 KO mice consume glucose at the same levels than sperm from wild type mice. These results suggest that, in addition to the CatSper Ca2+ channel complex, other sources of Ca2+ are active during sperm capacitation as previously suggested (Luque et al., 2018). These additional Ca2+ sources could be due to a Na+/Ca2+ exchanger (Wennemuth, Babcock, & Hille, 2003), to internal Ca2+ stores (Ho & Suarez, 2003) and/or to other Ca2+ channels not yet fully characterized by electrophysiology (for review see Beltran et al. 2016). Altogether, these results suggest that a crosstalk between cAMP and Ca2+ signaling pathways are required for maximum use of glycolytic resources and that intracellular Ca2+ is not exclusively dependent on the CatSper Ca2+ channel complex (see Fig. 5 for a simplified model).
Figure 5. Simplified model of the crosstalk between cAMP, calcium and glycolytic pathways in mouse spermatozoa.
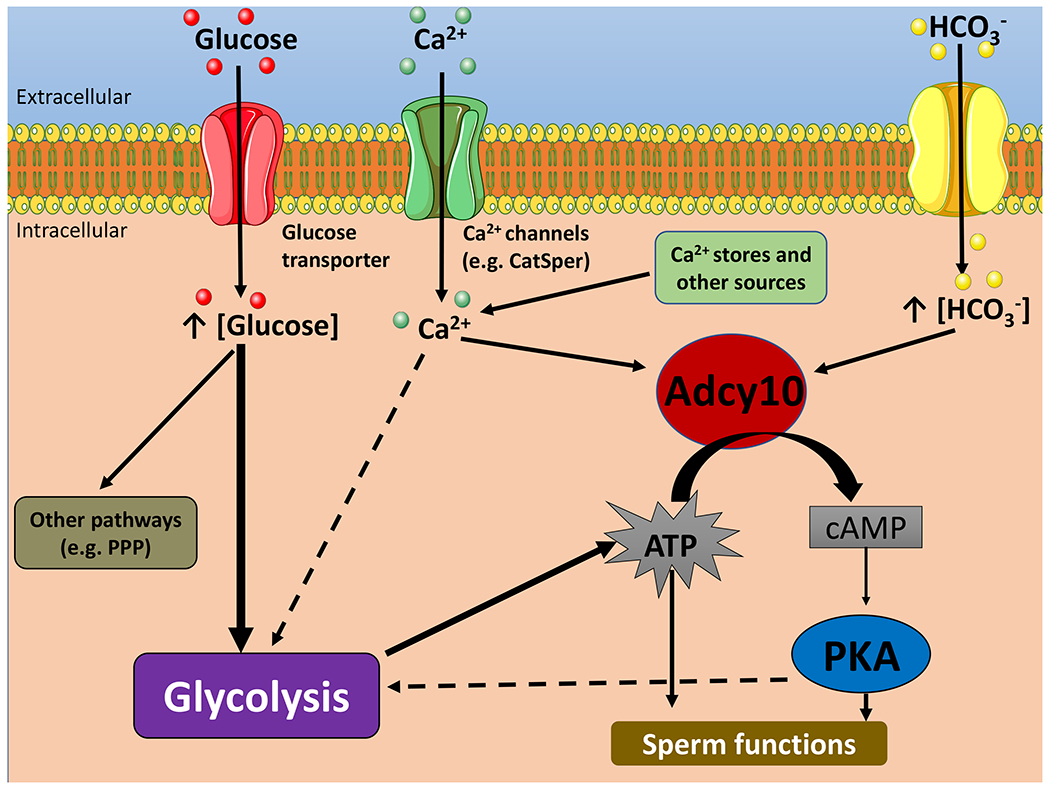
HCO3− stimulates Adcy10 and increase cAMP levels which activates PKA. PKA activation regulates glycolysis as well as other sperm pathways. Ca2+ modulates Adcy10 activity; in addition, Ca2+ ions modulate glycolysis either downstream or independently of the cAMP pathway. To simplify this model, glucose and ion transport are represented with generic terms. Single arrows indicate activation. Dotted arrows indicate hypothetic pathways. The intention of this working model is only to illustrate hypotheses. Although no depicted here, it is clear that the interactive network is more complex. Pentose Phosphate Pathway: PPP.
In the present manuscript, we investigated how glucose consumption is regulated during capacitation in mouse sperm. However, although glycolysis is one of the major pathways using glucose in the sperm, it is not the only one (for review see Rodriguez-Gil 2006). The pentose phosphate pathway it is also present in mammalian sperm and it has been proposed to play relevant roles in the production of NADPH and consequently in the regulation of redox pathways in these cells (Urner & Sakkas, 1999). Also, the fate of pyruvate (and/or lactate) produced at the end of glycolysis is not well-established. In most cell types, pyruvate is coupled to oxidative phosphorylation through conversion to acetyl Co-A. Because in sperm, mitochondria and glycolytic pathways are located in different flagellar compartments, it is not well-established the extent of crosstalk between these two metabolic pathways. In this regard, a recent study by Tourmente, Villar-Moya, Rial, and Roldan (2015) used Seahorse XF technology to compare metabolic pathways in different mouse species from the genus Mus. Their results revealed that, even in the same genus (Mus), the relative relevance of glycolysis and oxidative phosphorylation is species-specific (Tourmente et al., 2015). Thus, Tourmente et al. (2015) revealed that sperm incubated only in the presence of glucose (no pyruvate or lactate added) are able to consume oxygen suggesting that pyruvate and/or lactate produced by glycolysis can be shuttle to the flagellum mid-piece for mitochondria use. Our recent manuscript confirms this observation and also showed that both ECAR and OCR are enhanced when cAMP agonists are added to sperm incubated in the absence of HCO3− (Balbach et al., 2020).
In summary, the present study highlights the need to further investigate how metabolic pathways are regulated during capacitation. In this regard, our group has recently published evidence that manipulation of the metabolic state of the sperm in vitro can improve sperm functional parameters. In this work, mouse sperm were incubated in media depleted of external nutrients until they became motionless (starvation step), and thereafter individual nutrients were added back (rescue step). Starved and rescue sperm reach higher hyperactivation and fertilization rates in vitro than those incubated persistently in the presence of nutrients. Even more surprising was to find that upon fertilization, embryos derived from sperm treated with this starvation and rescue procedure improved embryo development and when transferred to pseudo-pregnant females produced significantly more pups (Navarrete et al., 2019). The molecular basis of the effects of starvation and rescue are not yet known; however, these findings underscore the need to improve our knowledge of sperm metabolic pathways and suggest that the study of sperm metabolic processes might have translational value in assisted reproductive technologies. We expect that the present studies contribute to this goal.
Supplementary Material
Supplementary Figure 1. Standard glucose concentration curves in the presence and absence of BSA. Glucose concentration curves were done in the presence (open squares) and absence (closed squares) of BSA as described in Methods. Relative light units (RLU) obtained in each independent experiment were averaged and the glucose concentration determined by using the linear equation of the glucose standard curve (y=mx + b), where “y” was RLU, “x” is glucose concentration, “m” is slope and “b” is y-intercept. The slope of each independent experiment was determined and the average ± SEM calculated for 10 independent experiments.
ACKNOWLEDGEMENTS
We are grateful to Dr. Jean-Ju Chung and Dr. David Clapham that originally donated CatSper1 KO mice.
Grants number:
This study was supported by NIH grants HD-038082 (to PEV) and HD088571 (to JB, LL and PEV). D Martin-Hidalgo was recipient of a post-doctoral Grant from the Government of Extremadura (Spain) and by Fondo Social Europeo (PO14005). AR is supported by a fellowship from Lalor Foundation.
Footnotes
DECLARATION OF INTERESTS
Dr. Visconti and Dr. Salicioni own equity interest in Sperm Capacitation Technologies Inc. a company with goals in improving assisted reproductive technologies. Drs. Buck and Levin own equity interest in CEP Biotech which has licensed commercialization of a panel of monoclonal antibodies directed against Adcy10.
REFERENCES
- Austin CR (1951). Observations on the penetration of the sperm in the mammalian egg. Australian Journal of Scientific Research. Ser. B: Biological Sciences, 4(4), 581–596. [DOI] [PubMed] [Google Scholar]
- Balbach M, Gervasi MG, Hidalgo DM, Visconti PE, Levin LR, & Buck J (2020). Metabolic changes in mouse sperm during capacitation. Biology of Reproduction. doi: 10.1093/biolre/ioaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistone MA, Alvau A, Salicioni AM, Visconti PE, Da Ros VG, & Cuasnicu PS (2014). Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation. Molecular Human Reproduction, 20(11), 1054–1066. doi: 10.1093/molehr/gau073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran C, Trevino CL, Mata-Martinez E, Chavez JC, Sanchez-Cardenas C, Baker M, & Darszon A (2016). Role of Ion Channels in the Sperm Acrosome Reaction. Advances in Anatomy, Embryology and Cell Biology, 220, 35–69. doi: 10.1007/978-3-319-30567-7_3 [DOI] [PubMed] [Google Scholar]
- Blackmore PF, & Eisoldt S (1999). The neoglycoprotein mannose-bovine serum albumin, but not progesterone, activates T-type calcium channels in human spermatozoa. Molecular Human Reproduction, 5(6), 498–506. doi: 10.1093/molehr/5.6.498 [DOI] [PubMed] [Google Scholar]
- Chang MC (1951). Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature, 168(4277), 697–698. [DOI] [PubMed] [Google Scholar]
- Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, . . . O’Brien DA (2010). Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biology of Reproduction, 82(1), 136–145. doi: 10.1095/biolreprod.109.079699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Raemy-Schenk AM, Troublé S, Gaillard P, Page P, Gotteland JP, Scheer A, … Yeow K (2006). A cellular assay for measuring the modulation of glucose production in H4IIE cells. Assay and Drug Development Technologies, 4(5), 525–533. doi: 10.1089/adt.2006.4.525 [DOI] [PubMed] [Google Scholar]
- Espinosa F, Lopez-Gonzalez I, Munoz-Garay C, Felix R, De la Vega-Beltran JL, Kopf GS, … Darszon A (2000). Dual regulation of the T-type Ca(2+) current by serum albumin and beta-estradiol in mammalian spermatogenic cells. FEBS Letters, 475(3), 251–256. doi: 10.1016/s0014-5793(00)01688-4 [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, … Gossen JA (2004). Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 2993–2998. doi: 10.1073/pnas.0400050101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi MG, & Visconti PE (2016). Chang’s meaning of capacitation: A molecular perspective. Molecular Reproduction and Development, 83(10), 860–874. doi: 10.1002/mrd.22663 [DOI] [PubMed] [Google Scholar]
- Goodson SG, Qiu Y, Sutton KA, Xie G, Jia W, & O’Brien DA (2012). Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biology of Reproduction, 87(3), 75. doi: 10.1095/biolreprod.112.102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, … Moss SB (2005). The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Developmental Cell, 9(2), 249–259. doi: 10.1016/j.devcel.2005.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HC, & Suarez SS (2003). Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biology of Reproduction, 68(5), 1590–1596. doi: 10.1095/biolreprod.102.011320 [doi];biolreprod.102.011320 [pii] [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, & Visconti PE (2010). Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. Journal of Biological Chemistry, 285(11), 7977–7985. doi: 10.1074/jbc.M109.085845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685. doi: 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Luque GM, Dalotto-Moreno T, Martin-Hidalgo D, Ritagliati C, Puga Molina LC, Romarowski A, … Buffone MG (2018). Only a subpopulation of mouse sperm displays a rapid increase in intracellular calcium during capacitation. Journal of Cellular Physiology. doi: 10.1002/jcp.26883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Briggiler CI, Jha KN, Chertihin O, Buffone MG, Herr JC, Vazquez-Levin MH, & Visconti PE (2005). Evidence of the presence of calcium/calmodulin-dependent protein kinase IV in human sperm and its involvement in motility regulation. Journal of Cell Science, 118(Pt 9), 2013–2022. doi: 10.1242/jcs.02326 [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, … O’Brien DA (2004). Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proceedings of the National Academy of Sciences of the United States of America, 101(47), 16501–16506. doi: 10.1073/pnas.0407708101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai C, & Travis AJ (2012). What sperm can teach us about energy production. Reproduction in Domestic Animals, 47 Suppl 4, 164–169. doi: 10.1111/j.1439-0531.2012.02071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, Aguila L, Martin-Hidalgo D, Tourzani DA, Luque GM, Ardestani G, … Visconti PE (2019). Transient Sperm Starvation Improves the Outcome of Assisted Reproductive Technologies. Front Cell Dev Biol, 7, 262. doi: 10.3389/fcell.2019.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, Alvau A, Lee HC, Levin LR, Buck J, Leon PM, … Visconti PE (2016). Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Scientific Reports, 6, 33589. doi: srep33589 [pii]; 10.1038/srep33589 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, Garcia-Vazquez FA, Alvau A, Escoffier J, Krapf D, Sanchez-Cardenas C, … Visconti PE (2015). Biphasic role of calcium in mouse sperm capacitation signaling pathways. Journal of Cellular Physiology, 230(8), 1758–1769. doi: 10.1002/jcp.24873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, & McKnight GS (2004). Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proceedings of the National Academy of Sciences of the United States of America, 101(37), 13483–13488. doi: 10.1073/pnas.0405580101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porambo JR, Salicioni AM, Visconti PE, & Platt MD (2012). Sperm phosphoproteomics: historical perspectives and current methodologies. Expert Review of Proteomics, 9(5), 533–548. doi: 10.1586/epr.12.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Espiritu L, Kleinboelting S, Navarrete FA, Alvau A, Visconti PE, Valsecchi F, … van den Heuvel J (2016). Discovery of LRE1 as a specific and allosteric inhibitor of soluble adenylyl cyclase. Nature Chemical Biology, 12(10), 838–844. doi: 10.1038/nchembio.2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, … Clapham DE (2001). A sperm ion channel required for sperm motility and male fertility. Nature, 413(6856), 603–609. doi: 10.1038/35098027 [doi];35098027 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gil JE (2006). Mammalian sperm energy resources management and survival during conservation in refrigeration. Reprod Domest Anim, 41 Suppl 2, 11–20. doi: 10.1111/j.1439-0531.2006.00765.x [DOI] [PubMed] [Google Scholar]
- Sakata Y, Saegusa H, Zong S, Osanai M, Murakoshi T, Shimizu Y, … Tanabe T (2002). Ca(v)2.3 (alpha1E) Ca2+ channel participates in the control of sperm function. FEBS Letters, 516(1–3), 229–233. doi: 10.1016/s0014-5793(02)02529-2 [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardenas C, Montoya F, Navarrete FA, Hernandez-Cruz A, Corkidi G, Visconti PE, & Darszon A (2018). Intracellular Ca2+ threshold reversibly switches flagellar beat off and on. Biology of Reproduction, 99(5), 1010–1021. doi: 10.1093/biolre/ioy132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno H, Krapf D, Hino T, Sanchez-Cardenas C, Darszon A, Yanagimachi R, & Visconti PE (2013). Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18543–18548. doi: 1317113110 [pii]; 10.1073/pnas.1317113110 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourmente M, Villar-Moya P, Rial E, & Roldan ER (2015). Differences in ATP Generation Via Glycolysis and Oxidative Phosphorylation and Relationships with Sperm Motility in Mouse Species. Journal of Biological Chemistry, 290(33), 20613–20626. doi: 10.1074/jbc.M115.664813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Yokoyama M, & Hosi T (1971). Studies on the fertilization of mouse eggs in vitro. The Japanese Journal of Animal Reproduction, 16(4), 147–157. doi: 10.1262/jrd1955.16.147 [DOI] [Google Scholar]
- Traba J, Miozzo P, Akkaya B, Pierce SK, & Akkaya M (2016). An Optimized Protocol to Analyze Glycolysis and Mitochondrial Respiration in Lymphocytes. J Vis Exp (117). doi: 10.3791/54918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urner F, & Sakkas D (1999). Characterization of glycolysis and pentose phosphate pathway activity during sperm entry into the mouse oocyte. Biology of Reproduction, 60(4), 973–978. doi: 10.1095/biolreprod60.4.973 [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, & Kopf GS (1995). Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development, 121(4), 1129–1137. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, … Kopf GS (1999). Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. Journal of Biological Chemistry, 274(5), 3235–3242. doi: 10.1074/jbc.274.5.3235 [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, & Kopf GS (1999). Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Developmental Biology, 214(2), 429–443. doi: 10.1006/dbio.1999.9428 [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Babcock DF, & Hille B (2003). Calcium clearance mechanisms of mouse sperm. Journal of General Physiology, 122(1), 115–128. doi: 10.1085/jgp.200308839 [doi];jgp.200308839 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, … Conti M (2006). Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Developmental Biology, 296(2), 353–362. doi: S0012-1606(06)00878-5 [pii]; 10.1016/j.ydbio.2006.05.038 [doi] [DOI] [PubMed] [Google Scholar]
- Yanagimachi R (1994). Mammalian fertilization In Knobil E & Neill J (Eds.), The Physiology of Reproduction. New York: Raven Press; (Reprinted from: Not in File). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Standard glucose concentration curves in the presence and absence of BSA. Glucose concentration curves were done in the presence (open squares) and absence (closed squares) of BSA as described in Methods. Relative light units (RLU) obtained in each independent experiment were averaged and the glucose concentration determined by using the linear equation of the glucose standard curve (y=mx + b), where “y” was RLU, “x” is glucose concentration, “m” is slope and “b” is y-intercept. The slope of each independent experiment was determined and the average ± SEM calculated for 10 independent experiments.


