Abstract
Exposure to high doses of alkylating agents is associated with increased risk of impaired spermatogenesis among non-irradiated male survivors of childhood cancer, but there is substantial variation in this risk. Here we conducted a genetic study for impaired spermatogenesis utilizing whole genome sequencing data from 167 non-irradiated male childhood cancer survivors of European ancestry from the St. Jude Lifetime Cohort treated with cyclophosphamide equivalent dose (CED)≥4000 mg/m2. Sperm concentration from semen analysis was assessed as the primary outcome. Common variants (MAF>0.05) were adjusted for age at cancer diagnosis, CED, and top principal components. Rare/low-frequency variants (MAF≤0.05) were evaluated jointly by various functional annotations and 4-kb sliding windows. A novel locus at 7q21.3 containing TAC1/ASNS was associated with decreased sperm concentration (rs7784118: P=3.5×10−8). This association was replicated in two independent samples of SJLIFE survivors of European ancestry, including 34 non-irradiated male survivors treated with 0<CED<4000 mg/m2 (P=3.1×10−4) and 24 male survivors treated with CED≥4000 mg/m2 and radiation therapy<40 Gray (P=0.012). No association was observed among survivors not exposed to alkylating agents included in the CED (P>0.29). rs7784118 conferred 3.48- and 9.73-fold increases in risk for clinically defined oligospermia and azoospermia and improved prediction of normospermic, oligospermic, and azoospermic states by 13.7%, 5.3%, and 21.7%. rs7784118 was associated with decreased testosterone level, increased levels of follicle-stimulating and luteinizing hormones, and 8.52-fold increased risk of Leydig cell failure. Additional research is warranted to determine how this SNP influences spermatogenesis and to assess its clinical utility in characterizing high-risk survivors and guiding intervention strategies.
Keywords: Impaired spermatogenesis, alkylating agents, childhood cancer survivors, whole-genome sequencing, ASNS, FOXC1
INTRODUCTION
Survival rates for childhood cancer have improved dramatically, with a current five-year survival probability of approximately 85%(1). However, many survivors are at risk of long-term therapy-related morbidity and mortality(2–7). In male survivors, radiotherapy and chemotherapy, in particular alkylating agents, used in cancer treatment may result in temporary, long-term, or permanent gonadal toxicity, which significantly impacts fertility(8). The alkylating agents produce adducts and cross-links in DNA and induce apoptosis through covalent binding of alkyl groups to cellular macromolecules(9). These result in death of stem spermatogonia and a block in their differentiation, which lead to prolonged reductions in sperm count and/or duration of azoospermia after cytotoxic therapy.
Using the Childhood Cancer Survivor Study (CCSS)(8), we have previously reported that male survivors aged 15 to 44 years, who were not surgically sterile, were 79% less likely to father a child compared to their siblings, with the risk strongly associated with testicular radiation of >7.5 Gray (Gy) and high cumulative cyclophosphamide dose (≥9360 mg/m2). In a report by the St. Jude Lifetime Cohort (SJLIFE) Study(4), approximately 66% of male survivors treated with alkylating agents and/or radiation therapy experienced gonadal toxicity. In another SJLIFE Study(10) including 214 non-irradiated male survivors exposed to alkylating agent chemotherapy, the prevalence of oligospermia or azoospermia was approximately 10% among those with a cyclophosphamide equivalent dose (CED) of <4000 mg/m2, which increased to 62% among those with CED≥4000 mg/m2. However, there was substantial variation in sperm concentration among survivors who received higher alkylating agent treatment, suggesting additional risk factors including genetic predisposition for greater cytotoxic damage in the testis and consequently decreased sperm concentration. Nevertheless, very little is known about the genetic factors that may influence fertility preservation after chemotherapy treatment among childhood cancer survivors.
To address this gap, we performed a genome-wide association (GWA) study using deep-coverage whole-genome sequencing (WGS) data in the SJLIFE study to comprehensively investigate the potential role of germline genetic factors on sperm concentration in non-irradiated adult male survivors of childhood cancer who had received high cumulative doses of alkylating agents. Genetic associations that achieved genome-wide significance were evaluated in two independent sets of samples from SJLIFE participants.
MATERIALS AND METHODS
Study population
The SJLIFE Study(11), initiated in 2007, is a retrospective cohort study with the prospective clinical follow-up and ongoing enrollment of survivors of childhood cancer treated at St. Jude Children’s Research Hospital (SJCRH) since 1962. Semen analysis was offered to men who had received gonadotoxic treatments (exposure to an alkylating agent, testicular irradiation [any dose], or hypothalamic–pituitary irradiation [≥40 Gy]). To be eligible for the current study, SJLIFE participants had to be ≥18 years of age, have semen analysis performed and have WGS data.
Whole-genome sequencing and quality control
To be included in the WGS(12), enrolled survivors were required to have consented for a SJLIFE clinical assessment before April 2016 and provided written consent for banking of a blood sample for use in future research. Survivors who had undergone allogeneic stem cell transplantation were excluded from sequencing. DNA was extracted from blood samples banked in the SJCRH biorepository using either the QIAamp DNA Blood Mini Kit (Qiagen cat#51106) or DNeasy Blood & Tissue Kit (cat# 69506). Following extraction, DNA concentration was fluorometrically measured using the Quant-it dsDNA Assay Kit (Life Technologies cat#Q33130) and DNA integrity verified visually by agarose gel electrophoresis (E-gel, Life Technologies, cat#G8008-01). Unique samples were barcoded and WGS was performed at the HudsonAlpha Institute for Biotechnology Genomic Services Laboratory (Huntsville, Alabama) using the Illumina HiSeq X10 sequencers to generate 360 million paired-ends reads, each 150 base pairs (bp) in length, for each sample. Whole-exome sequencing (WES) was performed internally at SJCRH by the genomics laboratory in the Department of Computational Biology using Illumina HiSeq 4000 sequencing platform and used for genomic concordance quality control (QC) checks for sample verification.
A total of 3026 DNA samples were sent to HudsonAlpha for WGS, and sequencing raw reads were received for all samples except for one that failed library preparation. Samples that failed the coverage QC, that is, >70% of coding exons covered at >20X, underwent “top off” to ensure the minimum coverage criteria were met. WES data of 3013 survivors were used to check for potential sample swaps and to confirm identification of coding variants from the WGS. A total of 19 samples failed additional QC filtering. The failures were attributed to contamination (n=5), gender discordance (n=7) and unexpected discordance with previously genotyped Affymetrix 6.0 data for the same sample leading to sample identity concerns (n=8). The mean coverage per sample was 36.8X.
Details of genotype data QC, mapping, variant identification and annotation among 3006 SJLIFE participants are described in the Supplementary Methods. Of the 2986 SJLIFE survivors with WGS data passing QC, sperm concentration was available for 742 male survivors who had not undergone vasectomy. No survivor underwent bilateral orchiectomy. We excluded 69 survivors receiving androgen treatment, leaving 673 survivors in the study. Of these, 529 survivors were of European ancestry based on their genotype data (Supplementary Figure 1 and Supplementary Methods), including 201 who had received alkylating agents without radiation, 219 who had received a combination of both alkylating agents and radiation therapy, and the remaining 109 who had not received alkylating agents with or without radiation therapy. Exposure to alkylating agents was calculated as CED(13) (Supplementary Methods). Among the 201 participants exposed to only alkylating agents, 167 received high CED exposure (≥4000 mg/m2), forming the discovery sample for the current GWAS. A flowchart of patient selection is shown in Figure 1. This study was approved by the institutional review board of the SJCRH and all participants provided a written informed consent.
Figure 1 |.

Flow chart of patient selection in the study.
Semen analysis
Procedures for sperm collection and semen analysis have been previously described(10). Briefly, sperm samples were collected by masturbation after a planned minimum of two days and a maximum of seven days of sexual abstinence and were processed within 30 minutes of collection following the WHO guidelines. Samples were allowed to liquefy and time to liquefaction was recorded. The fresh sperm sample was microscopically assessed before any freezing. If no sperm were detected, the sample was centrifuged, concentrated, and reassessed again before being classified as azoospermic. Specimens that contained more than zero and less than 15 million sperm/mL were classified as oligospermic and those with ≥15 million sperm/mL were classified as normospermic. In semen specimens that were not azoospermic, several additional characteristics including motility (%)(14), progressive motility (0-4)(15,16), and morphology (≥4% Kruger strict)(14) were assessed.
Hormone measurements
Serum testosterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) were measured using electro-chemiluminescent immunometric assay (Roche Cobas 6000 Analyzer; Roche Diagnostics, Indianapolis, IN); blood samples were drawn prior to 10 AM. Serum testosterone ≥250 ng/dL, FSH ≤11.6 IU/L, and LH ≤9.85 IU/L were considered normal. These cut-off values were validated using testosterone LH, and FSH levels measured in community controls who were not on sex hormone replacement (n=160; ages 18.3-62.7 years) (Supplementary Table 1). Specifically, the normal value for testosterone (≥250 ng/dL), also the suggested cutoff by the assay manufacturer, was between the 5th - 10th percentiles in controls and was consistent with clinical guidelines (17). The FSH of 11.6 IU/L and LH of 9.85 IU/L were the 97th percentile in controls and were used as the cutoffs in the absence of consensus regarding normal values for these hormones. Leydig cell failure (LCF) was defined as morning serum levels of total testosterone <250 ng/dL (or 8.67 nmol/L) and LH >9.85 IU/L(18). These hormone levels were examined as a secondary analysis following findings from the primary GWAS analysis of semen concentration. Among the 167 participants in the discovery sample, 128 (77%) survivors had their hormonal lab tests performed at the same time or within a week before the semen analysis. For 38 survivors, lab tests were performed more than a week (range: 1.01 to 7.20 years) before their semen analysis, and only one survivor had hormonal lab tests conducted almost a year after semen analysis.
Measurement of physical activity and dietary intake
Physical activity during the past seven days was assessed by asking survivors to complete the National Health and Nutrition Examination Survey Activity Questionnaire. Survivors were classified as physically active if they participated in vigorous physical activities, such as running, aerobics, wheelchair basketball, heavy yard work, or anything else that causes large increases in breathing or heart rate, for at least 150 minutes per week. Dietary intake was collected using a self-administered Blood 2005 Food Frequency Questionnaire asking about the usual dietary intake of 110 food items during the past 12 months. Diet was estimated using the Healthy Eating Index (HEI)-2010(19), which measures adherence to the 2010 Dietary Guidelines for Americans and has 12 components. For each component, intake of foods and nutrients is represented on a density basis (per 1000 kcal). For adequacy components (foods recommended for increased consumption for healthy nutrition [total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, and fatty acids]), a score of 0 is assigned for no intake, and the scores increase proportionately as intake increases to the recommended level. For moderation components (foods recommended for limited consumption for healthy nutrition [refined grains, sodium, and calories from added sugars and saturated fats]), intake not exceeding the recommended level is assigned the maximum score and the score decreases as intake increases. The total HEI-2010 score ranges from 0 (nonadherence) to 100 (perfect adherence).
GWA analysis
Association analyses were performed as described previously(20). Common variants (~6.6 million) were examined using linear regression, assuming an additive genetic model, adjusting for age at cancer diagnosis, CED and the top 20 principal components (PCs). PCs are eigenvectors obtained from principal component analyses of genotype data to represent the genetic ancestry of individuals. We chose to adjust for age at childhood cancer diagnosis and CED following our previous study(10) of spermatogenesis, which identified their associations with impaired spermatogenesis (P<0.10). We also adjusted for the top 20 PCs to account for finer population stratification among survivors of European ancestry(20,21). Burden test and Sequence Kernel Association Test (SKAT) were used initially with the large-sample-approximate test whose top results followed by randomization test with 100 million permutations, to assess associations of rare/low-frequency variants (~10.8 million), adjusting for the same covariates used in the analysis of common variants above. Rare/low-frequency variants were aggregated into four analytical sets with respect to the RefSeq gene model (protein truncating variants, non-synonymous-strict variants, non-synonymous-broad variants and regulatory variants), and 4-kb sliding window across the genome (Supplementary Methods). Common variants showing associations with P<5×10−8 were considered statistically significant at the genome-wide level. Rare/low-frequency variant associations were considered significant at P<9.6×10−7, accounting for the 26290 analytical variant sets created with respect to the RefSeq gene model and the two tests performed for each set. For the sliding window approach, we considered two statistical tests for 666920 contiguous non-overlapping windows, leading to a significance threshold at P<3.7×10−8.
Replication samples, analyses and meta-analysis
We utilized two replication datasets, both consisting of SJLIFE participants but independent of the discovery sample. The primary replication sample (n=34) fulfilled the same eligibility criteria as the participants in the discovery analysis, except that they were exposed to low doses of alkylating agents (CED<4000 mg/m2). The secondary replication sample (n=24) also fulfilled the same eligibility criteria as the survivors in the discovery sample, except they were also exposed to radiation therapy. To minimize the radiation exposure influences, we excluded: 1) survivors with radiation exposure to body regions previously known to have an impact on sperm concentration, namely, testes, brain/head, spine, pelvis, abdomen or total body irradiation(8,10,22); and 2) survivors who had a maximum radiation dose of ≥40 Gy to any body region (Supplementary Methods).
Replication analyses employed the same statistical framework and adjustment covariates including age at diagnosis, CED and top PCs except associations were further adjusted for the maximum radiation dose in secondary replication analyses. We combined the association results from discovery and replication analyses using the inverse variance-weighted fixed effect model in METAL(23). Heterogeneity of allelic associations was examined using Cochran’s Q Test(24) and the I2 index(25). For variants showing evidence of heterogeneity (Phet<0.05), meta-analysis was carried out using the Han-Eskin random-effects model (RE2) implemented in METASOFT(26).
Estimation of clinical utility of genetic findings
We used a polytomous logistic regression to evaluate associations for azoospermia and oligospermia relative to normospermia, adjusting for the same covariates used in the discovery analyses including age at diagnosis, CED and top 20 PCs. To assess the potential clinical utility of the genetic findings, we obtained predicted probabilities of the three sperm-concentration states for each survivor based on the polytomous logistic regression model with and without the genetic variants. A sperm-concentration state with a prediction probability>0.5 was considered the predicted state (note a survivor with a prediction probability ≤0.5 would not have a predicted state). We then performed McNemar’s test for assessing correct prediction of each sperm-concentration state (normospermia, oligospermia, or azoospermia) with and without the genetic variants in the prediction model.
Genetic associations with respect to endocrine parameters, age, physical activity and nutrition
Serum hormone levels are indirect markers of gonadal function and hence we first examined genetic associations with levels of testosterone, FSH, LH, and LCF in the discovery sample, adjusting for the same set of covariates as the sperm concentration analysis including age at diagnosis, CED and top 20 PCs. Second, the associations between the top SNP and sperm concentration were further adjusted, one at time, for testosterone, FSH, and LH values, to assess if the genetic associations with sperm concentration were confounded by serum hormone levels. Finally, stratified analyses were carried out with respect to normal cut-off values of hormonal levels to assess potential context-specific genetic effects. In addition, we performed subgroup analysis by the median age at semen analysis in the discovery sample to evaluate potential age-specificity of the top SNP’s association with sperm concentration. We also assessed potential confounding on the genetic finding by physical activity and nutrition, by further adding them as covariates in the model.
Genetic effect among survivors of childhood cancer not exposed to alkylating agents
To assess the specificity of the genetic findings identified among survivors exposed to alkylating agents included in the CED, we examined the top SNP’s association in an independent group of childhood cancer survivors of European ancestry who were not exposed to alkylating agents. There was a total of 109 survivors who also met the study eligibility criteria, including 21 non-irradiated survivors and 88 who were exposed to radiation therapy. Analyses were adjusted for age at diagnosis and top PCs among 21 non-irradiated survivors and with further adjustment for radiation dose in 88 survivors also treated with radiation therapy.
Data availability
Aligned binary map files for all the 334 survivors and the joint genotype calls are accessible through the St. Jude Cloud (https://stjude.cloud). All data generated or analyzed during the study are included in the published article (and its supplementary information files). Summary statistics from the discovery sample are available from the corresponding author upon request.
RESULTS
Demographic and treatment characteristics of all participants included in the discovery and replication cohorts are provided in Table 1 which also shows the characteristics of SJLIFE male survivors who met all the eligibility criteria of the discovery cohort but did not have both WGS and semen data available. The median time from cancer diagnosis to semen analysis was comparable across both discovery (21.2 years) and replication samples (primary replication sample: 20.4 years; and secondary replication sample: 19.8 years). Among the 201 non-irradiated participants exposed to alkylating agents, 167 survivors with a high CED dose (≥4000 mg/m2) in the discovery sample had a significantly lower adjusted mean sperm concentration (−38.44 million sperm/mL; 95% CI=−59.18,−17.70; P=3.7×10−4) than the 34 participants with a low CED dose (<4000 mg/m2) in the primary replication sample (Supplementary Table 2). Among survivors in the discovery sample, those with normal sperm concentration were more likely to have normal motility, progressive motility and morphology compared to those with oligospermia, although the difference was statistically significant only for progressive motility (P=6.5×10−5) (Supplementary Table 3).
Table 1 |.
Sociodemographic and treatment characteristics of SJLIFE survivors
| Variables | Discovery sample (n=167) | Primary replication sample (n=34) | Secondary Replication sample (n=24) | Non-participants* (n=109) |
|---|---|---|---|---|
| Age at diagnosis (yrs.) | ||||
| Median (range) | 5.4 (0.0-20.3) | 8.8 (0.7-19.6) | 12.6 (0.0-19.0) | 12.2 (0.0-23.6) |
| <=5 | 77 (46.1%) | 6 (17.6%) | 4 (16.7%) | 32 (29.4%) |
| >5-10 | 35 (21.0%) | 13 (38.2%) | 5 (20.8%) | 13 (11.9%) |
| >10-15 | 35 (21.0%) | 8 (23.5%) | 7 (29.2%) | 32 (29.4%) |
| >15 | 20 (12.0%) | 7 (20.6%) | 8 (33.3%) | 31 (28.4%) |
| Age at assessment (yrs.) | ||||
| Median (range) | 28.5 (18.9-56.1) | 29.5 (20.1-53.3) | 32.0 (19.7-51.5) | NA |
| Elapsed time from diagnosis to assessment (yrs.) | ||||
| Median (range) | 21.2 (9.7-43.9) | 20.4 (10.5-37.8) | 19.8 (11.4-38.8) | NA |
| Cyclophosphamide equivalent dose (CED) (mg/m2) | ||||
| Median (range) | 8060 (4149-31894) | 2268 (938-3553) | 12834 (4288-30216) | 7996 (4161-30290) |
| Maximum radiation exposure (Gray) | ||||
| Median (range) | NA | NA | 29.5 (2.0-38.0) | NA |
| Primary cancer diagnosis | ||||
| Acute lymphoblastic leukemia | 66 (39.5%) | 9 (26.5%) | 1 (4.2%) | 38 (34.9%) |
| Non-Hodgkin lymphoma | 30 (18.0%) | 0 (0.0%) | 0 (0.0%) | 20 (18.3%) |
| Neuroblastoma | 24 (14.4%) | 20 (58.8%) | 1 (4.2%) | 8 (7.3%) |
| Osteosarcoma | 19 (11.4%) | 3 (8.8%) | 0 (0.0%) | 24 (22.0%) |
| Retinoblastoma | 6 (3.6%) | 0 (0.0%) | 0 (0.0%) | 2 (1.8%) |
| Acute myeloid leukemia | 4 (2.4%) | 0 (0.0%) | 0 (0.0%) | 3 (2.8%) |
| Ewing sarcoma family of tumors | 4 (2.4%) | 0 (0.0%) | 12 (50%) | 2 (1.8%) |
| Germ cell tumor | 3 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Melanoma | 3 (1.8%) | 0 (0.0%) | 0 (0.0%) | 1 (0.9%) |
| Soft tissue sarcoma | 2 (1.2%) | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) |
| Central nervous system (CNS) tumors | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 1 (0.9%) |
| Histiocytosis | 1 (0.6%) | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) |
| Hodgkin lymphoma | 1 (0.6%) | 0 (0.0%) | 9 (37.5%) | 3 (2.8%) |
| Liver malignancies | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Other leukemia | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 1 (0.9%) |
| Rhabdomyosarcoma | 1 (0.6%) | 1 (2.9%) | 0 (0.0%) | 3 (2.8%) |
| Physical activity | ||||
| Active | 121 (72.5%) | 20 (58.8%) | 12 (50.0%) | 62 (56.9%) |
| Inactive | 40 (24.0%) | 13 (38.2%) | 12 (50.0%) | 41 (37.6%) |
| Missing | 6 (3.6%) | 1 (2.9%) | 0 (0.0%) | 6 (5.5%) |
| Education | ||||
| 1-8 years (grade school) | 3 (1.8%) | 0 (0.0%) | 0 (0.0%) | 2 (1.8%) |
| 9-12 years (high school), but did not graduate | 11 (6.6%) | 4 (11.8%) | 2 (8.3%) | 5 (4.6%) |
| Completed high school/GED | 26 (15.6%) | 4 (11.8%) | 2 (8.3%) | 32 (29.4%) |
| Training after high school, other than college | 10 (6.0%) | 1 (2.9%) | 1 (4.2%) | 7 (6.4%) |
| Some college | 45 (26.9%) | 8 (23.5%) | 5 (20.8%) | 27 (24.8%) |
| College graduate | 35 (21.0%) | 9 (26.5%) | 9 (37.5%) | 22 (20.2%) |
| Post-graduate level | 18 (10.8%) | 4 (11.8%) | 3 (12.5%) | 1 (0.9%) |
| Other | 6 (3.6%) | 1 (2.9%) | 0 (0.0%) | 3 (2.8%) |
| Missing | 13 (7.8%) | 3 (8.8%) | 2 (8.3%) | 10 (9.2%) |
| Median household income | ||||
| Less than $19,999 | 19 (11.4%) | 4 (11.8%) | 1 (4.2%) | 15 (13.8%) |
| $20,000-$39,999 | 38 (22.8%) | 4 (11.8%) | 4 (16.7%) | 28 (25.7%) |
| $40,000-$59,999 | 32 (19.2%) | 7 (20.6%) | 6 (25.0%) | 16 (14.7%) |
| $60,000-$79,999 | 19 (11.4%) | 7 (20.6%) | 2 (8.3%) | 9 (8.3%) |
| $80,000-$99,999 | 9 (5.4%) | 1 (2.9%) | 4 (16.7%) | 7 (6.4%) |
| Over $100,000 | 19 (11.4%) | 5 (14.7%) | 6 (25.0%) | 9 (8.3%) |
| Don’t know | 24 (14.4%) | 3 (8.8%) | 1 (4.2%) | 16 (14.7%) |
| Missing | 7 (4.2%) | 3 (8.8%) | 0 (0.0%) | 9 (8.3%) |
| Employment | ||||
| Unable to work due to illness or disability | 6 (3.6%) | 1 (2.9%) | 2 (8.3%) | 6 (5.5%) |
| Never had a job, or not currently working or unemployed and looking for work | 8 (4.8%) | 1 (2.9%) | 0 (0.0%) | 1 (0.9%) |
| Caring for home or family (not seeking paid work), or student, or retired, or working part or full-time | 129 (77.2%) | 30 (88.2%) | 18 (75%) | 80 (73.4%) |
| Others | 4 (2.4%) | 1 (2.9%) | 0 (0.0%) | 1 (0.9%) |
| Missing | 20 (12.0%) | 1 (2.9%) | 4 (16.7%) | 21 (19.3%) |
| Distance to St. Jude Children’s Research Hospital (miles) | ||||
| <100 | 31 (18.6%) | 5 (14.7%) | 7 (29.2%) | 17 (15.6%) |
| 100-299 | 49 (29.3%) | 9 (26.5%) | 7 (29.2%) | 32 (29.4%) |
| 300+ | 82 (49.1%) | 20 (58.8%) | 9 (37.5%) | 56 (51.4%) |
| Missing | 5 (3.0%) | 0 (0.0%) | 1 (4.2%) | 4 (3.7%) |
Non-participants fulfilled the same criteria as survivors in the discovery samples, except they did not have both WGS data and semen analysis
All survivors in the discovery and replication samples and the non-participants are of European ancestry based on their genotype data.
Genetic variants associated with sperm concentration
Common variant analyses identified three SNPs showing genome-wide significance. The quantile-quantile plot and genomic inflation factor of 1.01 (Supplementary Figure 2) indicate no or little influence of population stratification on the association results. These three SNPs represent two different loci on chromosome 7, including two SNPs (rs10225965 and rs2237572) on 7q21.2 and another SNP (rs7784118) on 7q21.3 (Table 2 and Figure 2a). The minor allele (T) of the top SNP on 7q21.2 (rs10225965) was associated with increased sperm concentration (39.14 million sperm/mL per minor allele; 95% CI=26.25,52.03; P=1.9×10−8). Another SNP rs2237572 on 7q21.2 in very high linkage disequilibrium (LD) with rs10225965 (r2=0.98) also showed a similar association with sperm concentration. SNP rs7784118 on 7q21.3 was associated with decreased sperm concentration (−37.30 million sperm/mL per minor allele; 95% CI=−49.83, −24.77; P=3.5×10−8; Figure 2b). Of the 167 survivors, over 50% carried the minor allele of rs7784118, including 78 and 7 survivors who were heterozygotes and homozygous for the minor allele (Supplementary Figure 3). Analyses of 10.8 million rare/low-frequency variants identified CDC42BPG and 13 sliding windows as significantly associated with sperm concentration (Supplementary Table 4). However, none of these remained significant based on the 100 million permutations.
Table 2 |.
Genome-wide significant loci on chromosome 7q21.3 for sperm concentration and their results in independent replication samples
| Discovery sample (n=167) | Primary replication sample (n=34) | Secondary replication sample (n=24) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | BP | MA/OA | MAF | Crude mean sperm concentration (OO/OM/MM) | Change in sperm concentration, million/mL (95% CI) | P | MAF | Change in sperm concentration, million/mL (95% CI) | P | MAF | Change in sperm concentration, million/mL (95% CI) | P |
| rs2237572 | 92630946 | C/T | 0.21 | 21.93/51.28/86.71 | 38.45 (25.44,51.46) | 4.2×10−8 | 0.16 | −73.51 (−183.60,36.58) | 0.22 | 0.26 | −0.22 (−13.31,12.86) | 0.974 |
| rs10225965 | 92644264 | T/C | 0.20 | 21.52/53.22/86.71 | 39.14 (26.25,52.03) | 1.9×10−8 | 0.16 | −73.51 (−183.60,36.58) | 0.22 | 0.24 | 0.80 (−13.11,14.70) | 0.912 |
| rs7784118 | 97627780 | A/G | 0.28 | 52.26/17.99/6.00 | −37.30 (−49.83,−24.77) | 3.5×10−8 | 0.34 | −73.27 (−99.97,−46.58) | 3.1×10−4 | 0.36 | −14.88 (−25.11,−4.64) | 0.012 |
Chr, chromosome; Genomic positions are shown relative to GRCh38 (hg38); MA, minor allele; OA, other (major) allele; MAF, minor allele frequency; OO, survivors homozygous for other allele; OM, survivors with heterozygote genotypes; MM, survivors homozygous for the minor allele; CI, confidence interval
Figure 2 |.

Results from the GWA analysis showing (a) Manhattan plot for genome-wide associations with sperm concentration in the discovery sample. The horizontal axis shows the chromosomal position, and the vertical axis shows the statistical significance of the tested autosomal common variants, using multivariable linear regression. Genome-wide significant threshold (P=5×10−8) is shown as red horizontal line. LocusZoom plot of the novel risk locus for sperm concentration at the 7q21.3 locus showing association with the sperm concentration expressed as −log10(P value) obtained from analysis of common variants in the discovery sample is shown in (b). Results for the SNPs within +/− 500kb of the top SNP rs7784118 are shown. SNPs are shown as circles, with the top SNP represented by a purple diamond. All other SNPs are color coded according to the strength of LD with the top SNP (as measured by r2 in the European 1000 Genomes project data).
Replication results
Association of rs7784118 at 7q21.3 locus was replicated in both primary (−73.27 million sperm/mL per minor allele; 95% CI=−99.97,−46.58; P=3.1×10−4) and secondary (−14.88 million sperm/mL per minor allele; 95% CI=−25.11,−4.64, P=0.012) replication samples (Table 2); results were also consistent in the combined analyses (Supplementary Table 5). In contrast, the 7q21.2 locus did not show associations in both primary and secondary replication samples.
Effect of endocrine parameters, age, physical activity and nutrition on the 7q21.3-sperm concentration
We observed associations between the top SNP rs7784118 on the 7q21.3 locus with the levels of various hormones among the 167 survivors in the discovery sample. After adjusting for age at diagnosis, CED and the top 20 PCs, the minor allele showed associations with decreased testosterone level (−70.38 ng/dL; P=1.8×10−3) and increased levels of both FSH (4.60 IU/L; P=7.5×10−4) and LH (1.55 IU/L; 95% CI=1.08-2.02; P=1.2×10−3). Six out of the 167 survivors had LCF and the SNP rs7784118 showed a significant association with an increased risk of LCF (OR=8.52; P=0.0082).
The association between rs7784118 and sperm concentration remained consistent even after adjusting for serum levels of testosterone (−34.72 million sperm/mL per minor allele; P=4.9×10−7), FSH (−28.52 million sperm/mL per minor allele; P=1.2×10−6) and LH (−33.08 million sperm/mL per minor allele; P=7.3×10−6). These observations were further supported by the results from stratified analyses which showed the rs7784118 was predominantly associated with sperm concentration among survivors with normal hormone levels of testosterone (−44.34 million sperm/mL per minor allele; P=7.6×10−8), FSH (−45.48 million sperm/mL per minor allele; P=4.7×10−8), and LH (−57.67 million sperm/mL per minor allele; P=8.7×10−9) (Supplementary Table 6). Except nominal statistical significance among survivors with abnormal FSH levels (7.41 million sperm/mL per minor allele; P=0.017), rs7784118 showed no statistically significant association among those with abnormal hormone levels.
Adjusting for age at diagnosis, CED and the top 20 PCs, we found similar associations between the rs7784118 and sperm concentration in survivors above or below the median age of 28.5 years at semen analysis, −39.88 million sperm/mL per minor allele (P=9.6×10−4) and −33.05 million sperm/mL per minor allele (P=1.8×10−4), respectively. Further adjusting for physical activity and nutrition did not materially change the association between the rs7784118 and sperm concentration (−39.13 million sperm/mL per minor allele; P=6.0×10−8).
Potential clinical utility of the 7q21.3 locus
In the discovery sample, 73 were normospermic, 48 were oligospermic, and 46 were azoospermic. Mean sperm concentrations were 3.43 million sperm/mL (range=0.001-14.5) for survivors with oligospermia and 76.24 million sperm/mL (range=17.0-245.0) for those with normospermia. SNP rs7784118 conferred increased risk of oligospermia (OR=3.48; 95% CI=1.46,8.33; P=4.9×10−3) and azoospermia (OR=9.73; 95% CI=3.64,26.02; P=5.8×10−6), respectively. Notably, the model with SNP rs7784118 successfully predicted 22 (47.8%) of the 46 azoospermic survivors, compared to only 12 individuals (26.1%) by the model without the SNP (Figure 3), and this improvement was statistically significant (P=0.016). The proportion of predicted survivors with oligospermia was also higher, albeit not statistically significant (P=0.37), for the model with the SNP (20.8%), relative to that of the model without the SNP (14.6%). Of the 73 male survivors with normospermia, 55 (75.3%) were correctly predicted by the model with the SNP, whereas only 45 (61.6%) were predicted to be normospermic by the model without the SNP. The improvement in prediction of normospermia was also statistically significant (P=0.044). Results in survivors not exposed to CED showed that rs7784118 was not associated with sperm concentration in 21 non-irradiated survivors not exposed to CED (25.56 million sperm/mL per minor allele; P=0.29) and in 88 survivors exposed to radiation therapy but not exposed to CED (9.98 million sperm/mL per minor allele; P=0.52).
Figure 3 |.
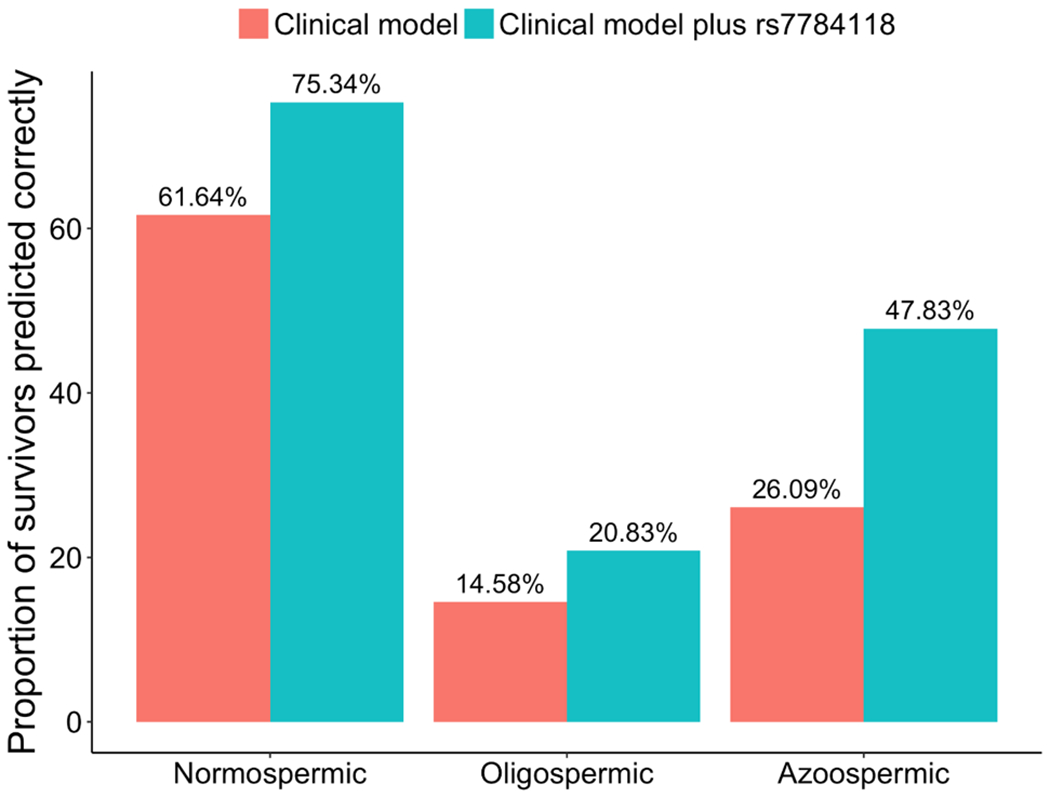
Proportion of male survivors in the discovery sample predicted (with >50% probabilities) to be normospermic, oligospermic or azoospermic by the clinical model including only non-genetic factors (age at diagnosis, CED and top principal components) and by the clinical model plus the top SNP rs7784118 at the 7q21.3 locus.
DISCUSSION
Expanding upon our previous findings(10), we performed the first comprehensive genetic investigation for impaired spermatogenesis in the high-risk clinical population of survivors of pediatric cancer, and identified a novel locus on chromosome 7q21.3 showing genome-wide significance with sperm concentration and replicated this finding in two independent samples. Our results showed appreciable improvement in the prediction of both impaired and normal spermatogenesis using the 7q21.3 locus, thereby supporting potential clinical utility in identifying patients at high-risk for impaired spermatogenesis. We also found a statistically significant (P=5.5×10−4) interaction between rs7784118 at the 7q21.3 locus and rs10225965 at the 7q21.2 locus in the discovery sample, but this finding was not replicated in either of the replication samples (both P>0.40). Further investigation in a larger sample size is required to accurately assess these non-additive genetic effects contributing to impaired spermatogenesis in survivors.
Magnitudes of the rs7784118 associations in the primary and secondary replication samples were approximately two-fold and half-fold, respectively, of that observed in the discovery sample. Furthermore, rs7784118 was not associated with sperm concentration in survivors of childhood cancer not exposed to CED, suggesting its specificity with decreased sperm concentration and impaired spermatogenesis in survivors exposed to CED. To assess this specificity more directly, we conducted an interaction test assessing a modification of the rs7784118 association with sperm concentration by alkylating agent exposure status. Specifically, we tested the SNP×CED-exposure interaction in a combined analysis of survivors exposed to CED (both discovery sample and primary replication sample) and those not exposed to CED: rs7784118 showed a significant interaction with CED exposure (beta = −36.06; P = 4.6×10−3). These data suggest rs7784118 may be associated with sperm concentration only in survivors with exposure to alkylating agents, although additional investigation in a larger sample is needed to confirm this observation and to further delineate the contribution of genetic risk factors and alkylating agents in impaired spermatogenesis.
While semen analysis remains the gold standard of fertility assessment, serum hormone levels provide an indirect assessment(27), although serum markers do not consistently correlate with sperm concentration(28). We demonstrate significant associations of rs7784118 with decreased levels of testosterone and increased levels of FSH and LH, the levels suggestive of LCF and consistent with gonadal injury(18,29). Our results show that the association between rs7784118 and sperm concentration was not confounded by serum levels of testosterone, FSH and LH, and the genetic association was observed chiefly among the survivors with normal hormone levels. The rs7784118’s association with sperm concentration was not modified by age at semen analysis, nor was it confounded by physical activity or nutrition. While residual confounding by factors we did not measure or adjust for, including unmeasured elements of physical activities and nutrition, these data suggest that the underlying mechanisms by which the 7q21.3 locus increases risk of impaired spermatogenesis in survivors are likely independent of the hormonal influence, physical activity and nutrition. Furthermore, rs7784118 showed 3.48-fold and 9.73-fold increased risk of oligospermia and azoospermia, respectively. The clinical prediction model with the rs7784118 genotype was able to correctly predict an additional 13.7%, 5.3% and 21.7% male survivors with normospermia, oligospermia and azoospermia, respectively, relative to the clinical model that did not include the SNP. However, our results of the clinical prediction model with the SNP should be interpreted with caution because performance of this model could not be evaluated in an independent cohort of survivors due to the unavailability of a comparable dataset.
SNP rs7784118 is located near TAC1 (tachykinin precursor 1) and ASNS (asparagine synthetase) and overlaps with the FOXC1 binding site. FOXC1 is a member of the forkhead family of transcription factors that is involved in a broad range of cellular and development of processes and certainly could influence spermatogenesis. ASNS converts aspartate and glutamine to asparagine and glutamate(30), influencing the glutamine - glutamate ratio and exchange between cells (referred to as the glutamine glutamate cycle). We speculate that ASNS could affect the germ cell supply of glutamine and influence spermatogenesis. Research is ongoing to elucidate the complete molecular mechanisms underlying the 7q21.3 association.
Limitations should be considered when interpreting the results of this study. GWASs have been very successful in identifying genotype-phenotype associations for thousands of complex traits and diseases over the past decade(31). These associations have provided insights into the genetic architecture of disease susceptibility, identification of new therapeutics, and risk prediction and optimization of therapies based on genotype. However, GWASs have limitations, such as their inability to identify all genetic determinants of complex traits, especially the rare variants, and the difficulty in defining the true causal associations. Sample size of this GWAS was not sufficiently large to identify genetic associations with modest effect size or with lower allele frequencies, and hence future GWASs are needed to discover rarer variants contributing to impaired spermatogenesis in male survivors of childhood cancer. Our study relied upon a single semen sample because of the logistics of the SJLIFE study, which requires many participants to travel long distances to the St. Jude Children’s Research Hospital for multiple days (average 3 days) of evaluation. However, a handful of survivors in the discovery sample had subsequent semen analysis and the results were generally consistent with those from the previous semen analysis used in this study (Supplementary Table 7). Our study population represents a highly selected group of long-term male survivors diagnosed and treated at a single institution, whose study eligibility was based on their previous cancer treatment (i.e., absence of radiation therapy and exposure to CED≥4000 mg/m2). Participation in the sperm analysis might have been affected by other factors such as history of established fertility, known infertility, inability or unwillingness to provide a sample, and being a SJLIFE participant, which may be affected by residing closer to St. Jude.
In summary, we identified a novel risk locus at 7q21.3 associated with sperm concentration in survivors of childhood cancer treated with alkylating agents. The top SNP rs7784118 also showed appreciable improvements on identification of male survivors with impaired spermatogenesis, and, if confirmed in independent datasets, the 7q21.3 locus may have potential utility in identifying male childhood cancer patients who are at increased risk of impaired spermatogenesis and guiding intervention strategies including pretreatment patient counselling and use of fertility preservation services. For genotype-informed high-risk male cancer patients treated with alkylating agents, options for preservation of fertility, including choice of less gonadotoxic chemotherapy regimens (as feasible), semen banking in postpubertal patients and cryopreservation of stem spermatogonia and/or testicular tissue in prepubertal patients for later use, may be discussed.
Supplementary Material
Statement of Significance:
The identified genetic markers harbor potential clinical utility in characterizing high-risk survivors and guiding intervention strategies including pretreatment patient counselling and use of fertility preservation services.
ACKNOWLEDGEMENTS/FUNDING
The St. Jude Lifetime Cohort (SJLIFE) study is supported by the United States National Cancer Institute (NCI) of the National Institutes of Health (NIH) (U01 CA195547: MMH and LLR, Principal Investigators; Cancer Center Support CORE grant CA21765: C. Roberts, Principal Investigator). This study is also supported by NIH/NCI R01 CA216354 (YY and JZ, Principal Investigators) from the National Cancer Institute and the American Lebanese Syrian Associated Charities, Memphis, Tennessee. We thank William H Kutteh, M.D., Ph.D. and Raymond W Ke, M.D. for providing the semen analysis data.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Howlader N NA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014, National Cancer Institute; Bethesda, MD, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 2.Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. New Engl J Med 2016;374:833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S, Sklar C. Second cancers in survivors of childhood cancer. Nat Rev Cancer 2002;2:124–32 [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA 2013;309:2371–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen JH, Moller T, Anderson H, Langmark F, Sankila R, Tryggvadottir L, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst 2009;101:806–13 [DOI] [PubMed] [Google Scholar]
- 6.Reulen RC, Frobisher C, Winter DL, Kelly J, Lancashire ER, Stiller CA, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 2011;305:2311–9 [DOI] [PubMed] [Google Scholar]
- 7.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2010;28:332–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meistrich ML. Male gonadal toxicity. Pediatr Blood Cancer 2009;53:261–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol 2014;15:1215–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidem Biomar 2017;26:666–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang ZM, Wilson CL, Easton J, Thrasher A, Mulder H, Liu Q, et al. Genetic Risk for Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. Journal of Clinical Oncology 2018;36:2078–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The Cyclophosphamide Equivalent Dose as an Approach for Quantifying Alkylating Agent Exposure: A Report From the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014;61:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department of Reproductive Health and Research W. WHO laboratory manual for the examination and processing of human semen. 5th edn World Health Organization; Geneva: 2010 [Google Scholar]
- 15.Bollendorf A, Check JH, Lurie D. Evaluation of the effect of the absence of sperm with rapid and linear progressive motility on subsequent pregnancy rates following intrauterine insemination or in vitro fertilization. J Androl 1996;17:550–7 [PubMed] [Google Scholar]
- 16.Comhaire FH, Vermeulen L, Hinting A, Schoonjans F. Accuracy of Sperm Characteristics in Predicting the Invitro Fertilizing-Capacity of Semen. J in Vitro Fertil Em 1988;5:326–31 [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society* Clinical Practice Guideline. J Clin Endocr Metab 2018;103:1715–44 [DOI] [PubMed] [Google Scholar]
- 18.Chemaitilly W, Liu Q, van Iersel L, Ness KK, Li Z, Wilson CL, et al. Leydig Cell Function in Male Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J Clin Oncol 2019:JCO1900738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, et al. The Healthy Eating Index-2010 Is a Valid and Reliable Measure of Diet Quality According to the 2010 Dietary Guidelines for Americans. J Nutr 2014;144:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sapkota Y, Cheung YT, Moon W, Shelton K, Wilson CL, Wang Z, et al. Whole-Genome Sequencing of Childhood Cancer Survivors Treated with Cranial Radiation Therapy Identifies 5p15.33 Locus for Stroke: A Report from the St. Jude Lifetime Cohort Study. Clin Cancer Res 2019;25:6700–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapkota Y, Turcotte LM, Ehrhardt MJ, Howell RM, Arnold MA, Wilson CL, et al. Genome-Wide Association Study in Irradiated Childhood Cancer Survivors Identifies HTR2A for Subsequent Basal Cell Carcinoma. J Invest Dermatol 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green DM, Zhu L, Wang M, Chemaitilly W, Srivastava D, Kutteh WH, et al. Effect of cranial irradiation on sperm concentration of adult survivors of childhood acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort Studydagger. Hum Reprod 2017;32:1192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics 1954;10:101–29 [Google Scholar]
- 25.Ioannidis JPA, Patsopoulos NA, Evangelou E. Heterogeneity in Meta-Analyses of Genome-Wide Association Investigations. Plos One 2007;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han B, Eskin E. Random-Effects Model Aimed at Discovering Associations in Meta-Analysis of Genome-wide Association Studies. Am J Hum Genet 2011;88:586–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelsey TW, McConville L, Edgar AB, Ungurianu AI, Mitchell RT, Anderson RA, et al. Follicle Stimulating Hormone is an accurate predictor of azoospermia in childhood cancer survivors. Plos One 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DM, Zhu L, Zhang N, Sklar CA, Ke RW, Kutteh WH, et al. Lack of Specificity of Plasma Concentrations of Inhibin B and Follicle-Stimulating Hormone for Identification of Azoospermic Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. Journal of Clinical Oncology 2013;31:1324–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson RA, Wallace EM, Groome NP, Bellis AJ, Wu FC. Physiological relationships between inhibin B, follicle stimulating hormone secretion and spermatogenesis in normal men and response to gonadotrophin suppression by exogenous testosterone. Hum Reprod 1997;12:746–51 [DOI] [PubMed] [Google Scholar]
- 30.Lomelino CL, Andring JT, McKenna R, Kilberg MS. Asparagine synthetase: Function, structure, and role in disease. J Biol Chem 2017;292:19952–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 2019;47:D1005–D12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aligned binary map files for all the 334 survivors and the joint genotype calls are accessible through the St. Jude Cloud (https://stjude.cloud). All data generated or analyzed during the study are included in the published article (and its supplementary information files). Summary statistics from the discovery sample are available from the corresponding author upon request.


