Summary
During the last two decades, there has been a dramatic increase in so-called non-B non-C hepatocellular carcinoma (NBNC HCC) in Japan. Majority of NBNC HCC are considered as so-called metabolic HCC and some could be related to occult HBV infection. Although there have been some reports on histological features predominant in metabolic HCC, very few specific driver genes for NBNC HCC have been reported. Most of the NBNC HCC are found incidentally and are relatively large in size. Since liver function is generally normal or subnormal, such patients have a higher chance for undergoing curative surgery. Although there has been slightly conflicting long-term outcomes reported for NBNC HCC, slightly better outcomes may be expected compared to other etiologies after curative surgery. However, risk of recurrence depends on the background liver. NBNC HCC in cirrhotic patients have a persistently higher risk of tumor recurrence requiring a long-term postoperative surveillance. It would be safe to conclude at this moment that NBNC HCCs should be treated using the same surgical strategy as HCCs with viral origin, same operative indications and same follow-up protocol.
Keywords: non-B non-C HCC, hepatitis, epidemiology, surgical strategy, recurrence
Introduction
More than 70% of cases with hepatocellular carcinoma (HCC) in Japan have long been from hepatitis C origin (C-HCC) followed by hepatitis B (B-HCC) and other etiology. These etiological features in Japan have been very unique in East Asia where most of the HCCs are derived from hepatitis B. Since the century changed, however, the proportion of so-called non-B and non-C HCC (NBNC HCC) has dramatically increased whereas C-HCC decreased significantly and B-HCC is unchanged (Figure 1) (1). Acting antivirals (DAA) treatment for hepatitis C virus (HCV) was introduced with the coverage by social insurance and the special subsidy program for anti-viral treatment around 2014, and most of the infected patients in Japan have enjoyed the benefit of DAA treatment. Incidence of de novo HCC in HCV infected patients is therefore expected to decrease in the next decade.
Figure 1.
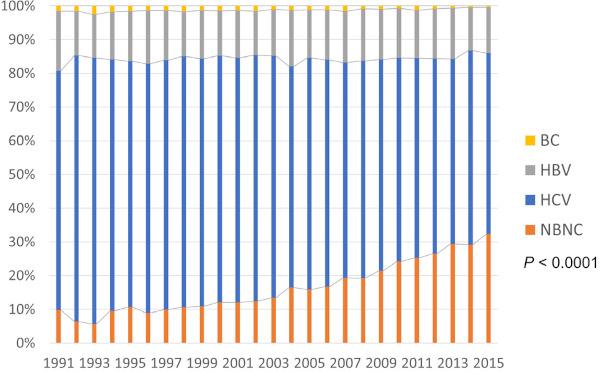
Trend in background liver disease for HCC in Japan (1).
Another significant change in HCC patient background is aging. According to nation-wide biannual HCC survey by the Liver Cancer Study Group of Japan (LCSGJ), average age of new HCC patients has been steadily increasing up to 69 years-old in the latest survey (Figure 2). Since average age of HCV derived HCC patients is one of the oldest among all etiology subgroups (2,3) and incidence of carcinogenesis increases by age (4), HCV-HCC population who benefits by curative surgery may shrink rapidly in the near future. In contrast, proportion of NBNC HCC in surgical candidates has been steadily increasing and have outnumbered HCV-HCC patients in the recent few years (Figure 3).
Figure 2.
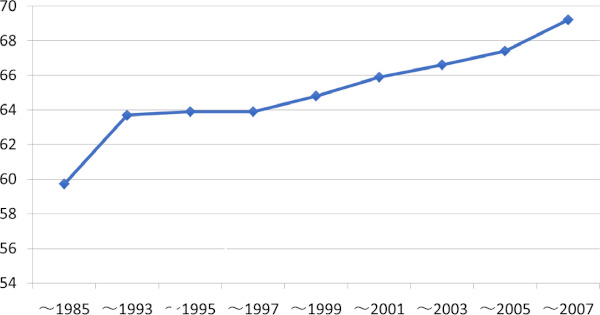
Average age of HCC patients diagnosed in Japan (Nation-wide survey by Japanese Study Group of Liver Cancer 1984-2007).
Figure 3.
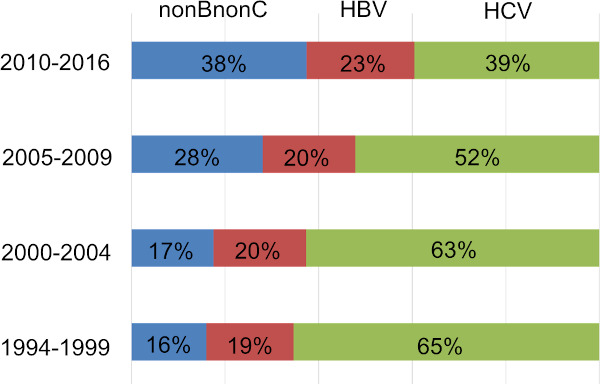
Etiological trend in HCC patients undergoing liver resection at the University of Tokyo (1994-2016).
NASH/NAFLD as a cause of NBNC HCC
According to the INUYAMA NOBLESSE Study group report covering 5,326 NBNC HCC patients in Japan, major etiologies for NBNC HCC were alcohol (27%), nonalcoholic fatty liver disease (NAFLD) (11%) followed by primary biliary cholangitis (PBC) (6%) and autoimmune hepatitis (AIH) (6%) (5). However, more than half (54%) of the cases has an unclassified etiology and many of them could be so-called metabolic HCC related to metabolic syndrome including diabetes mellitus, central obesity, hypertension, and dyslipidemia. Globally speaking, prevalence of obesity has not been relatively high in Japan with median body mass index (BMI) under 25 in both sexes (6). However, because of recent Westernization of food and other habits, increasing obesity and metabolic related diseases (metabolic syndrome) have been recent issues in Japan especially in males. Actually, prevalence of NAFLD jumps up when BMI exceeds 26 (6). Thirty to 40 % of Japanese males are estimated to have NAFLD and fibrosis may progress very slowly in approximately 30% of cases (7). Once cirrhotic liver is established, annual HCC development is estimated to be around 2%.
Occult HBV infection as a cause of NBNC HCC
Another important etiology for NBNC HCC is so-called occult HBV. Occult HBV or history of previous HBV infection is defined by positive anti-hepatitis B core antibody (HBcAb) with negative hepatitis B surface antigen (HBsAg). Although HBcAb is not routinely tested in daily practice in most of the specialized institutions for HCC in Japan, occult HBV infection may consist of 30-40% of NBNC HCC in Japan (5). It is well known that even a history of HBV infection, i.e. positive HBcAb and negative HBsAg, can be a risk factor for HCC among patients with negative serology results for active HBV infection (8-10). Since 2002, we have started to routinely test HBc antibody for all patients undergoing liver resection at the University of Tokyo Hospital. Incidence of occult HBV infection among surgical candidates was 29.0% (45/155) (2).
Genomic study
Although various etiologies and the number of gene signatures that have been identified recently (11), the global landscape of the genetic changes in HCC genomes underpinning different epidemiological backgrounds still remains uncharted (12). Recent HCC genome sequencing research covering 503 liver cancer genomes uncovered 30 candidate driver genes and 11 core pathway modules. They found that telomerase reverse transcriptase (TERT) is a central driver gene and promising molecular target. Furthermore, they identified frequently mutated genes including TP53 and CTNNB1 (b-catenin) (13). The Cancer Genome Atlas (TCGA) projects have revealed that HCC contains intra- and inter-tumor heterogeneity with numerous passenger mutations (14). It is thus suggested that different combinations of mutations contribute to the development of HCC. There are very few altered genes specific to NBNC HCC. They only reported that ARID1A mutation was more frequent while AXIN1 mutation was infrequent in NBNC HCC genomes. At present, there are no molecular targeted agents specifically effective to NBNC HCC. By using genome-wide random mutagenesis with Sleeping Beauty transposons, Kodama et al. reported that Sav1 is one of the candidate driver genes in steatohepatitis-based HCC (15). Integrated analysis, covering exome, transcriptome, methylome and high-throughput screens shed light on inhomogeneous profiles of individual HCC nodules in the whole liver, resulting in the establishment of next-stage etiology-specific molecular targeted therapy against HCC.
Steatohepatitic HCC
Steatohepatitic HCC (SH-HCC) is a recently established histological subcategory of HCC associated with the patients metabolic condition and the presence of steatosis or steatohepatitis in the background liver. Histological diagnostic for SH-HCC is made when the tumor fulfills four of the following five criteria: steatosis (> 5% tumor cells), ballooning or Mallory-Denk body formation, interstitial fibrosis and inflammatory infiltrates (16,17). SH-HCC is not equal to either NBNC-HCC, metabolic HCC, or NAFLD derived HCC. However, patients with SH-HCC were characterized by a higher frequency of diabetes mellitus and hypertension, along with higher serum levels of cholesterol and triglycerides, than those with conventional HCC (18). The background liver of SH-HCC patients showed steatosis and steatohepatitis more frequently. Although there has not been sufficient evidence to conclude prognostic features of SH-HCC, so far, there seems to be no significant differences compared to other histological types of HCC.
Surgical strategy for NBNC HCC
Surgical indications for NBNC HCC have been essentially the same as that for HCC with other etiologies. R0 resection with negative surgical margin is a basic strategy and anatomic resection is recommended whenever possible because of potential eradication of micrometastases in the tumor-bearing portal territory (19). Substantial surgical margin is not always achievable and because of the non-invasive and well-encapsulated nature of HCC, minimal or zero surgical margin without tumor exposure is considered acceptable with a non-inferior surgical outcome (20). Safety limit of liver resection is determined by the degree of liver functional reserve represented by the presence of ascites and jaundice, and the indocyanine green (ICG) retention test. These criteria are known as so called "Makuuchi criteria" and they assure a very low surgical mortality (Figure 4) (21,22).
Figure 4.
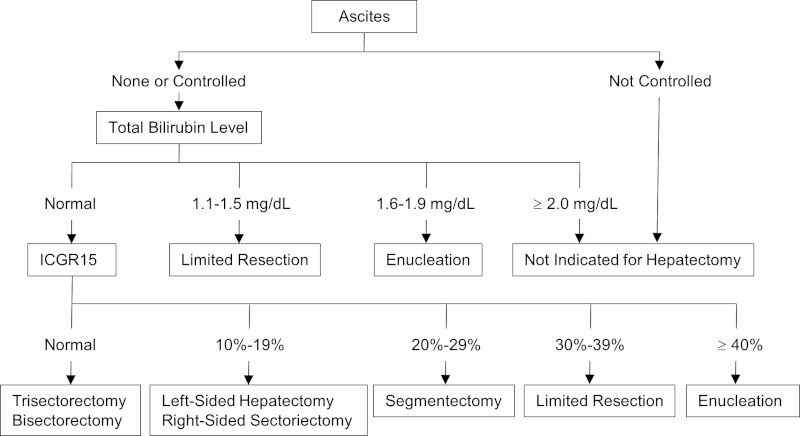
Makuuchi Criteria: Decision criterion for selection of operative procedures in patients with impaired liver function reserve. To convert total bilirubin from milligrams per deciliter to micromoles per liter, multer, multiply by 17.1 ICG15 indicates indocyanine green retention rate at 15 minutes (21,22).
Typical recent cases
Case 1: A 67-year-old male on a routine medical checkup and chest X-ray examination revealed an elevation of right diaphragm. Subsequent CT showed a tumor, 10 cm in diameter, in the right liver (Figure 5A). He was diabetic (HbA1c: 9.7%) and hypertensive. Alfa fetoprotein (AFP) was normal, but DCP (PIVKA-II) was 3,517 mAu/mL. HBcAb was negative. Child-Pugh score 5 and ICG retention rate at 15 min (ICG-R15) was 6.8%. He underwent right hepatectomy. Pathological findings were: Moderately to poorly differentiated hepatocellular carcinoma, 11 × 8 × 7 cm, eg, fc(-), sf(+), vp0, vv0, b0. Background liver was normal. He is fine with no evidence of disease for 21 months.
Figure 5.
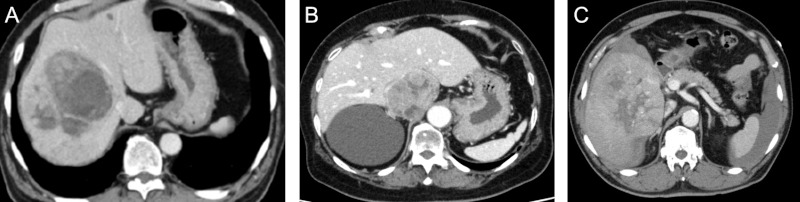
Contrast enhanced CT. (A), Case 1 showing a large HCC with mosaic pattern; (B), Case 2 showing a 6 cm sized tumor in the caudate lobe; and (C), Case 3 showing a large HCC in the right liver and intra-abdominal fluid collection.
Case 2: A 72-year-old female was diagnosed to have a renal cyst and follow-up US detected a liver tumor. AFP was 9,303 ng/mL and DCP (PIVKA-II) was 672 mAu/mL. HBcAb was negative. A contrast enhanced CT revealed a 6 cm mass in the caudate lobe (Figure 5B). She had a history of hypertension and hyperlipidemia. She underwent left hepatectomy. Pathological findings were: Well- to moderately differentiated hepatocellular carcinoma, 6.7 × 5.5 × 4.9 cm, eg, fc(+), sf(+), vp0, vv0, b0. Background liver was mild fibrosis (F1). She is fine with no evidence of disease for 11 months.
Case 3: A 68-year-old male had a history of hypertension and hyperlipidemia. He suddenly developed severe abdominal pain and he was transferred by ambulance to ER. An emergent contrast enhanced CT revealed a large enhancing mass, 17 cm in diameter, in the right liver and intraabdominal fluid collection (Figure 5C). AFP was 4,237 ng/mL and DCP (PIVKA-II) was 111,669 mAu/mL. Rupture of an HCC was suspected and he underwent TACE followed by right hepatectomy with a 3-day interval. Pathological findings were: Moderately differentiated hepatocellular carcinoma, 11.8 × 10.5 × 8.0 cm, eg, fc(+), sf(+), vp0, vv1, b0. Background liver was chronic hepatitis with mild fibrosis (F1, A2). He is fine with no evidence of disease for 8 months.
Short-term outcome after liver resection
Operative morbidity or mortality depends on liver function and comorbidities. Since NBNC HCC patients are frequently associated with obesity and so-called metabolic diseases including diabetes mellitus, central obesity, hypertension, and dyslipidemia, presence of such comorbidities may affect short-term outcomes. Yoshida et al. reported significantly higher incidence of postoperative complications in patients with metabolic HCC compared with those with cryptogenic HCC (40.0 vs. 22.7 %, p = 0.049) (Yoshida (23)). Several authors in the US have reported a near two-fold increased risk of complications among patients with obesity and metabolic HCC (Bhayani (24), Mathur (25), Pawlik (26)). As a basic perioperative management, all comorbidities in NBNC HCC patients should be precisely evaluated and controlled in a multi-discipline approach.
Long-term outcome after liver resection
Although there have been a number of reports on the long-term outcome of NBNC HCC or metabolic HCC, whether the long-term outcome of such HCCs is better than or comparable to that of HCC with other etiologies has not been conclusive (Wakai (27), Reddy (28), Kaneda (29), Yoshida (23), Vigano (30)). These discrepancies in the literature may be because of differences in background liver and in the etiologies of the control groups.
According to a nation-wide survey in Japan covering 57,450 HCC patients (Jan. 2000 to Dec. 2005), 9,307 patients were identified as NBNC HCC. Occult HBV infection was not excluded because HBc Ab was not routinely tested in most of the centers. After excluding those with extrahepatic disease or Child-Pugh C status and those undergoing other treatments, 4,741 NBNC HCC patients underwent the 3 major treatments, liver resection (HR: 2,827 cases) Radiofrequency ablation (RFA: 432 cases), and trans-arterial chemoembolization (TACE: 1,437 cases). As expected, the degree of liver damage in the HR group was significantly lower than that in the RFA and TACE groups. On the other hand, the HR and TACE groups had significantly more advanced tumors than the RFA group. The 5-year survival rates after HR, RFA, and TACE were 66%, 49%, and 32%, respectively. Stratifying the survival rates, according to the TNM stage and the Japan Integrated Staging (JIS) score (Kudo (31)), showed the HR group to have a significantly better long-term outcome than the RFA group in the stage II and in the JIS scores "1" and "2." Figures 6 A and B shows comparisons of overall survival according to treatment modalities in stage II with multiple tumors (A) or in stage II with > 2 cm tumors (B). HR offered significant prognostic advantages over TACE and RFA (Utsunomiya 2014 (3)).
Figure 6.
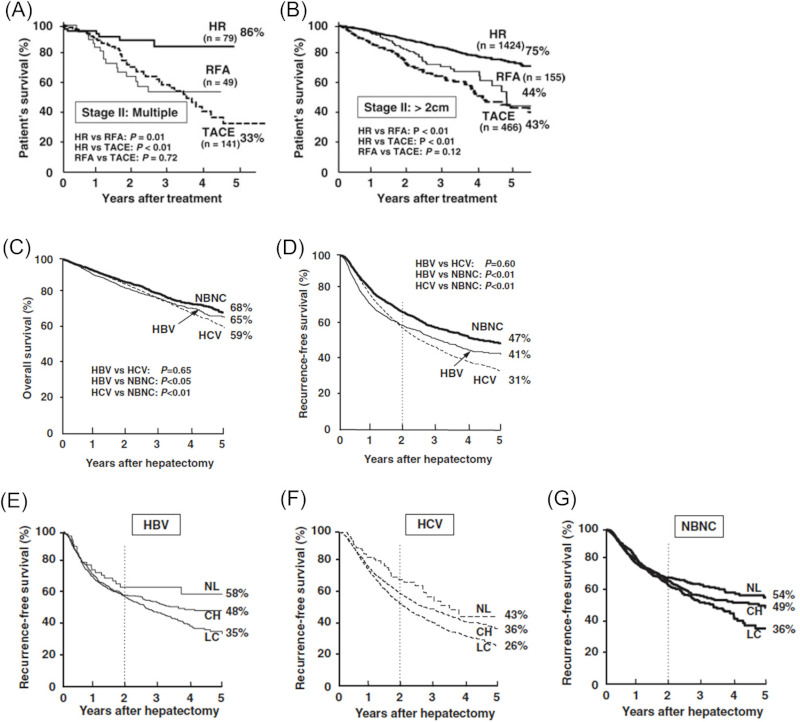
(A,B), OS for NBNC HCC (multiple or > 2cm) according to treatment options in Japan; (C,D), OS and RFS after liver resection for HCC according to viral status in Japan; (E,F, and G), Impact of background liver on RFS after liver resection for HCC according to viral status in Japan (Nationwide survey by Japanese Study Group of Liver Cancer 2000-2005 ) (3,32).
Using the same national registry data, patient outcomes after HR was analyzed according to the etiology of HCC (Utsunomiya 2015 (32)). Of the 11,950 HCC patients undergoing HR, 2,194 were HBV derived HCC, 7,018 were HCV derived HCC, and 2,738 were NBNC HCC. Patients with both HBV and HCV infection (n = 309) were excluded. Liver function in the HCV positive group was significantly worse than that in the HBV positive and NBNC HCC groups. The NBNC HCC group had significantly more advanced disease than other groups probably because they are not on the surveillance program. The 5-year overall survival rates after HR in the HBV-HCC, HCV-HCC, and NBNC HCC groups were 65%, 59%, and 68%, respectively. The 5-year recurrence-free survival (RFS) rates in these 3 groups were 41%, 31%, and 47%, respectively (Figures 6 C, D). After stratification according to the TNM stage, the NBNC HCC group had a significantly better RFS than the HBV HCC group in stages II, III, and IVA, and significantly better than the HCV-HCC group in stages I and II. Multivariate analysis revealed a significantly better RFS in the NBNC HCC group. They concluded that patients with NBNC HCC had a significantly lower risk of tumor recurrence than those with HBV and HCV derived HCC. However, tumor recurrence significantly depended on the histology of background liver regardless of the etiology. RFS curves of NBNC HCC in cirrhotic liver persistently went down to 36% at 5 years suggesting the need for long-term surveillance after resection (Figure 6 E, F, G).
Figure 7.
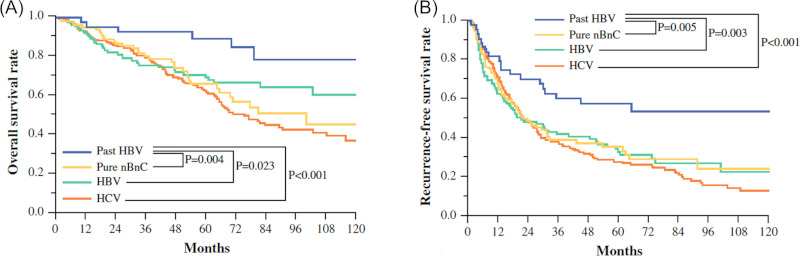
OS (A) and RFS (B) after liver resection for HCC according to viral status at the University of Tokyo (2002-2010) (2).
Conclusion
In conclusion, NBNC HCC is increasing in Japan particularly in surgical candidates. The majority of NBNC HCC in Japan are considered as so-called metabolic HCC and some could be related to occult HBV infection. Most of the NBNC HCC are found incidentally and are relatively large in size. Liver function is generally normal or subnormal thus providing a higher chance for curative surgery. Slightly better long-term outcomes may be expected compared to other etiologies, however, risk of recurrence depends on background liver. So far, NBNC HCCs should be treated with the same surgical strategy as HCCs of viral origin, same operative indications and same follow-up protocol.
References
- 1. Tateishi R, Uchino K, Fujiwara N, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011-2015 update. J Gastroenterol. 2019; 54:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Omichi K, Shindoh J, Yamamoto S, Matsuyama Y, Akamatsu N, Arita J, Kaneko J, Sakamoto Y, Hasegawa K, Kokudo N. Postoperative outcomes for patients with non-B non-C hepatocellular carcinoma: A subgroup analysis of patients with a history of hepatitis B infection. Ann Surg Oncol. 2015; 22 Suppl 3:S1034-1040. [DOI] [PubMed] [Google Scholar]
- 3. Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, Kokudo N; Liver Cancer Study Group of Japan. Nationwide study of 4741 patients with non-B non-C hepatocellular carcinoma with special reference to the therapeutic impact. Ann Surg. 2014; 259:336-345. [DOI] [PubMed] [Google Scholar]
- 4. Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010; 52:518-527. [DOI] [PubMed] [Google Scholar]
- 5. Tateishi R, Okanoue T, Fujiwara N, Okita K, Kiyosawa K, Omata M, Kumada H, Hayashi N, Koike K. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: A large retrospective multicenter cohort study. J Gastroenterol. 2015; 50:350-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T; JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J Gastroenterol. 2012; 47:586-595. [DOI] [PubMed] [Google Scholar]
- 7. Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009; 24:248-254. [DOI] [PubMed] [Google Scholar]
- 8. Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999; 341:22-26. [DOI] [PubMed] [Google Scholar]
- 9. Cho EJ, Kwack MS, Jang ES, You SJ, Lee JH, Kim YJ, Yoon JH, Lee HS. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion. 84 (Suppl 1):17-22. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka H, Iwasaki Y, Nouso K, Kobayashi Y, Nakamura S, Matsumoto E, Toshikuni N, Kaneyoshi T, Ohsawa T, Takaguchi K, Fujio K, Senoh T, Ohnishi T, Sakaguchi K, Shiratori Y. Possible contribution of prior hepatitis B virus infection to the development of hepatocellular carcinoma. J Gastroenterol Hepatol. 2005; 20:850-856. [DOI] [PubMed] [Google Scholar]
- 11. Hoshida Y, Moeini A, Alsinet C, Kojima K, Villanueva A. Gene signatures in the management of hepatocellular carcinoma. Semin Oncol. 2012; 39:473-485. [DOI] [PubMed] [Google Scholar]
- 12. Totoki Y, Tatsuno K, Covington KR, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genetics. 2014; 46:1267-1273. [DOI] [PubMed] [Google Scholar]
- 13. Schulze K, Imbeaud S, Letouzé E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nature Gen. 2015; 47:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017; 169:1327-1341. e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kodama T, Yi J, Newberg JY, Tien JC, Wu H, Finegold MJ, Kodama M, Wei Z, Tamura T, Takehara T, Johnson RL, Jenkins NA, Copeland NG. Molecular profiling of nonalcoholic fatty liver disease-associated hepatocellular carcinoma using SB transposon mutagenesis. Proc Natl Acad Sci U S A. 2018; 115:E10417-E10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salomao M, Yu WM, Brown RS Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): A distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010; 34:1630-1636. [DOI] [PubMed] [Google Scholar]
- 17. Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum. Pathol. 2012; 43:737-746 [DOI] [PubMed] [Google Scholar]
- 18. Shibahara J, Ando S, Sakamoto Y, Kokudo N, Fukayama M. Hepatocellular carcinoma with steatohepatitic features: A clinicopathological study of Japanese patients. Histopathology. 2014; 64:951-962. [DOI] [PubMed] [Google Scholar]
- 19. Shindoh J, Makuuchi M, Matsuyama Y, Mise Y, Arita J, Sakamoto Y, Hasegawa K, Kokudo N. Complete removal of the tumor-bearing portal territory decreased local recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016; 64:594-600. [DOI] [PubMed] [Google Scholar]
- 20. Aoki T, Kubota K, Hasegawa K, Kubo S, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, Nakashima O, Matsuyama Y, T. Murakami, Kudo M. on behalf of the Liver Cancer Study Group of Japan. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg. 2019; doi: 10.1002/bjs.11329. [DOI] [PubMed] [Google Scholar]
- 21. Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993; 9:298-304. [DOI] [PubMed] [Google Scholar]
- 22. Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003; 138:1198-1206. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida N, Takayama T, Midorikawa Y, Higaki T, Nakayama H, Moriguchi M, Tsuji S. Surgical outcomes in patients with hepatocellular carcinoma associated with metabolic syndrome. World J Surg. 2015; 39:471-477. [DOI] [PubMed] [Google Scholar]
- 24. Bhayani NH, Hyder O, Frederick W, Schulick RD, Wolgang CL, Hirose K, Edil B, Herman JM, Choti MA, Pawlik TM. Effect of metabolic syndrome on perioperative outcomes after liver surgery: A National Surgical Quality Improvement Program (NSQIP) analysis. Surgery. 2012; 152:218-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathur AK, Ghaferi AA, Osborne NH, Pawlik TM, Campbell DA, Englesbe MJ, Welling TH. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010; 14:1285-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pawlik TM, Soubrane O. Metabolic syndrome-associated hepatocellular carcinoma: questions still unanswered. J Hepatol. 2015; 63:8-9. [DOI] [PubMed] [Google Scholar]
- 27. Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011; 15:1450-1458. [DOI] [PubMed] [Google Scholar]
- 28. Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012; 55:1809-1819. [DOI] [PubMed] [Google Scholar]
- 29. Kaneda K, Kubo S, Tanaka H, Takemura S, Ohba K, Uenishi T, Kodai S, Shinkawa H, Urata Y, Sakae M, Yamamoto T, Suehiro S. Features and outcome after liver resection for non-B non-C hepatocellular carcinoma. Hepatogastroenterology. 2012; 59:1889-1892. [DOI] [PubMed] [Google Scholar]
- 30. Vigano L, Conci S, Cescon M, Fava C, Capelli P, D'Errico A, Torzilli G, Di Tommaso L, Giuliante F, Vecchio FM, Salizzoni M, David E, Pinna AD, Guglielmi A, Capussotti L. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter case-control study with HCV-related HCC. J Hepatol. 2015; 63:93-101. [DOI] [PubMed] [Google Scholar]
- 31. Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: The JIS score compared with the CLIP score. Hepatology. 2004; 40:1396-1405. [DOI] [PubMed] [Google Scholar]
- 32. Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, Takayama T, Kokudo N; Liver Cancer Study Group of Japan. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma. A nationwide study of 11,950 patients. Ann Surg. 2015;261:513-520. [DOI] [PubMed] [Google Scholar]
- 33. Yeo W, Mo FK, Chan SL, Leung NW, Hui P, Lam WY, Mok TS, Lam KC, Ho WM, Koh J, Tang JW, Chan AT, Chan PK. Hepatitis B viral load predicts survival of HCC patients undergoing systemic chemotherapy. Hepatology. 2007; 45:1382-1399. [DOI] [PubMed] [Google Scholar]
- 34. Zhou HB, Li QM, Zhong ZR, Hu JY, Jiang XL, Wang H, Wang H, Yang B, Hu HP. Level of hepatitis B surface antigen might serve as a new marker to predict hepatocellular carcinoma recurrence following curative resection in patients with low viral load. Am J Cancer Res. 2015; 5:756-771. [PMC free article] [PubMed] [Google Scholar]


