Abstract
Magnetic resonance imaging (MRI)-based quantification of the blood-oxygenation-level-dependent (BOLD) effect allows oxygen extraction fraction (OEF) mapping. The multi-parametric quantitative BOLD (mq-BOLD) technique facilitates relative OEF (rOEF) measurements with whole brain coverage in clinically applicable scan times. Mq-BOLD requires three separate scans of cerebral blood volume and transverse relaxation rates measured by gradient-echo (1/T2*) and spin-echo (1/T2). Although the current method is of clinical merit in patients with stroke, glioma and internal carotid artery stenosis (ICAS), there are relaxation measurement artefacts that impede the sensitivity of mq-BOLD and artificially elevate reported rOEF values.
We posited that T2-related biases caused by slice refocusing imperfections during rapid 2D-GraSE (Gradient and Spin Echo) imaging can be reduced by applying 3D-GraSE imaging sequences, because the latter requires no slice selective pulses. The removal of T2-related biases would decrease overestimated rOEF values measured by mq-BOLD. We characterized effects of T2-related bias in mq-BOLD by comparing the initially employed 2D-GraSE and two proposed 3D-GraSE sequences to multiple single spin-echo reference measurements, both in vitro and in vivo. A phantom and 25 participants, including young and elderly healthy controls as well as ICAS-patients, were scanned. We additionally proposed a procedure to reliably identify and exclude artefact affected voxels. In the phantom, 3D-GraSE derived T2 values had 57% lower deviation from the reference. For in vivo scans, the formerly overestimated rOEF was reduced by −27% (p < 0.001). We obtained rOEF = 0.51, which is much closer to literature values from positron emission tomography (PET) measurements. Furthermore, increased sensitivity to a focal rOEF elevation in an ICAS-patient was demonstrated.
In summary, the application of 3D-GraSE improves the mq-BOLD-based rOEF quantification while maintaining clinically feasible scan times. Thus, mq-BOLD with non-slice selective T2 imaging is highly promising to improve clinical diagnostics of cerebrovascular diseases such as ICAS.
Keywords: Oxygen extraction fraction OEF, Multi-parametric quantitative BOLD, mq-BOLD, T2, R2’, 3D GraSE
1. Introduction
As the brain has high energy demands without oxygen storage capacities, cerebral oxygen supply is crucial (Hyder, 2009). An important parameter of oxygen supply is the oxygen extraction fraction (OEF), which is defined as the ratio of oxygen consumed by the brain to oxygen delivered. OEF has high potential to improve diagnosis of cerebrovascular diseases (CVD) as a biomarker of hemodynamic function (Donahue et al., 2018). In patients with internal carotid artery stenosis (ICAS), correlations between higher OEF and increased stroke risks were found (Baron et al., 1981; Derdeyn et al., 2002; Powers et al., 2011) as well as local flow-metabolism uncoupling (Goettler et al., 2019). Furthermore, OEF is of interest for neuroscientific applications (Epp et al., 2019), as cerebral oxygen consumption supports neuronal activity (Hyder et al., 2002; Shu et al., 2016a, 2016b; Smith et al., 2002).
OEF measurements were originally established by 15O labeled water PET (Donahue et al., 2018). However, its application is limited due to the administration of short-lived radioactive 15O-tracers, invasive arterial blood sampling and restricted availability of PET-facilities with an onsite cyclotron. Thus, several non-invasive MRI-based alternatives have been proposed (Blockley et al., 2012; Pike, 2012). An easily applicable technique with full brain coverage is multi-parametric quantitative BOLD (mq-BOLD) (Hirsch et al., 2014). This approach relies on the biophysical model of Yablonskiy and Haacke (1994) and derives relative OEF (rOEF) based on three separate measurements of transverse relaxation times by spin-echo (T2) and gradient-echo (T2*) as well as the relative cerebral blood volume (rCBV) (Hirsch et al., 2014). Mq-BOLD is highly promising in several pathologies, such as stroke (Gersing et al., 2015), glioma (Preibisch et al., 2017; Toth et al., 2013; Wiestler et al., 2016) and ICAS (Goettler et al., 2019; Kaczmarz et al., 2020a).
However, systematic errors still limit quantitative interpretations and impede the clinical usability of mq-BOLD. Measured rOEF = 0.6–0.7 in healthy GM was systematically elevated (Goettler et al., 2019; Kaczmarz et al., 2020b) compared to physiologically expected OEF = 0.35–0.56 (Donahue et al., 2018; Marchal et al., 1992), and was thus appropriately named relative OEF (Hirsch et al., 2014). In that regard, T2 overestimations can occur by 2D-GraSE (Gradient and Spin Echo) (Hirsch et al., 2014) as well as 2D-TSE (Turbo Spin Echo) imaging (Seiler et al., 2019). A known issue in T2-mapping is stimulated echoes, which arise from imperfect matching between the excitation and refocusing pulse profiles (Hennig, 1988; Uddin et al., 2013). This is specific to 2D-acquisitions because of slice-selection pulse imperfections near slice edges. To overcome this limitation, non-slice selective (Prasloski et al., 2012a) and 3D-acquisition techniques are advisable (Prasloski et al., 2012b; Whittall et al., 1997), making 3D-GraSE (Oshio and Feinberg, 1991) ideal to overcome T2-related bias in mq-BOLD.
The aim of this study was therefore to improve rOEF mapping by mq-BOLD towards lower, physiologically more meaningful values. We hypothesized significantly reduced T2-related bias by applying 3D-GraSE. We further proposed a procedure to reliably identify and exclude artefact voxels to enhance the sensitivity to pathophysiological focal rOEF increases. To this end, T2 and rOEF were compared between mq-BOLD with 2D-GraSE and 3D-GraSE in a phantom and in 25 subjects, including ICAS-patients.
2. Methods
Quantitative T2-mapping and its impact on mq-BOLD were compared for the initially applied 2D-GraSE and two proposed 3D-GraSE sequences (Fig. 1). The echo timings and scan time of 3D-GraSE-I were similar to 2D-GraSE, whereas 3D-GraSE-II used shorter echo spacing (16 ms→10 ms) with more echoes (8 → 16) and prolonged echo train (128 ms→160 ms). Evaluations were conducted in four steps. First, in a phantom compared with multiple single spin echoes (single-SE) as a reference. Second, R2’ was calculated with additionally acquired T2*-maps in young healthy controls (YHC). In the last two steps, rOEF by mq-BOLD was evaluated in elderly healthy controls (EHC) and ICAS-patients based on the different T2-sequences with additional T2* and rCBV mapping.
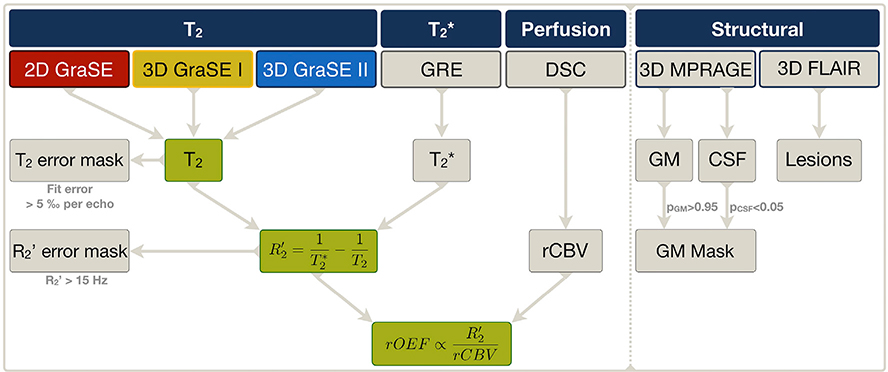
Fig. 1. Overview of applied MRI sequences and derived parameters.
The main purpose of this study was to compare the impact of three different GraSE sequences on T2 mapping, R2’ and rOEF calculations by mq-BOLD. We therefore measured the formerly applied 2D-GraSE (red) as well as the proposed 3D-GraSE-I (yellow) and II sequences (blue). Quantitative T2* imaging was performed by multi-echo gradient echo (GRE) imaging. R2’ was subsequently calculated from T2 and T2*, separately for all three GraSE sequences. Masks of elevated T2 fit-errors (T2 error mask) and of R2’ elevations (R2’ error mask) were generated to exclude artefact voxels. Dynamic susceptibility contrast (DSC) imaging was applied to obtain relative cerebral blood volume (rCBV) maps. By applying the mq-BOLD model to rCBV and R2’, each voxel’s rOEF was calculated for each T2 GraSE sequence, respectively. The impact of the three different GraSE sequences on T2, R2’ and rOEF was evaluated (green). Besides, restrictive GM masks excluding CSF were generated and FLAIR lesions evaluated by structural imaging.
2.1. Phantom and participants
The gel phantom contained six flasks with different T2 relaxation times covering typical GM values (see inlay in Fig. 2B and Supplemental Table 1). For in vivo evaluations, 25 volunteers participated in this prospective study. Participants were enrolled by word-of-mouth advertisement from March until October 2017. Ten YHC (4 females, mean age 28.4 ± 4.1 years, range 21–35 years), twelve EHC (7 females, mean age 71.8 ± 5.3 years, range 63–78 years) and three patients with unilateral, high-grade, asymptomatic, extracranial ICAS were scanned (2 females, mean age 63.0 ± 9.6 years, range 52–70 years). The study was approved by the medical ethical board of the Klinikum rechts der Isar, in line with Human Research Committee guidelines of the Technical University of Munich (TUM). All participants provided informed consent in accordance with the standard protocol approvals. Data of two YHC needed to be excluded due to technical problems during data acquisition.
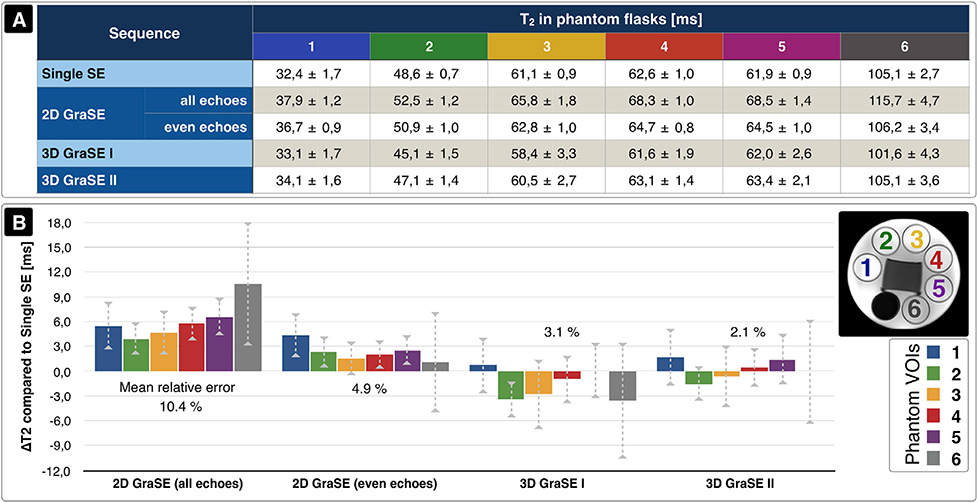
Fig. 2. Comparison of quantitative T2 values measured by different sequences in a phantom.
The phantom contained six flasks with different T2 relaxation times. (A) Average T2 values within each flask are summarized for the multiple single spin echo (Single SE) reference sequence, 2D-GraSE with all and even echoes fitted, 3D-GraSE-I and 3D-GraSE-II (mean ± standard deviation) with all echoes fitted. (B) T2 values derived from single-SE data were used as references and compared to the GraSE results. This ΔT2 is plotted for all phantom flasks and GraSE sequences and scaled in ms. Corresponding values within each flask are shown by consistent color coding (see inlay in B). The average T2 deviation of each GraSE sequence is noted in percent. Best accordance was found for 3D-GraSE-II.
2.2. Image acquisition
Scanning was performed on a 3T Philips Ingenia MR-Scanner (Philips Healthcare, Best, The Netherlands) on software release R5.1.8 with a custom patch. Standard 32-channel head-receive and 16-channel head/neck-receive coils were used. The following imaging protocol was applied (see Fig. 1):
2D-GraSE: 8 echoes; TE1 = ΔTE = 16 ms; TR = 8596 ms; EPI-factor = 7; α = 90°; 180° refocusing control; 30 slices; 0.3 mm gap; voxel size 2.0 × 2.1 × 3.0 mm3; matrix 112 × 91; acq. time 2:23 min.
3D-GraSE-I: 8 echoes; TE1 = ΔTE = 16 ms; TR = 251 ms; oversampling 1.3; EPI-factor = 7; TSE-factor = 8; α = 90°; 180° refocusing control; 30 slices; voxel size 2.0 × 2.1 × 3.0 mm3; matrix 112 × 91; acq. time 2:08 min.
3D-GraSE-II: 16 echoes, TE1 = ΔTE = 10 ms; TR = 487 ms; oversampling 1.3; EPI-factor = 7; TSE-factor = 16; α = 90°; 180° refocusing control; 30 slices; voxel size 2.0 × 2.1 × 3.0 mm3; matrix 112 × 91; acq. time 4:09 min.
Single-SE for phantom reference measurements: TE = 60, 70, 80, 100, 120, 140, 160 ms; TR = 3000 ms, each; 5 slices, acquired voxel size 3.5 × 4.0 × 4.0 mm3; acq. time 2:36 min per TE.
Multi-echo gradient echo (GRE): 12 echoes, TE1 = ΔTE = 5 ms, TR = 1950 ms, α = 30°, 30 slices, matrix 112 × 92, voxel size 2.0 × 2.0 × 3.0 mm3, total acq. time 6:08 min.
DSC-MRI: single-shot GRE-EPI, 80 volumes during injection of weight-adjusted Gd-DOTA bolus (concentration 0.5 mmol/ml; dose 0.1 mmol/kg; minimum 7.5 mmol per subject; flow rate 4 ml/s) with TE = 30 ms, TR = 1513 ms; α = 60°; 26 slices; voxel size 2.0 × 2.0 × 3.5 mm3, acq. time 2:01 min.
MPRAGE: 3D acquisition, TE = 4 ms; TR = 9 ms; α = 8°; TI = 1000 ms; shot interval 2300 ms; SENSE AP/RL 1.5/2.0; 170 slices; matrix 240 × 238; voxel size 1.0 × 1.0 × 1.0 mm3; acq. time 5:59 min
FLAIR: 3D acquisition, TE = 289 ms; TR = 4800 ms; TI = 1650 ms; α = 90°; TSE-factor = 167; 163 slices; matrix 224 × 224; voxel size 1.1 × 1.1 × 1.1 mm3; acq. time 4:34 min.
2.3. Image analysis
Data evaluations were performed using MATLAB R2016b (The MathWorks Inc., Natick, USA) and SPM12 (v6225) (Penny et al., 2011) with custom programs. Quantitative T2 parameter maps were derived by mono-exponential fittings of all echoes for 3D-GraSE and only even echoes for 2D-GraSE in vivo, as initially implemented to reduce stimulated echoes (Hirsch et al., 2014). Multi-echo GRE data were corrected for macroscopic background gradients (Baudrexel et al., 2009; Hirsch and Preibisch, 2013) and motion (Magerkurth et al., 2011) before mono-exponential fitting for T2* and spatial coregistration to T2. Both, T2* and T2-maps were smoothed with a 3D Gaussian filter-kernel of 3 mm prior to the calculation of
| [1] |
DSC data was processed as described previously (Hedderich et al., 2019; Kluge et al., 2016) with CBV normalization to 2.5% in normal appearing white matter (NAWM) (Leenders, 1994), yielding relative CBV (rCBV). Following the mq-BOLD approach, rOEF was calculated as
| [2] |
with , B0 = 3T and Δχ = Hct·Δχ0 = 0.35·0.264·10−6 = 0.924·10−7 (Hirsch et al., 2014).
To investigate the impact of the different T2-mapping sequences, R2’ and rOEF were calculated with the same T2* and rCBV (Fig. 1). For quality assessment, all parameter maps were screened specifically for motion artefacts and spatial misregistration (raters: SK, CP).
2.4. Artefact removal
To account for mismatches between the measured data and mono-exponential fittings of T2 and T2*, fit-errors were evaluated on a voxel-wise basis and normalized by the number of acquired echoes. Empirically set thresholds were applied and checked carefully (SK, CP). Voxels with fit-errors>5‰ per echo were excluded from data evaluation (Supplemental Fig. 1). An additional threshold of R2′<15 s−1 was applied (Kaczmarz et al., 2020b) to exclude areas with iron-induced focal R2’ increases, especially in deep GM regions. This also excluded areas with higher macroscopic background gradients, as corrections are only reliable up to approximately 220 μT/m (Hirsch and Preibisch, 2013).
2.5. Statistical analyses
Phantom measurements were evaluated by averaging T2-values within VOIs in each flask. 2D-GraSE was compared with 3D-GraSE-I, II and single-SE reference values (Prasloski et al., 2012b). For in-vivo evaluations, restrictive GM masks were generated from MPRAGE segmentations (pGM>0.95) with additional CSF exclusion (pCSF<0.05). Within these masks, average T2, T2*, R2’, rCBV, and rOEF values were calculated. The impact of 2D vs. 3D-GraSE on T2, R2’ and rOEF values in GM on group level was illustrated by paired scatter plots. Significance of group mean value differences was tested by ANOVA, homogeneity of variance asserted using Levene’s test and pairwise correlations corrected for multiple comparisons with Tukey or Games-Howell post-hoc analysis in SPSS (v26, IBM Corp., Armonk, USA). Values of p < 0.05 were considered statistically significant. Distributions of the fit-errors and parameter values were compared by histograms.
3. Results
In the phantom, single-SE reference measurements revealed average transverse T2 relaxation times between 32.4 and 105.1 ms within the six flasks. By comparison, the original 2D-GraSE sequence overestimated T2 by 10.4% with all echo fitting and 4.9% with even echoes only. The 3D-GraSE-I and II sequences reduced overestimations to 3.1% and 2.1%, respectively (Fig. 2).
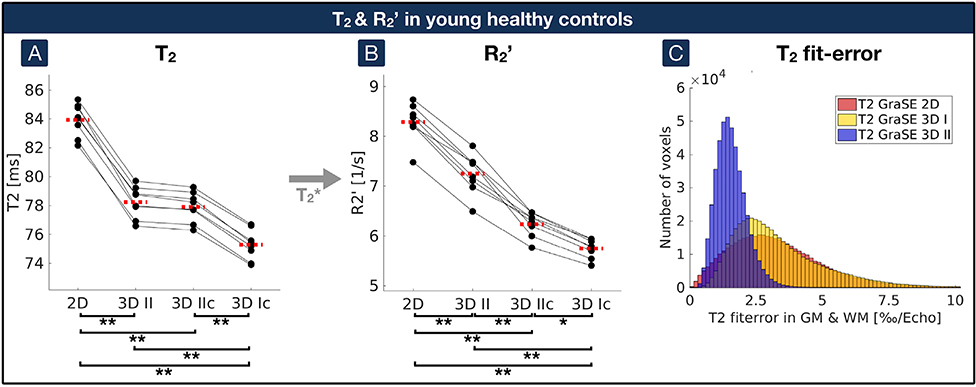
In YHC, quantitative T2-mapping by 2D-GraSE with even echo fitting yielded T2 = 83.9 ± 1.1 ms. 3D-GraSE-I and II decreased T2 by −8.8% and −6.8%, respectively (T2 = 76.5 ± 1.2 ms and 78.2 ± 1.1 ms; p < 0.001, each). Consequently, R2’ decreased by −14.5% and −13.3% for 3D-GraSE-I and II (R2′ = 6.9 ± 0.3 s−1 and 7.2 ± 0.4 s−1) compared to R2′ = 8.3 ± 0.4 s−1 by 2D-GraSE with T2* = 53.9 ± 1.7 ms (Table 1). Additional artefact exclusion further reduced the T2 and R2 values (3D-GraSE-I: T2 = 75.2 ± 1.0 ms and R2′ = 5.6 ± 0.2 s−1; 3D-GraSE-II: T2 = 77.9 ± 1.0 ms and R2′ = 6.1 ± 0.2 s−1). While artefact exclusion effects on T2-values were comparably weak (p > 0.5; Fig. 3A), R2’ values decreased by up to −32.5% (p < 0.001; Fig. 3B). Fit-errors of 3D-GraSE-II were much lower (0.1%) compared to 3D-GraSE-I (19.6%) and 2D-GraSE (15.3%, Fig. 3C; Table 2). For 3D-GraSE-II, most excluded voxels were affected by T2* fit-errors (83.3%), followed by R2’ elevations (51.5%) and only minor T2 fit-errors (0.9%). The fraction of voxels in GM excluded due to R2’ thresholding was similar between 2D (9.7%) and 3DGraSE-II (7.3%). Due to lowest errors and time restrictions, 3D-GraSE-II was applied in the following evaluations in EHC as well as ICAS-patients and compared with 2D-GraSE.
Table 1. Summary of average parameter values for all groups.
Quantitative parameter values were compared for YHC, EHC and ICAS-patients. The number of scanned participants is shown for each group. Two YHC were excluded due to data acquisitions problems. Average T2 values in GM were calculated for all GraSE sequences (group mean ± standard deviation), each with and without artefact exclusion (“corrected”). Displayed 2D-GraSE values were generated by fitting of even echoes only. Resulting R2’ values were calculated from GraSE-based T2 values and GRE-based T2*. For EHC and ICAS-patients, rOEF values were calculated from R2’ and additionally acquired DSC-based rCBV maps. Note, 3D-GraSE-I and II decreased T2, R2’ and rOEF towards physiologically more realistic values for all subject groups.
| Participants |
Average T2 in GM [ms] |
|||||||
| Group | n | 2D GraSE | 2D GraSE corrected | 3D GraSE I | 3D GraSE I corrected | 3D GraSE II | 3D GraSE II corrected | |
| YHC | 10 | 83.9 ± 1.1 | 81.6 ± 0.9 | 76.5 ± 1.2 | 75.2 ± 1.0 | 78.2 ± 1.1 | 77.9 ± 1.0 | |
| EHC | 12 | 89.2 ± 3.1 | 85.4 ± 2.5 | – | – | 81.6 ± 1.9 | 81.2 ± 2.0 | |
| ICAS | 3 | 89.6 ± 1.3 | 85.9 ± 1.8 | – | – | 82.9 ± 0.7 | 82.2 ± 1.2 | |
|
Participants |
Average R2’ in GM [1/s] |
T2* [ms] |
||||||
| Group | n | 2D GraSE | 2D GraSE corrected | 3D GraSE I | 3D GraSE I corrected | 3D GraSE II | 3D GraSE II corrected | |
| YHC | 10 | 8.3 ± 0.4 | 6.9 ± 0.3 | 6.9 ± 0.3 | 5.6 ± 0.2 | 7.2 ± 0.4 | 6.1 ± 0.2 | 53.9 ± 1.7 |
| EHC | 12 | 8.5 ± 0.7 | 7.7 ± 0.6 | – | – | 7.4 ± 0.7 | 6.6 ± 0.6 | 55.7 ± 4.0 |
| ICAS | 3 | 8.4 ± 0.7 | 7.3 ± 0.4 | – | – | 7.6 ± 0.9 | 6.6 ± 0.5 | 56.5 ± 2.4 |
| Participants | Average rOEF in GM [] | rCBV [%] | ||||||
| Group | n | 2D GraSE | 2D GraSE corrected | 3D GraSE I | 3D GraSE I corrected | 3D GraSE II | 3D GraSE II corrected | |
| YHC | 10 | – | – | – | – | – | – | – |
| EHC | 12 | 0.70 ± 0.08 | 0.61 ± 0.06 | – | – | 0.59 ± 0.08 | 0.51 ± 0.06 | 4.71 ± 0.27 |
| ICAS | 3 | 0.65 ± 0.05 | 0.59 ± 0.02 | – | – | 0.58 ± 0.06 | 0.52 ± 0.03 | 4.68 ± 0.04 |
Fig. 3. Impact of GraSE sequences and artefact exclusion on T2 and R2’ compared by paired scatterplots in young healthy controls.
(A) Quantitative T2 values in GM were compared between the 2D-GraSE (”2D”), 3D-GraSE-II (”3D II”), 3D-GraSE-II with additional artefact exclusion (”3D IIc”) and 3D-GraSE-I with artefact exclusion (”3D Ic”). Voxels with elevated T2 fit-errors or R2’ elevations were excluded by artefact exclusion. (B) R2’ was calculated based on T2 values from the different GraSE sequences with the same quantitative T2* map. (A, B) Single participant’s average parameter values in GM are represented by black dots. Corresponding values of the same participant are connected by black lines. Median values on group level are indicated by red dashed lines for each parameter and acquisition technique. Asterisks indicate significant differences with p < 0.03, double asterisks p < 0.001 with correction for multiple comparisons. (C) Errors of the T2 fits were compared for the three GraSE sequences. Average errors within GM of all participants are shown in the histogram. Voxels with fit-errors > 5‰ per echo were excluded from the artefact-corrected analyses (”3D Ic” and ”3D IIc”). Note much lower fit-errors in 3D-GraSE-II, indicating better fitting quality (blue).
Table 2. Summary of average fit-errors for all groups.
Fit-errors were compared for YHC, EHC and ICAS-patients. The number of scanned participants is shown for each group. Fit-errors in GM were evaluated for the 2D-GraSE, 3D-GraSE-I and 3D-GraSE-II, scaled in permille per echo (group mean ± standard deviation). Voxels with fit-errors>5‰ per echo were excluded. The corresponding fraction of excluded GM voxels due to T2 fit-errors are compared for all sequences and groups. Note, clearly decreased errors by 3D-GraSE-II with only few excluded voxels (≤1%).
| Participants |
Average T2 fit-error in GM [‰/echo] |
Fraction of excluded GM voxels (with error > 5‰/echo) [%] |
|||||
|---|---|---|---|---|---|---|---|
| Group | n | 2D GraSE | 3D GraSE I | 3D GraSE II | 2D GraSE | 3D GraSE I | 3D GraSE II |
| YHC | 10 | 3.2 ± 0.2 | 3.5 ± 0.3 | 1.5 ± 0.1 | 15.3 | 19.6 | 0.1 |
| EHC | 12 | 4.0 ± 0.3 | – | 1.8 ± 0.1 | 26.4 | – | 0.3 |
| ICAS | 3 | 4.0 ± 0.2 | – | 1.8 ± 0.2 | 28.4 | – | 1.0 |
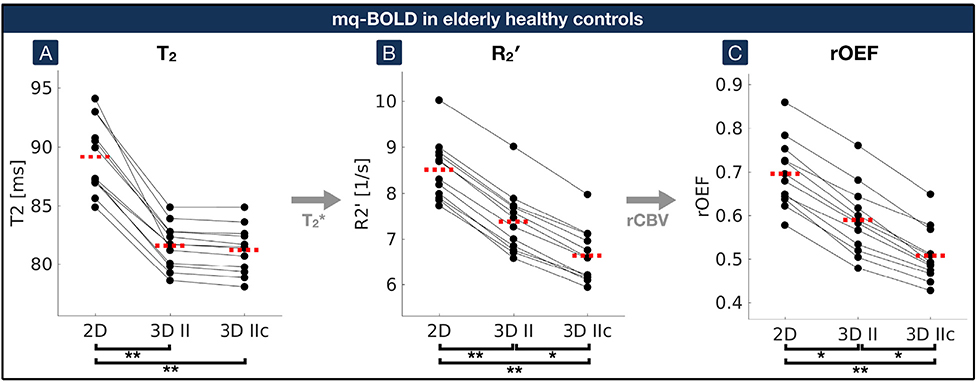
In EHC, 2D-GraSE yielded T2-values of 89.2 ± 3.1 ms. Average GM values of R2′ = 8.5 ± 0.7 s−1 and rOEF = 0.70 ± 0.08 were calculated from additional measurements of T2* (55.7 ± 4.0 ms) and rCBV (4.71 ± 0.27%) (Table 1). The application of 3D-GraSE-II significantly decreased T2 by −8.5% (T2 = 81.6 ± 1.9 ms; p < 0.001), R2’ by −12.9% (R2′ = 7.4 ± 0.7 s−1, p < 0.001) and rOEF by −15.7% (rOEF = 0.59 ± 0.08, p < 0.03). Artefact exclusion further decreased R2’ and rOEF by up to −27.1% in total (R2′ = 6.6 ± 0.6 s−1; rOEF = 0.51 ± 0.06; p < 0.03; Fig. 4). FLAIR lesion gradings with an average Fazekas-score of 1.3 indicated only minor microangiopathic changes (Fazekas et al., 1987). None of the participants had subacute or older territorial infarct lesions.
Fig. 4. Impact of 2D and 3D-GraSE-II sequences on mq-BOLD parameters by paired scatterplots in elderly healthy controls.
(A) Quantitative T2-values acquired by 2D-GraSE (”2D”), 3D-GraSE-II (”3D II”) and with additional artefact correction (”3D IIc”) were compared. (B) R2’ was calculated based on T2 values obtained by the different GraSE sequences and the quantitative T2*-map. (C) R2’ values were combined with DSC-based rCBV to calculate rOEF according to the mq-BOLD model. Single participant’s average parameter values in GM are represented by black dots. Corresponding values of the same participant are connected by black lines. Median values on group level are indicated by red dashed lines for each parameter and sequence. Asterisks indicate significant differences with p < 0.03, double asterisks p < 0.001 with correction for multiple comparisons. While artefact correction of 3D-GraSE (”3D IIc”) has a comparably low impact on T2, corresponding R2’ and rOEF values were significantly decreased.
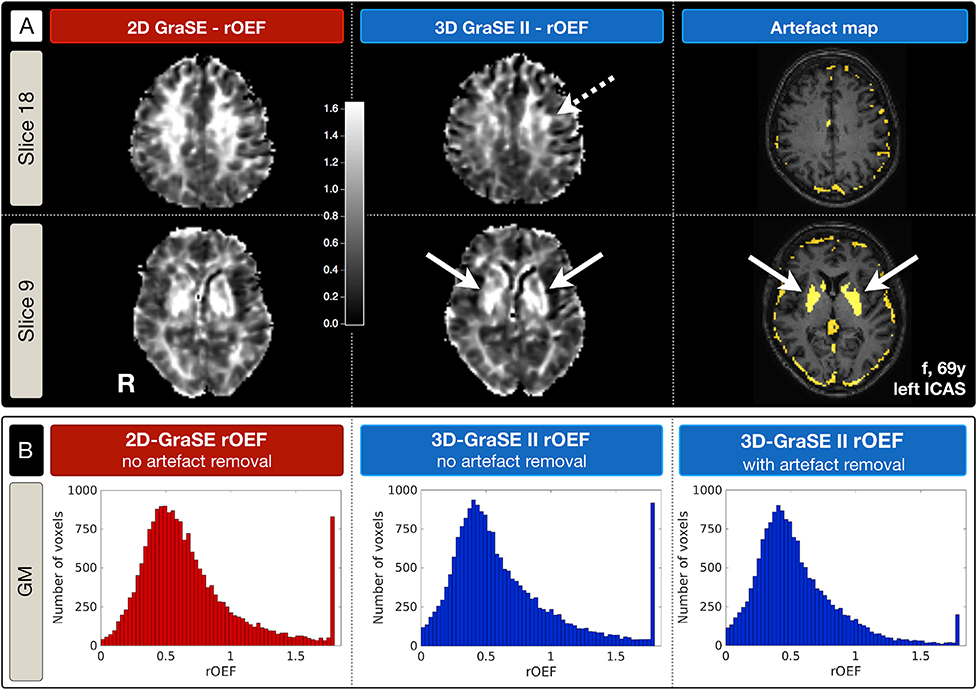
In ICAS, all parameter values decreased with 3D-GraSE-II and artefact exclusion, yielding similar values as in EHC (Table 1). Furthermore, focal rOEF hyperintensities ipsilateral to the stenosis were enhanced and only recognizable by 3D-GraSE-II (Fig. 5A). Two regions stand out in the artefact maps. First, well-known iron deposition in the striatum corresponds to maximum rOEF-values. Second, artefact voxels occur along the brains’ surface towards the cranial bone (Fig. 5A). Artefact removal in 3D-GraSE-II improved rOEF towards lower values and additionally decreased the number of voxels with maximum rOEF values (Fig. 5B).
Fig. 5. Exemplary rOEF and artefact maps of a left-sided ICAS-patient comparing 2D-GraSE vs. 3D-GraSE-II.
(A) rOEF-maps derived by 2D-GraSE (indicated in red) vs. 3D-GraSE-II (blue) and the corresponding artefact map of 3D-GraSE-II are compared in two axial slices. All rOEF-maps are displayed within the same colormap scaling (0 to 1.6). Note that focal rOEF hyperintensities ipsilateral to the stenosis, potentially related to pathophysiological effects, are located at the perfusion territories border zone (Supplemental Fig. 4) and only apparent by 3D-Grase-II based rOEF (dashed arrow). Artefact voxels with elevated fitting errors of T2 and T2* or R2’ increases are shown in yellow. The striatum with high-iron content is clearly visible in the artefact-map and corresponds to maximum rOEF values (solid arrows). (B) rOEF value distributions are compared by histograms. They highlight the lower rOEF values by 3D-GraSE-II compared to 2D-GraSE. Moreover, additional artefact removal of 3D-GraSE-II reduced the frequency of maximum values of rOEF = 1.8.
4. Discussion
T2-mapping by 2D-vs 3D-GraSE was compared with regard to their impact on rOEF values modeled by mq-BOLD. Formerly overestimated T2, R2’ and rOEF values significantly improved by 3D-GraSE, as hypothesized. Remarkably, 3D-GraSE-II also improved the fit quality, lowering the number of excluded artefact voxels due to T2 fit-errors in ICAS-patients by the factor 30. The specific impact of 3D-GraSE on T2, R2’ and rOEF are discussed below.
4.1. Impact on T2
The phantom measurements confirmed T2 overestimations by 2D-GraSE (Hirsch et al., 2014). 3D-GraSE lowered T2-values, as hypothesized, only deviating 2.1% from single-SE values. Similarly, comparisons in YHC confirmed overestimations by 2D-GraSE, whereas lower average GM values of T2 = 76.5 ms were measured with 3D-GraSE-I. This agrees well with literature values at 3T of T2 = 73.5 ms in YHC by single-SE (Hirsch et al., 2014) and T2 = 76.2 ms in EHC by multi-SE (Christen et al., 2012). The proposed artefact voxel exclusion further improved T2-values. While both 3D-GraSE sequences improved T2, 3D-GraSE-II performed best with regards to lowest fit errors, due to its improved echo sampling. Evaluations of average fit-errors revealed improvements in 3D-GraSE vs. 2D-GraSE by a factor up to 2 (Supplemental Fig. 2) and reduced voxel exclusions due to T2 fit-errors up 150 times (Table 2).
Literature values of alternative T2-mapping by TSE yielded much higher healthy average GM values of 119 ms (Sedlacik et al., 2014; Wagner et al., 2012, 2015), which necessitates sophisticated quantitative T2 post-processing corrections (Noth et al., 2017). Thus, 3D-GraSE is ideal for fast, quantitative T2-mapping with full brain coverage (Prasloski et al., 2012b; Whittall et al., 1997).
4.2. Impact on R2′
R2’ was calculated based on T2 and additional T2*-mapping. As for T2, average R2’ values decreased with 3D-GraSE to R2′ = 6.1 s−1 in YHC, with artefact exclusion. This is in good agreement with literature values by GRE and TSE of similar aged healthy participants in frontal cortex with R2′ = 7.4 s−1 (Sedlacik et al., 2014) and R2′ = 7.9 s−1 (Wagner et al., 2012). Remaining deviations may be due to fittings of only 3 and 5 echoes for T2 in those studies, respectively, which might be insufficient (Whittall et al., 1997). In general, reported average R2’ values vary. While much higher average GM values of R2′ = 12.0 s−1 have been reported by GRE and TSE (Wagner et al., 2015), other methods reported lower values, specifically R2′ = 5.1 s−1 by GRE (Ulrich and Yablonskiy, 2015), R2′ = 3.0 s−1 and 4.4 s−1 by asymmetric spin echo (ASE) (An and Lin, 2003; Blockley and Stone, 2016), R2′ = 2.9 s−1 by GESSE (He and Yablonskiy, 2007) and R2′ = 2.7 s−1 by Gradient Echo Sampling of FID and Echo (GESFIDE) (Ni et al., 2014).
The proposed artefact exclusion had stronger effects on average R2’ than T2 values, due to removal of R2’ elevations, which were caused by strong susceptibility gradients at the borders and in fronto-basal and temporal brain regions as well as iron deposition in the striatum (Fig. 5A and Supplemental Fig. 3). As R2’ values decreased with 3D-GraSE-II compared to 2D-GraSE, slightly fewer voxels were excluded by R2’ thresholding. Iron concentration increases with age could explain increased number of artefact voxels in EHC vs. YHC. The observed R2’ increases with age also agree with literature (Sedlacik et al., 2014).
4.3. Impact on rOEF
Maps of rOEF were calculated in EHC and ICAS-patients based on R2’ and additional rCBV measurements. 2D-GraSE yielded rOEF = 0.70 in accordance with previously reported overestimations (Hirsch et al., 2014). But rOEF was significantly lower with 3D-GraSE. Artefact exclusion further decreased the average GM value to rOEF = 0.51 in EHC. Overall, rOEF values decreased by −27.1% by 3D-GraSE and artefact exclusion, which is more similar to literature values from PET measurements (Donahue et al., 2018; Marchal et al., 1992). While a similar mq-BOLD implementation using the same model yielded much lower average OEF = 0.33 in healthy controls (Christen et al., 2012), they restricted T2 imaging echo-times to maximum 55 ms. Together with comparably high CBV values, this explains their systematically lower OEF values (see Eqs. (1) and (2)).
Values of T2, R2’ and rOEF with 3D-GraSE were comparable in EHC and ICAS-patients. This is in line with previously observed unaffected rOEF on group level in high-grade stenosis patients (Bouvier et al., 2015; Goettler et al., 2019). Nevertheless, focal rOEF increases have been found (Kaczmarz et al., 2020a), which potentially have a high clinical relevance as an indicator of misery perfusion to assess individual stroke risks (Baron et al., 1981). Interestingly, those focal rOEF elevations were only visible with 3D-GraSE (Fig. 5A). Pathophysiological origins of rOEF elevations are supported by their localization at the border zone between perfusion territories (Supplemental Fig. 4), measured by super-selective arterial spin labeling (Helle et al., 2010). This increased sensitivity of mq-BOLD with 3D-GraSE in an ICAS-patient, as a proof-of-principle, is highly promising for the detection of even subtle oxygenation changes.
4.4. Applicability and limitations
An obvious strength of this study is its potential for widespread clinical applications due to standard sequences. Minor remaining T2 variations may be attributed to diffusion effects, especially for single-SE (Carr and Purcell, 1954), and known echo timing dependencies, which are also related to diffusion (Poon and Henkelman, 1992; Whittall et al., 1999). In vivo scans can be additionally affected by partial volume effects (PVE), especially in presence of CSF contamination (Whittall et al., 1999), and multi-compartmental tissue structures (MacKay et al., 2006). Nevertheless, mono-exponential fittings were applied to achieve full brain volume coverage within clinically feasible scan times, while multi-exponential fittings would require higher SNR (Whittall et al., 1997).
Measured rOEF values may be slightly higher than literature PET values due to PVE, especially with CSF (He and Yablonskiy, 2007; Stone and Blockley, 2017). Smoothing may further enhance those effects, but was applied as spatial resolutions of the sequences were only harmonized as far as possible, while maintaining parameters of standard clinical protocols. Those effects were accounted for by restrictive GM thresholding and CSF exclusion.
Furthermore, the mq-BOLD implementation neglects intravascular signals (Hirsch et al., 2014; Yablonskiy and Haacke, 1994), even though effects on T2* might be non-negligible at 3T (Donahue et al., 2011; Li and van Zijl, 2020). While profound investigations based on a recent model (Berman and Pike, 2018) found minor intravascular effects on q-BOLD parameter estimates in ASE, intravascular effects were demonstrated in simulations of a GESSE sequence (Stone et al., 2019). Thus, future consideration of intravascular signal contributions in mq-BOLD may be beneficial (He and Yablonskiy, 2007). Other potential confounders are neglects of vessel size dependent hematocrit variations and imperfect SE refocusing (Berman et al., 2018). While 3D-GraSE lowered whole brain rOEF values (Fig. 5A), evaluations were restricted to GM due to known artefactual GM-WM rOEF contrast, mainly caused by approximating venous CBV by total rCBV (Hirsch et al., 2014) and, although minor on average, vessel orientation effects in WM (Kaczmarz et al., 2020b). CBV normalization to NAWM might limit sensitivity to global differences between subjects, groups and in longitudinal studies. While the threshold of R2′<15 s−1 was applied based on previous work, lower thresholds, in general, directly result in lower average rOEF values. Thus, excluded voxels were carefully evaluated to avoid potential confounds.
4.5. Outlook
The presented improvements by 3D-GraSE are also highly promising for R2′-based calibrated BOLD measurements as a viable alternative to complex gas challenges (He and Yablonskiy, 2007; Kida et al., 2000; Liu et al., 2019; Shu et al., 2016a). Physiological underpinnings of the BOLD signal could hereby be measured in activation studies (Blockley et al., 2012).
5. Conclusions
We demonstrated the successful implementation of 3D-GraSE-based T2-imaging in mq-BOLD for whole brain rOEF mapping within clinically applicable scan times. Measured T2 values with 3D-GraSE were in excellent agreement with the literature. With additional artefact exclusion, formerly overestimated rOEF decreased up to −27%. Measured average rOEF = 0.51 in GM is considerably closer to literature values. Interestingly, focal rOEF increases in an ICAS-patient only became apparent by 3D-GraSE, which shows great promise for future clinical applications of mq-BOLD.
Supplementary Material
Acknowledgments
We would like to thank Dr. Jens Göttler, Dr. Nico Sollmann and Ilias Tsiachristos for their support in participant recruitment and during the MRI measurements. We are very grateful to Dr. Andreas Hock from Philips Healthcare for his support regarding the MR-sequences and PD Dr. Michael Helle, also from Philips Healthcare, for his support on the perfusion territory mapping. We also thank Prof. Dr. Ralf Deichmann from the Goethe University in Frankfurt/Main for his support to correct T2* parameter maps for motion and macroscopic background gradients. We thank all our study participants for their efforts to take part in this study.
This work was supported by the Friedrich-Ebert-Stiftung (grant to SK), the Dr.-Ing. Leonhard Lorenz-Stiftung (grant to SK 971/19) and the German Research Foundation (DFG) – Project number PR 1039/6-1 (grant to CP). FH was supported by NIH grants (R01 MH-067528, R01 NS-100106, P30 NS-052519).
Abbreviations
- ANOVA
Analysis of variance
- ASE
Asymmetric spin echo
- BOLD
Blood-oxygenation-level-dependent
- CBV
Cerebral blood volume
- CSF
Cerebrospinal fluid
- DSC
Dynamic susceptibility contrast
- EHC
Elderly healthy control
- EPI
Echo planar imaging
- FID
Free induction decay
- FLAIR
Fluid-attenuated inversion recovery
- GESFIDE
Gradient Echo Sampling of FID and Echo
- GESSE
Gradient Echo Sampling of Spin Echo
- GM
Gray matter
- GraSE
Gradient and spin echo
- GRE
Gradient echo
- ICAS
Internal carotid artery stenosis
- MPRAGE
Magnetization prepared rapid acquisition gradient echo
- mq-BOLD
Multi-parametric quantitative BOLD
- MRI
Magnetic resonance imaging
- NAWM
Normal appearing white matter
- OEF
Oxygen extraction fraction
- PET
Positron Emission Tomography
- PVE
Partial volume effect
- q-BOLD
Quantitative BOLD
- rCBV
Relative cerebral blood volume
- rOEF
Relative oxygen extraction fraction
- single-SE
Single spin echo
- TE
Echo time
- TI
Inversion time
- TR
Repetition time
- TSE
Turbo spin echo
- VOI
Volume of interest
- WM
White matter
- YHC
Young healthy control
Footnotes
Declaration of conflicting interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.117095.
Data and code availability
For reasons of ethics and privacy issues of the acquired clinical data, the data is only available via a request to the authors. Institutional restrictions of patient privacy then require a formal data sharing agreement. The applied MATLAB code is available upon request. Sharing of applied sequence modifications is limited by a nondisclosure agreement. The applied sequence changes have been published (Hirsch and Preibisch, 2013) and further information will be shared on request. Custom MATLAB code for post-processing of mq-BOLD MRI data for neuro-scientific studies is available at https://doi.org/10.5281/zenodo.3909300 and https://gitlab.lrz.de/nmrm_lab/public_projects/mq-BOLD.
References
- An H, Lin W, 2003. Impact of intravascular signal on quantitative measures of cerebral oxygen extraction and blood volume under normo- and hypercapnic conditions using an asymmetric spin echo approach. Magn. Reson. Med 50, 708–716. [DOI] [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P, 1981. Reversal of focal misery-perfusion syndrome by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke 12, 454–459. [DOI] [PubMed] [Google Scholar]
- Baudrexel S, Volz S, Preibisch C, Klein JC, Steinmetz H, Hilker R, Deichmann R, 2009. Rapid single-scan T 2*-mapping using exponential excitation pulses and image-based correction for linear background gradients. Magn. Reson. Med 62, 263–268. [DOI] [PubMed] [Google Scholar]
- Berman AJL, Mazerolle EL, MacDonald ME, Blockley NP, Luh WM, Pike GB, 2018. Gas-free calibrated fMRI with a correction for vessel-size sensitivity. Neuroimage 169, 176–188. [DOI] [PubMed] [Google Scholar]
- Berman AJL, Pike GB, 2018. Transverse signal decay under the weak field approximation: theory and validation. Magn. Reson. Med 80, 341–350. [DOI] [PubMed] [Google Scholar]
- Blockley NP, Griffeth VEM, Buxton RB, 2012. A general analysis of calibrated BOLD methodology for measuring CMRO(2) responses: comparison of a new approach with existing methods. Neuroimage 60, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blockley NP, Stone AJ, 2016. Improving the specificity of R2′ to the deoxyhaemoglobin content of brain tissue: prospective correction of macroscopic magnetic field gradients. Neuroimage 135, 253–260. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Detante O, Tahon F, Attye A, Perret T, Chechin D, Barbieux M, Boubagra K, Garambois K, Tropres I, Grand S, Barbier EL, Krainik A, 2015. Reduced CMRO2 and cerebrovascular reserve in patients with severe intracranial arterial stenosis: a combined multiparametric qBOLD oxygenation and BOLD fMRI study. Hum. Brain Mapp 36, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr HY, Purcell EM, 1954. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys. Rev 94, 630–638. [Google Scholar]
- Christen T, Schmiedeskamp H, Straka M, Bammer R, Zaharchuk G, 2012. Measuring brain oxygenation in humans using a multiparametric quantitative blood oxygenation level dependent MRI approach. Magn. Reson. Med 68, 905–911. [DOI] [PubMed] [Google Scholar]
- Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, Powers WJ, 2002. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain 125, 595–607. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Achten E, Cogswell PM, De Leeuw FE, Derdeyn CP, Dijkhuizen RM, Fan AP, Ghaznawi R, Heit JJ, Ikram MA, Jezzard P, Jordan LC, Jouvent E, Knutsson L, Leigh R, Liebeskind DS, Lin W, Okell TW, Qureshi AI, Stagg CJ, van Osch MJ, van Zijl PC, Watchmaker JM, Wintermark M, Wu O, Zaharchuk G, Zhou J, Hendrikse J, 2018. Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. J. Cerebr. Blood Flow Metabol 38, 1391–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Hoogduin H, van Zijl PC, Jezzard P, Luijten PR, Hendrikse J, 2011. Blood oxygenation level-dependent (BOLD) total and extravascular signal changes and DeltaR2* in human visual cortex at 1.5, 3.0 and 7.0 T. NMR Biomed. 24, 25–34. [DOI] [PubMed] [Google Scholar]
- Epp S, Preibisch C, Andrews-Hanna J, Riedl V, 2019. Towards a Metabolic Baseline for Default Mode Network Activations and Deactiviations. OHBM, Rome, Italy. [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA, 1987. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol 149, 351–356. [DOI] [PubMed] [Google Scholar]
- Gersing AS, Ankenbrank M, Schwaiger BJ, Toth V, Janssen I, Kooijman H, Wunderlich S, Bauer JS, Zimmer C, Preibisch C, 2015. Mapping of cerebral metabolic rate of oxygen using dynamic susceptibility contrast and blood oxygen level dependent MR imaging in acute ischemic stroke. Neuroradiology 57, 1253–1261. [DOI] [PubMed] [Google Scholar]
- Goettler J, Kaczmarz S, Kallmayer M, Wustrow I, Eckstein HH, Zimmer C, Sorg C, Preibisch C, Hyder F, 2019. Flow-metabolism uncoupling in patients with asymptomatic unilateral carotid artery stenosis assessed by multi-modal magnetic resonance imaging. J. Cerebr. Blood Flow Metabol 39, 2132–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Yablonskiy DA, 2007. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn. Reson. Med 57, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderich D, Kluge A, Pyka T, Zimmer C, Kirschke JS, Wiestler B, Preibisch C, 2019. Consistency of normalized cerebral blood volume values in glioblastoma using different leakage correction algorithms on dynamic susceptibility contrast magnetic resonance imaging data without and with prebolus. J. Neuroradiol 46 (1), 44–51. [DOI] [PubMed] [Google Scholar]
- Helle M, Norris DG, Rüfer S, Alfke K, Jansen O, van Osch MJP, 2010. Superselective pseudocontinuous arterial spin labeling. Magn. Reson. Med 64, 777–786. [DOI] [PubMed] [Google Scholar]
- Hennig J, 1988. Multiecho imaging sequences with low refocusing flip angles. J. Magn. Reson 78, 397–407, 1969. [Google Scholar]
- Hirsch NM, Preibisch C, 2013. T2* mapping with background gradient correction using different excitation pulse shapes. AJNR Am. J. Neuroradiol 34, E65–E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch NM, Toth V, Forschler A, Kooijman H, Zimmer C, Preibisch C, 2014. Technical considerations on the validity of blood oxygenation level-dependent-based MR assessment of vascular deoxygenation. NMR Biomed. 27, 853–862. [DOI] [PubMed] [Google Scholar]
- Hyder F, 2009. Dynamic imaging of brain function. Methods Mol. Biol 489, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG, 2002. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc. Natl. Acad. Sci. U. S. A 99, 10771–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarz S, Gottler J, Petr J, Hansen MB, Mouridsen K, Zimmer C, Hyder F, Preibisch C, 2020a. Hemodynamic impairments within individual watershed areas in asymptomatic carotid artery stenosis by multimodal MRI. J. Cerebr. Blood Flow Metabol 271678×20912364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarz S, Gottler J, Zimmer C, Hyder F, Preibisch C, 2020b. Characterizing white matter fiber orientation effects on multi-parametric quantitative BOLD assessment of oxygen extraction fraction. J. Cerebr. Blood Flow Metabol 40, 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida I, Kennan RP, Rothman DL, Behar KL, Hyder F, 2000. High-resolution CMRO2 mapping in rat cortex: a multiparametric approach to calibration of BOLD image contrast at 7 tesla. J. Cerebr. Blood Flow Metabol 20, 847–860. [DOI] [PubMed] [Google Scholar]
- Kluge A, Lukas M, Toth V, Pyka T, Zimmer C, Preibisch C, 2016. Analysis of three leakage-correction methods for DSC-based measurement of relative cerebral blood volume with respect to heterogeneity in human gliomas. Magn. Reson. Imaging 34, 410–421. [DOI] [PubMed] [Google Scholar]
- Leenders KL, 1994. PET: blood flow and oxygen consumption in brain tumors. J. Neuro Oncol 22, 269–273. [DOI] [PubMed] [Google Scholar]
- Li W, van Zijl PCM, 2020. Quantitative theory for the transverse relaxation time of blood water. NMR Biomed. 33, e4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu EY, Guo J, Simon AB, Haist F, Dubowitz DJ, Buxton RB, 2019. The potential for gas-free measurements of absolute oxygen metabolism during both baseline and activation states in the human brain. Neuroimage, 116342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay A, Laule C, Vavasour I, Bjarnason T, Kolind S, Mädler B, 2006. Insights into brain microstructure from the T2 distribution. Magn. Reson. Imag 24, 515–525. [DOI] [PubMed] [Google Scholar]
- Magerkurth J, Volz S, Wagner M, Jurcoane A, Anti S, Seiler A, Hattingen E, Deichmann R, 2011. Quantitative T*2-mapping based on multi-slice multiple gradient echo flash imaging: retrospective correction for subject motion effects. Magn. Reson. Med 66, 989–997. [DOI] [PubMed] [Google Scholar]
- Marchal G, Rioux P, Petit-Tabouè M-C, Sette G, Travère J-M, Le Poec C, Courtheoux P, Derlon J-M, Baron J-C, 1992. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch. Neurol 49, 1013–1020. [DOI] [PubMed] [Google Scholar]
- Ni W, Christen T, Zun Z, Zaharchuk G, 2014. Comparison of R2′ measurement methods in the normal brain at 3 tesla. Magn. Reson. Med 73, 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth U, Shrestha M, Schure JR, Deichmann R, 2017. Quantitative in vivo T2 mapping using fast spin echo techniques - a linear correction procedure. Neuroimage 157, 476–485. [DOI] [PubMed] [Google Scholar]
- Oshio K, Feinberg DA, 1991. GRASE (Gradient-and Spin-Echo) imaging: a novel fast MRI technique. Magn. Reson. Med 20, 344–349. [DOI] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, 2011. Statistical Parametric Mapping: the Analysis of Functional Brain Images. Elsevier Science. [Google Scholar]
- Pike GB, 2012. Quantitative functional MRI: Concepts, issues and future challenges. Neuroimage 62, 1234–1240. [DOI] [PubMed] [Google Scholar]
- Poon CS, Henkelman RM, 1992. Practical T2 quantitation for clinical applications. J. Magn. Reson. Imag 2, 541–553. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Clarke WR, Grubb RL Jr., Videen TO, Adams HP Jr., Derdeyn CP, Investigators C, 2011. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. J. Am. Med. Assoc 306, 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasloski T, Madler B, Xiang QS, MacKay A, Jones C, 2012a. Applications of stimulated echo correction to multicomponent T2 analysis. Magn. Reson. Med 67, 1803–1814. [DOI] [PubMed] [Google Scholar]
- Prasloski T, Rauscher A, MacKay AL, Hodgson M, Vavasour IM, Laule C, Madler B, 2012b. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. Neuroimage 63, 533–539. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Shi K, Kluge A, Lukas M, Wiestler B, Gottler J, Gempt J, Ringel F, Al Jaberi M, Schlegel J, Meyer B, Zimmer C, Pyka T, Forster S, 2017. Characterizing hypoxia in human glioma: a simultaneous multimodal MRI and PET study. NMR Biomed. 30. [DOI] [PubMed] [Google Scholar]
- Sedlacik J, Boelmans K, Löbel U, Holst B, Siemonsen S, Fiehler J, 2014. Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T. Neuroimage 84, 1032–1041. [DOI] [PubMed] [Google Scholar]
- Seiler A, Lauer A, Deichmann R, Noth U, You SJ, Pfeilschifter W, Singer OC, Pilatus U, Wagner M, 2019. Complete restitution of the ischemic penumbra after successful Thrombectomy : a pilot study using quantitative MRI. Clin. Neuroradiol 29 (3), 415–423. [DOI] [PubMed] [Google Scholar]
- Shu CY, Herman P, Coman D, Sanganahalli BG, Wang H, Juchem C, Rothman DL, de Graaf RA, Hyder F, 2016a. Brain region and activity-dependent properties of M for calibrated fMRI. Neuroimage 125, 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu CY, Sanganahalli BG, Coman D, Herman P, Rothman DL, Hyder F, 2016b. Quantitative beta mapping for calibrated fMRI. Neuroimage 126, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F, 2002. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc. Natl. Acad. Sci. U. S. A 99, 10765–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Blockley NP, 2017. A streamlined acquisition for mapping baseline brain oxygenation using quantitative BOLD. Neuroimage 147, 79–88. [DOI] [PubMed] [Google Scholar]
- Stone AJ, Holland NC, Berman AJL, Blockley NP, 2019. Simulations of the effect of diffusion on asymmetric spin echo based quantitative BOLD: an investigation of the origin of deoxygenated blood volume overestimation. Neuroimage 201, 116035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth V, Forschler A, Hirsch NM, den Hollander J, Kooijman H, Gempt J, Ringel F, Schlegel J, Zimmer C, Preibisch C, 2013. MR-based hypoxia measures in human glioma. J. Neuro Oncol 115, 197–207. [DOI] [PubMed] [Google Scholar]
- Uddin MN, Marc Lebel R, Wilman AH, 2013. Transverse relaxometry with reduced echo train lengths via stimulated echo compensation. Magn. Reson. Med 70, 1340–1346. [DOI] [PubMed] [Google Scholar]
- Ulrich X, Yablonskiy DA, 2015. Separation of cellular and BOLD contributions to T2* signal relaxation. Magn. Reson. Med 75, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Helfrich M, Volz S, Magerkurth J, Blasel S, Porto L, Singer OC, Deichmann R, Jurcoane A, Hattingen E, 2015. Quantitative T2, T2*, and T2’ MR imaging in patients with ischemic leukoaraiosis might detect microstructural changes and cortical hypoxia. Neuroradiology 57, 1023–1030. [DOI] [PubMed] [Google Scholar]
- Wagner M, Magerkurth J, Volz S, Jurcoane A, Singer OC, Neumann-Haefelin T, Zanella FE, Deichmann R, Hattingen E, 2012. T2’- and PASL-based perfusion mapping at 3 Tesla: influence of oxygen-ventilation on cerebral autoregulation. J. Magn. Reson. Imag 36, 1347–1352. [DOI] [PubMed] [Google Scholar]
- Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW, 1997. In vivo measurement of T2 distributions and water contents in normal human brain. Magn. Reson. Med 37, 34–43. [DOI] [PubMed] [Google Scholar]
- Whittall KP, MacKay AL, Li DK, 1999. Are mono-exponential fits to a few echoes sufficient to determine T2 relaxation for in vivo human brain? Magn. Reson. Med 41, 1255–1257. [DOI] [PubMed] [Google Scholar]
- Wiestler B, Kluge A, Lukas M, Gempt J, Ringel F, Schlegel J, Meyer B, Zimmer C, Förster S, Pyka T, Preibisch C, 2016. Multiparametric MRI-based differentiation of WHO grade II/III glioma and WHO grade IV glioblastoma. Sci. Rep 6, 35142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonskiy DA, Haacke EM, 1994. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn. Reson. Med 32, 749–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For reasons of ethics and privacy issues of the acquired clinical data, the data is only available via a request to the authors. Institutional restrictions of patient privacy then require a formal data sharing agreement. The applied MATLAB code is available upon request. Sharing of applied sequence modifications is limited by a nondisclosure agreement. The applied sequence changes have been published (Hirsch and Preibisch, 2013) and further information will be shared on request. Custom MATLAB code for post-processing of mq-BOLD MRI data for neuro-scientific studies is available at https://doi.org/10.5281/zenodo.3909300 and https://gitlab.lrz.de/nmrm_lab/public_projects/mq-BOLD.