Summary
Antimicrobial-resistant bacteria that are spreading all over the world have caused significant problems. While these problems are becoming bigger, the development of new antimicrobials has remained stagnant, and some essential antimicrobials for treatment may be depleted in the near future. This is a significant problem that may jeopardize the sustainability of healthcare itself. In Japan, the National Action Plan on Antimicrobial Resistance was established in April 2016. Based on the five strategic objectives set out in the World Health Organization's Global Action Plan on Antimicrobial Resistance, and by adding a 6th objective of international cooperation as a Japanese unique goal, the goals, strategies and specific actions of each of these six fields are presented. This Action Plan is significantly characterized by setting numerical targets as a performance index to be achieved by 2020.
Keywords: Antimicrobial resistance (AMR), surveillance, monitoring, prevention, public awareness, international cooperation
Introduction
Antimicrobial-resistant bacteria are spreading globally and have become a problem. However, while the issue of antimicrobial resistance (AMR) is becoming more significant, the development of new antibiotics has stagnated. It is estimated that if no countermeasures are taken, 10 million people will annually die of AMR by 2050, which would largely exceed the number who die of cancer. To establish a sustainable healthcare environment, we have to take countermeasures against AMR now to ensure the continuous use of antibiotics in the future.
At the World Health Assembly in May 2015, a Global Action Plan to tackle AMR was endorsed. All member states are urged to develop and establish a national action plan on AMR within two years. In Japan, the National Action Plan on Antimicrobial Resistance was released in April 2016 (1). Japan's National Action Plan on Antimicrobial Resistance 2016-2020 includes "International Cooperation" as a unique 6th pillar additionally set in reference to the 5 pillars of the Global Action Plan, and a goal, strategy and countermeasures have been set for each area (Figure 1).
Figure 1.

Six areas and their Goals for Japan's National Action Plan on Antimicrobial Resistance (AMR). Japan's National Action Plan on Antimicrobial Resistance (AMR) 2016-2020 includes "International Cooperation" as a unique 6th pillar additionally set in reference to the 5 pillars of the Global Action Plan.
Current status and challenges of antibiotic treatment in Japan
The use of antibiotics in Japan is not particularly higher compared to that in Europe or the USA. However, in Japan oral antibiotics account for 92.4% of the total daily usage, and consists of third generation cephalosporins, macrolides and fluoroquinolone derivatives, which are so-called broad-spectrum antibiotics. Therefore, it is considered that the problem in Japan is the use of such broad-spectrum oral antibiotics (1). Higashi, et al. examined the national prescription database for the period from January to March 2005 and reported that approximately 60% of patients with non-bacterial upper respiratory tract infections were given antibiotics. The breakdown of the prescriptions was third generation cephalosporins (46%), macrolides (27%) and quinolones (16%) in descending order of frequency, and were prescribed more often at clinics than hospitals (2).
Antibiotics may be prescribed to patients with the common cold for the purpose of pneumonia prophylaxis. Regarding this, a study was conducted to investigate how many patients with acute respiratory tract infection, including those with the common cold, need to be treated with antibiotics to prevent one patient from developing complications including pneumonia (3). The results showed that one case of complications could be prevented if 4,000 patients with acute respiratory tract infection would be given antibiotics. Considering the cost of antibiotics prescribed to 4,000 patients and the risks of adverse drug reactions and resistant bacteria, the prophylactic use of antibiotics to prevent complications is not recommended because the demerits outweigh the benefits.
Background and Outline of the Ministry of Health, Labour and Welfare (MHLW) "Manual of Antimicrobial Stewardship (1st Edition)"
Under these circumstances, Japan first decided to promote the appropriate outpatient handling of conditions that do not normally require antibiotic treatment such as viral upper respiratory tract infections and infectious enteritis. To achieve this, appropriate medical handling has to be promoted based on an official guideline. Therefore, the MHLW compiled "Manual of Antimicrobial Stewardship (1st Edition)" (4). This manual is mainly created for healthcare professionals who provide consultations to outpatients. It targets patients who are of school age and older and who do not have concomitant diseases. The covered diseases are acute respiratory tract infections and acute diarrheal diseases, for which it is considered that unnecessary antibiotics are generally used based on the above-mentioned study. Children aged 5 years and under, babies and infants are excluded from the manual because particular clinical conditions need to be considered in such cases. Highly complicated cases that require an expert's judgement are also excluded from the manual, including those with concomitant diseases. For cases that are not covered by the manual, it is necessary to refer to the existing guidelines of academic societies or to consult experts.
Acute respiratory tract infection is a concept that includes acute upper respiratory tract infections (acute upper respiratory tract inflammation) and acute lower respiratory tract infections (acute bronchitis). For those conditions, such terms as the "common cold" etc. are generally used. Among these terms, "common cold" is used with various meanings from "acute upper respiratory tract infections" to "acute lower respiratory tract infections". In addition, patients often describe acute pyrexia, malaise and various feelings of being unwell as "I caught a cold". Therefore, when a patient presents with the complaint of "I caught a cold", it is important to examine the condition in detail, because a significantly wide range of diseases should be considered.
Viruses account for approximately 90% of the causative organisms of acute respiratory tract infections, and include the rhinovirus and coronavirus. Bacteria are involved in only a limited number of cases of acute respiratory tract infections, and in such cases, acute pharyngitis is mostly caused by group A β-hemolytic streptococcus (GAS) and acute bronchitis by Mycoplasma or Chlamydophila.
The American College of Physicians provides a useful classification to differentiate cases of acute upper respiratory tract infections that require antibiotics from those that do not (5). It classifies acute respiratory tract infections into four disease types, namely, the common cold (nonspecific upper respiratory tract inflammation, ordinary common cold), acute rhinosinusitis, acute pharyngitis and acute bronchitis, based on the three areas of symptoms such as nasal symptoms (nasal discharge, nasal congestion), pharyngeal symptoms (pharyngalgia) and lower respiratory tract symptoms (cough, sputum). In this manual explanations are also given based on this classification (Figure 2). The common cold is an acute viral respiratory tract infection in which symptoms of all three areas, namely, nasal symptoms (nasal discharge, nasal congestion), pharyngeal symptoms (pharyngalgia) and lower respiratory tract symptoms (cough, sputum) present "simultaneously" and "at a similar level", regardless of whether pyrexia is present or not. In the natural clinical course of the common cold, slight fever, malaise and pharyngalgia develop in the beginning, followed by nasal discharge and nasal congestion, and then by cough and sputum. Around the third day after onset the symptoms peak, and overall the symptoms subside in 7 to 10 days after the onset. However, coughing often remains for about three weeks with the common cold, and physicians should be aware of this. However, even if coughing persists, it does not necessarily mean that the clinical condition requires an antibiotic. On the other hand, if symptoms worsen as a deviation from the natural course, or in case of the re-exacerbation of once-subsided symptoms, the complication of secondary infection should be considered. This manual recommends that antibiotics should basically not be given to any acute respiratory tract infections if there are no complications. For example, this manual recommends not to give antibiotics to patients with the common cold.
Figure 2.
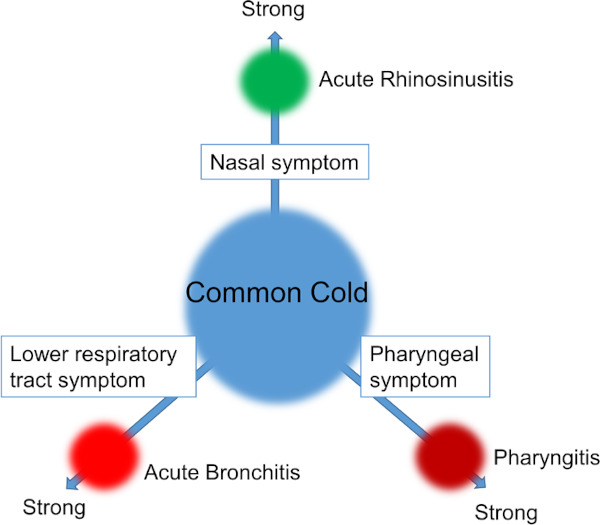
The four disease types of acute rhinosinusitis, acute pharyngitis and acute bronchitis (extracted from MHLW "Manual of Antimicrobial Stewardship (1st Edition)"(4). The common cold is an acute viral respiratory tract infection in which symptoms of all three areas, namely, nasal symptoms (nasal discharge, nasal congestion), pharyngeal symptoms (pharyngalgia) and lower respiratory tract symptoms (cough, sputum) present "simultaneously" and "at a similar level", regardless of whether pyrexia is present or not. MHLW, Ministry of Health, Labour and Welfare.
Knowledge, awareness and attitude of the public towards AMR
To control antimicrobial-resistant bacteria, it is necessary to encourage public understanding of the threat of AMR and to change the way antibiotics are handled. Concerning public awareness of AMR, an awareness survey of 3,390 people in Japan was conducted (6). The results of the survey indicated that 46.8% and 40.6% of respondents, respectively, believed that the following statements were correct: "antibiotics kill viruses" and "antibiotics are effective against the common cold and influenza", while 38.8% of all respondents were aware that antibiotics may cause adverse reactions. This figure was lower than that found with a preceding survey conducted in European Union countries in 2016 (Special Eurobarometer 445) (7). The results indicated that enlightenment on AMR is an urgent task in Japan. In the UK and Northern European countries, the effectiveness of such measures as active campaigns targeting the general public have been revealed.
Our "Antibiotics Awareness Survey 2018" conducted on 721 people nationwide in Japan revealed that many patients could not distinguish antibiotics from other medicines intended to control symptoms such as antipyretics or from antivirals. In addition, when the respondents were asked what medicine they would like to be prescribed for the common cold, the most common answer was an antitussive, followed by an antipyretic, medicine that suppresses nasal discharge, and antibiotics followed as the fourth most common answer (8). The above-mentioned Special Eurobarometer 445 revealed that accurate knowledge of AMR has been spreading among the public over time (7). The joint committee of the Japanese Society of Chemotherapy and the Japanese Association of Infectious Diseases conducted a nationwide questionnaire survey of clinic physicians in February last year, and 50.4% of the respondents stated that when a patient with a common cold or his/her family requests an antibiotic to be prescribed, "I would prescribe (an antibiotic) if they are not convinced by my explanation" (9). There is also a study that revealed that physicians tend to feel that "the patient wishes to receive an antibiotic" when a patient is not convinced by the treatment options the physician offers. These facts indicated that there are often gaps between patient's desire and physician's considerations, and that antibiotics are prescribed as a result.
Courteous explanation to patients will change the medical care for acute respiratory tract infections
In this context, an effective explanation to patients is drawing attention. It is known that accurate explanations to patients with acute respiratory tract infections increase their satisfaction. Giving only dismissive explanations such as "It is a viral infection. There are no effective treatment options available" or "You don't need antibiotics" could cause dissatisfaction. Positive explanations such as "I will prescribe medication to alleviate your symptoms" or "Lukewarm drinks will ease your nasal congestion" tend to be better accepted. When comparing three ways of explaining; positive explanation alone, dismissive explanation alone and both types of explanations, the patients who were given both types of explanations received fewer prescriptions of antibiotics and showed a higher level of satisfaction.
Since there is great concern about whether it will be possible to change medical practice, we hereby present some encouraging facts. First, there is a study with positive results for Japan. The study was conducted on 3,390 people in Japan, and 58.9% answered that the knowledge they acquire about antibiotics and AMR will change their behavior (8). This percentage is much higher than that seen in a similar study conducted in Europe. This result indicates that there is a high probability that people in Japan will change their behavior by acquiring knowledge. Therefore, we, as healthcare professionals must carefully consider how information should be delivered to individuals when conducting awareness-raising activities. Second, when Japan's medical fee was revised in April 2018, additional fee points were set out to support the appropriate use of antibiotics for pediatric patients. Last, we would like to introduce a preceding study. The study was conducted during a four-month period from October 2004 among physicians from 5 clinics in Japan on 691 adults with acute respiratory tract infections (excluding influenza) without underlying diseases. The breakdown of conditions included nonspecific upper respiratory inflammations in 80%, acute rhinosinusitis in 2%, acute pharyngitis in 13% and acute bronchitis in 5%. It was reported that when the physicians conducted examinations and treatment of those patients in accordance with the American College of Physicians guideline, 5% required antibiotics at the time of the initial consultation, and 2% needed antibiotics later (10).
It is possible to change people's mindset and medical practice if it is supported by medical fee revision. These facts showed that Japan's environment is considerably favorable to achieve changes of the examination and treatment practice of acute respiratory tract infections.
Research and Surveillance: Introduction of Japan Surveillance for Infection Prevention and Healthcare Epidemiology (J-SIPHE)
To accurately present the current AMR situation in Japan and to ensure measuring the progress of the action plan, appropriate statistics are essential. In the future, AMR countermeasures must also be promoted in hospitals, clinics and other healthcare settings than hospitals, such as facilities for elderly people and in the home care setting. Data sharing in these places will also be required.
Japan has a surveillance system that monitors the status of AMR and healthcare-associated infections called Japan Nosocominal Infections Surveillance (JANIS) (11). This in principle is the inpatients data from medical institutions with inpatient wards. In addition, Japan Antimicrobial Consumption Surveillance (JACS) was established by the Science Research Grant Project that serves as the database of antibiotics consumption in hospitals (12). Besides establishing these systems, owing to the efforts made by our pharmacists in Japan, it is now possible to calculate the standardized usage of antibiotics at many medical institutions.
One common problem with the conduct of surveillance is the burden placed on the individuals who are in charge. Developing a surveillance method that lowers the burden on healthcare professionals is possible by digitalization and automation of data collection and analysis by using available healthcare information such as electronic medical charts and NDB. It is necessary to carefully consider the way the surveillance is conducted. For example, regarding the appropriate use of antibiotics, surveillance was conventionally conducted based on the consumption of antibiotics at each medical institution. However, it is difficult to determine whether antibiotics are used appropriately based only on their consumption levels. To evaluate the appropriate use of antibiotics, we also need another surveillance that investigates the appropriate use of the antibiotics as such, and that means to collect indicators of the process and outcomes of medical practice for infectious diseases.
Therefore we have the Japan Surveillance for Infection Prevention and Healthcare Epidemiology (J-SIPHE), which is a platform for surveillance data of infectious disease countermeasures (Figure 3).
Figure 3.
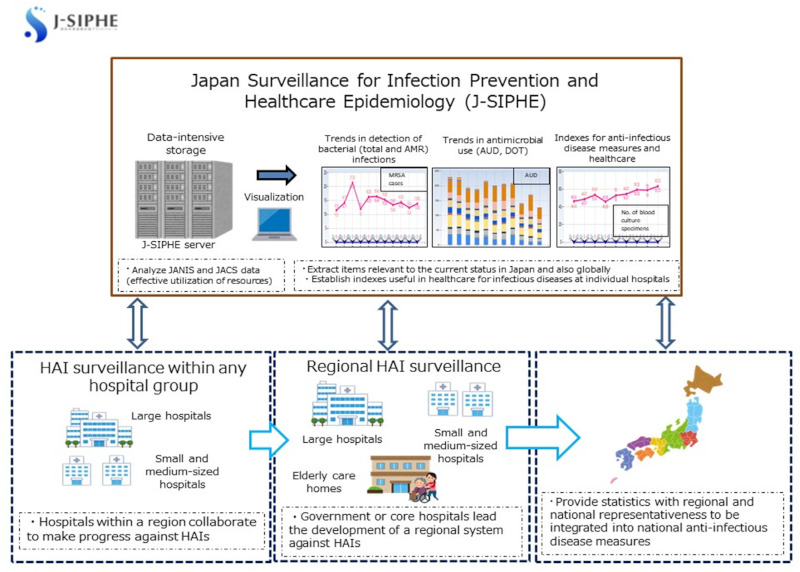
Japan Surveillance for Infection Prevention and Healthcare Epidemiology (J-SIPHE). The Japan Surveillance for Infection Prevention and Healthcare Epidemiologyh is a platform for surveillance data of infectious disease countermeasures.
The most significant characteristic of J-SIPHE is that it imports the information returning from JANIS to each medical institution and the data in the same format as that registered to JACS. It also collects data that have already been collected at each medical institution as part of nosocomial infection measures and/or the promotion of appropriate antibiotics use, and presents the data in an easily understandable manner for individuals who are in charge of the implementation of such measures. Another significant point is that these data can be shared with other medical institutions. J-SIPHE allows sharing of data within a randomly formed group of medical institutions. J-SIPHE started full-scale operations in January 2019.
Progress and challenges of the AMR action plan: Trend of antibiotics use
The AMR action plan sets numerical targets (Table 1). We now investigate the progress and challenges.
Table 1. Target value of antibiotics usage and drug resistant organism*.
| Index | 2020 (Target Value)** |
|---|---|
| Proportion of penicillin-resistance in Streptococcus pneumoniae from CSF sample | ≤ 15% |
| Proportion of penicillin-resistance in Streptococcus pneumoniae from samples other than CSF | |
| Proportion of fluoroquinolone resistance in Escherichia coli | ≤ 25% |
| Proportion of methicillin resistance in Staphylococcus aureus | ≤ 20% |
| Proportion of carbapenem resistance in Pseudomonas aeruginosa (Imipenem) | ≤ 10% |
| Proportion of carbapenem (Meropenem) resistance in Pseudomonas aeruginosa | ≤ 10% |
| Proportion of carbapenem (Imipenem) resistance in Escherichia coli | ≤ 0.2% |
| Proportion of carbapenem (Meropenem) resistance in Escherichia coli | ≤ 0.2% |
| Proportion of carbapenem (Imipenem) resistance in Klebsiella pneumoniae | ≤ 0.2% |
| Proportion of carbapenem (Meropenem) resistance in Klebsiella pneumoniae | ≤ 0.2% |
*Data extracted from Japans National Action Plan on Antimicrobial Resistance 2016-2020 (1). **Proportion of resistant isolates of specific indicator microorganisms in humans (%).
In the action plan, it is stipulated that use must be reduced to less than 2/3rds of that in 2013. An interim report of sales volumes of antibiotics in the medical care setting has become available. The sales volume for 1,000 individuals times the daily sales volume (DDD/1,000 inhabitant days = DID) was calculated by correcting the volume of the antibiotics, the WHO Defined Daily Dose (daily use by a person weighing 70 kg). The total sales volume of antibiotics was 14.9 DID in 2013, 14.5 DID in 2014, 14.6 DID in 2015, 14.6 DID in 2016, 13.8 DID in 2017 and 13.3 DID in 2018, showing a 10.7% reduction in 2018 compared to 2013 (13). However, the use of injectable antibiotics increased slightly during the same period of time.
The use of antibiotics in the dental care setting has also been revealed. The AMR clinical reference center tabulated the use of antibiotics that were prescribed in the dental care setting for the period from 2013 to 2016 by using the National Database of Health Insurance Claims and Specific Health Checkup of Japan (NDB), and disclosed it. It revealed that the usage of antibiotics in the dental care setting was less than 1/10th that in the medical care setting, that oral antibiotics accounted for a significant majority, and that other β-lactam antibiotics than penicillin were most frequently used (14).
In the process of implementing the action plan, the use of antibiotics in medical care in Japan has been revealed (15). Major peaks of the use of oral and injectable antibiotics lie in pediatric care for those aged 0 to 9 years and in geriatric care for those aged 70 years and older. However, it is characteristic of Japan that there is another low peak seen in those aged from 30 to 34 years. People around these ages tend to have a low possibility to be affected by diseases that require antibiotics. Therefore, it is assumed that there is some situation in which those people would require antibiotics. In addition, another study calculated the total use of antibiotics stratified by age for children aged 15 years and younger (16). Among these children aged from 0 to 15 years, the highest total use of antibiotics was found among those aged 1 year. Immunity is not fully developed in children around that age and they are considerably susceptible to acute respiratory tract infections including the common cold. The above-mentioned results are therefore considered to reflect such a tendency. The use of injectable antibiotics is particularly characteristic. The highest use of injectable antibiotics is seen among those aged 85 years and older (15). It is considered that when people of this age contract an infection, they are more frequently hospitalized and then receive treatment with injectable antibiotics.
It is considered a favorable trend that the total sales volume of antibiotics had decreased by 10.7% by the end of the third year of the five-year action plan. However, close investigation of the causes of the slight increase in the use of injectable antibiotics is necessary. When the appropriate use of antibiotics is promoted, it is occasionally observed that the use of antibiotics actually increased in medical institutions. This is considered because the dosage and regimen are followed appropriately. Injectable antibiotics are mainly used for inpatients. Promotion of the appropriate use of injectable antibiotics for inpatients shows actual difficulties due to the various individual inpatient conditions. For example, a decrease in oral antibiotics use can be promoted by a clear strategy such as "to reduce the administration of unnecessary antibiotics". However, since the conditions of inpatients vary, it is not an easy task to identify unnecessary administration of antibiotics. In the future, specified measures need to be taken by defining the meaning of "appropriate use" in detail and clarifying the target population for the appropriate use of antibiotics. Regarding the evaluation index, discussion is needed on whether use should be continued or another index be employed
Progress and challenges of the AMR action plan: Trend of resistant bacteria
With regard to the trend of resistant bacteria, Nippon AMR One Health Report (NAOR) 2018 (17) stated that "In Japan, the carbapenem resistance rate in Enterobacteriaceae, particularly Escherichia coli and Klebsiella pneumoniae remained below 1% during the observed period, despite its global increase in humans. Likewise, the proportion of vancomycin-resistant enterococci in humans remained less than 1%. Penicillin resistance (non-susceptibility rate) in Streptococcus pneumoniae also has been on the decline in recent years. While the criteria for assessing carbapenem resistance in Pseudomonas aeruginosa changed in 2014, the resistance rate was trending downward". On the other hand, it is also reported that "The rate of resistance against the third-generation cephalosporins and fluoroquinolones among Escherichia coli, however, was increasing. Although the percentage of methicillin-resistant Staphylococcus aureus (MRSA) has been declining in recent years, levels remained high" (17). Furthermore, the prevalence of fluoroquinolone-resistant E. coli (%) was compared between humans and animals using JANIS, which involves surveillance of the medical care setting and the Japanese Veterinary Antimicrobial Resistance Monitoring System (JVARM), which comprises nationwide monitoring of resistant bacteria involved in the livestock care setting. The results showed that although fluoroquinolone-resistant E. coli remained at a low level in the livestock care setting during the period from 2011 to 2015, it has been increasing in the human medical care setting year after year (18).
The possibility of transmission of resistant bacteria between humans and animals has been discussed (19). However, the prevalence in humans has been demonstrating an upward trend year by year compared to that in animals, which indicates that there is a unique situation in the human area and that the issue has not been resolved yet. Concerning this issue, further epidemiological research is necessary to explain the situation, and interventions based on the results are necessary.
Prepare for "import of resistant bacteria" via healthcare practice
A man aged 84 years was hospitalized in Cairo due to septic shock while staying in Cairo during his 15- day trip to Turkey and Egypt. His condition improved after receiving intensive care and he was transferred to the Center Hospital of the National Center for Global Health and Medicine. As it is well known that resistant bacteria are highly prevalent in countries surrounding the Mediterranean Sea including Egypt, specimens such as feces were investigated. The examination identified Klebsiella pneumoniae exhibiting a MIC of 4 μg/mL of imipenem, a carbapenem antibiotic. Further investigation revealed that this bacterium was Klebsiella pneumoniae that produced the enzyme OXA-48, which inactivates carbapenems (20). It is also known that there was an outbreak of this bacterium in European countries, particularly in the Mediterranean Sea area. Based on such factors as quarantine of the patient right from the start and efforts of healthcare professionals, no secondary or horizontal infections occurred. Since then, screening tests have been performed on all patients who had undergone health consultations or were hospitalized overseas within the previous one-year period. The results have revealed that approximately more than half of the patients had some resistant bacteria that required quarantine of the patient (21). In recent years, outbreaks of resistant bacteria that were brought from overseas have occurred in medical institutions nationwide one after another. The import of resistant bacteria through healthcare practice is a problem for the entire country of Japan. The Disease Control and Prevention Center developed and disclosed a guideline to prevent the import of such resistant bacteria (22).
Conclusion
In Japan, the Action Plan on AMR released in April 2016 prompted a vigorous debate, not only because of its high-level goals, but also because provided guidelines would significantly change the conventional concept of medical care. Three years have already passed since it was implemented, and there are areas that have steadily shown results. On the other hand, it became clear that there are areas that have hardly shown improvement such as fluoroquinolone-resistant and third-generation cephalosporin-resistant E. coli. To solve these issues, it is not adequate to take approaches only from the medical care point of view, and it is necessary to clarify the structure of problems from the One Health approach, and set effective countermeasures accordingly. We have also learned that the issue of resistant bacteria is inherently a fundamental problem of the healthcare/medical care system itself. Therefore, from this point onwards, we should not regard the issue of resistant bacteria only as a problem that is limited to the area of infectious diseases, but as a problem of the entire healthcare system, and eventually the entire society.
References
- 1. Antimicrobial Resistance (AMR) Action Plan. 2016. https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000138942.pdf (Accessed July 20, 2019).
- 2. Higashi T, Fukuhara S. Antibiotic prescriptions for upper respiratory tract infection in Japan. Intern Med. 2009; 48:1369-1375. [DOI] [PubMed] [Google Scholar]
- 3. Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007; 335:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manual of Antimicrobial Stewardship (1st Edition). 2016. https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000193504.pdf (Accessed July 21, 2019).
- 5. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016; 164:425-434. [DOI] [PubMed] [Google Scholar]
- 6. Kamata K, Tokuda Y, Gu Y, Ohmagari N, Yanagihara K. Public knowledge and perception about antimicrobials and antimicrobial resistance in Japan: A national questionnaire survey in 2017. PLoS One. 2018; 13:e0207017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Special Eurobarometer 445 Report Antimicrobial Resistance April 2016. https://ec.europa.eu/commfrontoffice/publicopinion/index.cfm/ResultDoc/download/DocumentKy/74168 (Accessed July 22, 2019).
- 8. Antibiotics Awareness Survey 2018. http://amr.ncgm.go.jp/pdf/20181026_ig_vol8_report.pdf (Accessed July 25, 2019) (in Japanese).
- 9. Gu Y, Fujitomo Y, Soeda H, Nakahama c, Hasegawa N, Maesaki S, Maeda) M, Matsumoto T, Miyairi I, Ohmagari N. A Nationwide Questionnaire Survey of Clinic Doctors on Antimicrobial Stewardship in Japan. Japanese Journal of Chemotherapy 2018; 66:205 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 10. Tomii K, Matsumura Y, Maeda K, Kobayashi Y, Takano Y, Tasaka Y. Minimal use of antibiotics for acute respiratory tract infections: validity and patient satisfaction. Inter Med. 2007; 46:267-272. [DOI] [PubMed] [Google Scholar]
- 11. Japan Nosocominal Infections Surveillance JANIS. https://janis.mhlw.go.jp/ (Accessed July 28 2019) (in Japanese).
- 12. Japan Antimicrobial Consumption Surveillance (JACS). https://www.jacs.asia/ (Accessed July 28, 2019) (in Japanese).
- 13. AMR Clinical Reference Center. Press Release: National antibiotics sales volume survey 2018 data released on March 15, 2019. http://amr.ncgm.go.jp/pdf/20190315_ig_vol9-pressrelease.pdf (Accessed July 30, 2019) (in Japanese).
- 14. AMR Clinical Reference Center. Antibiotics usage surveillance in the dental care based on National Database of Health Insurance Claims and Specific Health Checkup of Japan (NDB) 2019. http://amrcrc.ncgm.go.jp/surveillance/010/20190315dentist_NDB_fig_JAPAN.pdf (Accessed July 30, 2019) (in Japanese).
- 15. Yamasaki D, Tanabe M, Muraki Y, Kato G, Ohmagari N, Yagi T. The first report of Japanese antimicrobial use measured by national database based on health insurance claims data (2011-2013): comparison with sales data, and trend analysis stratified by antimicrobial category and age group. Infection. 2018; 46:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinoshita N, Morisaki N, Uda K, Kasai M, Horikoshi Y, Miyairi I. Nationwide study of outpatient oral antimicrobial utilization patterns for children in Japan (2013-2016). J Infect Chemother. 2019; 25:22-27. [DOI] [PubMed] [Google Scholar]
- 17. Nippon AMR One Health Report (NAOR) 2018. https://www.mhlw.go.jp/content/10900000/000530087.pdf (Accessed August 1, 2019).
- 18. Trends in the proportion (%) of Fluoroquinolones-resistant Escherichia coli [the proportion of antimicrobial resistance in humans and animals]. 2018. https://amr-onehealth.ncgm.go.jp/en/statistics/1198/ (Accessed July 30, 2019).
- 19. Suzuki S. Current status and future prospects of antimicrobial-resistant bacteria in Japan. Japanese Journal of Food Microbiology. 2018; 35:69-80. [Google Scholar]
- 20. Hashimoto A, Nagamatsu M, Ohmagari N, Hayakawa K, Kato Y, Kirikae T. Isolation of OXA-48 carbapenemase-producing Klebsiella pneumoniae ST101 from an overseas traveler returning to Japan. Jpn J Infect Dis. 2014; 67:120-121. [DOI] [PubMed] [Google Scholar]
- 21. Hayakawa K, Mezaki K, Sugiki Y, Nagamatsu M, Miyoshi-Akiyama T, Kirikae T, Kutsuna S, Takeshita N, Yamamoto K, Katanami Y, Ohmagari N. High rate of multidrug-resistant organism colonization among patients hospitalized overseas highlights the need for preemptive infection control. Am J Infect Control. 2016; 44:e257-e259. [DOI] [PubMed] [Google Scholar]
- 22. Guidance for medical institutions to countermeasure highly resistant bacteria imported from overseas. http://dcc.ncgm.go.jp/prevention/resource/resource05.pdf (Accessed August 1, 2019) (in Japanese).


