Summary
COVID-19, that emerged in December 2019 in the city of Wuhan, China and is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly evolved into a pandemic. Italy has become one of the largest epicentres outside Asia, accounting now for at least 80,539 infections (cumulative incidence of 95.9/100,000) and 8,165 deaths (case fatality rate 10.1%). It has seriously affected people above the age of 60 years. The International Health Regulations (IHR) revised in 2005 bind governments to disclose vital information regarding the identification and detection of new disease outbreaks regardless of its causative agent. In contrast to the previous SARS epidemic, China timely informed the world about the onset of a new outbreak. It also soon disclosed the clinical characteristics of patients with COVID-19. Unfortunately, despite the fast recognition of the Chinese epidemic, the application of the 2005 IHR was not followed by an effective response in every country and most health authorities failed to rapidly perceive the threat posed by COVID-19. To further complicate matters, IHR implementation, which relies primarily on self-reporting data rather than on an external review mechanism, was limited in speed and further hindered by high costs. The response in Italy suffered from several limitations within the health system and services. The action against this threat must instead be quick, firm and at the highest trans-national level. The solution lies in further strengthening countries' preparedness through a clear political commitment, mobilization of proper resources and implementation of a strict surveillance and monitoring process.
Keywords: COVID-19, SARS-CoV-2, global health, pandemic
COVID-19 pandemic: how it began
Future generations will probably remember 2020 as the year when countries' strategies for epidemic preparedness and response were tested and failed in containing the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 probably spilled over to humans from bats in November 2019 (1,2) and caused a pneumonia outbreak in the Chinese town of Wuhan (Hubei, China) starting in December 2019 (3). Only a few days later, on 10 January 2020, the causative agent was identified and is now known as SARS-CoV-2. Its genome was rapidly sequenced and shared with the global community.
The Chinese government implemented draconian measures to contain the spread of the virus by declaring the lockdown of sixteen towns in the Hubei province (~60 million people) starting from Wuhan on 23 January (4).
Meanwhile, Chinese researchers started to disclose data on clinical characteristics of COVID-19 patients. Age and sex disaggregated crude fatality rates (CFRs), and treatments used were reported. The period of incubation was estimated in the range of 2-14 days (median 4-5 days after exposure) (5). Clinical evidence showed that 80% of those infected develop a flu-like syndrome, or a pauci-symptomatic or even asymptomatic disease; 15% have severe pneumonia; and 5% need support in an intensive care unit (ICU). Overall, the CFR for COVID-19 was described as ranging from 3.0 to 4.0% increasing proportionally with age, pre-existing comorbidities and disease severity. It reached 14.8% in patients aged ≥ 80 years and almost 50% in critical cases admitted to ICU (6).
The transmissibility of this virus, expressed as a basic reproduction number (R0) varying between 2.0 and 3.6 (7-9), was considered similar to that of other respiratory pathogens with spreading possibly favored by the presence of asymptomatic carriers (10,11). Evidence suggests that the virus is transmitted by droplets and can persist for a few hours in the environment on surfaces (11). Unfortunately, in the absence of a vaccine, the current treatment is based on drugs (e.g. lopinavir/ ritonavir, remdesivir, beta-interferon, and chloroquine) developed for different pathogens that still have to prove their efficacy against COVID-19. The mainstay of COVID-19 control is therefore based on the prevention of its spreading through classic public health measures: hand and respiratory hygiene, quarantine of those infected and of contacts of infectious cases and use of personal protective equipment (12).
The role of globalization
The theory of the evolution of virulence in emerging infectious diseases predicts that selection for pathogen virulence and horizontal transmission is higher at the onset of an epidemic but decreases thereafter, as the epidemic depletes the pool of susceptible hosts (13). The 1918 influenza pandemic followed this pattern as it was characterized by three different waves (spring of 1918, fall of 1918, and winter 1918-19), of which the second one was the most virulent. It seemed to have partially protected the previously affected populations and regions from the third wave, which was less severe (14).
However, the Spanish flu pandemic occurred in an era when the world was much less inter-connected with travel that was difficult and expensive. Besides its challenges, globalization brought prosperity as well as the opportunity for people to benefit from the progressive erosion of boundaries and easier transport and communication. Globalization, however, also has a profound impact on the epidemiology of infectious diseases. The traditional patterns of disease spread, and the evolution and conventional responses of health systems was deeply shaped by the dawning of globalization. The continuous increase of the human population and the economic development ongoing in low- and middle-income countries may further exacerbate these dynamics. The phenomena resulting from globalization and unregulated urbanization were already influential in the emergence and spread of some of the most relevant pathogens of the 20th century, such as the agents of SARS, swine flu and Ebola (15), not underestimating how the human immunodeficiency virus itself was favored in its global spread (16).
Globalization of transport and human movement are likely the main reason underpinning the spread of COVID-19 which has infected, to date, at least 413,467 people in more than 150 countries in all continents except Antarctica and has been responsible for 18,433 deaths (CFR of 4.5%) (17).
Increasing the political commitment
Hard lessons were learned following the SARS epidemic in 2003, when the Chinese government delayed key information regarding a novel infectious outbreak in the province of Guangdong (China). After that epidemic, the Member States of the World Health Organization (WHO) revisited the International Health Regulations (IHR) (18) which were eventually adopted by the World Health Assembly in 2005 to be implemented in all countries by mid-2007. The new IHR bind governments to disclose vital information regarding the identification and detection of new disease outbreaks regardless of its causative agent. In this occasion, such mechanism resulted in China publicly and rapidly sharing the information and viral genome of SARS-CoV-2.
Unfortunately, in the case of the COVID-19 pandemic, the application of the 2005 IHR that allowed rapid reporting by China was not followed by an immediate response in every country. Governments failed to rapidly perceive the threat posed by COVID-19. In addition, health systems in some countries were ill-prepared to face an emergency such as COVID-19 despite the commitment made by delegates of all countries in 2005, when approving the revised IHR, and their renewed promise in 2015 at the signing off at the highest political level of the United Nations (UN) Sustainable Development Goals (SDG) (19). SDG 3 (devoted to health), target 3.D specifies that all countries must strengthen their capacity for 'early warning, risk reduction and management of national and global health risks'. Without strong commitment that translates international resolutions into a well-financed plan on health emergencies, countries remain heavily exposed to the risk of pandemics. To oversee countries' strategic preparedness against emergencies two instruments have been developed: the WHO's IHR Core Capacity Monitoring Framework and the Joint External Evaluation process. However, both tools assess the level of coordination between Public Health and Security authorities, but do not measure how interventions are conducted and their effectiveness. To further complicate matters, IHR rely primarily on self-reporting data rather than on an external review mechanism which would be hindered by higher costs and subsequently limited in speed and frequency.
In any case, governments that have not properly prepared are currently struggling in an attempt to build last-minute epidemic responses addressing the COVID-19 pandemic (20,21). They essentially miss a strategic plan for epidemic response ready to be adapted and implemented against the current threat. There is therefore the need to establish proper national preparedness plans and to finance them adequately even if the next epidemic may not appear for some years. Given the easiness with which any epidemic may spread across borders today and the inter-connections existing among countries worldwide, there is an even more urgent need to build an international accountability system that monitors progress by governments in building and financing those plans. This would require regular reporting to the highest possible political level: the UN General Assembly (UNGA).
Lack in epidemic preparedness and delayed response: the case of Italy
Data from the Chinese COVID-19 outbreak, together with the declaration of a Public Health Emergency of International Concern from the WHO's Director-General on 30 January 2020, should have warned governments early on the need to take serious measures to prevent secondary transmission of SARS-CoV-2. However, things happened differently as a second large outbreak of COVID-19 emerged rapidly in Italy on 21 February 2020 (22).
At the beginning, the Italian COVID-19 cases were attributed to a single cluster of transmission occurring mainly in the little town of Codogno (Lodi province, Region Lombardy). The city was rapidly locked down together with another ten neighboring villages. Regrettably, the identification of the very first Italian case of COVID-19 failed as, based on some preliminary evidence, the virus appeared to have been circulating unnoticed in Lombardy since mid-January 2020 (23). If this were indeed the case, it would suggest that the original guidance provided by the health authorities (24) and focused on the recognition of the respiratory syndrome mainly on the basis of recent travels to, or business with, China, or known contact with a case, or the work in a health facility could have misled practitioners preventing them from suspecting COVID-19 in the absence of those conditions. This may have delayed the identification of the epidemic while it was already spreading in the community. Subsequently, efforts to precisely describe the origin of the cluster have been unsuccessful. Meanwhile, the health system and the public services in Lombardy were rapidly placed under serious stress (25) and local and national public health authorities were eventually obliged to make unprecedented decisions to pursue reduction of the R0 of SARS-CoV-2 through progressive restriction of citizens' movements up to a total lockdown of the country regardless of the epidemiology of the disease in different parts of Italy.
At the same time, while the Italian public health authorities at regional and national level tried to cope with the growing epidemic, the highly fragmented health systems resulted in a complex situation that became difficult to manage in the absence of a coherent strategy. For instance, some hospitals, especially those in Lombardy, applied the existing plans for responding to maxi-emergencies. These plans unfortunately are meant mainly for short-term natural disasters rather than for infectious epidemics of the COVID-19 type requiring intensive respiratory support. As a result, the effectiveness of response was compromised and as of now in Italy at least 80,539 have been infected with SARS-CoV-2 (cumulative incidence of 95.9/100,000 population) and 8,165 have died (CFR of 10.1%) (26). Notably, the cumulative incidence of COVID-19 cases increases with age (Figure 1), and mortality starts to rise from those aged 40-49 years old (Figure 2) (27).
Figure 1.
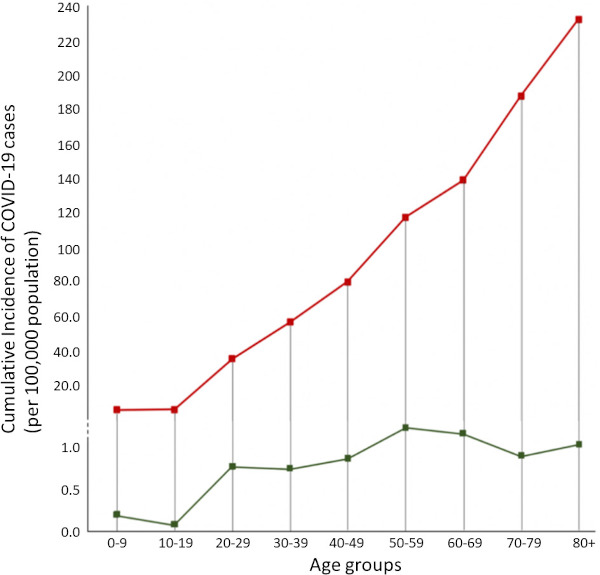
COVID-19 cumulative incidence rates (per 100,000 population) for Italy (red) (27) and Japan (green) (28) per each age group.
Figure 2.
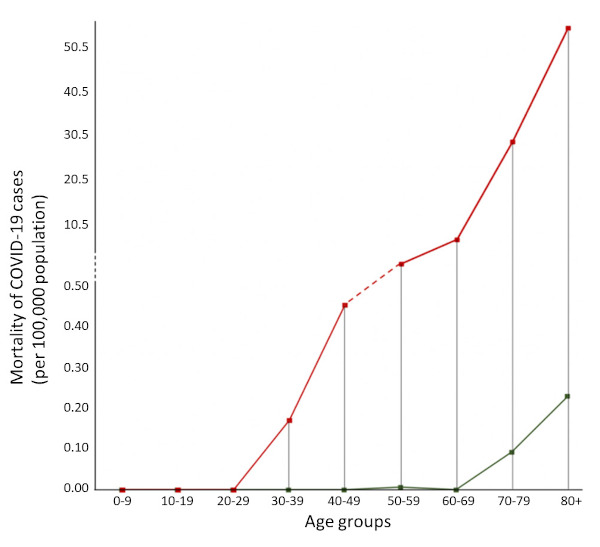
COVID-19 mortality rates (per 100,000 population) for Italy (red) (27) and Japan (green) (28) per each age group.
The Italian epicentre is located in Lombardy (where the CFR overall is 14.9%), and especially in two provinces: Bergamo (7,458 cases) and Brescia (6,931 cases) (Figure 3). In those cities, like in the capital city of Milan, hospitals are gasping due to the sudden waves of hospitalizations and many patients, not solely among the elderly, requiring admission to ICU and mechanical ventilation. Hospital administrations are now struggling to reform their assets by designating facilities dedicated only to COVID-19 patients while maintaining other wards fully COVID-19-free in order to limit nosocomial transmission. In addition, due to exhaustion of critical tools for a respiratory outbreak, such as facemasks and respirators, the government is now trying to urgently import them from other countries, including China.
Figure 3.
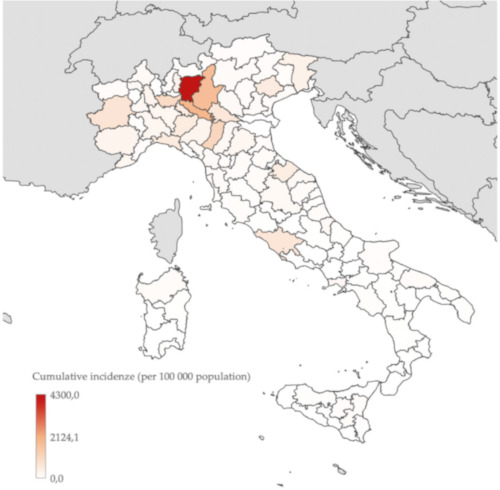
COVID-19 cumulative incidence (per 100,000 population) in Italy by province (n = 67,044), 26 March 2020 (26).
The omen of a large scale COVID-19 epidemic, from its epicentre in China, was almost immediately high due to the presence of an international airport nearby. The first imported cases of COVID-19 were soon documented in Japan (CFR, cumulative incidence, and mortality are reported in Table 1, Figures 1 and 2, respectively) (28), Republic of Korea, and Thailand. Nevertheless, political inaction together with the high transmissibility of SARS-CoV-2 have resulted in emergence of multiple clusters of local transmission worldwide. Hence, the WHO definition of COVID-19 as a pandemic on 11th of March 2020 also in the hope to shake the mind of political authorities and motivate governments to ensure people adhere to the international and national policies in all affected countries (29).
Table 1. COVID-19 reported deaths and case-fatality rates (CFRs) in Italy and Japan.
| Items | Italy (27) |
Japan (28) |
||
|---|---|---|---|---|
| Reported deaths, n (%) | CFR, % | Reported deaths, n (%) | CFR, % | |
| Age | ||||
| 0-9 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 |
| 10-19 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 |
| 20-29 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 |
| 30-39 | 12 (0.2) | 0.3 | 0 (0.0) | 0.0 |
| 40-49 | 41 (0.8) | 0.6 | 0 (0.0) | 0.0 |
| 50-59 | 168 (3.3) | 1.5 | 1 (2.3) | 0.5 |
| 60-69 | 541 (10.8) | 5.2 | 0 (0.0) | 0.0 |
| 70-79 | 1,768 (35.2) | 15.6 | 15 (34.9) | 9.2 |
| 80+ | 2,488 (49.6) | 23.7 | 26 (60.5) | 21.0 |
| ND | 1 (0.0) | - | 1 (2.3) | - |
| Total | 5,019 (100.0) | 8.7 | 43 (100.0) | 4.0 |
Conclusions
The COVID-19 pandemic will have epochal economic, social and cultural consequences and a dramatic toll of human lives, but lessons can already be learned. Events like the current one will occur again in the future, will have unpredictable characteristics, and will represent a major threat for all countries from a health, an economic, and a social perspective. The response against this threat must be quick, firm and at the highest trans-national level. A possible solution lies in further strengthening countries' preparedness by obtaining the political commitment required and implementing a strict monitoring process. This, for example, could imply the establishment of a mechanism that ensures reporting of progress at the level of the UNGA where heads of States make decisions, rather than limiting accountability to often powerless and ill-financed health ministries. Whatever the mechanism, innovative thinking is necessary to face health emergencies effectively. SARS-CoV-2 is a pandemic teaching us once more that microorganisms do not respect borders and spread quickly thus having the potential to disrupt economies and societies as a whole.
Acknowledgements
This paper is dedicated to all those people who are bravely fighting the SARS-CoV-2.
References
- 1. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020. 579: 265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai A, Bergna A, Acciarri C, Galli M, Zehender G. Early phylogenetic estimate of the effective reproduction number of 2019-nCoV. J Med Virol. 2020; doi: 10.1002/jmv.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wuhan Municipal Health Commission. Briefing on the pneumonia epidemic situation, 31 December 2019. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989 (accessed March 12, 2020).
- 4. The New York Times. Coronavirus death toll climbs in China, and a lockdown widens. The New York Times, Januray 23, 2020. https://www.nytimes.com/2020/01/23/world/asia/china-coronavirus.html (accessed March 23, 2020).
- 5. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; doi:10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020; 382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, Lou Y, Gao D, Yang L, He D, Wang MH. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020. 92:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020. 395: 689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020; 382:970-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020; 27 pii: taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: interim guidance. Geneva: World Health Organization 2020. https://apps.who.int/iris/handle/10665/330674 (accessed March 25, 2020).
- 13. Berngruber TW, Froissart R, Choisy M, Gandon S. Evolution of virulence in emerging epidemics. PloS Pathog. 2013; 9:e1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patterson KD, Pyle GF. The geography and mortality of the 1918 influenza pandemic. Bull Hist Med.1991; 65:4-21. https://www.jstor.org/stable/44447656 (accessed March 25, 2020). [PubMed] [Google Scholar]
- 15. Suzuki Y, Gojobori T. The origin and evolution of Ebola and Marburg viruses. Mol Biol Evol. 1997; 14:800-806. [DOI] [PubMed] [Google Scholar]
- 16. Sharp PM, Hahn BH. The evolution of HIV-1 and the origin of AIDS. Philos Trans R Soc B Biol Sci. 2010; 365:2487-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 65. Geneva: World Health Organization 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200325-sitrep-65-covid-19.pdf?sfvrsn=ce13061b_2 (accessed March 25, 2020).
- 18. World Health Organization. The International Health Regulations (2005): third edition. Geneva: World Health Organization 2005. https://apps.who.int/iris/bitstream/handle/10665/246107/9789241580496-eng.pdf?sequence=1 (accessed March 25, 2020).
- 19. United Nations General Assembly. Transforming our world: the 2030 Agenda for Sustainable Development. Resolution A/RES/70/1. New York: United Nations 2015. https://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed March 25, 2020).
- 20. The Economist. COVID-19 - The politics of pandemics. The Economist, Mar 12, 2020. https://www.economist.com/leaders/2020/03/12/the-politics-of-pandemics (accessed March 17, 2020).
- 21. The Lancet. COVID-19: too little too late? Lancet. 2020; 395:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 33. Geneva: World Health Organization 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200222-sitrep-33-covid-19.pdf?sfvrsn=c9585c8f_4 (accessed March 25, 2020).
- 23. Devlin H, Beaumont P, Tondo L, Burke J. Coronavirus may have been in Italy for weeks before it was detected. The Guardian Feb 28, 2020. https://www.theguardian.com/world/2020/feb/28/coronavirus-may-have-been-in-italy-for-weeks-before-it-was-detected (accessed March 17, 2020).
- 24. Ministero della Salute, Direzione Generale della Prevenzione Sanitaria. Circolare del 27.01.2020: Polmonite da nuovo coronavirus (2019-nCoV) in Cina. http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=72847&parte=1%20&serie=null (accessed March 25, 2020).
- 25. Grasselli G, Pesenti A, Cecconi M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA, 2020; doi:10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 26. Data from Dipartimento della Protezione Civile: COVID-19 Italia - Monitoraggio della situazione. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1 (accessed March 26, 2020).
- 27. Data from Istituto Superiore di Sanità. https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati (accessed March 23, 2020).
- 28. Data from the Japanese Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/english/ (accessed March 25, 2020).
- 29. World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed March 18, 2020).


