Summary
The whole world is now facing an unprecedented pandemic with over 1.8 million confirmed cases and more than one hundred thousand deaths. To counter the pandemic, Shenzhen created a central command and control structure based on the only designated hospital- Shenzhen Third People's Hospital which is a large general hospital specialized on infectious diseases in the bay area. The hospital has taken many decisive and effective actions to respond to the epidemic. Here, we will describe and share healthcare experiences from Shenzhen and call for international cooperation and collaboration.
Keywords: COVID-19, public health emergency, Shenzhen, China
Once the lockdown of Wuhan ended on April 8th, efforts to combat COVID-19 in China have earned a stage achievement. The entire process of fighting the epidemic is a huge test for the Chinese healthcare system. As an extremely large city in the Guangdong-Hong Kong- Macau Greater Bay Area, Shenzhen has a large number of residents and migrant workers from Hubei Province, and the need for epidemic prevention and control has brought enormous pressure on the city. Shenzhen Third People's Hospital is the only designated hospital for COVID-19 epidemic in the city, and it is also the "National Clinical Research Center for Infectious Diseases" (1). The first patient infected with COVID-19 was admitted on January 11, 2020; this was the earliest family-concentrated case in Guangdong Province. This case provided direct and solid evidence for the existence of person to person transmission and thus blew the whistle in the whole province.
As of the afternoon of April 11, 456 COVID-19 cases have been seen at the hospital, involving 225 males and 231 females (Figure 1A). A total of 423 patients were discharged from the hospital and 3 patients died, representing a mortality rate of 0.65%. That rate was significantly lower than the rate reported in other areas of China affected by COVID-19 (2). Moreover, there were "no infections" among medical personnel.
Figure 1.
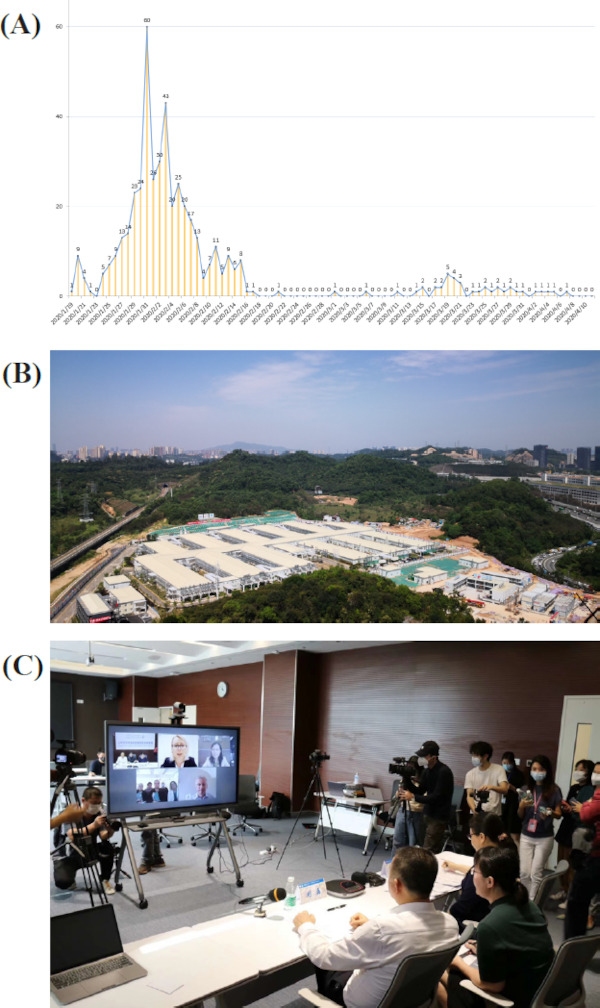
(A), A total of 456 confirmed cases admitted in Shenzhen Third People's Hospital from January 19, 2020 to April 11, 2020 (Data are from the Shenzhen CDC); (B), Photo of the newly-built emergency hospital area; (C), Video conference to share experience fighting the epidemic with colleagues overseas.
In order to deal with a potential epidemic, the hospital vacated 1,000 beds, and built an emergency hospital area similar to Wuhan Huoshenshan Hospital, providing more than 1,000 beds of reserves (Figure 1B); Shenzhen Health Commission and the hospital prepared seven extracorporeal membrane oxygenation (ECMO) machines to save the lives of severely ill patients. The hospital was also the first to use antibodies extracted from the plasma of recovered patients as well as lung transplantation to treat severe cases, and this approach has yielded good results (3,4). At the same time, Shenzhen Third People's Hospital organized and summarized its clinical experience in the Shenzhen version of COVID-19 diagnosis and treatment guidelines based on national and provincial treatment protocols for COVID-19. In addition, the Shenzhen version has been constantly updated and adjusted in accordance with changes in clinical practice. Furthermore, Shenzhen Third People's Hospital released a new version of the guidelines for newborns, infants, and children and an English version to share Shenzhen's experience with the world (Figure 1C).
Shenzhen Third People's Hospital published as many as 27 academic articles related to COVID-19, providing reliable references for colleagues around the world (5-8). Our hospital, the National Clinical Research Center for Infectious Diseases, was the first facility to detect live coronavirus in the stool of patients, indicating the possibility of fecal-oral transmission of COVID-19 (9). The hospital's experience in treating severely ill patients with plasma from recovered patients was featured in the authoritative international journal JAMA (4). The hospital conducted scientific research in cooperation with Tsinghua University's School of Medicine, the Chinese Academy of Medical Science, and the Southern University of Science and Technology to screen the blood of recovered patients and isolate B lymphocytes that can secrete high-affinity antibodies and to establish monoclonal antibody cell lines. The technique devised by that research can now be used clinically, and a related scientific paper has been published (10).
At present, China's prevention and control of COVID-19 has achieved important initial results. Outside China, however, the pandemic continues to spread, and Shenzhen faces increasing risks of imported cases from overseas. Viruses have no borders, so we should enhance international cooperation and jointly combat this global pandemic.
References
- 1. Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020; doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 2. Liang WH, Guan WJ, Li CC, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a nationwide analysis of China. Eur Respir J. 2020; doi: 10.1183/13993003.00562-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for COVID-19-related pulmonary fibrosis. Chin Med J. 2020; doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically III patients with COVID-19 with convalescent plasma. JAMA. 2020; doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, Li YJ, Xie XJ, Feng C, Liu L. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020; doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, Xin N, Huang Z, Liu L, Zhang G, Wang J. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020; doi: 10.1136/ bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. An J, Liao X, Xiao T, et al. Clinical characteristics of the recovered COVID-19 patients with re-detectable positive RNA test. medRxiv. 2020; doi: https://doi.org/10.1101/2020.03.26.20044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ju B, Zhang Q, Ge X, et al. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection. bioRxiv. 2020; doi: https://doi.org/10.1101/2020.03.21.990770. [DOI] [PubMed] [Google Scholar]


