Purpose of the review
The aim of this article is to summarize recent data on rubella virus (RuV) vaccine in chronic inflammation focusing on granulomas in individuals with primary immunodeficiencies (PIDs).
Recent findings
The live attenuated RuV vaccine has been recently associated with cutaneous and visceral granulomas in children with various PIDs. RuV vaccine strain can persist for decades subclinically in currently unknown body site(s) before emerging in granulomas. Histologically, RuV is predominately localized in M2 macrophages in the granuloma centers. Multiple mutations accumulate during persistence resulting in emergence of immunodeficiency-related vaccine-derived rubella viruses (iVDRVs) with altered immunological, replication, and persistence properties. Viral RNA was detected in granuloma biopsies and nasopharyngeal secretions and infectious virus were isolated from the granuloma lesions. The risk of iVDRV transmissibility to contacts needs to be evaluated. Several broad-spectrum antiviral drugs have been tested recently but did not provide significant clinical improvement. Hematopoietic stem cell transplantation remains the only reliable option for curing chronic RuV-associated granulomas in PIDs.
Summary
Persistence of vaccine-derived RuVs appears to be a crucial factor in a significant proportion of granulomatous disease in PIDs. RuV testing of granulomas in PID individuals might help with case management.
Keywords: chronic inflammation, granuloma, immunodeficiency-related vaccine-derived rubella viruses, primary immunodeficiency, rubella virus
INTRODUCTION
Recent advances in next-generation sequencing have led to the identification of a considerable number of primary immunodeficiency diseases (PIDs), which now comprise 406 distinct disorders associated with 430 genetic defects of the immune system [1]. Although each individual disorder is rare, collectively PID disorders are not uncommon, and at least 1/1200, or 6.5 million, individuals are currently living with PIDs [2]. Cutaneous granuloma, a serious complication in individuals with diverse PIDs, has been long thought to be largely because of immune dysregulation and therapy has focused on immune suppression [3,4]. The purpose of this review is to highlight recent findings of the association of vaccine-derived rubella virus (RuV) persistence in the inflamed tissues of PID patients and granulomas.
Prevalence of granulomas in patients with primary immunodeficiency diseases
A granuloma is a compact immunological structure, which concentrates macrophages, lymphocytes, and signaling molecules around persisting inflammatory triggers, both infectious and noninfectious [5]. This structure provides the opportunity for activation of immune effector cells that may limit infection, kill the pathogen, and then repair tissue injury. Granulomas can occur in different tissues and may be self-limited or progress to a chronic inflammatory disorder if the antigenic trigger is not eliminated. Granulomas have been described commonly in children with PIDs and can be a presenting sign of PIDs [6,7]. Until recently, PID granulomas were considered to be sterile because no microorganisms had been consistently detected [3,4].
Recently, the prevalence of granulomas in individuals with PID was determined using two data sources: MarketScan database of US national healthcare claims and a PID patient disease registry, United States Immunodeficiency Network (USIDNET) [8]. Skin granuloma was the most common type, but other organs were also involved. The proportion with granulomas was similar across age groups for the MarketScan population (0.8–1.7%). In the USIDNET registry, the proportion whoever had granulomas ranged from 2 to 9%, with the lowest proportion in PID patients aged 0–19 years and highest proportion in those aged 35–44 years.
Overall, the estimated granuloma prevalence in PID patients was 1–4% comprising between 65 000 and 260 000 individuals worldwide.
Box 1.
no caption available
ASSOCIATION BETWEEN RUBELLA VIRUS VACCINE AND GRANULOMA
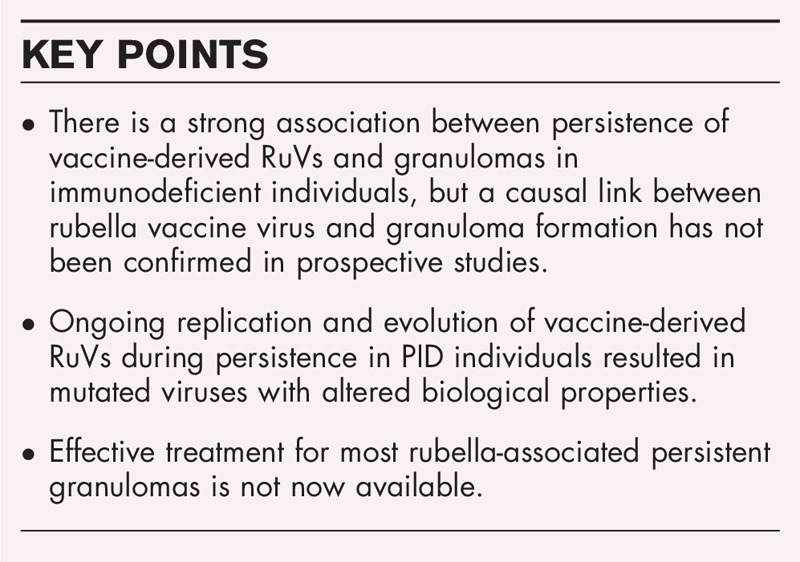
The first evidence for the association between RuV vaccine and granulomas was obtained by deep sequencing of samples from in a three-case series of children with PID [9]. The RA27/3 vaccine sequences were detected in granuloma lesions but not in the normal skin of the same individuals. The association between RuV and granulomas in skin lesions in PID was further confirmed in a larger blinded study (n = 19) by using a different detection method (fluorescent immunohistochemical staining for the RuV capsid protein) and a different sample type (formalin-fixed paraffin-embedded tissue slides) covering a broad spectrum of PIDs [10]. Table 1 summarizes data on the identification of RuV antigen and/or RNA in a wide spectrum of PIDs with granulomas of unknown cause for published [9,10,11–14,15▪,16] and unpublished cases (Perelygina, personal communication). Approximately 70% of cases, who largely had prominent T-cell defects with concomitant antibody deficiency, had granulomas positive for RuV. Many cases shared a diagnosis of a DNA repair disorder [13]. We are currently evaluating different granuloma-associated conditions in both in PID patients and immunologically normal individuals for the presence of RuV in granulomatous lesions.
Table 1.
Defection of RuV in granulomas of unknown cause in PID patients
RUBELLA VIRUS: BASIC VIROLOGY
RuV is a small, enveloped virus belonging to genus Rubivius, which has been moved in 2019 from the Togaviridae family to the newly created Matonaviridae family. The RuV genome is a 9.7-kb linear single-stranded RNA of positive polarity, which encodes for three structural proteins (envelope glycoproteins E1 and E2, and capsid protein C) and two nonstructural replicase proteins p150 and p90 [17]. The capsid protein serves as a package for genomic RNA forming nucleocapsid. The nucleocapsid is surrounded by an envelope, which is decorated by spikes consisting of E1 and E2 heterodimers. These heterodimers facilitate RuV entry and are targets for neutralizing antibody. RuV is transmitted via direct or droplet contact with respiratory secretions and is highly infectious (estimated basic reproduction number R0 = 5–7) [18]. Nasopharyngeal mucosal epithelia appear to be the portal of entry and primary site of virus replication and shedding.
RuV is divided into two clades, Clade 1 and 2, which were subdivided into 10 and 3 genotypes, respectively. Clades and genotypes were identified by sequence analysis. Only 4 genotypes 1E, 1J, 1G and 2B are currently circulating worldwide with 1E and 2B being most frequently detected [19]. RuV does not interfere significantly with host cell metabolism and is not cytocidal in many cell types, resulting in the establishment of persistent infections [20,21]. Although acute rubella is a mild and often subclinical disease, persistent rubella infections can lead to chronic diseases, which were predominately associated with immune-mediated pathologies. In postnatal infections, RuV can establish persistent infections in immune-privileged body sites leading to a spectrum of clinical manifestations including encephalitis, chronic uveitis, and chronic arthritis [22–25]. RuV can also persist in developing fetal organs causing multiple birth defects [collectively known as congenital rubella syndrome (CRS)] [26,27].
RUBELLA VACCINE
To prevent CRS, several live-attenuated rubella vaccines have been developed. The RA27/3 vaccine strain originated from a Clade 1, genotype 1a RuV isolated from a CRS affected fetus in 1961 [28]. RA27/3 is commonly used in combination with other vaccines, such as measles, measles–mumps (MMR), or measles–mumps–varicella. RA27/3 has been in use in the United States and many countries worldwide for the last 40 years. The vaccine is efficacious, safe, and induces long-lasting immunity [28]. To prevent rubella and CRS, 168 countries offer rubella vaccine with a 69% worldwide coverage for rubella vaccination (www.who.int/news-room/fact-sheets/detail/rubella). Rubella and CRS has been eliminated from the United States since 2004 and was declared eliminated in the Americas in 2015 (www.paho.org).
Live-attenuated virus vaccines are contraindicated for severely immunocompromised persons (e.g., from hematologic tumors, chemotherapy, long-term immunosuppressive therapy, and persons with primary or acquired immunodeficiency) because it can lead to a severe disease. According to Advisory Committee on Immunization Practices General Best Practice Guidelines on Immunizations, the assessment of severe immunosuppression is often based on the CD4+ T cell counts (https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/contraindications.html). Unfortunately, in several PID disorders, T cells can be dysfunctional whereas CD4+ cell counts remain within normal limits complicating PID diagnosis and risk stratification. Furthermore, a large proportion of PID individuals are diagnosed after 1 year of age, when MMR is often given.
HISTOPATHOLOGICAL FEATURES OF RUBELLA VIRUS-ASSOCIATED GRANULOMAS
The severity of the RuV-associated granulomatous inflammation can vary from a few superficial cutaneous plaques or nonulcerated nodules located predominately on face and limbs to deep ulcerated lesions with necrosis covering large areas and leading to tissue destruction [29]. In addition to skin, RuV antigen can be found in granulomas in multiple visceral organs including lung, spleen, kidney, lymph nodes, bone marrow, and liver [12,13].
Histologically, RuV-associated granulomas are predominantly sarcoidal epithelioid type consisting of M2 type (CD68+/CD206+ and CD68+/CD163+) macrophages harboring RuV antigen at the granuloma center surrounded by lymphocytes (Fig. 1) [10]. Many viruses utilize M2 macrophages for virus replication and dissemination in tissues [30]. M2 macrophages are involved in tissue repair and persist in chronic inflammatory conditions presenting attractive long-term reservoir for virus persistence. In addition to macrophages, RuV can occasionally be detected in epidermal keratinocytes; the epidermal skin layer becomes damaged and ulcers occur [10].
FIGURE 1.
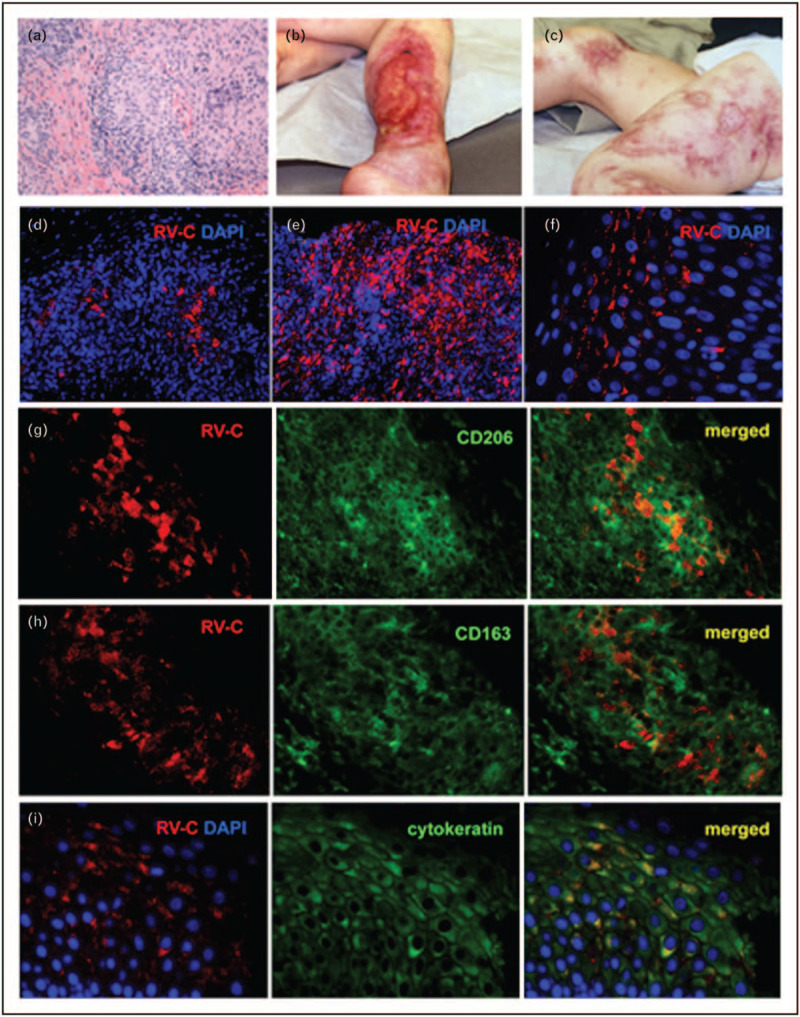
Cutaneous granulomas in PID patients. (a) Hematoxylin and eosin staining of a cutaneous granuloma from case 1. A well-formed granuloma is centrally located. (b, c) Cutaneous skin lesions from case 3. Acute and chronic ulcers are observed. (d–f) Distribution of infected cells in skin samples of PID patients. Histological immunofluorescent staining showing focal (d, case 1) or widespread (e, case 2) distribution of RuV capsid in granulomas and focal capsid localization in the epidermis (f, case 5). Activation status of macrophages in granulomas (g–i, case 6). Double immunofluorescent staining of granulomas with RuV capsid antibody (red) and M2 macrophage specific antibodies, CD206 (g, green) or CD163 (h, green). (i) RuV antigen expression in the suprabasal cell layer of skin epidermis (case 1). Double immunofluorescent staining with RuV capsid antibody (red) and keratinocyte specific antibody (cytokeratin, green). Nuclei were counterstained with DAPI, 4′,6-diamidino-2-phenylindole. Previously published in Ref. [8].
Both noncaseating granulomas and caseating granulomas with necrotic center may be present in the same lesion although noncaseating granulomas usually predominate [29]. RuV positive multinucleated giant cells (macrophage syncytia or Langhans cells) can also be seen in some lesions. A distinctive feature of chronic cutaneous granulomas in PID is the predominance of CD8+ over CD4+ T cells [6,31]. CD4+ T cells have been shown to play a critical role in resolution of Mycobacterium-induced granulomas in tuberculosis [32]. It has yet to be determined whether insufficient CD4+ cells contribute to the inability of individuals with PID to resolve RuV associated granulomas.
The onset of RuV positive inflammatory lesions varied considerably between patents, from two months to 14 years (average two years) after receiving MMR [13]. The timing between vaccination and granuloma development likely depends on the magnitude of immune system dysfunction and additional host factors, such as the receipt of systemic immunosuppressive therapies.
EVOLUTION OF RUBELLA VIRUS VACCINE IN PRIMARY IMMUNODEFICIENCY DISEASE PATIENTS
Vaccine-derived RuVs can persist for decades in PID patients and the longer the persistence lasts, the more viral mutations accumulate. To date, six full-length or near full-length sequences of RuV genomic RNA from skin granuloma biopsies and from nasopharyngeal (NP) secretions of one PID patient have been published [9,10,15▪]. All sequences were derivatives of the RA27/3 vaccine strain (Fig. 2) with multiple nucleotide and amino acid substitutions and, therefore, they were designated immunodeficiency-related vaccine-derived rubella viruses (iVDRVs). It is unknown whether any of these substitutions are back mutations as attenuating mutations of the RA27/3 vaccine have not been characterized. A positive linear relationship between the number of mutations in iVDRV genomes and times after vaccination strongly indicate ongoing replication and evolution of persisting vaccine viruses in PID patients [15▪]. An overall rate of sequence evolution was estimated to be 1.8 × 10−3 nt substitutions/site/year, or 18 nt substitutions/genome/year, which is within the observed range for many RNA viruses.
FIGURE 2.
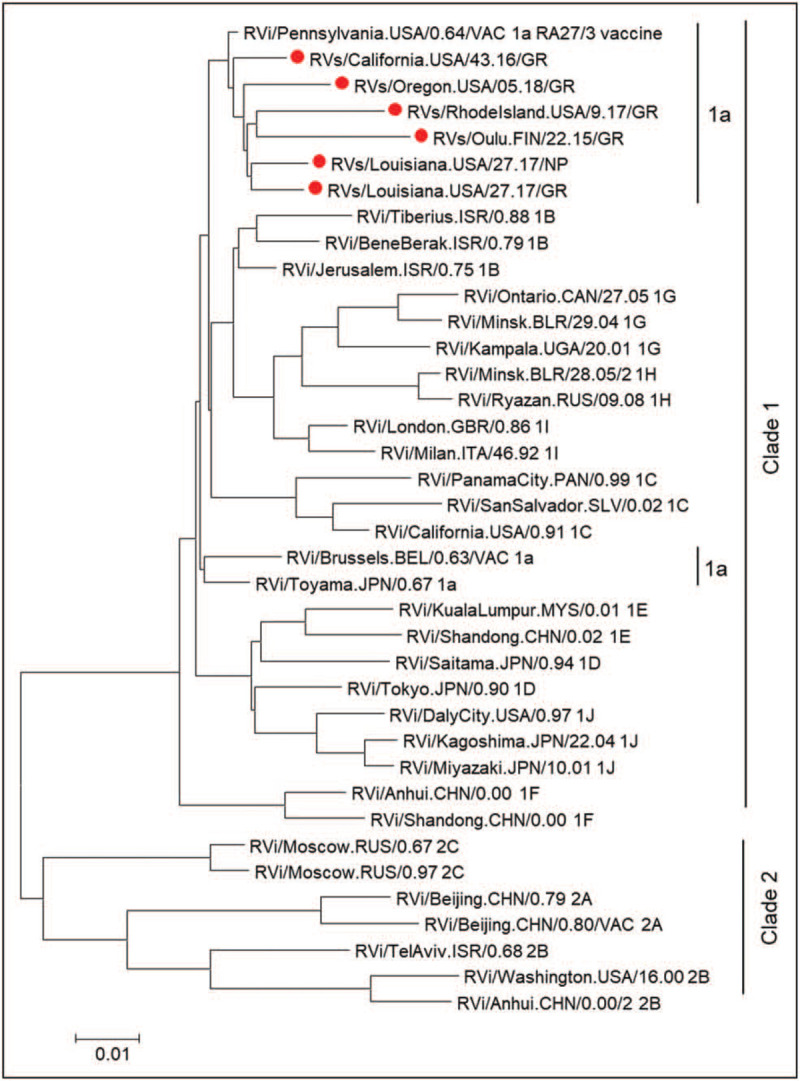
Phylogenetic tree of iVDRV. The genetic relationships between the consensus genome sequences from each original granuloma sample and the whole genomes of the WHO reference viruses were inferred using the maximum likelihood method in molecular evolutionary genetics analysis 7. All taxa are labeled with WHO names with iVDRV sequences marked with red dots. The genetic distances were computed using the maximum composite likelihood method. The scale bar indicates the number of base substitutions per site. RA27/3 and iVDRVs represent a separate branch on the tree with RA27/3 being basal. Previously published in Ref. [15▪].
PUBLIC HEALTH-RELATED ISSUES
There are several important questions from a public health standpoint: Are persisting iVDRVs infectious? Can iVDRVs be shed and transmitted to nonimmune contacts? Can iVDRVs cause CRS in nonimmune pregnant women? Is the vaccine-induced immune response protective against iVDRVs?
Some of the questions have been recently addressed. Infectious iVDRVs viruses were recovered from four out of five cutaneous granuloma biopsies, but no virus was detected in a swab from the lesion surface by reverse-transcriptase-polymerase-chain-reaction in one of those individuals [15▪]. RuV is a respiratory virus, which is predominantly shed into nasopharynx. iVDRV RNA was found in two out of five sequential NP swabs in one granuloma case out of three tested, but infectious virus was not detected [15▪]. The frequency and levels of virus shedding into the NP cavity and onto the lesion surface, as well as transmissibility of iVDRVs to nonimmune contacts has yet to be determined in a large study group.
Reduced immune pressure by the defective immune system in PIDs may be responsible for the emergence of iVDRV mutant viruses capable of low-level, decades-long persistence. Most amino acid mutations in iVDRVs occurred in the structural proteins, including mutations in the neutralizing epitopes of the E1 envelope glycoprotein and CD8+ cytotoxic T lymphocyte epitopes of the capsid protein [10,15▪]. The data on poor neutralization of iVDRV strains by sera from vaccinated healthy individuals raise the concern that some of these mutant viruses may be poorly recognized by the rubella vaccine-induced immunity. Importantly, multiple mutations have resulted in altered biological properties of the iVDRV strains compared with the parental RA27/3 vaccine strain [15▪]. The iVDRV strains were less cytopathic in cell culture, produced lower amounts of viral RNA, proteins, and infectious virions and, unlike RA27/3, can persist in primary cultures of fibroblasts, presumably the initial target cells following vaccination. Unfortunately, the lack of available animal models makes it difficult to evaluate iVDRV pathogenic properties in vivo.
PROGNOSTIC SEROLOGICAL MARKERS FOR GRANULOMAS
Persisting rubella immunoglobulin M and very high levels of RuV-neutralizing antibodies were found in PID patients with RuV-associated granulomas [15▪]. It remains to be determined whether these are serological markers for RuV persistence and/or predict an appearance of granulomas in vaccinated individuals.
CAUSAL RELATIONSHIP BETWEEN RUBELLA VIRUS AND GRANULOMAS
Establishment of casual relationships between persisting viruses and chronic diseases has always been problematic, especially for viruses that persist subclinically before causing disease [33]. It is even more problematic, when a disease is immune-mediated and not a result of direct virus cytopathology. Nonetheless, most of Hill's epidemiological criteria for causation [34,35] have been met for the causal link between RuV vaccine and granuloma development in PID individuals. RuV is present in 70% of granulomas in a broad range of PIDs (strength of association). The association between RuV and granuloma has been demonstrated by multiple laboratories in different countries using different study groups, different types of samples and by different study designs (consistency). Two other live-attenuated viruses in MMR, measles and mumps, have never been detected in RuV-positive granulomas; RuV is the only infectious agent detected in the lesions by NextGen sequencing (specificity). RuV is present in cutaneous granuloma lesions but absent in healthy skin (a biologic gradient). MMR vaccination precedes the granuloma development (temporality). Infectious iVDRVs have been isolated from granulomas and, histologically, rubella antigen has been found in macrophages in the middle of granulomas, where the granuloma causative agent is expected to be localized (biological plausibility). RuV vaccine persistence has been associated with other inflammatory diseases, such as uveitis, encephalitis, and arthritis (analogy). Nevertheless, prospective natural history studies might provide additional strong evidence of causality.
TREATMENT STRATEGIES
No effective specific therapy is currently available to cure RuV infections. The drugs with known broad antiviral properties, nitazoxanide, ribavirin and interferon, have been largely unsuccessful for treatments of patients with RuV positive granulomas [13,29,36,37]. Intravenous immunoglobulin (IVIG) therapy does not eliminate persisting RuV but may provide moderate improvement and may prevent systemic virus spread. Unfortunately, the levels of RuV neutralizing antibody in immunoglobulin preparations are unknown as well as the role of neutralizing antibody in resolution of RuV associated inflammation. Antibody-dependent enhancement of RuV disease has never been seen as a concern, but since RuV was found in macrophages in granuloma lesions, there is a theoretical potential of IVIG supplementation to amplify the infection. Current therapy for PID granulomas has focused on immune suppression, which may reduce tissue inflammation but are mostly ineffective in resolving granulomatous disease [38–40]. Caution must be given to the use of systemic steroid drugs, as it may lead to more severe rubella systemic infection. Hematopoietic stem cell transplantation is the only known effective treatment, which usually leads to complete remission. Several immunomodulatory drugs have been recently evaluated (rapamycin, rituximab, infliximab, interleukin-2) but provided only a moderate effect in the limited number of patients [13,29].
CONCLUSION
The strong association between persistence of vaccine-derived RuVs and development of granulomatous disease in individuals with various PIDs has been recently established. Chronic lesions of unknown cause in such patients should be investigated for the presence of RuV, which may impact proper diagnosis and consideration of treatment strategies for this condition. Currently used granuloma treatments are only moderately effective with exception of hematopoietic stem cell transplantation, which is not feasible for all PID patients. Identifying the precise mechanisms that contribute to long-term asymptomatic persistence of RuVs and recognizing the risk factors that trigger the development of RuV-associated granulomas will be critical for the development of more effective targeted strategies for granuloma treatments in persons with PID.
Acknowledgements
None.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention.
Financial support and sponsorship
This work of L.P. and J.I. was supported by core funding from the Centers for Disease Control and Prevention. K.E.S. was supported by the Wallace Chair of Pediatrics and by a grant from the National Institute of Health (R21-AI130967-01A1).
Conflicts of interest
None.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a “work of the United States Government” for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government
REFERENCES
- 1.Bousfiha A, Jeddane L, Picard C, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol 2020; 40:66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousfiha AA, Jeddane L, Ailal F, et al. Primary immunodeficiency diseases worldwide: more common than generally thought. J Clin Immunol 2013; 33:1–7. [DOI] [PubMed] [Google Scholar]
- 3.Chiam LY, Verhagen MM, Haraldsson A, et al. Cutaneous granulomas in ataxia telangiectasia and other primary immunodeficiencies: reflection of inappropriate immune regulation? Dermatology 2011; 223:13–19. [DOI] [PubMed] [Google Scholar]
- 4.Nanda A, Al-Herz W, Al-Sabah H, Al-Ajmi H. Noninfectious cutaneous granulomas in primary immunodeficiency disorders: report from a national registry. Am J Dermatopathol 2014; 36:832–837. [DOI] [PubMed] [Google Scholar]
- 5.Pagan AJ, Ramakrishnan L. The formation and function of granulomas. Annu Rev Immunol 2018; 36:639–665. [DOI] [PubMed] [Google Scholar]
- 6.Harp J, Coggshall K, Ruben BS, et al. Cutaneous granulomas in the setting of primary immunodeficiency: a report of four cases and review of the literature. Int J Dermatol 2015; 54:617–625. [DOI] [PubMed] [Google Scholar]
- 7.Shoimer I, Wright N, Haber RM. Noninfectious granulomas: a sign of an underlying immunodeficiency? J Cutan Med Surg 2016; 20:259–262. [DOI] [PubMed] [Google Scholar]
- 8.Leung J, Sullivan KE, Perelygina L, et al. Prevalence of granulomas in patients with primary immunodeficiency disorders, United States: data from national healthcare claims and the US Immunodeficiency Network Registry. J Clin Immunol 2018; 38:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodemer C, Sauvage V, Mahlaoui N, et al. Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clin Microbiol Infect 2014; 20:O656–O663. [DOI] [PubMed] [Google Scholar]
- 10.Perelygina L, Plotkin S, Russo P, et al. Rubella persistence in epidermal keratinocytes and granuloma M2 macrophages in patients with primary immunodeficiencies. J Allergy Clin Immunol 2016; 138:1436–1439.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first blinded study confirming the association of rubella virus persistence and granulomas in a broad variety of PIDs and identifies M2 macrophages as a rubella virus persistence site.
- 11.Deripapa E, Balashov D, Rodina Y, et al. Prospective study of a cohort of Russian Nijmegen breakage syndrome patients demonstrating predictive value of low kappa-deleting recombination excision circle (KREC) numbers and beneficial effect of hematopoietic stem cell transplantation (HSCT). Front Immunol 2017; 8:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neven B, Perot P, Bruneau J, et al. Cutaneous and visceral chronic granulomatous disease triggered by a rubella virus vaccine strain in children with primary immunodeficiencies. Clin Infect Dis 2017; 64:83–86. [DOI] [PubMed] [Google Scholar]
- 13.Buchbinder D, Hauck F, Albert MH, et al. Rubella virus-associated cutaneous granulomatous disease: a unique complication in immune-deficient patients, not limited to DNA repair disorders. J Clin Immunol 2019; 39:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhossche J, Johnson L, White K, et al. Cutaneous granulomatous disease with presence of rubella virus in lesions. JAMA Dermatol 2019; 155:859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Perelygina L, Chen MH, Suppiah S, et al. Infectious vaccine-derived rubella viruses emerge, persist, and evolve in cutaneous granulomas of children with primary immunodeficiencies. PLoS Pathog 2019; 15:e1008080. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first report of isolation of infectious vaccine-derived rubella viruses from cutaneous granulomatous lesions of PID patients and the first demonstration of altered biological properties of iVDRVs.
- 16.Murguia-Favela L, Hiebert J, Haber RM. ‘Noninfectious’ cutaneous granulomas in primary immunodeficiency patients and association with rubella virus vaccine strain. J Cutan Med Surg 2019; 23:341–342. [DOI] [PubMed] [Google Scholar]
- 17.Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet 2015; 385:2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edmunds WJ, Gay NJ, Kretzschmar M, et al. The prevaccination epidemiology of measles, mumps and rubella in Europe: implications for modelling studies. Epidemiol Infect 2000; 125:635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivailler P, Abernathy E, Icenogle J. Genetic diversity of currently circulating rubella viruses: a need to define more precise viral groups. J Gen Virol 2017; 98:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham AL, Fraser JR. Persistent rubella virus infection of human synovial cells cultured in vitro. J Infect Dis 1985; 151:638–645. [DOI] [PubMed] [Google Scholar]
- 21.Perelygina L, Zheng Q, Metcalfe M, Icenogle J. Persistent infection of human fetal endothelial cells with rubella virus. PLoS One 2013; 8:e73014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howson CP, Katz M, Johnston RB, Jr, Fineberg HV. Chronic arthritis after rubella vaccination. Clin Infect Dis 1992; 15:307–312. [DOI] [PubMed] [Google Scholar]
- 23.Chaari A, Bahloul M, Berrajah L, et al. Childhood rubella encephalitis: diagnosis, management, and outcome. J Child Neurol 2014; 29:49–53. [DOI] [PubMed] [Google Scholar]
- 24.Kreps EO, Derveaux T, De Keyser F, Kestelyn P. Fuchs’ uveitis syndrome: no longer a syndrome? Ocul Immunol Inflamm 2015; 24:348–357. [DOI] [PubMed] [Google Scholar]
- 25.Tingle AJ, Mitchell LA, Grace M, et al. Randomised double-blind placebo-controlled study on adverse effects of rubella immunisation in seronegative women. Lancet 1997; 349:1277–1281. [DOI] [PubMed] [Google Scholar]
- 26.Rawls WE. Viral persistence in congenital rubella. Prog Med Virol 1974; 18:273–288. [PubMed] [Google Scholar]
- 27.Plotkin S, Reef S, Cooper L, Alford CA. J. R, Klein J, Wilson C, et al. Rubella. Infectious diseases of the fetus and newborn infant. Philadelphia, PA: Elsevier; 2011. 861–898. [Google Scholar]
- 28.Plotkin SA. The history of rubella and rubella vaccination leading to elimination. Clin Infect Dis 2006; 43: Suppl 3: S164–S168. [DOI] [PubMed] [Google Scholar]
- 29.Leclerc-Mercier S, Moshous D, Neven B, et al. Cutaneous granulomas with primary immunodeficiency in children: a report of 17 new patients and a review of the literature. J Eur Acad Dermatol Venereol 2019; 33:1412–1420. [DOI] [PubMed] [Google Scholar]
- 30.Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci 2018; 19:2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jager M, Blokx W, Warris A, et al. Immunohistochemical features of cutaneous granulomas in primary immunodeficiency disorders: a comparison with cutaneous sarcoidosis. J Cutan Pathol 2008; 35:467–472. [DOI] [PubMed] [Google Scholar]
- 32.Saunders BM, Frank AA, Orme IM, Cooper AM. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell Immunol 2002; 216:65–72. [DOI] [PubMed] [Google Scholar]
- 33.Knobler S. The infectious etiology of chronic diseases: defining the relationship, enhancing the research, and mitigating the effects: workshop summary. 2004; Washington, DC: National Academies Press, xvii, 215 p. [PubMed] [Google Scholar]
- 34.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965; 58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol 2005; 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perelygina L, Hautala T, Seppanen M, et al. Inhibition of rubella virus replication by the broad-spectrum drug nitazoxanide in cell culture and in a patient with a primary immune deficiency. Antiviral Res 2017; 147:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perelygina L, Buchbinder D, Dorsey MJ, et al. Outcomes for nitazoxanide treatment in a case series of patients with primary immunodeficiencies and rubella virus-associated granuloma. J Clin Immunol 2019; 39:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woelke S, Valesky E, Bakhtiar S, et al. Treatment of granulomas in patients with ataxia telangiectasia. Front Immunol 2018; 9:2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra A, Gooi J, Darling J, Newton-Bishop JA. Infliximab in the treatment of a child with cutaneous granulomas associated with ataxia telangiectasia. J Am Acad Dermatol 2011; 65:676–677. [DOI] [PubMed] [Google Scholar]
- 40.Boursiquot JN, Gerard L, Malphettes M, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol 2013; 33:84–95. [DOI] [PubMed] [Google Scholar]


