Summary
Mechanisms accounting for sex differences in the incidence of adverse events caused by fluoropyrimidine treatments, and histologic differences in efficacy are insufficiently understood. We determined differences between the sexes in terms of the safety of S-1 plus oxaliplatin (SOX)/bevacizumab-versus-l-leucovorin, 5-fluorouracil (5-FU) and oxaliplatin (FOLFOX)/bevacizumab, and the impact of histology on their therapeutic effects, in 512 unresectable metastatic colorectal cancer patients from the SOFT phase III study. Nausea (OR: 2.88, P < 0.001) and vomiting (OR: 3.04, P = 0.005) occurred more frequently in females than males treated with SOX/bevacizumab, while nausea (OR: 2.12, P = 0.006), vomiting (OR: 3.26, P = 0.004), leukopenia (OR: 2.61, P < 0.001), neutropenia (OR: 2.92, P < 0.001), and alopecia (OR: 4.13, P < 0.001) were higher in females on FOLFOX/bevacizumab. Mean relative dose intensities (RDIs) of S-1 during all cycles of SOX/bevacizumab were significantly lower in females (73.9%) than males (81.5%) (P < 0.001), while RDIs of continuous infusion of 5-FU in the FOLFOX/bevacizumab regimen were 75.0% in females and 80.5% in males (P = 0.005). No significant differences in efficacy with regard to overall survival (OS) and progression-free survival (PFS) were identified between the sexes for either SOX/bevacizumab or FOLFOX/bevacizumab treatment. Patients with poorly-differentiated adenocarcinoma had significantly worse OS (HR: 2.72, 95% CI: 1.67-4.44, P < 0.0001) and PFS (HR: 1.89, 95% CI: 1.18-3.02, P = 0.0079) than patients with well- or moderately-differentiated adenocarcinoma. Female patients experienced more frequent and severe adverse reactions to SOX/bevacizumab and FOLFOX/bevacizumab and a worse prognosis for poorly-differentiated adenocarcinoma were confirmed in this phase III study. This warrants further translational research to identify the responsible mechanisms.
Keywords: gender, fluorouracil, S-1, poorly differentiated adenocarcinoma, bevacizumab
Introduction
Colorectal cancer is the second leading cause of cancer-related deaths worldwide (1). Fluoropyrimidines and their biochemical modulators have been key drugs used in strategies for treating patients with metastatic colorectal cancer for more than 6 decades. FOLFOX (leucovorin, 5-fluorouracil (5-FU), and oxaliplatin) or FOLFIRI (leucovorin, 5-FU, and irinotecan) plus bevacizumab have been widely used as first-line treatment options for metastatic colorectal cancer (2,3). The SOFT trial showed that oral S-1 and oxaliplatin (SOX) plus bevacizumab was non-inferior to FOLFOX plus bevacizumab and the TRICOLORE (4) study showed that S-1 and irinotecan plus bevacizumab was non-inferior to FOLFOX or capecitabine/oxaliplatin (CapeOX) plus bevacizumab for progression-free survival (PFS), thereby establishing the therapeutic usefulness of this agent (5-7). S-1 is an oral anticancer drug that combines tegafur, a prodrug of 5-FU, with two modulators. The first of these, gimeracil, reversibly inhibits DPD, the primary metabolizing enzyme of 5-FU, and thus maintains higher 5-FU levels in the blood for a longer period of time. The second is oteracil potassium, which suppresses, and thereby decreases, the activity and toxicity of 5-FU for normal gastrointestinal tissue (8). In patients with compromised renal function, gimeracil clearance is decreased, leading to high concentrations of 5-FU in blood and an increased risk of 5-FU-related side effects (9). Previously, we found that the incidence of grade 3 or higher diarrhea in metastatic colorectal cancer patients in the SOFT trial who were treated with SOX and bevacizumab depended on renal function (5). Thus, the incidence of diarrhea in patients with a creatinine clearance (Ccr) of < 70 mL/ min before treatment exceeded 20% and tended to be higher than in patients with a Ccr of ≥ 70 mL/min.
It had been previously established that female patients treated with fluoropyrimidines developed leukopenia, stomatitis, diarrhea, nausea, vomiting, and alopecia more often and more severely than males (10-15). It was suggested that poorer clearance of 5-FU, reduced activity of dihydropyrimidine dehydrogenase (DPD; the initial enzyme in the catabolism of 5-FU (10,16)), and polymorphism of DPD or thymidylate synthase (13,17) are possible causes of such sex-related differences in adverse events during fluoropyrimidine treatment. However, the fundamental cause of this perceived sex difference is not yet known. DPD expression and activity in human liver did not reveal any sex-related differences (13). Dose modification and the administration schedule of 5-FU, and changing optimal supportive therapies for female patients, are not usually considered and are not implemented in clinical practice. Sex differences in the toxicity of anti-cancer agents are not only observed for 5-FU but also cisplatin, doxorubicin, and other anti-cancer agents (18). Female patients had significantly higher rates of nausea and vomiting, but the cause of the sex discrepancy is also unknown (19). We need to investigate any sex differences in disease and biological response in comparison of males and females genetically and epigenetically.
Several reports have documented a poor prognosis for advanced resectable colorectal cancer with poorly-differentiated adenocarcinoma histology relative to well-or moderately-differentiated adenocarcinoma. However, whether this also applies to unresectable metastatic cancer during palliative chemotherapy was not known (20,21). Poorly-differentiated adenocarcinoma is closely associated with the presence of microsatellite instability (MSI) and is found more often in females. Microsatellite unstable poorly-differentiated adenocarcinoma (23%, 12/53), which is characterized as having a right colon predilection, larger size, and infrequent lymph node metastasis, has a better prognosis than microsatellite-stable poorly-differentiated adenocarcinoma (74%, 41/53) (22,23).
In the present study, we aimed to evaluate and compare the safety of the SOX plus bevacizumab and FOLFOX plus bevacizumab regimens in female and male patients with metastatic colorectal cancer and the impact on histological tumor type treatment efficacy.
Patients and Methods
Patients
The SOFT trial was a randomized, open-label, phase III study that compared the efficacy and safety of the SOX/ bevacizumab and FOLFOX/bevacizumab regimens in patients with unresectable advanced or recurrent metastatic colorectal cancer (5). SOX/bevacizumab was confirmed to be non-inferior to FOLFOX/bevacizumab in 512 randomized patients. In the SOX/bevacizumab regimen, S-1 was given orally for the first 2 weeks of a 3-week cycle, oxaliplatin at a dose of 130 mg/ m2 and bevacizumab at 7.5 mg/kg infused on day 1. In the FOLFOX/bevacizumab regimen, patients received a 5 mg/kg intravenous infusion of bevacizumab and a simultaneous intravenous infusion of 85 mg/m2 oxaliplatin, 200 mg/m2 l-leucovorin, 400 mg/m2 bolus fluorouracil, and 2,400 mg/m2 infused fluorouracil (46 h) delivered with an infusion pump on day 1 to 2 of a 2-week cycle. A 5-hydroxytryptamine-3 (5-HT3) receptor antagonist and dexamethasone were usually given to patients treated with either SOX or FOLFOX. The Ccr was estimated using the Cockcroft-Gault equation.
Statistical analysis
We did analyses of survival by modified intention to treat: we excluded individuals who underwent randomization but who were subsequently shown not to meet inclusion criteria. Patients who received at least one dose of the assigned study drugs were included in analyses of safety.
The incidence of adverse events during the first 8 weeks and then all periods was compared between the two regimens using Fisher's exact test and logistic regression for males and females separately. Multivariate analyses for toxicities were also carried out using a logistic regression model. Adverse events were assessed in accordance with the Common Terminology Criteria for Adverse Events version 3.0. Median OS and PFS were estimated using the Kaplan-Meier method. Statistical significance was considered to be P < 0.05. Multivariate analyses by Cox proportional hazards model was used to estimate hazard ratios (HRs) of prognostic factors for OS and PFS. Treatment delivery was evaluated for females and males in both treatment groups.
Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Baseline characteristics of all patients enrolled in the SOFT study were similar in the two sexes and in the treatment groups, although the proportion of patients with liver metastases in the SOX/bevacizumab group was higher in females than males. The proportion of female patients treated with SOX/bevacizumab and FOLFOX/bevacizumab was 33% (83/250) and 37% (93/249), respectively, for primary analysis (Table 1).
Table 1. Baseline characteristics of male and female patients.
| Items | SOX/Bev (n = 250) |
P | FOLFOX/Bev (n = 249) |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 167) | Female (n = 83) | Male (n = 156) | Female (n = 93) | |||||||
| n | % | n | % | n | % | n | % | |||
| Age | ||||||||||
| < 70 | 120 | 71.9 | 67 | 80.7 | 0.164 | 116 | 74.4 | 69 | 74.2 | 0.164 |
| ≥ 70 | 47 | 28.1 | 16 | 19.3 | 40 | 25.6 | 24 | 25.8 | ||
| Primary lesion | ||||||||||
| Colon | 80 | 47.9 | 48 | 57.8 | 0.422 | 76 | 48.7 | 47 | 50.5 | 0.422 |
| Rectosigmoid | 30 | 18.0 | 14 | 16.9 | 21 | 13.5 | 19 | 20.4 | ||
| Rectum | 55 | 32.9 | 20 | 24.1 | 59 | 37.8 | 27 | 29.0 | ||
| Others | 2 | 1.2 | 1 | 1.2 | 0 | 0 | ||||
| Differentiation assessed by histology | ||||||||||
| Well or moderate | 148 | 88.6 | 67 | 80.7 | 0.223 | 135 | 86.5 | 77 | 82.8 | 0.223 |
| Poorly | 6 | 3.6 | 5 | 6.0 | 4 | 2.6 | 6 | 6.5 | ||
| Other | 13 | 7.8 | 11 | 13.3 | 17 | 10.9 | 10 | 10.8 | ||
| Adjuvant chemotherapy for colorectal cancer | ||||||||||
| No | 142 | 85.0 | 72 | 86.7 | 0.849 | 128 | 82.0 | 83 | 89.2 | 0.849 |
| Yes | 25 | 15.0 | 11 | 13.3 | 28 | 18.0 | 10 | 10.8 | ||
| Target lesion | ||||||||||
| No | 12 | 7.2 | 9 | 10.8 | 0.340 | 11 | 7.0 | 11 | 11.8 | 0.340 |
| Yes | 155 | 92.8 | 74 | 89.2 | 145 | 93.0 | 82 | 88.2 | ||
| Liver metastases | ||||||||||
| No | 45 | 26.9 | 38 | 45.8 | 0.004 | 53 | 34.0 | 34 | 36.6 | 0.004 |
| Yes | 122 | 73.1 | 45 | 54.2 | 103 | 66.0 | 59 | 63.4 | ||
| Lung metastases | ||||||||||
| No | 93 | 55.7 | 50 | 60.2 | 0.501 | 87 | 55.8 | 47 | 50.5 | 0.501 |
| Yes | 74 | 44.3 | 33 | 39.8 | 69 | 44.2 | 46 | 49.5 | ||
| Lymph node metastases | ||||||||||
| No | 123 | 73.7 | 66 | 79.5 | 0.350 | 119 | 76.3 | 67 | 72.0 | 0.350 |
| Yes | 44 | 26.3 | 17 | 20.5 | 37 | 23.7 | 26 | 28.0 | ||
| Other metastases | ||||||||||
| No | 135 | 80.8 | 53 | 63.9 | 0.005 | 136 | 87.2 | 70 | 75.3 | 0.005 |
| Yes | 32 | 19.2 | 30 | 36.1 | 20 | 12.8 | 23 | 24.7 | ||
| Metastatic organs | ||||||||||
| 1 | 73 | 43.7 | 38 | 45.8 | 0.788 | 83 | 53.2 | 44 | 47.3 | 0.788 |
| ≥ 2 | 94 | 56.3 | 45 | 54.2 | 73 | 46.8 | 49 | 52.7 | ||
P: Fisher's exact test. Bev, bevacizumab; FOLFOX, 5-FU/l-leucovorin plus oxaliplatin; SOX, S-1 plus oxaliplatin.
Safety
The most common hematologic adverse events of grade 3 or higher were leukopenia in 21 (8%) of 249 patients given FOLFOX/bevacizumab vs. 6 (2%) of 250 given SOX/bevacizumab (P = 0.0029) and neutropenia in 84 (34%) vs. 22 (9%) (P < 0.0001). Grade 3 or higher diarrhea in 23 (9%) vs. 7 (3%) (P = 0.0040) were significantly more common in patients given SOX/bevacizumab than in those given FOLFOX6/bevacizumab. Adverse events are shown in Table 2 (Appendix). Female patients treated with SOX/bevacizumab developed nausea and vomiting significantly more frequently than males, while females treated with FOLFOX/bevacizumab exhibited more leukopenia, neutropenia, nausea, vomiting, and alopecia 8 weeks after the beginning of each treatment cycle and over the entire treatment period. The difference between the sexes in the incidence of nausea and vomiting after FOLFOX/bevacizumab was more marked in patients with Ccr > 70 mL/min. According to multivariate analysis, sex was an independent predictive factor for nausea and vomiting due to SOX/bevacizumab, and for leukopenia, neutropenia, nausea, vomiting, and alopecia due to FOLFOX/ bevacizumab at 8 weeks and over all cycles (Table 3, Appendix). Thrombocytopenia with SOX/bevacizumab and FOLFOX/bevacizumab was more frequent in patients with Ccr < 70 mL/min and lower body mass index (BMI) after 8 weeks. Thrombocytopenia after FOLFOX/bevacizumab also developed more often in patients ≥ 70 years of age.
Table 2. Adverse events of any grade after 8 weeks of therapy and over the entire treatment period with S-1 plus oxaliplatin and bevacizumab or 5-FU/l-LV plus oxaliplatin and bevacizumab in patients stratified by creatinine clearance.
| SOX/Bev (n = 250) | FOLFOX/Bev (n = 249) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (n = 83) |
Male (n = 167) |
Female (n = 93) |
Male (n = 156) |
||||||||||||
| n | % | n | % | Fisher P(a) | OR [95% CI] | P | n | % | n | % | Fisher P(a) | OR [95% CI] | P | ||
| 8 weeks | |||||||||||||||
| Leukopenia | 30 | 36.1 | 47 | 28.1 | 0.244 | 1.45 [0.83-2.53] | 0.198 | 63 | 67.7 | 70 | 44.9 | <0.001 | 2.58 [1.51-4.41] | <0.001 | |
| CCr 70 mL/min > | 9 | 50.0 | 12 | 40.0 | 0.558 | 1.50 [0.46-4.87] | 0.500 | 22 | 75.9 | 13 | 44.8 | 0.031 | 3.87 [1.26-11.9] | 0.018 | |
| 70 mL/min ≦ | 21 | 32.3 | 35 | 25.5 | 0.319 | 1.39 [0.73-2.65] | 0.317 | 41 | 64.1 | 57 | 44.9 | 0.014 | 2.19 [1.18-4.07] | 0.013 | |
| Neutropenia | 26 | 31.3 | 50 | 29.9 | 0.884 | 1.07 [0.60-1.89] | 0.823 | 61 | 65.6 | 63 | 40.4 | <0.001 | 2.81 [1.65-4.80] | <0.001 | |
| CCr 70 mL/min > | 10 | 55.6 | 10 | 33.3 | 0.147 | 2.50 [0.75-8.30] | 0.135 | 18 | 62.1 | 11 | 37.9 | 0.114 | 2.68 [0.93-7.74] | 0.069 | |
| 70 mL/min ≦ | 16 | 24.6 | 40 | 29.2 | 0.614 | 0.79 [0.40-1.55] | 0.497 | 43 | 67.2 | 52 | 40.9 | <0.001 | 2.95 [1.57-5.55] | <0.001 | |
| Thrombocytopenia | 21 | 25.3 | 55 | 32.9 | 0.245 | 0.69 [0.38-1.25] | 0.218 | 25 | 26.9 | 51 | 32.7 | 0.394 | 0.76 [0.43-1.34] | 0.336 | |
| CCr 70 mL/min > | 8 | 44.4 | 16 | 53.3 | 0.766 | 0.70 [0.22-2.27] | 0.552 | 11 | 37.9 | 14 | 48.3 | 0.596 | 0.66 [0.23-1.86] | 0.427 | |
| 70 mL/min ≦ | 13 | 20.0 | 39 | 28.5 | 0.230 | 0.63 [0.31-1.28] | 0.201 | 14 | 21.9 | 37 | 29.1 | 0.305 | 0.68 [0.34-1.38] | 0.286 | |
| Nausea | 50 | 60.2 | 55 | 32.9 | <0.001 | 3.09 [1.79-5.32] | <0.001 | 48 | 51.6 | 55 | 35.3 | 0.012 | 1.96 [1.16-3.30] | 0.012 | |
| CCr 70 mL/min > | 10 | 55.6 | 12 | 40.0 | 0.375 | 1.88 [0.58-6.12] | 0.297 | 11 | 37.9 | 8 | 27.6 | 0.577 | 1.60 [0.53-4.85] | 0.403 | |
| 70 mL/min ≦ | 40 | 61.5 | 43 | 31.4 | <0.001 | 3.50 [1.89-6.48] | <0.001 | 37 | 57.8 | 47 | 37.0 | 0.009 | 2.33 [1.26-4.31] | 0.007 | |
| Vomiting | 19 | 22.9 | 14 | 8.4 | 0.003 | 3.24 [1.53-6.87] | 0.002 | 18 | 19.4 | 11 | 7.1 | 0.007 | 3.16 [1.42-7.04] | 0.005 | |
| CCr 70 mL/min > | 7 | 38.9 | 4 | 13.3 | 0.074 | 4.14 [1.00-17.1] | 0.049 | 5 | 17.2 | 1 | 3.4 | 0.194 | 5.83 [0.64-53.5] | 0.119 | |
| 70 mL/min ≦ | 12 | 18.5 | 10 | 7.3 | 0.028 | 2.88 [1.17-7.06] | 0.021 | 13 | 20.3 | 10 | 7.9 | 0.018 | 2.98 [1.23-7.25] | 0.016 | |
| Diarrhea | 31 | 37.3 | 56 | 33.5 | 0.575 | 1.18 [0.68-2.05] | 0.551 | 27 | 29.0 | 29 | 18.6 | 0.061 | 1.79 [0.98-3.27] | 0.058 | |
| CCr 70 mL/min > | 12 | 66.7 | 15 | 50.0 | 0.369 | 2.00 [0.59-6.73] | 0.263 | 9 | 31.0 | 5 | 17.2 | 0.358 | 2.16 [0.62-7.49] | 0.225 | |
| 70 mL/min ≦ | 19 | 29.2 | 41 | 29.9 | 1.000 | 0.97 [0.51-1.85] | 0.920 | 18 | 28.1 | 24 | 18.9 | 0.195 | 1.68 [0.83-3.39] | 0.148 | |
| Stomatitis | 17 | 20.5 | 35 | 21.0 | 1.000 | 0.97 [0.51-1.86] | 0.931 | 31 | 33.3 | 43 | 27.6 | 0.390 | 1.31 [0.75-2.29] | 0.336 | |
| CCr 70 mL/min > | 4 | 22.2 | 3 | 10.0 | 0.400 | 2.57 [0.50-13.1] | 0.256 | 9 | 31.0 | 12 | 41.4 | 0.585 | 0.64 [0.22-1.88] | 0.414 | |
| 70 mL/min ≦ | 13 | 20.0 | 32 | 23.4 | 0.718 | 0.82 [0.40-1.69] | 0.593 | 22 | 34.4 | 31 | 24.4 | 0.172 | 1.62 [0.84-3.13] | 0.148 | |
| Alopecia | 3 | 3.6 | 1 | 0.6 | 0.108 | 6.23 [0.64-60.8] | 0.116 | 28 | 30.1 | 14 | 9.0 | <0.001 | 4.37 [2.16-8.85] | <0.001 | |
| CCr 70 mL/min > | 0 | 0 | 0 | 0 | - | - | - | 11 | 37.9 | 3 | 10.3 | 0.030 | 5.30 [1.29-21.7] | 0.021 | |
| 70 mL/min ≦ | 3 | 4.6 | 1 | 0.7 | 0.099 | 6.58 [0.67-64.5] | 0.106 | 17 | 26.6 | 11 | 8.7 | 0.002 | 3.81 [1.66-8.75] | 0.002 | |
| Sensory neuropathy | 60 | 72.3 | 128 | 76.6 | 0.534 | 0.80 [0.44-1.45] | 0.453 | 62 | 66.7 | 106 | 67.9 | 0.889 | 0.94 [0.55-1.63] | 0.834 | |
| CCr 70 mL/min > | 14 | 77.8 | 22 | 73.3 | 1.000 | 1.27 [0.32-5.03] | 0.731 | 20 | 69.0 | 18 | 62.1 | 0.783 | 1.36 [0.46-4.03] | 0.581 | |
| 70 mL/min ≦ | 46 | 70.8 | 106 | 77.4 | 0.383 | 0.71 [0.36-1.38] | 0.311 | 42 | 65.6 | 88 | 69.3 | 0.625 | 0.85 [0.45-1.60] | 0.263 | |
|
All periods | |||||||||||||||
| Leukopenia | 49 | 59.0 | 96 | 57.5 | 0.892 | 1.07 [0.63-1.82] | 0.815 | 76 | 81.7 | 99 | 63.5 | 0.003 | 2.57 [1.39-4.78] | 0.003 | |
| CCr 70 mL/min > | 13 | 72.2 | 20 | 66.7 | 0.757 | 1.30 [0.36-4.68] | 0.688 | 24 | 82.8 | 22 | 75.9 | 0.747 | 1.53 [0.42-5.52] | 0.518 | |
| 70 mL/min ≦ | 36 | 55.4 | 76 | 55.5 | 1.000 | 1.00 [0.55-1.80] | 0.990 | 52 | 81.3 | 77 | 60.6 | 0.005 | 2.81 [1.37-5.79] | 0.005 | |
| Neutropenia | 50 | 60.2 | 98 | 58.7 | 0.892 | 1.07 [0.62-1.82] | 0.814 | 76 | 81.7 | 104 | 66.7 | 0.013 | 2.24 [1.20-4.17] | 0.011 | |
| CCr 70 mL/min > | 12 | 66.7 | 16 | 53.3 | 0.546 | 1.75 [0.52-5.89] | 0.367 | 24 | 82.8 | 23 | 79.3 | 1.000 | 1.25 [0.34-4.68] | 0.738 | |
| 70 mL/min ≦ | 38 | 58.5 | 82 | 59.9 | 0.879 | 0.94 [0.52-1.72] | 0.850 | 52 | 81.3 | 81 | 63.8 | 0.013 | 2.46 [1.19-5.08] | 0.015 | |
| Thrombocytopenia | 52 | 62.7 | 123 | 73.7 | 0.080 | 0.60 [0.34-1.05] | 0.075 | 45 | 48.4 | 90 | 57.7 | 0.189 | 0.69 [0.41-1.15] | 0.155 | |
| CCr 70 mL/min > | 12 | 66.7 | 23 | 76.7 | 0.513 | 0.61 [0.17-2.22] | 0.452 | 16 | 55.2 | 21 | 72.4 | 0.274 | 0.47 [0.16-1.40] | 0.175 | |
| 70 mL/min ≦ | 40 | 61.5 | 100 | 73.0 | 0.106 | 0.59 [0.32-1.11] | 0.101 | 29 | 45.3 | 69 | 54.3 | 0.284 | 0.70 [0.38-1.27] | 0.240 | |
| Nausea | 58 | 69.9 | 72 | 43.1 | <0.001 | 3.06 [1.75-5.36] | <0.001 | 62 | 66.7 | 77 | 49.4 | 0.009 | 2.05 [1.20-3.50] | 0.008 | |
| CCr 70 mL/min > | 12 | 66.7 | 14 | 46.7 | 0.237 | 2.29 [0.68-7.70] | 0.182 | 16 | 55.2 | 13 | 44.8 | 0.600 | 1.52 [0.54-4.26] | 0.432 | |
| 70 mL/min ≦ | 46 | 70.8 | 58 | 42.3 | <0.001 | 3.30 [1.75-6.21] | <0.001 | 46 | 71.9 | 64 | 50.4 | 0.005 | 2.52 [1.32-4.80] | 0.005 | |
| Vomiting | 28 | 33.7 | 23 | 13.8 | <0.001 | 3.19 [1.69-6.00] | <0.001 | 29 | 31.2 | 21 | 13.5 | 0.001 | 2.91 [1.54-5.50] | 0.001 | |
| CCr 70 mL/min > | 8 | 44.4 | 5 | 16.7 | 0.049 | 4.00 [1.05-15.2] | 0.042 | 10 | 34.5 | 3 | 10.3 | 0.056 | 4.56 [1.10-18.9] | 0.036 | |
| 70 mL/min ≦ | 20 | 30.8 | 18 | 13.1 | 0.004 | 2.94 [1.43-6.06] | 0.004 | 19 | 29.7 | 18 | 14.2 | 0.019 | 2.56 [1.23-5.32] | 0.012 | |
| Diarrhea | 48 | 57.8 | 85 | 50.9 | 0.347 | 1.32 [0.78-2.25] | 0.301 | 41 | 44.1 | 55 | 35.3 | 0.180 | 1.45 [0.86-2.45] | 0.167 | |
| CCr 70 mL/min > | 14 | 77.8 | 16 | 53.3 | 0.127 | 3.06 [0.82-11.5] | 0.097 | 13 | 44.8 | 10 | 34.5 | 0.592 | 1.54 [0.54-4.45] | 0.422 | |
| 70 mL/min ≦ | 34 | 52.3 | 69 | 50.4 | 0.881 | 1.08 [0.60-1.95] | 0.797 | 28 | 43.8 | 45 | 35.4 | 0.274 | 1.42 [0.77-2.62] | 0.265 | |
| Stomatitis | 31 | 37.3 | 72 | 43.1 | 0.415 | 0.79 [0.46-1.35] | 0.384 | 48 | 51.6 | 75 | 48.1 | 0.603 | 1.15 [0.69-1.93] | 0.590 | |
| CCr 70 mL/min > | 5 | 27.8 | 8 | 26.7 | 1 | 1.06 [0.29-3.92] | 0.933 | 13 | 44.8 | 16 | 55.2 | 0.600 | 0.66 [0.24-1.86] | 0.432 | |
| 70 mL/min ≦ | 26 | 40.0 | 64 | 46.7 | 0.449 | 0.76 [0.42-1.38] | 0.370 | 35 | 54.7 | 59 | 46.5 | 0.289 | 1.39 [0.76-2.54] | 0.284 | |
| Alopecia | 6 | 7.2 | 9 | 5.4 | 0.579 | 1.37 [0.47-3.98] | 0.565 | 36 | 38.7 | 25 | 16.0 | <0.001 | 3.31 [1.82-6.02] | <0.001 | |
| CCr 70 mL/min > | 0 | 0 | 2 | 6.7 | 0.521 | - | 0.952 | 14 | 48.3 | 3 | 10.3 | 0.003 | 8.09 [2.00-32.8] | 0.003 | |
| 70 mL/min ≦ | 6 | 9.2 | 7 | 5.1 | 0.357 | 1.89 [0.61-5.86] | 0.271 | 22 | 34.4 | 22 | 17.3 | 0.011 | 2.50 [1.25-4.99] | 0.009 | |
| Sensory neuropathy | 74 | 89.2 | 154 | 92.2 | 0.479 | 0.69 [0.28-1.70] | 0.423 | 85 | 91.4 | 139 | 89.1 | 0.665 | 1.30 [0.54-3.14] | 0.561 | |
| CCr 70 mL/min > | 16 | 88.9 | 26 | 86.7 | 1.000 | 1.23 [0.20-7.51] | 0.822 | 27 | 93.1 | 27 | 93.1 | 1.000 | 1.00 [0.13-7.62] | 1.000 | |
| 70 mL/min ≦ | 58 | 89.2 | 128 | 93.4 | 0.403 | 0.58 [0.21-1.64] | 0.306 | 58 | 90.6 | 112 | 88.2 | 0.807 | 1.30 [0.48-3.51] | 0.612 | |
(a) Fishers exact test; comparing frequency of adverse events.
Bev, bevacizumab; CCr, creatinine clearance rate; FOLFOX, 5-FU/l-leucovorin plus oxaliplatin; OR, odds ratio; SOX, S-1 plus oxaliplatin; 95% CI, 95% confidence interval.
Table 3. Multivariate analyses for adverse events after 8 weeks and all periods from the beginning of treatment with S-1 plus oxaliplatin with bevacizumab or 5-FU/l-LV plus oxaliplatin with bevacizumab.
| Objective variables | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Explanatory variables |
Base category |
Leukopenia | Neutropenia | Thrombo-cytopenia | Nausea | Vomiting | Diarrhea | Stomatitis | Alopecia | Sensory neuropathy | |||
| 8 weeks | SOX/Bev | Sex | male | OR | 1.54 | 1.15 | 0.62 | 2.88 | 3.04 | 1.18 | 0.95 | 5.94 | 0.72 |
| Female vs. male | 95% CI | 0.86-2.74 | 0.64-2.07 | 0.33-1.15 | 1.66-5.01 | 1.41-6.56 | 0.67-2.09 | 0.49-1.83 | 0.60-58.80 | 0.39-1.33 | |||
| P value | 0.147 | 0.642 | 0.131 | <0.001 | 0.005 | 0.572 | 0.871 | 0.128 | 0.291 | ||||
| CCr | 70 mL/min > | OR | 0.61 | 0.70 | 0.31 | 0.66 | 0.37 | 0.36 | 1.45 | - | 0.77 | ||
| 70 mL/min ≦ vs. 70 mL/min > | 95% CI | 0.31-1.22 | 0.35-1.41 | 0.15-0.62 | 0.32-1.35 | 0.15-0.91 | 0.18-0.71 | 0.58-3.60 | - | 0.35-1.67 | |||
| P value | 0.165 | 0.314 | 0.001 | 0.255 | 0.031 | 0.003 | 0.424 | 0.958 | 0.502 | ||||
| BMI | median > | OR | 0.96 | 1.01 | 1.93 | 1.24 | 1.37 | 0.79 | 1.00 | 2.55 | 1.57 | ||
| Median ≦ vs. median > per sex | 95% CI | 0.55-1.69 | 0.57-1.76 | 1.08-3.45 | 0.73-2.12 | 0.63-2.98 | 0.46-1.37 | 0.54-1.87 | 0.26-25.45 | 0.87-2.83 | |||
| P value | 0.899 | 0.986 | 0.028 | 0.428 | 0.429 | 0.406 | 0.995 | 0.426 | 0.139 | ||||
| Age | 70 > | OR | 1.92 | 2.36 | 1.25 | 0.49 | 0.82 | 1.14 | 0.61 | - | 0.56 | ||
| 70 ≦ vs. 70 > | 95% CI | 1.02-3.61 | 1.26-4.42 | 0.65-2.41 | 0.25-0.95 | 0.32-2.08 | 0.60-2.16 | 0.27-1.37 | - | 0.29-1.09 | |||
| P value | 0.043 | 0.007 | 0.497 | 0.035 | 0.670 | 0.686 | 0.226 | 0.954 | 0.09 | ||||
| FOLFOX/Bev | Sex | male | OR | 2.61 | 2.92 | 0.68 | 2.12 | 3.26 | 1.82 | 1.25 | 4.13 | 0.98 | |
| Female vs. male | 95% CI | 1.51-4.51 | 1.69-5.02 | 0.38-1.24 | 1.24-3.64 | 1.45-7.35 | 0.99-3.36 | 0.71-2.20 | 2.02-8.43 | 0.56-1.71 | |||
| P value | <0.001 | <0.001 | 0.212 | 0.006 | 0.004 | 0.055 | 0.437 | <0.001 | 0.945 | ||||
| CCr | 70 mL/min > | OR | 0.89 | 1.21 | 0.46 | 1.72 | 1.63 | 0.96 | 0.62 | 0.70 | 1.06 | ||
| 70 mL/min ≦ vs. 70 mL/min > | 95% CI | 0.46-1.75 | 0.62-2.35 | 0.23-0.93 | 0.87-3.41 | 0.58-4.59 | 0.45-2.06 | 0.31-1.24 | 0.31-1.58 | 0.54-2.09 | |||
| P value | 0.739 | 0.580 | 0.030 | 0.121 | 0.355 | 0.914 | 0.176 | 0.391 | 0.868 | ||||
| BMI | median > | OR | 1.16 | 1.13 | 1.86 | 1.01 | 0.65 | 1.35 | 1.16 | 0.70 | 1.54 | ||
| Median ≦ vs. median > per sex | 95% CI | 0.68-1.98 | 0.67-1.92 | 1.03-3.36 | 0.59-1.71 | 0.29-1.47 | 0.73-2.51 | 0.66-2.05 | 0.34-1.43 | 0.89-2.67 | |||
| P value | 0.575 | 0.643 | 0.039 | 0.984 | 0.300 | 0.345 | 0.599 | 0.321 | 0.124 | ||||
| Age | 70 > | OR | 1.65 | 1.17 | 2.04 | 0.77 | 1.07 | 1.20 | 0.78 | 0.94 | 1.23 | ||
| 70 ≦ vs. 70 > | 95% CI | 0.88-3.09 | 0.63-2.18 | 1.08-3.83 | 0.41-1.45 | 0.41-2.80 | 0.59-2.44 | 0.40-1.52 | 0.41-2.17 | 0.64-2.37 | |||
| P value | 0.120 | 0.614 | 0.027 | 0.426 | 0.897 | 0.621 | 0.466 | 0.888 | 0.531 | ||||
| all | SOX/Bev | Sex | male | OR | 1.06 | 1.07 | 0.57 | 2.87 | 3.00 | 1.32 | 0.76 | 1.29 | 0.65 |
| Female vs. male | 95% CI | 0.61-1.82 | 0.62-1.85 | 0.32-1.01 | 1.63-5.07 | 1.58-5.71 | 0.77-2.27 | 0.44-1.33 | 0.44-3.83 | 0.26-1.62 | |||
| P value | 0.848 | 0.796 | 0.053 | <0.001 | <0.001 | 0.307 | 0.342 | 0.642 | 0.355 | ||||
| CCr | 70 mL/min > | OR | 0.59 | 1.06 | 0.72 | 0.73 | 0.55 | 0.66 | 1.89 | 1.31 | 1.33 | ||
| 70 mL/min ≦ vs. 70 mL/min > | 95% CI | 0.29-1.19 | 0.54-2.09 | 0.34-1.52 | 0.36-1.49 | 0.24-1.23 | 0.33-1.31 | 0.91-3.92 | 0.26-6.50 | 0.46-3.85 | |||
| P value | 0.140 | 0.862 | 0.391 | 0.387 | 0.144 | 0.232 | 0.090 | 0.745 | 0.355 | ||||
| BMI | median > | OR | 1.11 | 0.94 | 1.25 | 1.20 | 0.99 | 0.88 | 1.55 | 2.06 | 1.18 | ||
| Median ≦ vs. median > per sex | 95% CI | 0.66-1.86 | 0.56-1.56 | 0.72-2.18 | 0.71-2.03 | 0.51-1.89 | 0.53-1.47 | 0.92-2.61 | 0.67-6.32 | 0.48-2.89 | |||
| P value | 0.693 | 0.800 | 0.429 | 0.507 | 0.964 | 0.626 | 0.098 | 0.205 | 0.724 | ||||
| Age | 70 > | OR | 1.20 | 1.00 | 0.78 | 0.51 | 0.54 | 1.05 | 0.87 | 0.80 | 0.57 | ||
| 70 ≦ vs. 70 > | 95% CI | 0.65-2.23 | 0.54-1.83 | 0.41-1.49 | 0.27-0.96 | 0.23-1.26 | 0.58-1.93 | 0.46-1.62 | 0.21-3.09 | 0.22-1.51 | |||
| P value | 0.560 | 0.992 | 0.448 | 0.036 | 0.156 | 0.865 | 0.657 | 0.741 | 0.258 | ||||
| FOLFOX/Bev | Sex | male | OR | 2.55 | 2.23 | 0.66 | 2.16 | 2.92 | 1.45 | 1.15 | 3.29 | 1.21 | |
| Female vs. male | 95% CI | 1.35-4.79 | 1.18-4.21 | 0.38-1.13 | 1.25-3.73 | 1.52-5.58 | 0.85-2.48 | 0.68-1.94 | 1.80-6.02 | 0.49-2.95 | |||
| P value | 0.004 | 0.014 | 0.127 | 0.006 | 0.001 | 0.170 | 0.602 | <0.001 | 0.680 | ||||
| CCr | 70 mL/min > | OR | 0.76 | 0.64 | 0.60 | 1.45 | 0.77 | 0.84 | 0.86 | 0.96 | 0.54 | ||
| 70 mL/min ≦ vs. 70 mL/min > | 95% CI | 0.35-1.66 | 0.29-1.42 | 0.30-1.17 | 0.74-2.81 | 0.35-1.73 | 0.43-1.64 | 0.45-1.64 | 0.46-2.02 | 0.16-1.78 | |||
| P value | 0.491 | 0.275 | 0.134 | 0.277 | 0.534 | 0.616 | 0.648 | 0.921 | 0.310 | ||||
| BMI | median > | OR | 1.03 | 1.39 | 1.73 | 0.77 | 1.24 | 1.34 | 1.24 | 0.92 | 0.87 | ||
| Median ≦ vs. median > per sex | 95% CI | 0.57-1.83 | 0.77-2.50 | 1.01-2.95 | 0.45-1.30 | 0.64-2.40 | 0.78-2.27 | 0.74-2.07 | 0.50-1.69 | 0.37-2.04 | |||
| P value | 0.933 | 0.277 | 0.045 | 0.323 | 0.527 | 0.287 | 0.420 | 0.782 | 0.750 | ||||
| Age | 70 > | OR | 2.08 | 1.78 | 2.24 | 0.61 | 0.51 | 0.75 | 0.77 | 1.30 | 0.60 | ||
| 70 ≦ vs. 70 > | 95% CI | 0.99-4.35 | 0.85-3.74 | 1.18-4.24 | 0.33-1.12 | 0.22-1.20 | 0.40-1.41 | 0.42-1.41 | 0.64-2.63 | 0.23-1.53 | |||
| P value | 0.052 | 0.127 | 0.013 | 0.112 | 0.122 | 0.367 | 0.397 | 0.464 | 0.280 | ||||
The median BMI of female patients was 21.3 kg/m2, and the BMI of males was 22.0 kg/m2; -, not evaluable Bev, bevacizumab; BMI, body mass index; CCr, creatinine clearance; CI, confidence interval; FOLFOX, 5-FU/l-leucovorin plus oxaliplatin; OR, odds ratio; SOX, S-1 plus oxaliplatin.
The mean relative dose intensities (RDIs) of S-1 during all cycles of SOX/bevacizumab were significantly lower in females (73.9%) than males (81.5%) (P < 0.001), while the RDIs of continuous infusion of 5-FU in FOLFOX/bevacizumab were 75.0% in females and 80.5% in males (P = 0.005) (Table 4, Appendix). The RDIs of oxaliplatin were not significantly different between female and male patients treated with either SOX/bevacizumab or FOLFOX/bevacizumab.
Table 4. Total dose and relative dose intensity.
| SOX/Bev (n = 250) | FOLFOX/Bev (n = 249) | ||||||||
| Male (n = 167) |
Female (n = 83) |
P | Male (n = 156) |
Female (n = 93) |
P | ||||
| Bevacizumab | Median | 90.6 | 85.7 | 0.166 | a | 83.3 | 83.4 | 0.466 | a |
| Range | 0-100 | 0-100 | 0-100 | 36.7-100 | |||||
| Mean | 85.2 | 81.6 | 0.134 | b | 78.9 | 79.4 | 0.832 | b | |
| SD | 16.3 | 20.4 | 19.1 | 14.6 | |||||
| Oxaliplatin | Median | 77.6 | 71.9 | 0.141 | a | 64.8 | 58.3 | 0.202 | a |
| Range | 5.3-100 | 27.7-100 | 21.4-100 | 18.2-100 | |||||
| Mean | 74.3 | 70.2 | 0.157 | b | 65.3 | 61.7 | 0.219 | b | |
| SD | 21.4 | 20.7 | 23.0 | 20.7 | |||||
| l-leucovorin | Median | 87.8 | 84.8 | 0.017 | a | ||||
| Range | 48.6-100 | 55.6-100 | |||||||
| Mean | 86.4 | 82.9 | 0.017 | b | |||||
| SD | 11.0 | 11.8 | |||||||
| 5-FU,bolus | Median | 82.8 | 70.0 | 0.001 | a | ||||
| Range | 36.8-100 | 34.8-100 | |||||||
| Mean | 77.8 | 69.9 | <0.001 | b | |||||
| SD | 17.6 | 17.7 | |||||||
| 5-FU,ci | Median | 84.1 | 74.9 | 0.005 | a | ||||
| Range | 44.4-100 | 41.7-100 | |||||||
| Mean | 80.5 | 75.0 | 0.005 | b | |||||
| SD | 14.9 | 15.0 | |||||||
| S-1 | Median | 82.2 | 76.0 | 0.003 | a | ||||
| Range | 16.7-104.2 | 0-100 | |||||||
| Mean | 81.5 | 73.9 | <0.001 | b | |||||
| SD | 14.9 | 19.9 | |||||||
a: Wilcoxon rank sum test; b: t test.
ci, continuous infusion; FOLFOX/Bev, l-leucovorin, 5-fluorouracil, oxaliplatin, and bevacizumab; SOX/Bev, S-1, oxaliplatin, and bevacizumab.
Efficacy
No significant differences in efficacy with regard to OS and PFS were identified between the sexes. The worse prognostic factor was poorly differentiated adenocarcinoma for OS (P < 0.0001) and PFS (P = 0.0079) (Table 5 and Table 6 (Appendix), Figure 1).
Table 5. Prognostic factors for overall survival in patients treated with S-1 plus oxaliplatin with bevacizumab or 5-FU/l-LV plus oxaliplatin with bevacizumab.
| Variables | Base category | SOX/Bev | FOLOX/Bev | All patients | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |||
| Sex | Female vs. male | Male | 0.97 | 0.69-1.35 | 0.84 | 1.25 | 0.90-1.73 | 0.17 | 1.12 | 0.89-1.41 | 0.32 |
| Age | 70 ≦ vs. 70 > | 70 > | 1.14 | 0.80-1.62 | 0.47 | 0.95 | 0.64-1.38 | 0.77 | 1.06 | 0.82-1.36 | 0.66 |
| Primary lesion | Rectosigmoid vs. colon | Colon | 0.78 | 0.50-1.20 | 0.26 | 0.86 | 0.54-1.38 | 0.54 | 0.84 | 0.61-1.15 | 0.26 |
| Rectum vs. colon | Colon | 0.96 | 0.66-1.40 | 0.85 | 0.95 | 0.67-1.35 | 0.78 | 0.97 | 0.75-1.25 | 0.82 | |
| Histology | Poorly vs. well/ moderate | Well/ moderate | 2.61 | 1.32-5.15 | 0.0056 | 2.41 | 1.12-5.17 | 0.024 | 2.72 | 1.67-4.44 | < 0.0001 |
| Others vs. well/ moderate | Well/ moderate | 0.90 | 0.52-1.53 | 0.70 | 1.25 | 0.75-2.04 | 0.39 | 1.07 | 0.74-1.53 | 0.72 | |
| Adjuvant chemotherapy | Yes vs. No | No | 0.97 | 0.61-1.53 | 0.89 | 0.73 | 0.45-1.18 | 0.20 | 0.83 | 0.59-1.15 | 0.25 |
| Target lesion | Yes vs. No | No | 1.22 | 0.65-2.25 | 0.53 | 1.7 | 0.83-3.44 | 0.15 | 1.40 | 0.88-2.22 | 0.15 |
| Liver metastases | Yes vs. No | No | 1.12 | 0.69-1.80 | 0.63 | 1.46 | 0.88-2.38 | 0.14 | 1.27 | 0.90-1.78 | 0.16 |
| Lung metastases | Yes vs. No | No | 0.98 | 0.63-1.50 | 0.91 | 1.48 | 0.88-2.46 | 0.13 | 1.21 | 0.88-1.66 | 0.23 |
| Lymph node metastases | Yes vs. No | No | 1.25 | 0.76-2.06 | 0.38 | 1.41 | 0.89-2.20 | 0.14 | 1.33 | 0.95-1.84 | 0.092 |
| Other metastases | Yes vs. No | No | 1.29 | 0.79-2.09 | 0.31 | 1.32 | 0.78-2.223 | 0.30 | 1.26 | 0.89-1.77 | 0.18 |
| Metastatic organs | 2 ≦ vs. 1 | 1 | 0.90 | 0.52-1.55 | 0.72 | 0.98 | 0.54-1.75 | 0.94 | 0.96 | 0.65-1.39 | 0.82 |
| Treatment | SOX/Bev vs. FOLFOX/Bev | FOLFOX/Bev | - | - | - | - | - | - | 1.02 | 0.82-1.26 | 0.88 |
FOLFOX/Bev, l-leucovorin, 5-fluorouracil, oxaliplatin, and bevacizumab; HR, hazard ratio; SOX/Bev, S-1, oxaliplatin, and bevacizumab; 95% CI, 95% confidence interval.
Table 6. Prognostic factors for progression-free survival in patients treated with S-1 plus oxaliplatin with bevacizumab or 5-FU/l-LV plus oxaliplatin with bevacizumab.
| Variables | Base category | SOX/Bev | FOLOX/Bev | All patients | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |||
| Sex | Female vs. male | Male | 0.90 | 0.68-1.20 | 0.49 | 1.09 | 0.82-1.44 | 0.56 | 0.97 | 0.79-1.18 | 0.72 |
| Age | 70 ≦ vs. 70 > | 70 > | 1.05 | 0.77-1.43 | 0.77 | 0.81 | 0.58-1.12 | 0.20 | 0.89 | 0.71-1.11 | 0.30 |
| Primary lesion | Rectosigmoid vs. colon | Colon | 0.85 | 0.58-1.24 | 0.40 | 0.94 | 0.63-1.38 | 0.74 | 0.89 | 0.68-1.16 | 0.40 |
| Rectum vs. colon | Colon | 0.94 | 0.67-1.30 | 0.71 | 0.81 | 0.59-1.11 | 0.19 | 0.85 | 0.68-1.06 | 0.15 | |
| Histology | Poorly vs. well/ moderate | Well/ moderate | 3.33 | 1.74-6.35 | 0.0003 | 1.30 | 0.63-2.68 | 0.48 | 1.89 | 1.18-3.02 | 0.0079 |
| Others vs. well/ moderate | Well/ moderate | 1.07 | 0.66-1.71 | 0.78 | 0.87 | 0.55-1.37 | 0.55 | 0.91 | 0.65-1.25 | 0.55 | |
| Adjuvant chemotherapy | Yes vs. No | No | 0.68 | 0.45-1.02 | 0.059 | 0.75 | 0.51-1.11 | 0.15 | 0.75 | 0.57-0.99 | 0.038 |
| Target lesion | Yes vs. No | No | 0.85 | 0.51-1.40 | 0.52 | 1.17 | 0.70-1.95 | 0.58 | 0.96 | 0.67-1.37 | 0.83 |
| Liver metastases | Yes vs. No | No | 1.05 | 0.69-1.59 | 0.81 | 0.97 | 0.62-1.50 | 0.88 | 1.03 | 0.77-1.39 | 0.82 |
| Lung metastases | Yes vs. No | No | 1.19 | 0.82-1.72 | 0.35 | 1.26 | 0.82-1.94 | 0.29 | 1.24 | 0.94-1.62 | 0.12 |
| Lymph node metastases | Yes vs. No | No | 1.00 | 0.63-1.58 | 0.99 | 0.87 | 0.58-1.32 | 0.52 | 0.98 | 0.73-1.30 | 0.87 |
| Other metastases | Yes vs. No | No | 0.99 | 0.64-1.52 | 0.95 | 1.02 | 0.64-1.63 | 0.92 | 1.04 | 0.76-1.42 | 0.80 |
| Metastatic organs | 2 ≦ vs. 1 | 1 | 1.08 | 0.67-1.74 | 0.74 | 0.99 | 0.61-1.60 | 0.96 | 1.00 | 0.72-1.37 | 0.98 |
| Treatment | SOX/Bev vs. FOLFOX/Bev | FOLFOX/Bev | - | - | - | - | - | - | 1.06 | 0.88-1.28 | 0.51 |
FOLFOX/Bev, l-leucovorin, 5-fluorouracil, oxaliplatin, and bevacizumab; HR, hazard ratio; SOX/Bev, S-1, oxaliplatin, and bevacizumab; 95% CI, 95% confidence interval.
Figure 1.
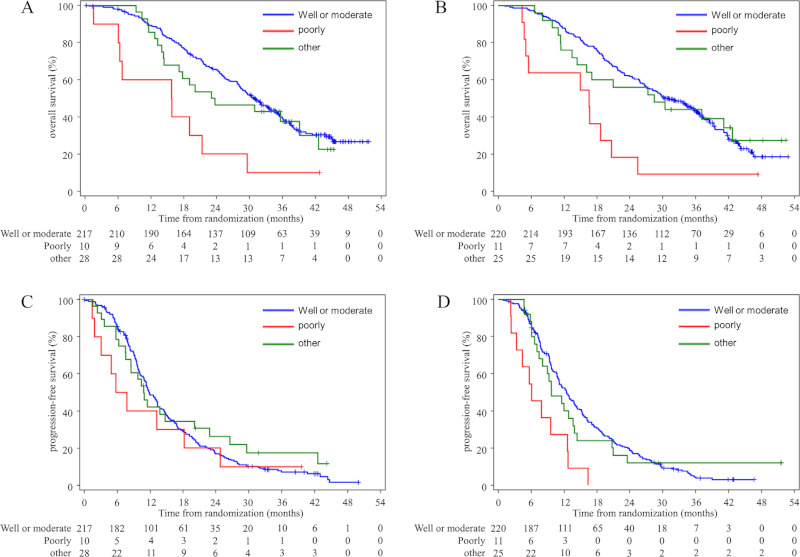
Kaplan-Meier estimates of overall survival according to histology of colorectal cancer treated with FOLFOX/ Bev (A) or SOX/Bev (B) and progression-free survival with FOLFOX/Bev (C) and SOX/Bev (D) in the full dataset.
Discussion
Nausea and vomiting due to treatment with SOX/ bevacizumab, and leukopenia, neutropenia, nausea, vomiting and alopecia due to FOLFOX/bevacizumab were more frequent in female patients than males in multivariate analysis. Sex differences in response to fluoropyrimidines and irinotecan combination therapy were also reported in a recent randomized trial, PETACC-3. These findings document a statistically significant and clinically relevant greater risk of nonhematological and objectively measurable hematological adverse events in female patients (24).
Nausea and vomiting are the most common adverse reactions associated with chemotherapy that can significantly diminish patient quality of life. To mitigate this, the use of 5-HT3receptor antagonists and dexamethasone have been recommended by the guidelines from the Japanese Society of Clinical Oncology (JSCO), the American Society of Clinical Oncology (ASCO), and the Multinational Association of Supportive Care in Cancer / European Society for Medical Oncology (MASCC/ESMO) (25-27). A Japanese phase III randomized controlled trial, the SENRI trial, was conducted in > 400 colorectal cancer patients treated with oxaliplatin-based chemotherapy. This trial established that a combination of 5-HT3receptor antagonists, dexamethasone and aprepitant/ fosaprepitant was superior to the combination of 5-HT3receptor antagonists and dexamethasone alone in controlling nausea and vomiting over the entire treatment period, especially in the late phase (28). Other recent Japanese phase III trials have documented a superior efficacy of dexamethasone on day 2 to 3, and olanzapine at a dose of 5 mg plus standard antiemetic therapy with 5-HT3receptor antagonists, aprepitant, and dexamethasone on day 1 (29). Female sex is a well-known risk factor for chemotherapy-induced nausea and vomiting, and we should therefore consider treatment options employing consecutive dexamethasone on day 2 to 3 and olanzapine for female patients receiving oxaliplatin-based regimens as is done for treatment with highly emetogenic chemotherapeutic agents (25,30).
Significantly more patients given FOLFOX/ bevacizumab had grade 3 or higher leukopenia, neutropenia, and any grade of alopecia, than patients given SOX/bevacizumab, in a first analysis (5). In addition, females treated with FOLFOX/bevacizumab suffered leukopenia, neutropenia, and alopecia significantly more frequently than male patients. The proportions of patients with grade 3 or higher sensory neuropathy did not differ significantly between the groups. Ruzzo et al. recently reported that interactions of gene polymorphisms and sex on hematological toxicity of adjuvant therapy with FOLFOX or CapeOX were detected for MTHFR rs1801133 (31). In female patients, the ERCC1 rs11615 CC genotype worsened grade 3 or more neurological toxicity, as did XPD rs13181G, for example. Genomic effects have rarely been analyzed by sex, but such approaches may reveal sex differences in adverse events in the near future. Leukopenia and neutropenia occurred more often in patients ≥ 70 years of age, according to multivariate analysis. Decreased hematopoietic capacity and proliferation of monoclonal hematopoietic cells in the elderly may affect the higher incidence of leukopenia and neutropenia despite relatively mild SOX/bevacizumab effects on bone marrow suppression (32). Thrombocytopenia after SOX/ bevacizumab was more frequent in patients with Ccr < 70 mL/min and lower BMI 8 weeks from the beginning of chemotherapy. Thrombocytopenia after FOLFOX/ bevacizumab was also more common in patients with a lower Ccr, lower BMI, and aged ≥ 70 years. Cespedes Feliciano et al. reported that a higher proportion of patients in the lowest versus highest tertile of muscle mass experienced neutropenia (55% vs. 38%, P = 0.008) and thrombocytopenia (13% vs. 5%; P = 0.02) (33). Low muscle mass was associated with poor chemotherapy outcomes in that severe adverse events were more likely, either because patients with low muscle mass are over-dosed or because they are more frail or have an older functional age, conferring a higher risk of toxicity. However, no significant sex differences were observed in the incidence of subjective adverse reactions like stomatitis because patients could temporarily stop oral S-1 by themselves, depending on their symptoms, following their education in adequate self-administration routines. This is in contrast to infusions of 5-FU that patients cannot control themselves. The proportions of patients with grade 3 or greater diarrhea was significantly higher in the group given SOX/bevacizumab than in those given FOLFOX/bevacizumab, especially in patients with lower Ccr. On the other hand, there was no sex difference in the incidence of diarrhea.
A significantly more frequent incidence of nausea, vomiting, neutropenia, thrombocytopenia, and alopecia was seen with bolus 5-FU compared with its protracted venous infusion, similar to S-1 in terms of pharmacokinetics (34,35). 5-FU clearance is significantly lower in females than in males regardless of age and the dose given (10). Females receive supra-optimal doses compared with males (36) and higher plasma 5-FU concentrations are significantly related to more severe neutropenia and stomatitis (37). It would be difficult to explain the higher incidence of toxicities of 5-FU by rare DPYD variants. Only 3-5% of Caucasians have reduced DPD activity (38-40) and patients without a DPYD variant resulting in decreased or lack of function may still experience severe toxicity due to additional genetic, environmental, or other factors (41,42).
Although a significantly worse prognosis for poorly-differentiated adenocarcinoma was observed here, similar to previous reports (20,21,43), genomic analyses of somatic mutations or MSI were not carried out in the SOFT trial. Information on tumor location (right or left side) was also not recorded in this trial, other than whether the tumor was in the colon or rectum. Therefore, nothing can be said on this topic. Intensive anti-emetic therapy should be considered at least because of the higher incidence of nausea and vomiting in SOX/ bevacizumab and FOLFOX/bevacizumab-treated female patients. It is difficult to argue for reducing the starting dose of SOX for female patients to compensate for the higher incidence of adverse events compared with males because severe toxicities are rarely induced by SOX. In conclusion, sex differences regarding adverse reactions during treatment with SOX/bevacizumab or FOLFOX/ bevacizumab were confirmed in the SOFT study. This warrants further fundamental research to pursue the underlying cause.
Acknowledgements
This study was funded by Taiho. We wish to thank all the patients, clinicians, and support staff who participated in this study.
Conflict of interest
YY has received honoraria from Taiho, Chugai, Nipponkayaku, Japan. KM has received honoraria from Eli Lilly, Chugai, Takeda, Ono, Taiho, Sanofi, Bristol- Myers Squibb, and Bayer; and research funding from Parexel International, Merck Serono, Daiichi-Sankyo, Sumitomo-Dainippon Pharma, Shionogi, Pfizer, Mediscience Planning, and Solasia Pharma. HB has received honoraria from Taiho and Chugai; and research funding from Taiho, Chugai and Yakult Honsha. KY has received honoraria from Ono, Taiho, Chugai, Bayer, Bristol-Myers Squibb, and Eli Lilly; and research funding from Quintiles MS, NanoCarrier, Eli Lilly, Sumitomo- Dainippon Pharma, Takeda, Taiho, Chugai, MSD, Daiichi-Sankyo, and Ono. TS receives a department support grant from Chugai and Yakult Honsha; honoraria from Taiho and Chugai. MG has received honoraria from Taiho and Chugai. HM has received research funding from Chugai. YS has received honoraria from Yakult Honsha and Taiho. SM has received honoraria from Taiho. NT and TH were employees of Taiho. The other authors declare that they have no conflicts of interest.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019; 144:1941-1953. [DOI] [PubMed] [Google Scholar]
- 2. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004; 22:23-30. [DOI] [PubMed] [Google Scholar]
- 3. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229-237. [DOI] [PubMed] [Google Scholar]
- 4. Yamada Y, Denda T, Gamoh M, et al. S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): a randomized, open-label, phase III, noninferiority trial. Ann Oncol. 2018; 29:624-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamada Y, Tahara M, Miya T, Satoh T, Shirao K, Shimada Y, Ohtsu A, Sasaki Y, Tanigawara Y. Phase I/ II study of oxaliplatin with oral S-1 as first-line therapy for patients with metastatic colorectal cancer. Br J Cancer. 2008; 98:1034-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamada Y, Takahari D, Matsumoto H, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013; 14:1278-1286. [DOI] [PubMed] [Google Scholar]
- 7. Muro K, Boku N, Shimada Y, et al. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol. 2010; 11:853-860. [DOI] [PubMed] [Google Scholar]
- 8. Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, Unemi N, Fukushima M. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996; 56:2602- 2606. [PubMed] [Google Scholar]
- 9. Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, Tatsuta M, Kawasaki T, Satomi T. Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor agent in animal model and in patients with impaired renal function. Cancer Chemother Pharmacol. 2002; 50:25-32. [DOI] [PubMed] [Google Scholar]
- 10. Milano G, Etienne MC, Cassuto-Viguier E, Thyss A, Santini J, Frenay M, Renee N, Schneider M, Demard F. Influence of sex and age on fluorouracil clearance. J Clin Oncol. 1992; 10:1171-1175. [DOI] [PubMed] [Google Scholar]
- 11. Sloan JA, Goldberg RM, Sargent DJ, Vargas-Chanes D, Nair S, Cha SS, Novotny PJ, Poon MA, O'Connell MJ, Loprinzi CL. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol. 2002; 20:1491-1498. [DOI] [PubMed] [Google Scholar]
- 12. Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010; 28:4086-4093. [DOI] [PubMed] [Google Scholar]
- 13. Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J, Kerb R, Blievernicht J, Fischer J, Hofmann U, Bokemeyer C, Eichelbaum M; German 5-FU Toxicity Study Group. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-fluorouracil Toxicity Study Group. J Clin Oncol. 2008; 26:2131-2138. [DOI] [PubMed] [Google Scholar]
- 14. Chansky K, Benedetti J, Macdonald JS. Differences in toxicity between Men and Women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer. 2005; 103:1165-1171. [DOI] [PubMed] [Google Scholar]
- 15. Yamada Y, Koizumi W, Nishikawa K, et al. Sex differences in the safety of S-1 plus oxaliplatin and S-1 plus cisplatin for patients with metastatic gastric cancer. Cancer Sci. 2019; 110:2875-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987; 47:2203-2206. [PubMed] [Google Scholar]
- 17. Rosmarin D, Palles C, Church D, et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis. J Clin Oncol. 2014; 32:1031-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh S, Parulekar W, Murray N, Feld R, Evans WK, Tu D, Shepherd FA. Influence of sex on toxicity and treatment outcome in small-cell lung cancer. J Clin Oncol. 2005; 23:850-856. [DOI] [PubMed] [Google Scholar]
- 19. Liaw CC, Wang CH, Chang HK, Liau CT, Yeh KY, Huang JS, Lin YC. Gender discrepancy observed between chemotherapy-induced emesis and hiccups. Support Care Cancer. 2001; 9:435-441. [DOI] [PubMed] [Google Scholar]
- 20. Shibata J, Kawai K, Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, Hata K, Nozawa H, Kazama S, Yamaguchi H, Ishihara S, Sunami E, Kitayama J, Sugihara K, Watanabe T. Prognostic impact of histology type in curatively resected stage IV colorectal cancer: A Japanese multicenter retrospective study. Ann Surg Oncol. 2015; 22 Suppl 3:S621-629. [DOI] [PubMed] [Google Scholar]
- 21. Kanada M, Oba K, Aoyama T, Kashiwabara K, Mayanagi S, Maeda H, Honda M, Hamada C, Sadahiro S, Sakamoto J, Saji S, Yoshikawa T; Japanese Foundation for Multidisciplinary Treatment of Cancer. Clinical signatures of mucinous and poorly differentiated subtypes of colorectal adenocarcinomas by a propensity score analysis of an independent patient database from three phase III trials. Dis Colon Rectum. 2018; 61:461-471. [DOI] [PubMed] [Google Scholar]
- 22. Kazama Y, Watanabe T, Kanazawa T, Tanaka J, Tanaka T, Nagawa H. Microsatellite instability in poorly differentiated adenocarcinomas of the colon and rectum: relationship to clinicopathological features. J Clin Pathol. 2007; 60:701-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiao H, Yoon YS, Hong SM, Roh SA, Cho DH, Yu CS, Kim JC. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013; 140:341-347. [DOI] [PubMed] [Google Scholar]
- 24. Cristina V, Mahachie J, Mauer M, Buclin T, Van Cutsem E, Roth A, Wagner AD. Association of patient sex with chemotherapy-related toxic effects: a retrospective analysis of the PETACC-3 trial conducted by the EORTC Gastrointestinal Group. JAMA Oncol. 2018; 4:1003-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takeuchi H, Saeki T, Aiba K, et al. Japanese Society of Clinical Oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. Int J Clin Oncol. 2016; 21:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017; 35:3240-3261. [DOI] [PubMed] [Google Scholar]
- 27. Roila F, Warr D, Hesketh PJ, Gralla R, Herrstedt J, Jordan K, Aapro M, Ballatori E, Rapoport B. 2016 updated MASCC/ESMO consensus recommendations: Prevention of nausea and vomiting following moderately emetogenic chemotherapy. Support Cancer Care. 2017; 25:289-294. [DOI] [PubMed] [Google Scholar]
- 28. Nishimura J, Satoh T, Fukunaga M, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled phase 3 trial. Eur J Cancer. 2015; 51:1274-1282. [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020; 21:242-249. [DOI] [PubMed] [Google Scholar]
- 30. Takemoto H, Nishimura J, Komori T, et al. Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea. Int J Clin Oncol. 2017; 22:88-95. [DOI] [PubMed] [Google Scholar]
- 31. Ruzzo A, Graziano F, Galli F, et al. Sex-related differences in impact on safety of pharmacogenetic profile for colon cancer patients treated with FOLFOX-4 or XELOX adjuvant chemotherapy. Sci Rep. 2019; 9:11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019; 366:eaan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH, Xiao J, Castillo AL, Caan BJ. Muscle mass at diagnosis of non-metastatic colon cancer and early discontinuation of chemotherapy, delays and dose reductions on adjuvant FOLFOX: The C-SCANS Study. Cancer. 2017; 123:4868-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, Hill M, Hickish T, Lofts F, Jodrell D, Webb A, Oates JR. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005; 16:549-557. [DOI] [PubMed] [Google Scholar]
- 35. Yamada Y, Hamaguchi T, Goto M, Muro K, Matsumura Y, Shimada Y, Shirao K, Nagayama S. Plasma concentrations of 5-fluorouracil and F-β-alanine following oral administration of S-1, a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, as compared with protracted venous infusion of 5-fluorouracil. Br J Cancer. 2003; 89:816-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaldate RR, Haregewoin A, Grier CE, Hamilton SA, McLeod HL. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. The Oncologist. 2012; 17:296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider M, Etienne MC, Milano G, et al. Phase II trial of cisplatin, fluorouracil, and pure l folinic acid for locally advanced head and neck cancer: a pharmacokinetic and clinical survey. J Clin Oncol. 1995; 13:1656-62. [DOI] [PubMed] [Google Scholar]
- 38. Froehlich TK, Amstutz U, Aebi S, Joerger M, Largiadèr CR. Clinical importance of risk variants in the dihydropyrimidine dehydrogenase gene for the prediction of early-onset fluoropyrimidine toxicity. Int J Cancer. 2015; 136:730-739. [DOI] [PubMed] [Google Scholar]
- 39. Amstutz U, Henricks LM, Offer SM, Barbarino J, Schellens JHM, Swen JJ, Klein TE, McLeod HL, Caudle KE, Diasio RB, Schwab M. Clinical pharmacogenetics implementation consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 update. Clin Pharmacol Ther. 2018; 103:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Etienne MC, Lagrange JL. Dassonville O, Fleming R, Thyss A, Renée N, Schneider M, Demard F, Milano G. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol. 1994; 12:2248-2253. [DOI] [PubMed] [Google Scholar]
- 41. Maekawa K, Saeki M, Saito Y, et al. Genetic variations and haplotype structure of the DPYD gene encoding dihydropyrimidine dehydrogenase in Japanese and their ethnic differences. J Hum Genet. 2007; 52:804-819. [DOI] [PubMed] [Google Scholar]
- 42. Accounting for sex in the genome. Nat Med. 2017; 23:1243. [DOI] [PubMed] [Google Scholar]
- 43. Sasaki Y, Hamaguchi T, Yamada Y, et al. Value of KRAS, BRAF, and PIK3CA Mutations and survival benefit from systemic chemotherapy in colorectal peritoneal carcinomatosis. Asian Pac J Cancer Prev. 2016; 17:539-543. [DOI] [PubMed] [Google Scholar]


