Abstract
Background and Objectives:
It is unknown whether the degree of response to preoperative therapy correlates with locoregional recurrence (LR) or distant recurrence (DR) after resection of gastric cancer.
Methods:
Patients who underwent resection of gastric adenocarcinoma following chemotherapy and chemoradiation (1995–2015) were reviewed. The tumor regression grade (TRG) was defined by the percentage of viable tumor cells in the specimen (TRG0 = 0%, TRG1 = 1–2%, TRG2 = 3–50%, TRG3 ≥ 50%). The relationships among TRG, recurrence-free survival (RFS), LR, and DR were examined.
Results:
Two hundred forty-seven patients met the inclusion criteria (TRG0, 52 [21%]; TRG1, 49 [20%]; TRG2, 98 [40%]; TRG3, 48 [19%]). LR and DR occurred in 6.1% and 32.0% of patients, respectively. No patient with TRG0 experienced LR. R1 resection (6–15%) and LR (6–8%) rates were similar among TRG1–3 patients. R1 resection was associated with LR (hazard ratio [HR], 17.85; P < 0.001). ypN status (HR, 2.44; P =0.004) and linitis plastica (HR, 2.90; P < 0.001) were associated with DR. TRG was not independently associated with RFS, LR, or DR.
Conclusions:
TRG0 imparted excellent local control. However, TRG1–3 patients had similar R1 resection rates and therefore similar LR. DR is associated with ypN status and linitis plastica, not TRG.
Keywords: Gastric cancer, tumor regression grade, recurrence, neoadjuvant, radiation therapy
1 |. INTRODUCTION
The results of randomized clinical trials have firmly established multimodal therapy as the standard of care for patients with localized gastric cancer [1–3]. In particular, the use of chemotherapy with or without chemoradiation (CXRT) prior to gastrectomy has increased in recent years [4]. Thus, understanding the effect of preoperative therapy on outcome for patients with gastric cancer is increasingly necessary. The post-neoadjuvant therapy staging system (ypTNM) in the eighth edition of the AJCC Cancer Staging Manual provides one framework for the assessment of response to preoperative therapy, and has been previously validated in gastric cancer patients treated in this manner [5,6].
Tumor regression grade (TRG) is another well-described tool for evaluating histological response to therapy in resected tumor specimens. In contrast to ypT category, which is determined by the deepest level of invasion at which viable tumor is present, the TRG is based on the percentage of overall residual viable tumor cells. Although both ypT category and TRG assess the post-treatment status of the primary tumor, it remains unclear how the two interact to predict outcome in any given patient. For instance, given a ypT3 lesion with 10% residual viable tumor cells and a ypT2 lesion with 90% residual viable tumor cells, it is not clear which represents the higher risk tumor.
Landmark randomized trials have clearly demonstrated improved locoregional control in gastric cancer patients receiving adjuvant CXRT, and decreased risk of distant failure in patients receiving perioperative systemic therapy [1–3,7,8]. Whereas gastric cancer patients with pathological complete response (pCR) experience better survival than do those with residual viable disease, the relationship between TRG and survival is unclear [2,6,9–12]. Even less clear is the impact of TRG on the likelihood of recurrence after curative-intent gastrectomy, which occurs frequently despite the use of perioperative systemic therapy (26–37%) [13,14]. Moreover, in an era in which preoperative CXRT is often given to patients with gastric cancer, the specific clinical implication of TRG after CXRT has not been well studied in these patients. Therefore, we undertook the present study to determine whether the degree of response to preoperative CXRT—as measured by TRG—is related to the risk of recurrence after resection of gastric cancer.
2 |. METHODS
2.1 |. Patient population and definition of variables
With Institutional Review Board approval, we reviewed a prospectively maintained database of patients with primary gastric or Siewert type III gastroesophageal adenocarcinoma who received therapy prior to resection at The University of Texas MD Anderson Cancer Center from 1995 to 2015. The treatment strategy for individual patients with gastric cancer are generally determined by multidisciplinary conference. Our institutional preference is to treat patients with cT2 and more advanced tumors with induction chemotherapy followed by CXRT. As has been previously published, induction chemotherapy typically consists of a multiagent regimen including 5-fluorouracil and a platinum agent (cisplatin, oxaliplatin) with or without a taxane (docetaxol, paclitaxel) [13]. CXRT most commonly consists of a radiation dose of 45Gy with concurrent 5-fluorouracil based chemosensitization, the technical details of which have also been previously described [15]. To limit potential confounding factors related to the local effects of radiation therapy, we excluded patients that received chemotherapy alone as a preoperative treatment; all patients were required to have received CXRT. Patients with prior gastrectomy were also excluded. The following variables were collected: age, sex, race/ethnicity, date of diagnosis, date of surgery, date of recurrence (if any), date of death or last follow-up visit, histological grade, signet ring cell morphology, tumor location, linitis plastica, clinical TNM stage, type of surgical resection, extent of lymph node dissection (D1 versus D1+/D2), number of lymph nodes examined, need for concomitant organ resection, and post-treatment pathology. Our method of determining clinical TNM stage based on preoperative endoscopic ultrasonography, computed tomography, and positron emission tomography as well as the routine performance of pretreatment staging laparoscopy was described previously [6]. Similarly, we have defined linitis plastica as gastric wall thickening and lack of distensibility involving at least 1/3 of the stomach as identified on endoscopy, preoperative CT, and by diagnostic laparoscopy [16]. No patients with preoperatively suspected metastatic disease were eligible for resection; patients with M1 disease upon final pathology were included.
Our standard protocol for the pathologic assessment of post-treatment gastrectomy specimens is to embed the entire tumor or ulcer bed at 3–5 millimeter intervals, in order to ensure complete histologic evaluation of the tumor (or previous tumor site). All H&E sections were examined as part of routine pathologic assessment and the percentage of viable tumor and TRG were reported. TRG was defined by the percentage of viable tumor cells in the resected primary tumor (TRG0 = 0%, TRG1 = 1–2%, TRG2 = 3–50%, TRG3 = >50%). These criteria were adopted to reflect the recommendations of the College of American Pathologists (CAP), which designate a four-category system for scoring treatment effect: 0 = complete response, 1 = single cells or rare small groups of cells (near-complete response), 2 = residual cancer with evident tumor regression, 3 = extensive residual cancer with no evident tumor regression [17]. However, the CAP recommendations do not define specific percentages of viable tumor cells for each category. A cutoff of less than 1–2% viable tumor cells was therefore selected to closely reflect the criterion of single cells or rare small groups of cells (TRG1). A cutoff of >50% viable tumor cells was selected to closely reflect the criterion of extensive residual cancer with no evident tumor regression (TRG3), based on previously reported literature that >50% residual disease portends worse outcome [12].
Locoregional recurrence (LR) was defined as an anastomotic recurrence, recurrence within the surgical bed, or recurrence within the regional lymph node basin. Distant recurrence (DR) was defined as any recurrence within the peritoneum, liver, lungs, bone, brain, or distant lymph node basin.
2.2 |. Statistical analysis
The demographic and clinical characteristics of the TRG groups were compared using a chi-square test or the Fisher exact test as appropriate. Time to recurrence (overall, LR, and DR) was calculated from the date of diagnosis to the date of recurrence or last follow-up visit. Overall recurrence-free survival (RFS) was estimated using the Kaplan-Meier method; the overall RFS curves for the TRG groups were compared using the log-rank test. Cox regression models were used to estimate hazard ratios (HRs) for factors associated with overall RFS, LR, and DR. Each variable was run in a univariable model and retained if the P value was less than 0.2; stepwise selection of variables was then performed to make the final multivariable model. As the variable of interest, TRG was included in the multivariable model in all instances. For the sake of comparision, variables independently associated with overall RFS were also included in the multivariable models for LR and DR if the initial P < 0.2 threshold was not met. P values less than 0.05 were considered significant. All analyses were conducted using SAS Enterprise Guide software (version 7.1; SAS Institute, Cary, NC).
3 |. RESULTS
We identified 247 patients who met the inclusion criteria. Patient demographic, clinical, and pathological characteristics stratified according to the TRG are shown in Table 1. The majority of the patients were younger than 65 years (64.4%), male (59.5%), and white (55.5%). Tumors were predominantly poorly differentiated (70.9%), located in the gastric body (40.9%), and had clinical T category 3/4a (78.1%). Nearly half of the patients had signet ring cell features (49.0%). The clinical nodal status was positive in 57.1% of the patients.
TABLE 1.
Characteristics of gastric cancer patients undergoing curative-intent gastrectomy after preoperative chemotherapy and CXRT stratified according to TRG.
| n (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | All (n = 247) | TRG | ||||
| 0 (n = 52) | 1 (n = 49) | 2 (n = 98) | 3 (n = 48) | P value | ||
| Age | 0.205 | |||||
| <65 years | 159 (64.4) | 28 (53.8) | 33 (67.3) | 69 (70.4) | 29 (60.4) | |
| ≥65 years | 88 (35.6) | 24 (46.2) | 16 (32.7) | 29 (29.6) | 19 (39.6) | |
| Sex | 0.041 | |||||
| Male | 147 (59.5) | 37 (71.2) | 34 (69.4) | 51 (52.0) | 25 (52.1) | |
| Female | 100 (40.5) | 15 (28.9) | 15 (30.6) | 47 (48.0) | 23 (47.9) | |
| Race/ethnicity | 0.543 | |||||
| White | 137 (55.5) | 31 (59.6) | 30 (61.2) | 53 (54.1) | 23 (47.9) | |
| Black | 22 (8.9) | 3 (5.8) | 5 (10.2) | 9 (9.2) | 5 (10.4) | |
| Asian | 61 (24.7) | 13 (25.0) | 10 (20.4) | 21 (21.4) | 17 (35.4) | |
| Hispanic/Latino | 27 (10.9) | 5 (9.6) | 4 (8.2) | 15 (15.3) | 3 (6.3) | |
| Tumor histology | 0.710 | |||||
| Moderately differentiated | 52 (21.1) | 13 (25.0) | 9 (18.4) | 22 (22.5) | 8 (16.7) | |
| Poorly differentiated | 175 (70.9) | 33 (63.5) | 35 (71.4) | 71 (72.5) | 36 (75.0) | |
| Unknown | 20 (8.1) | 6 (11.5) | 5 (10.2) | 5 (5.1) | 4 (8.3) | |
| Signet ring cell features | <0.001 | |||||
| No | 126 (51.0) | 42 (80.8) | 25 (51.0) | 43 (43.9) | 16 (33.3) | |
| Yes | 121 (49.0) | 10 (19.2) | 24 (49.0) | 55 (56.1) | 32 (66.7) | |
| Linitis plastica | 0.150 | |||||
| No | 223 (90.3) | 50 (96.2) | 43 (87.8) | 90 (91.8) | 40 (83.3) | |
| Yes/suspected | 24 (9.7) | 2 (3.9) | 6 (12.2) | 8 (8.2) | 8 (16.7) | |
| Tumor location | 0.030 | |||||
| Gastroesophageal junction | 67 (27.1) | 16 (30.8) | 19 (38.8) | 26 (26.5) | 6 (12.5) | |
| Cardia/fundus | 18 (7.3) | 4 (7.7) | 3 (6.1) | 9 (9.2) | 2 (4.2) | |
| Body | 101 (40.9) | 18 (34.6) | 14 (28.6) | 43 (43.9) | 26 (54.2) | |
| Distal | 50 (20.2) | 14 (26.9) | 9 (18.4) | 18 (18.4) | 9 (18.8) | |
| Total | 11 (4.5) | 0 (0.0) | 4 (8.2) | 2 (2.0) | 5 (10.4) | |
| Margin status | 0.213a | |||||
| R0 | 229 (92.7) | 52 (100.0) | 44 (89.8) | 92 (93.9) | 41 (85.4) | |
| R1 | 18 (7.3) | 0 (0.0) | 5 (10.2) | 6 (6.1) | 7 (14.6) | |
| Postoperative chemotherapy | 0.148 | |||||
| No | 238 (96.4) | 51 (98.1) | 48 (98.0) | 91 (92.9) | 48 (100.0) | |
| Yes | 9 (3.6) | 1 (1.9) | 1 (2.0) | 7 (7.1) | 0 (0.0) | |
| Organ resection | 0.129 | |||||
| No | 207 (83.8) | 44 (84.6) | 45 (91.8) | 76 (77.6) | 42 (87.5) | |
| Yes | 40 (16.2) | 8 (15.4) | 4 (8.2) | 22 (22.5) | 6 (12.5) | |
| Extent of lymph node dissection | 0.442 | |||||
| D0/D1 | 28 (11.3) | 8 (15.4) | 7 (14.3) | 6 (6.1) | 7 (14.6) | |
| D1+/D2 | 218 (88.3) | 44 (84.6) | 42 (85.7) | 91 (92.9) | 41 (85.4) | |
| Unknown | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | |
| Number of lymph nodes examined | 0.026 | |||||
| <16 | 68 (27.5) | 17 (32.7) | 19 (38.8) | 17 (17.4) | 15 (31.3) | |
| ≥16 | 179 (72.5) | 35 (67.3) | 30 (61.2) | 81 (82.7) | 33 (68.8) | |
| cT category | 0.714 | |||||
| 1 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | |
| 2 | 30 (12.2) | 5 (9.6) | 9 (18.4) | 9 (9.2) | 7 (14.6 | |
| 3/4a | 193 (78.1) | 41 (78.9) | 38 (77.6 | 78 (79.6) | 36 (75.0) | |
| 4b | 23 (9.3) | 6 (11.5) | 2 (4.1) | 10 (10.2) | 5 (10.4) | |
| cN status | 0.492 | |||||
| Negative | 106 (42.9) | 19 (36.5) | 23 (46.9) | 46 (46.9) | 18 (37.5) | |
| Positive | 141 (57.1) | 33 (63.5) | 26 (53.1) | 52 (53.1) | 30 (62.5) | |
| ypT category | <0.001 | |||||
| 0 | 52 (21.1) | 52 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 1a/1b | 40 (16.2) | 0 (0.0) | 18 (36.7) | 19 (19.4) | 3 (6.3) | |
| 2 | 48 (19.4) | 0 (0.0) | 14 (28.6) | 24 (24.5) | 10 (20.8) | |
| 3 | 85 (34.4) | 0 (0.0) | 16 (32.7) | 44 (44.9) | 25 (52.1) | |
| 4a/4b | 22 (8.9) | 0 (0.0) | 1 (2.0) | 11 (11.2) | 10 (20.8) | |
| ypN status | <0.001 | |||||
| ypN- | 161 (65.2) | 46 (88.5) | 34 (69.4) | 63 (64.3) | 18 (37.5) | |
| ypN+ | 86 (34.8) | 6 (11.5) | 15 (30.6) | 35 (35.7) | 30 (62.5) | |
| LR | 0.892 | |||||
| No | 232 (93.9) | 52 (100.0) | 46 (93.9) | 90 (91.8) | 44 (91.7) | |
| Yes | 15 (6.1) | 0 (0.0) | 3 (6.1) | 8 (8.2) | 4 (8.3) | |
| DR | 0.057 | |||||
| No | 168 (68.0) | 42 (80.8) | 35 (71.4) | 64 (65.3) | 27 (56.3) | |
| Yes | 79 (32.0) | 10 (19.2) | 14 (28.6) | 34 (34.7) | 21 (43.8) | |
| Vital status | 0.418 | |||||
| Alive | 131 (53.0) | 27 (51.9) | 24 (49.0) | 58 (59.2) | 22 (45.8) | |
| Dead | 116 (47.0) | 25 (48.1) | 25 (51.0) | 40 (40.8) | 26 (54.2) | |
| Type of resection | 0.233 | |||||
| Total gastrectomy | 140 (56.7) | 26 (50.0) | 31 (63.3) | 52 (53.1) | 31 (64.6) | |
| Subtotal gastrectomy | 93 (37.7) | 22 (42.3) | 13 (26.5) | 42 (42.9) | 16 (33.3) | |
| Proximal gastrectomy | 14 (5.7) | 4 (7.7) | 5 (10.2) | 4 (4.1) | 1 (2.1) | |
The TRG distribution was as follows: TRG0, 52 patients (21%); TRG1, 49 patients (20%); TRG2, 98 patients (40%); and TRG3, 48 patients (19%). Chi-square analysis identified significant differences in sex, presence of signet ring cells, tumor location, number of lymph nodes examined, ypT category, and ypN category among TRG0–3 groups (see Table 1). As the degree of response to preoperative therapy decreased (from TRG0 to TRG3), the percentage of patients with signet ring cell features increased in a stepwise manner (from 19.2% to 66.7%). Similarly, the rate of post-treatment nodal positivity (ypN+) increased as the response to therapy decreased (TRG0 = 11.5%, TRG1 = 30.6%, TRG2 = 35.7%, TRG3 = 62.5%).
3.1 |. Overall RFS
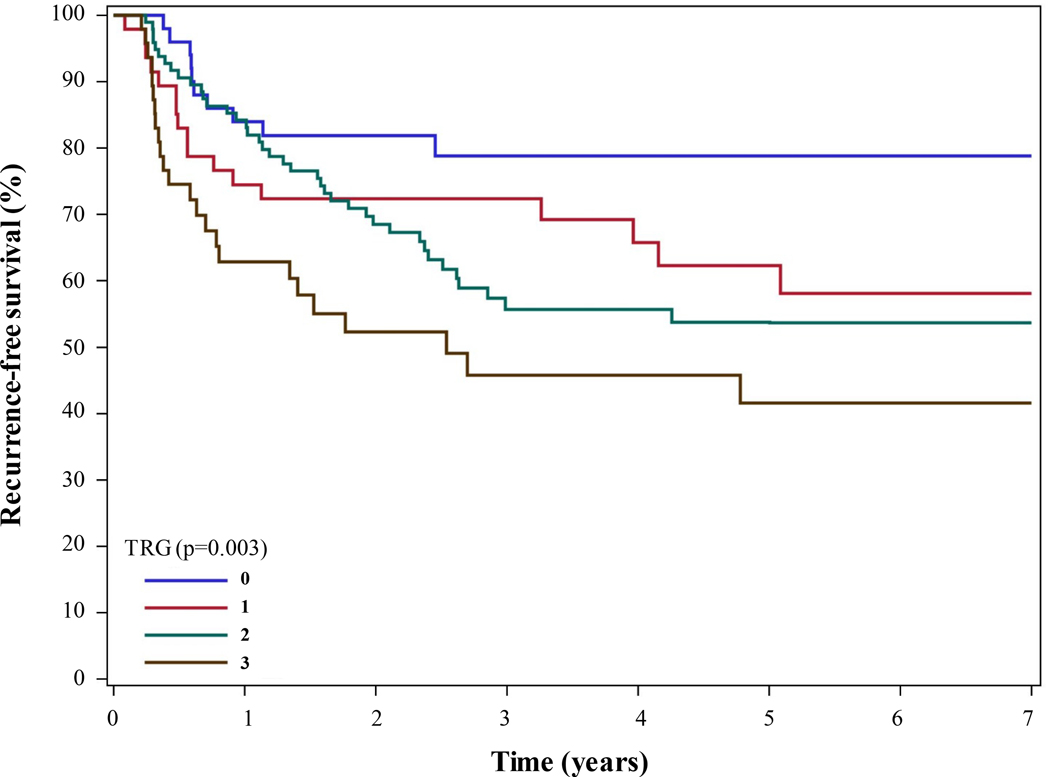
The 5-year overall RFS rates were 79%, 62%, 54%, and 42% in the TRG0, TRG1, TRG2, and TRG3 groups, respectively (P = 0.003 [log-rank test]) (Figure 1). Additional factors associated with overall RFS in univariable Cox regression analysis include ypN status, ypT category, tumor location, signet ring cell features, linitis plastica, R1 resection, and ypM1 status (Supplementary Table S1). Creation of a multivariable model for overall RFS identified R1 resection (HR, 3.11 [95% CI, 1.68–5.78]; P < 0.001), ypN status (HR, 2.72 [95% CI, 1.74–4.24]; P < 0.001), and linitis plastica (HR, 1.89 [95% CI, 1.05–3.37]; P = 0.033) as being independently associated with RFS. The TRG did not independently predict RFS. We performed sensitivity analyses treating the TRG as a binary variable in additional models, in which TRG was never independently associated with overall RFS. Specifically, the HR for TRG2/3 vs. TRG0/1 was 1.27 (95% CI, 0.79–2.03; P = 0.321), and that for TRG1/2/3 vs. TRG0 was 1.36 (95% CI, 0.68–2.72; P = 0.392).
FIGURE 1.
Kaplan-Meier RFS curve for gastric cancer patients undergoing curative-intent gastrectomy after preoperative chemotherapy and CXRT stratified according to TRG.
3.2 |. LR
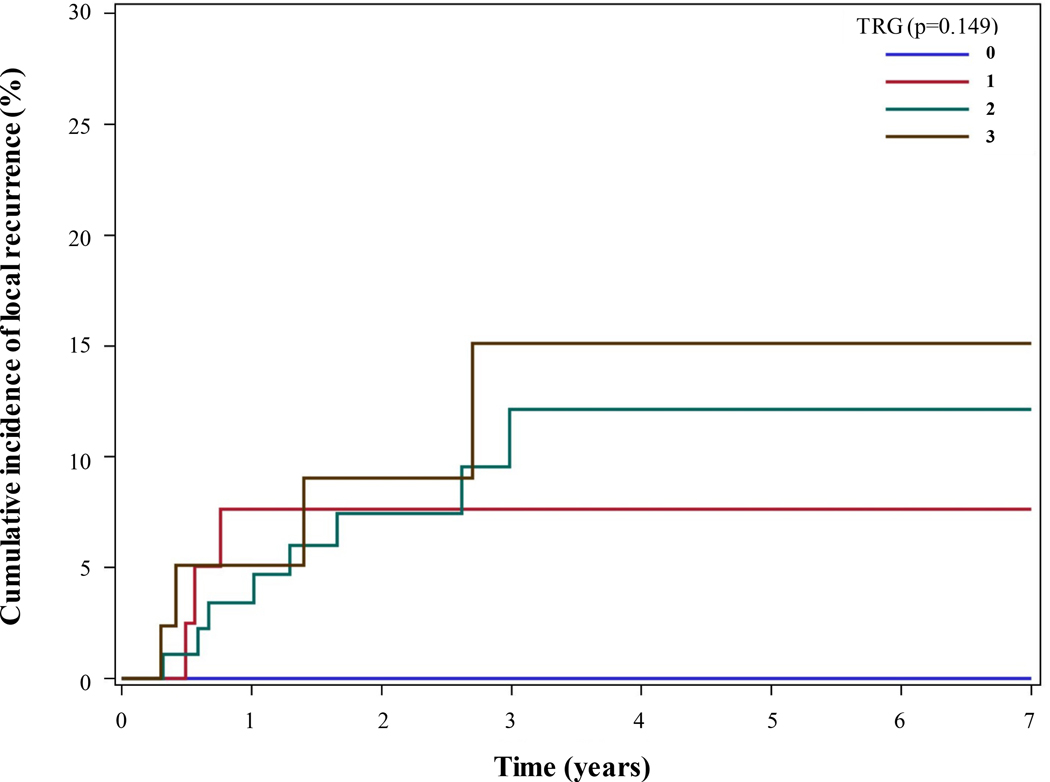
LR occurred in 6.1% of the patients (n = 15) during a median follow-up duration of 2.62 years. No patients in the TRG0 group experienced LR. In comparison, 6.5% (n = 3) of the TRG1 group, 8.2% (n = 8) of the TRG2 group, and 8.3% (n = 4) of the TRG3 group experienced LR. The cumulative incidence of LR over time is shown in Figure 2, stratified according to TRG. We found no statistically significant differences in the incidence of LR among the TRG groups as analyzed by chi-square and log-rank tests.
FIGURE 2.
Cumulative incidence curve of LR for gastric cancer patients undergoing curative-intent gastrectomy after preoperative chemotherapy and CXRT stratified according to TRG.
Univariable Cox regression analysis of risk factors for LR identified ypT4a/b category (HR, 14.40 [95% confidence interval (CI), 1.58–130.92]; P = 0.018) and R1 resection (HR, 22.12 [95% CI, 7.78–62.90]; P < 0.001) as potential predictors of LR (Table 2). After stepwise selection of variables to create a multivariable model, only R1 resection was independently associated with LR (HR, 17.85 [95% CI, 6.06–52.57]; P < 0.001).
TABLE 2.
Univariable and multivariable Cox regression analysis of factors associated with LR in gastric cancer patients treated with preoperative chemotherapy and CXRT.
| Univariable | ||
|---|---|---|
| Variable | HR (95% CI) | P value |
| TRG | 0.895 | |
| 0 (ref) | ref | |
| 1 | - | |
| 2 | - | |
| 3 | - | |
| ypN status (ypN+ vs. ypN-) | 1.75 (0.62–4.92) | 0.291 |
| ypT category | 0.074 | |
| 0 | - | |
| 1 (ref) | ref | |
| 2 | 2.39 (0.25–22.9) | 0.451 |
| 3 | 4.08 (0.50–33.16) | 0.189 |
| 4a/b | 14.38 (1.58–130.92) | 0.018 |
| Age at diagnosis (≥65 vs. <65 years) | 1.24 (0.44–3.49) | 0.679 |
| Sex (female vs. male) | 0.93 (0.33–2.62) | 0.895 |
| Race/ethnicity | 0.846 | |
| White (ref) | ref | |
| Black | 1.38 (0.30–6.37) | 0.683 |
| Asian | 0.73 (0.20–2.70) | 0.637 |
| Hispanic | 0.54 (0.07–4.29) | 0.563 |
| Tumor location | 0.257 | |
| Gastroesophageal junction | ref | |
| Cardia/fundus | 2.21 (0.37–13.25) | 0.385 |
| Body | 0.84 (0.19–3.77) | 0.824 |
| Antrum | 1.75 (0.39–7.83) | 0.463 |
| Total | 5.25 (0.87–31.58) | 0.070 |
| Tumor histology | 0.987 | |
| Moderately differentiated | ref | |
| Poorly differentiated | 1.11 (0.31–3.94) | 0.872 |
| Unknown | - | |
| Signet ring cell features (yes vs. no) | 3.03 (0.96–9.51) | 0.058 |
| Linitis plastica (yes/suspected vs. no) | 1.97 (0.44–8.79) | 0.373 |
| Concomitant organ resection | 2.08 (0.66–6.53) | 0.210 |
| Adjuvant therapy (yes vs. no) | 1.94 (0.26–14.73) | 0.523 |
| Number of lymph nodes examined <16 | 1.59 (0.45–5.67) | 0.468 |
| Extent of lymph node dissection | 0.880 | |
| D0/D1 | ref | |
| D1+/D2 | 0.68 (0.15–3.02) | 0.613 |
| Unknown | - | |
| R1 resection | 22.12 (7.78–62.90) | <0.001 |
| ypM1 status | 6.65 (0.86–51.16) | 0.069 |
| Type of resection | 0.503 | |
| Total gastrectomy | ref | |
| Subtotal gastrectomy | 0.85 (0.28–2.60) | |
| Proximal gastrectomy | 2.23 (0.47–10.52) | |
| Multivariable | ||
| Variable | HR (95% CI) | P value |
| TRG | 0.972 | |
| 0 (ref) | Ref | |
| 1 | >99.99 | |
| 2 | >99.99 | |
| 3 | >99.99 | |
| ypN status (ypN+ vs. ypN-) | 0.75 (0.15–3.64) | 0.721 |
| Linitis plastica (yes/suspected vs. no) | 1.31 (0.45–3.83) | 0.625 |
| R1 resection | 17.85 (6.06–52.57) | <0.001 |
3.3 |. DR
DR occurred in 32.0% of the patients (n = 79). DR occurred in 19.2% (n = 10) of TRG0 patients, 28.6% (n = 14) of TRG1 patients, 34.7% (n = 34) of TRG2 patients, and 43.8% (n = 21) of TRG3 patients. Differences in the incidence of DR among the TRG groups approached but did not meet statistical significance by chi-square analysis (P = 0.057), but were statistically significant by log-rank test (P =0.024, Supplementary Figure S1).
Univariable Cox regression analysis of risk factors identified numerous potential predictors of DR (Table 3). TRG overall (P = 0.025) and TRG3 in particular (HR, 3.04 [95% CI, 1.43–6.46]; P = 0.004) were associated with DR. Other factors identified as potentially predictive of DR included ypN status, ypT category, linitis plastica, R1 resection, and the presence of intraoperatively discovered and resected ypM1 disease. Stepwise selection of variables and the creation of a multivariable model identified ypN status (HR, 2.44 [95% CI, 1.34–4.45]; P = 0.004) and linitis plastica (HR, 2.90 [95% CI, 1.79–4.69]; P < 0.001) as being independently associated with DR. In this model, the TRG did not independently predict DR.
TABLE 3.
Univariable and multivariable Cox regression analysis of factors associated with DR in gastric cancer patients treated with preoperative chemotherapy and CXRT.
| Univariable | ||
|---|---|---|
| Variable | HR (95% CI) | P value |
| TRG | 0.025 | |
| 0 (ref) | ref | |
| 1 | 1.55 (0.69–3.49) | 0.292 |
| 2 | 1.89 (0.93–3.82) | 0.077 |
| 3 | 3.04 (1.43–6.46) | 0.004 |
| ypN status (ypN+ vs. ypN-) | 3.20 (2.05–4.99) | <0.001 |
| ypT category | <0.001 | |
| 0 | 1.13 (0.43–2.96) | 0.811 |
| 1 (ref) | ref | |
| 2 | 1.48 (0.59–3.71) | 0.403 |
| 3 | 2.95 (1.31–6.63) | 0.009 |
| 4a/b | 6.12 (2.42–15.47) | <0.001 |
| Age at diagnosis (≥65 vs. <65 years) | 0.74 (0.45–1.21) | 0.233 |
| Sex (female vs. male) | 1.05 (0.67–1.64) | 0.837 |
| Race/ethnicity | 0.723 | |
| White (ref) | ref | |
| Black | 0.76 (0.33–1.78) | 0.527 |
| Asian | 0.74 (0.42–1.30) | 0.293 |
| Hispanic | 0.89 (0.44–1.82) | 0.757 |
| Tumor location | 0.223 | |
| Gastroesophageal junction | ref | |
| Cardia/fundus | 0.62 (0.21–1.78) | 0.371 |
| Body | 0.84 (0.48–1.45) | 0.525 |
| Antrum | 1.08 (0.58–2.01) | 0.820 |
| Total | 2.21 (0.89–5.45) | 0.087 |
| Tumor histology | 0.616 | |
| Moderately differentiated | ref | |
| Poorly differentiated | 0.85 (0.50–1.44) | 0.542 |
| Unknown | 0.64 (0.25–1.60) | 0.336 |
| Signet ring cell features (yes vs. no) | 1.44 (0.92–2.25) | 0.110 |
| Linitis plastica (yes/suspected vs. no) | 2.99 (1.70–5.28) | <0.001 |
| Concomitant organ resection | 1.54 (0.90–2.64) | 0.113 |
| Adjuvant therapy (yes vs. no) | 1.07 (0.34–3.39) | 0.909 |
| Number of lymph nodes examined <16 | 0.81 (0.51–1.30) | 0.386 |
| Extent of lymph node dissection | 0.237 | |
| D0/D1 | Ref | |
| D1+/D2 | 0.65 (0.34–1.23) | 0.185 |
| Unknown | 2.01 (0.26–15.58) | 0.505 |
| R1 resection | 2.53 (1.21–5.28) | 0.014 |
| ypM1 status | 7.38 (3.36–16.19) | <0.001 |
| Type of resection | 0.264 | |
| Total gastrectomy | ref | |
| Subtotal gastrectomy | 0.67 (0.42–1.10) | |
| Proximal gastrectomy | 0.71 (0.26–1.96) | |
| Multivariable | ||
| Variable | HR (95% CI) | P value |
| TRG | 0.851 | |
| 0 (ref) | Ref | |
| 1 | 1.01 (0.44–2.35) | |
| 2 | 1.21 (0.58–2.54) | |
| 3 | 1.33 (0.58–3.06) | |
| ypN status (ypN+ vs. ypN-) | 2.44 (1.34–4.45) | 0.004 |
| Linitis plastica (yes/suspected vs. no) | 2.90 (1.79–4.69) | <0.001 |
| R1 resection | 1.73 (0.80–378) | 0.167 |
4 |. DISCUSSION
In this analysis of gastric cancer patients who underwent curative-intent gastrectomy after chemotherapy and CXRT, primary tumor pCR (TRG0) occurred in 21% of the patients, none of whom experienced LR. For all other patients (TRG1–3), the degree of response to preoperative therapy was not independently associated with either LR or DR. Instead, R1 resection defined the risk of LR for gastric cancer patients given preoperative therapy, and the rate of R1 resection did not vary among the TRG1, TRG2, and TRG3 groups. Because ypN status and linitis plastica were the only variables defining the risk of DR, this study re-emphasizes the clinical importance of these factors in patients with resectable gastric cancer. The TRG is seemingly correlated with aggressive tumor biology but does not appear to add predictive value with regard to the risk of recurrence when compared with routinely analyzed factors such as ypN and margin status.
In the era preceding widespread use of preoperative therapy, the incidence rates for overall recurrence of resected gastric cancer ranged from 30% to 42%, with as many as half of patients experiencing LR [18–20]. The 6.1% incidence rate for LR and 32.0% incidence rate for DR in the present study are consistent with recently reported patterns of recurrence in the era of preoperative therapy for resectable gastric cancer. Mokadem et al recently reported on a cohort of patients in the Netherlands Cancer Registry, in which 408 gastric cancer patients from 18 centers received chemotherapy prior to resection. They found that the 5-year risk of recurrence was 36.8% overall and that the vast majority (81.6%) of recurrences were distant. The rate of LR as the sole site of recurrence was 11.2% [14]. We previously reported on the pattern of first recurrence for all patients with resected gastric cancer at our institution, in whom the most common sites of recurrence were the peritoneum (49%), liver (21%), and locoregional (15%). Sixty-one percent of these patients received preoperative therapy (with and without CXRT) [13].
In an early report of phase 2 clinical trials of treatment of resectable gastric cancer at MD Anderson, response to chemotherapy was found to be the most important predictor of overall survival (OS) in a binary fashion inasmuch as responders (<50% viable tumor cells) fared better than did nonresponders (>50% viable tumor cells), with actuarial 5-year OS rates of 83% and 31%, respectively (P < 0.001 [log-rank test]) [21]. Similarly, a prospective trial of patients receiving neoadjuvant CXRT showed that patients who had at least a partial response to therapy had longer survival than did those who did not [22]. Since then, numerous retrospective studies have investigated TRG or histological response grade as a predictor of OS in gastric cancer patients. Despite the known association between pCR and improved outcome, the literature does not support a linear relationship between the degree of response to therapy and OS. In a cohort of 168 patients with gastric cancer treated with preoperative chemotherapy only at Memorial Sloan Kettering Cancer Center, Mansour and colleagues found that histological response grade did not independently predict disease-specific survival [12]. Similarly, in a Japanese cohort of 70 resected gastric cancer patients given preoperative chemotherapy alone, histological response grade was neither independently associated with OS for all patients nor associated with OS in the cohort of patients with “advanced” (N2-N3) disease. In these cohorts, only ypN status independently predicted OS. Of note, in the cohort of patients with N0-N1 disease (n = 35), response to therapy was independently associated with OS for patients who had less than 33% remaining viable tumor cells (HR 17.24, [95% CI 2.1–141.3]; P = 0.008) [23]. In addition, a retrospective study of 45 patients given MAGIC trial regimen chemotherapy (epirubicin, cisplatin, and 5-fluourouracil/capecitabine) demonstrated that patients with major response to chemotherapy (<10% residual viable tumor; n = 9 [17.3%]) experienced 5-year disease-specific survival time of 47.6 months compared with 16.8 months in patients with lesser response to therapy. However, this finding was not significant (P = 0.53), perhaps because of the limited sample size [24]. Finally, a study of a large cohort of 850 patients with predominantly gastroesophageal junction tumors demonstrated significant association of TRG with tumor grade, clinical response to therapy, and Lauren classification but not an independent association of it with OS [11].
Few studies have investigated the relationship between response of gastric cancer to therapy and recurrence. A 2011 retrospective review of gastric cancer patients at Memorial Sloan Kettering Cancer Center who underwent chemotherapy with and without CXRT followed by resection (n = 609) found that the 5-year overall risk of recurrence for patients with pCR (n = 60) was significantly less than that for patients with residual viable tumor (27% vs. 51%; P = 0.01 [log-rank test]). The reviewers also found no difference in the pattern of recurrence—43% for LR and 57% for DR—between the two groups. Of note, 6 of 14 patients with pCR had LR, all of whom had received CXRT [9]. In the review recently reported by Mokadem and colleagues, tumor regression data were available for 180 of 408 patients. Of these, 43 experienced complete or subtotal tumor regression, and the remaining 137 had partial or no tumor regression. Partial/no tumor regression was independently associated with RFS in a multivariable Cox regression analysis (HR, 2.63 [95% CI, 1.22–5.64]; P = 0.013). However, ypN+ status was the factor most strongly associated with RFS (HR, 4.92 [95% CI, 3.35–7.24]; P ≤ 0.001). Other independent factors associated with RFS included three (vs. more than six) cycles of preoperative chemotherapy, R1 resection, fewer than 15 lymph nodes examined, and diffuse-type gastric cancer[14]. In the present study, we performed a uniquely dedicated investigation of the degree of response of gastric cancer to therapy and recurrence. TRG did not independently correlate with LR, DR, or RFS. Instead, LR was predicted by R1 resection, DR was predicted by ypN status and the presence of linitis plastica, and RFS was predicted by all three. Our results therefore add to the growing body of literature regarding the importance of ypN status in gastric cancer patients receiving preoperative therapy and corroborate studies demonstrating the importance of linitis plastica and histological subtype in determining the risk of gastric cancer recurrence [25–27].
Using the National Cancer Database, we previously demonstrated that CXRT leads to higher rates of primary tumor pCR than does chemotherapy alone [28]. Because all patients received preoperative CXRT in this study, it provides important insight into the relationship between response to CXRT and local control. No LR occurred in patients with TRG0, indicating excellent local control when pCR occurs. However, the rate of LR did not vary among the TRG1, TRG2, and TRG3 groups, nor did the rate of R1 resection. This may indicate that only patients who have primary tumor pCR (TRG0) experience a local benefit from preoperative CXRT. This is a clinically significant finding insofar as the rationale for CXRT is often predicated on perceived improvement in local control. Thus the role of preoperative CXRT in patients with resectable gastric cancer will continue to generate significant debate pending the results of two forthcoming randomized trials poised to address this important question (TOPGEAR and CRITICS-II) [29,30]. In addition, future studies with specific attention to molecular profiling may allow us to identify a priori which patients are likely to respond to preoperative CXRT.
The present study has some limitations in addition to the risk of selection bias inherent in its retrospective design. We extracted TRG data directly from medical records, not from a re-review of specimens specifically for this study. Although specialized gastrointestinal pathologists performed all pathology, tumor regression grading may be subjectively dependent on the pathologist. There is a risk of sampling error when determing pathologic response; however, as a percentage of viable cells in the entire reviewed section, TRG is likely to be representative of the entire tumor and therefore less subject to sampling error than traditional measurements like ypT category. The specific percentage of viable tumor cell values selected to define TRG0–3 groups in this study is novel, and may render comparison with the established literature more challenging. However, the system used is very similar to the one recommended by the College of American Pathologists and the one established by Becker et al [31,32]. Our values were carefully selected to differentiate between patients with pCR (TRG0) and those with near-complete response (TRG1).
We excluded patients who received chemotherapy alone as preoperative treatment to avoid potential confounding factors related to the local effects of CXRT and because evidence demonstrates that CXRT induces primary tumor pCRs more frequently than does chemotherapy alone [9,28]. Although TRG does not correlate with LR, DR, or RFS in patients who have received CXRT, we cannot draw this conclusion regarding patients who receive preoperative chemotherapy alone based on the results of this study. In particular, because CXRT exerts a local effect on the primary tumor, TRG may correlate better with DR or RFS for patients who have received chemotherapy alone. In the latter scenario, TRG is the sole result of a systemic therapy to which all disease—both local and distant micrometastatic—is subjected.
In conclusion, the degree of response to preoperative CXRT as measured according to the TRG does not predict risk of recurrence of resected gastric cancer. However, local control is excellent when TRG0 is achieved. Therefore, strategies that increase the likelihood of primary tumor pCR are likely to improve local control of this cancer. Adherence to the maxim of margin-negative resection of gastric cancer remains critical to mitigating the risk of LR even as near-complete pathological response is approached, as the rate of R1 resection is similar among patients with TRG1–3. ypN status is the most important factor associated with DR and overall RFS in gastric cancer patients given preoperative therapy, and strategies to achieve ypN0 status are the most likely to improve overall outcomes in this patient population.
Supplementary Material
SUPPLEMENTARY FIGURE S1 Kaplan-Meier curve of DR for gastric cancer patients undergoing curative-intent gastrectomy after preoperative chemotherapy and CXRT stratified according to TRG.
SYNOPSIS.
It is unknown whether the degree of response to preoperative therapy—as quantified by tumor regression grade (TRG)—correlates with the risk of locoregional recurrence (LR) and distant recurrence (DR) after resection of gastric cancer. No patient with TRG0 experienced LR, indicating excellent local control. However, TRG did not otherwise correlate with LR or DR; instead R1 resection continues to define the risk for LR, and linitis plastica and ypN status define the risk for DR.
Acknowledgments
Grant support: Supported in part by the NIH/NCI under award number P30CA016672 and used the Clinical Trials Support Resource.
Footnotes
Disclosures: Prajnan Das has received an honorarium from Adlai Nortye, Ltd.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and regional) and with the Helsinki Declaration of 1975, as revised in 1983. This is a retrospective study of de-identified patients, no informed consent was required. Ethics approval for this work has been obtained.
REFERENCES
- 1.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 2.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. [DOI] [PubMed] [Google Scholar]
- 4.Greenleaf EK, Hollenbeak CS, Wong J. Trends in the use and impact of neoadjuvant chemotherapy on perioperative outcomes for resected gastric cancer: Evidence from the American College of Surgeons National Cancer Database. Surgery. 2016;159(4):1099–1112. [DOI] [PubMed] [Google Scholar]
- 5.Amin MB, Edge SB, Greene FL. AJCC Cancer Staging Manual. 8th ed New York: Springer; 2017. [Google Scholar]
- 6.Ikoma N, Blum M, Estrella JS, et al. Evaluation of the American Joint Committee on Cancer 8th edition staging system for gastric cancer patients after preoperative therapy. Gastric Cancer. 2018;21(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. [DOI] [PubMed] [Google Scholar]
- 9.Fields RC, Strong VE, Gonen M, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104(12):1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackham AU, Greenleaf E, Yamamoto M, et al. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J Surg Oncol. 2016;114(4):434–439. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt T, Sicic L, Blank S, et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer. 2014;110(7):1712–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansour JC, Tang L, Shah M, et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol. 2007;14(12):3412–3418. [DOI] [PubMed] [Google Scholar]
- 13.Ikoma N, Chen HC, Wang X, et al. Patterns of Initial Recurrence in Gastric Adenocarcinoma in the Era of Preoperative Therapy. Ann Surg Oncol. 2017;24(9):2679–2687. [DOI] [PubMed] [Google Scholar]
- 14.Mokadem I, Dijksterhuis WPM, van Putten M, et al. Recurrence after preoperative chemotherapy and surgery for gastric adenocarcinoma: a multicenter study. Gastric Cancer. 2019;22(6):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikoma N, Das P, Blum M, et al. Preoperative Chemoradiation Therapy Does Not Increase Risk of Anastomotic Leak in Patients With Gastric Cancer. Int J Radiat Oncol Biol Phys. 2017;99(3):660–666. [DOI] [PubMed] [Google Scholar]
- 16.Ikoma N, Agnes A, Chen HC, et al. Linitis Plastica: a Distinct Type of Gastric Cancer. J Gastrointest Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 17.ZG E, IG O, AS R, IB N, AB L Monogenec Arrhythmic Syndromes: From Molecular and Genetic Aspects to Bedside. Acta Naturae. 2016;8(2):62–74. [PMC free article] [PubMed] [Google Scholar]
- 18.D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240(5):808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9(4):394–400. [DOI] [PubMed] [Google Scholar]
- 20.Spolverato G, Ejaz A, Kim Y, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. J Am Coll Surg. 2014;219(4):664–675. [DOI] [PubMed] [Google Scholar]
- 21.Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229(3):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22(14):2774–2780. [DOI] [PubMed] [Google Scholar]
- 23.Fujitani K, Mano M, Hirao M, Kodama Y, Tsujinaka T. Posttherapy nodal status, not graded histologic response, predicts survival after neoadjuvant chemotherapy for advanced gastric cancer. Ann Surg Oncol. 2012;19(6):1936–1943. [DOI] [PubMed] [Google Scholar]
- 24.Mingol F, Gallego J, Orduna A, et al. Tumor regression and survival after perioperative MAGIC-style chemotherapy in carcinoma of the stomach and gastroesophageal junction. BMC Surg. 2015;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikoma N, Estrella JS, Hofstetter W, et al. Nodal Downstaging in Gastric Cancer Patients: Promising Survival if ypN0 is Achieved. Ann Surg Oncol. 2018;25(7):2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren Histologic Type Is the Most Important Factor Associated With Pattern of Recurrence Following Resection of Gastric Adenocarcinoma. Ann Surg. 2018;267(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stark AP, Ikoma N, Chiang YJ, et al. Characteristics and Survival of Gastric Cancer Patients with Pathologic Complete Response to Preoperative Therapy. Ann Surg Oncol. 2019;26(11):3602–3610. [DOI] [PubMed] [Google Scholar]
- 28.Ikoma N, Das P, Hofstetter W, et al. Preoperative chemoradiation therapy induces primary-tumor complete response more frequently than chemotherapy alone in gastric cancer: analyses of the National Cancer Database 2006–2014 using propensity score matching. Gastric Cancer. 2018;21(6):1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer. 2015;15:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slagter AE, Jansen EPM, van Laarhoven HWM, et al. CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer. 2018;18(1):877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253(5):934–939. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Sun Y, Hu S, et al. Comparison of five tumor regression grading systems for gastric adenocarcinoma after neoadjuvant chemotherapy: a retrospective study of 192 cases from National Cancer Center in China. BMC Gastroenterol. 2017;17(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE S1 Kaplan-Meier curve of DR for gastric cancer patients undergoing curative-intent gastrectomy after preoperative chemotherapy and CXRT stratified according to TRG.