Summary
The advent of preoperative 3-dimensional (3D) simulation software has made a variety of unprecedented surgical simulations possible. Since 2004, we have performed more than 2,000 preoperative simulations in the University of Tokyo Hospital, and they have enabled us to obtain a great deal of information, such as the detailed shape of liver segments, the precise volume of each segment, and the volume of hepatic venous drainage areas. As a result, we have been able to perform more aggressive and complicated surgery safely. The next step is to create a navigation system that will accurately reproduce the preoperative plan. Real-time virtual sonography (RVS) is a navigation system that provides fusion images of ultrasonography and reconstructed computed tomography images or magnetic resonance images. The RVS system facilitates the surgeon's understanding of interpretation of ultrasound images and the detection of tumors that are difficult to find by ultrasound alone. In the near future, surgical navigation systems may evolve to the point where they will be able to inform surgeons intraoperatively in real time about not only intrahepatic structures, such as vessels and tumors, but also the portal territory, hepatic vein drainage areas, and resection lines that have been planned preoperatively.
Keywords: liver simulation, liver navigation, real-time virtual sonography (RVS), fusion imaging
Introduction
Hepatectomy is one of the most curative treatments for patients with various malignant liver tumors, but the high post-hepatectomy morbidity and mortality rates were once obstacles to the adoption of this choice of treatment. By the contribution of the establishment of surgical criteria (1,2) and the development of surgical devices (3-5), the surgical outcomes have improved over the last three decades.
Around the year 2000, one such advancement, 3-dimensional (3D) simulation software, was developed and facilitated understanding of intrahepatic structures by visualizing 3D imaging, even for novice hepatic surgeons. In addition, 3D simulation software makes it possible to calculate not only the volume of the liver as a whole but also the volume of future liver remnants or the areas perfused by intrahepatic vessels.
Since 2004, more than 2,000 preoperative simulations have been performed at our institution, and the additional information they have yielded has provided surgeons with new knowledge that has enabled safer surgery. The next step in preoperative simulation, intraoperative navigation may help surgeons intraoperatively to perform liver resections as planned, and it may be necessary to perform safer liver resection.
In this article, we review achievements that have been made with this 3D simulation system and consider the future development of intraoperative navigation systems.
Identification of the shape of liver segments
Couinaud's description of liver anatomy (6), which is based on portal vein branching, has been widely used by hepatologists and surgeons. Anatomic liver resection (7,8), i.e., removal of portal venous territory, was proposed to spare the future liver remnant without impairing oncological outcomes for patients with hepatocellular carcinoma (HCC). Anatomic liver resection is sometimes required even in patients with colorectal cancer liver metastasis (CRLM) to avoid a large ischemic area when the portal vein adjacent to the tumor is resected. The first step in anatomic liver resection is identification of the boundaries of the segment. Makuuchi et al. reported a dye staining technique in which the portal vein is punctured and indigo-carmine is injected under ultrasonographic guidance (9) , and clamping or dividing the corresponding portal vein makes it possible to visualize the boundaries of the segment as a demarcation line (10,11). However, these methods only allow visualization of the portal territory on the liver surface, and the border deep in the liver parenchyma is unclear. Injection of the objective portal vein with an ultrasound contrast-enhanced agent and ultrasound examination of the area of the parenchyma stained has been reported as a means of identifying the boundary of the liver parenchyma (12,13).
After 3D simulation software became available, it became possible to calculate the shape of a segment by considering the portal vein dominant area. Shindoh et al. used 3D simulation software and were the first to identify the shape of intersegmental planes and show that intersegmental planes are not always flat (14). They found that the right portal scissura is not always flat, but appeared to have a concave shape when viewed from the dorsal side in 54% of the cases examined. Anatomical liver resections may be incomplete, if surgeons do not pay attention to the shape of segmental borders, and the same technical pitfall has been reported in the use of right lateral sector grafts during living donor liver transplantation (LDLT) (15,16). The 3D simulation validation re-realized that intersegmental plane always located along the hepatic vein, and exposing the landmark vein was important for accurate resection (14). Indocyanine green (ICG) fluorescence imaging has recently been used as a staining method to improve identification of segmental borders (17-19). Because the ICG fluorescence persists for a long time during liver parenchyma transection, it is possible to correctly identify the shape of liver segments intraoperatively.
It is especially difficult to identify the 3D shape of segment 1 without using simulation software, because the anatomy of the portal vein perfusing segment 1 is variable, and, usually, any major hepatic vein runs on the border of the territory. Although caudate lobectomy can be performed by using the counterstaining technique to identify the boundary of a segment (20,21), additional information regarding the 3D shape of segment 1 obtained preoperatively using 3D simulation software may play a significant role in identifying the shape of segment 1. Maki et al. stated that the acaval vein runs between the paracaval portion and segment 7 or 8 in 48% of the cases (22). 3D simulation also demonstrated the cranial margin of the paracaval portion, which reached the diaphragmatic surface in 30% of the cases, and it alerted the surgeon to the fact that if the root of the right hepatic vein or middle hepatic vein were exposed during segmentectomy of segment 7 or 8, the paracaval portion might be resected at the same time (22).
Volumetry of portal territory
Preoperative volumetry is now recognized as a technique that is essential to ensuring a safe hepatic resection by estimating the volume of the future liver remnant. Makuuchi et al. established criteria for maximum resection volume classified according to the ICG retention rate (1), and Kubota et al. established indications for portal vein embolization according to the volume of the future liver remnant (2). Liver volumetry had conventionally been achieved by manually tracing computed tomography (CT) images and dividing the liver parenchyma along the lines formed by the routes of the hepatic veins (2,23). This technique allowed limited portal territories, such as the anterior sector, posterior sector, segment 4, and left liver, which are partitioned by hepatic veins or the falciform ligament, to be calculated, but accurate calculation of the volume of Couinaud segments (6) is impossible because of the loss of clear dividing lines in the liver (2,24).
After the development of 3D simulation software, simply creating 3D images made automatic liver volumetry possible, and it also became possible to measure liver volume corresponding to any branch of the portal vein. Several studies reported finding that the volume of segments calculated with the simulation software correlated well with the weight of the actual resected specimens (19,25-28). Mise et al. reported a detailed analysis of Couinaud segment volumes based on their study of 107 LDLT donors (29). They found that segment 8 was the largest segment, occupying a quarter of the whole liver (almost 26%), that it was followed by segment 7 (almost 17%), and that these volumes were comparable to one section of left liver (1). In addition, since the volume of each segment varies significantly, it is important to estimate the volume of the hepatic remnant in each individual case.
Moreover, anatomic resection of the territory of the portal triad branches distal to the Couinaud segments may be optional. The hepatectomy procedure in each patient should be planned according to the balance between the hepatic functional reserve and estimated volume of the hepatic remnant in that patient. Precise volumetry measurements of portal territories with simulation software may aid in planning an accurate operative strategy.
Hepatic vein anatomy and criteria for hepatic venous reconstruction
The postoperative impact of hepatic congestion that results from resecting hepatic veins was unknown until the early 2000s. Although a venous communication may be formed after hepatectomy combined with resection of hepatic vein (30,31), it is unclear whether this phenomenon occurs in all cases. It has also been reported that portal regurgitation occurs in congested areas (32), and that such areas are susceptible to necrosis (33). Akamatsu et al., on the other hand, found that adequate venous drainage during the first month after liver transplantation was important for liver regeneration (34). Mise et al. demonstrated safe standards for venous reconstruction criteria (35). In their study, hepatic vein reconstruction was required, if non-congested future liver remnant was not maintained at least 40-50% of total liver volume, and it reduced surgical invasiveness without influencing the postoperative course. By following this criteria, we have previously reported performing aggressive but safe hepatectomy combined with hepatic vein resection and reconstruction (36,37). These detailed examinations are largely dependent on the ability of simulation software to calculate the area drained by the hepatic vein. Tani et al. created a "venous drainage map" that was drawn by using 3D simulation software, and it informed surgeons about the congested volume in the hepatic remnant after hepatectomy combined with hepatic vein resection (38). For example, the venous drainage map showed that extended right hemihepatectomy combined with middle hepatic vein resection would be accompanied by partial congestion of segment 4. Simulation software enables preoperative estimations of such congested volumes that would otherwise be impossible.
How to evaluate liver function in congested areas is also unclear. In a study in which Hashimoto et al. used near-infrared spectroscopy to detect ICG uptake they showed that sinusoidal perfusion in veno-occlusive regions was reduced by about half that in non-veno-occlusive regions (39), and in a study using ICG fluorescent imaging Kawaguchi et al. showed that portal uptake function in veno-occlusive regions was approximately 40% of that in non-occlusive regions (40). Gadoxetate disodium-enhanced magnetic resonance imaging (MRI) has also attracted attention as a method of evaluating liver function (41,42), and differences in transporter expression, which can be considered an indicator of liver function, between congested and non-congested areas has been investigated (43). Site by site liver function assessment, such as congested area or non-congested area, may be possible by verifying gadoxetate disodium-enhanced MRI. In the future, objective studies using simulation software, ICG fluorescent imaging, gadoxetate disodium-enhanced MRI, etc., will be necessary to collect more evidence to clarify liver function in congested areas.
Achievements of virtual hepatectomy
Preoperative examination of 3D images containing images of tumors and intrahepatic vasculature assists liver surgeons in their attempt to acquire an anatomical understanding of liver. Lamade et al. described the advantages of referring to 3D simulation images as a means of understanding tumor locations and planning resections, even for surgeons in training (44). Saito et al. found that preoperative simulations were useful in achieving negative surgical margins (26). Lang etal. have stated that preoperative simulations helped them prevent postoperative liver failure by accurately preserving the vascularity of the hepatic remnant something, which 2D CT is incapable of doing (45). Takamoto et al. reported that the image of segment border by preoperative simulation was helpful when intraoperative staining was unclear (28). Furthermore, Oshiro et al. developed new 3D simulation software called "Liversim" that takes liver deformation during liver resection into account, and they have described this new simulation system as a useful means of preoperative imaging (46,47). The Liversim software allows simulation of the whole liver transection procedure, from the start of liver transection to removal of the resected specimen, and provides gradually changing images of the transection plane and exposed vasculature.
Mise et al. summarized 1,194 preoperative simulation cases focusing on LDLT and hepatectomy for HCC/ colorectal liver metastasis (48). Preoperative evaluation of liver volume and the area drained by the middle hepatic vein of living liver transplant donors by 3D simulations enabled aggressive harvesting of right liver grafts while maintaining donor safety. Precise segment volumetry by 3D simulation software in HCC cases allows increased anatomical resection in impaired liver function patients, and it may improve long-term outcomes. Similarly, by enabling surgeons to easily understand the relationship between the tumor and vessels, preoperative 3D simulations in CRLM patients has helped surgeons perform aggressive hepatectomy for advanced tumors by means of complex resections.
Application to intraoperative navigation
The preoperative simulation systems described above are useful for preoperative planning of hepatic resection, but they do not provide the surgeon with a navigation tool during the actual operation. To enable surgeons to use systems to navigate during operations, there are several reports that enable reflection of the preoperative simulation in the intraoperative field. For example, a 3D printing model of the intrahepatic vessels prepared on the basis of 3D simulation images has been introduced to help surgeons understand intrahepatic anatomy in 3D intraoperatively (49), and Nishino et al. recently reported having devised a method of real-time navigation surgery that uses projection mapping (50). Their system makes it possible to project ICG fluorescence images onto the surface of the liver during liver transections and differentiate between areas that need to be resected and those that do not need to be resected. Augmented reality (AR) techniques have also been developed and provide 3D images of intrahepatic vessels and tumors by using a projector beam to display the images on the patient's body or organ surface (51), or by using stereovision eyeglasses to display on the monitor (52,53).
Real-time virtual sonography (RVS)
RVS is a novel fusion imaging technology that has recently been developed and that simultaneously provides ultrasonography images and virtual sonographic images reconstructed from CT or MRI scans by using an electromagnetic system, and RVS is regarded as one of the "navigation" systems that help surgeons perform intraoperatively. In addition to being useful when performing liver surgery, RVS has been reported to be useful when performing radiofrequency ablation (54,55) and breast cancer biopsies (56-59). Sato et al. have used RVS when performing liver surgery and have demonstrated its usefulness for visualizing the relationships between resection lines and tumors (60).
Although the ideal navigation system would be easy to use for persons who are unfamiliar with intrahepatic anatomy, the current RVS system requires manual adjustment using bifurcation of intrahepatic vessels which takes time and requires some understanding of intrahepatic anatomy (61). Ang et al. reported finding that RVS adjustment took a median time of 3 minutes (range 1-12 minutes) (62), and our own measurements showed that it took a median time of 105 seconds (range, 51-245 seconds) (61). A new RVS equipped with an automated adjustment system that enables quick, easy adjustments is currently being developed (63).
RVS has an error between the ultrasonography image and the reconstructed CT image, and we showed that the error is less than 10 mm (61) This error is considered permissible for use while comparing the two images alternately. One of the advantages of using this system is the ability to identify hepatic tumors that are difficult to identify by intrahepatic ultrasonography (IOUS) alone. Two representative cases in which RVS technology was effective in detecting small liver tumors are summarized below.
Case 1
A 67-year-old male underwent hepatic resection for HCC; the tumor measured 30 mm in diameter and was located in segment 8. Preoperative late-phase CT images revealed a low-density nodule, 8 mm in diameter, in segment 4 (Figure 1). At laparotomy, B-mode IOUS clearly demonstrated the tumor in segment 8, and a slightly hyperechoic nodule was seen in segment 4. However, the nodule in segment 4 was too small to conclude that it was the same as the nodule that had been detected in the preoperative late-phase CT images. Intraoperative RVS synchronized with preoperative CT was performed to accurately locate the tumor in segment 4. Since the nodule was seen between the two tributaries of the middle hepatic vein in the preoperative CT images, the RVS was adjusted to the bifurcation point of the middle hepatic vein. Meticulous examination by RVS confirmed that the nodule detected by the IOUS was identical to the tumor detected in the preoperative late-phase CT images. Then, RVS in which contrast-enhanced IOUS (CE-IOUS) was performed using Sonazoid (gaseous perflubutane; GE Healthcare, Oslo, Norway) was synchronized with preoperative CT images was performed 15 minutes after the contrast medium injection, and the nodule was ultimately judged to be benign, because it was isoechoic with the surrounding liver (Figure 2, Video S1, https:// www.globalhealthmedicine.com/site/supplementaldata.html?ID=6) (64,65). Based on the above findings, only the tumor in segment 8 was resected. Postoperative follow-up CT for two years showed no changes in the size of the nodule in segment 4, thereby confirming that it was not a malignant tumor.
Figure 1.
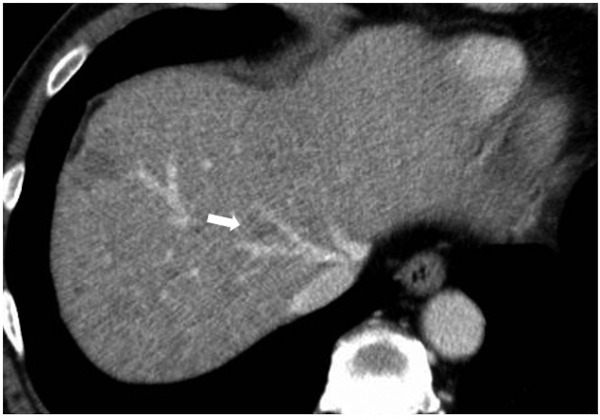
Preoperative CT scan in Case 1. The arrow points to a low-density nodule that was suspected of being a tumor preoperatively.
Figure 2.
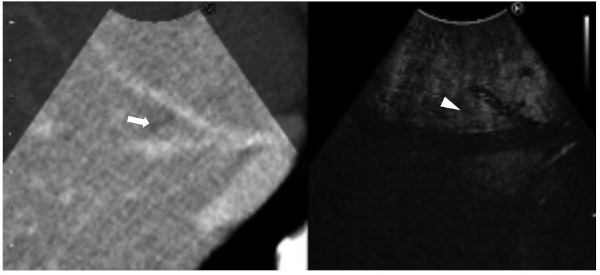
Still intraoperative real-time virtual sonography image in Case 1. Preoperative CT (left side) and contrast-enhanced intraoperative ultrasound (CE-IOUS; right side) were synchronized. The nodule was diagnosed as benign, because there was no hypo-echoic nodule at the same site on the CE-IOUS scan (arrowhead) where the low-density nodule was seen on the CT scan (arrow).
Case 2
A 76-year-old female was diagnosed with rectal cancer and there were numerous cysts in the liver (Figure 3A). The diffusion-weighted MRI (DW-MRI) and positron-emission tomography (PET) findings led to suspicion of a synchronous liver metastasis, 7 mm in diameter, in segment 5 (Figure 3B and 3C). Since contrast-enhanced imaging was not performed because of the patient's underlying renal dysfunction, it was rather difficult to precisely identify the tumor. Low anterior resection of the rectum and synchronous liver resection were planned. At laparotomy, the multiple cysts interfered with localization of the tumor in segment 5 both by B-mode IOUS and by CE-IOUS. Intraoperative RVS in which CE-IOUS was synchronized with DW-MRI was performed to locate the tumor, and it demonstrated a hypoechoic nodule at the site where the suspected liver metastasis had been identified by DW-MRI (Figure 4, Video S2, https://www.globalhealthmedicine.com/site/supplementaldata.html?ID=7). B-mode IOUS ruled out the possibility of the nodule being a cyst, because, in contrast to the other cysts, it was isoechoic. The tumor was resected with a negative surgical margin, and histopathology confirmed it to be a liver metastasis from the rectal cancer.
Figure 3.
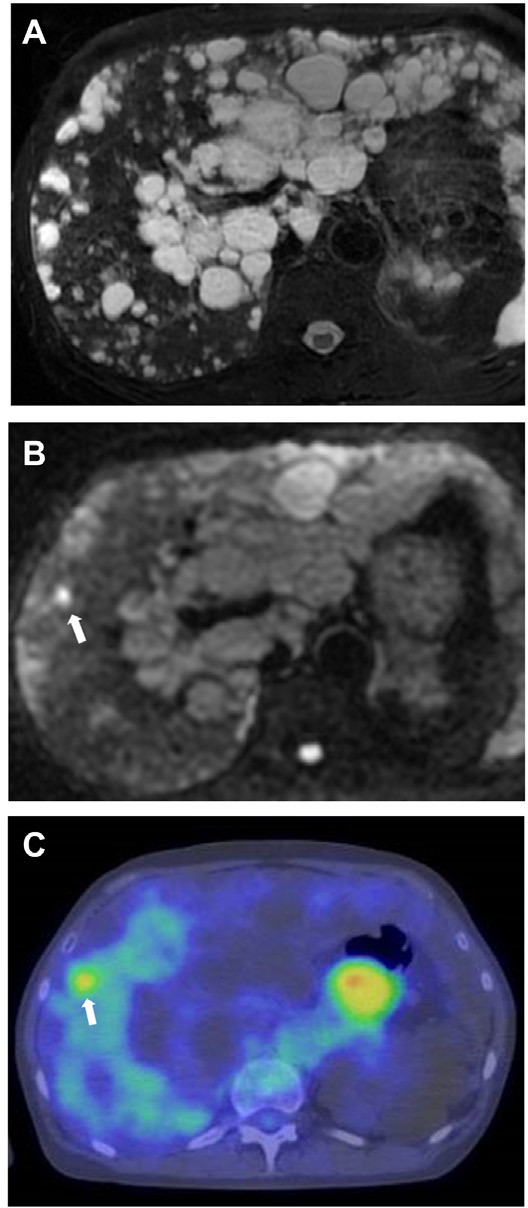
Preoperative magnetic resonance imaging and positron-emission tomography in Case 2. (A) T2-weighted image revealing multiple cysts in the liver. Diffusion-weighted magnetic resonance imaging (B) and positron-emission tomography (C) were able to detect tumor (arrow).
Figure 4.
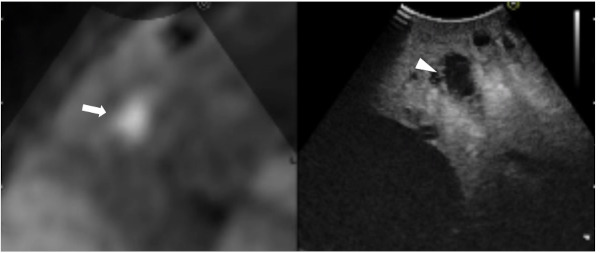
Still intraoperative real-time virtual sonography image in Case 2. Preoperative diffusion-weighted magnetic resonance imaging (DW-MRI; left side) and CE-IOUS (right side) were synchronized. The CE-IOUS image demonstrated a hypo-echoic nodule (arrowhead) at the same site where DW-MRI demonstrated a lesion that was strongly suspected of being a liver metastasis (arrow).
Another fusion imaging system that uses the optical tracking system has also been reported (66). In this system, the position and direction of the ultrasound probe are identified using a marker attached on the probe through the optical tracking camera placed above the site of the laparotomy. However, this system is limited by the fact that it will not work if there is an obstruction between the camera and the marker, and during liver surgery the echo probe is sometimes positioned deep in the diaphragm, which obstructs visualization of the marker. Electromagnetic navigation systems, such as the RVS system, on the other hand, can be used in narrow places without worrying about shielding, and can be applied to laparoscopic surgery.
Conclusions
The advent of 3D simulation software has made safer and more aggressive surgery possible. In the future, we need to disseminate new evidence brought by simulation, and expect the development of a new surgical navigation system that will help surgeons performing planned operations.
References
- 1. Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993; 9:298-304. [DOI] [PubMed] [Google Scholar]
- 2. Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997; 26:1176-1181. [DOI] [PubMed] [Google Scholar]
- 3. Saiura A, Yamamoto J, Koga R, Sakamoto Y, Kokudo N, Seki M, Yamaguchi T, Muto T, Makuuchi M. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. Am J Surg. 2006; 192:41-45. [DOI] [PubMed] [Google Scholar]
- 4. Saiura A, Yamamoto J, Koga R, Seki M, Yamaguchi T. Liver transection using the LigaSure sealing system. HPB (Oxford). 2008; 10:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ichida A, Hasegawa K, Takayama T, Kudo H, Sakamoto Y, Yamazaki S, Midorikawa Y, Higaki T, Matsuyama Y, Kokudo N. Randomized clinical trial comparing two vessel-sealing devices with crush clamping during liver transection. Br J Surg. 2016; 103:1795-1803. [DOI] [PubMed] [Google Scholar]
- 6. Couinaud C. Liver lobes and segments: notes on the anatomical architecture and surgery of the liver. Presse Med. 1954; 62:709-712. (in French) [PubMed] [Google Scholar]
- 7. Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005; 242:252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford). 2013; 15:31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985; 161:346-350. [PubMed] [Google Scholar]
- 10. Takasaki K, Kobayashi S, Tanaka S, Saito A, Yamamoto M, Hanyu F. Highly anatomically systematized hepatic resection with Glissonean sheath code transection at the hepatic hilus. Int Surg. 1990; 75:73-77. [PubMed] [Google Scholar]
- 11. Takasaki K. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg. 1998; 5:286-291. [DOI] [PubMed] [Google Scholar]
- 12. Arita J, Kokudo N, Zhang K, Makuuchi M. Three-dimensional visualization of liver segments on contrast-enhanced intraoperative sonography. AJR Am J Roentgenol. 2007; 188:W464-466. [DOI] [PubMed] [Google Scholar]
- 13. Shindoh J, Seyama Y, Umekita N. Three-dimensional staining of liver segments with an ultrasound contrast agent as an aid to anatomic liver resection. J Am Coll Surg. 2012; 215:e5-10. [DOI] [PubMed] [Google Scholar]
- 14. Shindoh J, Mise Y, Satou S, Sugawara Y, Kokudo N. The intersegmental plane of the liver is not always flat ― tricks for anatomical liver resection. Ann Surg. 2010; 251:917-922. [DOI] [PubMed] [Google Scholar]
- 15. Sugawara Y, Makuuchi M, Takayama T, Mizuta K, Kawarasaki H, Imamura H, Hashizume K. Liver transplantation using a right lateral sector graft from a living donor to her granddaughter. Hepatogastroenterology. 2001; 48:261-263. [PubMed] [Google Scholar]
- 16. Sugawara Y, Makuuchi M, Takayama T, Imamura H, Kaneko J. Right lateral sector graft in adult living-related liver transplantation. Transplantation. 2002; 73:111-114. [DOI] [PubMed] [Google Scholar]
- 17. Aoki T, Yasuda D, Shimizu Y, Odaira M, Niiya T, Kusano T, Mitamura K, Hayashi K, Murai N, Koizumi T, Kato H, Enami Y, Miwa M, Kusano M. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J Surg. 2008; 32:1763-1767. [DOI] [PubMed] [Google Scholar]
- 18. Inoue Y, Arita J, Sakamoto T, Ono Y, Takahashi M, Takahashi Y, Kokudo N, Saiura A. Anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. Ann Surg. 2015; 262:105-111. [DOI] [PubMed] [Google Scholar]
- 19. Miyata A, Ishizawa T, Tani K, Shimizu A, Kaneko J, Aoki T, Sakamoto Y, Sugawara Y, Hasegawa K, Kokudo N. Reappraisal of a Dye-Staining Technique for Anatomic Hepatectomy by the Concomitant Use of Indocyanine Green Fluorescence Imaging. J Am Coll Surg. 2015; 221:e27-36. [DOI] [PubMed] [Google Scholar]
- 20. Takayama T, Makuuchi M, Watanabe K, Kosuge T, Takayasu K, Yamazaki S, Hasegawa H. A new method for mapping hepatic subsegment: counterstaining identification technique. Surgery. 1991; 109:226-229. [PubMed] [Google Scholar]
- 21. Takayama T, Tanaka T, Higaki T, Katou K, Teshima Y, Makuuchi M. High dorsal resection of the liver. J Am Coll Surg. 1994; 179:72-75. [PubMed] [Google Scholar]
- 22. Maki H, Sakamoto Y, Kawaguchi Y, Akamatsu N, Kaneko J, Arita J, Hasegawa K, Kokudo N. Anatomical boundary between the caudate lobe of the liver and adjacent segments based on three-dimensional analysis for precise resections. J Gastrointest Surg. 2018; 22:1709-1714. [DOI] [PubMed] [Google Scholar]
- 23. Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979; 90:185-187. [DOI] [PubMed] [Google Scholar]
- 24. Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004; 135:404-410. [DOI] [PubMed] [Google Scholar]
- 25. Ariizumi S, Takahashi Y, Kotera Y, Omori A, Yoneda G, Mu H, Katagiri S, Egawa H, Yamamoto M. Novel virtual hepatectomy is useful for evaluation of the portal territory for anatomical sectionectomy, segmentectomy, and hemihepatectomy. J Hepatobiliary Pancreat Sci. 2013; 20:396-402. [DOI] [PubMed] [Google Scholar]
- 26. Saito S, Yamanaka J, Miura K, Nakao N, Nagao T, Sugimoto T, Hirano T, Kuroda N, Iimuro Y, Fujimoto J. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005; 41:1297-1304. [DOI] [PubMed] [Google Scholar]
- 27. Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007; 31:1249-1255. [DOI] [PubMed] [Google Scholar]
- 28. Takamoto T, Hashimoto T, Ogata S, Inoue K, Maruyama Y, Miyazaki A, Makuuchi M. Planning of anatomical liver segmentectomy and subsegmentectomy with 3-dimensional simulation software. Am J Surg. 2013; 206:530-538. [DOI] [PubMed] [Google Scholar]
- 29. Mise Y, Satou S, Shindoh J, Conrad C, Aoki T, Hasegawa K, Sugawara Y, Kokudo N. Three-dimensional volumetry in 107 normal livers reveals clinically relevant inter-segment variation in size. HPB (Oxford). 2014; 16:439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sano K, Makuuchi M, Miki K, Maema A, Sugawara Y, Imamura H, Matsunami H, Takayama T. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. 2002; 236:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lasinski W, Zientarski B. The anastomotic system of sub-hepatic veins in man. Bull Assoc Anat (Nancy). 1976; 60:559-566. (in French) [PubMed] [Google Scholar]
- 32. Sano K, Makuuchi M, Takayama T, Sugawara Y, Imamura H, Kawarasaki H. Technical dilemma in living-donor or split-liver transplant. Hepatogastroenterology. 2000; 47:1208-1209. [PubMed] [Google Scholar]
- 33. Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K, Koh K, Han S, Choi K, Hwang K, Makuuchi M, Sugawara Y, Min P. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001; 71:812-814. [DOI] [PubMed] [Google Scholar]
- 34. Akamatsu N, Sugawara Y, Nagata R, Kaneko J, Aoki T, Sakamoto Y, Hasegawa K, Kokudo N. Adult right living-donor liver transplantation with special reference to reconstruction of the middle hepatic vein. Am J Transplant. 2014; 14:2777-2787. [DOI] [PubMed] [Google Scholar]
- 35. Mise Y, Hasegawa K, Satou S, Aoki T, Beck Y, Sugawara Y, Makuuchi M, Kokudo N. Venous reconstruction based on virtual liver resection to avoid congestion in the liver remnant. Br J Surg. 2011; 98:1742-1751. [DOI] [PubMed] [Google Scholar]
- 36. Miyata A, Sakamoto Y, Yamamoto S, Akamatsu N, Arita J, Kaneko J, Hasegawa K, Kokudo N. Aggressive hemihepatectomy combined with resection and reconstruction of middle hepatic vein for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016; 23:494-500. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto M, Akamatsu N, Hayashi A, Togashi J, Sakamoto Y, Tamura S, Hasegawa K, Fukayama M, Makuuchi M, Kokudo N. Safety and efficacy of venous reconstruction in liver resection using cryopreserved homologous veins. J Hepatobiliary Pancreat Sci. 2017; 24:511-519. [DOI] [PubMed] [Google Scholar]
- 38. Tani K, Shindoh J, Akamatsu N, Arita J, Kaneko J, Sakamoto Y, Hasegawa K, Kokudo N. Venous drainage map of the liver for complex hepatobiliary surgery and liver transplantation. HPB (Oxford). 2016; 18:1031-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hashimoto T, Miki K, Imamura H, Sano K, Satou S, Sugawara Y, Kokudo N, Makuuchi M. Sinusoidal perfusion in the veno-occlusive region of living liver donors evaluated by indocyanine green and near-infrared spectroscopy. Liver Transpl. 2008; 14:872-880. [DOI] [PubMed] [Google Scholar]
- 40. Kawaguchi Y, Ishizawa T, Miyata Y, Yamashita S, Masuda K, Satou S, Tamura S, Kaneko J, Sakamoto Y, Aoki T, Hasegawa K, Sugawara Y, Kokudo N. Portal uptake function in veno-occlusive regions evaluated by real-time fluorescent imaging using indocyanine green. J Hepatol. 2013; 58:247-253. [DOI] [PubMed] [Google Scholar]
- 41. Yamada A, Hara T, Li F, Fujinaga Y, Ueda K, Kadoya M, Doi K. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology. 2011; 260:727-733. [DOI] [PubMed] [Google Scholar]
- 42. Shimamoto D, Nishie A, Asayama Y, Ushijima Y, Takayama Y, Fujita N, Shirabe K, Hida T, Kubo Y, Honda H. MR prediction of liver function and pathology using Gd-EOB-DTPA: effect of liver volume consideration. Biomed Res Int. 2015; 2015:141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shimizu A, Kobayashi A, Motoyama H, Sakai H, Yamada A, Yoshizawa A, Momose M, Kadoya M, Miyagawa S. Features of acute liver congestion on gadoxetate disodium-enhanced MRI in a rat model: Role of organic anion-transporting polypeptide 1A1. J Magn Reson Imaging. 2015; 42:828-836. [DOI] [PubMed] [Google Scholar]
- 44. Lamade W, Glombitza G, Fischer L, Chiu P, Cardenas CE, Sr., Thorn M, Meinzer HP, Grenacher L, Bauer H, Lehnert T, Herfarth C. The impact of 3-dimensional reconstructions on operation planning in liver surgery. Arch Surg. 2000; 135:1256-1261 [DOI] [PubMed] [Google Scholar]
- 45. Lang H, Radtke A, Hindennach M, Schroeder T, Fruhauf NR, Malago M, Bourquain H, Peitgen HO, Oldhafer KJ, Broelsch CE. Impact of virtual tumor resection and computer-assisted risk analysis on operation planning and intraoperative strategy in major hepatic resection. Arch Surg. 2005; 140:629-638; discussion 638. [DOI] [PubMed] [Google Scholar]
- 46. Oshiro Y, Yano H, Mitani J, Kim S, Kim J, Fukunaga K, Ohkohchi N. Novel 3-dimensional virtual hepatectomy simulation combined with real-time deformation. World J Gastroenterol. 2015; 21:9982-9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oshiro Y, Ohkohchi N. Three-dimensional liver surgery simulation: computer-assisted surgical planning with three-dimensional simulation software and three-dimensional printing. Tissue Eng Part A. 2017; 23:474-480. [DOI] [PubMed] [Google Scholar]
- 48. Mise Y, Hasegawa K, Satou S, Shindoh J, Miki K, Akamatsu N, Arita J, Kaneko J, Sakamoto Y, Kokudo N. How has virtual hepatectomy changed the practice of liver surgery?: experience of 1194 virtual hepatectomies before liver resection and living donor liver transplantation. Ann Surg. 2018; 268:127-133 [DOI] [PubMed] [Google Scholar]
- 49. Kuroda S, Kobayashi T, Ohdan H. 3D printing model of the intrahepatic vessels for navigation during anatomical resection of hepatocellular carcinoma. Int J Surg Case Rep. 2017; 41:219-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nishino H, Hatano E, Seo S, Nitta T, Saito T, Nakamura M, Hattori K, Takatani M, Fuji H, Taura K, Uemoto S. Real-time navigation for liver surgery using projection mapping with indocyanine green fluorescence: development of the novel medical imaging projection system. Ann Surg. 2018; 267:1134-1140. [DOI] [PubMed] [Google Scholar]
- 51. Volonté F, Pugin F, Bucher P, Sugimoto M, Ratib O, Morel P. Augmented reality and image overlay navigation with OsiriX in laparoscopic and robotic surgery: not only a matter of fashion. J Hepatobiliary Pancreat Sci. 2011; 18:506-509. [DOI] [PubMed] [Google Scholar]
- 52. Tang R, Ma LF, Rong ZX, Li MD, Zeng JP, Wang XD, Liao HE, Dong JH. Augmented reality technology for preoperative planning and intraoperative navigation during hepatobiliary surgery: A review of current methods. Hepatobiliary Pancreat Dis Int. 2018; 17:101-112. [DOI] [PubMed] [Google Scholar]
- 53. Quero G, Lapergola A, Soler L, Shabaz M, Hostettler A, Collins T, Marescaux J, Mutter D, Diana M, Pessaux P. Virtual and augmented reality in oncologic liver surgery. Surg Oncol Clin N Am. 2019; 28:31-44. [DOI] [PubMed] [Google Scholar]
- 54. Minami Y, Kitai S, Kudo M. Treatment response assessment of radiofrequency ablation for hepatocellular carcinoma: usefulness of virtual CT sonography with magnetic navigation. Eur J Radiol. 2012; 81:e277-280. [DOI] [PubMed] [Google Scholar]
- 55. Nakai M, Sato M, Sahara S, Takasaka I, Kawai N, Minamiguchi H, Tanihata H, Kimura M, Takeuchi N. Radiofrequency ablation assisted by real-time virtual sonography and CT for hepatocellular carcinoma undetectable by conventional sonography. Cardiovasc Intervent Radiol. 2009; 32:62-69. [DOI] [PubMed] [Google Scholar]
- 56. Uematsu T, Takahashi K, Nishimura S, Watanabe J, Yamasaki S, Sugino T, Oishi T, Kakuda Y, Sato M, Hayashi T. Real-time virtual sonography examination and biopsy for suspicious breast lesions identified on MRI alone. Eur Radiol. 2016; 26:1064-1072. [DOI] [PubMed] [Google Scholar]
- 57. Nakano S, Kousaka J, Fujii K, Yorozuya K, Yoshida M, Mouri Y, Akizuki M, Tetsuka R, Ando T, Fukutomi T, Oshima Y, Kimura J, Ishiguchi T, Arai O. Impact of real-time virtual sonography, a coordinated sonography and MRI system that uses an image fusion technique, on the sonographic evaluation of MRI-detected lesions of the breast in second-look sonography. Breast Cancer Res Treat. 2012; 134:1179-1188. [DOI] [PubMed] [Google Scholar]
- 58. Uematsu T. Real-time virtual sonography (RVS)-guided vacuum-assisted breast biopsy for lesions initially detected with breast MRI. Jpn J Radiol. 2013; 31:826-831. [DOI] [PubMed] [Google Scholar]
- 59. Fausto A, Rizzatto G, Preziosa A, Gaburro L, Washburn MJ, Rubello D, Volterrani L. A new method to combine contrast-enhanced magnetic resonance imaging during live ultrasound of the breast using volume navigation technique: a study for evaluating feasibility, accuracy and reproducibility in healthy volunteers. Eur J Radiol. 2012; 81:e332-337. [DOI] [PubMed] [Google Scholar]
- 60. Satou S, Aoki T, Kaneko J, Sakamoto Y, Hasegawa K, Sugawara Y, Arai O, Mitake T, Miura K, Kokudo N. Initial experience of intraoperative three-dimensional navigation for liver resection using real-time virtual sonography. Surgery. 2014; 155:255-262. [DOI] [PubMed] [Google Scholar]
- 61. Miyata A, Arita J, Shirata C, Abe S, Akamatsu N, Kaneko J, Kokudo N, Hasegawa K. Quantitative assessment of the accuracy of real-time virtual sonography for liver surgery. Surg Innov. 2020; 27:60-67. [DOI] [PubMed] [Google Scholar]
- 62. Lv A, Li Y, Qian HG, Qiu H, Hao CY. Precise navigation of the surgical plane with intraoperative real-time virtual sonography and 3D simulation in liver resection. J Gastrointest Surg. 2018; 22:1814-1818. [DOI] [PubMed] [Google Scholar]
- 63. Takamoto T, Mise Y, Satou S, Kobayashi Y, Miura K, Saiura A, Hasegawa K, Kokudo N, Makuuchi M. Feasibility of intraoperative navigation for liver resection using real-time virtual sonography with novel automatic registration system. World J Surg. 2018; 42:841-848. [DOI] [PubMed] [Google Scholar]
- 64. Hatanaka K, Kudo M, Minami Y, Maekawa K. Sonazoid-enhanced ultrasonography for diagnosis of hepatic malignancies: comparison with contrast-enhanced CT. Oncology. 2008; 75 Suppl 1:42-47. [DOI] [PubMed] [Google Scholar]
- 65. Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, Takahashi S, Inoue T, Hagiwara S, Chung H, Ueshima K, Maekawa K. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology. 2008; 51 Suppl 1:61-69. [DOI] [PubMed] [Google Scholar]
- 66. Kingham TP, Scherer MA, Neese BW, Clements LW, Stefansic JD, Jarnagin WR. Image-guided liver surgery: intraoperative projection of computed tomography images utilizing tracked ultrasound. HPB (Oxford). 2012; 14:594-603. [DOI] [PMC free article] [PubMed] [Google Scholar]


