Abstract
Marijuana (MJ) use and post-traumatic stress disorder (PTSD) have both been associated with abnormalities in brain white matter tracts, including the cingulum and the anterior thalamic radiations (ATR), which project from subcortical regions to frontal cortex. Studies have not assessed the integrity of these tracts in patients with comorbid PTSD and MJ use. To examine effects of PTSD and MJ use on brain structure, we performed diffusion tensor imaging scans on seventy-two trauma-exposed participants, categorized into four groups: those with PTSD who used MJ at least weekly (PTSD+MJ; n = 20), those with PTSD with no regular MJ use (PTSD; n = 19), trauma-exposed controls without PTSD who used MJ (TEC+MJ; n = 14) and trauma-exposed controls with no PTSD or MJ use (TEC; n = 19). White matter integrity was evaluated by calculating fractional anisotropy (FA). Results showed that while FA values in the right ATR and the cingulum differed across groups, there were no significant interactions between PTSD and MJ in any white matter tracts, indicating that MJ exposure neither normalizes nor worsens white matter abnormalities in those with PTSD. Further study is needed to evaluate the impact of MJ use on other neurobiological markers of PTSD.
Keywords: addiction, marijuana, diffusion tensor imaging, fractional anisotropy, PTSD
1. Introduction
Post-traumatic stress disorder (PTSD) has the highest lifetime prevalence of all anxiety disorders (8–9%) (Grinage, 2003) and is often comorbid with other anxiety disorders, depression, and eating disorders (Bryant, 2001; Kearns et al., 2012; Wolitzky-Taylor et al., 2010). PTSD is characterized by symptoms such as re-experiencing of a stressful or traumatic experience through flashbacks and nightmares, emotional numbness or avoidance, and episodic symptoms of hyper-arousal. These symptoms often cause significant distress and impact social and occupational functioning. Unfortunately, even effective therapies, such as exposure-based therapy, leave many people with PTSD with significant symptomatology; over one-third of patients who complete evidence-based therapies retain a diagnosis of PTSD at treatment completion (Cukor et al., 2010).
Increasingly, those with PTSD are seeking alternative treatments, such as marijuana (MJ), that lack an evidence base for safety and efficacy (Chilcoat and Breslau, 2019; Lepage et al., 2018; Vlahov et al., 2019). There have been recent reports of increased prevalence of MJ use among those with PTSD patients for flashbacks, recurring traumatic memories or disturbing dreams (Yarnell, 2015). Many with PTSD develop problems from MJ use; the Veterans Health Administration estimated the prevalence of cannabis use disorder (CUD) in PTSD to be approximately 22% in 2014 (Marcel and Glenna). As legalization of the commercial MJ market spreads throughout the United States, it is expected that prevalence of MJ use by those with PTSD hoping for symptom relief will continue to increase. Thus, it is critical to understand the effects of MJ on the brain in those with PTSD, particularly in regions shown to have structural abnormalities in those with PTSD.
There is a growing consensus that PTSD is associated with abnormalities in brain white matter tracts, which consist of myelinated axons of neurons that connect brain regions, subserving rapid information transfer throughout the brain (Mehta et al., 2009). White matter tracts implicated in PTSD include the cingulum bundle, which projects from the cingulate gyrus to the hippocampus and medial temporal cortex, as well as from the thalamus to the frontal cortex, and the anterior thalamic radiations (ATR), which projects from the thalamus to frontal cortex (Coenen et al., 2012). Integrity of these tracts are critical for emotion regulation, and these regions are thought to contribute to the emotion dysregulation underlying symptoms of PTSD (Bierer et al., 2015; Sanjuan et al., 2013). It should be noted the direction of these changes are unclear, with some studies showing greater and other studies showing reduced connectivity of these fibers associated with PTSD. Studies have reported lower white matter integrity of the cingulum in individuals with high exposure to trauma (Kim et al., 2006) and veterans with combat trauma (Rinne-Albers et al., 2016; Schuff et al., 2011). Decreased white matter integrity of the cingulum has been reported in women with high exposure to trauma (Fani et al., 2015; Fani et al., 2012), Afghanistan and Iraq war veterans with PTSD (Sanjuan et al., 2013), and in adults (Rinne-Albers et al., 2016) and adolescents (Daniels et al., 2013) with a history of trauma and abuse. Others have reported that individuals with PTSD have higher FA in the anterior cingulate bundle (Abe et al., 2006), superior frontal gyrus (Zhang et al., 2012), and bilateral posterior cingulum gyrus (Zhang et al., 2012) compared to healthy controls. A recent meta-analysis of 14 studies of adult-onset PTSD with traumatic experience in adulthood (Siehl et al., 2018) found that the most commonly reported changes in white matter were in the cingulum (both decreased and increased FA) and frontal regions. Further research is needed to determine whether decreases or increases in FA in the cingulum are detrimental. It should also be noted that the age of trauma may affect the nature of these white matter changes. It is possible that trauma-induced white matter alterations only occur during the early, formative years of brain development in the case of childhood trauma (see (Daniels et al., 2013), and it is also possible that the neurotoxicity of PTSD itself, even in adulthood, may be driving white matter alterations. To date, only a handful of studies have attempted to parse out these differences.
White matter tract integrity is commonly measured using diffusion tensor imaging (DTI), a non-invasive magnetic resonance imaging (MRI) technique that capitalizes on diffusion properties of water through brain tissue (Alexander et al., 2007; Seckfort et al., 2008). DTI methodology characterizes parameters of white matter microstructure, yielding measures of its directional organization (fractional anisotropy: FA), averaged water diffusion in all directions (mean diffusivity: MD), and water diffusion perpendicular to the primary fiber orientation (radial diffusivity: RD). DTI studies have largely demonstrated that stress and trauma impact white matter integrity (Eluvathingal et al., 2006) as excessive exposure to stress hormones, especially during critical windows of brain development, can impair white matter pathway formation (Richert et al., 2006). White matter integrity is particularly impaired within circuits such as the cingulum that support memory formation and emotion regulation (Ayling et al., 2012; Eluvathingal et al., 2006; Hanson et al., 2010; Jackowski et al., 2008; Seckfort et al., 2008). As the cingulum is highly involved in memory, and particularly memories specific to negative affect and pain (Shackman et al., 2011), we hypothesized that, consistent with much of the literature, we would see increased WM connectivity in the cingulum in those with PTSD. This increased WM connectivity could potentially be a mechanistic explanation for why increased WM connectivity could lead to PTSD symptoms such as flashbacks and nightmares.
Emerging evidence suggests that MJ use may disrupt the developmental trajectory of white matter organization (Gruber et al., 2014; Gruber and Yurgelun-Todd, 2005). Most cross-sectional DTI studies indicate lower FA in MJ users in various white matter regions, including the superior longitudinal fasciculus (Ashtari et al., 2009; Hedges et al., 2007; Jacobus et al., 2009; Lorenzetti et al., 2010), arcuate fasciculus (Ashtari et al., 2009), frontal white matter adjacent to the anterior cingulate cortex (Gruber et al., 2014) and hippocampus (Zalesky et al., 2012), internal capsule (Gruber et al., 2014), and the corpus callosum (Arnone et al., 2008). It should be noted that other cross-sectional studies have found higher FA in these regions (Bava et al., 2010; DeLisi et al., 2006; Filbey et al., 2014; Jacobus et al., 2009), indicating that the direction of change needs to be further clarified with longitudinal studies that can assess FA before and after MJ use. One such longitudinal study that assessed DTI measures at baseline and at a 2-year follow-up in young adult MJ users and non-using controls found that MJ users demonstrated reduced longitudinal growth in FA in several frontal and parietal regions, including the posterior cingulum and the right anterior thalamic radiation (Becker et al., 2015). Together, these studies suggested that MJ may contribute to disruptions of white matter fiber tracts. However, little is known about how MJ use affects white matter pathways in those with comorbid psychiatric conditions such as PTSD. In this study, our intention was to investigate white matter tracts of participants with PTSD with and without MJ use, in order to determine whether MJ use is associated with white matter changes in this group.
No studies to date to our knowledge have assessed the impact of MJ exposure on the integrity of white matter tracts in those with PTSD. As both PTSD and MJ exposure have been shown to be associated with increases in white matter integrity, those with both PTSD and MJ use may show more severe increases in FA. On the other hand, though evidence is mostly limited to anecdotal experiences, case reports, and observational studies (Shishko et al., 2018), if MJ does indeed reduce core symptoms of PTSD, it is possible that MJ could ameliorate abnormalities in FA in those with PTSD. Here we aimed to evaluate the impact of PTSD, MJ exposure, and their combination on white matter integrity in the cingulum and ATR using a 2×2 factorial design, investigating the following four groups; those with PTSD who used MJ at least weekly (PTSD+MJ), those with PTSD with no regular MJ use (PTSD), trauma-exposed controls without PTSD who used MJ (TEC+MJ) and trauma-exposed controls with no PTSD or MJ use (TEC). We hypothesized independent effects of MJ use and PTSD on white matter integrity in these white matter tracts involved in emotion regulation. Specifically, we hypothesized that (1) increased FA in the cingulum and the anterior thalamic radiations would be associated with PTSD, and (2) this effect would be additive in those with MJ use.
2. Methods
2.1. Participants
All participants gave written informed consent and were compensated for their participation in the study. This study was approved by the Partners Human Research Committee Institutional Review Board.
Seventy-two adults, aged 18–58 who had experienced a traumatic event were enrolled. Participants were recruited using local advertising. Exclusion criteria included unstable major medical illness, current or past psychotic disorder, active substance use disorder other than cannabis use disorder, clinically significant head injury or traumatic brain injury, or contraindications to MRI scanning. A urine drug screen was performed for THC, amphetamines, cocaine, barbiturates, methamphetamines, benzodiazepines, opiates, phencyclidine, oxycodone, and methadone. A positive result was not exclusionary; however, any participant with a positive screen on the day of the scan for any drug other than THC was rescheduled.
2.2. Assessments
The following assessments were administered during a screening visit:
PTSD Checklist for DSM-5
(PCL-5; (Blevins et al., 2015) (American Psychiatric Association, 2013). The PCL is a 20-item self-report measure that assesses past-month DSM-5 symptoms of PTSD. This questionnaire includes a life event checklist (LEC), which is a self-report measure that assesses exposure to 16 potentially traumatic events in a respondent’s lifetime. We used Criterion A, the diagnostic criteria for PTSD, to determine what constitutes a “traumatic event.” The PCL also assesses severity of PTSD symptoms, from “not at all” (0), “a little bit” (1), “moderately” (2), “quite a bit” (3), and “extremely” (4). Total scores range from 0–80.
Timeline follow-back interview
(TLFB; (Sobell et al., 1996)). The TLFB was used to assess MJ and alcohol use in the past 90 days.
Group Assignment
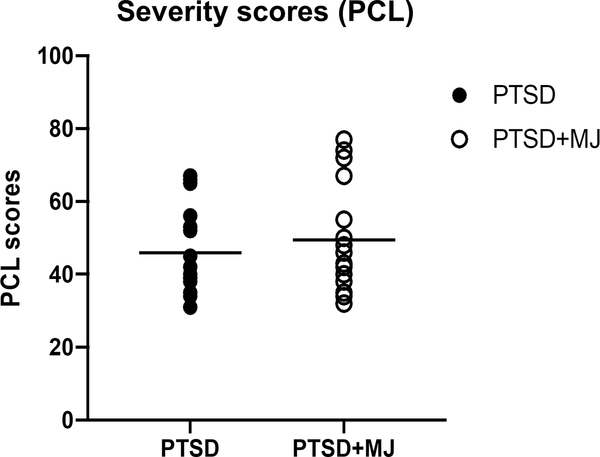
Participants were divided into four subgroups; those with trauma and PTSD who used MJ regularly (PTSD+MJ; n = 20), those with trauma and PTSD with no regular MJ use (PTSD; n = 19), trauma-exposed controls without PTSD who used MJ at least weekly (TEC+MJ; n = 14) and trauma-exposed controls with no PTSD or MJ use (TEC; n = 19). Participants were categorized based on responses to the PCL and the TLFB. The PTSD and PTSD+MJ participants had PCL severity scores greater than 30, indicative of likely PTSD, while the TEC participants were required to have PCL scores under 20 (Weathers, 2013). The MJ groups (TEC+MJ and PTSD+MJ), also reported current MJ use of greater than 3 times per week on average on the TLFB, and more than 100 lifetime MJ occasions. PTSD and TEC participants reported MJ use of less than once a month, and less than 50 lifetime occasions. PCL scores were matched between PTSD+MJ and PTSD groups (see Fig 1).
Figure 1.
PCL-5 score of PTSD and PTSD+MJ groups.
All enrolled participants participated in a neuroimaging session on a separate day. Participants were asked to refrain from using substances on the day of the study.
2.3. MRI acquisition
Neuroimaging data was acquired on a 3T Skyra Trio Siemens scanner using a 32-channel head coil at the Martinos Center for Biomedical Imaging. Scans were acquired using parameters based on the Human Connectome Project (HCP). A whole-brain T1-weighted structural scan was acquired using the following parameters: TA = 7:38min, voxel size = 0.7×0.7×0.7mm3, GRAPPA acceleration factor 2, A-P phase encoding, 256 slices, 224mm FOV read, slice thickness=0.70mm, TR= 2400ms, TE=2.02ms, TI=1000ms, echo spacing 7.6ms, bandwidth = 270 Hz/Px. A 3D multi-echo T2 SPACE sequence (160 axial slices, 256 mm FOV, TR = 4880 ms, 2x GRAPPA acceleration, TE = 2.64/4.7/6.32/8.65 msec, BW 320 Hz/px, Tacq = 8.23 min) was also acquired. Diffusion weighted images were acquired using single spin echo EPI with 10 non-diffusion weighted (b = 0 s/mm2) images, and one non-zero b-values (700 s/mm2) each with 60 directions; TR/TE = 3000 ms/66 ms, 2.0 mm3 isotropic resolution, 256 mm FOV, total scan time = 3:24 min:sec; echo spacing is 0.78ms, readout bandwidth is 1490 Hz/px; the total echo train length (ETL) is 84.42ms.
A top up scan for diffusion tensor images was acquired to reduce field distortion due to EPI sequences. All raw and processed data was visually inspected and determined to be of good-to-excellent quality.
2.4. DTI analysis
Diffusion tensor data were processed to delineate major white matter pathway using Tracts Constrained by Underlying Anatomy (TRACULA) toolbox (Yendiki et al., 2011), which uses global probabilistic tractography based on known white matter anatomy acquired from brain parcellation toolbox in each subject. We performed TRACULA by automated streamlines in the Freesurfer (Dale et al., 1999; Fischl et al., 2001) software (version: 6.0) that contains two parts: 1) parcellation on high-resolution T1 and T2 weighted images using automatic segmentation function; and 2) tractography on 60 directions diffusion-weighted images using TRACULA toolbox in the Freesurfer. The T1 weighted image was first parcellated into 34 cortical regions based on Deskikan/Killiany atlas (Desikan et al., 2006). The T2 weighted image was used to improve segmentation accuracy with better subcortical contrasts (Wisse et al., 2014). Diffusion tensor images were then input into TRACULA to estimate the probability of diffusion property using “ball and stick model” (Behrens et al., 2003) with given prior knowledge of white matter segmentation from the first step. Our a priori tract of interest was the cingulum–cingulate gyrus bundle (CCG); however, exploratory analyses also examined the other pathways derived from TRACULA: corpus callosum-forceps major (fmajor), corpus callosum–forceps minor (fminor), and bilateral parts of following track: superior longitudinal fasciculus-parietal terminations (SLFP), superior longitudinal fasciculus-temporal terminations (SLFT), inferior longitudinal fasciculus (ILF), uncinate fasciculus (UNC), anterior thalamic radiation (ATR), cingulum–angular bundle (CAB), and corticospinal tract (CST).
For quality assessment, four measures of head movement were calculated from the DWI and the output of the prior eddy-current correction procedures (average translation (unit: mm), average rotation (unit: mm), percent “bad” slices (%), and percent dropout score (%). Any participant with > 1.5 mm of moment (translation or rotation), or with >2% of bad slices or dropout was excluded. There was no significant difference in motion between groups (all p’s > 0.10), and no participants were excluded due to excessive motion.
2.5. Statistical analysis
After the TRACULA processing, white matter pathways were reconstructed, and mean value of fractional anisotropy (FA) and tract length at each point were computed to allow group comparison of diffusivity measurements voxel by voxel. We selected FA as our primary proxy of white matter integrity. Other measures (radial diffusivity, mean diffusivity and axonal diffusivity) are reported in Supplementary Table 1.
Voxel-wise analyses
The FA along tracts were extracted (as described in https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TraculaStatistics) from each subject for further statistical analyses. For each point, group effects were estimated using analysis of covariance (ANCOVA), with MJ use and PTSD conditions as independent variables, and motion factors (translation and rotation) as covariates. All tests were carried out at a two-sided significance level α of 5%. All analyses were evaluated according to FSL’s Permutation Analysis of Linear models (PALM) (Winkler et al., 2016), with 5000 permutations. Though we primarily investigated the cingulum and the anterior thalamic radiation, exploratory analyses were conducted assessing differences among groups for other tracts (Corticospinal tract, cingulum angular bundle, inferior longitudinal fasciculus, uncinate fasciculus, corpus callosum, forceps major, forceps minor, and superior longitudinal fasciculus).
ROI analyses
For tracts that showed significant PALM-corrected group differences, values were extracted and averaged to a single value per ROI for each subject. Next, we ran two-way ANOVA models with PTSD (No- PTSD [TEC, TEC+MJ] vs. PTSD [PTSD, PTSD+MJ]) and MJ use (Non-users [TEC, PTSD] vs. MJ users [TEC+MJ, PTSD+MJ]) as group factors and their interaction as independent variables. These were followed by Sidak’s multiple comparisons tests, with corrected p-values for pairwise tests. Linear regressions, covarying for motion, were conducted to assess relationships between FA and PCL scores. To control for within-subject dependencies between hemispheres, measures for the left hemisphere were regressed onto the right, and the resulting residuals were extracted and converted into z-scores. These scores represent the unique variance not shared between hemispheres. Then, correlations between PCL symptom severity scores and the decorrelated measures were computed. All statistical tests were performed using R, version 3.5.1 (R Foundation for Statistical Computing, Vienna Austria). To adjust for multiple comparisons, we used the false discovery rate adjustment by Benjamini and Hochberg (Benjamini and Hochberg, 1995).
3. Results
3.1. Demographics
The PTSD+MJ, PTSD, TEC+MJ, and TEC groups did not significantly differ in age, gender, race, IQ, or years of education (Table 1). The average age of onset of MJ use was 19.5 years (SD = 5.6) for PTSD+MJ and 20.1 years (SD = 6.8) for TEC+MJ. PTSD+MJ used cannabis more frequently than TEC+MJ group (p = 0.003) and reported higher CUDIT scores (p = 0.004). There were no significant group differences in average weekly alcohol consumption or AUDIT scores. In both the PTSD+MJ and the PTSD groups, 37 of 39 participants met DSM-5 criteria for a current PTSD diagnosis. All four groups were matched on age of trauma and years since trauma occurred; as childhood trauma was not exclusionary, approximately 30% of participants in each group experienced trauma before the age of 18 (age range = 0.5 years – 30 years).
Table 1.
Baseline Participant Characteristics
| Participant Group | |||||
|---|---|---|---|---|---|
| TEC (n=19) | PTSD (n=19) | PTSD+MJ (n=20) | TEC+MJ (n=14) | p-value | |
|
Demographics | |||||
| Age | 28.6 (6.1) | 27.7 (9.5) | 28.9 (6.7) | 35.6 (12.6) | 0.06 |
| Sex (% Male, n) | 15.8%, 3 | 5.3%, 1 | 15.0%, 3 | 21.4%, 3 | 0.59 |
| Race (%, n) | 0.13 | ||||
| White | 63.2%, 12 | 73.7%, 14 | 80.0%, 16 | 71.4%, 10 | |
| Black | 10.5%, 2 | 21.1%, 4 | 15.0%, 3 | 14.3%, 2 | |
| Other | 26.3%, 5 | 5.3%, 1 | 5.0%, 1 | 14.3%, 2 | |
| Ethnicity (% Hispanic, n) | 10.5%, 2 | 0.0%, 0 | 10.0%, 2 | 7.1%, 1 | 0.91 |
| Years of Education | 16.5 (1.7) | 16.0 (2.0) | 15.3 (1.6) | 17.5 (4.0) | 0.06 |
| WTAR Standard Score | 115.3 (8.7) | 116.8 (8.2) | 116.3 (8.2) | 118.6 (8.4) | 0.74 |
|
Cannabis Use Characteristics | |||||
| Age of Initiation | -- | -- | 19.5 (5.6) | 20.1 (6.8) | 0.94 |
| Frequency of Use (Days per Week) | -- | -- | 5.9 (1.3) | 3.7 (2.1) | 0.001 |
| Frequency of Use (Times per Day) | -- | -- | 1.7 (0.8) | 1.3 (0.5) | 0.08 |
| Frequency of Use (Times per Week) | -- | -- | 10.8 (6.4) | 4.8 (3.4) | 0.003 |
| CUDIT | 0.6 (1.2) | 0.8 (1.6) | 12.1 (5.6) | 7.1 (2.5) | 0.004 |
|
Alcohol Use Characteristics | |||||
| Frequency of Use (Times per Week) | 1.2 (1.1) | 1.1 (1.6) | 1.5 (1.4) | 1.9 (1.9) | 0.53 |
| AUDIT | 3.1 (1.8) | 4.4 (3.3) | 6.1 (4.5) | 4.2 (2.6) | 0.19 |
|
Psychiatric Characteristics | |||||
| Age of Trauma | 19.5 (8.7) | 16.5 (6.7) | 16.8 (8.9) | 14.6 (10.2) | 0.43 |
| Years Since Trauma Ended | 9.1 (8.4) | 11.2 (12.9) | 12.6 (13.1) | 16.2 (13.7) | 0.46 |
| PCL-5 Total | 8.0 (4.8) | 43.8 (12.8) | 49.9 (13.7) | 6.8 (6.5)§ | 0.86 |
Note. All values are means and standard deviations at screening unless otherwise noted. For cannabis use characteristics, p-values from comparisons between the TEC+MJ and PTSD+MJ groups only. For PCL-5 total, p-values come from comparisons between the PTSD and PTSD+MJ groups only.
PCL scores are missing for 3 TEC+MJ participants
Abbreviations: AUDIT, Alcohol Use Disorders Identification Test; CUDIT, Cannabis Use Disorder Identification Test; PCL-5, PTSD Checklist for DSM-5; PTSD, Post-Traumatic Stress Disorder; WTAR, Wechsler Test of Adult Reading.
3.2. Voxelwise Analyses
Voxelwise analyses indicated significant group differences (p < 0.05 corrected) in FA values in the bilateral dorsal cingulum (cluster size = 25% on the right, 6% for the left) and in the bilateral anterior thalamic radiation (cluster size = 19% on the right, 18% on the left). There were no significant group differences in any other tracts evaluated in exploratory analyses (superior longitudinal fasciculus-parietal terminations, superior longitudinal fasciculus-temporal terminations, inferior longitudinal fasciculus, uncinate fasciculus, and cingulum–angular bundle).
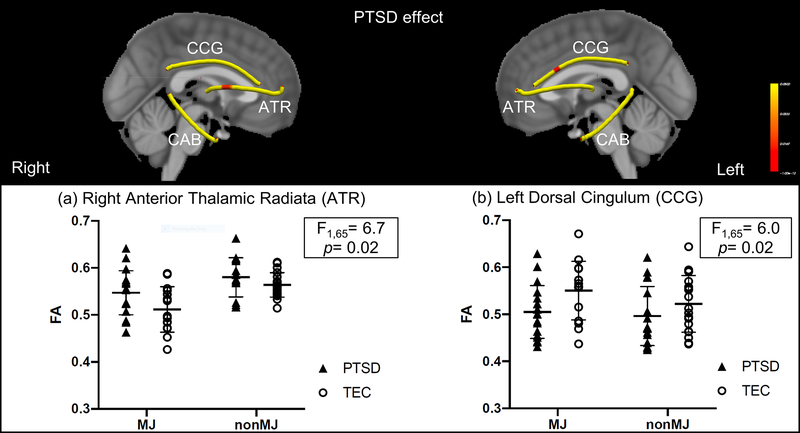
3.3. PTSD Effect
There was a main effect of PTSD in FA in the right anterior thalamic radiation (F(1,65) = 6.7; p = 0.02), with PTSD participants showing higher FA than TEC, regardless of MJ use (Fig 2a). A main effect of PTSD was also observed in left dorsal cingulum (F(1,65) = 6; p = 0.02) with PTSD participants showing lower FA than TEC (Fig 2b). There were no significant interactions between PTSD and MJ in these tracts.
Figure 2.
Voxel-wise analysis of PTSD effect. Yellow indicates the fiber skeleton created by the center of fiber tracts derived from TRACULA. Red indicates significant differences at p < 0.05 (corrected). PTSD was associated with higher FA compared to TEC in right anterior thalamic radiation (ATR) and lower FA compared to TEC in left dorsal cingulum (CCG).
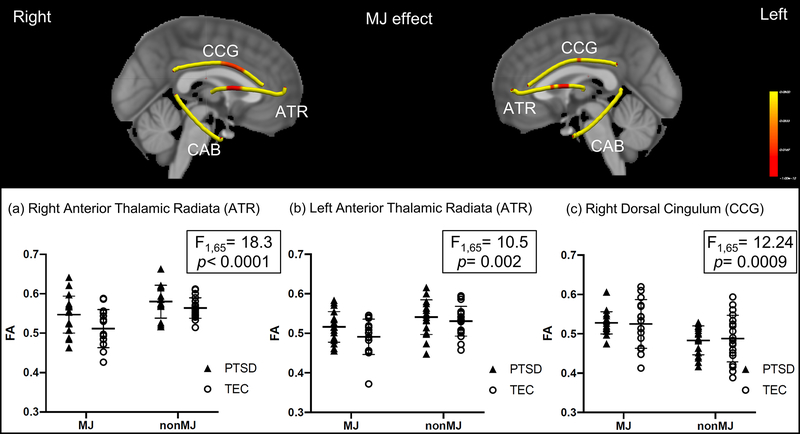
3.4. MJ effect
There was a significant main effect of MJ on FA in the right dorsal cingulum (F(1,65) = 12.24, p < 0.001), with MJ showing greater FA than non-MJ using groups, regardless of PTSD status (Fig 3c). There was also an MJ effect in the right anterior thalamic radiation (F(1,65) = 18.3, p < 0.001) (Fig 3a), and the left anterior thalamic radiation (F(1,65) = 10.05, p = 0.002) (Fig 3b), with MJ showing reduced FA values than non-MJ using groups, regardless of PTSD (Table 2). There were no significant interactions between PTSD and MJ in these tracts.
Figure 3.
Voxel-wise analysis of MJ effect. MJ was associated with lower FA in bilateral anterior thalamic radiation (ATR) and higher FA in right dorsal cingulum (CCG). * indicates a significant difference between groups. Yellow indicates the fiber skeleton created by the center of fiber tracts derived from TRACULA. Red indicates significant differences at p < 0.05 (corrected).
Table 2.
Anatomical Locations of Between-Group Differences (p < 0.05 corrected) in FA between PTSD, PTSD+MJ, TEC and TEC+MJ Participants
| Fiber Tract | Tract length (%) | Direction | MNI Coordinates |
|---|---|---|---|
| PTSD - TEC | |||
| Left Dorsal Cingulum | 6% | ↓ | −8, −2, −34 |
| Right Anterior Thalamic Radiata | 10% | ↑ | 18, 3, 12 |
| MJ - NON-MJ | |||
| Right Dorsal Cingulum | 25% | ↑ | 7, 3, 32 |
| Right Anterior Thalamic Radiata | 19% | ↓ | 19, 7, 12 |
| Left Anterior Thalamic Radiata | 18% | ↓ | −18, 4, 12 |
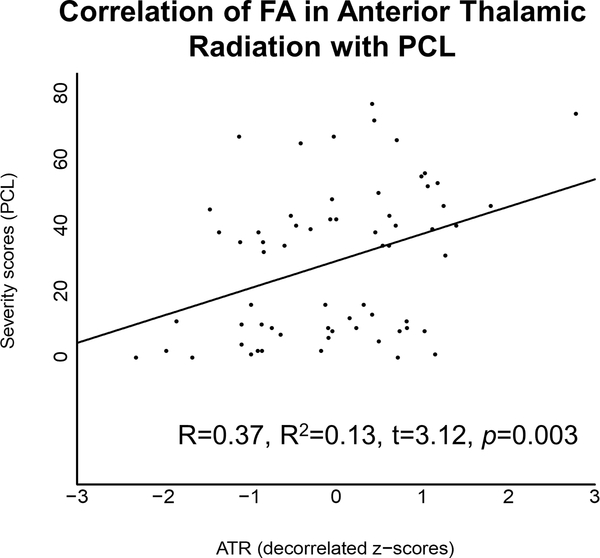
3.5. Relationship analysis between FA and PTSD severity
PTSD symptom severity (PCL) scores were positively correlated with FA in the anterior thalamic radiations (R = 0.37, R2 = 0.13, t = 3.12, p = 0.003) (Fig 4). There was no correlation between PCL scores and FA in the dorsal cingulum (R = 0.06, R2 = 0.00, t = 0.51, p = 0.615).
Figure 4.
PCL scores were positively associated with FA in the anterior thalamic radiations (R = 0.37, R2 = 0.13, t = 3.12, p = 0.003).
4. Discussion
This study provides evidence that PTSD and MJ use are independently associated with altered FA in the right thalamic radiation and left cingulum, with no significant interactions between PTSD and MJ detected. PTSD was associated with increased FA in the right anterior thalamic radiation, while MJ use was associated with decreased FA bilaterally in the anterior thalamic radiation. Increased FA in the anterior thalamic radiation, but not the cingulum, was associated with higher PTSD symptom severity ratings. There were no significant interactions between PTSD and MJ use, thus this study did not find support for MJ normalization of PTSD-related white matter abnormalities.
4.1. Effect of PTSD on white matter integrity
This finding of a significant PTSD effect on the right anterior thalamic radiation (ATR) is consistent with previous reports that regions connected by this tract (e.g. the dorsal thalamus and medial frontal cortex) showed greater cerebral blood flow during traumatic recall as compared to a condition of simple fear in those with PTSD (Huber et al., 2001), as increased connectivity in these regions could underlie flashbacks and nightmares. ATR afferent fibers from thalamic nucleus to the cingulate area and medial cortex are important for memory-related function (Maddock, 1999). Increased connectivity in the ATR in the PTSD group is also consistent with recent reports that fronto-thalamic circuitry is preferentially affected in PTSD (Yin et al., 2011) and in emotional systems underlying reward-seeking and punishment (Coenen et al., 2012).
We also report a significant PTSD effect in the left dorsal cingulum, with the PTSD group showing decreased FA compared with the non-PTSD group. Several studies suggest that the cingulum bundle, which is involved in emotion regulation, may underlie the pathological fear processing characteristic of PTSD symptomatology (O’Doherty et al., 2018; Sanjuan et al., 2013). It should be noted that whether increased or decreased connectivity underlie pathological PTSD symptoms remains unclear, and the current study did not support our hypothesis that we would observe increased FA in this region. In a meta-analysis of seven DTI studies in trauma-exposed adults, authors identified significant FA decreases in nine clusters and FA increases in six clusters in the trauma-exposed groups, with the largest identified cluster pertained to an FA decrease in the right cingulum, and with further increases and decreases identified bilaterally in different sections of the cingulum (Daniels et al., 2013). It is also possible that, as hypothesized by Daniels et al., age of trauma exposure could impact the direction of white matter change with PTSD, with perhaps greater decreases occurring if the trauma occurred during childhood or adolescence, and increases occurring during adulthood as a consequence of PTSD. In the current study, trauma exposure occurred across the lifespan, which may partly explain why we observed overall decreases in FA.
4.2. Effect of MJ on white matter integrity
Regular (at least weekly) MJ use was associated with lower FA in the anterior thalamic radiation and higher FA in the dorsal cingulum, with no interactions between MJ use and PTSD. Though there are few studies of MJ exposure on white matter integrity, altered white matter in the arcuate fasciculus (Ashtari et al., 2009), right fimbria of the hippocampus (fornix), and splenium of the corpus callosum and commissural fibers (Zalesky et al., 2012) has been reported in MJ users. A recent DTI study showed significant increases in FA in the corpus callosum in MJ users compared to controls (Mandelbaum and de la Monte, 2017). It should also be noted that the direction of change, like in the PTSD literature, is unclear; one study found that early MJ onset was associated with lower FA (Gruber et al., 2014), and one of the few longitudinal studies of DTI and MJ use found that more MJ use was correlated with reduced longitudinal growth in FA in the superior longitudinal fasciculus and the corticospinal tract (Becker et al., 2015). These inconsistencies in the literature underscore the importance of evaluating groups that are well-matched on MJ use parameters.
4.3. Limitations
Although we attempted to match MJ use between the TEC+MJ and PTSD+MJ groups, the PTSD+MJ group used significantly greater amounts of MJ, and not surprisingly, had higher CUDIT scores. Therefore, the MJ effects on WM detected in this study could have been largely driven by the PTSD+MJ group. Future studies could recruit trauma-exposed, non-PTSD participants with greater frequency of use of MJ to better disentangle this (though it should be noted that all MJ participants were fairly heavy users, e.g. using, on average, at least 3 times per week). Additionally, the small sample size in each group, especially of males, limits our ability to examine interactions between WM integrity and other factors such as sex, age of trauma, or type of trauma. We also recruited participants with a mix of childhood and adult-onset trauma, obscuring our ability to determine whether the FA decreases are associated with specific timing of trauma. Further, though most participants began MJ initiation after the trauma had occurred, a sizable minority (about 30%) has already initiated MJ use before their traumatic event. Therefore, we are limited in our ability to draw conclusions about whether MJ was a causal factor in any associations that we observed between FA and MJ use in those with PTSD. Finally, because this study is cross-sectional, we cannot determine whether FA alterations associated with either PTSD or MJ use predated these disorders; longitudinal studies are needed to better understand the relationship between PTSD, MJ, and white matter structure. Future longitudinal studies are also needed to more precisely map the time course of white matter changes in those with PTSD and/or MJ use, to more explicitly discover whether MJ use changes white matter integrity in those with PTSD.
5. Conclusion:
The current study demonstrates that PTSD and MJ use are independently associated with altered FA in the right thalamic radiation and left cingulum. No interactions between PTSD and MJ were detected, indicating that MJ use in those with PTSD neither significantly ameliorates nor worsens white matter abnormalities detected in PTSD. Future studies are needed to determine whether MJ use normalizes (or worsens) any other neurobiological markers of PTSD. However, this study does not provide evidence that MJ use normalizes PTSD-associated white matter abnormalities. Therefore, the risks and benefits of medical MJ use should be carefully weighed in light of available evidence suggesting that whole plant marijuana may increase the risk for psychosis and substance use disorders (Filbey et al., 2014). Thus, there is an urgent need to better understand associations between MJ use and PTSD, and to understand how MJ use in various psychiatric populations may affect brain structure and function.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge and thank Karestan Koenen for her assistance with the protocol development.
Role of funding source: This work was supported by NIDA K01 DA034093 (JG), NIDA R01DA042043 (JG), and NIDA K24 DA030443 (AEE). These funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the manuscript for publication.
6. References:
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, Ohtani T, Masutani Y, Kato N, Ohtomo K, 2006. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. Neuroimaging 146, 231–242. 10.1016/j.pscychresns.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS, 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT, 2008. Corpus callosum damage in heavy marijuana use: Preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage 41, 1067–1074. 10.1016/j.neuroimage.2008.02.064 [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S, 2009. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research 43, 189–204. 10.1016/j.jpsychires.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A.P., 2013. Cautionary statement for forensic use of DSM-5, 5 ed. 10.1176/appi.books.9780890425596 [DOI]
- Ayling E, Aghajani M, Fouche JP, van der Wee N, 2012. Diffusion tensor imaging in anxiety disorders. Curr Psychiatry Rep 14, 197–202. 10.1007/s11920-012-0273-z [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF, 2010. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 72, 347–354. 10.1016/j.bandc.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M, 2015. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci 16, 23–35. 10.1016/j.dcn.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott C.a.M., Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM, 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci 6, 750–757. 10.1038/nn1075 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. www.jstor.org/stable/2346101 [Google Scholar]
- Bierer LM, Ivanov I, Carpenter DM, Wong EW, Golier JA, Tang CY, Yehuda R, 2015. White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: A pilot study. Psychoneuroendocrinology 51, 567–576. 10.1016/j.psyneuen.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL, 2015. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress 28, 489–498. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- Bryant RA, 2001. Posttraumatic Stress Disorder and Mild Brain Injury: Controversies, Causes and Consequences. J. Clin. Exp. Neuropsychol 23, 718–728. 10.1076/jcen.23.6.718.1024 [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N, 2019. Posttraumatic Stress Disorder and Drug Disorders: Testing Causal Pathways. Arch. Gen. Psychiatry 55, 913–917. 10.1001/archpsyc.55.10.913 [DOI] [PubMed] [Google Scholar]
- Coenen VA, Panksepp J, Hurwitz TA, Urbach H, Madler B, 2012. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J. Neuropsychiatry Clin. Neurosci 24, 223–236. 10.1176/appi.neuropsych.11080180 [DOI] [PubMed] [Google Scholar]
- Cukor J, Olden M, Lee F, Difede J, 2010. Evidence-based treatments for PTSD, new directions, and special challenges. Ann. N. Y. Acad. Sci 1208, 82–89. 10.1111/j.1749-6632.2010.05793.x [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Daniels JK, Lamke J-P, Gaebler M, Walter H, Scheel M, 2013. White Matter Integrity and Its Relationship to Ptsd and Childhood Trauma—a Systematic Review and Meta-Analysis. Depression and Anxiety 30, 207–216. 10.1002/da.22044 [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA, 2006. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal 3, 17 10.1186/1477-7517-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Chugani DC, Makki M, 2006. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117, 2093–2100. 10.1542/peds.2005-1727 [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Norrholm SD, Bradley B, Ressler KJ, Jovanovic T, 2015. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 64, 249–259. 10.1016/j.cortex.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ, 2012. White Matter Integrity in Highly Traumatized Adults With and Without Post-Traumatic Stress Disorder. Neuropsychopharmacology 37, 2740–2746. 10.1038/npp.2012.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J, 2014. Long-term effects of marijuana use on the brain. Proc. Natl. Acad. Sci. U. S. A 111, 16913–16918. 10.1073/pnas.1415297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM, 2001. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 20, 70–80. 10.1109/42.906426 [DOI] [PubMed] [Google Scholar]
- Grinage BD, 2003. Diagnosis and management of post-traumatic stress disorder. Am. Fam. Physician 68, 2401–2408. http://www.ncbi.nlm.nih.gov/pubmed/14705759 [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE, 2014. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology 231, 1455–1465. 10.1007/s00213-013-3326-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA, 2005. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Cognitive Brain Research 23, 107–118. 10.1016/j.cogbrainres.2005.02.016 [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD, 2010. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J. Neurosci 30, 7466–7472. 10.1523/JNEUROSCI.0859-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DW, Thatcher GW, Bennett PJ, Sood S, Paulson D, Creem-Regehr S, Brown BL, Allen S, Johnson J, Froelich B, Bigler ED, 2007. Brain integrity and cerebral atrophy in Vietnam combat veterans with and without posttraumatic stress disorder. Neurocase 13, 402–410. 10.1080/13554790701851551 [DOI] [PubMed] [Google Scholar]
- Huber M, Siol T, Herholz K, Lenz O, Köhle K, Heiss WD, 2001. Activation of thalamo-cortical systems in post-traumatic flashbacks: A positron emission tomography study. Traumatology (Tallahass. Fla.) 7, 131–141. 10.1177/153476560100700402 [DOI] [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH, Kaufman J, 2008. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Research: Neuroimaging 162, 256–261. 10.1016/j.pscychresns.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF, 2009. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol. Teratol 31, 349–355. 10.1016/j.ntt.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO, 2012. Early Interventions for Ptsd: A Review. Depression and Anxiety 29, 833–842. 10.1002/da.21997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Provenzale JM, Law M, 2006. Magnetic resonance and diffusion tensor imaging in pediatric white matter diseases. Top. Magn. Reson. Imaging 17, 265–274. 10.1097/01.rmr.0000248665.84211.0f [DOI] [PubMed] [Google Scholar]
- Lepage C, de Pierrefeu A, Koerte IK, Coleman MJ, Pasternak O, Grant G, Marx CE, Morey RA, Flashman LA, George MS, McAllister TW, Andaluz N, Shutter L, Coimbra R, Zafonte RD, Stein MB, Shenton ME, Bouix S, 2018. White matter abnormalities in mild traumatic brain injury with and without post-traumatic stress disorder: a subject-specific diffusion tensor imaging study. Brain Imaging Behav. 12, 870–881. 10.1007/s11682-017-9744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yücel M, 2010. Structural MRI Findings in Long-Term Cannabis Users: What Do We Know? Subst. Use Misuse 45, 1787–1808. 10.3109/10826084.2010.482443 [DOI] [PubMed] [Google Scholar]
- Maddock RJ, 1999. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 22, 310–316. [DOI] [PubMed] [Google Scholar]
- Mandelbaum DE, de la Monte SM, 2017. Adverse Structural and Functional Effects of Marijuana on the Brain: Evidence Reviewed. Pediatr. Neurol. 66, 12–20. 10.1016/j.pediatrneurol.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel OB-M, Glenna SR, Marijuana Use and PTSD among Veterans - PTSD: National Center for PTSD; https://www.ptsd.va.gov/professional/treat/cooccurring/marijuana_ptsd_vets.asp [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJ, 2009. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J. Child Psychol. Psychiatry 50, 943–951. 10.1111/j.1469-7610.2009.02084.x [DOI] [PubMed] [Google Scholar]
- O’Doherty DCM, Ryder W, Paquola C, Tickell A, Chan C, Hermens DF, Bennett MR, Lagopoulos J, 2018. White matter integrity alterations in post-traumatic stress disorder. Hum. Brain Mapp 39, 1327–1338. 10.1002/hbm.23920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert KA, Carrion VG, Karchemskiy A, Reiss AL, 2006. Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress. Anxiety 23, 17–25. 10.1002/da.20131 [DOI] [PubMed] [Google Scholar]
- Rinne-Albers MA, van der Werff SJ, van Hoof MJ, van Lang ND, Lamers-Winkelman F, Rombouts SA, Vermeiren RR, van der Wee NJ, 2016. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: a DTI study. Eur Child Adolesc Psychiatry 25, 869–878. 10.1007/s00787-015-0805-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A, 2013. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res. 214, 260–268. 10.1016/j.pscychresns.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, Mueller SG, Wang Z, Marmar CR, Weiner MW, Neylan TC, 2011. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. Neuroimage 54, S62–S68. 10.1016/j.neuroimage.2010.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckfort DL, Paul R, Grieve SM, Vandenberg B, Bryant RA, Williams LM, Clark CR, Cohen RA, Bruce S, Gordon E, 2008. Early Life Stress on Brain Structure and Function Across the Lifespan: A Preliminary Study. Brain Imaging and Behavior 2, 49 10.1007/s11682-007-9015-y [DOI] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ, 2011. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci 12, 154–167. 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishko I, Oliveira R, Moore TA, Almeida K, 2018. A review of medical marijuana for the treatment of posttraumatic stress disorder: Real symptom re-leaf or just high hopes? Ment Health Clin 8, 86–94. 10.9740/mhc.2018.03.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl S, King JA, Burgess N, Flor H, Nees F, 2018. Structural white matter changes in adults and children with posttraumatic stress disorder: A systematic review and meta-analysis. Neuroimage Clin 19, 581–598. 10.1016/j.nicl.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB, 1996. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 42, 49–54. https://www.ncbi.nlm.nih.gov/pubmed/8889403 [DOI] [PubMed] [Google Scholar]
- Vlahov D, Galea S, Resnick H, Ahern J, Boscarino JA, Bucuvalas M, Gold J, Kilpatrick D, 2019. Increased Use of Cigarettes, Alcohol, and Marijuana among Manhattan, New York, Residents after the September 11th Terrorist Attacks. Am. J. Epidemiol 155, 988–996. 10.1093/aje/155.11.988 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP, 2013. Using the PTSD Checklist for DSM_IV (PCL). 4.
- Winkler AM, Webster MA, Brooks JC, Tracey I, Smith SM, Nichols TE, 2016. Non-parametric combination and related permutation tests for neuroimaging. Hum. Brain Mapp 37, 1486–1511. 10.1002/hbm.23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM, Biessels GJ, Geerlings MI, 2014. A Critical Appraisal of the Hippocampal Subfield Segmentation Package in FreeSurfer. Front. Aging Neurosci. 6 10.3389/fnagi.2014.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitzky-Taylor KB, Castriotta N, Lenze EJ, Stanley MA, Craske MG, 2010. Anxiety disorders in older adults: a comprehensive review. Depression and Anxiety 27, 190–211. 10.1002/da.20653 [DOI] [PubMed] [Google Scholar]
- Yarnell S, 2015. The Use of Medicinal Marijuana for Posttraumatic Stress Disorder: A Review of the Current Literature. Prim. Care Companion CNS Disord. 17. 10.4088/PCC.15r01786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, Jbabdi S, Gollub R, Fischl B, 2011. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform 5, 23 10.3389/fninf.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, Chen H, Feng B, Jiang T, Jin H, Wong C, Gong Q, Li L, 2011. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: A functional magnetic resonance imaging study. Brain Res. 1411, 98–107. 10.1016/j.brainres.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Zalesky A, Solowij N, Yücel M, Lubman DI, Takagi M, Harding IH, Lorenzetti V, Wang R, Searle K, Pantelis C, Seal M, 2012. Effect of long-term cannabis use on axonal fibre connectivity. Brain 135, 2245–2255. 10.1093/brain/aws136 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li W, Shu N, Zheng H, Zhang Z, Zhang Y, He Z, Hou C, Li Z, Liu J, Wang L, Duan L, Jiang T, Li L, 2012. Increased white matter integrity of posterior cingulate gyrus in the evolution of post-traumatic stress disorder. Acta Neuropsychiatrica 24, 34–42. 10.1111/j.1601-5215.2011.00580.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.