Summary
Immune checkpoint inhibitors have entered clinical practice for the treatment of hepatocellular carcinoma (HCC). Several previous studies for other cancers have revealed that tumor mutation burden, tumor PD-L1 expression and cytotoxic T-cell infiltration are predictive of treatment response. The genetic analysis of HCC has shown that β-catenin mutation might be a biomarker predicting the poor response against immune checkpoint inhibitors. β-catenin is a transcription factor downstream of WNT signaling and somatic mutations of this gene are the third most common in HCC. WNT signaling is an important signal for organogenesis and is also involved in the maintenance of stem cells in several organs. Recently, clinical and basic studies have shown the specific roles of WNT/β-catenin signaling in many aspects of hepatic function and carcinogenesis including metabolic zonation and inflammation, and sub-classification and radiologic features of HCC. Base on the review on the recent advances of research investigating WNT/β-catenin signaling associated with hepatocytes, we speculate the clinical role of this signal on the immunotherapy for HCC, which suggests that an era of genetic mutation profiles may be coming to add to the HCC treatment algorithm.
Keywords: hepatocellular carcinoma (HCC), immune checkpoint inhibitor, WNT, β-catenin
Immune checkpoint inhibitors have entered clinical practice for the treatment of hepatocellular carcinoma (HCC): first-line trials of nivolumab (1) and second-line trials of pembrolizumab (2) initially showed promise of efficacy, but neither of these trials failed to demonstrate statistical efficacy. However, in 2020, the combination of the PD-L1 antibody atezolizumab with the angiogenesis inhibitor VEGF antibody, bevacizumab, showed a significant overall survival benefit in a trial comparing it to sorafenib, which has long played an important role in the treatment of HCC as a first-line treatment (3).
Treatment of other cancers with immune checkpoint inhibitors is controversial because they are very effective in 20-30% of cases and can reduce progression of disease over time, but have little or no effect in some cases. Because of the high cost of the drug, it is required to discover the predictive factor to narrow down the list of patients who might benefit from treatment. The best known predictor of response is the tumor mutation burden (4). Mutation-produced neo-antigens are thought to induce active tumor immunity. Pembrolizumab has been used in patients with MSI-high mutations due to deterioration of mismatch repair enzymes, regardless of the type of cancer. In addition to tumor mutation burden, tumor PD-L1 expression and cytotoxic T-cell infiltration into the tumor tissue may also be predictive of treatment response (5).
The recent genetic analysis of HCC has shown that β-catenin mutation might be a biomarker predicting the poor response against immune checkpoint inhibitors. As WNT/β-catenin signaling has been studied as an important signal for hepatic organogenesis and carcinogenesis, we review recent basic and clinical findings on this signal as a factor that may be relevant to immunotherapy, which is coming soon against advanced HCC.
β-catenin mutation in HCC
Harding et al. performed a clinical sequence of 127 patients with HCC treated with molecularly targeted therapy using NGS. Of the 31 patients treated with immune checkpoint inhibitors, those with β-catenin mutations had a significantly lower DCR (0% vs. 53%) and PFS (2.0 vs. 7.4 months) (6). The first report of β-catenin mutations in HCC was published in 1998, showing that 20-30% of patients carry a genetic mutation in exon 3, which contains a functionally repressive phosphorylation sequence, and that this mutation suppresses β-catenin degradation and leads to increased function (7,8). Comprehensive analysis by next generation sequence has confirmed that it is the third most frequent genetic mutation after hTERT promoter and TP53 (9).
Binding of WNTs to the plasma membrane activates intracellular signaling and β-catenin translocates into the nucleus, where it activates the expression of target genes. In the absence of ligand binding, WNTs are localized in the vicinity of the plasma membrane to form a complex with APCs, which is phosphorylated and then degraded (10). It is well known that APC mutations are highly prevalent in colorectal cancer, but β-catenin mutations are more common in HCC.
WNT/β-catenin signaling in hepatic zonation
WNT signaling has been reported to play an important role in the maintenance of metabolic zonation in hepatic lobes and the formation of liver tissue. It is known that WNT/β-catenin signaling is activated in zone 3 around the central vein, and GS (Glutamine synthetase), which is often used as a marker of zone 3, is a representative target gene for this signal, and many metabolic genes involved in hepatocyte function are among its targets. On the other hands, hepatocytes located in periportal area (zone 1) are initially exposed to nutrients and oxygen supplied from portal veins and hepatic arteries, as well as bacterial and viral pathogens (11). Hepatic zonation is also critical for hepatic regeneration, and the discussion about location of hepatocyte stem cells has been at the center of the debate. Although hepatocyte stem cells have long been thought to be oval cells in the canals of Hering near zone 1, recent research using lineage-tracing experiments of mice has raised the possible liver progenitors of Axin2-positive cells in zone 3 (12). Axin2 is a factor associated with WNT/β-catenin signaling, and it has been shown that WNT ligands from endothelial cells of central vein may be involved in zonation and stem cell maintenance. Recent studies suggest that, while oval cells may be the source of regenerative hepatocytes during liver injury, pericentral cells may be the source of regeneration in the absence of liver injury (13).
WNT/β-catenin signaling in sub-classification and clinical features of HCC
Because the constitutively activation of WNT/β-catenin signaling in HCC confers distinct characteristics, HCC with β-catenin mutation is sub-classified as a single cluster by gene expression profiles. Its clinical characteristics include relatively slow progression and good prognosis. In addition, β-catenin mutation may associate with interesting radiologic feature in clinical settings (14). Gadoxetic acid-enhanced magnetic resonance imaging (EOB-MRI) are frequently applied to HCC high-risk patients because of the high detectability. In this imaging modality, most of HCC show the decreased uptake of EOB comparing with normal liver tissue in the hepatobiliary phase, because the expression of the transporter, OATP1B3 decreases in cancer cells. Rather, a small portion of HCC nodules are reported to uptake more EOB. According to expression and mutation analysis, cancers with β-catenin mutation increase EOB uptake (15,16), and especially increased uptake prove to be due to activation of both HNF4a and β-catenin (17).
WNT/β-catenin signaling associated immunosuppressive phenotype
RNA seq data have been used to classify HCC by gene expression signature involved in immunity. Fujita et al. classified them into four groups, tumor-associated macrophage (TAM), β-catenin, cytolytic activity (CYT), and regulatory T cells (Treg) (18). Shimada et al. also divided them to three groups, including mitogenic and stem cell-like tumors with chromosomal instability, β-catenin-mutated tumors displaying immune suppression, metabolic disease-associated tumors (19). Both reports classified into a single cluster of HCC with β-catenin mutations as immunosuppressive phenotype. Expression analysis showed less infiltration of immune cells, suggesting that the tumors are immunologically cold and may be related to immune checkpoint inhibitor responsiveness. Expression analysis has also been performed in cancers other than HCC, and analysis of expression data for 31 solid tumors from The Cancer Genome Atlas (TCGA) showed that activation of β-catenin was inversely correlated with the expression signature of T-cell-inflamed tumors (20). This has been examined in detail in basic studies in malignant melanoma. Malignant melanoma, the earliest cancer type for which immune checkpoint inhibitors were used clinically (21), has also been found in some of these tumors with β-catenin mutations (22). In a mouse model of carcinogenesis expressing knock-in activated β-catenin, there was less infiltration of T cells and less response to immune checkpoint inhibitors (23). Similarly, in a mouse model of HCC, β-catenin-activated cancers canceled the therapeutic effect of PD-1 antibodies (24). These basic findings may explain the clinical investigation in HCC.
An era of genetic mutation profiles coming to add to the HCC treatment algorithm
WNT/β-catenin is activated in normal hepatocyte around central vein in hepatic lobule and some proportion of cancer cells. In contrast, periportal area, which is more likely to be exposed to external pathogens such as bacteria or virus, may be prepared to induce inflammatory and immune cells, which is easily recalled by the pathological findings that inflammation is located in this area in the cases of viral hepatitis (Figure 1). In addition, inflammatory cells in tumor tissue are often involved in the invasion of cancer, and it is possible that such cancers are more aggressive and have a worse prognosis. On the other hand, immune checkpoint inhibitors may be more effective in cancer tissues with abundant inflammatory cell infiltration. If immune checkpoint inhibitors are less effective in β-catenin mutated cancers that are less invasive and have a relatively good prognosis, then the therapeutic indications for local treatment, such as resection or ablation, may be expanded in these cancers. This suggests that an era of genetic mutation profiles may be coming to add to the HCC treatment algorithm.
Figure 1.
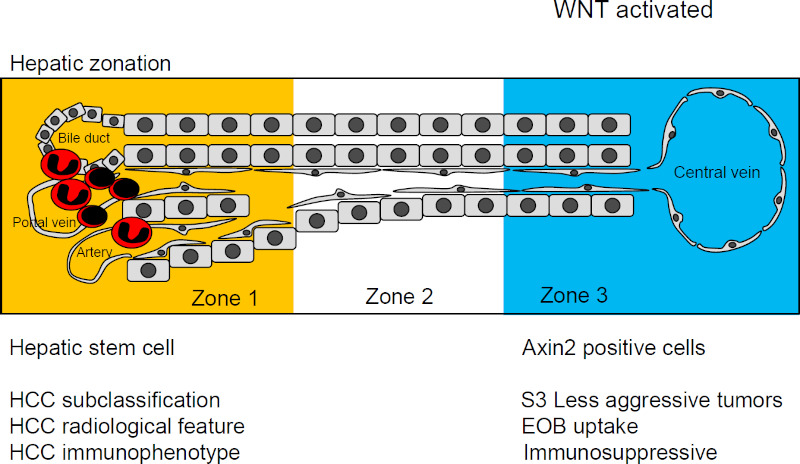
Basic and clinical features associated with WNT activation.
Funding: None.
Conflict of Interest
Y. A. received consulting fees from Eisai Co., Ltd.
References
- 1. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020; 38:193-202. [DOI] [PubMed] [Google Scholar]
- 3. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894-1905. [DOI] [PubMed] [Google Scholar]
- 4. Diaz LA Jr, Le DT. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 373:1979. [DOI] [PubMed] [Google Scholar]
- 5. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015; 372:2018-2028. [DOI] [PubMed] [Google Scholar]
- 6. Harding JJ, Nandakumar S, Armenia J, et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019; 25:2116-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998; 95:8847-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y. Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res. 1998; 58:2524-2527. [PubMed] [Google Scholar]
- 9. Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017; 169:1327-1341.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell JO, Monga SP. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu Rev Pathol. 2018; 13:351-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birchmeier W. Orchestrating Wnt signalling for metabolic liver zonation. Nat Cell Biol. 2016; 18:463-465. [DOI] [PubMed] [Google Scholar]
- 12. Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015; 524:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li W, Li L, Hui L. Cell plasticity in liver regeneration. Trends Cell Biol. 2020; 30:329-338. [DOI] [PubMed] [Google Scholar]
- 14. Goossens N, Sun X, Hoshida Y. Molecular classification of hepatocellular carcinoma: potential therapeutic implications. Hepat Oncol. 2015; 2:371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ueno A, Masugi Y, Yamazaki K, et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol. 2014; 61:1080-1087. [DOI] [PubMed] [Google Scholar]
- 16. Kitao A, Matsui O, Yoneda N, et al. Hepatocellular carcinoma with β-catenin mutation: imaging and pathologic characteristics. Radiology. 2015; 275:708-717. [DOI] [PubMed] [Google Scholar]
- 17. Yamashita T, Kitao A, Matsui O, et al. Gd-EOB- DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology. 2014; 60:1674-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujita M, Yamaguchi R, Hasegawa T, et al. Classification of primary liver cancer with immunosuppression mechanisms and correlation with genomic alterations. EBioMedicine. 2020; 53:102659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimada S, Mogushi K, Akiyama Y, et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine. 2019; 40:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 2019; 25:3074-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013; 369:122-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997; 275:1790-1792. [DOI] [PubMed] [Google Scholar]
- 23. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015; 523:231-235. [DOI] [PubMed] [Google Scholar]
- 24. Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019; 9:1124-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]


