Abstract
Background
G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins plays an important role in the cellular regulation of responses to external stimuli. Despite intensive structural research, the mechanism underlying the receptor–G protein coupling of closely related subtypes of Gαi remains unclear. In addition to the structural changes of interacting proteins, the interactions between lipids and proteins seem to be crucial in GPCR-dependent cell signaling due to their functional organization in specific membrane domains. In previous works, we found that Gαs and Gαi3 subunits prefer distinct types of membrane-anchor lipid domains that also modulate the G protein trimer localization. In the present study, we investigated the functional selectivity of dopamine D2 long receptor isoform (D2R) toward the Gαi1, Gαi2, and Gαi3 subunits, and analyzed whether the organization of Gαi heterotrimers at the plasma membrane affects the signal transduction.
Methods
We characterized the lateral diffusion and the receptor–G protein spatial distribution in living cells using two assays: fluorescence recovery after photobleaching microscopy and fluorescence resonance energy transfer detected by fluorescence-lifetime imaging microscopy. Depending on distribution of data differences between Gα subunits were investigated using parametric approach–unpaired T-test or nonparametric–Mann–Whitney U test.
Results
Despite the similarities between the examined subunits, the experiments conducted in the study revealed a significantly faster lateral diffusion of the Gαi2 subunit and the singular distribution of the Gαi1 subunit in the plasma membrane. The cell membrane partitioning of distinct Gαi heterotrimers with dopamine receptor correlated very well with the efficiency of D2R-mediated inhibition the formation of cAMP.
Conclusions
This study showed that even closely related subunits of Gαi differ in their membrane-trafficking properties that impact on their signaling. The interactions between lipids and proteins seem to be crucial in GPCR-dependent cell signaling due to their functional organization in specific membrane domains, and should therefore be taken into account as one of the selectivity determinants of G protein coupling.
Video abstract
Keywords: Heterotrimeric G proteins, Dopamine D2 receptor, Functional selectivity, Protein–membrane interaction, FLIM–FRET, FRAP
Background
Dopamine D2 receptor (D2R) is one of the class A G-protein-coupled receptors (GPCRs), whose interaction with the heterotrimeric GTP-binding proteins (or G proteins) induces various cellular responses. GPCR proteins are the most recognized target by about 35% of the available drugs [1]. For example, the classical drugs used in the treatment of schizophrenia or Parkinson’s disease represent the ligands of dopamine receptors. Based on the cellular response induced by their activation, dopamine receptors are divided into two groups: D1-like (D1, D5) and D2-like (D2, D3, D4). The D2R long isoform, which is studied in the present work, is a member of the D2-like group. The characteristic feature of this group is the inhibition of adenylyl cyclase, which leads to a decrease in the level of cAMP via interaction with the Gαi/o class of G proteins. By contrast, the D1-like group induces the opposite effect on the level of cAMP by interacting with the Gαs subunits, which in turn activates adenylyl cyclase [2, 3].
Depending on the activity of the Gα subunit, the most recognized partners of GPCRs —heterotrimeric G proteins—are divided into four classes as follows: Gi/o, Gs, Gq, and G12/13. The heterotrimeric G proteins consist of three components—α, β, and γ—forming a trimer in an inactive state, which binds the GDP nucleotide. After activation by a receptor, the bound GDP nucleotide is exchanged for GTP, triggering the dissociation of the trimer into a Gα subunit and a Gβγ dimer; both these components induce various downstream effects leading to many cellular responses. The Gα subunit is composed of two domains: a GTPase domain, which is responsible for autoregulation via GTP hydrolysis; and a helical domain, which interacts with partners such as the RGS proteins (regulator of G protein signaling), effectors, and the Gβγ dimer. Changes in the conformation of the helical domain are also implicated in the enzymatic cycle and the overall activity of the G proteins [4]. It has been identified that N-terminus is the site of lipidation which enables docking at the surface of the lipid bilayer, while Gγ prenylation influences the plasma membrane localization of the G proteins. Furthermore, lipid modifications of the Gα subunit differ among various classes of G proteins. In the case of Gαi, N-myristylation and S-palmitoylation occur, whereas in Gαs N- and S-palmitoylation take place. N-acylation of the lipid moiety is irreversible, but it may be insufficient to allow stable docking at the surface of the lipid bilayer [5]. The second reversible modification occurring at the cysteine residue is proposed as one of the mechanisms that regulate the localization and performance of the G proteins. It has been postulated that activation triggered deprivation of cysteine modification can lead to depletion or enrichment of the protein population at different stages of signal transduction [6–10].
Gi/o class consists of two subclasses: Gαi and Gαo. The Gαi subclass is composed of Gαi1, Gαi2, and Gαi3 (genes GNAI1, GNAI2, GNAI3), and the Gαo subclass is composed of Gαo1 and Gαo2 (genes GNAO1, GNAO2). These proteins show profound homology (approximately 70% amino acid sequence identity) but vary in other features such as electrostatic properties [11]. While D2R is expressed mostly in the basal ganglia (as well as in other brain regions such as the midbrain, thalamus, hypothalamus, and cerebral cortex), none of the three Gαi subunits show any regional specificity in the brain and are present in the regions where D2R is expressed. However, the mRNA levels of these subunits vary—Gαi2 has a similar prevalence as Gαo1—whereas the levels of Gαi1 and Gαi3 are relatively lower [12].
The following three regions of the Gα subunit are identified to be involved in the interaction with receptor: C-terminal helix, α4–β6 loop, and to a lesser extent, αN–β1 loop [13, 14]. The last six amino acids in the C-terminus appear to have the most profound impact as a determinant of selectivity in the G protein–receptor interaction [15, 16]. However, the role of the C-terminus of Gα in interactions with receptors is heterogeneous among GPCRs. In particular, in the case of receptors interacting with Gαi, the other regions of this subunit are thought to reduce the impact of its C-terminus in the interaction with the receptor (the Gαi subunits show high similarity in the C-terminal residues, and only Gαi3 differs in the identity of amino acids in two positions). Furthermore, in contrast to other GPCRs, the Gαi-interacting receptors exhibit selectivity toward specific Gα subunits to a greater extent [17]. On the other hand, the second and third intracellular loops (2ICL, 3ICL), together with the transmembrane helix (TM)—TM3, TM5, and TM6, are recognized as the most relevant regions of GPCRs in terms of their interaction with suitable G proteins [18]. Even closely related receptors show differences in the secondary structure of these regions. Changes in the secondary structure of 2ICL and the length of 3ICL are proposed to influence the selectivity toward G proteins [19]. Nevertheless, in the case of both G proteins and GPCRs, these determinants are not fully recognized yet. In addition to the C-terminus of Gα subunit which acts as a determinant of selectivity of receptor–G protein coupling, there exist other determinants (although not so well documented) that are equally, and sometimes even more important in the recognition of G protein by many receptors [16, 17, 20, 21]. The following factors may affect this process: ligand used to stimulate the receptor, time of stimulation, interacting partners (such as RGS, AGS (receptor-independent activators of G protein signaling), and others), receptors oligomerization, and the lipid composition of the cell membrane [15, 22–24].
Dopamine D2R is capable of coupling more than one G protein while modulating the formation of cyclic AMP. The ability to inhibit the activity of adenylate cyclase depends on the ability to couple one, or more, of the Gi/o subunits [25–27]. However, the mechanism by which the receptor can selectively discriminate between the closely related subtypes of G proteins and involvement of other factors still remains unclear. It has been shown earlier that dopamine D2R may differentially couple the Gαi and Gαo subtypes in a receptor agonist-dependent manner, leading to diverse functional outcomes [25, 27]. However, most of these interactions were analyzed using a system that measures intracellular events (e.g. cAMP accumulation, calcium mobilization) or in isolated membrane fractions (e.g. radioligand binding studies). Since the Gαi and Gαo proteins are closely related, it is further difficult to separate their signaling when working on isolated membrane fractions. Most of the studies exploring the role of protein–protein interactions neglect the interactions of the signaling proteins with lipids, as well as the participation of the lipid bilayer itself in the processes of signal transduction. One of the important aspects that have not been fully explored is the impact of the plasma membrane on the efficiency and selectivity of the G proteins signaling. The mutual influence of lipids and membrane proteins along with cytoskeleton is considered as a factor that may promote their nanoclustering and organization into dynamic signaling platforms [28, 29].
Similarly, in the case of trimeric G proteins, partitioning occurs in different regions of the cell membrane. A review of the published data indicated that the Gαi proteins reside in the ordered parts of the membrane that are rich in cholesterol and sphingolipids [30, 31]. It is assumed that the partitioning process is driven not only by the lipid moieties attached to proteins but also by interaction with other components residing in such clusters (i.e. caveolins) [31, 32]. Moreover, the specific membrane targeting of G proteins is affected by the Gβγ dimer which seems to determine the preference toward the less-ordered segments of the lipid bilayer [31, 33, 34]. These observations indicate that localization may change in different states of the signal transduction process when the G protein trimer dissociates or associates. In addition, it has been postulated that ligand binding induces changes in the localization of GPCRs [35, 36]. Regardless of the signals within proteins, such as palmitoylation which is a signal stated to localize in the ordered membrane regions [37], other factors may also influence the overall outcome in the compartmentalization process.
In the present study, we analyzed the behavior of the three closely related Gαi proteins and dopamine D2R in a lipid bilayer environment in the context of activation selectivity. We monitored the dynamics and the mutual proximity of D2R and Gαi1, Gαi2, or Gαi3, as well as their heterotrimers formed with the Gβ1γ2 dimer, using two highly selective and sensitive assays: fluorescence resonance energy transfer (FRET) and fluorescence recovery after photobleaching (FRAP). These approaches allowed comparing the receptor and G proteins directly at the living cell membrane in their native dynamic environment, without relying on downstream signals such as the production of second messengers. Surprisingly, although the Gαi proteins showed high similarity, our results revealed significant differences not only in their rate of lateral diffusion within the plasma membrane but also in their colocalization with dopamine D2R. We found that the cell membrane partitioning of particular Gαi heterotrimers and dopamine D2R showed a good correlation with the efficiency of D2R-mediated inhibition of cAMP. These results suggest that the membrane distribution of signaling partners can be investigated in depth in terms of how it contributes to the selectivity of the G protein–receptor coupling. To the best of our knowledge, this is the first report to show that the Gαi subunits differ in their membrane-trafficking properties that impact on their signaling, as the membrane localization of the Gαi1, Gαi2, and Gαi3 subunits has been considered to be identical so far.
Methods
Site-directed mutagenesis
All the genes encoding human Gα subunits (GNAI1, GNAI2, GNAI3, GNAS) and dopamine D2R long isoform (DRD2L) were purchased from UMR cDNA Resource Center (Bloomsburg, PA, USA), and the sequences of fluorescent proteins (FP) were obtained from Clontech (Mountain View, CA, USA).
The sequences coding for mCitrine or mGFP were inserted into the αb–αc loop of the human Gαi subunits through Overlap Extension PCR Cloning [38]. In the case of Gαi1 and Gαi2, the sequences were inserted after Ala121, while for Gαi3, they were inserted after Ala114 [39]. The sequence of FP was flanked by Ser–Gly and Gly–Ser linkers. The mCitrine and mGFP sequences were obtained as described previously [40]. In the Gαs subunit, the FP sequence was incorporated between the helical and GTPase domains as described previously [40]. The sequences of all the Gα subunit fusion proteins were obtained in a pcDNA3.1+ vector (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The D2R-mCherry construct (with mCherry fused to the C-terminus of D2R) was prepared by introducing restriction sites to DRD2 through polymerase chain reaction and then by cloning the dopamine receptor gene into the pmCherry-N1 vector (Clontech) using NheI and XhoI enzymes. Additionally, to ensure the correct location of the described fusion protein in the membrane, it was necessary to extend the linker between the proteins, which was achieved using a 35-amino acid linker with a flexible character consisting of repeated GGSG sequences.
Cell culture and transfection
The human embryonic kidney 293 cells (HEK293) (ATCC, Manassas, VA, USA) were cultured in Minimum Essential Medium (MEM) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS) (Sigma Aldrich, Poznań, Poland) under 5% CO2 at 37 °C. For imaging experiments, the cells were seeded onto sterile glass coverslips and cultured in 30-mm plates, while for determining the levels of cAMP, the cells were seeded onto six-well plates coated with 0.5% gelatin (Type A; BioShop Canada Inc., Montréal, Canada). Transient transfection was performed using the TransIT-X2® Dynamic Delivery System (Mirus Bio, Madison, WI, USA) according to the manufacturer’s instruction. The amounts of DNA used for each experiment were as follows: determination of cAMP levels—0.9 or 1.7 μg DNA per well; FLIM–FRET and FRAP—0.1–0.45 μg DNA per dish. The ratio of DNA (Gα-D2R) used was as follows: determination of cAMP levels: 1–1.25; FLIM–FRET and FRAP: 1:1.5; in case of overexpression of trimer, Gβ, Gγ and Gα were used in equimolar DNA amounts. All the experiments were performed 2 or 3 days after transfection.
Live-cell imaging microscopy
Leica SP5 II SMD confocal microscope (Leica Microsystems, Mannheim, Germany) or Leica TCS SP5 confocal scanning microscope (Leica Microsystems, Mannheim, Germany) with a 63 × 1.4 numerical aperture and a oil-corrected objective lens was used for the observation of cells. Fluorescence of mCitrine or mGFP was acquired at 495–570 nm with an excitation wavelength of 488 nm (argon ion laser), and that of mCherry at 610–700 nm with an excitation wavelength of 594 nm (laser diode). During observation, the cells were kept at 37 °C in an air–steam cube incubator in Dulbecco’s Modified Eagle Medium (DMEM-F12; without phenol red) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 2% FBS.
cAMP level measurements
The concentration of cAMP was determined in cell lysates using cAMP ELISA chemiluminescence kit (STA-500; Cell Biolabs Inc., San Diego, CA, USA). Three days after transfection, the HEK293 cells were stimulated with 1 µM rotigotine hydrochloride (Sigma Aldrich, Poznań, Poland), a D2R agonist, for 10 min. Prior to stimulation, the cells transfected with Gαi were incubated in a medium containing 1 µM forskolin (Sigma Aldrich, Poznań, Poland) for 5 min. These prestimulation and stimulation procedures were conducted in MEM supplemented with 0.5% FBS. After stimulation, the cells were harvested and their cAMP concentration was determined according to the manufacturer’s instructions. In each case, four independent experiments were performed in duplicates. Nontransfected cells were used as controls, and the concentrations of cAMP in transfected cells were normalized in comparison with the values determined in controls in each experiment.
FLIM–FRET measurements
The cells were observed using Leica SP5 II SMD confocal microscope with an integrated module PicoHarp 300 Time-Correlated Single Photon Counting (TCSPC) system (PicoQuant, Berlin, Germany). The experiments were conducted as described earlier in detail [34]. Confocal images of the cells were collected prior to each FLIM measurement. mCitrine (energy donor) and mCherry (energy acceptor) were used as the FRET pair. The FLIM–FRET experiments were carried out on live HEK293 cells expressing appropriate levels of Gα-mCitrine (donor alone: Gα-mCitrine or Gα-mCitrine with Gβ1γ2) and D2R-mCherry (donor and acceptor: Gα-mCitrine with or without Gβ1γ2 and D2R-mCherry). Excitation was performed using a pulsed laser diode (Leica; 40 MHz) at 470 nm. Emission from 500 to 550 nm was collected with an avalanche photodiode using a fluorescence band-pass filter. All the images were recorded in 512 × 512 format with an acquisition time of approximately 3–4 min. In each experiment, the cells with only donor and those with donor–acceptor were observed, and the level of fluorescence of mCitrine and mCherry was estimated. In the case of cells treated with rotigotine (1 μM) for the stimulation of D2R, the ligand was added immediately after the imaging was started and the images were collected for up to 15 min after stimulation.
To quantify the apparent fluorescence lifetimes in the plasma membrane, we manually selected the regions of cell areas in each image and fitted the fluorescence lifetime histograms with double-exponential decay functions using SymPhoTime software (PicoQuant, Berlin, Germany). In the case of each image, the FRET efficiency was calculated for the FRETing state with the equation: by comparing the donor lifetimes in the presence (τDA) and absence (τD) of the acceptor [41].
FRAP measurements
All the FRAP experiments were performed and results were analyzed as described earlier [40]. As high photostability was required, mGFP-tagged fusion proteins of Gα subunits were used in these experiments. Briefly, the transiently transfected live HEK293 cells were incubated at 37 °C. Just before imaging, the culture medium was replaced with fresh DMEM-F12 medium enriched with 2% FBS. The FRAP images were collected for at least 100 s after the photobleaching impulse using Leica TCS SP5 confocal scanning microscope equipped with LAS AF software and a 63 × 1.4 NA oil-immersion lens.
Statistical analysis
Data distribution was determined using Shapiro–Wilk W test and skewness and kurtosis analysis. Depending on the approach applied (unpaired T-test for parametric data and Mann–Whitney U test for nonparametric data), the results are presented as mean ± standard error of the mean (SEM) or median ± median absolute deviation (MAD). The details of the statistical analysis were described previously [34].
Results
Functionality of created fusion proteins
The purpose of this work was to investigate the differences in the coupling selectivity of the three Gαi subunits of G proteins (Gαi1, Gαi2, Gαi3) toward dopamine D2R in living cells. Their mutual colocalization was observed in basal conditions without receptor stimulation and after stimulation with a full agonist—rotigotine. Two approaches were used for analyzing the proteins of interest: FLIM–FRET and FRAP measurements with the use of FPs (mCitrine, mGFP, or mCherry) as tags. Such approaches require more attention during the creation of fusion proteins. In addition, the incorporation of FPs may have a profound impact on the conformation of the investigated proteins and may also influence their functionality, localization and at the tail end—expression level. To address this last uncertainty, we examined the levels of mRNA of all Gα-FP fusion proteins used in co-expression with dopamine D2 receptor with or without company of Gβγ subunits. We saw, that the relative expression of studied proteins remained constant in each experimental set-up (Additional file 1).
In the case of Gαi proteins, mCitrine or mGFP was incorporated into the second loop (αb–αc) after A114 (Gαi3) or A121 (Gαi1, Gαi2), flanked with short linkers, based on the results reported by Gibson and Gilman for Gαi3 [34, 39]. This process minimizes the possibility of disruption of the interaction between the Gα subunit and D2R (via C-terminus of Gα) and the effect on their localization at the surface of the cell membrane which occurs via the N-terminus. All proteins were properly localized at the cell membrane; this is especially noticeable in the case of overexpression of the complete trimer (Fig. 1). Gαs, investigated as subunit non-interacting with D2R as well as with different characteristics, also exhibited proper cellular localization and a similar behavior as Gαi with reference to the influence of Gβ1γ2 on localization. The Gαs subunit was fused with mCitrine or mGFP cloned between the helical and GTPase domains by replacing amino acids 72–82 with an FP sequence and adding short linkers [42].
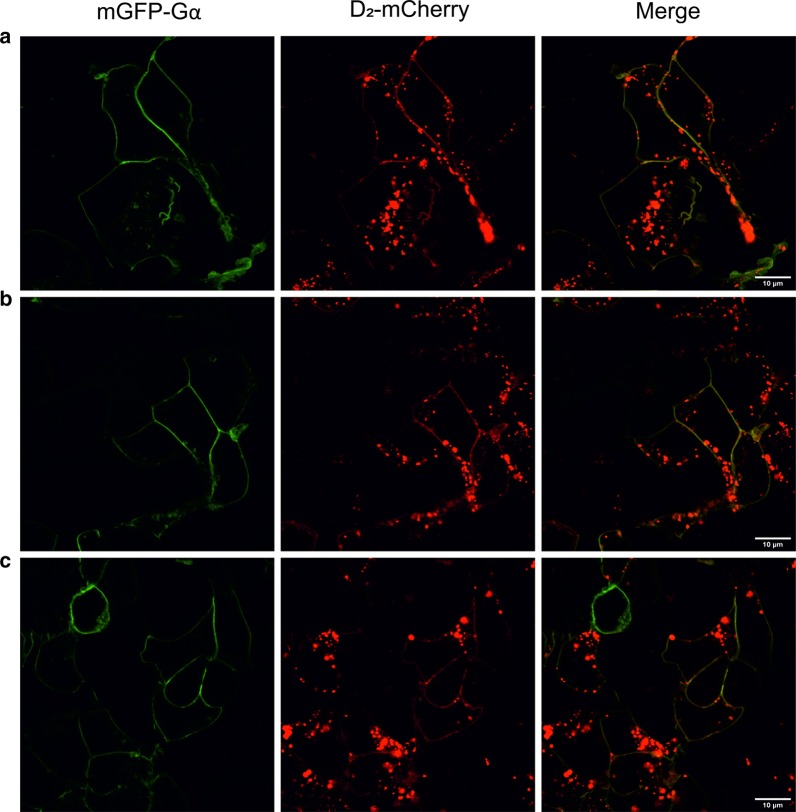
Fig. 1.
Cellular localization of Gαi subunit heterotrimers with dopamine D2 receptor. Representative confocal images of FP-tagged Gαi-mGFP subunits with Gβ1γ2 dimer and D2R-mCherry receptor in transiently cotransfected HEK293 cells. Localization of the investigated proteins: a Gαi1β1γ2-D2R, b Gαi2β1γ2-D2R, and c Gαi3β1γ2-D2R. Scale bar, 10 µm
The functionality of some of the G proteins prepared in this manner was already verified by other authors. They found that these insertions did not change the properties of the proteins, such as their interaction with adenylyl cyclase, localization at the surface of the cell membrane, or the process of nucleotides exchange [39, 42]. Nevertheless, the activity of all the proteins was investigated by taking into account their ability to inhibit adenylyl cyclase after the activation of D2R. Our previous studies have indicated that the differences in the response of Gαi3-mCitrine or Gαi3-mGFP fusion proteins are insignificant. Therefore, only the configurations with Gα-mCitrine were investigated further [34]. For the activation of D2R, rotigotine hydrochloride was used as a full agonist, which exhibits an equally functional response as dopamine [43]. Rotigotine is not a selective dopamine D2R agonist, but in the present study, the cellular response induced by stimulation with this compound enabled us to observe the differences between the Gαi subtypes (Fig. 2). It is noteworthy to mention that this agonist also binds efficiently to the dopamine D1-like receptors, which was confirmed by the measurements of cAMP levels. These results are in agreement with observations reported by other research groups [43–45].
Fig. 2.
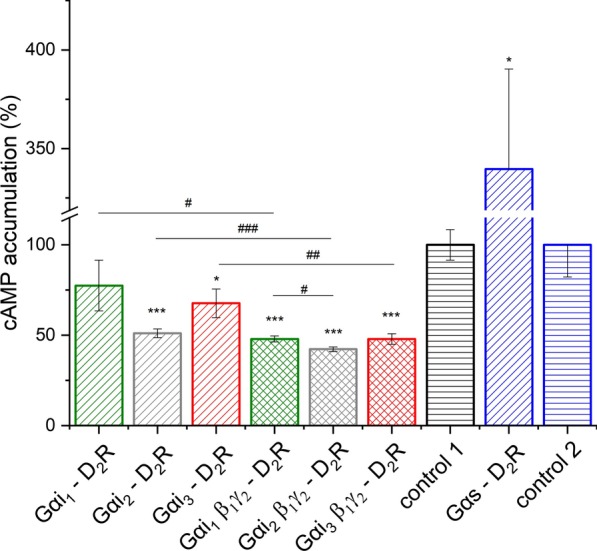
Changes in the intracellular cAMP level after stimulation of dopamine D2 receptor with rotigotine. HEK293 cells transiently transfected with Gα-mCitrine and D2R-mCherry or Gα-mCitrineβ1γ2 and D2R-mCherry were prestimulated with 1 μM of forskolin to enhance the basal levels of cAMP. After 5 min, rotigotine was added at a final concentration of 1 μM and the cells were incubated with the agonist for 10 min. In the case of Gαs, the prestimulation step with the use of forskolin was omitted. After stimulation, the cells were harvested and their intracellular cAMP concentration was determined. Data are presented as percentage of the cAMP levels in controls (nontransfected cells), and an appropriate control was used in every experiment. Control 1—Gαi; Control 2—Gαs. Error bars represent SEM; n = 4 experiments were performed in duplicates; unpaired T-test was used to evaluate the differences between samples. Comparison with adequate control: *p < 0.05 and ***p < 0.001; comparison within Gαi: #p < 0.05, ##p < 0.005, and ###p < 0.001
The ability of rotigotine to activate D2R was confirmed by the measurements of intracellular cAMP levels taken in all the investigated settings (Fig. 2). However, data on the ability of this agonist to inhibit or activate adenylyl cyclase are limited in the literature. Most of the available studies focus on the thermodynamic and kinetic properties associated with binding to the receptor, or the overall pharmacological effect [46–48]. By contrast, in the present study, we analyzed the response of different subtypes of Gαi and Gαs by changes in the intracellular cAMP level following the stimulation of cells in which D2R and Gα were induced for overexpression. In the case of overexpression of only Gαi and D2R, two Gα subunits were able to inhibit adenylyl cyclase at a statistically significant level (Gαi2: p < 0.001; Gαi3: p < 0.05), except Gαi1. Additionally, as assumed, full Gαi heterotrimers showed an increased ability to interact with D2R, which was indicated by statistically significant differences when the effects of Gαi–D2R and Gαiβ1γ2–D2R interactions were compared. The higher level of Gβ1γ2 dimer present in cells supported the formation of the whole trimer more efficiently and influenced the behavior of the Gαi subunits investigated. It is worth noticing that in comparison with control conditions, the differences between the Gαi subunits diminished upon additional overexpression of Gβ1γ2 and only comparison between Gαi1β1γ2 and Gαi2β1γ2 showed significant differences in the cAMP level (p < 0.01); in the case of Gαi3β1γ2, such a phenomenon was not observed. The obtained results indicate that Gαi2 has the most profound influence on the inhibition of adenylyl cyclase, in the case of both overexpression of only Gα subunit or additional overexpression of Gβ1γ2 dimers. These results are in agreement with other studies, which used agonists such as dopamine, quinpirole, or N-n-propylnorapomorphine (NPA) [2, 27, 49, 50]. Moreover, incubation of cells overexpressing Gαs and D2R with rotigotine confirmed the ability of this agonist to induce the activation of adenylyl cyclase (p < 0.05). The ability to increase the cAMP level most probably results from the presence of other isoforms of adenylyl cyclase which are activated by the Gβγ dimer [51].
Nanoscale distribution of Gαi and D2R monitored by FLIM–FRET and FRAP in living HEK293 cells
In the present study, we have shown that the FLIM–FRET and FRAP assays can be used to characterize the nanoscale spatial distribution of the closely related Gαi subunits in the plasma membrane, which in turn helped in assessing their role in the regulation of coupling preferences of D2R–Gαi proteins. Because of the unique spatial sensitivity, FRET was applied to elucidate the organization of the Gαi subunits and their heterotrimers formed with Gβ1γ2 in the plasma membrane. On the other hand, the FRAP technique was used to study the lateral dynamics of the investigated proteins in the cellular membrane. FRET was analyzed between the mCitrine-labeled Gαi or Gαs subunits (energy donor) and the mCherry-labeled D2R (energy acceptor). The emission spectrum of mCitrine was shown to overlap with the excitation spectrum of mCherry, making them a suitable donor–acceptor pair for FRET [52]. Following the energy transfer between the donor and the acceptor, the lifetime of mCitrine is shortened. This reduction in the fluorescence lifetime of the donor reflects the molecular proximity between the proteins that are linked to the fluorophores of the donor and acceptor. Thus, the FLIM–FRET technology allowed studying the membrane trafficking of Gαi as monomers and heterotrimers when co-expressed with D2R.
The fluorescence lifetime histograms obtained for mCitrine (Gαi1-mCitrine, Gαi2-mCitrine, Gαi3-mCitrine, Gαs-mCitrine) were fitted with a double-exponential decay function, and FLIM images showing the apparent lifetime of each pixel were generated. These images and the distribution of lifetimes with and without an acceptor in the HEK293 cells, which was estimated using SymPhoTime software, are shown in Fig. 3a–d. The FLIM images of the cells cotransfected with Gαi-mCitrine and D2R-mCherry showed a reduction in the apparent lifetime of the donor (change in color toward the blue hues across all pixels), compared to those expressing only Gαi-mCitrine. For example, the fluorescence lifetimes of mCitrine in the cells expressing Gαi2-mCitrine were estimated to be 2.76 ± 0.04 ns (τ1) and 3.23 ± 0.03 ns (τ2), with the amplitude of each of these lifetimes being approximately 40% and 60%, respectively. In the FRET system (cells additionally expressing D2R-mCherry), the donor emission curves were also fitted with the double-exponential decay model. However, shortening of the fluorescence lifetime that can be attributed to FRET was observed only in the case of the short component τ1, while the other component (τ2) remained almost unchanged. This indicates the involvement of only one donor species characterized by the lifetime τ1 in the energy transfer (FRETing donor state). Therefore, only the FRETing component was taken into account while calculating the FRET efficiency (Fig. 4b).
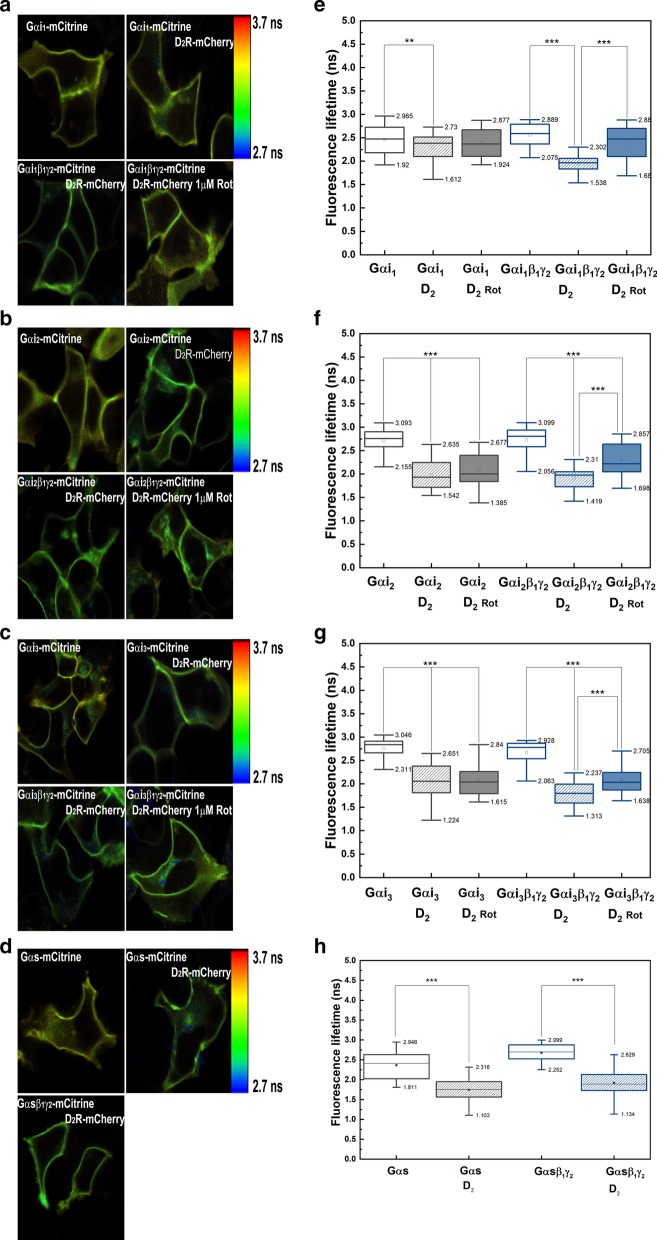
Fig. 3.
FLIM–FRET results. HEK293 cells were transiently transfected with Gα-mCitrine alone or both D2R-mCherry and Gα-mCitrine (donor and acceptor) with or without Gβ1γ2, or the donor in the presence of the acceptor after treatment with 1 μM rotigotine; mCitrine lifetime was measured: a Gαi1-mCitrine; b Gαi2-mCitrine; c Gαi3-mCitrine; d Gαs-mCitrine. Fluorescence lifetimes are presented in a continuous pseudo-color scale representing the time values ranging from 2.7 (blue) to 3.7 ns (red). e–h Box-and-whisker plots of the fluorescence lifetime τ1 of energy donor (Gα-mCitrine) and donor in the presence of acceptor (D2R-mCherry) are provided. The median is shown as a line in the box, while the bottom and top boundaries represent the lower and upper quartile, respectively. Statistical significance of the difference in the fluorescence lifetimes of the donor (τ1) was detected in the absence and presence of the energy acceptor using Mann–Whitney U test (**p < 0.005, ***p < 0.0001). Gαi1: n = 58; Gαi1 and Gβ1γ2: n = 40; Gαi1 and D2R with rotigotine: n = 34, without rotigotine: n = 61; Gαi1 and Gβ1γ2 and D2R with rotigotine: n = 26, without rotigotine: n = 58; Gαi2: n = 49; Gαi2 and Gβ1γ2: n = 44; Gαi2 and D2R with rotigotine: n = 41, without rotigotine: n = 47; Gαi2 and Gβ1γ2 and D2R with rotigotine: n = 31, without rotigotine: n = 46; Gαi3: n = 50; Gαi3 and Gβ1γ2: n = 54; Gαi3 and D2R with rotigotine: n = 33, without rotigotine: n = 68; Gαi3 and Gβ1γ2 and D2R with rotigotine: n = 33, without rotigotine: n = 79; Gαs: n = 39; Gαs and Gβ1γ2: n = 39; Gαs and D2R: n = 55; Gαs and Gβ1γ2 and D2R: n = 48
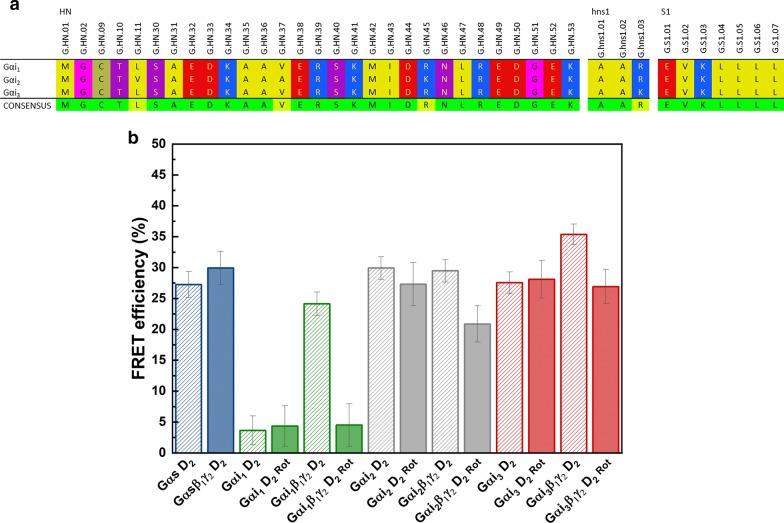
Fig. 4.
The difference in sequences of Gαi subunits and efficiency of energy transfer. a Multiple protein sequence alignment of human Gαi subunits encompassing residues of N-terminal fragment. The residues are colored to indicate their physicochemical properties: red—negatively charged amino acids, blue—residues with a positive charge, magenta indicates hydrogen bonding, and yellow shows hydrophobic aliphatic residues. In the consensus line, the positions in which there are differences in the sequence shown in yellow. Modified from gpcrdb.org (83). b A plot of calculated FRET efficiency percentage E derived from τ1; error bars represent standard errors
In the case of cells expressing Gαi2-mCitrine or Gαi3-mCitrine, cotransfection with D2R-mCherry significantly reduced the apparent lifetime of mCitrine to 1.93 ± 0.03 or 2.06 ± 0.04 ns, respectively (Fig. 3f, g). By contrast, the lifetime of Gαi1-mCitrine was decreased only slightly (2.39 ± 0.03 ns) in comparison to the τ1 estimated for the donor alone (2.48 ± 0.04 ns). The efficiencies of energy transfer between different Gαi subunits and D2R (Fig. 4b) calculated in the present study indicate that the spatial distribution of even closely related Gαi subunits differs. Here, we used Gαs subunit (not interacting with D2R) as a control, which has been reported to prefer localizing in the membrane region that differs from Gαi in lipid composition [53]; however, a significant FRET signal with D2R was also detected in this case (Fig. 4b). Combining these data, it can be concluded that dopamine D2R can exist in different membrane locations.
The apparent diffusion coefficients of monomeric subunits calculated in the study are summarized in Table 1. The lateral mobility of Gαi1-mGFP and Gαi3-mGFP was found to be similar (0.316 ± 0.012 and 0.338 ± 0.022 µm2 s−1, respectively). By contrast, the apparent diffusion coefficient of the Gαi2-mGFP subunit was much higher (0.474 ± 0.015 µm2 s−1). Interestingly, the diffusion characteristics of both Gαi2-mGFP and Gαi3-mGFP were not found to change significantly in the presence of dopamine D2R-mCherry at the cell membrane. However, for the Gαi1-mGFP subunit, mobility slightly increased (0.356 ± 0.014 µm2 s−1). This may have resulted from the competition between the receptor and the Gαi1-mGFP subunit for the membrane regions [36], which was confirmed by the results of the FRET experiments, with the poor efficiency of FRET between Gαi1-mGFP and D2R-mCherry indicating separate membrane localization.
Table 1.
Lateral diffusion characteristics of Gα subunits in HEK293 cells in the presence of Gβ1γ2 and/or dopamine D2 receptor
|
Dapp (µm2 s−1) |
Mf (%) |
N | |
|---|---|---|---|
| Gαsa | 0.130 ± 0.004 | 84.5 ± 1.5 | 49 |
| Gαs Gβ1γ2b | 0.246 ± 0.009 | 92.4 ± 0.8 | 143 |
| Gαs D2R | 0.232 ± 0.009 | 89.0 ± 1.0 | 88 |
| Gαsβ1γ2 D2R | 0.245 ± 0.009 | 90.6 ± 1.1 | 110 |
| Gαi1 | 0.316 ± 0.012 | 91.8 ± 1.4 | 55 |
| Gαi1 Gβ1γ2 | 0.237 ± 0.010 | 88.2 ± 1.6 | 53 |
| Gαi1 D2R | 0.356 ± 0.014 | 92.2 ± 1.4 | 45 |
| Gαi1β1γ2 D2R | 0.291 ± 0.012 | 91.0 ± 1.2 | 60 |
| Gαi2 | 0.474 ± 0.015 | 95.2 ± 0.7 | 120 |
| Gαi2 Gβ1γ2 | 0.526 ± 0.019 | 94.1 ± 1.3 | 50 |
| Gαi2 D2R | 0.467 ± 0.020 | 93.1 ± 1.4 | 58 |
| Gαi2β1γ2 D2R | 0.345 ± 0.013 | 90.9 ± 1.3 | 60 |
| Gαi3a | 0.338 ± 0.022 | 94.2 ± 1.7 | 34 |
| Gαi3 Gβ1γ2b | 0.424 ± 0.014 | 93.5 ± 0.9 | 66 |
| Gαi3 D2R | 0.358 ± 0.016 | 90.1 ± 1.5 | 60 |
| Gαi3β1γ2 D2R | 0.381 ± 0.014 | 91.0 ± 1.4 | 50 |
The activation of D2R with rotigotine did not influence the FRET signal observed between the monomeric Gα and the agonist-receptor complex. This suggests that even if heterotrimers were formed between Gαi-mCitrine and endogenous Gβγ dimers, these complexes had no influence on the measured FRET. On the contrary, in the intracellular cAMP assay, a reduction in the level of cAMP was observed for all Gαi monomers (Fig. 2). This might have been caused, at least partly, by the assembly of an additional heterotrimer complex of Gαi-mCitrine fusion proteins with an endogenous Gβγ dimer. However, most cells showed an almost unchanged FRET signal upon receptor activation, which indicates that the concentration of heterotrimers formed with endogenous Gβγ dimer remains relatively low. In cells expressing additional Gβ1γ2, D2R activation resulted in a significant reduction in the FRET efficiency (Fig. 4b).
Thus, the data obtained from the FLIM–FRET and FRAP assays provide new insights about the location of the closely related Gαi subunits in the plasma membrane: (i) FRAP analysis of fluorescently labeled Gαi2 showed its significantly faster lateral diffusion compared to that of Gαi3 and Gαi1; (ii) FRET of fluorescently labeled D2R with Gαi2 and Gαi3 in the plasma membrane was higher than that with Gαi1. These together suggest the different distributions of Gαi subunits in the plasma membrane.
Mapping the organization of Gαi heterotrimers in the plasma membrane
FRAP analysis was also performed for all the investigated subunits in the heterotrimeric system (Table 1). For this purpose, the HEK293 cells were cotransfected with additional vectors encoding Gβ1 and Gγ2 subunits to provide an excess of Gβγ dimers. As shown earlier, the Gβγ dimer was found to modulate the lateral diffusion of a heterotrimeric G protein compared to a monomeric Gα subunit [34]. In addition, the apparent diffusion coefficients of Gαi2 and Gαi3 with Gβ1γ2 complexes were estimated to be substantially higher compared to that of monomers (0.526 ± 0.019 and 0.424 ± 0.014 µm2 s−1, respectively). However, the presence of the Gβ1γ2 dimer had the opposite effect on the mobility of Gαi1 subunit, and the Dapp value was found to decrease to 0.237 ± 0.010 µm2 s−1. This observed effect is particularly interesting in the case of Gαi1 and Gαi3 subunits because, as monomers with a high sequence identity, these subunits were characterized by identical lateral mobility.
For heterotrimeric Gαiβ1γ2 complexes, the presence of dopamine D2R in the cell membrane caused a significant change in the lateral mobility of all the investigated subunits. The biggest difference was observed for the Gαi2 subunit, for which the Dapp value declined to 0.345 ± 0.013 µm2 s−1. Such a change in lateral diffusion may have resulted from an effective limitation of mobility to the receptor signaling platform areas such as the raft domains of the plasma membrane [54]. Although, for the Gαi1β1γ2 heterotrimer, a slight increase in Dapp was observed (0.291 ± 0.012 µm2 s−1) in the presence of D2R, whereas the mobility of Gαi3β1γ2 slightly decreased in the presence of D2R compared to the heterotrimer alone (0.381 ± 0.014 µm2 s−1). However, it should be noted that in the presence of D2R the differences in the lateral diffusion rates of the investigated Gαi heterotrimers diminished. This might be due to the colocalization of all heterotrimers with D2R, which was proved by the FRET measurements.
As shown in Fig. 3e, g, the association of Gαi1 and Gαi3 with Gβ1γ2 dimers caused a further reduction in the apparent fluorescence lifetime of mCitrine in the presence of mCherry-fused D2R (box chart), and thus, there was an increase in the FRET efficiencies. The highest FRET efficiency of 35.4 ± 1.6% was detected in the cells co-expressing D2R and Gαi3β1γ2, while the lowest efficiency of 11% was found for the Gαi1β1γ2 heterotrimer. By contrast, Gαi2β1γ2 exhibited the same FRET signal with D2R as the monomeric Gαi2. However, the FRET efficiency of this subunit was found to be relatively high. The lifetime of Gαi2-mCitrine in the heterotrimeric complex was estimated as 2.0 ± 0.03 ns, which amounted to an energy transfer efficiency of 29.5 ± 1.8% (versus 29.9 ± 1.8% with monomeric Gαi2). These different patterns of changes in the FRET efficiency further point toward the difference in the membrane distribution of distinct Gαi subunits and D2R. Interestingly, in appropriate FRET pairs, differences were observed in the contribution of the fluorescence decay times for Gαi-mCitrine in heterotrimeric complex and the monomeric Gαi. The amplitude of the FRETing component (τ1) decreased to 17% and 25% for Gαi1β1γ2 and Gαi3β1γ2, respectively, whereas it remained at the same level for Gαi2β1γ2, similar to that calculated for the D2R–Gαi2 pair. The simplest explanation that could be provided for this effect is that the subpopulations of Gαi1 and Gαi3, for which the energy transfer to D2R-mCherry was reduced, relocate within the membrane upon the formation of the heterotrimeric complex. Thus, based on the FLIM data, it can be concluded that the receptor and heterotrimeric G protein in the basal state (before receptor activation) localize at the cell membrane within the same area (signaling platform), promoting signal transduction.
FRET analysis revealed that all the Gαi heterotrimers responded to the agonist rotigotine. The activation of D2R with rotigotine caused a significant reduction in the FRET efficiency in the case of all heterotrimers (Fig. 4b). The most pronounced decrease was observed for Gαi1β1γ2, while the lowest decrease was noted for Gαi2β1γ2. However, the difference observed in the FRET signal between the G protein and D2R before and after receptor stimulation cannot be interpreted as the magnitude of the rotigotine effect. D2R activation by agonist leads to the activation of G protein, which is accompanied by the dissociation of Gα from the Gβγ dimer, followed by Gα translocation within the plasma membrane and the internalization of the receptor. Because the complexes formed between the agonist-bound D2R and the G proteins have short lifetimes [55], which are off the time scale of the FLIM measurement, the recorded FLIM signal comes dominantly from the further steps of the signaling cascade. Thus, the FRET signal obtained after receptor stimulation should be compared to the value of FRET between monomeric Gαi and D2R. According to which the most pronounced effect of rotigotine was observed for Gαi2β1γ2.
Discussion
Dopamine is a neurotransmitter that plays a critical role in controlling movement, cognition, and emotion. Dopamine receptors are expressed in neurons of the nigrostriatal pathway (motor-related), the mesolimbic-cortical pathway (reward system, emotional control) and tuberoinfundibular system [56]. Peripheral dopamine neurons are involved in renal and cardiovascular functions, and immune regulation. Dysfunction of dopaminergic pathways play an essential role in the pathophysiology of Parkinson's disease, schizophrenia, mood disorders, attention-deficit disorder, Huntington’s disease, Tourette's syndrome, Tardive dyskinesia, and other disorders. Therefore, insight into the selectivity of signal transduction between the dopamine receptor and G proteins is crucial for understanding of current therapies and development of new treatments. Interestingly, 21.9% of all GPCRs couple exclusively to the Gαi/o subfamily, another 5% couple to Gαi/o and of other G subfamilies [57]. All these receptors may couple differentially among various Gαi and Gαo isoforms, and individually prefer one specific isoform to the others [58–60].
The structural details behind the selectivity of receptor–G protein activation remain unclear and are an important subject of biochemical and biophysical studies [17, 23, 61, 62]. Studies dealing with the Gαs, Gαi, and Gαq families have shown their direct roles in regulating the levels of the secondary messenger and have provided substantial insight into the GPCR–G protein interface [63–65]. Despite that there is a plethora of data regarding the coupling specificity of various GPCRs, only a little is known about the potential receptor selectivity between the closely related members of the G protein families. In the present study, we have focused on the functional selectivity of dopamine D2R toward the Gαi1, Gαi2, and Gαi3 subunits, and analyzed whether the organization of Gαi heterotrimers in the plasma membrane can influence D2R signaling. This is particularly interesting in light of the current understanding of the complexity observed with the structural and functional organization of the cell membranes. The different lipid species present in membranes influence their properties, including the formation of membrane domains, as well as induce changes in the activity and density of the membrane proteins. However, it is not known whether the organization of G proteins in the plasma membrane influences their coupling with D2R or whether it might be one of the determinants of their coupling selectivity. Since it has been reported that there are differences in the plasma membrane targeting and trafficking pathways of the G proteins composed of Gα subunits belonging to different subfamilies, it is, therefore, reasonable to also evaluate the behavior of heterotrimers composed of closely related Gαi in the membranes, especially taking into account the already existing data [34, 53, 66, 67].
The membrane-binding area of Gα is limited to two sites on the surface of the protein and the membrane [67]. Its most critical membrane-binding determinant is the lipid anchors in conjunction with a polybasic motif at the N-terminus [66, 68, 69]. Depending on the specific subclass, the Gα subunits are palmitoylated and mostly myristoylated [70, 71]. All the Gαi subunits are N-myristoylated and S-palmitoylated, and the amino acid identity among them is high: Gαi1 and Gαi3 share a sequence identity of 94%, whereas Gαi2 has a lower identity of 87.5% and 85.5% to Gαi1 and Gαi3, respectively. We found two differences between the Gαi subunits in the positively charged motif at the N-terminus, which appear to be relevant (Fig. 4a). The first one concerns the position 21 where an R residue is present in Gαi3 and Gαi1, while a K residue is present in Gαi2. An additional substitution is found at position 32 of Gαi3, where K is present in the place of R in Gαi1 and Gαi2. However, when comparing the diffusion coefficients of Gαi3 and Gαi1, this position seems to be of lower importance in attaching Gαi to the membrane. Even if these substitutions did not appear to be significant, as they had no effect on the charge of the amino acid residues, it was assumed that they affect the interactions of Gαi with the membrane and might also influence the efficiency of translocation of Gα within the membrane. Our diffusion data suggest that the N-terminal residues of the Gα function as an essential signal to ensure the correct localization of the Gαi subunits at the plasma membrane.
The sequence differences in the polybasic motif between the Gαi subunits seemed to correlate well with the differences in their lateral diffusion coefficients detected by the FRAP experiments. The diffusion coefficient of the subunits increases in the following order: Gαi1 ≤ Gαi3 < Gαi2. Our data strongly suggest that the presence of the cluster of positively charged amino acids in the N-terminus of Gαi contributes to the membrane targeting of Gα, thus strengthening its affinity to the plasma membrane. The reduced membrane mobility of Gαi1 corresponds to the presence of a larger number of R residues in the polybasic motif. Both K and R function as basic residues; however, they differ in their geometric structure and possible interactions. Compared to the K residue, the R residue forms a higher number of electrostatic interactions, such as salt-bridges and hydrogen bonds, so it presumably results in stronger interactions than those generated by the lysine residue [72, 73]. The interactions that are observed for the positively charged residues include hydrogen bonds to the phosphate groups of phospholipids and electrostatic interactions to the negatively charged lipids at the cytosolic surface of the membrane [74]. Together, these interactions might cause retention of the positively charged residues on the cytoplasmic face of the membrane, slowing down the membrane mobility of the protein. In line with this hypothesis, in the present study, the Gαs subunit, which possesses a higher number of positively charged residues in the polybasic motif, showed the slowest membrane mobility among all the investigated Gα subunits.
On the other hand, the slower rate of lateral diffusion observed for Gα indicates that molecular motion is transiently confined, and such a protein resides within a particular region for a longer period of time. This in turn could enhance the FRET signal—in this case, the energy transfer between D2R and the slowest diffusing Gαi subunit (assuming a similar distribution for all the Gαi subunits across the membrane). As mentioned above, the FRET technique was applied to assess the trafficking of Gαi as monomers and heterotrimers when co-expressed with dopamine D2R and to analyze the corresponding changes in their relative membrane localization. Interestingly, we detected that the resonance energy transfer between D2R and the slowest diffusing Gαi subunit—Gαi1—had the lowest efficiency (almost none). The highest FRET signal was observed for the fastest diffusing Gαi2, while a slightly less efficient signal was recorded for Gαi3 (diffusion rates comparable to Gαi1) and Gαs (the slowest diffusion rate). These results indicate that the sequestration of Gα subunits, even those belonging to the same Gαi subfamily, in the plasma membrane may also vary.
In our earlier work, which investigated the distribution of Gαs and Gαi3 in the plasma membrane, we observed that these proteins were localized in different types of specific membrane domains [53]. It was found that the Gαs subunits preferred solid-like domains (insensitive to cholesterol, with a structure or composition of lipid rafts), while the Gαi3 subunit preferred the more fluid regions of the membrane and detergent-resistant domains such as lipid rafts. This suggests that distinct protein acylation may act as a signal for recruitment or retention into particular membrane regions/domains containing specific lipids. As already mentioned, despite that all the Gαi subunits had the same lipid anchors, the difference in the sequence of the polybasic region (for example, the presence of additional R residues) has an impact on the lipid preference and membrane localization of the subunits. A similar value of apparent diffusion coefficient observed for Gαi1 and Gαi3 in this study suggests their similar membrane localization. Therefore, it is tempting to conclude that Gαi1 also prefers detergent-resistant and cholesterol-dependent membrane domains (i.e. Lo-like domains) in the plasma membrane. However, the FRET data do not support this interpretation (as different FRET efficiency was estimated for the pairs D2R–Gαi1 and D2R–Gαi3). The main limitation of the FRAP studies is that it does not provide detailed information about where the species are present and the subpopulations in different locations cannot be simply identified. Another important aspect that needs to be considered is the nature of the interaction of the R residue with the membrane, as in some cases it leads to a local distortion of the bilayer around proteins [74]. This distortion is manifested in a high level of local water penetration inside the membrane, and can lead to a decrease in the thickness of the bilayer as well as affect the long-range interactions. We cannot rule out that the lower FRET signal observed between D2R and Gαi1 could result from the local deformation of the membrane induced by Gαi1, which affects the distribution and density of D2R in such an area in the membrane. This scenario is quite probable since the N-terminus of Gαi1 is arginine-rich, and the dopamine D2R is broadly distributed throughout the cell membrane, as supported by our FRET results. It has been shown by Sharma et al. that D2R exists in both detergent-soluble and detergent-insoluble fractions of the plasma membrane [36]. The similar plasma membrane localization of the Gαi1 and Gαi3 subunits cannot be excluded; however, faster diffusion of Gαi2 indicates that this subunit is localized within the membrane area which is composed of different types of lipids, a more fluid membrane area, and rich in D2R, as suggested by our FRET data.
As reported in our previous work, the diffusion of the Gαs and Gαi3 subunits speeds up upon the formation of heterotrimer [34, 40]. The Gβγ dimer is responsible for the rapid relocation of Gα from the lamellar membrane region where it resides as a monomer [67]. As expected, the membrane mobility of Gαi2β1γ2 and Gαi3β1γ2 changed in a similar way. The association of Gβ1γ2 with the GDP-bound Gαi2 or Gαi3 caused the G proteins to relocate into the more fluid membrane regions (the diffusion rate increased despite the increase in the molecular weight of the complex). However, an opposite behavior was observed for Gαi1. In the case of this subunit, when the trimer was formed, its membrane mobility slowed down the diffusion rate. This implies that—in contrast to Gαi2 and Gαi3—Gαi1 in the heterotrimer complex did not change its lipid environment or changed it only slightly (the formation of heterotrimers by Gαi1 did not involve the translocation of this protein into a more fluid region in the membrane as noted for the rest of the Gαi subunits). Alternatively, the observed slow-down of the heterotrimer diffusion could be a sustained effect of the structural perturbations of the lipid bilayers that were caused by the N-terminus of Gαi1, which also affected the diffusion of the full heterotrimeric complex. To clearly discriminate between these possibilities, structural in vitro studies in a model system (purified proteins and lipid bilayers) are required. However, the impact of the Gβ1γ2 dimer on the membrane distribution of the complete complex of Gαi1β1γ2 is unquestionable, as was indicated by the significant FRET signal between the complete heterotrimer and D2R compared to the almost undetectable signal of the monomeric Gαi1. Previous studies of fluorescence and electron paramagnetic resonance have also pointed out that the N-terminus of Gαi1 undergoes a conformational change upon Gβγ binding and activation [75].
Taken together, this new experimental evidence strengthens our earlier hypothesis that the Gβγ dimer alone does not define the affinity or specificity of the complete heterotrimer toward the membrane lipid phase [34]. In general, the distinct heterotrimeric combinations showed differences in their mobility characteristics (Table 1). Therefore, the interplay between the Gβγ and Gα subunits is critical for controlling the trafficking of the complete G protein heterotrimer. Moreover, we found that for some heterotrimers, Gα acts as a crucial modulator of the membrane localization. These findings appear to be in contradiction with the previously published results of the nuclear magnetic resonance-based studies (on purified proteins and liposomes), suggesting that only Gβγ dimer is responsible for the cellular localization of the heterotrimeric Gαi1 proteins, thereby masking the lamellar membrane affinity of Gαi1 [67].
Besides the observed changes in diffusion rates, the Gβ1γ2-dependent translocation of the Gαi1 and Gαi3 subunits also induced an increase in the FRET efficiency. The simplest explanation that can be given for this phenomenon is that the heterotrimers are localized within the D2R-rich membrane fraction and are waiting for the agonist-activated receptor. By contrast, we found that the FRET signal between Gαi2β1γ2 and D2R remained at the same level as that calculated for the D2R–Gαi2 pair. Since the monomeric Gαi2 is located in the D2R-rich membrane region, it is most likely that its spatial distribution undergoes only a slight change upon heterotrimer formation. Our data are in general agreement with the results published by Sharma et al. [36] who observed that the majority of the plasma membrane-expressed population of D2R was located within the detergent-resistant structures that do not correspond to classical lipid rafts. Treatment with an agonist led to the loss of both the detergent-soluble and detergent-resistant D2R fractions; however, the loss of detergent-resistant fraction was significantly greater.
In the present study, we found that the use of an antiparkinsonian drug, rotigotine, as a D2R agonist led to the inhibition of cAMP production and noticed a difference in the coupling selectivity of heterotrimers. The order of rotigotine potency (Gαi2 > Gαi3 = Gαi1) observed in the FRET experiments remains in general agreement with the results shown by direct measurement of the level of intracellular cAMP. Because the complexes formed between agonist-bound D2R and G proteins have short lives, the time-resolution of the FRET measurements allowed detecting only the further steps in the signaling cascade: dissociation of Gα from the trimer complex, followed by its relocation within the plasma membrane and receptor internalization. All these processes together were manifested as a decrease in the FRET efficiency, as compared to the basal signal (FRET for D2R–Gαi pair). It is noteworthy that in several earlier reports, Gαi2 was also indicated as selective toward D2R, causing maximal inhibition of adenylate cyclase [26, 76, 77]. However, the experimental data imply that the coupling selectivity of Gαi is regulated by the agonist-activated conformation of D2R. For example, stimulation of D2R with R(+)-3-PPP hydrochloride caused preferential coupling to Gαi3 rather than Gαi1 or Gαi2 [25]. The C-terminal Gα, as well as the movement magnitude of the sixth transmembrane helix of activated receptor, which varies from one receptor to another, has been predicted to be the main modulator of the selectivity of the G protein subtypes. Regarding the Gαi subunits, the sequence of C-terminal helix is almost identical for all proteins (two substitutions in Gαi3: 350D/E, 354F/Y). Furthermore, neither the amino acid sequences of β2–β3 loop nor the β6 sheet in the Ras-like domain—additional residues predicted as selectivity determinants—show any significant differences (one substitution in Gαi3, 195H/Y) [15, 23]. This clearly indicates that there must be other selectivity determinants for the coupling of Gαi heterotrimers. Our data support the new idea that membrane location can serve as an important selectivity determinant of downstream signaling. Considering that the ligated receptors might be clustered [78, 79] for longer durations within the given domains in the cell membrane, which also contain appropriate G protein, it seems likely that this combination fine-tunes the sensitivity and specificity of a given signaling pathway.
Conclusions
The concept of rapid translocation of the Gα monomers after dissociation from the Gβγ dimer and their localization to the lamellar structures, where they interact with effector molecules, is widely accepted and has also been confirmed by numerous studies [6, 80, 81]. The model of membrane localization-dependent signaling by G protein has been proposed over a decade ago. However, since then, the knowledge of membrane organization and functioning has significantly evolved [54, 82]. Therefore, some aspects of this model require revision. For instance, all the monomeric Gαi subunits are considered as identical in terms of their membrane coupling, and it has been postulated that they localize to the same type of membrane structures—lipid raft domains [31, 32]. In fact, as proved in the present study, even closely related subunits of Gαi differ in their membrane trafficking properties that influence their signaling. The interactions between lipids and proteins seem to be crucial in GPCR-dependent cell signaling due to their functional organization in specific membrane domains, and should therefore be taken into account as one of the selectivity determinants of G protein coupling.
Supplementary information
Additional file 1. Supplemental RT-qPCR experiments.
Abbreviations
- GPCR
G protein-coupled receptor
- D2R
Dopamine D2 receptor
- FRAP
Fluorescence recovery after photobleaching
- FRET
Fluorescence resonance energy transfer
- FLIM
Fluorescence-lifetime imaging microscopy
- HEK293
Human embryonic kidney 293 cells
- cAMP
3′,5′-Cyclic adenosine monophosphate
- GTP
Guanosine-5′-triphosphate
- GDP
Guanosine diphosphate
- ICL
Intracellular loops
- TM
Transmembrane helix
- RGS
Regulators of G protein signaling
- AGS
Receptor-independent activators of G-protein
- PCR
Polymerase chain reaction
- mGFP
Monomeric Green Fluorescent Protein
- FP
Fluorescent protein
- MEM
Minimum essential medium
- FBS
Fetal bovine serum
- NPA
N-n-propylnorapomorphine
- Dapp
Apparent diffusion coefficient
- Mf
Mobile fraction
Authors’ contributions
Conceptualization: A.P. and P.M. Formal analysis: A.P., P.M. and B.R. Funding acquisition: A.P. Investigation: Genetics constructs: P.M., B.R. FRAP experiments: P.M. FLIM–FRET experiments: A.P. and B.R. cAMP production experiments: B.R. RT-qPCR experiments: E.B. Project administration: A.P. Visualization: P.M., A.P. Writing—original draft: A.P., B.R. and P.M. Writing—review and editing: A.P., M.D.-W., P.M. and B.R. All authors read and approved the final manuscript.
Funding
This work was supported by a grant awarded by the Polish National Center for Science (NCN, no. 2016/23/B/NZ1/00530). The open-access publication of this article was funded by the Priority Research Area BioS under the program “Excellence Initiative–Research University” at the Jagiellonian University in Kraków.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Agnieszka Polit, Email: a.polit@uj.edu.pl.
Beata Rysiewicz, Email: beata.rysiewicz@doctoral.uj.edu.pl.
Paweł Mystek, Email: pawel.mystek@uj.edu.pl.
Ewa Błasiak, Email: ewa.blasiak@uj.edu.pl.
Marta Dziedzicka-Wasylewska, Email: marta.dziedzicka-wasylewska@uj.edu.pl.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-020-00685-9.
References
- 1.Sriram K, Insel PA. GPCRs as targets for approved drugs: How many targets and how many drugs? Mol Pharmacol. 2018;93:251–258. doi: 10.1124/mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine Receptor Signaling. J Recept Signal Transduct Res. 2004;24:3. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu J-M, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:1. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 4.Syrovatkina V, Alegre KO, Dey R, Huang XY. Regulation, signaling, and physiological functions of G-proteins. J Mol Biol. 2016;428:19. doi: 10.1016/j.jmb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, et al. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:1. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 6.Vögler O, Barceló JM, Ribas C, Escribá PV. Membrane interactions of G proteins and other related proteins. Biochim Biophys Acta Biomembr. 2008;1778:7–8. doi: 10.1016/j.bbamem.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Van Keulen SC, Rothlisberger U. Effect of N-terminal myristoylation on the active conformation of Gαi1-GTP. Biochemistry. 2017;56:1. doi: 10.1021/acs.biochem.6b00388. [DOI] [PubMed] [Google Scholar]
- 8.Loisel TP, Ansanay H, Adam L, Marullo S, Seifert R, Lagacé M, et al. Activation of the β2-adrenergic receptor-Gα(s) complex leads to rapid depalmitoylation and inhibition of repalmitoylation of both the receptor and Gα(s) J Biol Chem. 1999;274:43. doi: 10.1074/jbc.274.43.31014. [DOI] [PubMed] [Google Scholar]
- 9.Degtyarev MY, Spiegel AM, Jones TLZ. Palmitoylation of a G protein α(i) subunit requires membrane localization not myristoylation. J Biol Chem. 1994;269:49. [PubMed] [Google Scholar]
- 10.Moreira IS. Structural features of the G-protein/GPCR interactions. Biochem Biophys Acta. 2014;1840:1. doi: 10.1016/j.bbagen.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Baltoumas F, Theodoropoulou MC, Hamodrakas SJ. Interactions of the α-subunits of heterotrimeric G-proteins with GPCRs, effectors and RGS proteins: a critical review and analysis of interacting surfaces, conformational shifts, structural diversity and electrostatic potentials. J Struct Biol. 2013;182:3. doi: 10.1016/j.jsb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Sjöstedt E, Zhong W, Fagerberg L, Karlsson M, Mitsios N, Adori C, et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science. 2020;367:6482. doi: 10.1126/science.aay5947. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney JP, Sunahara RK. Mechanistic insights into GPCR–G protein interactions. Curr Opin Struct Biol. 2016;41:247–254. doi: 10.1016/j.sbi.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mnpotra JS, Qiao Z, Cai J, Lynch DL, Grossfield A, Leioatts N, et al. Structural basis of G protein-coupled receptor-Gi protein interaction: formation of the cannabinoid CB2 receptor-Gi protein complex. J Biol Chem. 2014;289:29. doi: 10.1074/jbc.M113.539916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flock T, Hauser AS, Lund N, Gloriam DE, Balaji S, Babu MM. Selectivity determinants of GPCR–G protein binding. Nature. 2017;545:7654. doi: 10.1038/nature22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue A, Raimondi F, Kadji FMN, Singh G, Kishi T, Uwamizu A, et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell. 2019;177:7. doi: 10.1016/j.cell.2019.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okashah N, Wan Q, Ghosh S, Sandhu M, Inoue A, Vaidehi N, et al. Variable G protein determinants of GPCR coupling selectivity. Proc Natl Acad Sci USA. 2019;116:24. doi: 10.1073/pnas.1905993116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatakrishnan AJ, Deupi X, Lebon G, Heydenreich FM, Flock T, Miljus T, et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature. 2016;536:7617. doi: 10.1038/nature19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glukhova A, Draper-Joyce CJ, Sunahara RK, Christopoulos A, Wootten D, Sexton PM. Rules of engagement: GPCRs and G Proteins. ACS Pharmacol Transl Sci. 2018;1:2. doi: 10.1021/acsptsci.8b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blahos J, Fischer T, Brabet I, Stauffer D, Rovelli G, Bockaert J, et al. A novel site on the Gα-protein that recognizes heptahelical receptors. J Biol Chem. 2001;276:5. doi: 10.1074/jbc.M004880200. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Matsukawa M, Mizutani Y, Iijima T, Matsumura H. Initial, transient, and specific interaction between G protein-coupled receptor and target G protein in parallel signal processing: a case of olfactory discrimination of cancer-induced odors. Med Res Arch. 2018;6:9. [Google Scholar]
- 22.Calebiro D, Jobin ML. Hot spots for GPCR signaling: lessons from single-molecule microscopy. Curr Opin Cell Biol. 2019;57:57–63. doi: 10.1016/j.ceb.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Van Eps N, Altenbach C, Caro LN, Latorraca NR, Hollingsworth SA, Dror RO, et al. Gi- and Gs-coupled GPCRs show different modes of G-protein binding. Proc Natl Acad Sci USA. 2018;115:10. doi: 10.1073/pnas.1721896115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuho I, Ostrovskaya O, Kramer GM, Jones CD, Xie KMK. Distinct profiles of functional discrimination among G proteins determine the actions of G protein–coupled receptors. Physiol Behav. 2015;176:1. doi: 10.1126/scisignal.aab4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazi L, Nickolls SA, Strange PG. Functional coupling of the human dopamine D 2 receptor with Gα i1, Gα i2, Gα i3 and Gα o G proteins: evidence for agonist regulation of G protein selectivity. Br J Pharmacol. 2003;138:5. doi: 10.1038/sj.bjp.0705116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senogless E, Spiegel M, Caron G. Specificity of receptor-G protein. Interactions. 1990;265:8. [PubMed] [Google Scholar]
- 27.Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA. 2001;98:6. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Parajo MF, Cambi A, Torreno-Pina JA, Thompson N, Jacobson K. Nanoclustering as a dominant feature of plasma membrane organization. J Cell Sci. 2014;127:23. doi: 10.1242/jcs.146340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guigas G, Weiss M. Effects of protein crowding on membrane systems. Biochim Biophys Acta Biomembr. 2016;1858:10. doi: 10.1016/j.bbamem.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:3. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:3. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 32.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains: GQ binds caveolin to concentrate in caveolae, whereas gi and GS target lipid rafts by default. Mol Biol Cell. 2001;12:3. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evanko DS, Thiyagarajan MM, Siderovski DP, Wedegaertner PB. Gβγ isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Gαs and Gαq. J Biol Chem. 2001;276:26. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- 34.Mystek P, Rysiewicz B, Gregrowicz J, Dziedzicka-Wasylewska M, Polit A. Gγ and Gα identity dictate a G-protein heterotrimer plasma membrane targeting. Cells. 2019;8:1246. doi: 10.3390/cells8101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fallahi-Sichani M, Linderman JJ. Lipid raft-mediated regulation of G-protein coupled receptor signaling by ligands which influence receptor dimerization: a computational study. PLoS ONE. 2009;4:8. doi: 10.1371/journal.pone.0006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma M, Celver J, Octeau JC, Kovoor A. Plasma membrane compartmentalization of D2 dopamine receptors. J Biol Chem. 2013;288:18. doi: 10.1074/jbc.M112.443945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levental I, Lingwood D, Grzybek M, Coskun Ü, Simons K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci USA. 2010;107:51. doi: 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryksin AV, Matsumura I. Overlap extension PCR cloning: A simple and reliable way to create recombinant plasmids. Biotechniques. 2010;48:6. doi: 10.2144/000113418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson SK, Gilman AG. Giα and Gβ subunits both define selectivity of G protein activation by α2-adrenergic receptors. Proc Natl Acad Sci USA. 2006;103:1. doi: 10.1073/pnas.0509763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mystek P, Tworzydło M, Dziedzicka-Wasylewska M, Polit A. New insights into the model of dopamine D1 receptor and G-proteins interactions. Biochim Biophys Acta (BBA) Mol Cell Res. 2015;1853:594–603. doi: 10.1016/j.bbamcr.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Lakowicz JR. Principles of fluorescent spectroscopy. 3. New York: Springer; 2006. [Google Scholar]
- 42.Yu JZ, Rasenick MM. Real-time visualization of a fluorescent Gαs: Dissociation of the activated G protein from plasma membrane. Mol Pharmacol. 2002;61:2. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
- 43.Wood M, Dubois V, Scheller D, Gillard M. Rotigotine is a potent agonist at dopamine D1 receptors as well as at dopamine D2 and D3 receptors. Br J Pharmacol. 2015;172:4. doi: 10.1111/bph.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cordeaux Y, Nickolls SA, Flood LA, Graber SG, Strange PG. Agonist regulation of D2 dopamine receptor/G protein interaction. J Biol Chem. 2001;276:31. doi: 10.1074/jbc.M008644200. [DOI] [PubMed] [Google Scholar]
- 45.Scheller D, Ullmer C, Berkels R, Gwarek M, Lübbert H. The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson’s disease. Naunyn-Schmiedeberg’s Arch Pharmacol. 2009;379:1. doi: 10.1007/s00210-008-0341-4. [DOI] [PubMed] [Google Scholar]
- 46.Van der Weide J, De Vries JB, Tepper PG, Horn AS. Pharmacological profiles of three new, potent and selective dopamine receptor agonists: N-0434, N-0437 and N-0734. Eur J Pharmacol. 1986;125:2. doi: 10.1016/0014-2999(86)90037-3. [DOI] [PubMed] [Google Scholar]
- 47.Adachi N, Yoshimura A, Chiba S, Ogawa S, Kunugi H. Rotigotine, a dopamine receptor agonist, increased BDNF protein levels in the rat cortex and hippocampus. Neurosci Lett. 2017;662:51. doi: 10.1016/j.neulet.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Horn AS, Tepper P, Van Der Weide J, Watanabe M, Grigoriadis D, Seeman P. Synthesis and radioreceptor binding activity of N-0437, a new, extremely potent and selective D2 dopamine receptor agonist. Pharm Weekbl Sci Ed. 1985;7:5. doi: 10.1007/BF02307578. [DOI] [PubMed] [Google Scholar]
- 49.Lane JR, Powney B, Wise A, Rees S, Milligan G. G protein coupling and ligand selectivity of the D2L and D 3 dopamine receptors. J Pharmacol Exp Ther. 2008;325:1. doi: 10.1124/jpet.107.134296. [DOI] [PubMed] [Google Scholar]
- 50.Senogles SE, Heimert TL, Odife ER, Quasney MW. A region of the third intracellular loop of the short form of the D2 dopamine receptor dictates Gi coupling specificity. J Biol Chem. 2004;279:3. doi: 10.1074/jbc.M309792200. [DOI] [PubMed] [Google Scholar]
- 51.Watts VJ, Neve KA. Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Mol Pharmacol. 1997;52:2. doi: 10.1124/mol.52.2.181. [DOI] [PubMed] [Google Scholar]
- 52.Bajar BT, Wang ES, Zhang S, Lin MZ, Chu J. A guide to fluorescent protein FRET pairs. Sensors (Switzerland) 2016;16:9. doi: 10.3390/s16091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mystek P, Dutka P, Tworzydło M, Dziedzicka-Wasylewska M, Polit A. The role of cholesterol and sphingolipids in the dopamine D1 receptor and G protein distribution in the plasma membrane. Biochim Biophys Acta Mol Cell Biol Lipids. 2016;1861:11. doi: 10.1016/j.bbalip.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 54.Kusumi A, Fujiwara TK, Tsunoyama TA, Kasai RS, Koichiro AL, Masanao MH, et al. Defining raft domains in the plasma membrane. Traffic. 2020;21:106. doi: 10.1111/tra.12718. [DOI] [PubMed] [Google Scholar]
- 55.Gupte TM, Ritt M, Dysthe M, Malik RU, Sivaramakrishnan S. Minute-scale persistence of a GPCR conformation state triggered by non-cognate G protein interactions primes signaling. Nature Communications. 2019;10:1. doi: 10.1038/s41467-019-12755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand. 1971;82:367. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- 57.Offermanns S, Rosenthal W. Encyclopedia of molecular pharmacology. 2. Berlin: Springer; 2008. [Google Scholar]
- 58.Jiang M, Bajpayee NS. Molecular mechanisms of Go signaling. Neurosignals. 2009;17:1. doi: 10.1159/000186688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Park S, Bajpayee NS, Nagaoka Y, Boulay G, Birnbaumer L, et al. Augmented glucose-induced insulin release in mice lacking Go2, but not Go1or Gi proteins. Proc Natl Acad Sci USA. 2011;108:4. doi: 10.1073/pnas.1018903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang G, Wang Y, Park S, Bajpayee NS, Vi D, Nagaoka Y, et al. G o2 G protein mediates galanin inhibitory effects on insulin release from pancreatic β cells. Proc Natl Acad Sci USA. 2012;109:7. doi: 10.1073/pnas.1200100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hilger D, Masureel M, Kobilka BK. Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol. 2018;25:1. doi: 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gacasan SB, Baker DL, Parrill AL. G protein-coupled receptors: the evolution of structural insight. AIMS Biophysics. 2017;4:3. doi: 10.3934/biophy.2017.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flock T, Ravarani CNJ, Sun D, Venkatakrishnan AJ, Kayikci M, Tate CG, et al. Universal allosteric mechanism for G a activation by GPCRs. Nature. 2015;524:7564. doi: 10.1038/nature14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandhu M, Touma AM, Dysthe M, Sadler F, Sivaramakrishnan S, Vaidehi N. Conformational plasticity of the intracellular cavity of GPCR−G-protein complexes leads to G-protein promiscuity and selectivity. Proc Natl Acad Sci USA. 2019;116:24. doi: 10.1073/pnas.1820944116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Semack A, Sandhu M, Malik RU, Vaidehi N, Sivaramakrishnan S. Structural elements in the Gαs and Gβq C termini that mediate selective G Protein-coupled Receptor (GPCR) signaling. J Biol Chem. 2016;291:34. doi: 10.1074/jbc.M116.735720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wedegaertner PB. G protein trafficking. Subcell Biochem. 2012;63:193–223. doi: 10.1007/978-94-007-4765-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogler O, Casas J, Capo D, Nagy T, Borchert G, Martorell G, et al. The Gβγ dimer drives the interaction of heterotrimeric Gi proteins with nonlamellar membrane structures. J Biol Chem. 2004;279:35. doi: 10.1074/jbc.M402061200. [DOI] [PubMed] [Google Scholar]
- 68.Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:26. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kosloff M, Elia N, Selinger Z. Structural homology discloses a bifunctional structural motif at the N-termini of Gα proteins. Biochemistry. 2002;41:49. doi: 10.1021/bi026729x. [DOI] [PubMed] [Google Scholar]
- 70.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 71.Kleuss C, Krause E. Gαs is palmitoylated at the N-terminal glycine Christiane. The EMBO journal. 2003;22:4. doi: 10.1093/emboj/cdg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borders CL, Broadwater JA, Bekeny PA, Salmon JE, Lee ANNS, Eldridge AM, et al. A structural role for arginine in proteins: multiple hydrogen bonds to backbone carbonyl oxygens. Protein Sci. 1994;3:541. doi: 10.1002/pro.5560030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donald JE, Kulp DW, Degrado WF. Salt bridges: geometrically specific, designable interactions. Proteins. 2011;79:3. doi: 10.1002/prot.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hristova K, Wimley WC. A look at arginine in membranes. J Membr Biol. 2011;23:1. doi: 10.1007/s00232-010-9323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medkova M, Preininger AM, Yu NJ, Hubbell WL, Hamm HE. Conformational changes in the amino-terminal helix of the G protein αi1 following dissociation from gβγ subunit and activation. Biochemistry. 2002;41:31. doi: 10.1021/bi0255726. [DOI] [PubMed] [Google Scholar]
- 76.Montmayeur JP, Guiramand J, Borrelli E. Preferential coupling between dopamine D2 receptors and G-proteins. Mol Endocrinol. 1993;7:2. doi: 10.1210/mend.7.2.7682286. [DOI] [PubMed] [Google Scholar]
- 77.Żuk J, Bartuzi D, Matosiuk D, Kaczor AA. Preferential coupling of dopamine d2s and d2l receptor isoforms with gi1 and gi2 proteins—in silico study. Int J Mol Sci. 2020;21:2. doi: 10.3390/ijms21020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scarselli M, Annibale P, McCormick PJ, Kolachalam S, Aringhieri S, Radenovic A, et al. Revealing G-protein-coupled receptor oligomerization at the single-molecule level through a nanoscopic lens: methods, dynamics and biological function. FEBS J. 2016;283:7. doi: 10.1111/febs.13577. [DOI] [PubMed] [Google Scholar]
- 79.Kasai RS, Ito SV, Awane RM, Fujiwara TK, Kusumi A. The Class-A GPCR dopamine D2 receptor forms transient dimers stabilized by agonists: detection by single-molecule tracking. Cell Biochem Biophys. 2018;76:1. doi: 10.1007/s12013-017-0829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chisari M, Saini DK, Cho JH, Kalyanaraman V, Gautam N. G protein subunit dissociation and translocation regulate cellular response to receptor stimulation. PLoS ONE. 2009;4:11. doi: 10.1371/journal.pone.0007797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abankwa D, Vogel H. A FRET map of membrane anchors suggests distinct microdomains of heterotrimeric G proteins. J Cell Sci. 2007;120:16. doi: 10.1242/jcs.001404. [DOI] [PubMed] [Google Scholar]
- 82.Weinberg ZY, Puthenveedu MA. Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic. 2019;20:2. doi: 10.1111/tra.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pándy-Szekeres G, Munk C, Tsonkov TM, Mordalski S, Harpsøe K, Hauser AS, et al. GPCRdb in 2018: adding GPCR structure models and ligands. Nucleic Acids Res. 2018;46:D1. doi: 10.1093/nar/gkx1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental RT-qPCR experiments.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.