Summary
Coronavirus disease 2019 (COVID-19) is a respiratory tract infection caused by SARS-CoV-2. As of March 30, 2020, there have been 693,224 reported patients with COVID-19 worldwide, with 1,446 in Japan. Currently, although aspects of the route of transmission are unclear, infection by contact and by inhaling droplets is considered to be the dominant transmission route. Inflammatory symptoms in the upper respiratory tract persist for several days to 1 week after onset, and in some patients symptoms of pneumonia worsen and become severe. The presence of underlying diseases and advanced age are risk factors for increased severity. Diagnosis is based on detection of SARS-CoV-2 by polymerase chain reaction (PCR) testing of nasopharyngeal swabs or sputum. Symptomatic management is the main treatment for this disease. Although the efficacy of several agents is currently being tested, at present there is no effective therapeutic agent. To prevent infection, in addition to standard preventive measures, measures that counteract infection by contact and droplet inhalation are important. In addition, if procedures that cause aerosolization of virus are used, then measures that prevent airborne infection should be implemented.
Keywords: SARS-CoV-2, Japan, COVID-19
Introduction
To date, we know of four types of coronavirus that can infect humans, including those that account for 10% to 15% of the causes of common cold (Table 1). Acute upper respiratory inflammation caused by human coronavirus is less frequent in summer and fall and increases in winter and spring (1,2). Large outbreaks are believed to occur in a cycle of 2 to 3 years (3). Re-infection with human coronavirus may occur, a finding considered to be due to a relatively rapid reduction in antibody titres (4). The proportion of asymptomatic infected patients varies depending on age and is considered to be about 30% in adults (5,6).
Table 1. Types of coronavirus and their characteristics.
| Coronavirus infection | Common cold | SARS | MERS | COVID-19 |
|---|---|---|---|---|
| Causative virus | Human coronavirus (four types) | SARS coronavirus | MERS coronavirus | SARS-CoV-2 |
| Year of outbreak | Every year | 2002 to 2003 | From 2012 | From December 2019 |
| Epidemic region | Worldwide | Guangdong Province, China | Arabian Peninsula including Saudi Arabia | First reported in Wuhan, China, currently becoming a pandemic worldwide |
| Host animal | Human | Horseshoe bat (Rhinolophus ferrumequinum) | Dromedary camel | Unknown |
| Number of infected patients | Account for 10% to 15% of the causes of common cold | 8,098 patients (by end of epidemic) | 2,494 patients (as of April 16, 2020) | 1,991,562 patients (as of April 16, 2020) |
| Mortality rate | Very rare | 9.4% | 34.4% | 6.1% |
| Route of transmission | Droplets and contact | Droplets, contact, and stool | Droplets and contact | Droplets and contact |
| Basic reproduction number | Many from 1 person | 2 to 5 from 1 person | Less than 1 from 1 person | 2.6 from 1 person |
| Incubation period | 2 to 4 days | 2 to 10 days | 2 to 14 days | Estimated to be 1 to 14 days |
| Infectious Diseases Control Law | None | Infectious Diseases Category II | Infectious Diseases Category II | Designated Infectious Disease |
Species-specific coronaviruses have also been identified (7), for example, in dogs, cats, pigs, camels, bats, and sparrows. However, since each of these animal-specific coronaviruses exhibits a high species-specificity, it has been thought that they would not infect other animals across the species barrier.
Severe acute respiratory syndrome (SARS), which first occurred in Guangdong Province, China in 2002, was considered to be caused by transmission of a coronavirus to humans from bats (or the palm civet cat, Paguma larvata). Here, human-to-human infection occurred, resulting in 8,098 infected patients and 774 deaths (a mortality rate of 9.6%) (8). In 2012, an outbreak of Middle East respiratory syndrome (MERS) was reported in the Middle East (9). The reservoirs of coronavirus that led to this disease were bats and dromedary camels, and infection was found to occur mainly by transmission of coronavirus from camels to humans (10). As of March 2020, 2,494 infected patients and 858 deaths have been reported (a mortality rate of 34.4%). We therefore knew of two pathogens that caused epidemic coronavirus infection diseases in humans: a coronavirus harbored in animals, especially bats, infected humans with subsequent human-to-human infection.
In December 2019, an outbreak of unidentified pneumonia then occurred in Wuhan City, Hubei Province, China and was found to be caused by a novel coronavirus (SARS-CoV-2) (11). Like SARS-CoV, SARS-CoV-2 is classified as a β-coronavirus - a subgenus of coronaviruses (Figure 1). The structure of the gene that encodes the receptor region required for binding is very similar to that of SARS-CoV, suggesting that SARS-CoV-2 enters cells by binding to the ACE-2 receptor, as does SARS-CoV.
Figure 1.
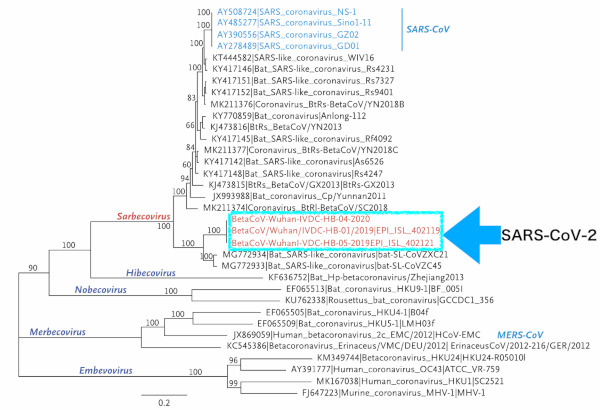
Phylogenetic analysis of SARS-CoV-2 and other beta-coronavirus genomes (Orthocoronavirinae subfamily) (11).
The host animal for this SARS-CoV-2 is unknown as of March 2020. However, in China it is evident that many patients were associated with the seafood market in Wuhan City, in the early part of the epidemic period (12). Based on this information, speculation suggests that an animal sold in the seafood market is the host animal for this virus. Phylogenetic analysis indicates that SARS-CoV-2 is closely related to coronaviruses harbored by bats. Bats are thus likely to be the primary host of SARS-CoV-2; however, it is unknown whether the virus was transmitted to humans directly from bats or via another intermediate host (13).
Epidemiology
On December 31, 2019, cases of unidentified pneumonia that occurred in Wuhan City, Hubei Province, China were reported to the WHO Office in China (14). Seven of 27 patients were severe and many of these patients were associated with a seafood market. By January 8, 2020, many of these patients were diagnosed as having pneumonia caused by a novel coronavirus (SARS-CoV-2) infection (15). According to reports on 41 confirmed cases, 66% of all patients were associated with the seafood market (for example, they worked there) (12). However, as early as December 2019, there were several cases not related to the seafood market, and human-to-human infection may already have occurred at this point.
Subsequently, the number of patients in Wuhan City continued to increase gradually, with 4 patients on January 18, 17 patients on January 19, and 136 patients on January 20. Cases imported from Wuhan City were reported in Thailand on January 12 and Japan on January 16 (16). In China, infected patients were reported in Guangdong Province and Beijing City, and the infection subsequently spread throughout China. On January 30, World Health Organization (WHO) declared a "Public Health Emergency of International Concern (PHEIC)".
As of March 30, 2020, there have been 693,224 reported patients with COVID-19 worldwide. Of these, 392,757 patients were in Europe (17). In Japan, as of March 30, there were 1,446 infected patients (1,420 domestic patients, 11 returnees on charter flights, and 15 patients under airport quarantine), 210 asymptomatic pathogen carriers (190 domestic patients, 4 returnees on charter flights, and 16 patients under airport quarantine), and 210 persons confirmed to be positive for coronavirus and under observation for the presence or absence of symptoms (210 domestic cases); based on data from the Tuberculosis and Infectious Disease Control Division, Ministry of Health, Labour and Welfare (Figure 2). On categorization by prefecture, urban regions such as Tokyo, Osaka, Aichi, Hokkaido, Chiba, Hyogo, Kanagawa, and Saitama account for 70% or more of infected patients.
Figure 2.

Change in the number of patients infected with a SARS-CoV-2 in Japan (published by the Ministry of Health, Labour and Welfare).
The greatest numbers of nosocomial infections have been reported from Tokyo and Oita. In Chiba, an outbreak in a welfare facility for the disabled has also been reported.
Mode of transmission
In Wuhan City, many of the infected patients were associated with a seafood market in the early epidemic period, suggesting that spread of infection had occurred from animals to humans (16). However, current transmission is mainly due to human-to-human infection.
At present, although there are some unknown aspects of human-to-human infection, infection by contact and by droplet inhalation is considered to be the dominant transmission route. The virus can also be detected in stool (18) and saliva (19); it is however unclear at present how much these route contribute to transmission of infection.
A substantial issue since the start of the epidemic is that few infected patients were identified on surveying contact persons. In China, just a few percent of close contacts were infected. Similarly, in USA, onset was seen in just 0.45% of 445 persons in contact with 10 confirmed patients (20). How therefore is infection spread?
One possibility is that infection may be spread from asymptomatic infected patients. Some cases of infection from asymptomatic infected patients have already been reported (21-23). If infection from such asymptomatic infected patients occurs, spreading infection can be explained even if persons are not infected through contact with symptomatic, infected patients. According to data from 44,672 Chinese patients infected with SARS-CoV-2, there are fewer infected patients under the age of 20, in comparison with the Chinese population distribution (24). As most infection in younger persons is asymptomatic or mild, it is possible that these individuals may not have been diagnosed. There is thus a possibility that infection may spread from younger persons with few symptoms. In addition, according to the calculations of Nishiura et al., the serial interval (the interval from the onset of infection in the first person to the onset of infection in the second person) is shorter than the incubation period, suggesting that persons in the pre-onset phase or those with onset but minimal symptoms may spread the infection (25).
It is also known that outbreaks occur more easily in certain circumstances. In Japan, outbreaks have been reported in "confined spaces with poor ventilation" such as buses, houseboats, live music clubs, and fitness centers. The outbreak on the Diamond Princess cruise ship can be considered representative of COVID-19 (26). In such specific circumstances, the super-spreading phenomenon is considered to occur.
With SARS and MERS, the existence of super-spreaders has been known (27). In 2015, when MERS became epidemic in Korea, the existence of super-spreaders was confirmed, with spread of infection from 1 person to 29 persons and from 1 person to 86 persons (28). With these infectious diseases, it is epidemiologically known that although approximately 80% of patients rarely infect others, infection spreads from 1 person to many in the remaining 20%. This "20/80 rule" (29) may be applicable to COVID-19. According to a survey of 110 domestic patients up to February 26, performed by the Cluster Response Team of the Novel Coronavirus Response Headquarters within the Ministry of Health, Labour and Welfare, about 80% of infected patients did not infect other persons and 20% did spread the infection (Figure 3). These 20% of infected patients spread the infection in "confined spaces with poor ventilation". Therefore, it is important to prevent such clusters in order to avoid the spread of infection (30).
Figure 3.
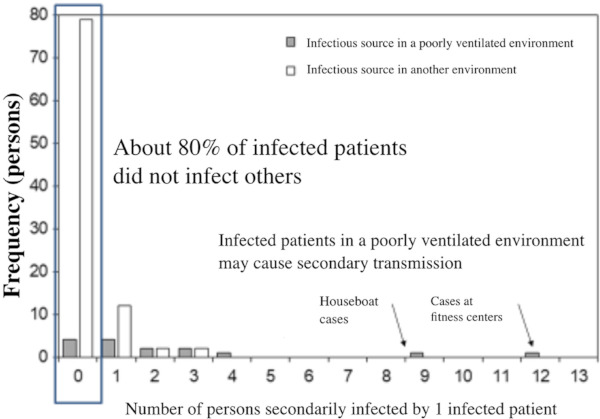
Number of persons secondarily infected by 1 infected patient (as of February 26, analytical result of 110 domestic patients) (Cluster Response Team of the Novel Coronavirus Response Headquarters within the Ministry of Health, Labour and Welfare).
Clinical manifestations
The incubation period is 14 days or less; in many cases the onset occurs about 5 days after exposure (15,24).
Many symptomatic patients have symptoms such as fever, respiratory symptoms (coughing and pharyngodynia), headache, and malaise. The frequency of runny nose and nasal congestion seems to be low (24). The frequency of digestive symptoms such as diarrhea and vomiting is less than 10% in many reports, which seems to be lower than that in SARS and MERS. The clinical manifestations are similar to those of influenza and the common cold, but some patients complain of dysosmia and taste abnormality. According to Giacomelli et al. (31), in a survey conducted on 59 of 88 patients with COVID-19, who could be interviewed, 33.9% of infected patients had either dysosmia or taste abnormality and 18.6% had both. With dysosmia and taste abnormality in addition to influenza-like symptoms, the possibility of COVID-19 may be higher.
In China, it was reported that the interval from onset to visiting hospital and to hospitalization was about 5 and 7 days, respectively (15). In some cases, the disease is thought to become severe over the course of about 1 week. In more severe cases, patients are likely to be admitted to ICU after Day 10 (12) (Figure 4). According to the data of 44,672 Chinese patients, severity was mild (no pneumonia or mild pneumonia) in 81% of infected patients, severe (dyspnea, hypoxemia, opacities caused by pneumonia appearing in 50% or more of the lungs, within 24 to 48 hours) in 14%, and very severe (respiratory failure, shock, and multi-organ failure) in 5%. Of these, 2.8% died, so the lives of nearly half of these most severe patients can be saved.
Figure 4.
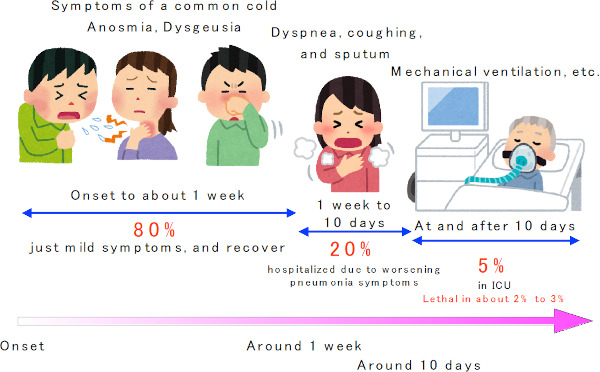
Typical course of the COVID-19.
Risk factors for severity are known to include advanced age and underlying diseases (cardiovascular disease, diabetes, malignancy, and chronic respiratory disease) (24,32).
The disease is less severe in patients aged less than 50 years, and the mortality rate increases with age in those aged 50 years or older. According to the data of 44,672 Chinese patients, the mortality rate is 14.8% in those aged 80 years or older (24) (Figure 5). The mortality rate is also clearly higher in patients with underlying diseases, compared with those without these diseases (Figure 6).
Figure 5.
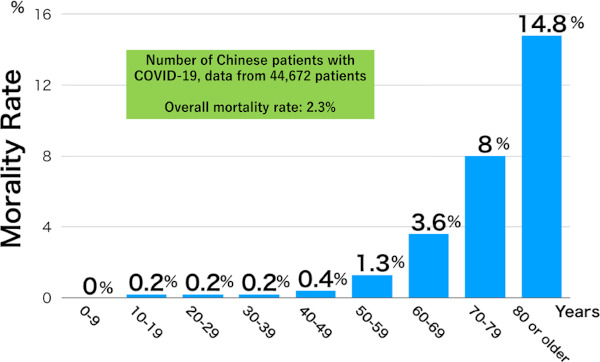
Mortality rate of the COVID-19, by age (24).
Figure 6.
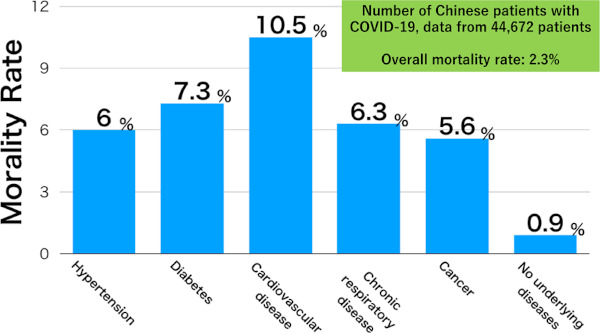
Mortality rate of the COVID-19, by underlying disease (24).
Severe cases have so far not been reported in pregnant women, nor has there been congenital infection (33,34). As SARS-CoV-2 was not detected in cord blood, amniotic fluid, and neonates (34,35), vertical transmission is considered not to occur. There are no reports of severe cases in infants (36).
Asymptomatic infected patients are known to occur at a certain rate. When the passengers of the Diamond Princess cruise ship were screened by PCR, about 17% were positive, and almost half of the positive passengers were asymptomatic (26).
Chest imaging findings are characterized by bilateral, peripheral infiltrative shadows, with ground glass attenuation (Figure 7). There are cases where despite pneumonia on chest CT, this cannot be diagnosed on chest X-ray. Chinese reports show that pneumonia was diagnosed on chest X-ray in just 59.1% of those with pneumonia, whereas chest CT provided a diagnosis in 86.2% (24). Although the author cannot directly extrapolate, because different patients may have undergone CT and X-ray, chest X-ray may miss 20% to 30% of patients with pneumonia. In cases where the pre-test probability is high (such as those with a history of contact with an infected person), even if pneumonia image is not observed on chest X-ray, a chest CT should be considered. After onset, the pneumonia image expands over time. However, the pneumonia image can be observed on chest CT even in asymptomatic infected patients (37). A significant pneumonia image may be observed even in asymptomatic infected patients without fever or respiratory symptoms, a characteristic of this disease.
Figure 7.
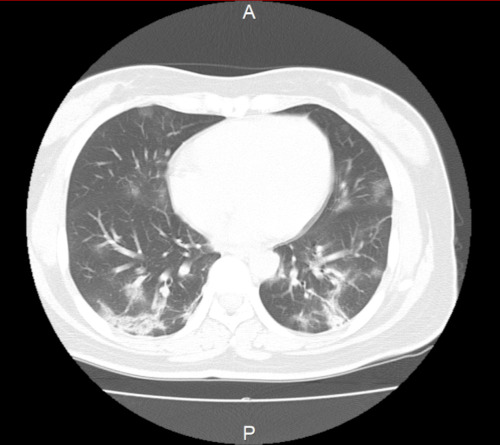
Chest CT image of the patient with the COVID-19 (case at the author's hospital).
Blood tests may show decreased lymphocytes, especially in severe cases (24).
Test and diagnosis
In Japan, the diagnosis is generally made by detecting SARS-CoV-2 using a PCR test.
The PCR test used to detect SARS-CoV-2 received insurance coverage from March 6. Consequently, in the Outpatient Services for Returnees and Contact Persons of approximately 800 medical institutions, the PCR test was covered by insurance when physicians wanted to investigate if the COVID-19 was present.
As PCR test samples, throat swabs or sputum, if available, was used until February 27. However, the viral load was demonstrated to be higher in the nasopharynx than in the pharynx (38). At present, a nasopharyngeal swab and sputum are the recommended samples (39). The WHO recommends that when an initial PCR test result is negative but infection is still strongly suspected, multiple samples should be collected and tested repeatedly (40). Many cases where an initial test result was negative despite a strong suspicion of COVID-19 based on CT findings have shown a positive result on repeated testing, including the case the author reported (41,42).
Because COVID-19 was designated in March as an infectious disease according to the Infections Diseases Control Law, if a diagnosis of COVID-19 is confirmed, the patient should in principle be hospitalized into an infectious disease bed at a designated medical institution for infectious diseases. In addition, the doctor who made the diagnosis must immediately report the case to a public health center.
Treatment
The CDC and WHO have their own treatment guidelines, the basis of which is to provide supportive treatment as needed, with appropriate prevention measures (43,44). In China, 1,099 reported patients received antimicrobial agents (58%), oseltamivir (35.8%), antifungal agents (2.8%), and glucocorticoids (18.6%) (24). They also received oxygen (41.3%), mechanical ventilation (6.1%: invasive 2.3% and non-invasive 5.1%), renal-replacement therapy (0.8%), and ECMO (extra-corporeal membrane oxygenation, 0.5%). Overall, 5% of patients were admitted to ICU.
Currently, there is no effective drug treatment for this COVID-19, but several candidate drugs are under investigation.
Lopinavir/Ritonavir (brand name: Kaletra) was believed to be effective for SARS and MERS, also caused by similar coronaviruses (45,46). In China, Kaletra has been used for this indication since the beginning of the epidemic of COVID-19. However, in a randomized, controlled study of 199 patients, lopinavir/ ritonavir was not found to be effective (47). Because of interactions, many drugs cannot be used in combination with Kaletra. Therefore, cautionary notes include the needs for prior confirmation of the types of medicine being taken, and an HIV test (if a patient is infected with HIV, there is a risk of resistance occurring).
Remdesivir has so far been used as a candidate therapeutic agent for Ebola hemorrhagic fever in other clinical trials. With Ebola hemorrhagic fever, where there is an ongoing epidemic in the Democratic Republic of the Congo, remdesivir was administered in a randomized controlled trial but was found to be less effective than two other drugs, MAb114 and REGN-EB3. Administration of remdesivir for Ebola hemorrhagic fever has been stopped at present (48). However, some have suggested that remdesivir may be an effective treatment for COVID-19. The Wuhan Institute of Virology has reported the efficacy of remdesivir for COVID-19 in Cell Research (49). On examination of the inhibitory effect of remdesivir on virus replication, 48 hours after cultured cells were infected with the SARS-CoV-2, marked inhibition was observed. In the first diagnosed case of COVID-19 in the United States, remdesivir was also administered (50). This patient recovered following administration, but it is unknown whether this was due to the efficacy of remdesivir. In Japan, a global investigator-initiated registration-directed trial of remdesivir has been started primarily at the National Center for Global Health and Medicine, from late March.
Favipiravir (brand name: Avigan) is a drug developed by FUJIFILM Toyama Chemical Co., Ltd. (Japan). In Japan, Avigan is approved as an anti-influenza drug and is stockpiled for outbreaks of a novel type of influenza. However, this drug is teratogenic and is not used for seasonal influenza. Since Avigan inhibits RNA polymerase, it is expected to be effective against a wide range of RNA viruses apart from influenza. As with remdesivir, Avigan has also been used for Ebola hemorrhagic fever. On examination of the results of treatment using Avigan for Ebola hemorrhagic fever in West Africa during outbreaks in 2014 to 2015, clear efficacy could not be shown (51,52). Based on a clinical trial that was conducted in Japan (unpublished results), Avigan may be effective for SFTS (Severe fever with thrombocytopenia syndrome), which has a high mortality rate of 27%, with approximately 80 infected patients being reported per year in Japan. Its efficacy for the SARS-CoV-2 is unknown. However, as a result of evaluation of Avigan in the above study of remdesivir published in Cell Research, which demonstrated a virus inhibitory effect of remdesivir, a certain virus inhibitory effect of Avigan was also exhibited at a level of laboratory. According a Chinese clinical trial registration site, clinical trials of Avigan to examine its efficacy for COVID-19 (ChiCTR2000029600 and ChiCTR2000029548) are currently underway (unpublished results). A Chinese report states that when interferon-α inhalation + lopinavir/ritonavir and interferon-α inhalation + Avigan were administered for 14 days to patients with COVID-19 in a non-randomized comparative study, the latter combination was superior in terms of time to virus negativity and improvement of pneumonia on CT on Day 14 (53).
The National Institute of Infectious Diseases reports that ciclesonide, an inhaled steroid agent for the treatment of asthma also indicated for bronchial asthma, has a specific anti-viral effect in COVID-19 (54). In Japan, 3 patients were reported to improve with ciclesonide (brand name: Alvesco) (55). There were however just 3 patients and the efficacy of ciclesonide for COVID-19 is not yet established. Ciclesonide is a steroid that is administered topically rather than systemically; therefore, it is less invasive and has fewer side effects. Since systemic administration of ciclesonide is not currently recommended (56), if efficacy is observed, ciclesonide will become a promising drug.
In China, the antimalarial drug chloroquine is used in addition to these drugs. Chloroquine was once used worldwide as a therapeutic agent for malaria. However, as chloroquine-resistant malaria has recently become more common, its use for the treatment of malaria is decreasing (57). In Japan, chloroquine is an unapproved drug. However, hydroxychloroquine (Plaquenil), which has a similar structure to chloroquine and has anti-inflammatory and immune regulatory effects, is used for systemic lupus erythematosus (SLE). Since chloroquine has the same effect, it may be effective for COVID-19. According to the report in Cell Research by Wuhan Institute of Virology, chloroquine shows the same inhibitory effect on SARS-CoV-2 at the laboratory level as remdesivir (49). In China, chloroquine was administered to 100 or more patients and was superior to the control treatment in suppressing progression of pneumonia, improving lung image findings, time to virus negativity, and disease duration (58). In addition, in a non-randomized controlled trial that included 36 French patients with COVID-19, the viral load 6 days after participating in the trial was significantly lower in the hydroxychloroquine group (59).
The use of convalescent plasma is one treatment option for emerging/reemerging infectious disease (60). This has been administered for SARS (61) and MERS (62). Convalescent plasma contains neutralizing antibody against SARS-CoV and MERS-CoV. Therefore, if administered to a patient, it would be expected to exert an antiviral effect. However, in using convalescent plasma there are issues that need to be addressed, including the procedures required to collect plasma from recovered patients, confirmation that they are negative for SARS-CoV-2, methods for confirming the production of sufficient antibody, and the need to screen for infection, as with blood transfusion. In the United States, it was reported that when convalescent plasma was administered to 5 patients with the COVID-19, all of these patients recovered (63).
Although there are various candidate therapeutic drugs, their efficacies have not been confirmed. Since most patients recover spontaneously, the use of effective drugs in all cases, or of a drug where the efficacy is unknown, is considered to be disadvantageous for patients. Therefore, it is believed that administration of drugs should be limited to severe cases or cases that have a high risk of becoming severe. In "Concept of Antiviral Drugs for COVID-19, first version (February 26, 2020)", proposed by the Japanese Association for Infectious Diseases, the following points are made:
I. Generally, if patients under 50 years of age develop pneumonia, they recover as part of the natural course of the disease. Therefore, follow-up may be performed without necessarily administering an antiviral drug.
II. Generally, patients aged 50 years or older have a high likelihood of developing serious respiratory failure and their mortality rates are high. Therefore, when hypoxemia occurs and oxygen administration is required, administration of an antiviral drug should be considered.
III. Patients with diabetes, cardiovascular disease, chronic lung disease, chronic obstructive pulmonary disease, or immunosuppression, etc. should also be treated according to Item 2 above.
IV. Regardless of age, administration of an antiviral drug should be considered in cases that exhibit respiratory failure that worsens when treated with oxygenation and symptomatic measures alone.
Conditions for discharge are as follows: On confirmation that a fever of 37.5°C or higher has not been observed for 24 hours or more and that respiratory symptoms are improving, a PCR test is conducted. For this, samples are collected 48 hours (first test) after the patient condition described above is confirmed, and a further 12 hours later (second test). If the results of both tests are negative, patients are able to leave the hospital (64). In asymptomatic infected patients, a PCR test is conducted using samples collected 48 hours (first test) after the day on which they were found to be positive and a further 12 hours later (second test). If the results of both tests are negative, patients are able to leave the hospital. There have been cases where SARS-CoV-2 is detected again by PCR testing in patients who have recovered from the COVID-19 and were confirmed to be negative by two PCR tests (65). At the present time, it is unknown what proportion of patients becomes PCR positive again following a negative test, or how long positivity lasts.
Measures for infection prevention
Protecting healthcare professionals from infection is one of the most important aspects of clinical practice for COVID-19. Among 138 patients reported from China, 43% were considered to be due to infection in hospital (66). Other coronavirus diseases, SARS and MERS, are known to be transmitted easily by nosocomial infection (67,68) and constitute a risk for healthcare professionals, who often have close contact with patients in confined spaces, in other words in hospital.
The route of transmission is considered to be infection by contact and inhaling droplets. The implementation of measures for airborne infection control is recommended in environments where aerosols are present. The WHO recommends implementation of measures for prevention of infection by contact and droplet inhalation, in addition to standard preventive measures. If a procedure is used that causes aerosolization of the virus, measures that prevent airborne infection should also be implemented (69). The personal protective equipment recommended for each activity is shown in Table 2.
Table 2. Personal protective equipment recommended for each target and activity (WHO. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19). https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf).
| Hospitalized patients | |||
|---|---|---|---|
| Ward | Healthcare professionals | Healthcare professionals who provide medical care | Surgical mask, gown, gloves, goggles or eye shield |
| Procedure that causes virus aerosolization | N95 mask, gown, gloves, goggles or eye shield | ||
| Sanitary workers | Entering a patient's room | Surgical mask, gown, thick gloves, goggles or eye shield (in case of a risk of inhaling droplets), boots | |
| Visitors | Entering a patient's room | Surgical mask, gloves | |
| Other areas patients may move to (hospital ward, hallway, etc.) | All staff | All activities except for contact with patients | Personal protective equipment is not needed |
| Triage | Healthcare professionals | Screening that does not necessitate direct contact with patients | Keep a distance of at least 1 m. Personal protective equipment is not needed |
| Patients with respiratory symptoms | All cases | Keep a distance of at least 1 m. Wear mask | |
| Patients without respiratory symptoms | All cases | Personal protective equipment is not needed | |
| Laboratory | Laboratory technician | Handling of respiratory specimens | Surgical mask, gown, gloves, goggles or eye shield (if there is a risk of inhaling droplets) |
| Target | Activity | Personal protective equipment | |
| Outpatients | |||
| Doctor's office | Healthcare professionals | Examination of patients with respiratory symptoms | Surgical mask, gown, gloves, protection of eyes |
| Healthcare professionals | Examination of patients without respiratory symptoms | Standard preventive measures | |
| Patients with respiratory symptoms | All cases | Wear surgical mask | |
| Patients without respiratory symptoms | All cases | Personal protective equipment is not needed | |
| Sanitary workers | After examination or between the examinations of patients with respiratory symptoms | Surgical mask, gown, thick globes, goggles or eye shield (if there is a risk of inhaling droplets), boots | |
| Waiting room | Patients with respiratory symptoms | All cases | Wear mask. Move immediately to an isolated room or a place distant from other patients. If this is impossible, keep a distance of at least 1 m from other patients |
| Patients without respiratory symptoms | All cases | Personal protective equipment is not needed | |
| Triage | Healthcare professionals | Screening that does not necessitate direct contact with patients | Keep a distance of at least 1 m. Personal protective equipment is not needed |
| Patients with respiratory symptoms | All cases | Keep a distance of at least 1 m. Personal protective equipment is not needed | |
| Patients without respiratory symptoms | All cases | Personal protective equipment is not needed | |
The CDC recommendations are similar, except for always implementing measures that prevent airborne infection (CDC. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Finfection-control.html). A point of similarity is that eye protection is emphasized and the use of eye guards is recommended.
In "Infection Control for COVID-19", proposed by the National Institute of Infectious Diseases and Disease Control and Prevention Center, National Center for Global Health and Medicine (https://www.niid.go.jp/niid/images/epi/corona/2019nCoV-01-200305.pdf), the followings are described (the contents are same as that those of the WHO):
I. Preventive measures for infection by contact and inhaled droplets should be implemented, in addition to the standard preventive measures.
II. Single rooms are desirable for doctors' offices and hospital rooms.
III. Doctors' offices and hospital rooms do not need a negative-pressure setting, but should be adequately ventilated.
IV. If a procedure is used that causes virus aerosolization, (for example, tracheal aspiration, endotracheal intubation, and lower respiratory tract specimen collection), an N95 mask (or DS2, equivalent mask), eye protector (goggles or face shield), long-sleeved lab coat, and gloves should be worn.
V. Patient movement should be limited to movement for essential purposes. Staff (information desk, attendant, and security guard) should comply with standard preventive measures.
References
- 1. Isaacs D, Flowers D, Clarke JR, Valman HB, MacNaughton MR. Epidemiology of coronavirus respiratory infections. Arch Dis Child. 1983; 58:500-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. 2010; 48:2940-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monto AS. Medical reviews. Coronaviruses. Yale J Biol Med. 1974; 47:234-251. [PMC free article] [PubMed] [Google Scholar]
- 4. Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990; 105:435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graat JM, Schouten EG, Heijnen ML, Kok FJ, Pallast EG, de Greeff SC, Dorigo-Zetsma JW. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol. 2003; 56:1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prill MM, Iwane MK, Edwards KM, Williams JV, Weinberg GA, Staat MA, Willby MJ, Talbot HK, Hall CB, Szilagyi PG, Griffin MR, Curns AT, Erdman DD. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr Infect Dis J. 2012; 31:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amer HM. Bovine-like coronaviruses in domestic and wild ruminants. Anim Health Res Rev. 2018; 19:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Outbreak of severe acute respiratory syndrome - worldwide, 2003. MMWR Morb Mortal Wkly Rep. 2003; 52:226-228. [PubMed] [Google Scholar]
- 9. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. The New England journal of medicine. 2012; 367:1814-20. [DOI] [PubMed] [Google Scholar]
- 10. Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al- Saeed MS, Hashem AM, Madani TA. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014; 370:2499-2505. [DOI] [PubMed] [Google Scholar]
- 11. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020; 382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perlman S. Another Decade, Another Coronavirus. N Engl J Med. 2020; 382:760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. Pneumonia of unknown cause - China. 2020. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (accessed April 16, 2019).
- 15. Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020; 382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Novel Coronavirus ( 2019- nCoV): situation report, 3. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200123-sitrep-3-2019-ncov.pdf?sfvrsn=d6d23643_8 (accessed April 16, 2019).
- 17. World Health Organization. Coronavirus disease 2019 [COVID-19] Situation Report - 70. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200330-sitrep-70-covid-19.pdf?sfvrsn=7e0fe3f8_4 (accessed April 16, 2019).
- 18. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020; doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. To KK, Tsang OT, Chik-Yan Yip C, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020; pii:ciaa149 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burke RM, Midgley CM, Dratch A, Fenstersheib M, Haupt T, Holshue M, Ghinai I, Jarashow MC, Lo J, McPherson TD, Rudman S, Scott S, Hall AJ, Fry AM, Rolfes MA. Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19 - United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:245-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020; 382:970-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu P, Zhu J, Zhang Z, Han Y, Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis. 2020; pii:jiaa077 doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020; doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020; doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020; 93:284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kakimoto K, Kamiya H, Yamagishi T, Matsui T, Suzuki M, Wakita T. Initial Investigation of Transmission of COVID-19 Among Crew Members During Quarantine of a Cruise Ship - Yokohama, Japan, February 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:312-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galvani AP, May RM. Epidemiology: dimensions of superspreading. Nature. 2005; 438:293-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015; 20:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agua-Agum J, Ariyarajah A, Aylward B, et al. Exposure Patterns Driving Ebola Transmission in West Africa: A Retrospective Observational Study. PLoS Med. 2016; 13:e1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Novel Coronavirus Q & A [for the public], Ministry of Health, Labour and Welfare, as of March 7, 2020. . http://japan.kantei.go.jp/98_abe/actions/202003/_00008.html (accessed April 16, 2019).
- 31. Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020; pii:ciaa330 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020; 21:335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, Zhou J, Cai H, Fang Q, Yu F, Fan J, Xu K, Chen Y, Sheng J. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg Infect Dis. 2020; 26:doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395:809-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020; pii:ciaa200 doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang Z-J. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020; doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020; 20:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020; 382:1177-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manual for Collection and Transportation of Samples from Suspected COVID-19 Patients, updated version [February 28, 2020], https://www.niid.go.jp/niid/images/pathol/pdf/2019-nCoV_200228.pdf (accessed April 16, 2019).
- 40. World Health Organization. Laboratory biosafety guidance related to coronavirus disease 2019 (COVID-19): interim guidance, 12 February 2020. https://apps.who.int/iris/handle/10665/331138 (accessed April 16, 2019).
- 41. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020; 12:200343 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B, Cao H, Li L. Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020; pii: ciaa199. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) Infection, Updated January 30, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed April 16, 2019).
- 44. World Health Organization. Novel Coronavirus (2019- nCoV) technical guidance: Patient management. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management (accessed April 16, 2019).
- 45. Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004; 59:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chong YP, Song JY, Seo YB, Choi JP, Shin HS. Antiviral Treatment Guidelines for Middle East Respiratory Syndrome. Infect Chemother. 2015; 47:212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir- Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020; doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mulangu S, Dodd LE, Davey RT, Jr, et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics N Engl J Med. 2019; 381:2293-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30:269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020; 382:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sissoko D, Laouenan C, Folkesson E, et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med. 2016; 13:e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bai CQ, Mu JS, Kargbo D, et al. Clinical and virological characteristics of Ebola virus disease patients treated with favipiravir (T-705)-sierra leone, 2014. Clin Infect Dis. 2016; 63:1288-1294. [DOI] [PubMed] [Google Scholar]
- 53. Cai Q, Yang M, Liu D, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering. 2020; doi: https://doi.org/10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, Shimojima M, Fukushi S. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. 2020; doi: https://doi.org/10.1101/2020.03.11.987016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, kato Y, Morishima T. Three patients with mild-to- moderate pneumonia caused by COVID-19, which was improved by ciclesonide inhalation. Posted in home page of the Japanese Association for Infectious Disease. http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_200302_02.pdf (accessed April 16, 2019). (in Japanese) .
- 56. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020; 395:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. World Health Organization. Guidelines for the treatment of malaria: World Health Organization; 2015. https://www.who.int/malaria/publications/atoz/9789241549127/en/ (accessed April 16, 2019) (in Japanese) .
- 58. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020; 14:72-73. [DOI] [PubMed] [Google Scholar]
- 59. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020; doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016; 14:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005; 24:44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015; 4:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shen C, Wang Z, Zhao F, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. HSB-IDCD Notification No. 0218-3, dated February 18, 2020. Handling of Discharge from Hospital and Restrictions on Work Attendance of COVID-19 Patients under the Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases [partial revision]. https://www.mhlw.go.jp/content/10900000/000597947.pdf (accessed April 16, 2019). (in Japanese) .
- 65. Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020; doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020; doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheng PK, Wong DA, Tong LK, Ip SM, Lo AC, Lau CS, Yeung EY, Lim WW. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004; 363:1699-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oboho IK, Tomczyk SM, Al-Asmari AM, Banjar AA, Al-Mugti H, Aloraini MS, Alkhaldi KZ, Almohammadi EL, Alraddadi BM, Gerber SI, Swerdlow DL, Watson JT, Madani TA. 2014 MERS-CoV outbreak in Jeddah - a link to health care facilities. N Engl J Med. 2015; 372:846-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. World Health Organization. Rational use of personal protective equipment for coronavirus disease 2019 [COVID-19]. https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf (accessed April 16, 2019).


